Zanamivir nasal in situ jellies with phase variation property and preparing method thereof
A technology of zanamivir and phase transition, which is applied in the direction of medical preparations containing non-active ingredients, medical preparations containing active ingredients, organic active ingredients, etc., which can solve the problems of low bioavailability, fast renal clearance, and drug clearance. Too fast and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
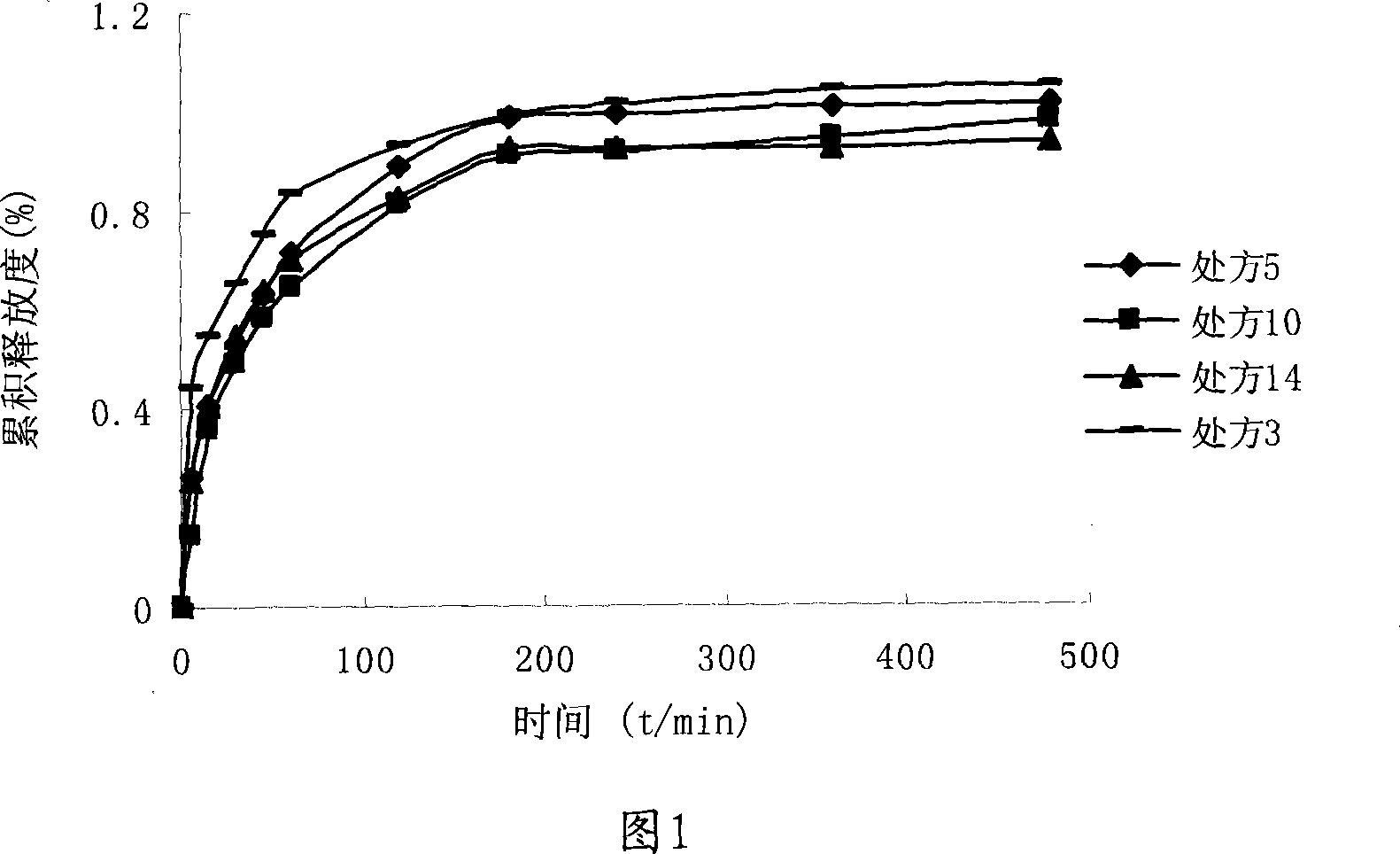
Image
Examples
Embodiment 1
[0022] Prepare ion-sensitive zanamivir nasal in situ gel, the prescription is as follows (see Table 1):
[0023] Table 1 ion-sensitive zanamivir nasal in situ gel prescription (wt%)
[0024] Prescription 1
Prescription 2
Prescription 3
Prescription 4
sodium alginate
deacetylated gellan gum
osmotic regulator
preservatives
pH regulator
Deionized water
1.2
6
Glycerin 1.8
Chlorobutanol 0.5
Adjust pH to 6.2 with HCL
margin
1.6
0.8
Sorbitol 4.5
Thimerosal 0.01
Adjust pH to 6.2 with HCL
margin
1.7
0.3
Glycerin 2
Chlorobutanol 0.5
Adjust pH to 6.2 with HCL
margin
1.1
1.5
Mannitol 5
Ethylparaben 0.02
Adjust pH to 6.2 with HCL
margin
[0025] Preparation Process:
[0026] The above ion-sensitive polymer was placed in an appropriate amount of deionized water, ...
Embodiment 2
[0032] Prepare temperature-sensitive zanamivir nasal in situ gel, the prescription is as follows (see Table 2):
[0033] Table 2 temperature-sensitive zanamivir nasal in situ gel prescription (wt%)
[0034] Prescription 5
Prescription 6
Prescription 7
Prescription 8
Poloxamer 407
Poloxamer 188
polyethylene glycol (molecule
Volume 6000~
11000)(PEG)
polyoxyethylene
(PEO)
prime (HPMC)
osmotic regulator
preservatives
pH regulator
Deionized water
1.6
20
5
2
---
---
NaOH adjustment
pH to 6.5
margin
1.6
35
---
---
---
2
Sorbitol 4.5
0.5
Regulate pH to 6.5
margin
1.6
---
28
...
Embodiment 3
[0040] Prepare pH sensitive zanamivir nasal in situ gel, the prescription is as follows (see Table 3):
[0041] Table 3 pH-sensitive zanamivir nasal in situ gel prescription (wt%)
[0042] Prescription 9
Prescription 10
Prescription 11
Zanamivir
Cellulose Acetate Phthalate (CAP)
Kabop 934
Hypromellose (HPMC)
Glyceryl Monooleate (GMO)
osmotic regulator
preservatives
1.6
30
---
---
0.5
---
Glycerin 1.5
Benzoic acid 0.02
1.6
---
0.8
---
1
---
Glycerin 2
Chlorobutanol 0.5
1.6
---
3
---
3
Mannitol 4.5
Thimerosal 0.01
[0043] pH regulator
Deionized water
Adjust pH to 4.0 with HCL
margin
Adjust pH to 4.0 with HCL
margin
Adjust pH to 4.0 with HCL
margin
[0044] Preparation:
[0045] Dissolve the pH-sensitive gel material...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com