Stable loteprednol etabonate and tobramycin compound composition
A technology of ecaroteprednol tobramycin and ecaroteprednol, which is applied in drug combinations, steroids, and pharmaceutical formulations, and can solve the problems of rapid growth of impurities and poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Invention Example 1 Preparation of etabacloteprednol monohydrate
[0050] Invention Example 1-1 Supercritical method for preparing etabac loteprednol monohydrate
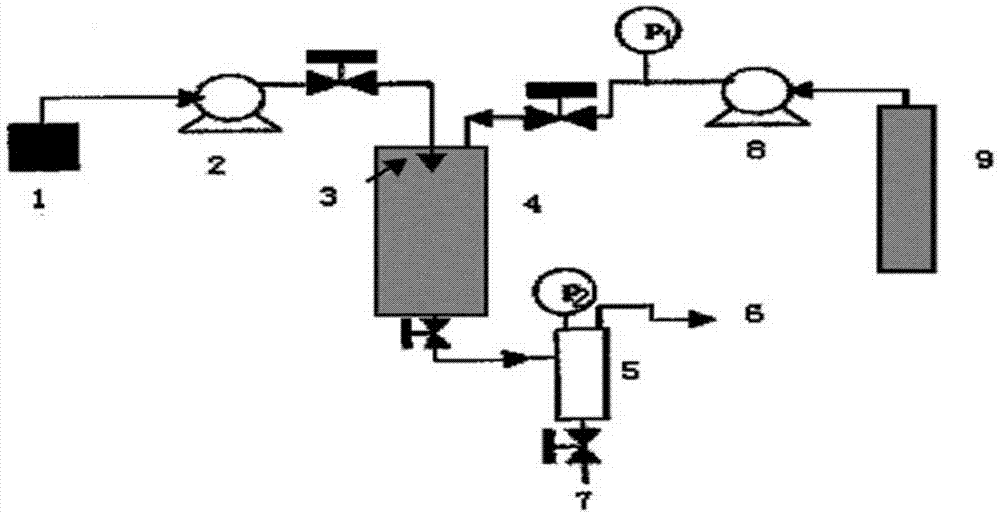
[0051] ⑴Configure etaborteprednol solution 1: Dissolve 5g etabaclotepdnol completely in a mixed solution of 300ml acetone and 30ml water at 50°C;
[0052] (2) The etabacloteprednol solution 1 configured in step (1) is connected to the solution pump 2, and the working pressure is controlled to be 15MPa;
[0053] (3) Carbon dioxide feed: the CO in the cylinder 2 Input the supercritical fluid anti-solvent equipment system through the booster pump 8, enter the crystallization kettle 4, control the flow rate at 10ml / min, control the starting temperature at 50°C, and the pressure at 15MPa;
[0054] (4) The etaban loteprednol solution 1 configured in the above steps (1) is rapidly sprayed into the crystallization kettle 4 through the nozzle 3 in the supercritical fluid antisolvent equipment system by the solution ...
Embodiment 1-2
[0059] Invention Example 1-2 Preparation of Eccarteprednol Monohydrate
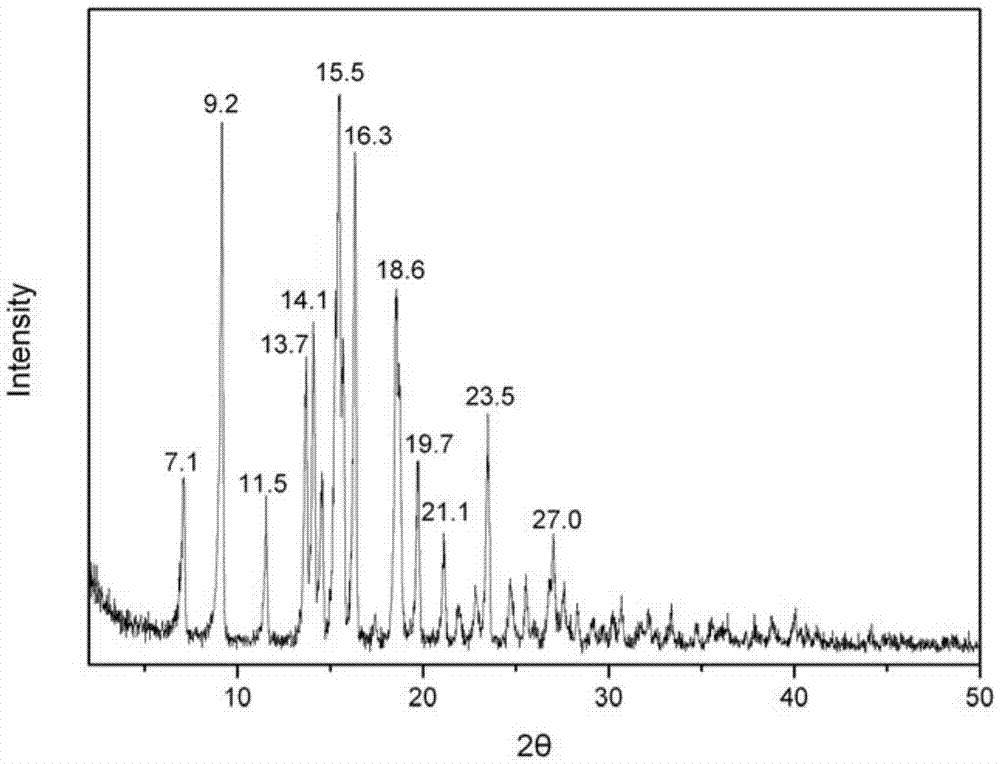
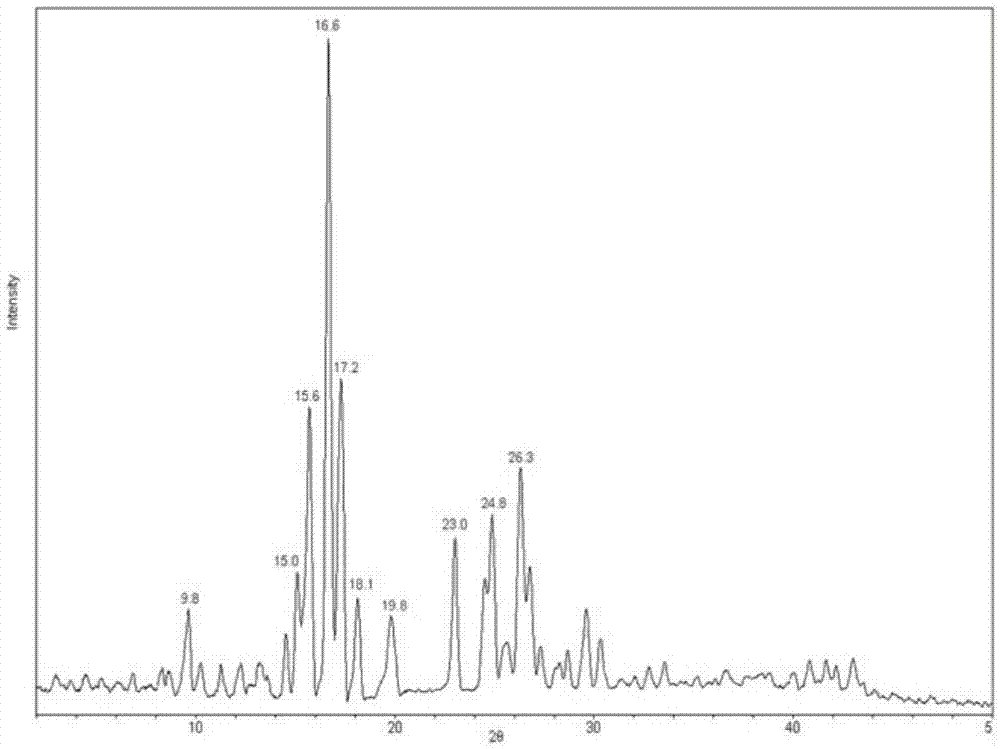
[0060] Take 5g of carteprednol and add 100ml of ethanol, 20ml of water, and 20ml of acetonitrile in a mixed solution, heat to 50°C, heat filter to remove insoluble matter, cool to 30°C (if crystals are precipitated, take the supernatant ), then add the seed crystals prepared in Example 1-1 of the invention, heat and stir for 30 minutes, a large amount of crystals are precipitated, cooled to 0-5°C, filtered, and dried. The dried crystals are analyzed by TG-DTA, and the weight loss is about 3.7%. , identified as etabacloteprednol monohydrate. The obtained crystal was subjected to X-ray powder diffraction measurement, and the measured characteristic peak positions were 2θ=9.8°, 15.0°, 15.6°, 16.6°, 17.2°, 18.1°, 19.8°, 23.0°, 24.8°, 26.3°.
Embodiment 1-3
[0061] Invention Example 1-3 Preparation of Eccarteprednol Monohydrate
[0062] Take 5g of carteprednol and add 100ml of ethanol, 20ml of water, and 30ml of acetonitrile and heat to 50°C, heat filter to remove insoluble matter, cool to 30°C (if crystals are precipitated, take the supernatant ), then add the seed crystals prepared in Example 1-1 of the invention, heat and stir for 30 minutes, a large amount of crystals are precipitated, cooled to 0-5°C, filtered, and dried. The dried crystals are analyzed by TG-DTA, and the weight loss is about 3.7%. , identified as etabacloteprednol monohydrate. The obtained crystal was subjected to X-ray powder diffraction measurement, and the measured characteristic peak positions were 2θ=9.8°, 15.0°, 15.6°, 16.6°, 17.2°, 18.1°, 19.8°, 23.0°, 24.8°, 26.3°.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com