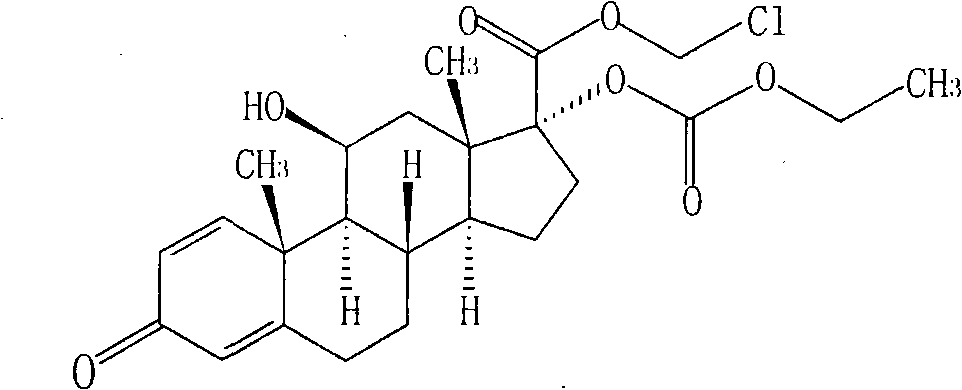
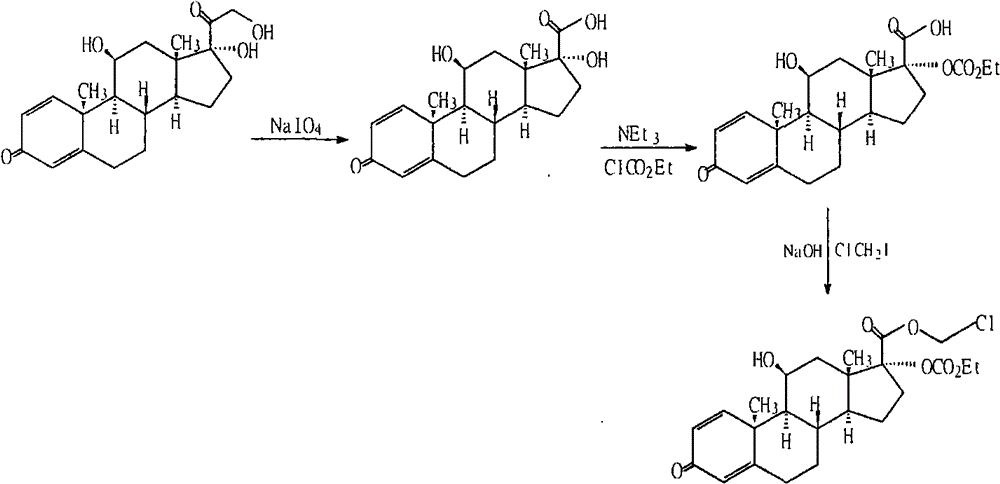
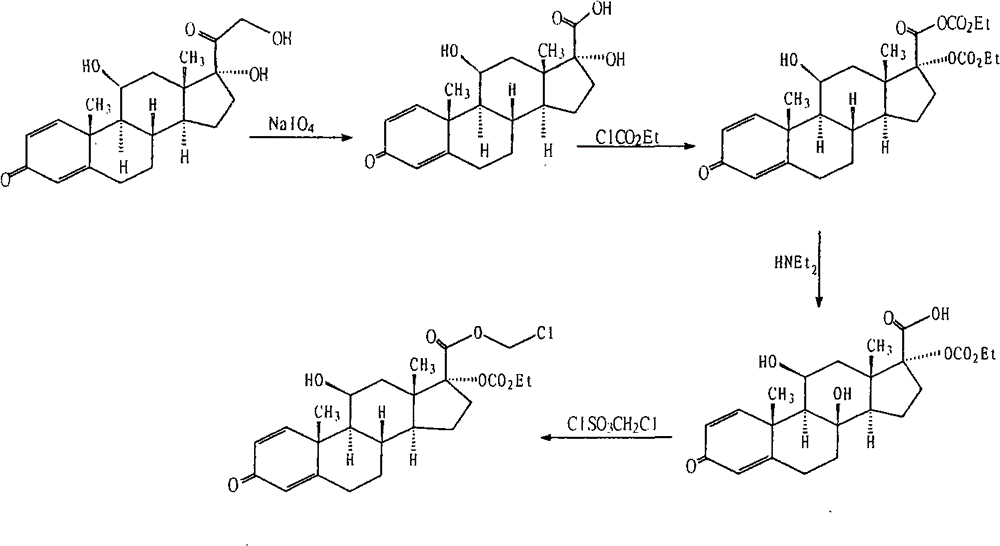
Purification method of loteprednol etabonate
A technique of loteprednol and a purification method, applied in the field of purification of loteprednol, can solve problems such as failure to meet medicinal requirements, difficult removal of impurities and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0024] Take 50 grams of loteprednol crude product, add it in a 1000 ml four-necked round bottom flask, add 500 ml of absolute ethanol, heat to reflux, the inner temperature is 78.5 ° C, stir and dissolve, cool down slightly, add 25 grams of activated carbon, continue Stir for 10 minutes, heat filter, the filtrate is naturally cooled to room temperature, natural crystallization, after airtight placement for 48 hours, suction filtration, washing with a small amount of ethanol, and vacuum drying at 50°C to obtain 43 grams of loteprednol fine product, the yield is 86%. HPLC detects that the content is 99.6%, and the content of the impurity peaks of related substances is all below 0.1%.
example 2
[0026] Take 50 grams of loteprednol crude product, add it in a 1000 milliliter four-neck round bottom flask, add 600 milliliters of isopropanol, heat to reflux, the inner temperature is 82.5 ℃, after stirring and dissolving, cool down slightly, add 25 grams of activated carbon, continue Stir for 10 minutes, heat filter, the filtrate is naturally cooled to room temperature, natural crystallization, after airtight placement for 48 hours, suction filtration, washing with a small amount of ethanol, and vacuum drying at 50°C to obtain 42 grams of loteprednol fine product, the yield is 84%. HPLC detects that the content is 99.5%, and the content of the impurity peaks of related substances is all below 0.1%.
example 3
[0028] Take 50 grams of loteprednol crude product, add it to a 1000 ml four-necked round-bottomed flask, add 700 ml of anhydrous, heat to reflux, the inner temperature is 64.5°C, stir and dissolve, then cool down slightly, add 30 grams of activated carbon, and continue stirring 10 minutes, hot filtration, filtrate naturally cooled to room temperature, natural crystallization, airtight place after 48 hours, suction filtration, a small amount of ethanol washing, 50 ℃ of vacuum drying, obtain loteprednol fine product 40 grams, yield is 80%, by HPLC Detection, the content is 99.5%, and the content of the impurity peaks of related substances is all below 0.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com