Synthetic method of 4-(N,N-dimethylamino) butyaldehyde dimethyl acetal
A technique for synthesizing butyraldehyde dimethyl acetal, which is applied in the field of synthesis of 4-(N,N-dimethylamino) butyraldehyde dimethyl acetal, and can solve the problems of troublesome processing, less than 20% yield, and high equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
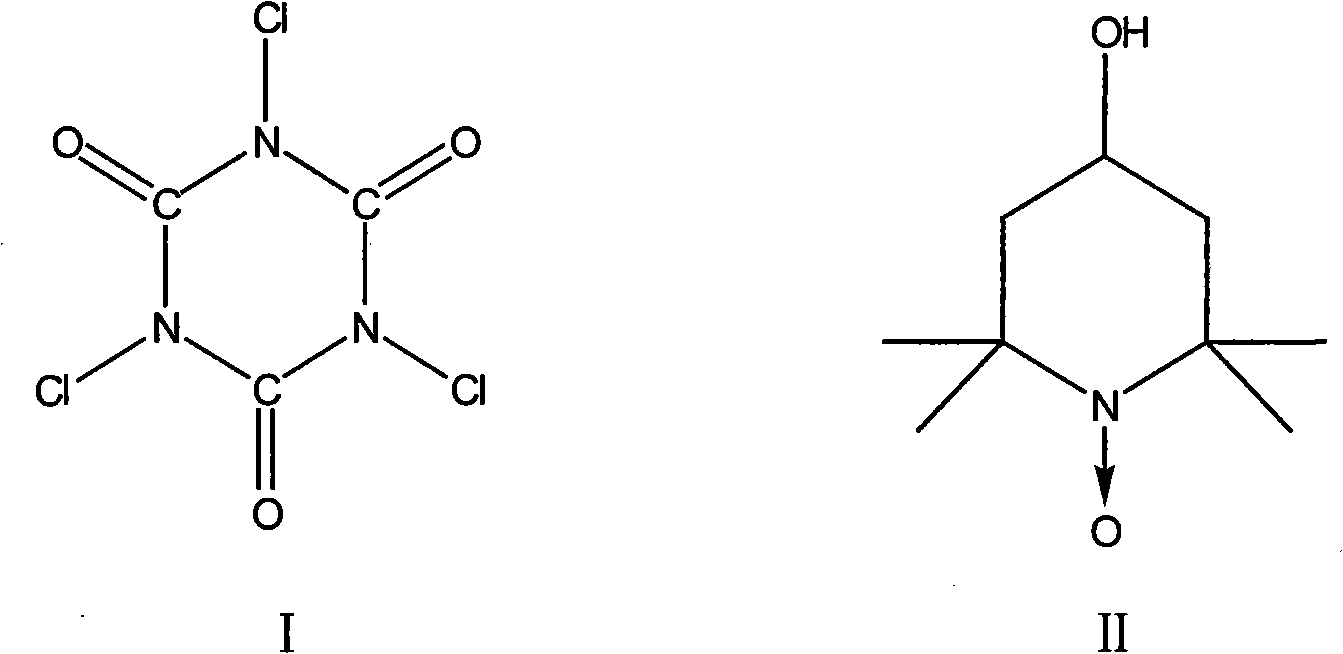
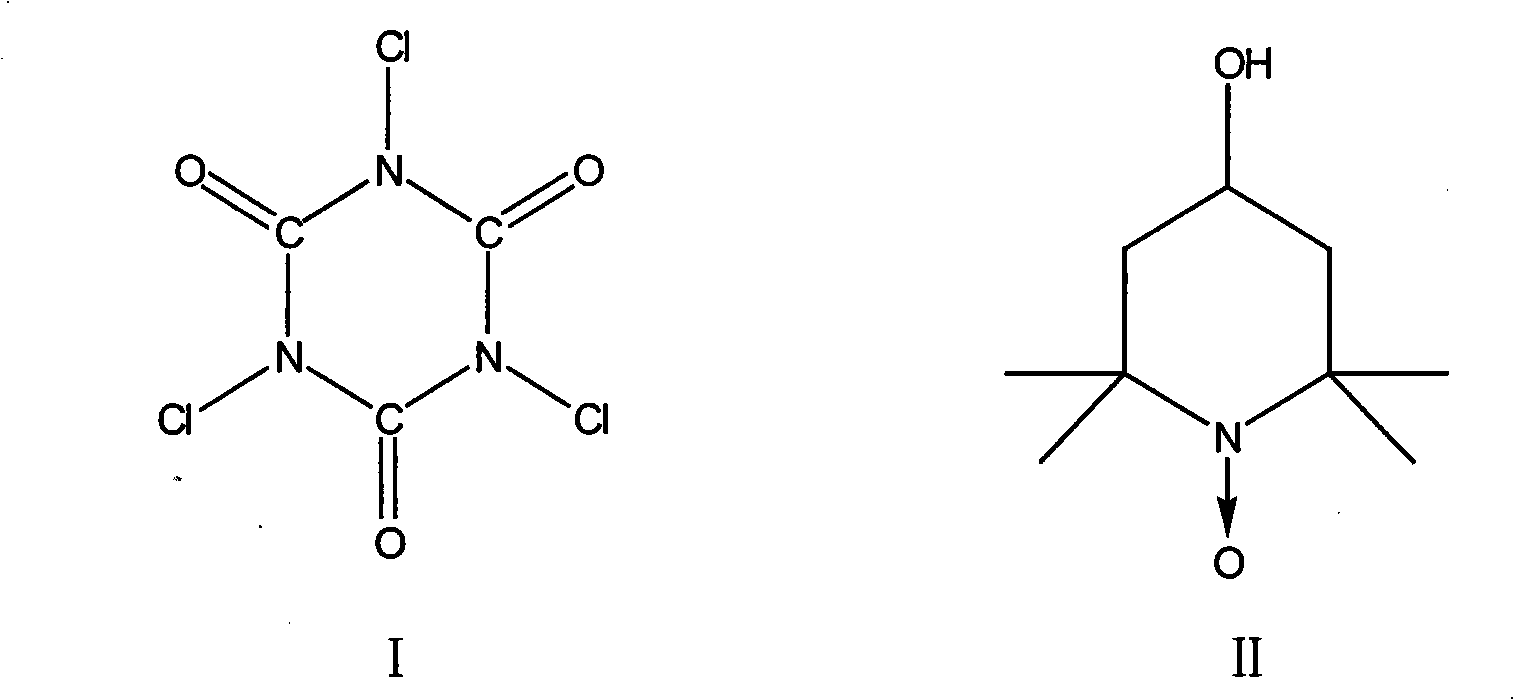
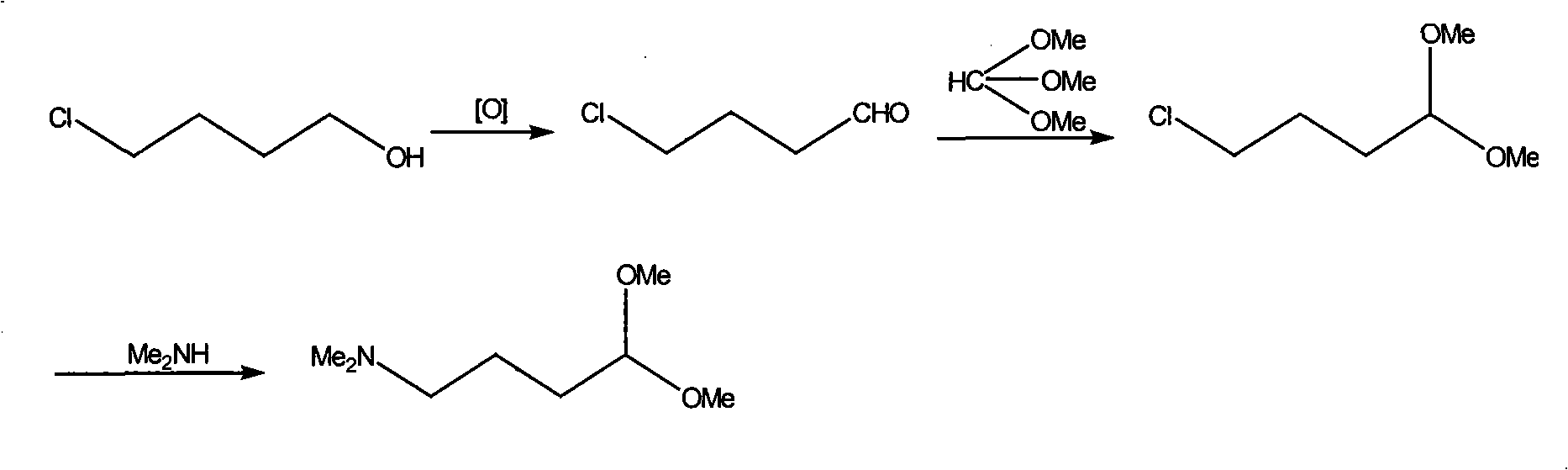
[0014] Add 80g 4-chlorobutanol (w=80%), 700mL methylene chloride and oxidizing agent successively in 1000mL four-necked bottle to be 180g trichloroisocyanuric acid, N 2 Add dropwise catalyst 0.28g 4-hydroxyl-2,2,6,6-tetramethylpiperidine-1-oxyl free radical (also known as nitroxide free radical piperidinol) in 25mL dichloromethane under protection and ice-water bath cooling The solution in , stop dropping when the temperature of the system rises, continue to react for 1 to 3 hours, filter and wash with dichloromethane. The washing liquid and the filtrate were combined, and 100 mL of trimethyl orthoformate was added, and stirred at room temperature for 2 to 4 hours. Wash with 200mL of 2% sodium carbonate solution, dry over anhydrous magnesium sulfate, concentrate, and then distill under reduced pressure to obtain 32g of product.
[0015] Add 11g of 4-chlorobutyraldehyde dimethyl acetal obtained in the previous step, 30mL of 33% dimethylamine aqueous solution, 4.0g of sodium ca...
Embodiment 2
[0017] Add 80g 4-chlorobutanol (w=80%), 700mL methylene chloride and oxidizing agent successively in 1000mL four-necked bottle to be 200g trichloroisocyanuric acid, N 2 Add dropwise catalyst 0.38g 4-hydroxyl-2,2,6,6-tetramethylpiperidine-1-oxyl free radical (also known as nitroxide free radical piperidinol) in 25mL dichloromethane under protection and ice-water bath cooling The solution in , stop dropping when the temperature of the system rises, continue to react for 1 to 3 hours, filter and wash with dichloromethane. The washing liquid and the filtrate were combined, and 100 mL of trimethyl orthoformate was added, and stirred at room temperature for 2 to 4 hours. Wash with 200mL of 2% sodium carbonate solution, dry over anhydrous magnesium sulfate, concentrate, and then distill under reduced pressure to obtain 33g of product.
[0018] Add 11.2g of 4-chlorobutyraldehyde dimethyl acetal obtained in the previous step, 30mL of 33% dimethylamine aqueous solution, 4.0g of sodium ...
Embodiment 3
[0020] In the 1000mL four-necked bottle, add 80g 4-chlorobutanol (w=80%), 700mL methylene chloride and oxidizing agent successively to be 220g trichloroisocyanuric acid, N 2 Under protection and ice-water bath cooling, add dropwise catalyst 0.42g 4-hydroxyl-2,2,6,6-tetramethylpiperidine-1-oxyl radical (also known as nitroxide radical piperidinol) in 25mL dichloromethane The solution in , stop dropping when the temperature of the system rises, continue to react for 1 to 3 hours, filter and wash with dichloromethane. The washing liquid and the filtrate were combined, and 100 mL of trimethyl orthoformate was added, and stirred at room temperature for 2 to 4 hours. Wash with 200mL of 2% sodium carbonate solution, dry over anhydrous magnesium sulfate, concentrate, and then distill under reduced pressure to obtain 35g of product.
[0021] Add 11.2g of 4-chlorobutyraldehyde dimethyl acetal obtained in the previous step, 30mL of 33% dimethylamine aqueous solution, 4.0g of sodium carb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com