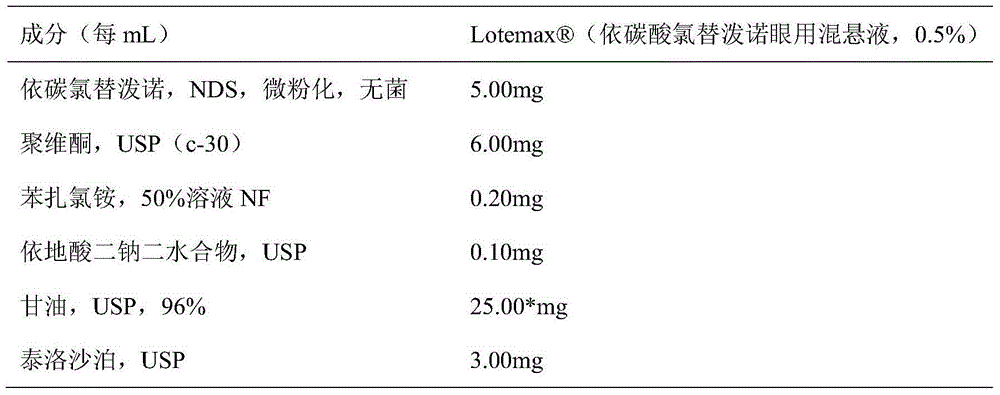
Loteprednol Etabonate suspension eye drops composition
A technology of loteprednol etabon and eye drops, applied in the directions of drug combination, steroid, liquid delivery, etc., can solve the problems of high requirements for drug storage conditions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Invention Example 1 Preparation of etabac loteprednol crystal form I
Embodiment 1-1
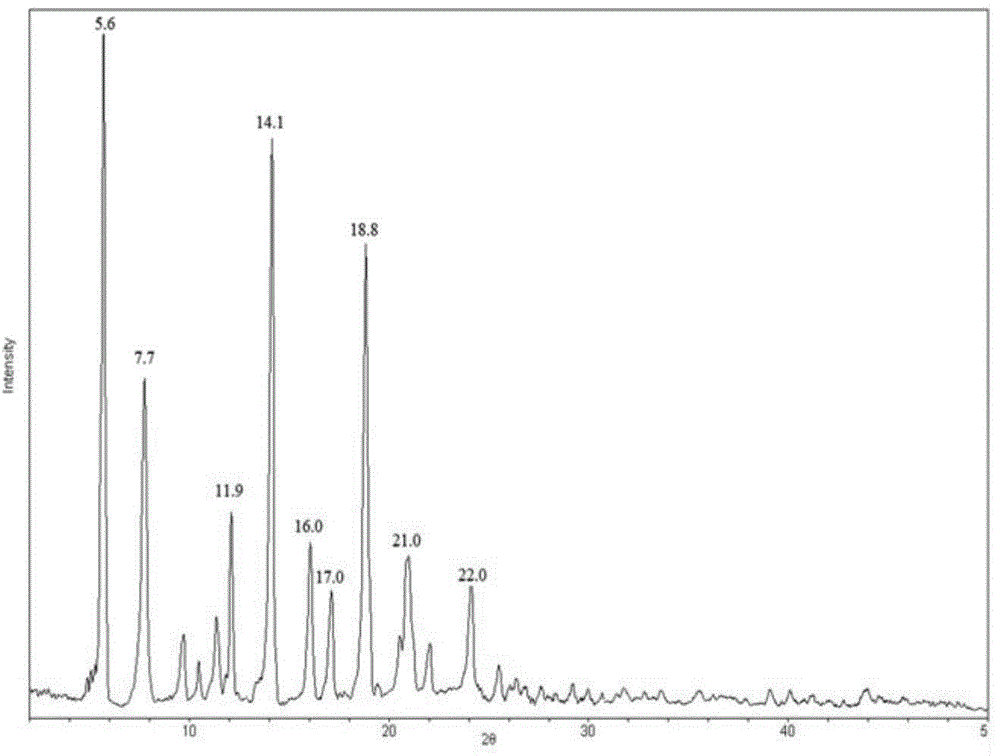
[0052] Get 12g of carteprednol and add 300ml of acetone, 60ml of n-hexane, and 60ml of acetonitrile in the mixed solution and heat until fully dissolved, put it in a 1000ml beaker, seal it with parafZlm sealing film, and pierce several small holes on the film, Place at room temperature at 20°C and evaporate slowly. After a few days, crystals precipitated out. Pour off the solution, let the crystal dry naturally, take it off carefully, pick a better crystal and send it as a sample for single crystal diffraction. Other crystals were crushed with a mortar and used as seeds. The following unit cell parameters were determined by single crystal diffraction structure analysis: α=90deg.; β=94.140(6)deg; γ=90deg; unit cell volume is The crystal belongs to monoclinic crystal system, space group, P2(1), and Z value is 2. The prepared single crystal I is subjected to X-ray powder diffraction measurement on the crystalline powder after crushing with a mortar, and the measured chara...
Embodiment 1-2
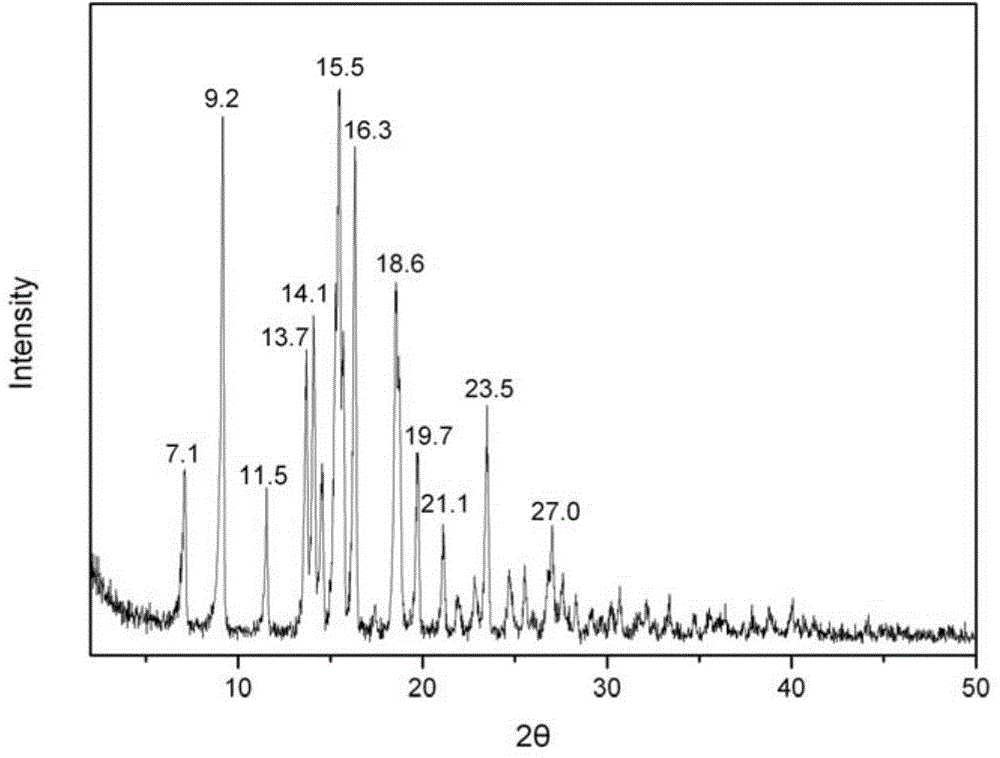
[0054] Take 5g of carteprednol and add 100ml of acetone, 20ml of n-hexane, and 20ml of acetonitrile in a mixed solution heated to 50°C, hot filter to remove insoluble matter, cool to 30°C (if any crystals are precipitated, take the supernatant solution), and then add the seed crystal prepared in Example 1-1 of the invention, keep stirring for 30 minutes, a large amount of crystals are precipitated, cooled to 0-5°C, filtered, dried, and the obtained crystals are subjected to X-ray powder diffraction measurement, and the obtained The characteristic peak positions are 2θ=7.1°, 9.2°, 11.5°, 13.7°, 14.1°, 15.5°, 16.3°, 18.6°, 19.7°, and it is confirmed as etabacloteprednol crystal form I.
[0055] Molecular formula: C24H31ClO7
[0056] Elemental analysis theoretical value: C, 61.73; H, 6.69; Cl, 7.59
[0057] Elemental analysis found values: C, 61.78; H, 6.75; Cl, 7.58
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com