Hyaluronic acid prodrug as well as preparation method thereof and application thereof in transdermal delivery
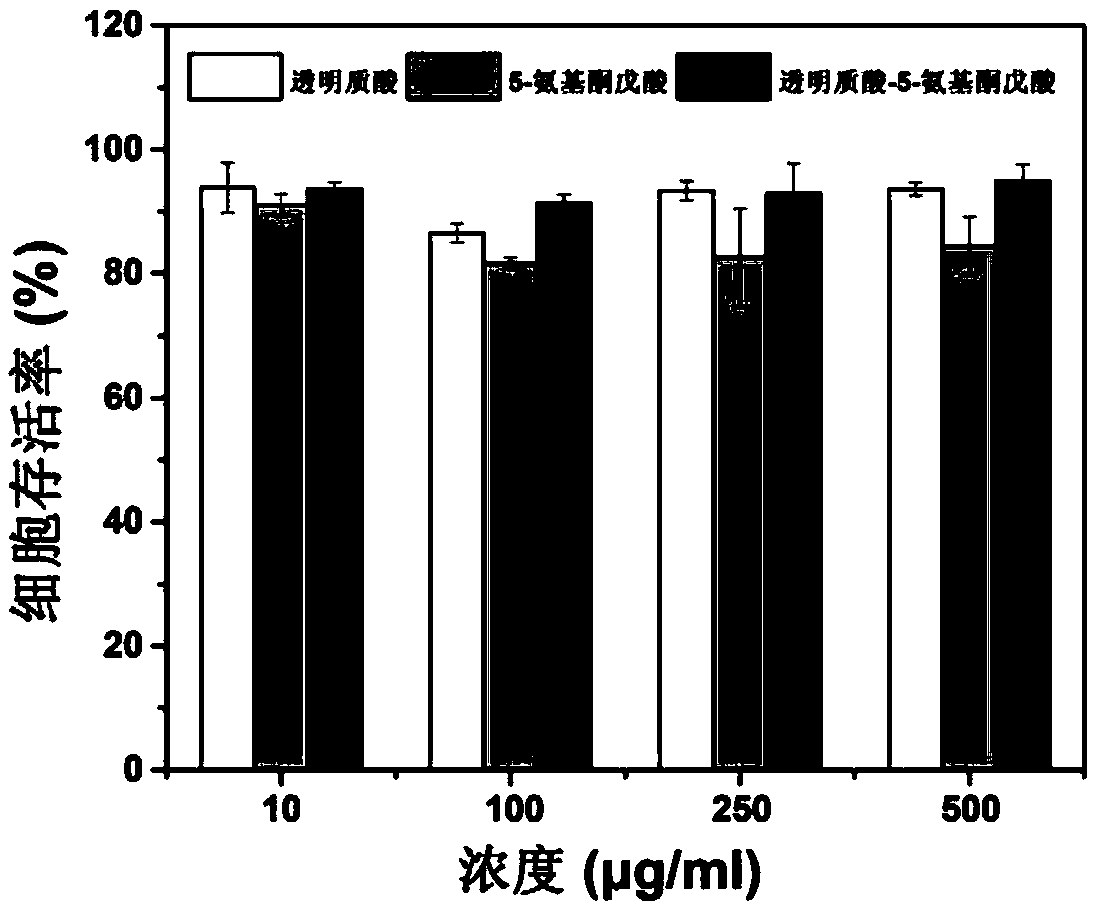
The technology of hyaluronic acid and prodrug is applied in the field of hyaluronic acid prodrug and its preparation, and can solve the problems such as the need to improve the biocompatibility of the carrier, the existence of skin irritation and allergy, the damage to the normal barrier function of the skin, and the like, To achieve the effect of improving percutaneous penetration, facilitating treatment and improving targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
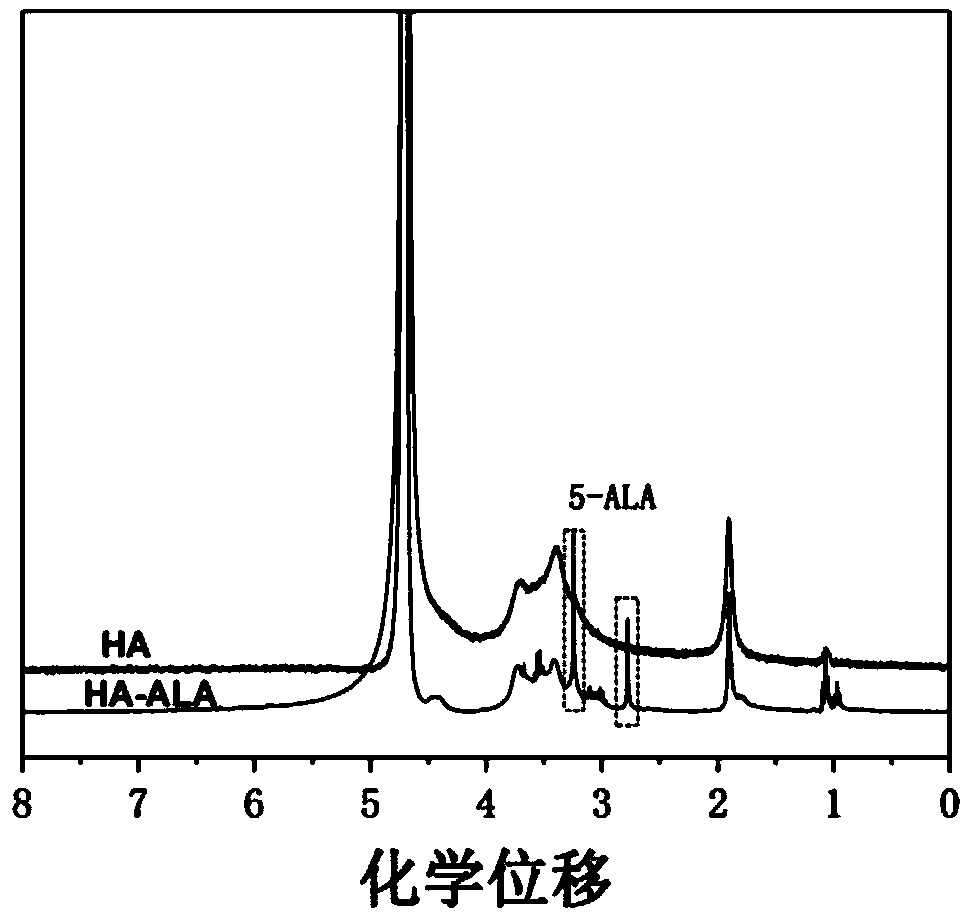
[0037] Embodiment 1: Preparation of hyaluronic acid grafted 5-aminolevulinic acid
[0038] (1) Weigh the corresponding raw materials: hyaluronic acid, drug molecule, N-hydroxysuccinimide, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, water residue quantity;
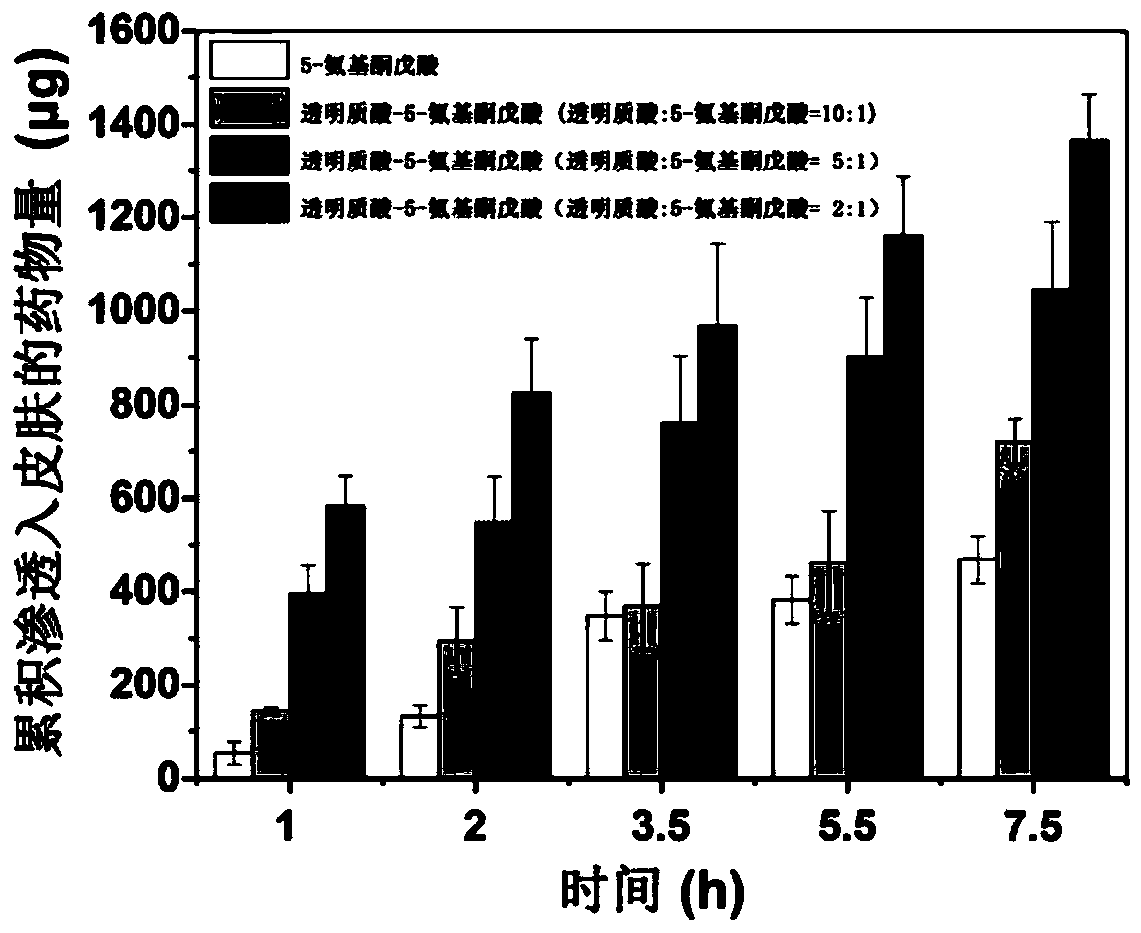
[0039] The mass ratio of described hyaluronic acid and drug molecule is 10:1, and hyaluronic acid, N-hydroxysuccinimide and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide salt The mass ratio of acid salt is: 4:2:1;
[0040] (2) Dissolve hyaluronic acid in water, then add N-hydroxysuccinimide and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, and place on a magnetic stirrer on, stirring at a speed of 200r / min for 1h;
[0041] (3) Dissolve the drug molecule in water, then add it into (2), and stir overnight; put the obtained sample into a dialysis bag (molecular weight cut-off is 500), and then immerse the dialysis bag in pure water and stir for 3 days , freeze-dried to obtain hyaluronic acid...
Embodiment 2
[0043] Embodiment 2: Preparation of hyaluronic acid grafted 5-aminolevulinic acid
[0044] (1) Weigh the corresponding raw materials: hyaluronic acid, drug molecule, N-hydroxysuccinimide, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, water residue quantity.
[0045] The mass ratio of hyaluronic acid to drug molecules is 5:1, hyaluronic acid, N-hydroxysuccinimide and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide salt The mass ratio of acid salt is: 4:2:1;
[0046] (2) Dissolve hyaluronic acid in water, then add N-hydroxysuccinimide and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, and place on a magnetic stirrer on, stirring at a speed of 200r / min for 1h;
[0047] (3) Dissolve the drug molecule in water, then add it into (2), and stir overnight; put the obtained sample into a dialysis bag (molecular weight cut-off is 500), and then immerse the dialysis bag in pure water and stir for 3 days , freeze-dried to obtain hyaluronic acid grafted with 5-a...
Embodiment 3
[0049] Embodiment 3: Preparation of hyaluronic acid grafted 5-aminolevulinic acid
[0050] (1) Weigh the corresponding raw materials: hyaluronic acid, drug molecule, N-hydroxysuccinimide, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, water residue quantity.
[0051] The mass ratio of the hyaluronic acid to the drug molecule is 2:1, and the hyaluronic acid, N-hydroxysuccinimide and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide salt The mass ratio of acid salt is: 4:2:1;
[0052] (2) Dissolve hyaluronic acid in water, then add N-hydroxysuccinimide and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, and place on a magnetic stirrer on, stirring at a speed of 200r / min for 1h;
[0053] (3) Dissolve the drug molecule in water, then add it into (2), and stir overnight; put the obtained sample into a dialysis bag (molecular weight cut-off is 500), and then immerse the dialysis bag in pure water and stir for 3 days , freeze-dried to obtain hyaluronic acid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com