Eye sterile suspension containing loteprednol etabonate and preparation method thereof
A technology of loteprednol etabonate and suspension, applied in the field of ophthalmic sterile suspension, can solve the problem of affecting crystal form and stability, failing to pass through a sterile filter, and not mentioning loteprednol etabonate. Nuo sterilization problems and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Loteprednol etabonate 5.0g
[0018] Tobramycin 3.0g
[0019] Glycerin 25.0g
[0020] Hypromellose 3.0g
[0021] Chlorobutanol 5.0g
[0022] Tween-80 2.0g
[0023] Edetate Disodium 0.5g
[0024] Appropriate amount of water for injection
[0025] Makes 1000ml
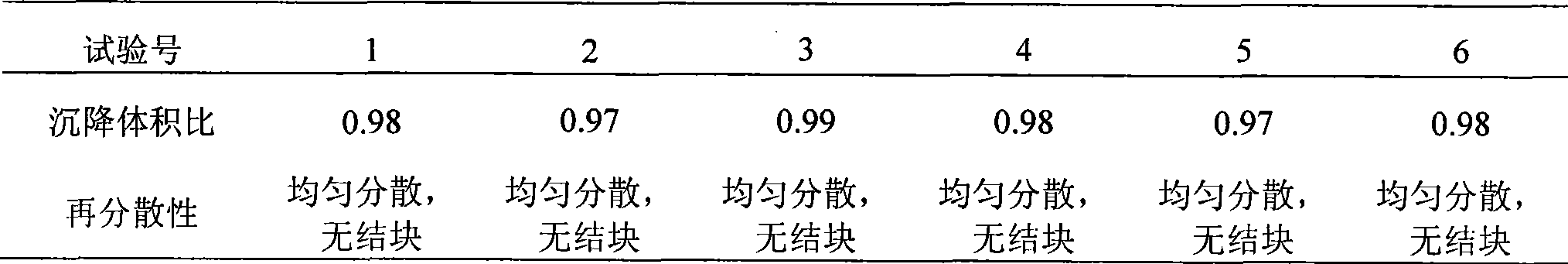
[0026] Micronized with loteprednol carbonate, sterilized by dry heat at 150°C; hypromellose was swollen with water, sterilized at 121°C for 20 minutes, cooled, and set aside; tobramycin, glycerin, chlorobutanol, Dissolve Tween-80 and edetate disodium in water, filter and sterilize with a 0.22 μm sterile filter membrane, add hypromellose to the aqueous solution, add loteprednol etabonate microcrystals while stirring, stir Evenly, adjust the pH value to 5-6. Subpackage.
Embodiment 2
[0028] Loteprednol etabonate 5.0g
[0029] Tobramycin 3.0g
[0031] Hypromellose 4.0g
[0032] Chlorobutanol 3.0g
[0033] Tween-80 3.0g
[0034] Edetate Disodium 0.6g
[0035] Appropriate amount of water for injection
[0036] Makes 1000ml
[0037] Micronized with loteprednol carbonate, sterilized by dry heat at 160°C; hypromellose was swollen with water, sterilized at 115°C for 30 minutes, cooled, and set aside; tobramycin, sodium chloride, chlorotert-butyl Dissolve alcohol, Tween-80, and edetate disodium in water, filter and sterilize with a 0.22 μm sterile filter membrane, add hypromellose to the aqueous solution, and add loteprednol etabonate microcrystals while stirring , stir well, and adjust the pH value to 5-6. Subpackage.
Embodiment 3
[0039] Loteprednol etabonate 5.0g
[0040] Glycerin 13g
[0041] Sodium chloride 4g
[0042] Hypromellose 2g
[0043] Chlorobutanol 0.6g
[0044] Tween-80 1.0g
[0045] Edetate Disodium 0.3g
[0046] Appropriate amount of water for injection
[0047] Makes 1000ml
[0048]Micronized with loteprednol carbonate, sterilized by dry heat at 160°C; hypromellose was swollen with water, sterilized at 121°C for 20 minutes, cooled, and set aside; glycerin, sodium chloride, chlorobutanol, spit Dissolve Wen-80 and edetate disodium in water, filter and sterilize with a 0.22 μm sterile filter membrane, add hypromellose to the aqueous solution, add loteprednol etabonate microcrystals while stirring, and stir well , adjust the pH value to 5-6.5. Subpackage.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com