Patents
Literature
96 results about "Chlorobutanol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chlorobutanol (trichloro-2-methyl-2-propanol) is a preservative, sedative, hypnotic and weak local anesthetic similar in nature to chloral hydrate. It has antibacterial and antifungal properties. Chlorobutanol is typically used at a concentration of 0.5% where it lends long term stability to multi-ingredient formulations. However, it retains antimicrobial activity at 0.05% in water. Chlorobutanol has been used in anesthesia and euthanasia of invertebrates and fishes. It is a white, volatile solid with a menthol-like odor.
Medicinal preparation containing exenatide
The invention provides a medicinal preparation containing exenatide suitable for multi-administration, which contains exenatide, buffer solution, pharmaceutically acceptable accessory and preservative, wherein the buffer solution can keep the pH value of the preparation in an aqueous solution state at 3.0 to 7.0; the accessory may be one or combination of glucose, sucrose, methionine, mannitol or glycine; and the preservative is selected from benzoic acid, sodium benzoate, potassium sorbate or acetone chloroform. The medicinal preparation has the advantages that the stability of physicochemical and biological activities of the exenatide is enhanced by adding a few components capable of being accepted by the human body, and then a preparation suitable for clinical use, particularly injection use is prepared.
Owner:HANGZHOU JIUYUAN GENE ENG
Preparation for Iontophoresis
InactiveUS20090163597A1Low absorption rateStable materialBiocideAntipyreticElectrical driveBiomedical engineering
A preparation used for iontophoresis in order to absorb a physiologically active substance via the skin or mucosa using electrical driving force and the preparation containing a local anesthetic, epinephrine or its salt, water and chlorobutanol.
Owner:TEIKOKU SEIYAKU KK TEIKOKU SEIYAKU CO LTD
Eye cyclosporin gel
InactiveCN1456350ANot easy to diluteGood water solubilitySenses disorderPharmaceutical delivery mechanismCyclosporinsWhole body
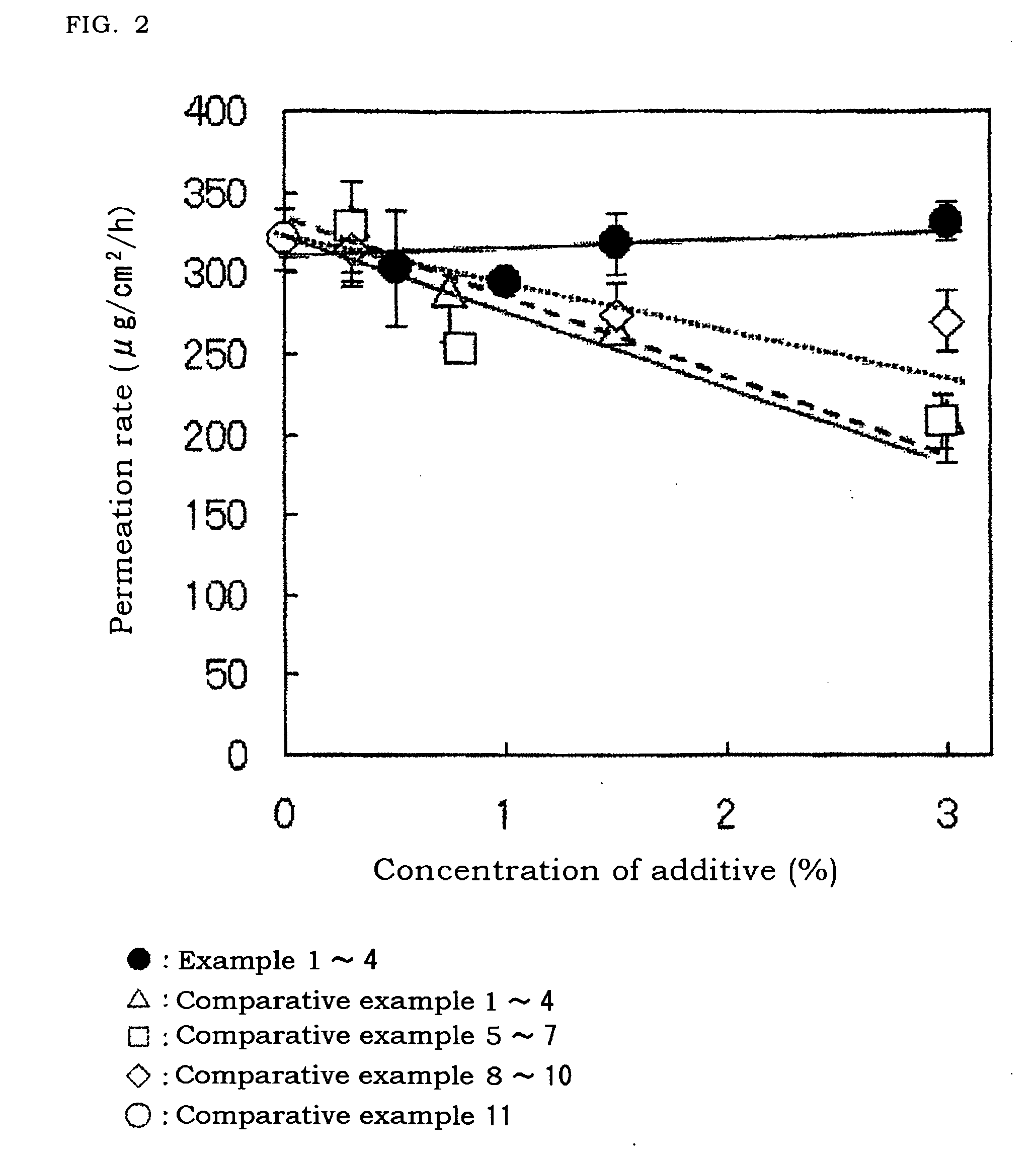
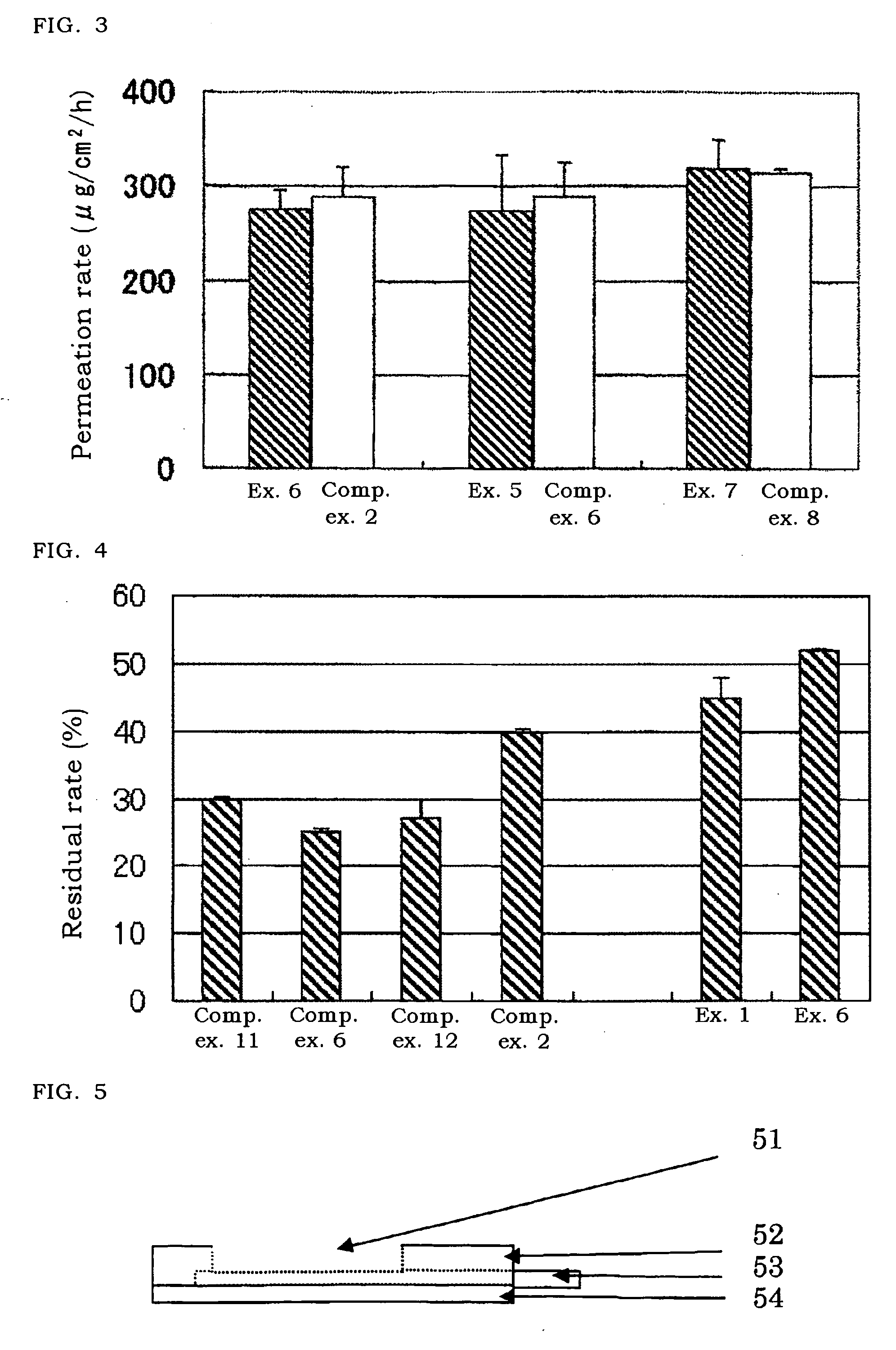
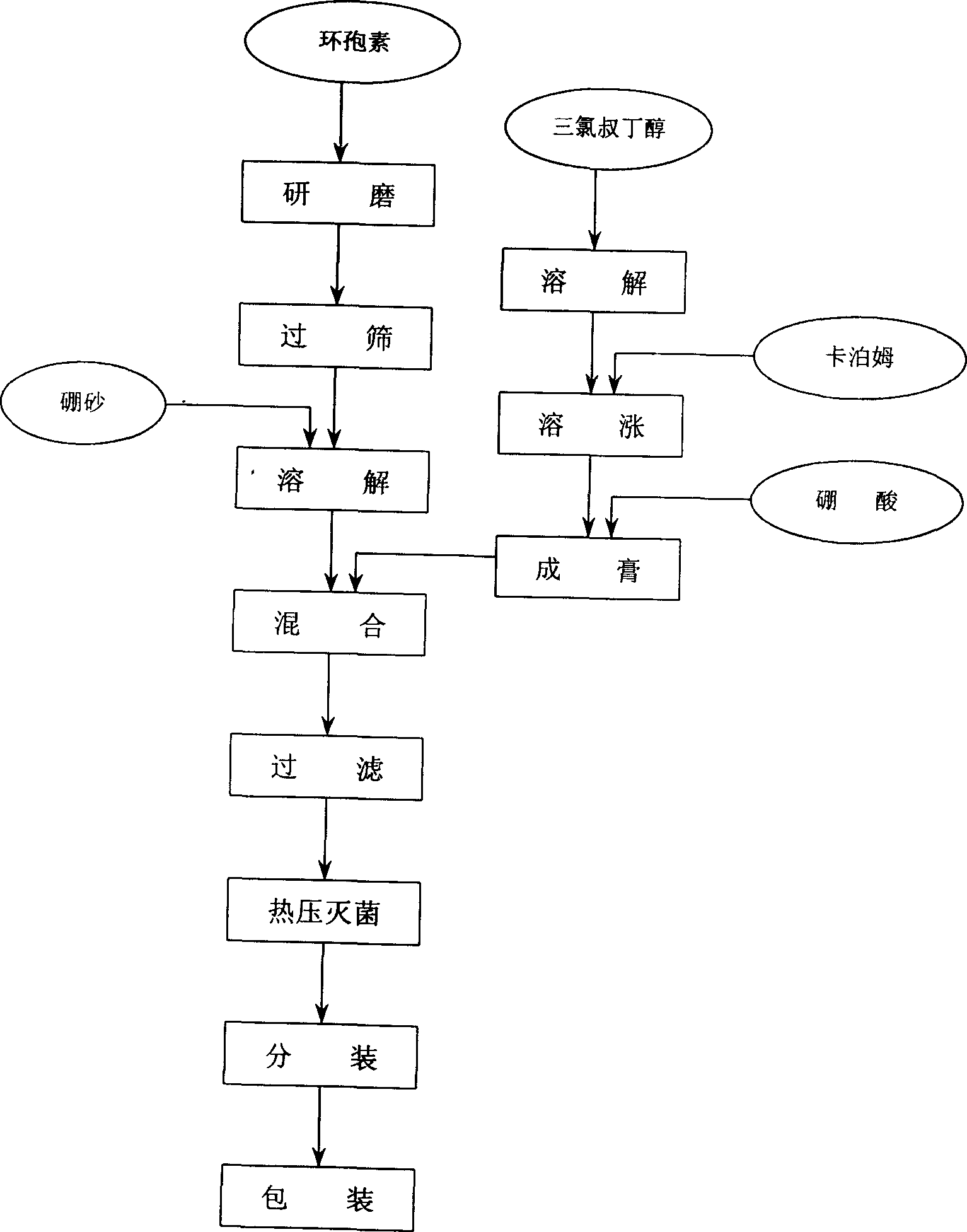
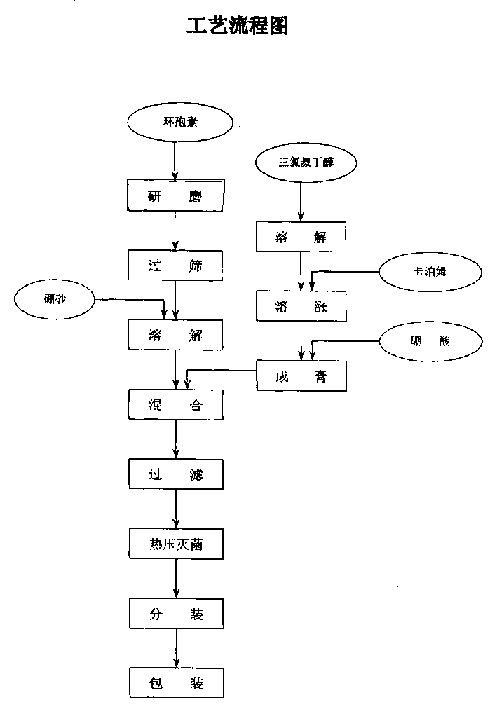
A cyclosporin eye gel for treating the rejection reaction of corneal transplantation, keratoconjunctival xerosis, catarrhal conjunctivitis, and chemical burn of eye is prepared from cyclosporin, boric acid, borax, trichloro-tert-butanol, etc. Its advantages are long stay time in eye and high curative effect.
Owner:刘继东
Preservative-containing virus formulations
InactiveUS20070148765A1Negligible lossHigh antibacterial activityMicrobiological testing/measurementPharmaceutical delivery mechanismViral VaccineGene
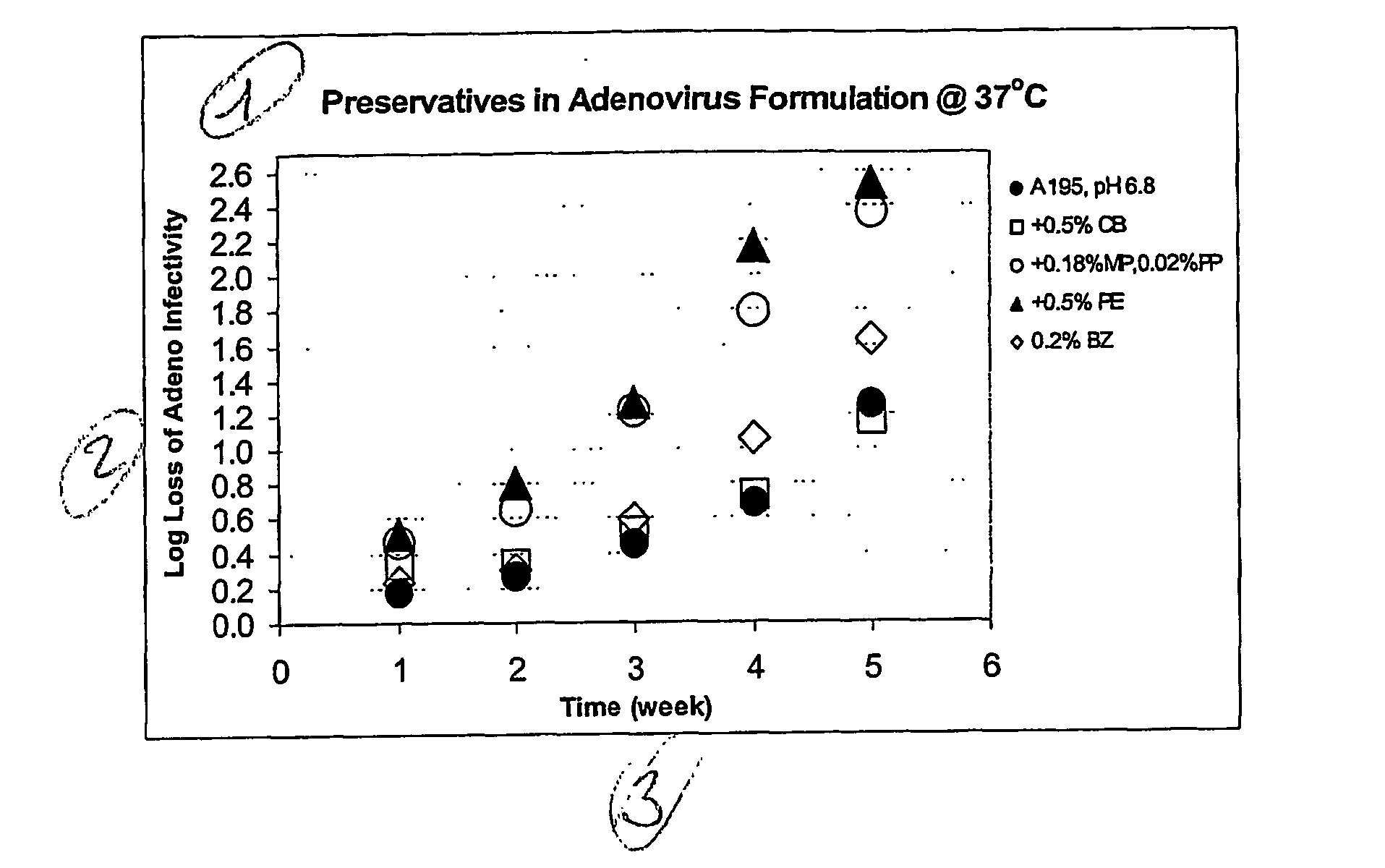
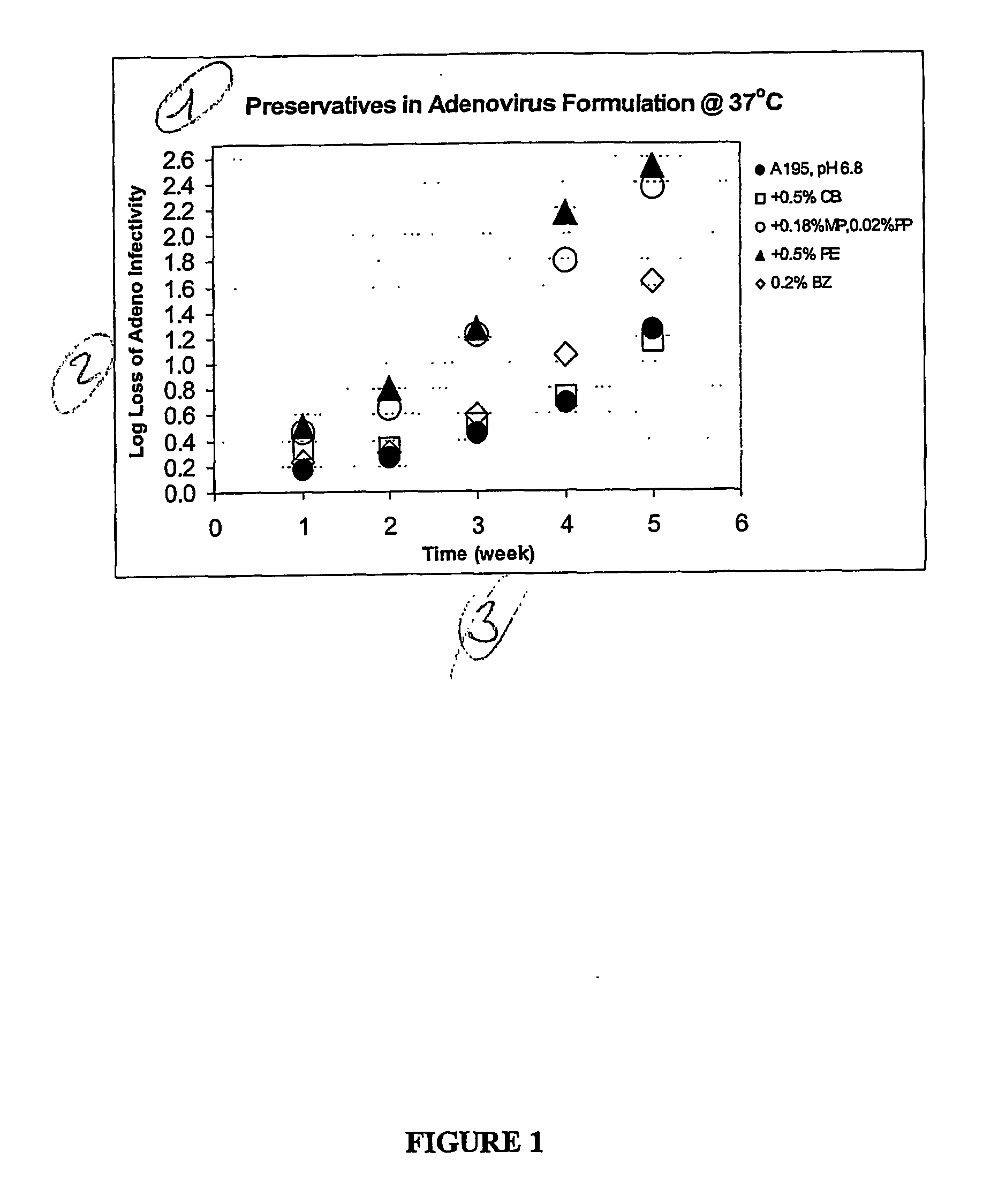
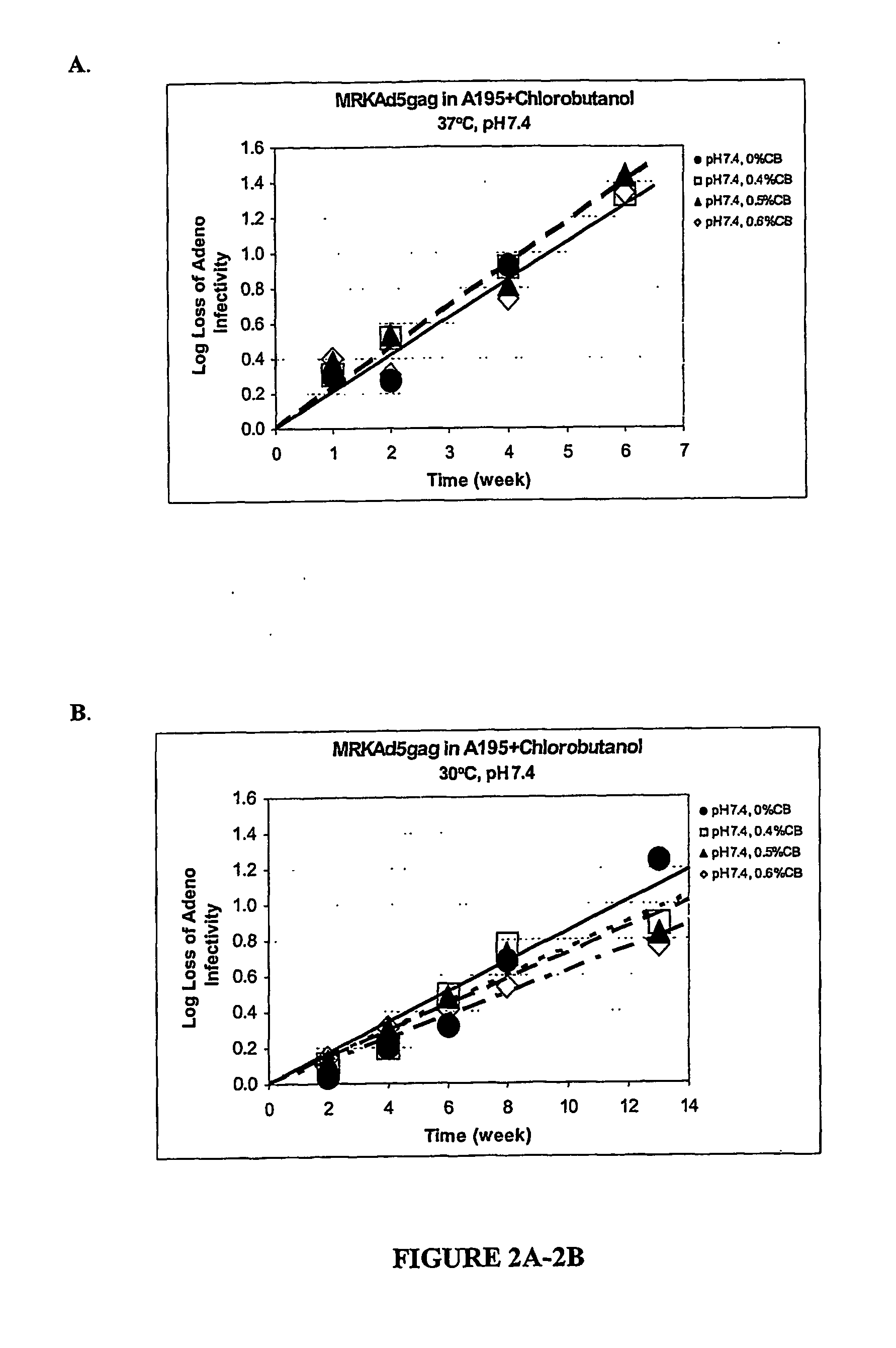
The preservation of live viral vaccines is disclosed. These liquid formulations comprise a live virus and a preservative, namely chlorobutanol. The preserved, live virus formulations of the present invention are (1) suitable for a vaccine or gene therapy product with a multi-dose image; (2) compatible with parenteral administration; and (3) are stable for extended periods of time with negligible loss of activity.
Owner:MERCK SHARP & DOHME CORP
Film-spraying agent for treating acute soft tissue injuries
InactiveCN102614287ASave resourcesIncrease profitHydroxy compound active ingredientsAntipyreticCellulosePyrrolidinones
The invention relates to an external preparation made from Chinese herbal medicines, specifically to a film-spraying agent for treating acute soft tissue injuries. 1000ml of the film-spraying agent in the invention is composed of the following raw materials: a Chinese herbal extract extracted from 20-60g of rheum officinale, 20-60g of Cacumen Platycladi, 10-30g of Cortex Phellodendri amurensis, 10-30g of Herba Lycopi and 10-30g of mint by ethanol, 2-6g of borneol, 0.4-1.2g of menthol, 2-6g of chlorobutanol, 30-60g of polyvinylpyrrolidone, 1-8g of hydroxypropyl methyl cellulose, and the rest ethanol water with a volume concentration of 30-40%. During application, the film-spraying agent of the invention is directly sprayed on the injured part and can form a film in 8 minutes. With good anti-inflammation, pain easing, stasis removing, acute soft tissue injury improving and microcirculation improving, as well as antibacterium effects, the film-spraying agent provided in the invention has an obvious effect in treating acute soft tissue injuries such as sprains, contusions and other local injuries, and redness, swelling, heat, pain, wound surface infection, as well as other aspects.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
Preparation method of ceftiofur long-acting injector
InactiveCN101416968AReduce stress responseGood curative effectAntibacterial agentsOrganic active ingredientsHalf-lifeTherapeutic effect
A ceftiofur long acting injection consists of the following substances: 2.5g to 5.0g of ceftiofur crude drugs, 0.5g to 3.0g of span 80, 0.1g to 0.3g of Vitamin E, 0.2g to 0.5g of aluminium stearate, 0.25g to 0.5g of chlorobutanol and soybean oil. The preparation method of the ceftiofur long acting injection comprises the following steps: (1) smashing the ceftiofur crude drugs till the particle size of the ceftiofur crude drugs is 5 Mum; (2) adding the span 80 into a mortar for grinding till the span 80 becomes even; (3) then, adding the aluminium stearate, the Vitamin E and the chlorobutanol into the mortar together; and (4) moving the mixture into a measuring vessel and adding the soybean oil into the measuring vessel till the total volume of the substances in the measuring vessel reaches 100ml. The ceftiofur long acting injection has the advantages of obvious therapeutic effect, long half-life period, less adverse reaction, reducing medication times, saving manual labor and time and the like.
Owner:PU LIKE BIO ENG
Stabilized thrombin compositions
ActiveUS8071090B2Peptide/protein ingredientsPharmaceutical non-active ingredientsSucroseThrombin activity
Owner:BAXTER INT INC
High-concentration preparation of soluble thrombomodulin
InactiveUS20060083733A1Improve stabilityHardly painAntibacterial agentsPowder deliveryHigh concentrationFreeze-drying
In preparing a soluble thrombomodulin solution having a concentration of as high as 10 mg / mL or above, foam-inhibiting effect can be attained by any method selected from among (a) incorporation of a nonionic surfactant, benzyl alcohol or chlorobutanol, (b) application of silicone coating on the inner wall of the vessel to be used in dissolving the freeze-dried preparation, and (c) evacuation of the vessel in dissolving the freeze-dried preparation. Further, a soluble thrombomodulin freeze-dried preparation excellent in stability is also provided which can be dissolved in 0.1 to 2 mL of an aqueous solution for dissolution to give a soluble thrombomodulin solution having a concentration of as high as 10 mg / mL or above and exhibiting an osmotic pressure ratio of 0.5 to 2.0.
Owner:ASAHI KASEI PHARMA
Ophthalmic composition for contact lens
Disclosed are; a method for suppressing adsorption of a refreshing agent and / or chlorobutanol by a contact lens in an aqueous ophthalmic composition for contact lens containing a refreshing agent and / or chlorobutanol, as well as for suppressing pH decline due to degradation of chlorobutanol, wherein the method comprises preparing the composition in the form of an oil-in-water type emulsion. An ophthalmic composition for contact lens in the form of an oil-in-water type emulsion containing a refreshing agent and / or chlorobutanol is also disclosed.
Owner:SENJU PHARMA CO LTD
Medicinal preparation containing exenatide
The invention provides a medicinal preparation containing exenatide suitable for multi-administration, which contains exenatide, buffer solution, pharmaceutically acceptable accessory and preservative, wherein the buffer solution can keep the pH value of the preparation in an aqueous solution state at 3.0 to 7.0; the accessory may be one or combination of glucose, sucrose, methionine, mannitol or glycine; and the preservative is selected from benzoic acid, sodium benzoate, potassium sorbate or acetone chloroform. The medicinal preparation has the advantages that the stability of physicochemical and biological activities of the exenatide is enhanced by adding a few components capable of being accepted by the human body, and then a preparation suitable for clinical use, particularly injection use is prepared.
Owner:HANGZHOU JIUYUAN GENE ENG
Synthesis method of high-purity 1,4-butane sultone
ActiveCN109293625AImprove the mixing effectShorten the sulfonation reaction timeOrganic chemistrySynthesis methodsFiltration
The invention discloses a synthesis method of high-purity 1,4-butane sultone. The synthesis method comprises the steps: 1, adding 4-chlorobutanol and a sodium sulfite solution into an alcohol solvent,raising the temperature until carrying out reflowing for 6 h, ending the reflowing to obtain a mixed solution A, concentrating the mixed solution A to recover the alcohol solvent, then, adding hydrochloric acid for acidification, carrying out concentration until a material becomes viscous, then, adding the alcohol solvent, separating out a sodium chloride crystal, carrying out filtration, and concentrating filtrate to recover the alcohol solvent so as to obtain 4-hydroxybutane sulfonic acid; 2, carrying out continuous flash evaporation dehydration on 4-hydroxybutane sulfonic acid at the vacuum degree of 1-8 mmHg and the temperature of 130-165 DEG C to obtain industrial-grade 1,4-butane sultone; and 3, adding an azeotrope into industrial-grade 1,4-butane sultone, carrying out normal-pressure fractional distillation to recover the azeotrope, then, carrying out reduced-pressure fractional distillation at the vacuum degree of 2-4 mmHg, and collecting fractions with the temperature of 120-121 DEG C to obtain high-purity 1,4-butane sultone. The method is simple and environment-friendly, the sulfonation yield is greatly increased, and the purity and yield of 1,4-butane sultone are greatly increased.
Owner:JINGCHU UNIV OF TECH
Corrosion-inhibiting transformer oil added with red phosphorus fire retardant and preparation method thereof
ActiveCN104130826AAvoid safety hazardsDelay the speed of oxidation and corrosionAdditivesSodium lactateAcrylic resin
Disclosed corrosion-inhibiting transformer oil added with a red phosphorus fire retardant is characterized by being prepared from the following raw materials in parts by weight: 1000-1500 parts of cycloalkyl base oil, 3-5 parts of aluminium nitride, 0.3-0.5 parts of 2,6-dimethylmorfolin, 0.2-0.5 parts of acrylic resin, 0.2-0.5 parts of a red phosphorus fire retardant, 0.3-0.5 parts of dodecenylsuccinic anhydride, 0.2-0.5 parts of benzotriazole, 0.2-0.5 parts of sodium lactate, 0.2-0.4 parts of chlorobutanol, and 0.4-0.8 parts of an auxiliary agent. By adding the red phosphorus fire retardant into the transformer oil, safety hidden trouble caused by inflammable property of transformer oil is effectively solved. Added dodecenylsuccinic anhydride helps to delay the oxidation corrosion speed of the transformer oil. The added auxiliary agent enables the transformer oil to be clarified, free of impurities, reduced in oxidation property, enhanced in stability, and prolonged in service life.
Owner:宝之川电气设备有限公司
Stabilized thrombin compositions
ActiveUS20080311104A1Peptide/protein ingredientsPharmaceutical non-active ingredientsSucroseThrombin activity
Stabilized thrombin compositions, processes for preparing them, and kits comprising them are disclosed. The compositions comprise thrombin, a bacteriostatically effective amount of benzyl alcohol or chlorobutanol, and 0.10%-5.0% (w / v) sucrose in aqueous solution. The compositions are stable when stored at 2° C.-8° C. for four weeks or more.
Owner:BAXTER INT INC
Cream composition and paper comprising same
The invention provides a cream composition which comprises bacteriostatic agents, glycerin and deionized water. The mass ratio of the bacteriostatic agents to the glycerin to the deionized water is 0.1-22:10-97:3-9. The bacteriostatic agents are at least one type of polyhexamethylene guanidine hydrochloride, polyhexamethylene biguanidine hydrochloride, iodopropynyl butylcarbamate, 2-methyl-4-amino-6-methoxy s-triazine, benzalkonium bromide, chloretone, sulfoacid pyrimidine sodium, diclofenac sodium, chlorhexidine and myristylpicolinum bromide. The invention further provides paper comprising the cream composition. The cream composition is formed on the outer surface of the paper, so that the paper realizes moisture retention, sterilization and bacteriostasis effects when used for wiping the skin of a user, and feels comfortable and soft.
Owner:GOLD HONG YE PAPER
Eye sterile suspension containing loteprednol etabonate and preparation method thereof
The invention relates to an ophthalmic sterile suspension containing loteprednol etabonate, which contains 0.5 percent of loteprednol etabonate minicrystal particles and takes 0.3 percent to 0.8 percent of chlorobutanol as a preservative, 0.1 percent to 0.3 percent of tween-80 as a surface active agent, 0.1 percent to 0.5 percent of hydroxypropylmethyl cellulose as a suspending agent, 0.03 percent to 0.08 percent of natrium adetate as a metal ion complexing agent, and glycerin and sodium chloride as an osmotic pressure regulator. The ophthalmic sterile suspension is prepared by respectively adopting different sterilization methods to the loteprednol etabonate and other components. Apart from the components, the ophthalmic sterile suspension also contains 0.3 percent of tobramycin.
Owner:马晶
Detection method for simultaneously determining various antibacterial agents in eye drops
The invention discloses a detection method for simultaneously determining various antibacterial agents in eye drops. Detection of eleven antibacterial agents (comprising chlorhexidine, benzyl alcohol, chlorobutanol, sorbic acid, benzoic acid, methyl p-hydroxybenzoate, ethyl p-hydroxybenzoate, propyl p-hydroxybenzoate, thimerosal, benzalkonium chloride and benzalkonium bromide) belonging to six types in the eye drops can be completed only through one-time experiment by adopting the method. The method adopting liquid chromatography has the advantages of good specialty, accurate qualitative and quantitative effects, high quantity of types and kinds of the simultaneously detected antibacterial agents, fast detection speed, suitableness for different brands of eye drops produced in different manufacturers, wide application range, and stable and reliable effect.
Owner:SHANGHAI INST FOR FOOD & DRUG CONTROL
Method for preparing capsaicin carrier
InactiveCN1827171AGood dispersionFinely dispersedOrganic active ingredientsAntipyreticAlcoholSide effect
This invention discloses a method for preparing capsaicin carrier. This method dissolves proper ratios of lecithin, sodium cholate, vitamin E and capsaicin with straight alcohol, embed the stator-rotor head of high-shearing dispersion uniformly emulsion machine into 45deg C aqueous solution( containing 0.5% 3-chloroisobutol), turn on the high-shearing dispersion uniformly emulsion machine, then drip the alcohol solution into the aqueous solution slowly from near stator-rotor head part, hold 45deg C, magnetic stirrer for 1 hour, the capsaicin carrier is obtained. This invention uses high-shearing dispersion uniformly emulsion machine to prepare capsaicin carrier, the equipment greatly reduces equipment cost (needs 6 thousand RMB only) and depresses preparation costú”At the same time, the prepared capsaicin carrier vesicle debased the toxic side effect in using and has good skin passing function.
Owner:广州玑本宫贸易有限公司
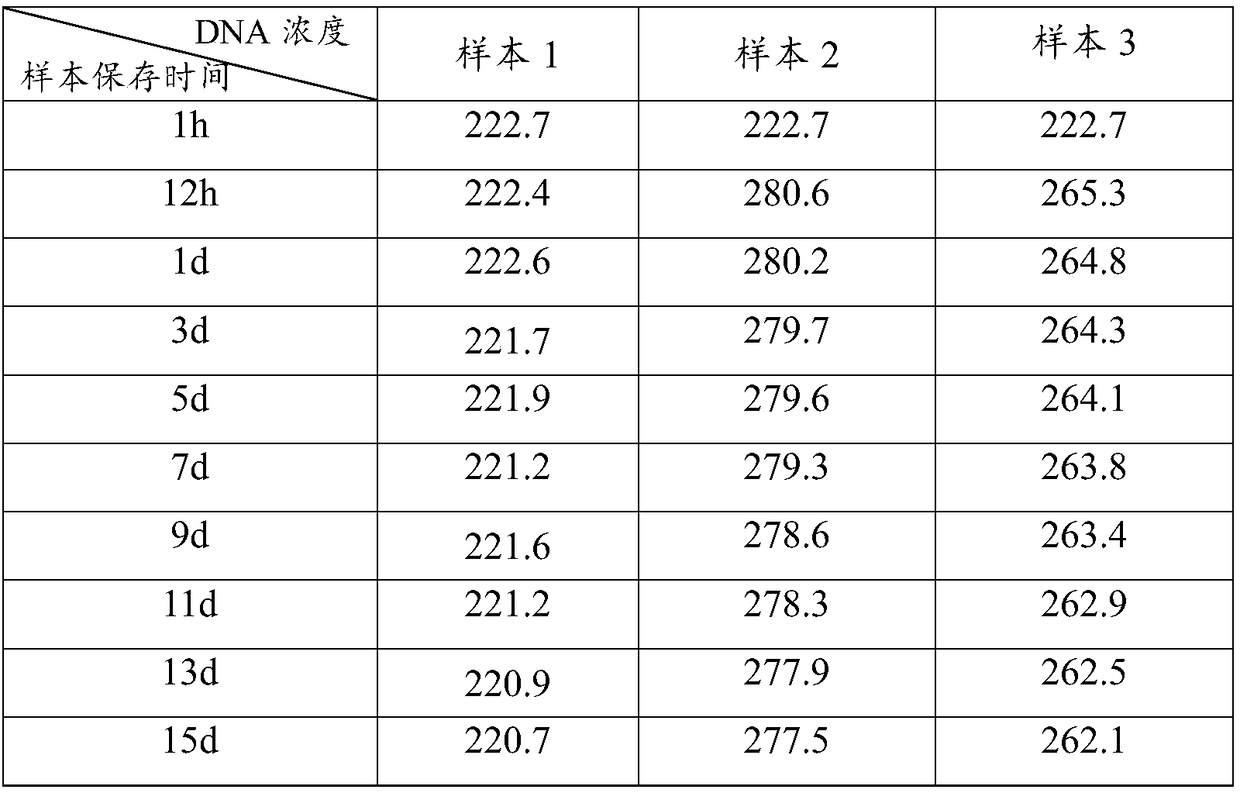
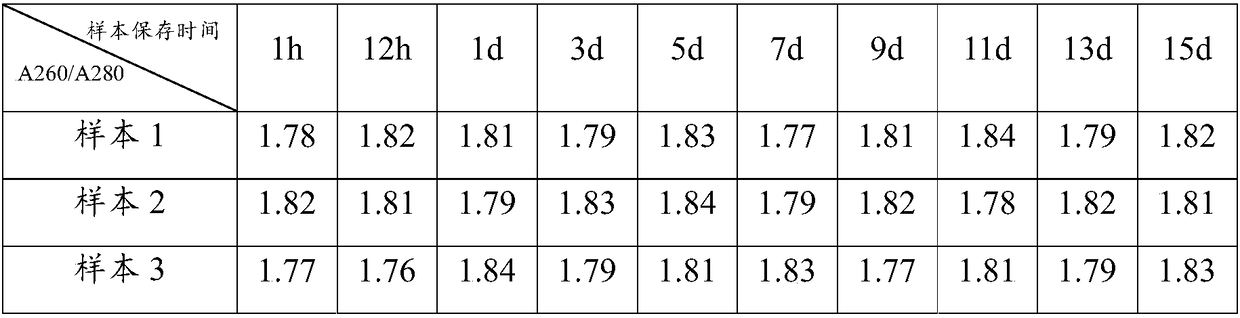
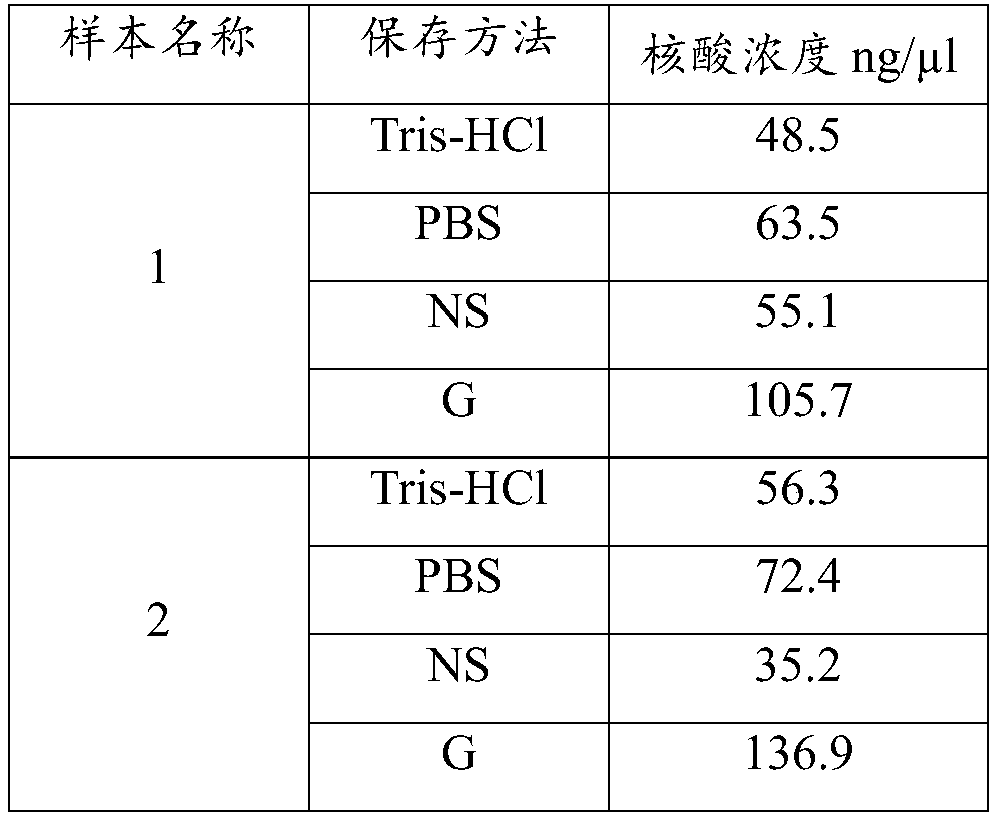
Kit for easy and odorless extraction of fecal nucleic acid and extraction method
The invention discloses a kit for easy and odorless extraction of fecal nucleic acid and an extraction method and relates to the field of molecular biotechnology. The kit includes a fecal stabilizingsolution containing Tris, guanidinium thiocyanate, ethylenediaminetetraacetic acid, sodium chloride, chlorobutanol, green tea extract, honeysuckle extract and an enzyme. The fecal stabilizing solutioncan store the DNA in the extracted fecal sample at a normal temperature of less than or equal to 40 DEG C for about 15 days, solve the problem of difficulty in transportation and storage and facilitate self-extraction and saving of the sample at home and is convenient and quick. The fecal stabilizing solution has the advantages of good deodorizing effect and use safety and can effectively removefecal odor gas such as ammonia gas and hydrogen sulfide thereby eliminating the odor and inhibiting the growth of bacteria.
Owner:SUREXAM BIO TECH
Veterinary amoxicillin-sulbactam suspension injection and preparation method thereof
InactiveCN103301060AImprove stabilitySimple processing technologyAntibacterial agentsSolution deliveryALUMINUM STEARATESAmoxicillin+Sulbactam
The invention relates to a veterinary drug, in particular to a stable, efficient and broad-spectrum veterinary amoxicillin-sulbactam suspension injection and a preparation method thereof. The pharmaceutical composition of the veterinary amoxicillin-sulbactam suspension injection comprises the following ingredients by volume weight: 5%-30% (w / v) of amoxicillin, 0.15%-5% (w / v) of sulbactam, 5%-20% (w / v) of suspending agent, 0.3%-8% (w / v) of antimicrobial preservative, and the balance of injection oil, wherein the suspending agent is aluminum stearate, and the antimicrobial preservative is benzyl alcohol or acetone chloroform. Specific raw materials and proportions are selected to greatly prolong the stability of the amoxicillin-sulbactam suspension injection, and meanwhile, the raw materials are simple and available, the processing technique is simple, the operation is convenient and the production cost is low.
Owner:CHONGQING FANGTONG ANIMAL PHARMA
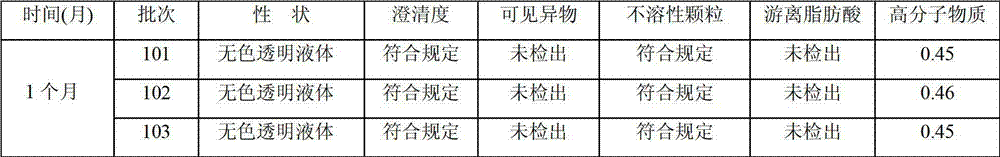
Synthetic method of 4-(N,N-dimethylamino) butyaldehyde dimethyl acetal
InactiveCN101307006AHigh yieldHigh purityOrganic compound preparationAmino-hyroxy compound preparationDimethyl acetalIsocyanuric acid
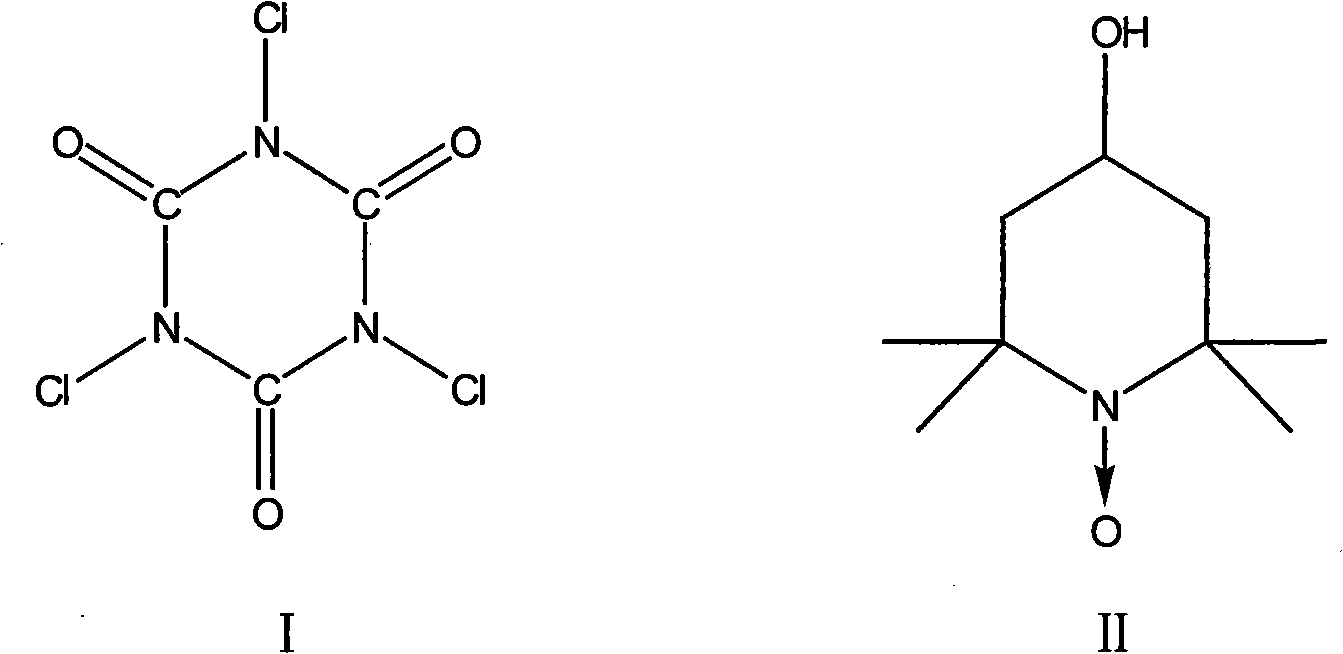
The invention discloses a method for synthesizing 4-(N, N- dimethylamino) butyaldehyde dimethyl acetal. The raw material of 4-chlorobutanol is subject to oxidation, aldolization and ammonolysis in sequence. In the process of the oxidation reaction, the used oxidizer is trichloride isocyanuric acid and the catalyst is 4-hydroxy-2,2,6,6-tetramethyl-1-Piperidinyloxy. The 4-(N, N- dimethylamino) butyaldehyde dimethyl acetal prepared by the method has high yield coefficient and purity quotient up to between 85 and 90 percent.
Owner:STAR LAKE BIOSCI CO INC ZHAOQING GUANGDONG
Pharmaceutical composition containing animal bone polypeptide
ActiveCN102920994AAvoid it happening againImprove securityPowder deliveryPeptide/protein ingredientsMedicineStock solution
The invention relates to a bone polypeptide product, and in particular relates to a pharmaceutical composition containing animal bone polypeptide. The pharmaceutical composition comprises the following components in parts by weight: 100 parts of polypeptide injection stock solution extracted from pig bones, cow bones and deer bones, 0.1-5 parts of chloretone and 0.5-5 parts of sodium dihydrogen phosphate. The pharmaceutical composition containing animal bone polypeptide is good in stability, extremely low in high polymer content, high in safety and extremely suitable for clinic application.
Owner:罗诚
Rhodiola root glycosides injection for treating cardiovascular/cerebrovascular diseases and preparation method thereof
InactiveCN1698627AQuickly control the diseaseAvoid first passOrganic active ingredientsPharmaceutical delivery mechanismDiseaseCurative effect
Disclosed is a rhodiola root glycosides injection for treating cardiovascular / cerebrovascular diseases and preparation method, wherein the constituents (by weight portion) comprises salidroside 0.2-2%, sodium chloride 0.5-1.5%, chlorobutanol 0.2-0.5%, and balancing water for injection. And the preparing process comprises the steps of loading 80% water for injection into a preparation container, charging in turn salidroside, sodium chloride, chlorobutanol, stirring till complete dissolving, charging water for injection to 1000ml, filtering with G3 sintered glass funnel, imbedding into 2ml ampoule, sterilizing 30 minutes at 100 deg. C.
Owner:SHANGHAI JIAO TONG UNIV
Preparation method of oxytocin injection
InactiveCN110339340ANot easy to oxidative degradationNot volatilePeptide/protein ingredientsPharmaceutical delivery mechanismMedicineChlorobutanol
The invention provides a preparation method of an oxytocin injection. According to the preparation method of the oxytocin injection, through control over the temperature in the preparation process anda nitrogen introduction mode and accurate adjustment for the pH value, chlorbutol in the injection is more stable and cannot be easily subjected to oxydative degradation and volatilized, and the oxytocin injection well corresponding to the labelled amount on a prescription is obtained and has practical application and popularization value.
Owner:CHENGDU HAITONG PHARMA
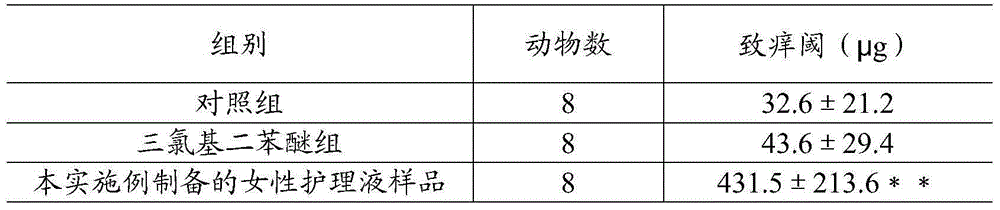
Female care solution and preparation method thereof
ActiveCN105193686AMaintain balanceMaintenance and repair effectCosmetic preparationsToilet preparationsSide effectPropolis
The invention relates to a female care solution and further relates to a preparation method of the female care solution. The female care solution is prepared from a propolis extract, collagen liquid, an aloe extract, a mint herb extract, a pectin solution, hyaluronic acid, sodium pyrrolidonecarboxylate, chlorobutanol preservative and the like. The female care solution does not contain a chemical bacteriostatic agent, is free of toxic and side effects, the active ingredient propolis has the inhibiting effect on harmful bacteria in the vagina and meanwhile has the protection effect on beneficial bacteria, and balance of normal flora of the vagina is kept. The female care solution is low in ethanol content and free of stimulatory effect and is stable.
Owner:HANGZHOU FENGQING TECH CO LTD
Tumor targeting haematoporphyrin injection for ultrasonic therapy and preparation method thereof
InactiveCN106924732AImprove tumor targetingImprove photostabilityOrganic active ingredientsPharmaceutical delivery mechanismTumor targetRetention time
The invention discloses a tumor targeting haematoporphyrin injection for ultrasonic therapy and a preparation method thereof. The preparation method comprises the following steps of firstly, enabling folate and aminocyclodextrin to react to generate folate-conjugated cyclodextrin; enabling the folate-conjugated cyclodextrin and haematoporphyrin to react, so as to obtain a folate-conjugated cyclodextrin haematoporphyrin inclusion compound; finally, adding the folate-conjugated cyclodextrin haematoporphyrin inclusion compound and acetone chloroform into injection water, and sterilizing, so as to obtain the tumor targeting haematoporphyrin injection for the ultrasonic therapy. The tumor targeting haematoporphyrin injection for ultrasonic therapy has the advantages that after the tumor targeting haematoporphyrin injection is injected into a tumor-bearing mice body, the in-vitro ultrasonic therapy is adopted, so that the targeting property of the haematoporphyrin for high expression ofexpressing the malicious tumor tissues in a folate receptor is obviously improved, the retention time of the drug in the tumor part is prolonged, and the tumor therapy effect is improved; the light stability of the haematoporphyrin is improved, the photosensitive toxic and side reaction of light sensitizing of the haematoporphyrin is decreased, and the wide application potential is realized in clinical application.
Owner:NANJING UNIV OF SCI & TECH
Ready-to-use oxytocin formulation and uses thereof
InactiveUS20190388498A1Long-term stabilityPharmaceutical delivery mechanismCyclic peptide ingredientsHigh concentrationMedicine
Owner:ETON PHARMA INC
Pituitrin solution and preparation method thereof
ActiveCN109608521ANo lossRaise quality standardsOxytocins/vasopressinsPeptide preparation methodsAcetic acidVirus inactivation
The invention discloses a pituitrin solution and a preparation method thereof. The preparation method of the pituitrin solution comprises the steps that in an extraction process of powdered pituitrinon acetic acid, chloretone is added, and the addition amount of the chloretone is 18-35% by mass of the powdered pituitrin. The pituitrin solution prepared by the preparation method disclosed by the invention has high titer (as high as 18.3u / ml ), and in the virus inactivation process, active components (the yield is as high as 1281 thousands u / kg) cannot lose, so that the pituitrin solution moreconforms to the quantity standard of biological products.
Owner:SPH NO 1 BIOCHEM & PHARMA CO LTD
Antibacterial care solution composition and application thereof
InactiveCN108522547AHas inhibitory effectGood sterilization effectBiocideDisinfectantsAdditive ingredientOcular prosthesis
The invention relates to the technical field of antibacterial agents and specifically relates to an antibacterial care solution composition and application thereof. The composition is prepared from the following ingredients of methylparaben, pyrrolidone sodium hydroxide, hexadecylpyridinium chloride, nano zinc, a freshener, a positive-ion surface active agent, acetone chloroform, polyethylene glycol, a buffer agent, a permeability regulator, taxus chinensis extractive and deionized water, wherein the taxu chinensis extractive is polypeptide compound which is extracted from taxus chinensis braches and leaves and has an antibacterial effect; the positive-ion surface active agent is benzalkonium chloride or benzalkonium bromide; the permeability regulator is a saturated sodium chloride, saturated potassium chloride or saturated glucose solution. The antibacterial care solution contains varieties of ingredients with antibacterial and sterilizing effects, the properties of chemical substances are stable, the property of the composition is gentle, and the antibacterial care solution composition can be applied to antibacterial and sterilizing storage of ocular prosthesis or contact lensesand has no irritation to the human body.
Owner:合肥昂诺新材料有限公司
Degradable slow-release invisible eye mask capable of eliminating eye fatigue and preparation method of degradable slow-release invisible eye mask
PendingCN111956791AEliminate dryness and discomfortEliminate eye fatigue and degrade dryness and discomfortSenses disorderHydroxy compound active ingredientsChlorobenzeneDisodium Edetate
The invention discloses a degradable slow-release invisible eye mask capable of eliminating eye fatigue and a preparation method of the degradable slow-release invisible eye mask, and relates to the technical field of invisible eye masks. Per each 10ml, the invisible eye mask comprises the following raw material components: 200mg-300mg of collagen peptide, 0.8-1.2mg of hyaluronic acid, 4-6mg of vitamin B6, 1.8-2.2mg of vitamin B12, 9-11 mg of dipotassium glycyrrhizinate, 0.18-0.22mg of napthoxylin hydrochloride, 0.28-0.32mg of neostigmine methylsulfate, 0.8-1.2mg of chlorpheniramine maleate, 80-150mg of glycerinum, 7-9mg of dexpanthenol, 4-6mg of menthol, 5-7mg of polysorbate 80, 5-7mg of borneol, 3-5mg of edetate disodium, 2-4mg of mint, 2-4mg of aminocaproic acid, 1-3mg of ethanol, 9-11mg of sodium chloride, 2-4 mg of benzalkonium chloride solution, and 3-5 mg of chlorobutanol solution; the mass fraction of the benzalkonium chloride solution is 0.01%; and the mass fraction of the chlorobutanol solution is 0.2%.
Owner:康洛信(广东)生物科技有限公司
Cooling agent for computer equipment
InactiveCN104927270AEnvironmentally friendlyImprove securityHeat-exchange elementsPolyvinyl alcoholSalicylic acid
The invention relates to a cooling agent for computer equipment. The cooling agent comprises the following raw materials in parts by weight: 40-60 parts of ethylene glycol monoethyl ether acetate, 40-45 parts of polyving akohol, 30-60 parts of triethanolamine, 10-15 parts of sodium tartrate, 3-5 parts of phosphoryl triethyl sodium borate, 2-3 parts of sodium borate, 3-4 parts of methylphenol, 3-4 parts of salicylic acid, 1-3 parts of octanedioic acid, 2-4 parts of adipic acid, 3-5 parts of acetone, 1-2 parts of isopropanol, 3-4 parts of chlorobutanol, 3-4 parts of styrene, 2-5 parts of polyvinyl alcohol, 3-4 parts of tert-butylhydroquinone, 3-5 parts of hydroxyethyl cellulose, 2-5 parts of castor oil, 1-2 parts of sorbitol, 2-3 parts of styrene, 8-10 parts of silicate ester, and 180-220 parts of deionized water. The cooling agent disclosed by the invention is environment-friendly, and high in safety and cost performance; the cooling agent can effectively protect the metal materials of cooling systems of the computer equipment, is harmless, and generates no pollution, so that the safe running of the systems of the computer equipment is guaranteed.
Owner:杨高林
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com