Tumor targeting haematoporphyrin injection for ultrasonic therapy and preparation method thereof
A porphyrin injection and tumor targeting technology, which is applied in the field of tumor-targeted hematoporphyrin injection for ultrasound therapy and its preparation, and can solve the problems of harsh clinical use conditions, low water solubility of hematoporphyrin, low tumor targeting, etc. problems, to achieve the effect of reducing photosensitivity toxicity, prolonging residence time, and wide application potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
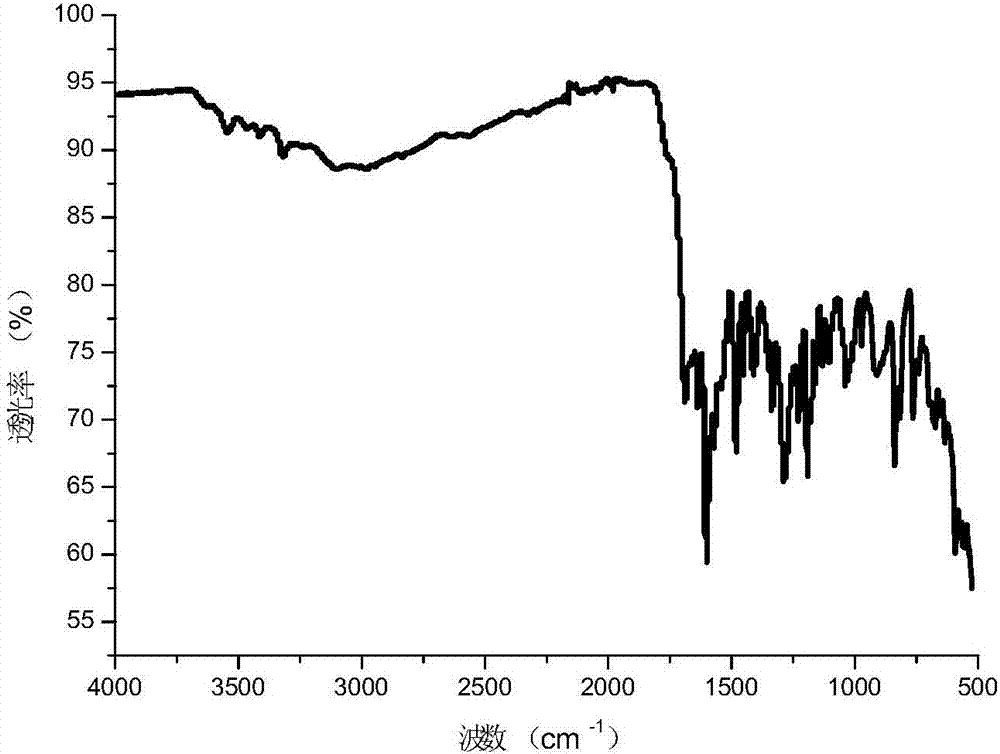
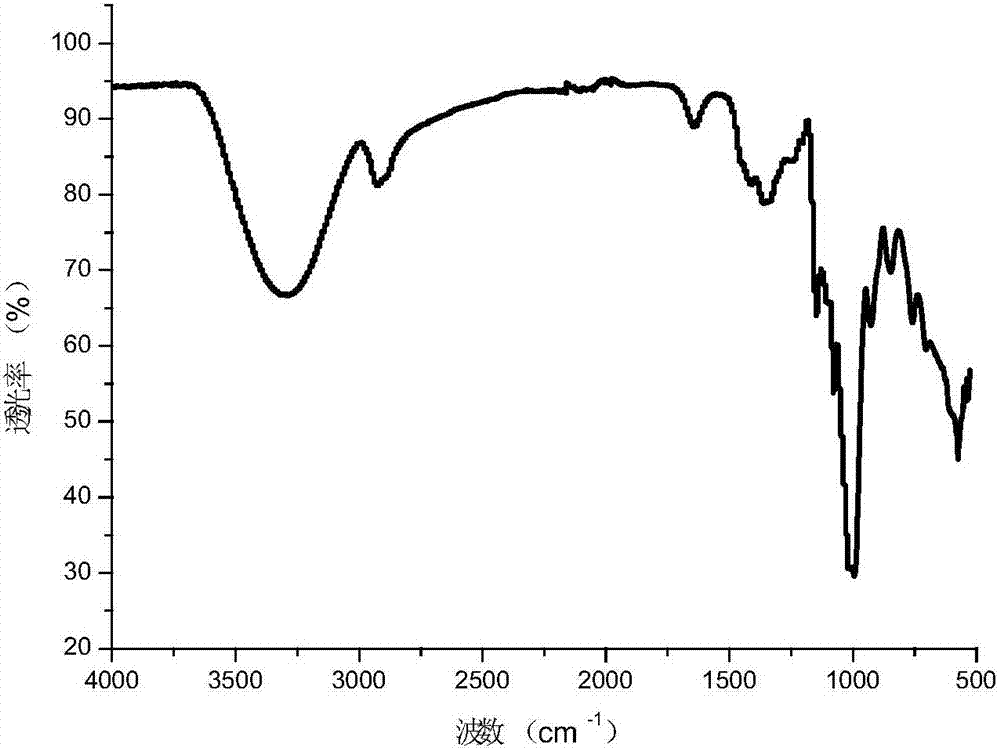
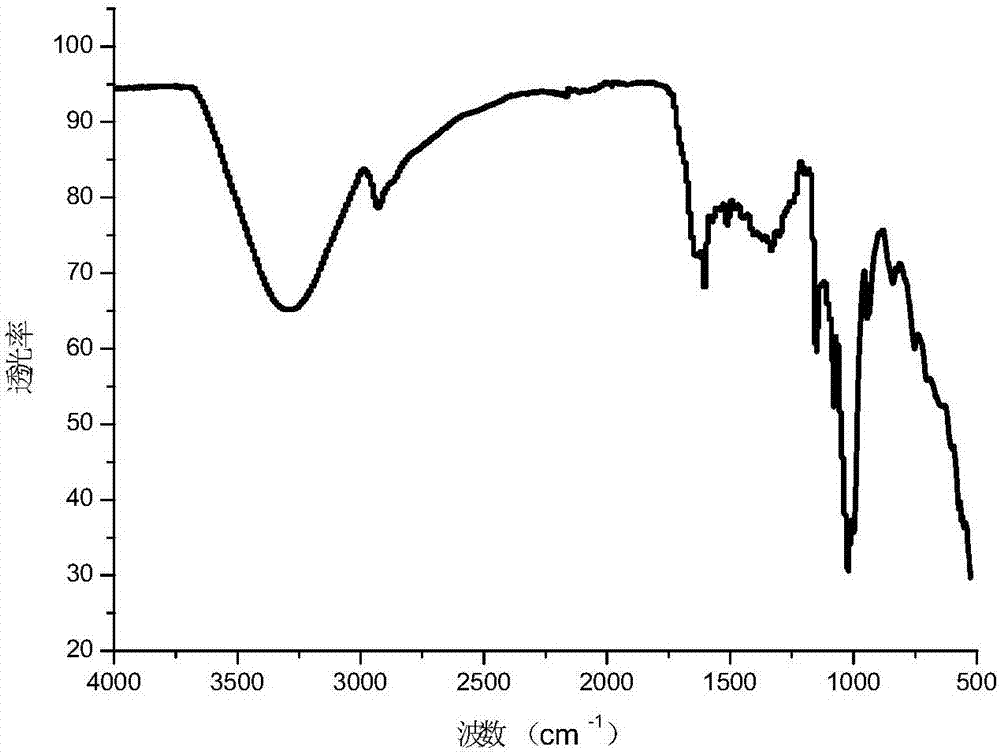
Image
Examples
Embodiment 1
[0023] Step 1, dissolve folic acid in N,N dimethylformamide, add N-hydroxysuccinimide and N,N'dicyclohexylcarbodiimide, stir and react at 5°C for 0.5h, then add amino Cyclodextrin continued to react for 12 hours, wherein the molar ratio of folic acid, N-hydroxysuccinimide, N,N'dicyclohexylcarbodiimide, and aminocyclodextrin was [1:1:1:10];
[0024] Step 2, the reaction solution obtained in step 1 is spun to dryness to obtain a dried product, which is dissolved in water and then filtered to obtain a clarified filtrate. After the spun-dried and clarified filtrate, the dried product is dissolved in acetone, and the filter cake is redissolved after filtration. In acetone, filter to obtain a filter cake, which is dried to obtain folic acid-coupled cyclodextrin;
[0025] Step 3, mix the folic acid-coupled cyclodextrin and hematoporphyrin obtained in step 2, dissolve them in water, and stir in the dark for 12 hours at 5°C, wherein the molar ratio of folic acid-coupled cyclodextrin an...
Embodiment 2
[0029] Step 1, dissolve folic acid in N,N dimethylformamide, add N-hydroxysuccinimide and N,N'dicyclohexylcarbodiimide, stir and react at 30°C for 2h, then add amino ring Dextrin continued to react for 24 hours, wherein the molar ratio of folic acid, N-hydroxysuccinimide, N,N'dicyclohexylcarbodiimide, and aminocyclodextrin was [5:1:1:10];
[0030] Step 2, the reaction solution obtained in step 1 is spun to dryness to obtain a dried product, which is dissolved in water and then filtered to obtain a clarified filtrate. After the spun-dried and clarified filtrate, the dried product is dissolved in acetone, and the filter cake is redissolved after filtration. In acetone, filter to obtain a filter cake, which is dried to obtain folic acid-coupled cyclodextrin;
[0031] Step 3, mix the folic acid-coupled cyclodextrin and hematoporphyrin obtained in step 2, dissolve them in water, and stir for 48 hours at 25° C. in the dark, where the molar ratio of folic acid-coupled cyclodextrin an...
Embodiment 3
[0035] Step 1, dissolve folic acid in N,N dimethylformamide, add N-hydroxysuccinimide and N,N'dicyclohexylcarbodiimide, stir and react at 50°C for 5h, then add amino ring Dextrin continued to react for 48 hours, wherein the molar ratio of folic acid, N-hydroxysuccinimide, N,N'dicyclohexylcarbodiimide, and aminocyclodextrin was [10:1:1:10];
[0036] Step 2, the reaction solution obtained in step 1 is spun to dryness to obtain a dried product, which is dissolved in water and then filtered to obtain a clarified filtrate. After the spun-dried and clarified filtrate, the dried product is dissolved in acetone, and the filter cake is redissolved after filtration. In acetone, filter to obtain a filter cake, which is dried to obtain folic acid-coupled cyclodextrin;
[0037]Step 3, mix the folic acid-coupled cyclodextrin and hematoporphyrin obtained in step 2, dissolve them in water, and stir for 96 hours in the dark at 50°C, wherein the molar ratio of folic acid-coupled cyclodextrin an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com