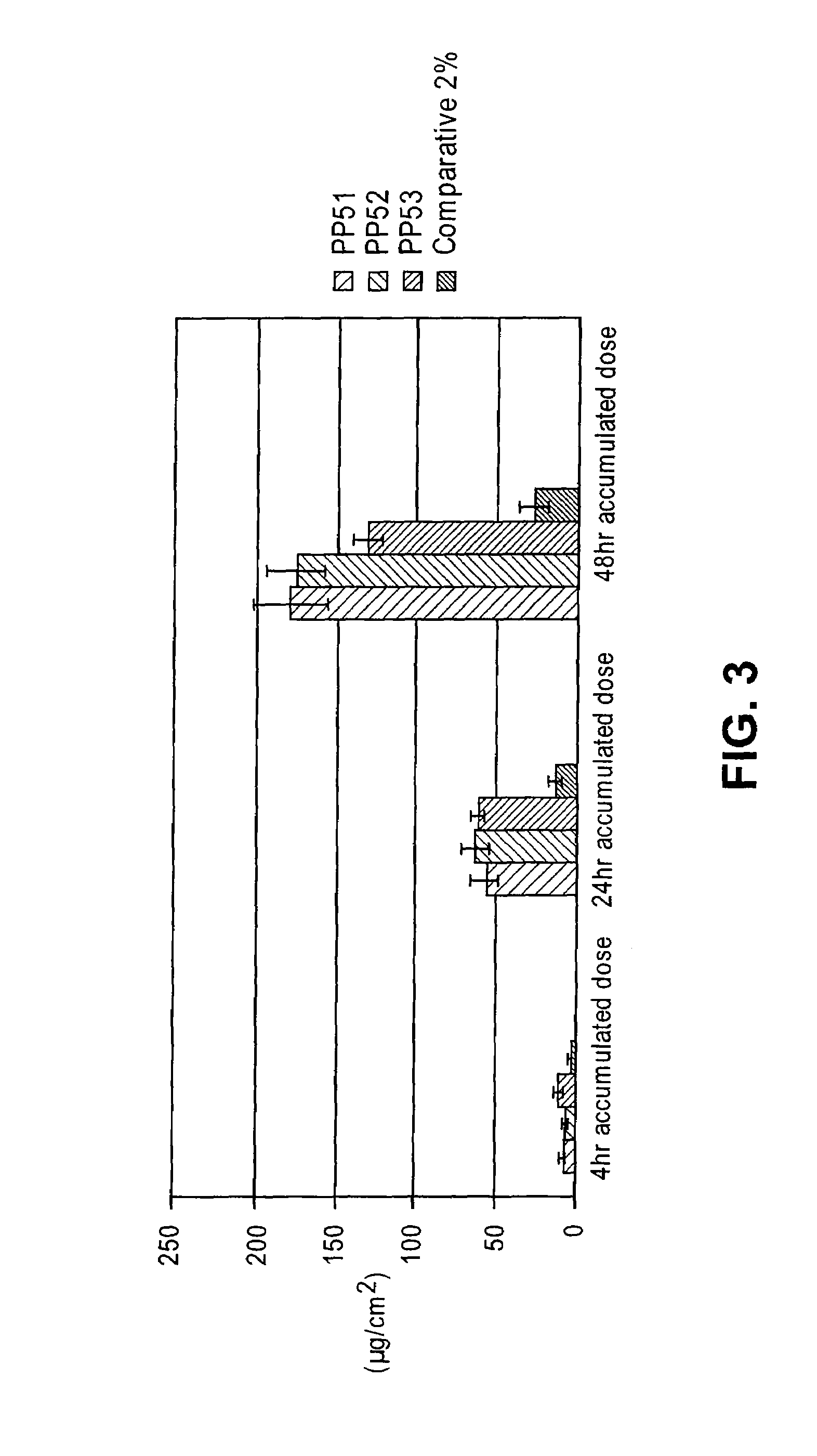
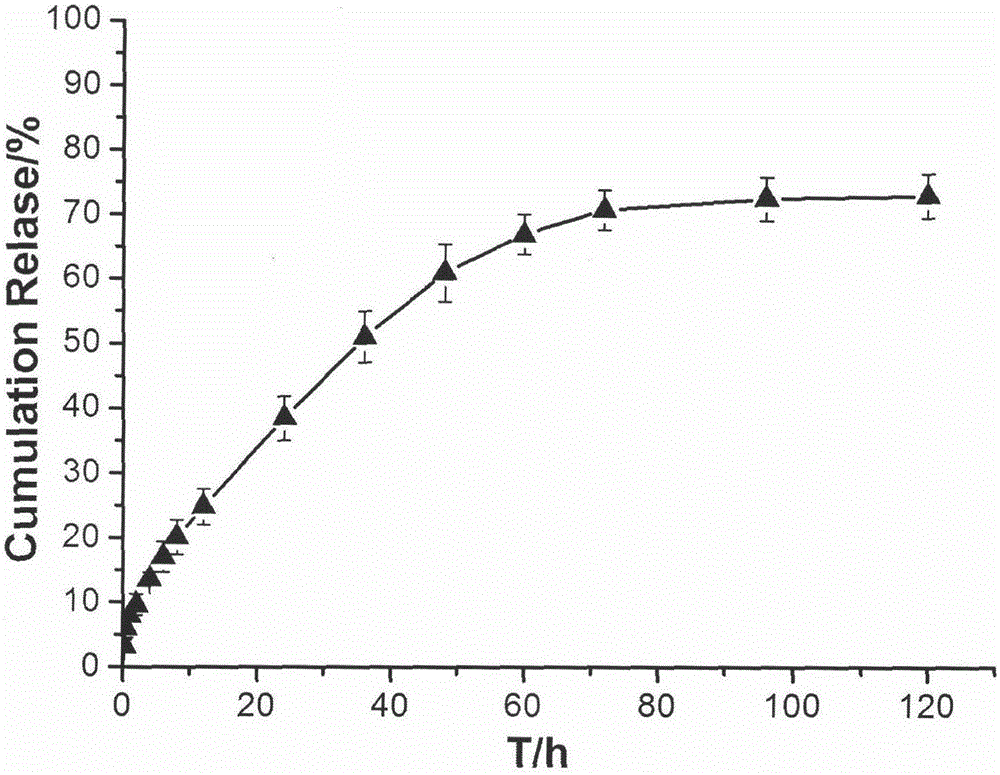
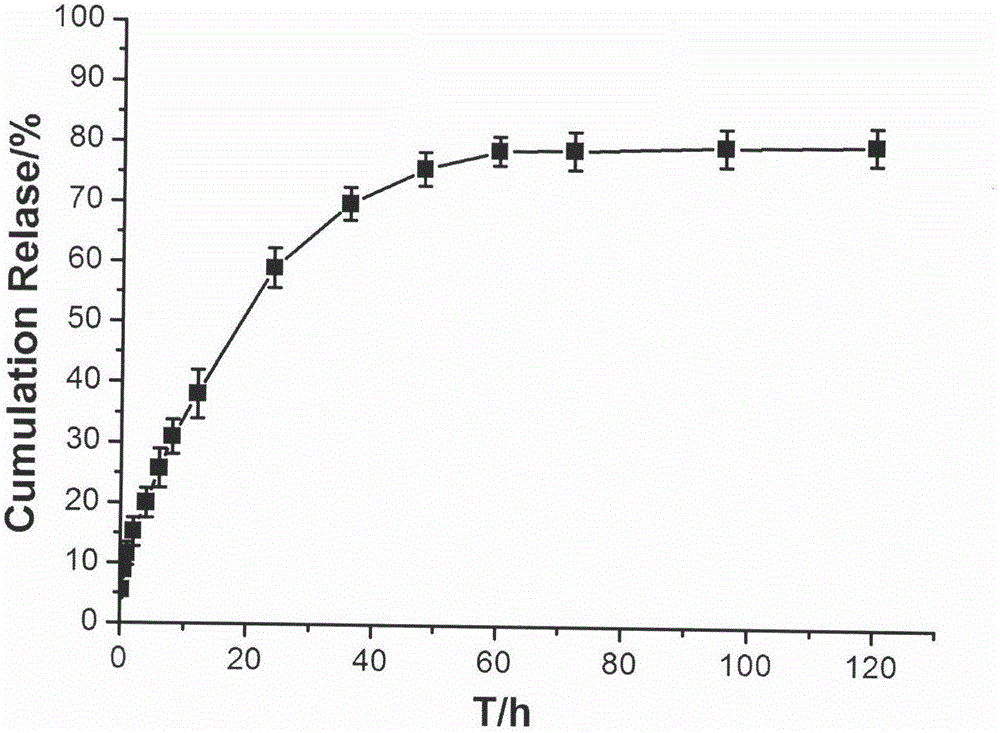
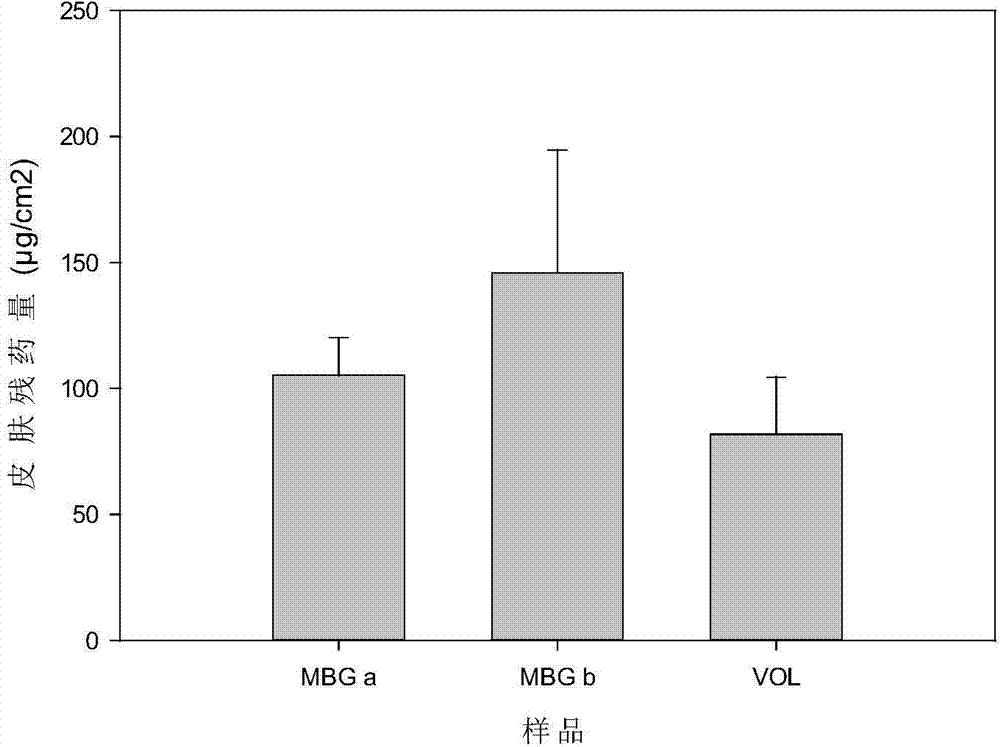
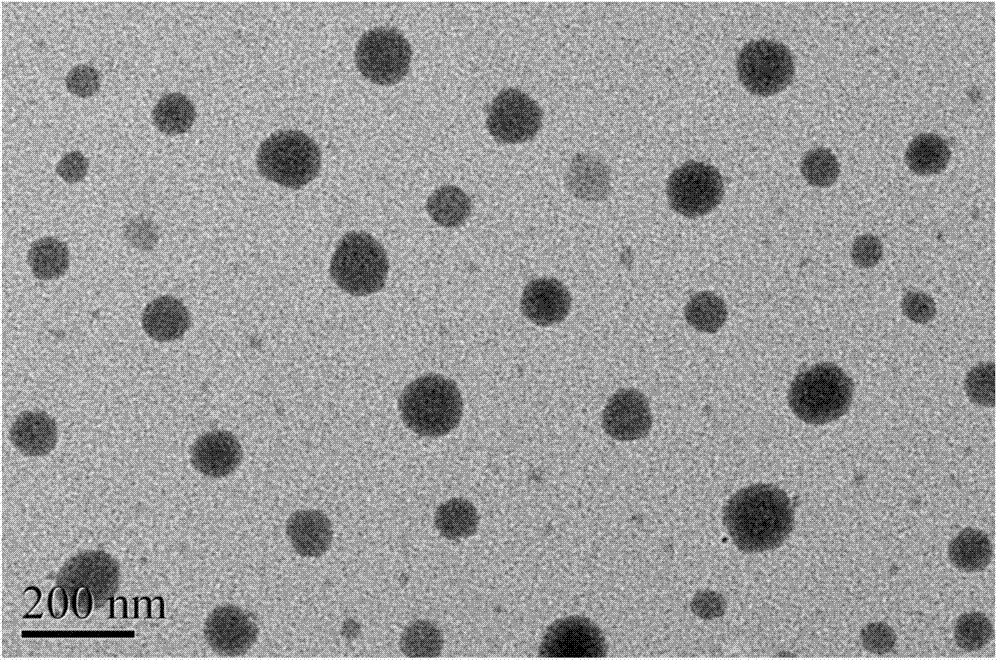
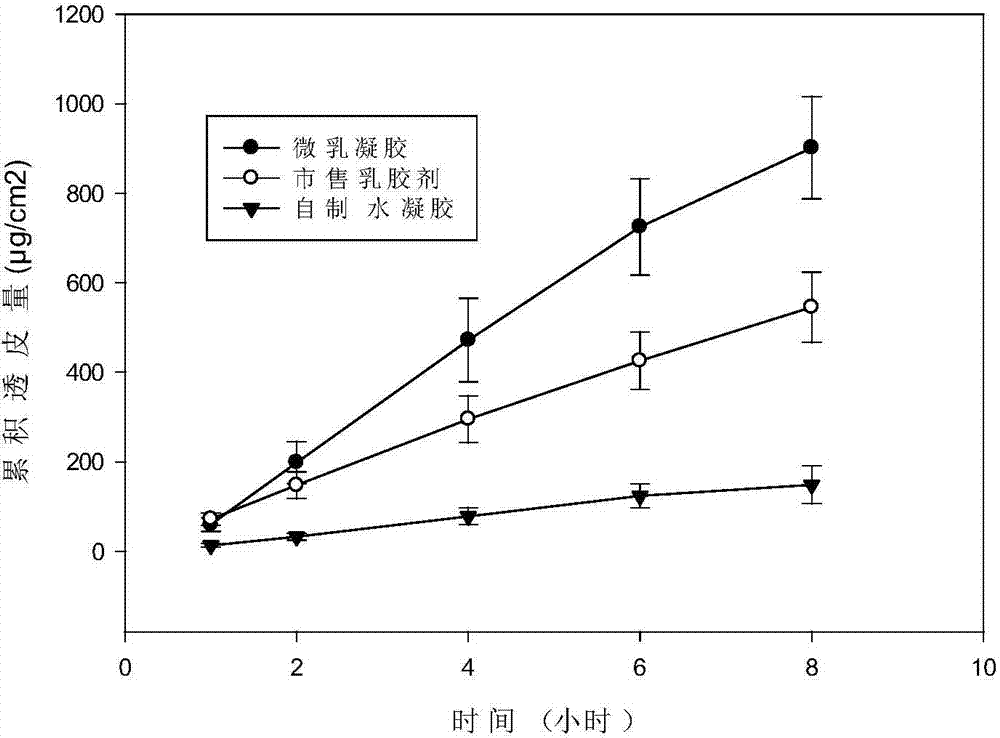
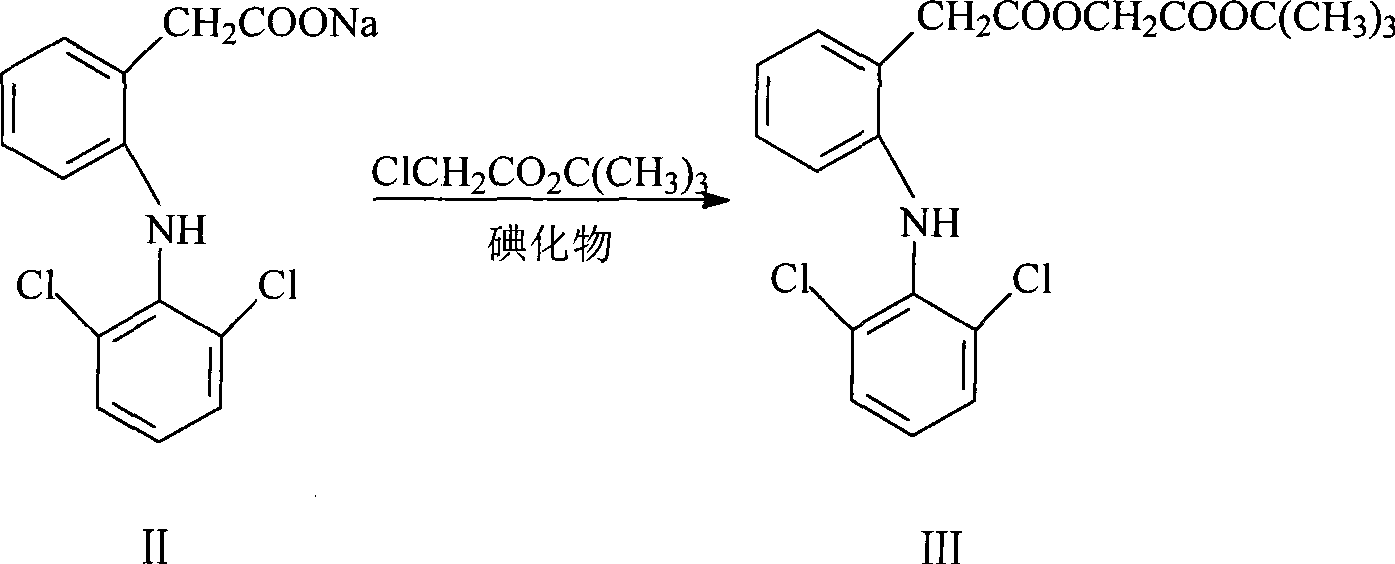
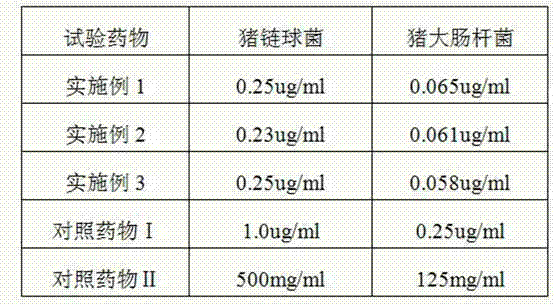
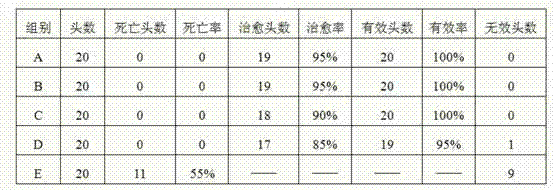
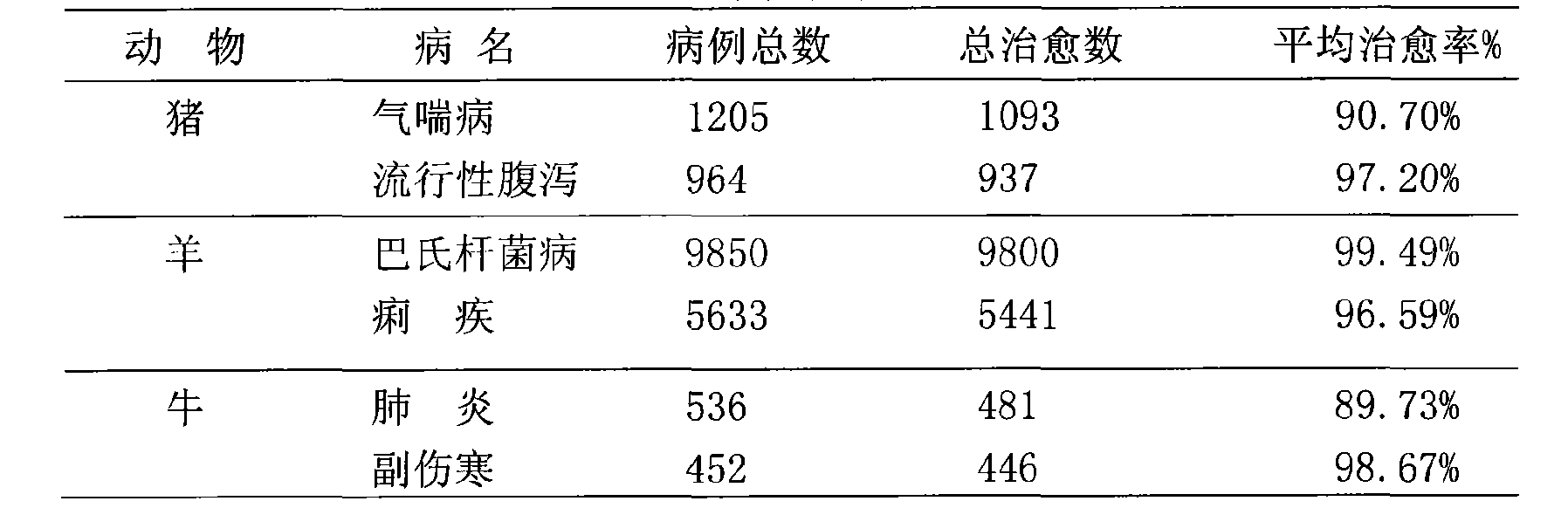
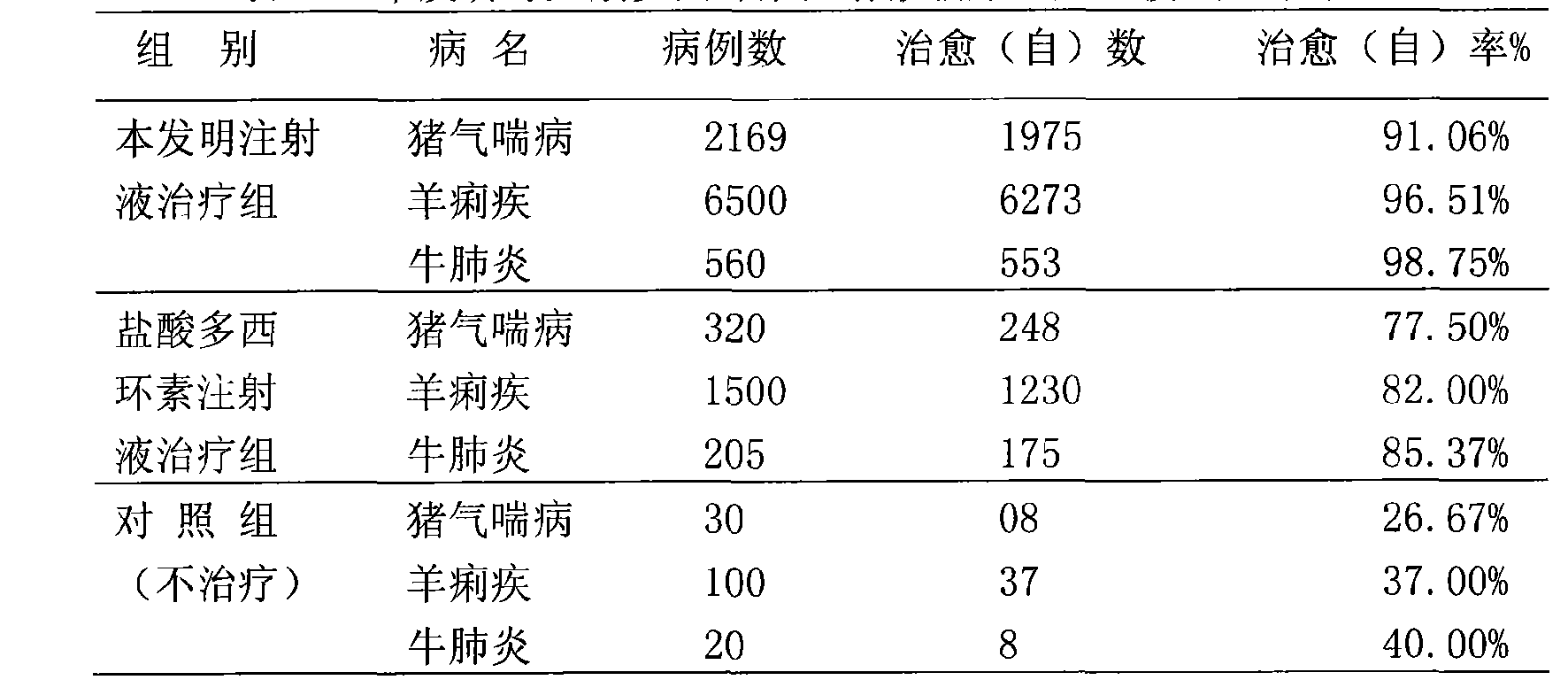
Patents
Literature
313 results about "Diclofenac Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The sodium salt form of diclofenac, a benzene acetic acid derivate and nonsteroidal anti-inflammatory drug (NSAID) with analgesic, antipyretic and anti-inflammatory activity. Diclofenac sodium is a non-selective reversible and competitive inhibitor of cyclooxygenase (COX), subsequently blocking the conversion of arachidonic acid into prostaglandin precursors. This leads to an inhibition of the formation of prostaglandins that are involved in pain, inflammation and fever.
Diclofenac gel
ActiveUS20080300311A1Extended drying timeHigh viscosityOrganic active ingredientsBiocideDiclofenac SodiumTopical treatment
The present invention provides a gel formulation comprising diclofenac sodium which has superior transdermal flux properties, which may be used for the topical treatment of pain, such as in osteoarthritis.
Owner:HORIZON THERAPEUTICS IRELAND DAC
Controlled and extended delivery of hyaluronic acid and comfort molecules via a contact lens platform
ActiveUS8388995B1Avoid effectivenessImprove bioavailabilityPharmaceutical delivery mechanismPharmaceutical non-active ingredientsCross-linkDiclofenac Sodium
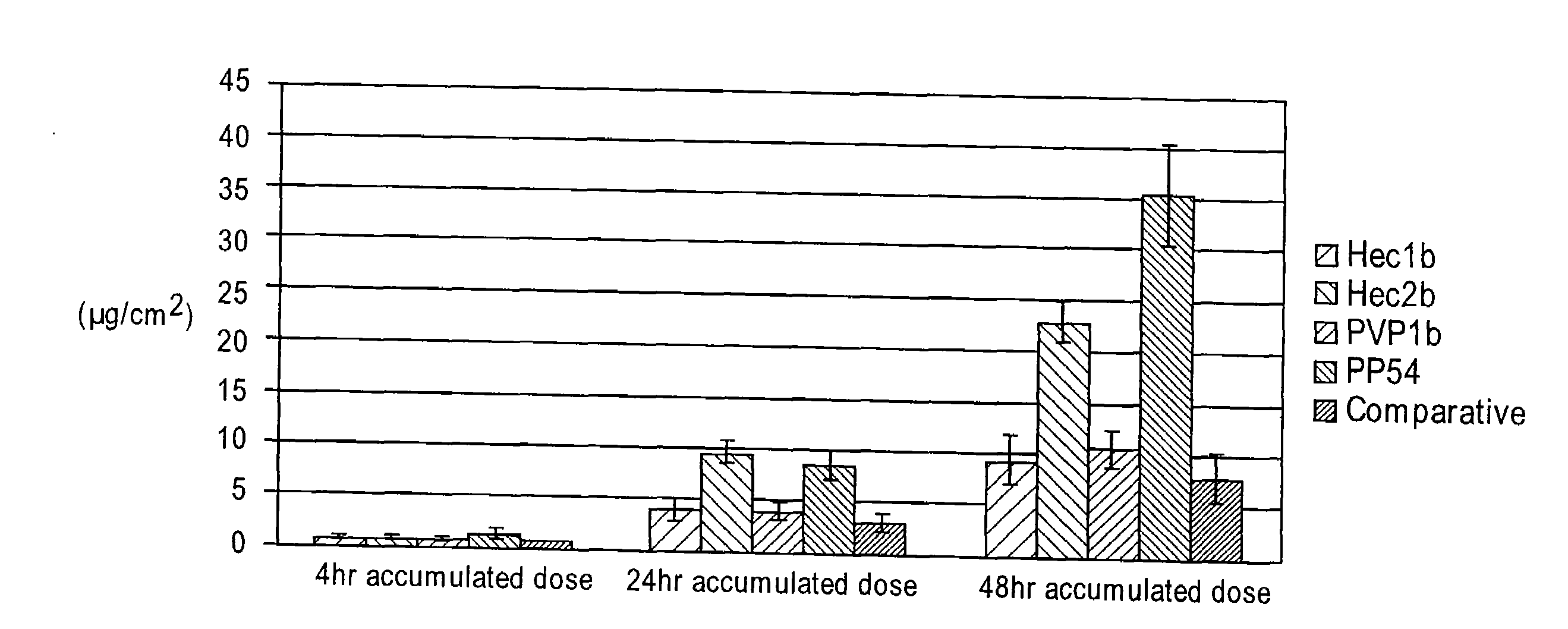
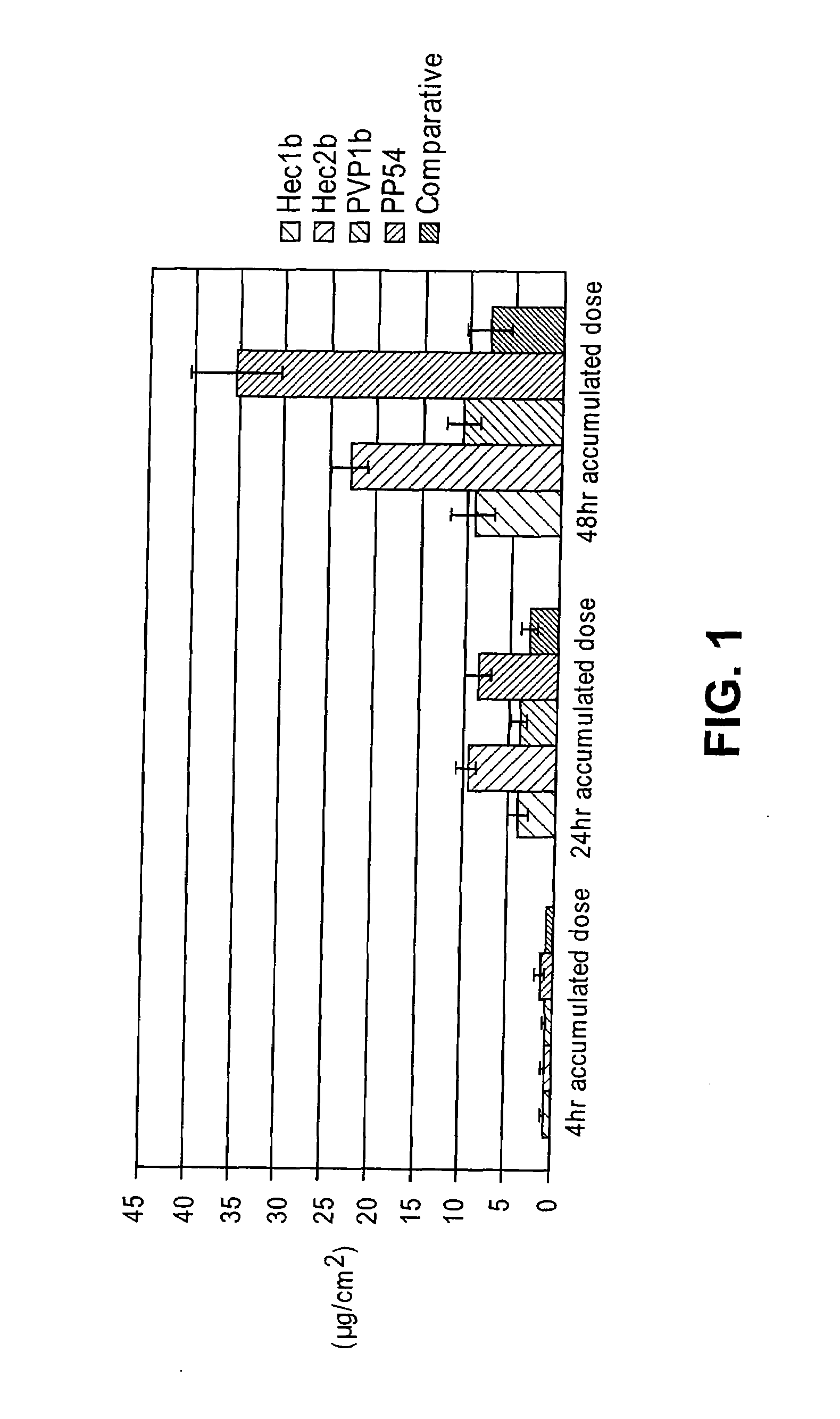
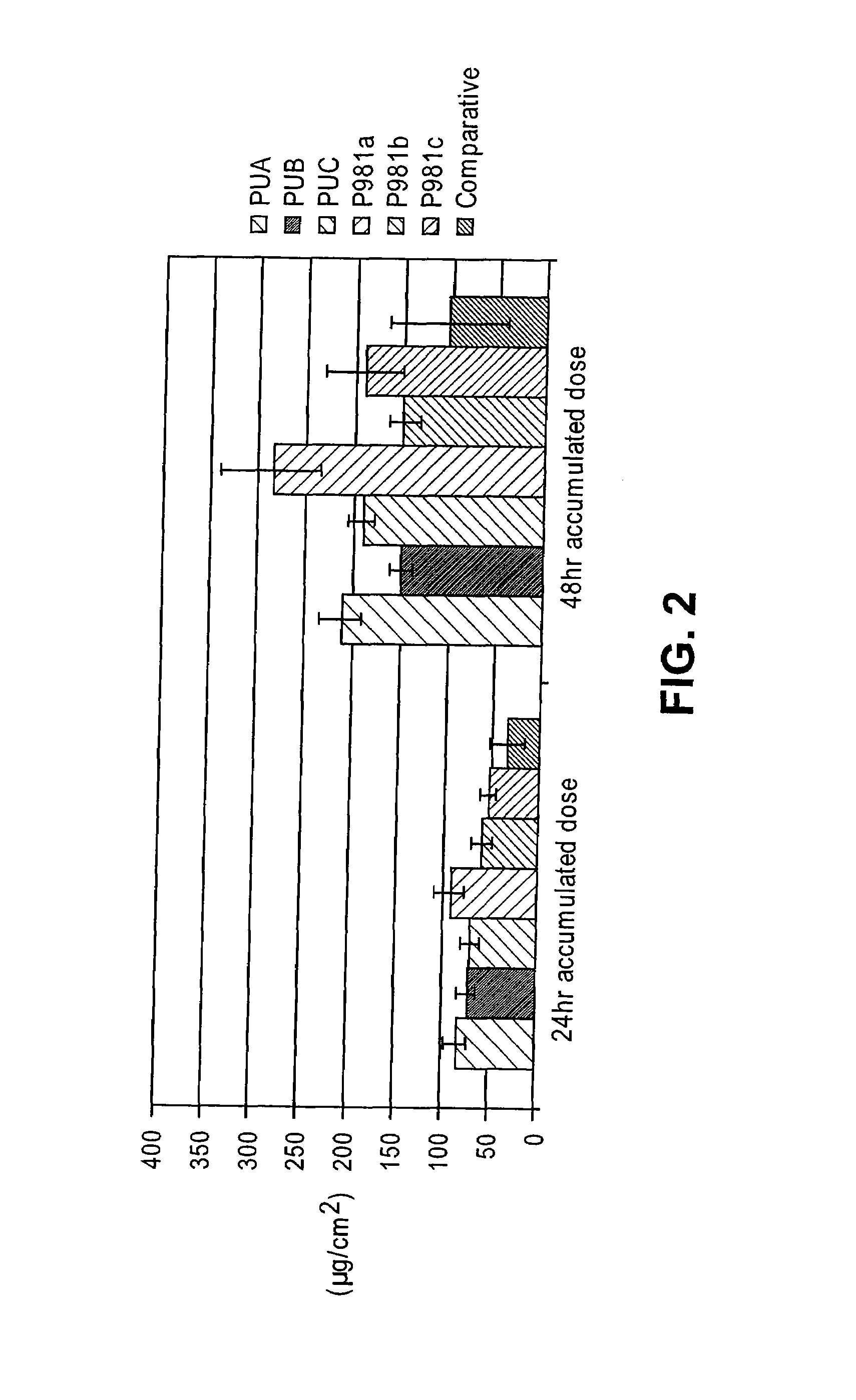
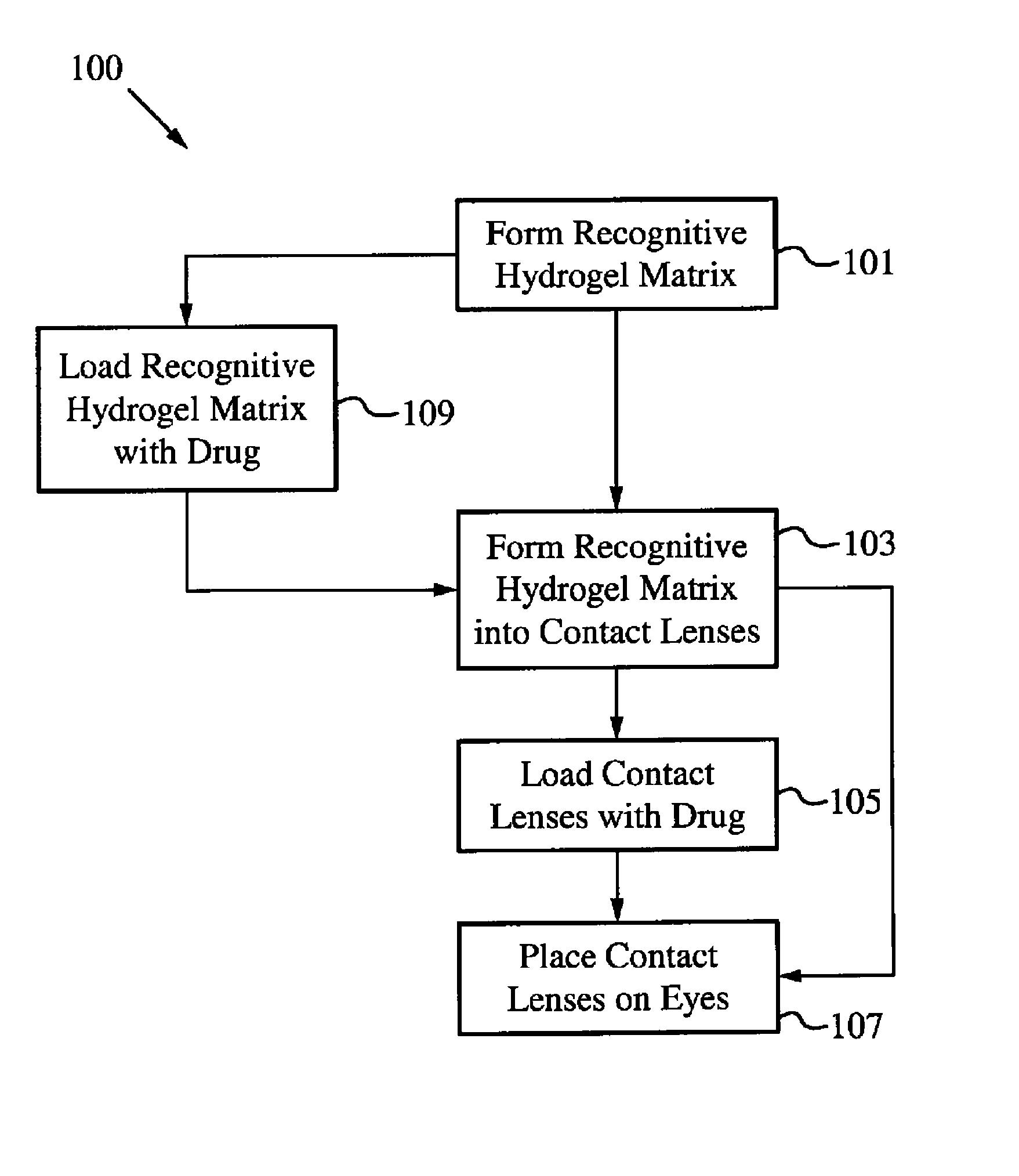
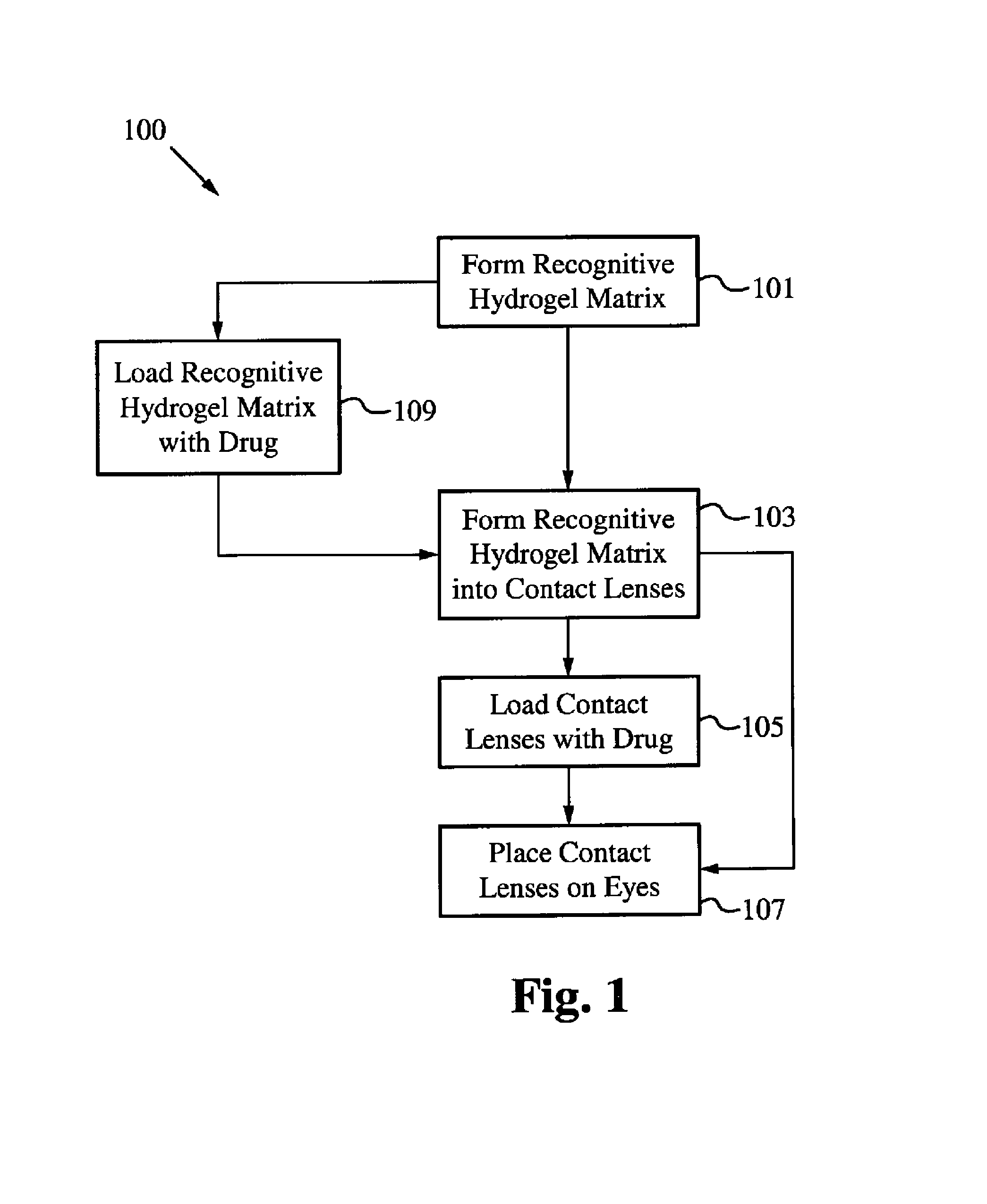
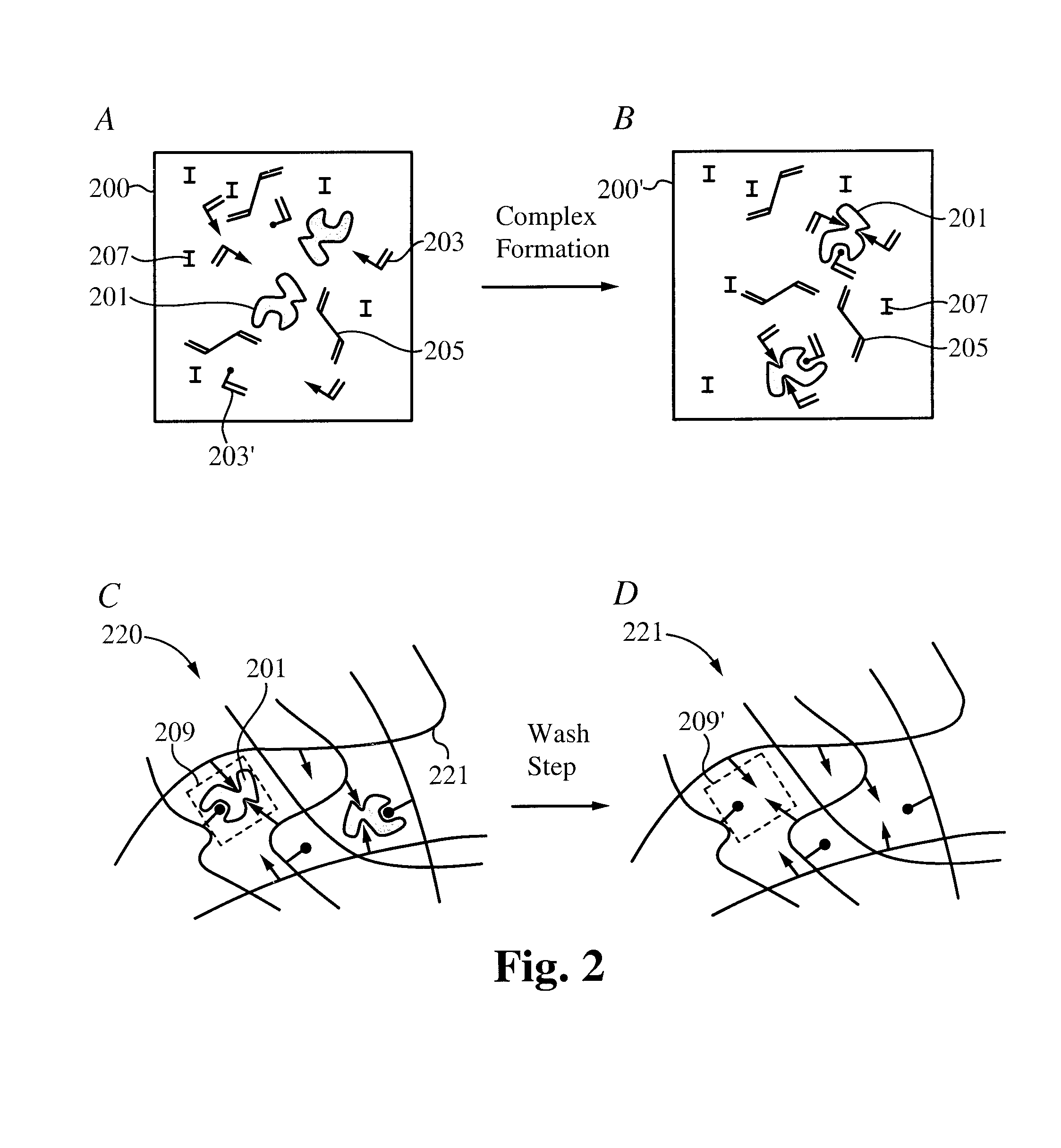
A drug delivery system is disclosed. The drug delivery system includes a recognitive polymeric hydrogel through which a drug is delivered by contacting biological tissue. The recognitive polymeric hydrogel is formed using a bio-template, which is a drug or is structurally similar to the drug, functionalized monomers, preferably having complexing sites, and cross-linking monomers, which are copolymerized using a suitable initiator. The complexing sites of the recognitive polymeric hydrogel that is formed mimic receptor sites of a target biological tissue, biological recognition, or biological mechanism of action. A system in accordance with some embodiments is a contact lens for delivering a drug through contact with an eye. In some embodiments, the drug is an anti-microbial, such as an anti-fungal agent for treatment of large animals. In some embodiments, a comfort molecule hyaluronic acid (HA) is delivered. In some embodiments, ketotifen fumarate (anti-histamine) and / or diclofenac sodium (anti-inflammatory) are delivered.
Owner:AUBURN UNIV
Diclofenac sodium pastes and preparation thereof
ActiveCN1489996AEasy to useImprove skin penetrationOrganic active ingredientsAntipyreticTectorial membraneOrganic solvent
A plaster of diclofenac sodium is composed of substrate, medicine layer containing diclofenac sodium, pressure sensitive adhesive and penetration promoter, and protecting film. Its advantages are high percutaneous effect and adhesion, and no irritation and sensitization to skin.
Owner:BENGBU BBCA MEDICINE SCI DEV +1
Diclofenac topical formulation
ActiveUS8252838B2Reduce deliveryConvenient timeOrganic active ingredientsBiocideDiclofenac SodiumTopical treatment
The present invention provides a gel formulation comprising diclofenac sodium which has superior transdermal flux properties, which may be used for the topical treatment of pain, such as in osteoarthritis.
Owner:HORIZON THERAPEUTICS IRELAND DAC
Temperature-sensitive in-situ gel preparation composition for anticular injection and preparation method thereof
InactiveCN104546691ASmall toxicityImprove complianceOrganic active ingredientsAntipyreticDiseaseGel preparation
The invention relates to a temperature-sensitive in-situ gel preparation composition for intra-articular injection and a preparation method thereof, and belongs to the field of medicine preparations. The preparation method comprises the following steps: preparing diclofenac sodium-carrying sodium alginate microspheres by combining a microsphere technology with a temperature-sensitive gel technology, and then carrying the medicine-carrying microspheres in a chitosan / beta-sodium glycerophosphate temperature-sensitive in-situ gel system to obtain a diclofenac sodium in-situ gel preparation composition. The composition is in a liquid state at a room temperature, capable of being converted to a semi-solid gel at a body temperature and forming a medicine storeroom at a medication part after being partially injected to enter an articular cavity, and beneficial to slowly releasing the medicine, prolonging the time of staying at the injection part, of the medicine, and enhancing the efficacy. The composition disclosed by the invention can be used for treating arthritis diseases of rheumatism, rheumatoid arthritis, osteoarthritis, synovitis and the like, as well as applicable to both diclofenac sodium and other arthritis treatment medicines of non-steroidal anti-inflammatory medicines, steroid hormone medicines, biological preparations and the like.
Owner:CHINA PHARM UNIV
Multifunctional microemlusion gel preparation and preparation process thereof
InactiveCN103655459AImprove skin penetrationImprove performanceAntimycoticsAntipyreticIndometacinActive agent
The invention discloses a multifunctional microemlusion gel preparation and a preparation process thereof, and belongs to the technical field of medicines. The preparation mainly comprises bulk pharmaceutical chemicals (such as non-steroidal anti-inflammatory drugs-diclofenac sodium, ibuprofen, indometacin, antifungal drugs-ornidazole, antiviral drugs-ganciclovir, hormone drugs-dexamethasone, local anesthesia drugs-lidocaine and irritants-menthol), a cationic polymer and a microemlusion, can further comprise gel or a thickener, and can be used for transdermal drug delivery and local drug delivery. The preparation process is simple, convenient, good in stability and pollution-free. Compared with existing cream and gel, the preparation has the advantages that a novel action mechanism is adopted, the accumulative penetration amount of unit area of drugs is remarkably increased, a certain slow-release effect is achieved, and the drug delivery frequency and the drug delivery amount can be reduced; a chemical penetration enhancer and a conventional preservative are not added, a certain bacterial inhibition effect is achieved, the skin irritation is avoided, and the use safety of the drugs is improved.
Owner:CHINA PHARM UNIV
Improved method for preparing aceclofenac
ActiveCN101531607AEasy to recycleShort reaction timeAntipyreticOrganic compound preparationIodideDiclofenac Sodium
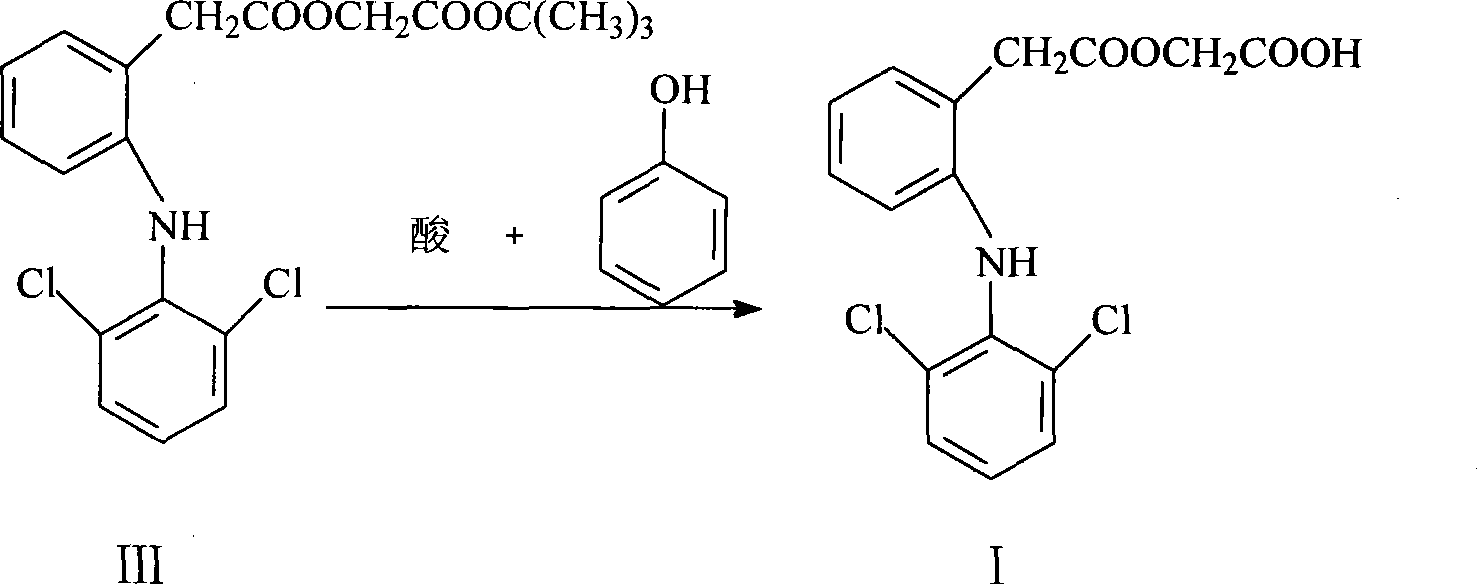
The invention provides an improved method used for preparing aceclofenac. The improved method is characterized in that (1) the method takes diclofenac sodium and tert-butyl chloroacetate as raw materials and iodide as a catalyst, and heats the substances to carry out condensation reaction; (2) the method takes tert-butyl aceclofenac as a raw material to carry out acidolysis reaction under the action of phenol and acid, and obtains aceclofenac crystal after post treatment and fine purification; and the total yield of both steps is above 88 percent and the content is over 99.2 percent (detected by HPLC). The improved method increases the yield and the content, and has short reaction time, simple and convenient operation and mild reaction conditions; and a reaction reagent is easy to recycle, so the improved method reaches the effects of lowering cost and reducing environment pollution.
Owner:LUNAN PHARMA GROUP CORPORATION
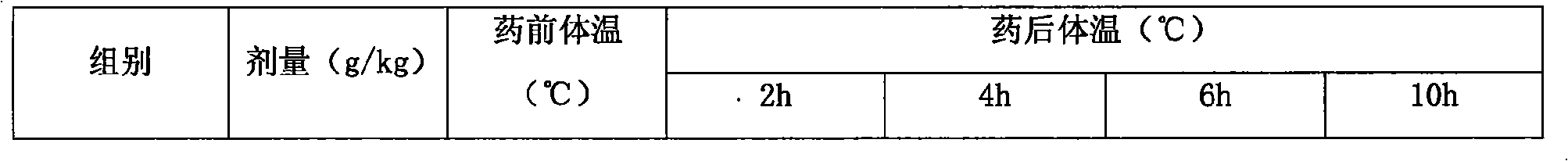
Compound paracetamol injection for livestock and process for preparing same
InactiveCN101015542AImprove survival rateIncrease weightOrganic active ingredientsAntipyreticDiclofenac SodiumPolyethylene glycol
The invention discloses a compound acetaminophen injection for livestock and its preparation method. the injection comprises acetaminophen 50-200, diclofenac sodium 10-50, polyethylene glycol-400 100-500, propylene glycol 100-500, and sodium bisulfite 10-30. the preparation method comprises (1) mixing polyethylene glycol-400 and propylene glycol, heating to 70-80deg.C, adding diclofenac sodium, stirring, adding acetaminophen, stirring; (2) heating water for injection to 30-40deg.C, adding sodium bisulfite, stirring; and (3) merging the solutions of steps 1 and 2, adjusting pH to 4.5-6.5 with hydrochloric acid or sodium hydroxide, and adding water for injection to 1000 to obtain the final product. The invention strengthen antipyretic and analgesic effects, is effective for treating pain and fever due to different kinds of inflammation, and can improve animal survival rate and increase animal body weight.
Owner:TIANJIN SHENGJI GRP CO LTD
Gargle for relieving pain for oral inflammation disease
InactiveCN101559077ANot pollutedGuaranteed chemical stabilityInorganic boron active ingredientsHydroxy compound active ingredientsDiseaseSodium bicarbonate
The invention belongs to the technical field of medicine, in particular to a drug composition of gargle which contains sodium dichlorophenolate and can relieve pain and be anti-inflammatory for the oral inflammation disease; wherein the drug composition comprises the following components by percentage: 0.10 to 30 percent of sodium dichlorophenolate hydroxypropyl-Beta-cyclodextrin inclusion (equal to 0.065 to 0.20 percent of concentration of sodium dichlorophenolate), 0.01 to 0.10 percent of lidocaine hydrochloride, 0.10 to 5.0 percent of borax, 0.1 to 2.0 percent of boric acid, 0.1 to 10 percent of glycerin, 0.10 to 5.0 percent of sodium bicarbonate, 0.001 to 0.10 percent of vitamin B12, 0.02 to 1.0 percent of tromethamine, 0.01 to 5.0 percent of sucralose, 0.10 to 5.0 percent of peppermint essence and 0.01 to 1.0 percent of sodium benzoate; a proper amount of pharmaceutical caramel color and water for injection is added till to reach the needed weight / volume concentration. All the above components are weighted by weight / volume percentage; the aqueous solution of the composition has the pH value of 6.5 to 9.0. The composition has little thrill to oral mucosa, stable storage for long time and good biological tolerance and has fast and long-lasting curative effect for relieving pain and being anti-inflammatory to the oral inflammation disease.
Owner:官培龙 +1
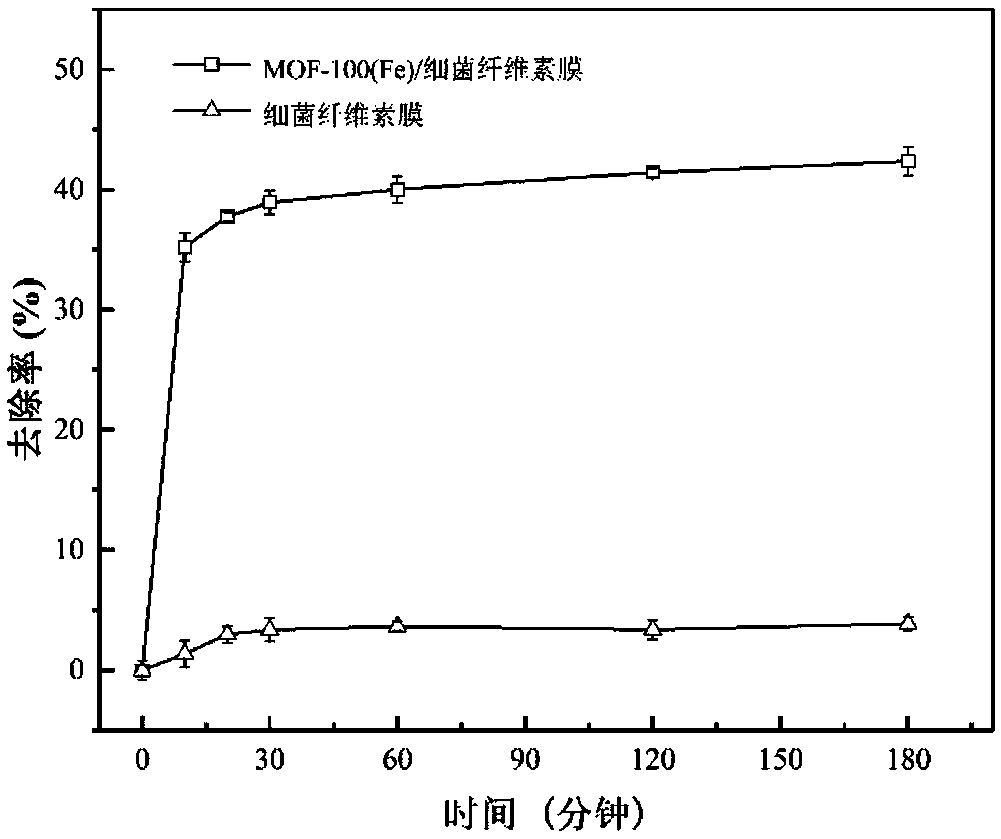
MOF-100(Fe)/bacterial cellulose composite material and preparation method and application thereof
ActiveCN108636368AEasy to useGood removal effectOther chemical processesWater contaminantsOscillatory reactionDiclofenac Sodium
The invention discloses a MOF-100(Fe) / bacterial cellulose composite material and a preparation method and application thereof. The preparation method comprises 1) activating a bacterial cellulose membrane in an alkali mixed solution to obtain an activated bacterial cellulose membrane, 2) orderly putting the activated bacterial cellulose membrane into an ethanol solution of ferric iron and an ethanol solution of trimesic acid, carrying out oscillation for a reaction and repeating oscillation multiple times to obtain the MOF-100(Fe) / bacterial cellulose composite material as a wet membrane, and 3) putting the wet membrane into an alkali solution for a reaction and carrying out washing to obtain the MOF-100(Fe) / bacterial cellulose composite material. The MOF-100(Fe) / bacterial cellulose composite material obtained through the cyclic oscillation method has good adsorption effects on organic pollutant diclofenac sodium and is easy to recover and recycle.
Owner:RENMIN UNIVERSITY OF CHINA
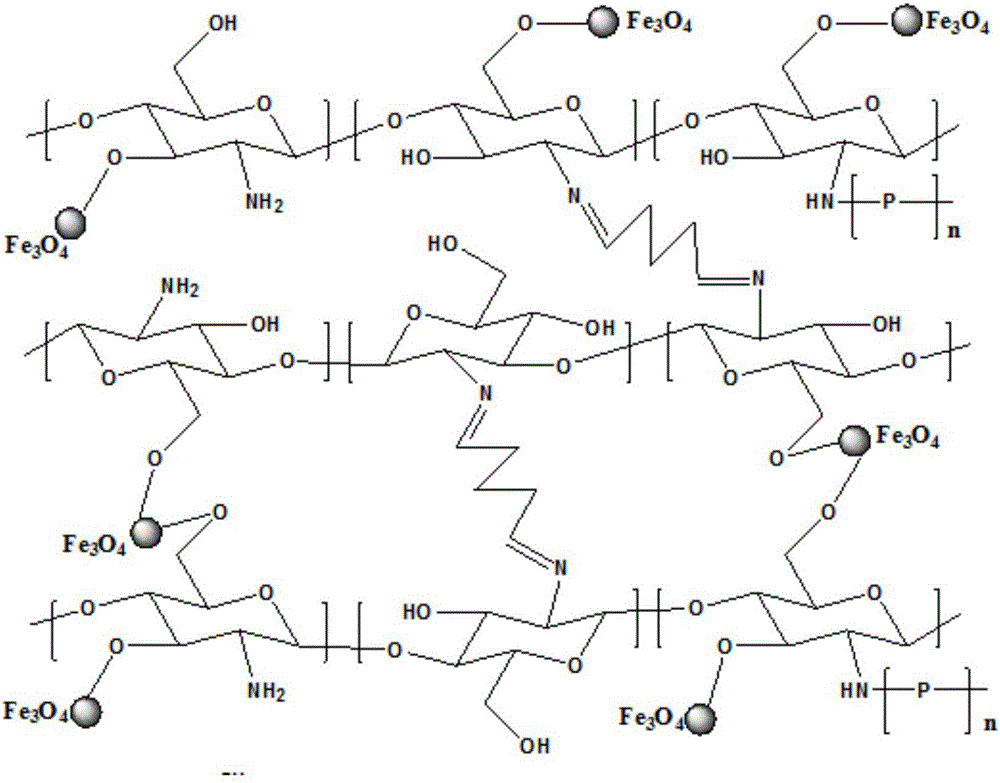
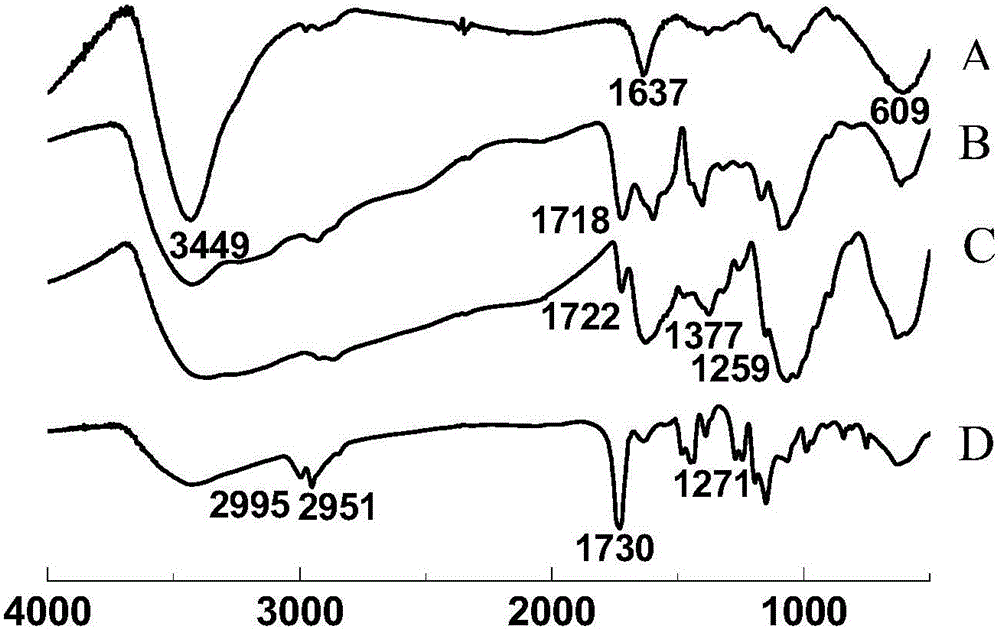
Chitosan magnetic composite spherule adsorbent with core-brush structure as well as preparation method and application thereof
InactiveCN106040194ALow costImprove adsorption capacityOther chemical processesWater contaminantsCrosslinked chitosanSorbent
The invention discloses a chitosan magnetic composite spherule adsorbent with core-brush structure as well as a preparation method and application thereof and belongs to the technical field of adsorbents. The preparation method comprises the following steps of adding a mixed liquor containing ferric ions and ferrous ions into a solution containing chitosan by use of a magnetic carrier technology, so as to obtain non-crosslinked chitosan magnetic composite spherules; then adding a glutaraldehyde solution so as to obtain crosslinked chitosan magnetic composite spherules; weighing the crosslinked chitosan magnetic composite spherule, triggering with potassium peroxodisulfate, and then adding a monomer (poly-methylacryloyl MAETAC, polyacrylic acid or polymethyl methacrylate), thereby obtaining the modified chitosan magnetic composite spherule adsorbent with the core-brush structure. According to the adsorption experiments, the synthetized modified chitosan magnetic composite spherule adsorbent with the core-brush structure has the characteristics of biodegradable property, easiness for separation from water, reusability, favorable adsorption effect on chemical diclofenac sodium and the like.
Owner:NANJING NORMAL UNIVERSITY
Medicine composition containing matrine class alkaloid, preparation method and pharmaceutical application
The invention provides a medicine composition containing matrine class alkaloid, a preparation method and pharmaceutical applications. The medicine composition is the combination of the matrine class alkaloid and inflammation-resisting pain-relieving class medicines. The inflammation-resisting pain-relieving medicine comprises the non-steroidal inflammation-resisting medicines of aspirin, acetaminophen, indometacin, ibuprofen, oxyphenbutazone, naproxen, mefenamic acid, diclofenac sodium, celecoxib, rofecoxib, valdecoxib and the like and also comprises the vegetable inflammation-resisting pain-relieving class medicines of escin, ferulic acid, berberine, wilfordine, ephedrine and the like and the pain-relieving class medicines of morphia, demerol and the like. The matrine class alkaloid and one or various of the inflammation-resisting pain-relieving class medicines can form a medicine composition used for the pharmaceutical applications of resisting cold, allaying a fever and treating the swelling and pain of the bone joint and the muscle, rheumatic diseases, cardiovascular diseases, arteriosclerotic diseases, tumors, anaphylactic diseases, senile dementia, mosquito bite, insect bite and the like.
Owner:QINGDAO QIYUAN BIO TECH CO LTD
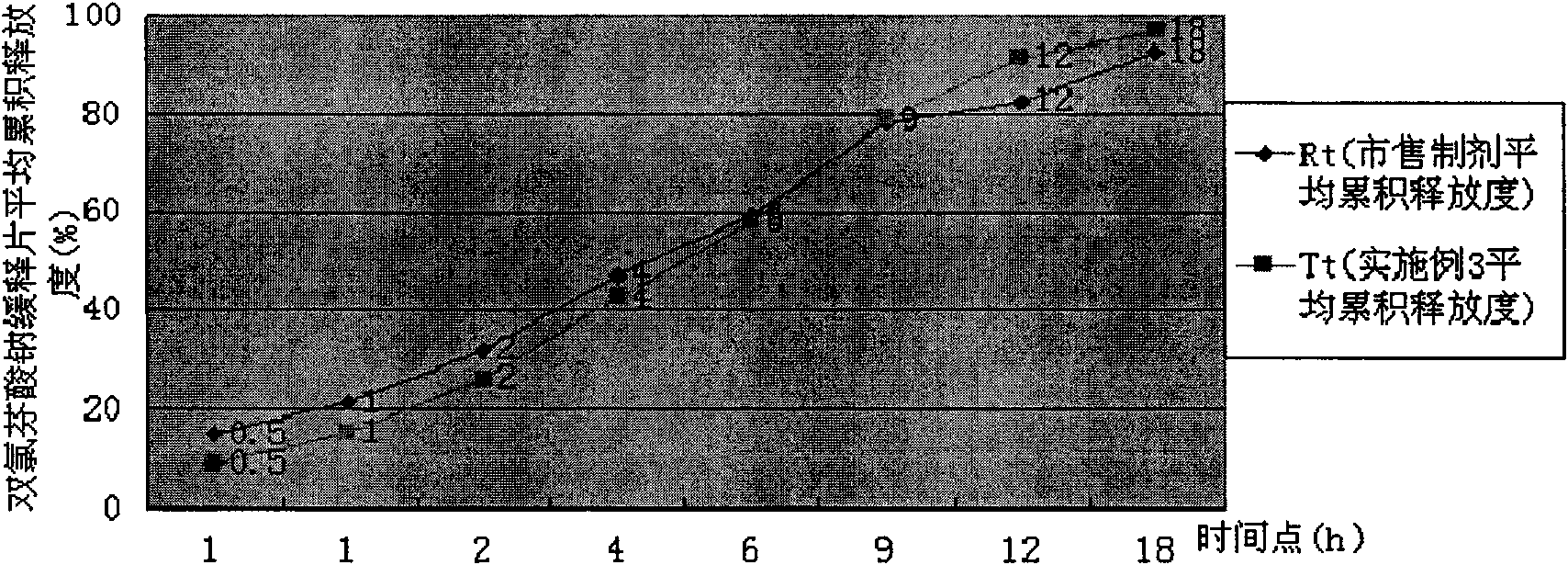
Sodium dichlorophenolate sustained-release tablet and method for controlling sustained-release of sodium dichlorophenolate sustained-release tablet
ActiveCN101574325ARaise quality standardsQuality assuranceOrganic active ingredientsAntipyreticSustained Release TabletDiclofenac Sodium
The invention discloses a sodium dichlorophenolate sustained-release tablet, and a method for controlling sustained-release of sodium dichlorophenolate sustained-release tablet. The sodium dichlorophenolate sustained-release tablet comprises sodium dichlorophenolate and HPMC high polymer material, the sodium dichlorophenolate accounts for 58.6-71.6% of the sum of the granules or the powder, wherein, the viscosity range of the granules or the powder of the prepared sodium dichlorophenolate sustained-release tablet is respectively 411-471centipoise and 955-1015centipoise. The sodium dichlorophenolate sustained-release tablet has quality conforming to the standard and similar release property with the preparation of the same type sold in markets, and the quality control is carried out before production, thus reducing re-doing, saving production cost, reducing energy consumption and improving productivity.
Owner:GUANGDONG HUANAN PHARMACEUTICAL GROUP CO LTD
Compound sulfamonomethoxine/compound sulfamonomethoxine sodium injection and preparation method
ActiveCN101810627AGood treatment effectAntibacterial agentsOrganic active ingredientsDiseaseSide effect
The invention discloses a compound sulfamonomethoxine / compound sulfamonomethoxine sodium injection which is prepared from main components and auxilary components, wherein the main components comprise 5-20 parts by weight of sulfamonomethoxine / sulfamonomethoxine sodium, 1-4 parts by weight of diclofenac sodium and 1-4 parts by weight of trimethoprim and the auxilary components comprise 0.2-0.4 part by weight of antioxidant, 40-70 parts by weight of organic solvent, 0.2-1 part by weight of regulating reagent and 10-20 parts by weight of water for injection. The injection prepared by the invention can provide effective treatment on secondary infection and mixed infection of streptococcicosis, toxoplasmosis, eperythrozoonosis, colibacillosis, asthma and other diseases, has obvious effect if applied for a long term, strong effect enhanced by several times or dozens of times, hardly generates drug resistance and has the advantages of less consumption, less application times, little irritation and quick treatment effect, can treat both symptoms and root causes, save production cost, improve product additional value and reduce toxic or side effects caused by mismatching of medicaments during treatment.
Owner:HENAN KANGXING PHARMA
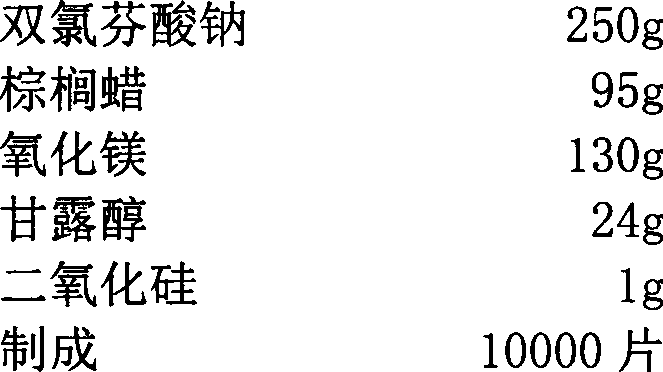
Diclofenac sodium sustained-release tablet and method for preparing same
ActiveCN103169680AReduce releaseInhibition releaseOrganic active ingredientsAntipyreticSide effectFiller Excipient
The invention relates to a diclofenac sodium sustained-release tablet and a method for preparing the same. By comprising the following components in percentage by weight: 15.0-60.0% of diclofenac sodium, 18.0-60.0% of retardant, 15.0-40.0% of filler, 3.0-20.0% of pore-foaming agent and 0.1-4.0% of lubricant, the diclofenac sodium sustained-release tablet is prepared by the processes of mixing the components, pelleting, thermally wrapping the retardant to the prepared particles, tabletting, heat treating, coating, and the like. The diclofenac sodium sustained-release tablet provided by the invention avoids the use dependence of hydroxypropyl methyl cellulose imported from abroad, reduces the irritation to the stomach when the clinical medication convenience is improved simultaneously, reduces the side effects, and is suitable for different age groups and in-vivo environments as the prepared sustained-release tablet can continuously release for 24hours. The preparation process of the product reduces the intensity of production. And the prepared product is safe and effective, and strong in convenience.
Owner:广东全瑞医药有限公司
Paddy rice rice blast-resistant selenium-rich yield-increasing agent and preparation method thereof
InactiveCN103641592AGood for balanced growthIncrease productionFertilizer mixturesSodium metasilicateBetaine
The invention discloses a paddy rice rice blast-resistant selenium-rich yield-increasing agent and a preparation method thereof. The paddy rice rice blast-resistant selenium-rich yield-increasing agent comprises the following raw materials by weight: 30-50 parts of ammonium biphosphate; 45-65 parts of monopotassium phosphate; 6-12 parts of boron fertilizer; 8-12 parts of selenium fertilizer; 3-6 parts of humic acid; 3-6 parts of lithium sulfate monohydrate; 10-20 parts of sodium metasilicate pentahydrate; 0.3-1.2 parts of diclofenac sodium; 0.1-0.3 parts of glycyrrhizic acid; 1-3 parts of p-chloro-m-xylenol; 0.5-1.5 parts of flucytosine; 6-9 parts of betaine; 0.2-0.4 parts of diphenhydramine; and 0.1-0.4 parts of cromolyn sodium. The paddy rice rice blast-resistant selenium-rich yield-increasing agent of the invention is strong in pertinency, solves various prominent problems in paddy rice plantation, and has various effects of rice blast resistance, seed setting rate increasing, thousand seed weight increasing, yield increasing, polished rice rate increasing, rice protein content increasing, rice selenium content increasing, and the like.
Owner:新疆久业富硒农业科技开发有限公司
Pharmaceutical composition for anti-inflammation ease-pain
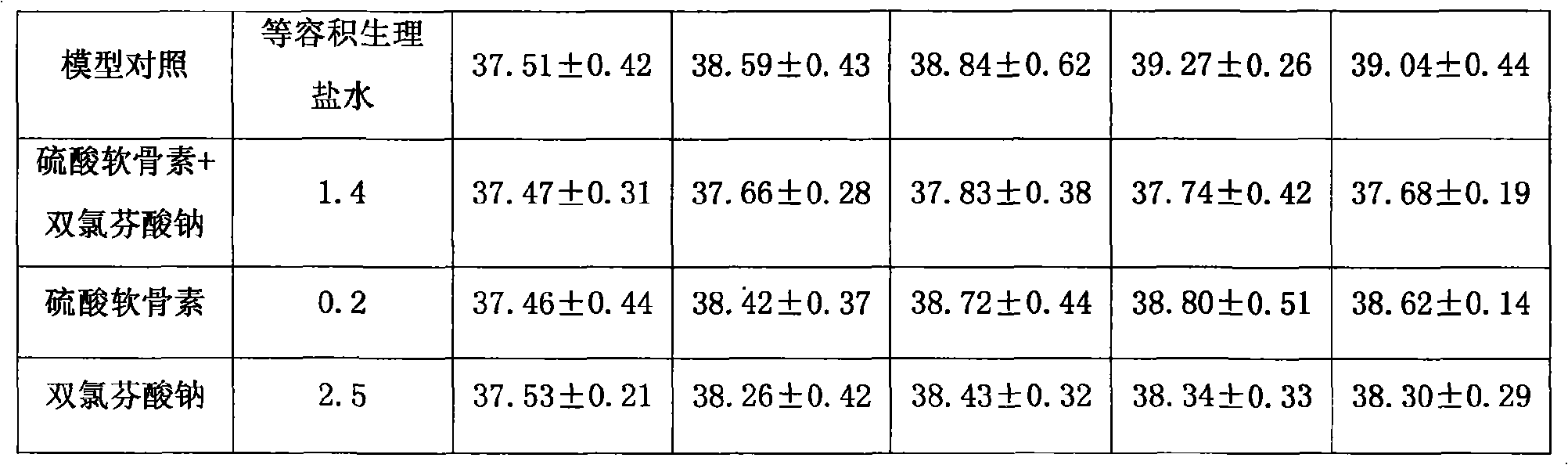
The invention provides an anti-inflammatory and pain-killing medical composition; chondroitin sulfate and a non-steroidal anti-inflammatory agent are used as active ingredients and mixed with a medical carrier to form the composition. Non-steroidal anti-inflammatory agent includes but not confines in diclofenac sodium (potassium), naprosyn and Lornoxicam; the composition exists in forms of squirt, freeze-dried powder and large-capacity transfusion for intravenous medication of a human body. The composition can be applied to symptomatic treatments of various febrile illnesses, various arthritises and inflammatory rheumatic diseases, soft tissue rheumatisms, neuralgia, arthralgia and pains, wounds relevant to degenerative diseases of spines, or postoperative pains.
Owner:FUKANGREN BIO PHARMA
Composition for external use for treating rheumatalgia and preparation method thereof
ActiveCN109316591AImprove playbackAccelerates Pain Relief EffectsOrganic active ingredientsAntipyreticLycopodiastrum casuarinoidesDiclofenac Sodium
The invention belongs to the technical field of traditional Chinese medicine, and discloses a composition for external use for treating rheumatalgia and a preparation method thereof. The traditional Chinese medicine composition is prepared from the following components in parts by weight: 1-3 parts of flos carthami, 2-6 parts of rhizoma chuanxiong, 1-4 parts of rhizoma seu radix notopterygii, 1-4parts of radix angelicae pubescentis, 4-10 parts of rhizoma zingiberis preparata, 4-10 parts of radix et rhizoma asari, 1-3 parts of radix paeoniae alba, 1-3 parts of herba lycopodii, 1-3 parts of lycopodiastrum casuarinoides, 2-6 parts of herba menthae, 4-10 parts of diclofenac sodium powder, 0.04-0.1 part of water-soluble capsaicin, 0.2-1 part of vanillyl butyl ether, 0.2-1 part of laurocapram and 0.2-1 part of wintergreen oil. The preparation method comprises the step that A, the diclofenac sodium powder, the water-soluble capsaicin, the vanillyl butyl ether, the laurocapram, the wintergreen oil, the flos carthami, the rhizoma chuanxiong, the rhizoma seu radix notopterygii, the radix angelicae pubescentis, the rhizoma zingiberis preparata, the radix et rhizoma asari, the radix paeoniaealba, the herba lycopodii, the lycopodiastrum casuarinoides and the herba menthae are added into white spirit for soaking and placement to obtain medicinal wine. The composition for the external use for treating the rheumatalgia and the preparation method thereof have the characteristics of promoting blood circulation for removing obstruction in collaterals, dispelling wind, eliminating dampness,relieving pain and being convenient to use.
Owner:李云昆
Magnetic microsphere capable of adsorbing diclofenac sodium
ActiveCN107583617AImprove adsorption removal effectImprove stabilityOther chemical processesWater contaminantsEthylenediamineSorbent
The invention provides a magnetic microsphere capable of adsorbing diclofenac sodium. The magnetic microsphere is prepared by the following steps: preparing nano Fe3O4 by a co-precipitation method, preparing a SiO2 coated magnetic nano particle Fe3O4@SiO2, preparing a chitosan compounded Fe3O4@SiO2 magnetic material, and preparing an ethylene diamine modified magnetic material. According to the preparation method, chitosan and magnetic particles are combined, then the microsphere is modified by ethylene diamine; the magnetic microsphere has more amino groups, the effect of adsorbing and removing diclofenac sodium in water is improved, moreover, the chitosan microsphere has a good magnetic responding performance, so the microsphere can be conveniently separated and recovered, and the magnetic microsphere is a green and sustainable absorbent.
Owner:ZHEJIANG OCEAN UNIV
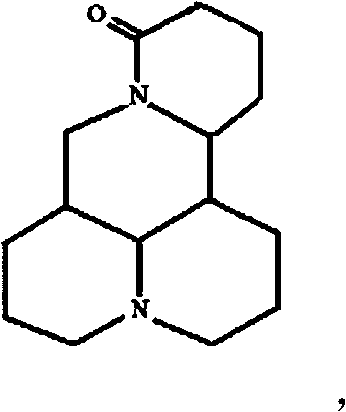
Method for synthesizing dichlofenac sodium
InactiveCN1580039AHigh yieldLow costOrganic compound preparationAmino-carboxyl compound preparationCyclohexanoneDiclofenac Sodium
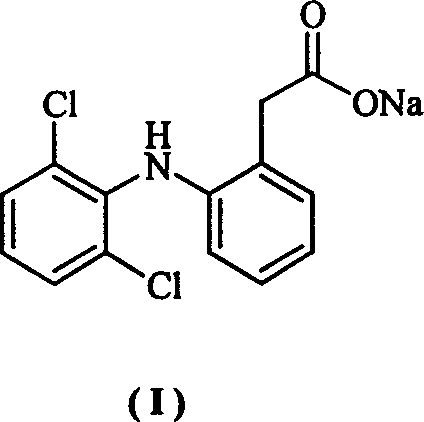
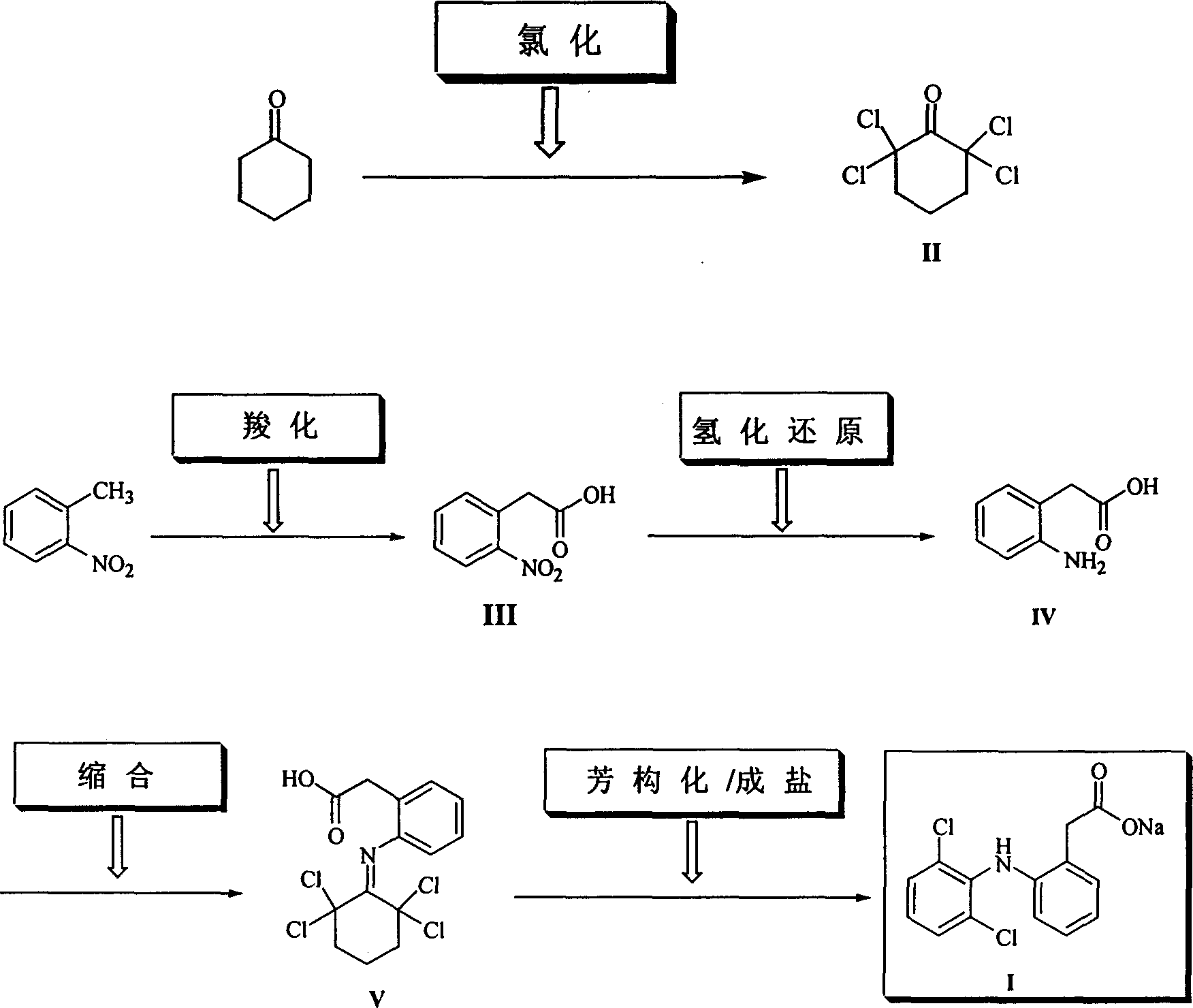
The invention provides a synthetic method of sodium diclofenac. The method includes the following steps: 1. Make the 2, 2, 6, 6-tetrachloride cyclohexanone (II) from the cyclohexanone through chlorination. 2. The carboxylation reaction occurs to the close nitrotoluene and carbon dioxide after the phase-transfer catalysis to get the close nitrophenyl acetic acid (III). 3. The hydrogenation reaction happens to the compound (III) catalysed by the polymer catalyst Pd / D-296 to produce the close aminophenyl acetic acid (IV). 4. The condensation reaction occurs to the compound IV and compound II to produce the compound of N-(2-carboxymerhy1 phenyl)-2,2,6,6-hexamethylene imine (V). 5. After the aromatization reaction and salifying happen to the compound V, we get the sodium diclofenac (I). By the close nitrotoluene, the yield is 85%. The invention is featured by facility of the raw material, simple operation, mild reation condition and easy industrialization.
Owner:FUDAN UNIV
Cream composition and paper comprising same
The invention provides a cream composition which comprises bacteriostatic agents, glycerin and deionized water. The mass ratio of the bacteriostatic agents to the glycerin to the deionized water is 0.1-22:10-97:3-9. The bacteriostatic agents are at least one type of polyhexamethylene guanidine hydrochloride, polyhexamethylene biguanidine hydrochloride, iodopropynyl butylcarbamate, 2-methyl-4-amino-6-methoxy s-triazine, benzalkonium bromide, chloretone, sulfoacid pyrimidine sodium, diclofenac sodium, chlorhexidine and myristylpicolinum bromide. The invention further provides paper comprising the cream composition. The cream composition is formed on the outer surface of the paper, so that the paper realizes moisture retention, sterilization and bacteriostasis effects when used for wiping the skin of a user, and feels comfortable and soft.
Owner:GOLD HONG YE PAPER
Method for determining content of vitamin B12 in complex vitamin B and diclofenac sodium tablet through high performance liquid chromatography
InactiveCN102226795AThe measurement result is accurateAccurate and reliable measurement resultsComponent separationDiclofenac SodiumVitamin B12
The invention relates to a method for determining content of vitamin B12 in a complex vitamin B and diclofenac sodium tablet through high performance liquid chromatography. The method comprises the following steps of: (1) preparing reference solution under a condition of keeping away from light; (2) removing wrapper of a complex vitamin B and diclofenac sodium tablet sample, porphyrizing the complex vitamin B and diclofenac sodium tablet sample, adding a mobile phase and dissolving the mixture ultrasonically, shaking up, centrifuging and precipitating, taking out liquid supernatant, weighing the liquid supernatant, heating the liquid supernatant to be boiling, and then cooling the liquid supernatant, dripping the mobile phase to recover the weight before boiling, shaking up, and filtering to get a test sample solution; and (3) providing chromatographic conditions, wherein a chromatographic column is selected from a octadecylsilane chemically bonded silica column, a mixture of methyl alcohol and 0.05mol / l monopotassium phosphate solution with volume ratio of 22:78 is used as the mobile phase, the pH value of the monopotassium phosphate solution is 6.4, column temperature is 35 DEG C, detection wavelength is 361nm, flow velocity is 1.0ml / min, and sample size is 20 microliter. The method provided by the invention is accurate, reliable and excellent in determination result, simple, convenient and scientific in means and accordant to requirements of Chinese pharmacopoeia.
Owner:NINGBO SHUANGWEI PHARMA
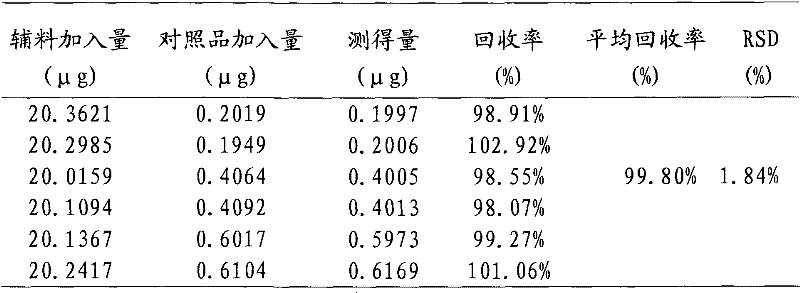
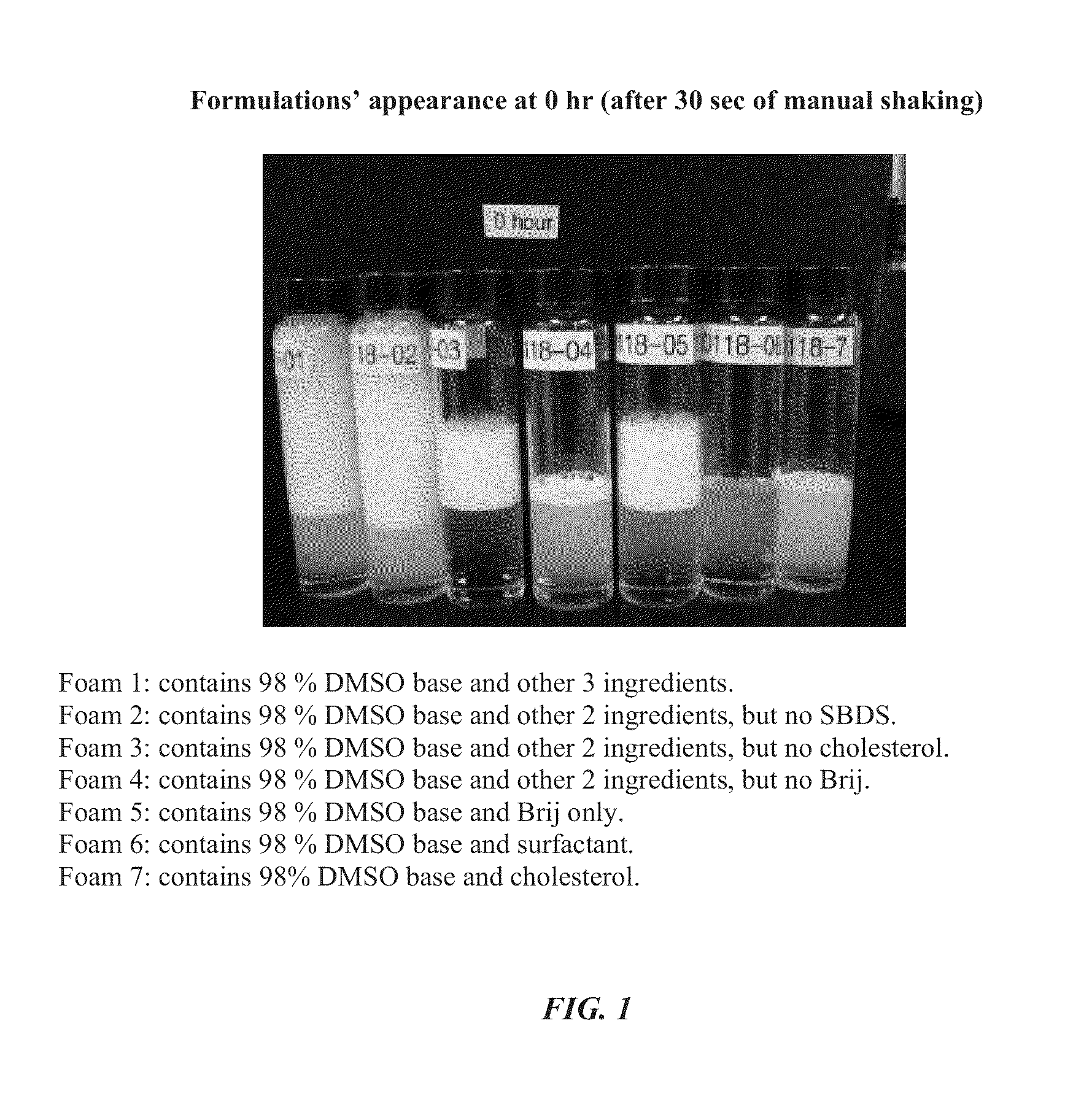
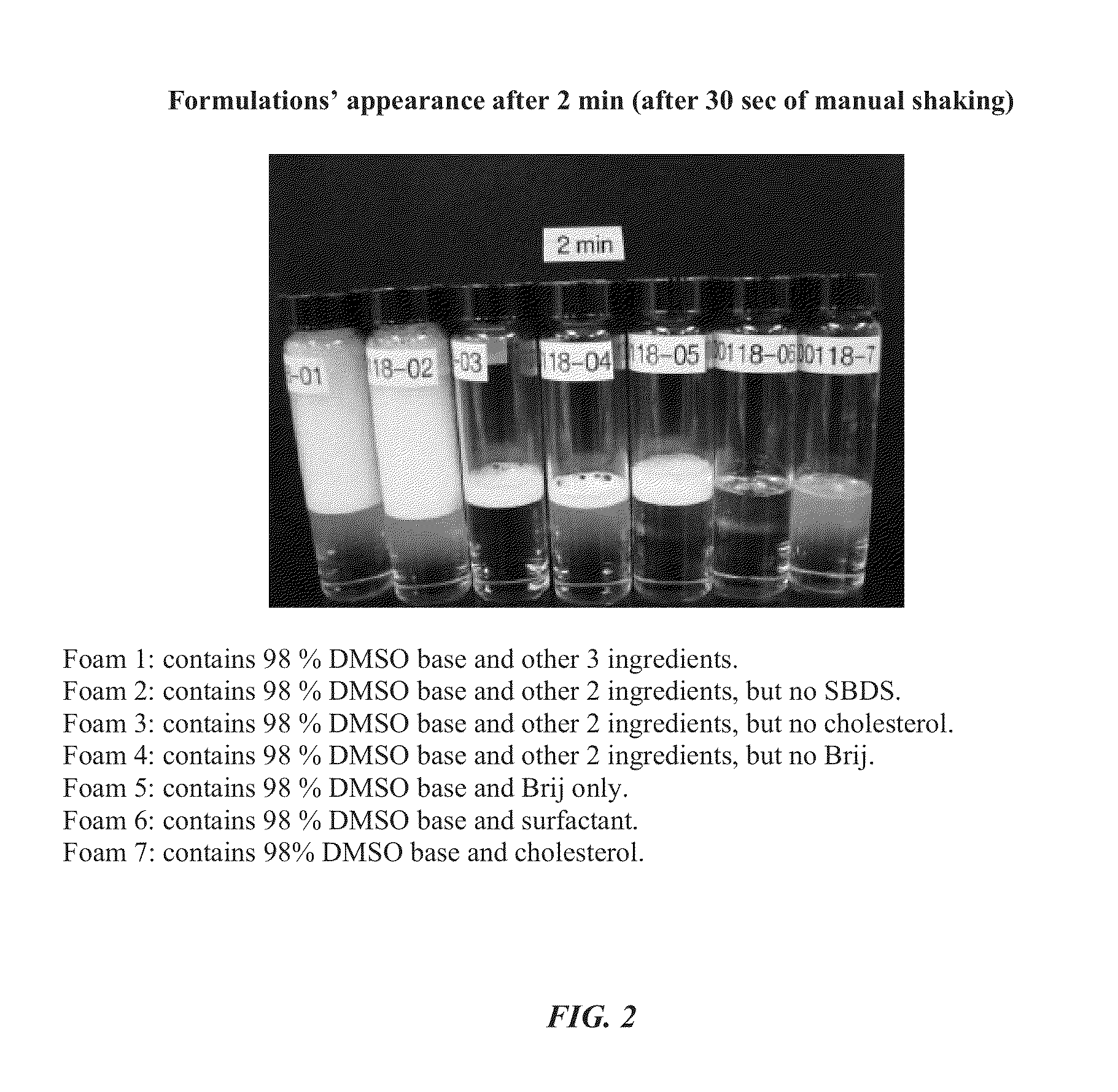
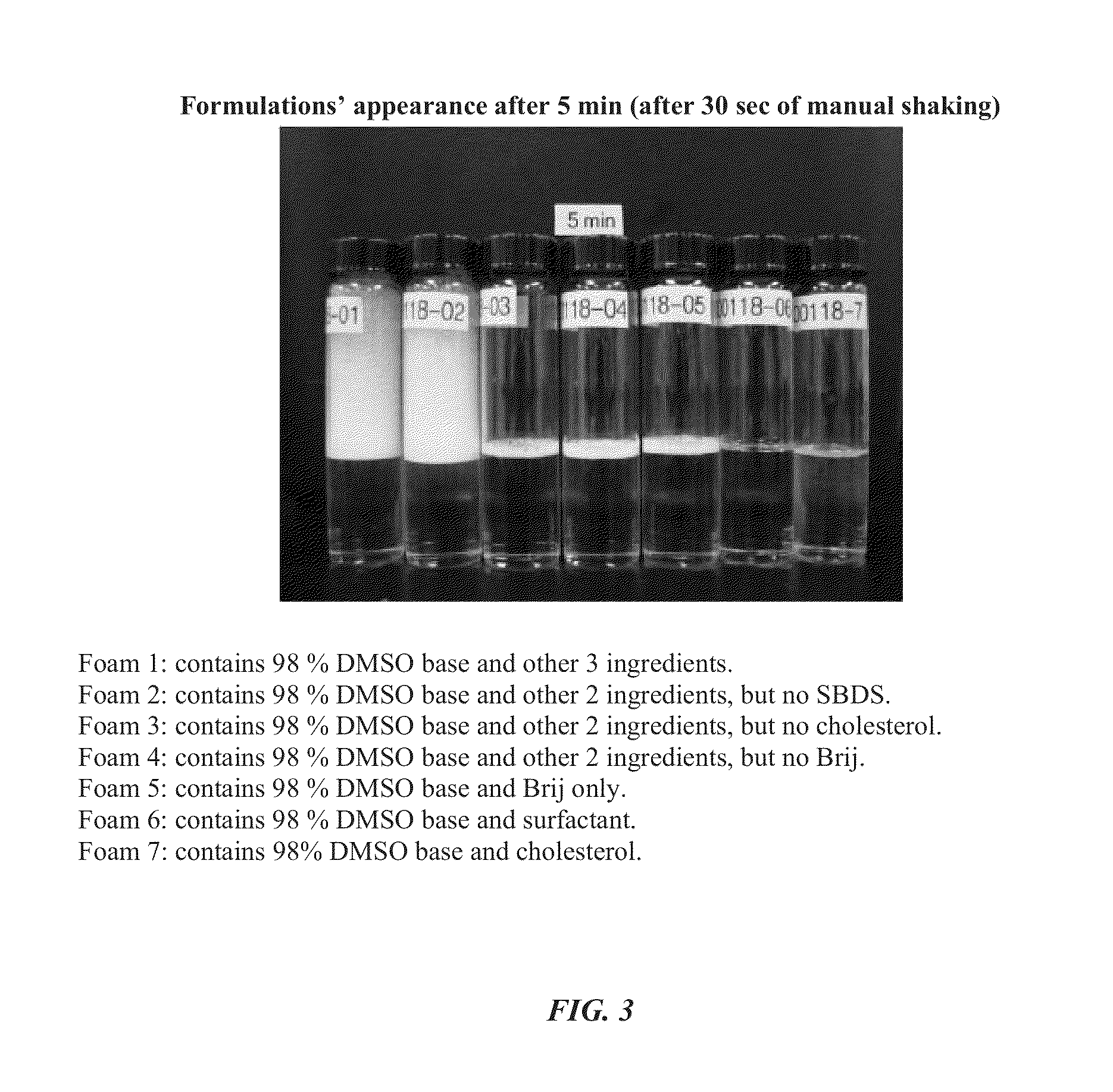
Foamable formulation
ActiveUS20130243701A1Relieve painAerosol deliverySulfur/selenium/tellurium active ingredientsAlcoholActive agent
The present invention provides DMSO-containing foamable formulations, methods for preparation, and methods of treatment. The formulations can provide good permeability and bioavailability at the target site. Preferably, the formulations are useful for treating osteoarthritis. In one embodiment, the invention provides a foamable formulation for topical use, said formulation comprising DMSO, polyalkylene glycol alkyl ether, an active agent, a monohydric lower alcohol, a diol, and water. Preferably, the active agent is a non-steroidal anti-inflammatory drug, such as diclofenac sodium or ibuprofen.
Owner:TRIBUTE PHARM CANADA INC
Medicinal composition containing diclofenac sodium and preparation method thereof
InactiveCN102871995ALess irritatingImprove complianceOrganic active ingredientsSenses disorderPatient complianceIrritation
The invention discloses a medicinal composition containing diclofenac sodium which is characterized in that the medicinal composition is an ophthalmic preparation with a pH value of 6.5 to 8.5; and 1mg / ml of diclofenac sodium is taken as a measuring standard, the medicinal composition contains 10 to 200mg / ml of a stabilizing agent, 10 to 100mg / ml of a cosolvent, 5 to 50mg / ml of a buffering agent, 45 to 50 mu g / ml of a preservative, a pH regulator and water for injection. The stabilizing agent is sulfobutylether-beta-cyclodextrin and a derivative of sulfobutylether-beta-cyclodextrin; the buffering agent is boric acid and / or borax; the preservative is benzalkonium chloride; the cosolvent is poloxamer; and the pH regulator is either sodium hydroxide or hydrochloric acid. The invention has the advantages that the medicinal composition provided by the invention is simple to operate and conditions are easy to implement. With the adoption of the medicinal composition containing diclofenac sodium obtained by the preparation method provided by the invention, irritation of diclofenac sodium eye drops is remarkably reduced and compliance of the patients is increased.
Owner:JIANGSU HANCHEN PHARMA
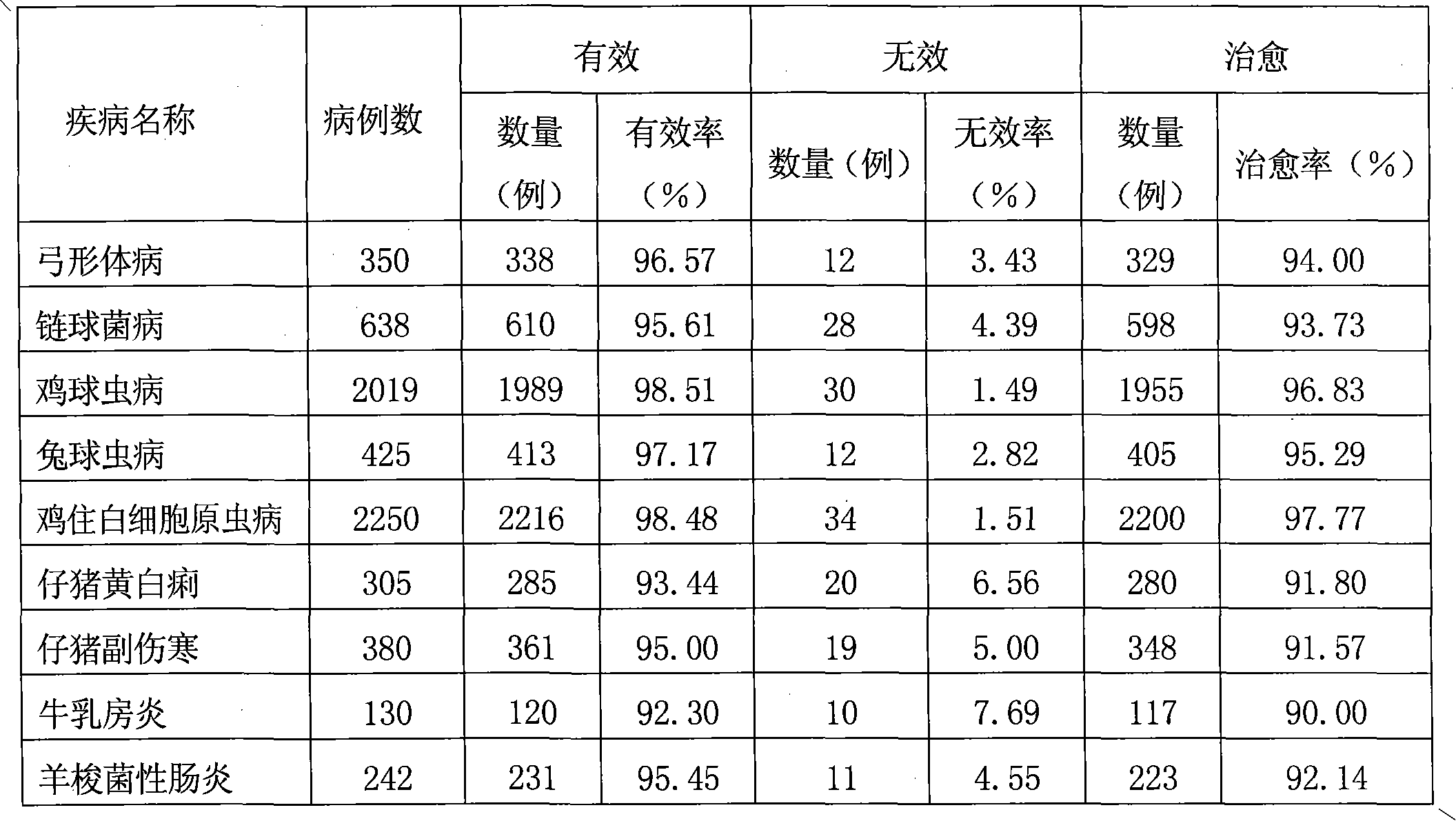
Injection for treating porcine mixed infection
ActiveCN102188436AImprove antibacterial propertiesGood antiviral effectAntibacterial agentsOrganic active ingredientsTrimethoprimSulfamonomethoxine
The invention discloses injection for treating porcine mixed infection. Each 100 liters of injection contains 10 to 15 kilograms of sulfamonomethoxine sodium, 2 to 5 kilograms of ofloxacin, 2 to 4 kilograms of diclofenac sodium, 1 to 2 kilograms of baicalin, 2 to 4 kilograms of trimethoprim, 0.1 to 0.2 kilogram of dexamethasone sodium phosphate, 0.1 to 0.2 kilogram of sodium thiosulfate, 3 to 6 liters of ethanolamine, 3 to 6 liters of phenyl carbinol, 30 to 60 liters of propylene glycol and the balance of water for injection. Compared with the conventional compound preparation for treating the porcine mixed infection, the injection effectively treats the porcine mixed infection and is convenient to use.
Owner:ZHENGZHOU BARY ANIMAL PHARMA
Preparation for injection of diclofenac sodium capable of being used for intravenous injection and preparation thereof
The invention provides an injection formulation of diclofenac sodium for intravenous injection and the preparation method, which is characterized in that the diclofenac sodium is included successfully with the inclusion material of propyl-Beta-cyclodextrin. The injection formulation has the advantages of improving the solubility of diclofenac sodium in the solution and reducing the adverse reaction in intravenous injection. The injection formulation exists in the form of injection, frozen powder, sterile powder and large-capacity infusion. The invention also describes the main technical parameters of adopting propyl- Beta -cyclodextrin to include the diclofenac sodium, such as inclusion method, the concentration of propyl-Beta-cyclodextrin, solution temperature in inclusion and inclusion time.
Owner:FUKANGREN BIO PHARMA
Diclofenac natrium slowly released capsule and its preparation method
InactiveCN1415290AStable concentrationBlood concentration fluctuates lessOrganic active ingredientsAntipyreticMedicinePlasticizer
A slowly-releasing diclofenac sodium capsule is prepared from diclofenac sodium (4.5-6.0 wt.%), excipient (60-90 wt.%), coating (4-10 wt.%), wtting agent (0-10 wt.%), surfactant (0-1 wt.%), plasticizer (0-2 wt.%) and opacifying agent (0-2 wt.%). Its advantages are high curative effect, low by-effect and high durability (12 hrs).
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
Compound doxycycline hydrochloride injection for animals and preparation method thereof
InactiveCN101380301AReduce the impactProof of StabilityAntipyreticTetracycline active ingredientsTrimethoprimDoxycycline hydrochloride
The invention relates to a compound doxycycline hydrochloride injection for veterinary use and a preparation method thereof. The raw materials comprise doxycycline hydrochloride, thiamphenicol, dexamethasone sodium phosphate, trimethoprim, diclofenac sodium, organic solvent and water for injection. As a special compound preparation for veterinary use, the injection has obvious curative effects on infectious diseases caused by gram-positive bacteria, gram-negative bacteria and mycoplasma, quickly takes effects after the injection is injected into livestock with the diseases, and has special efficacy on mixed infection and severe diseases caused by various pathogenic bacteria and viruses. After intramascular for animals, the injection is easily absorbed, takes effect for a long time and causes little local stimulation. The injection has the advantages of convenient use, short treatment course, low drug resistance, and the like.
Owner:陈建波
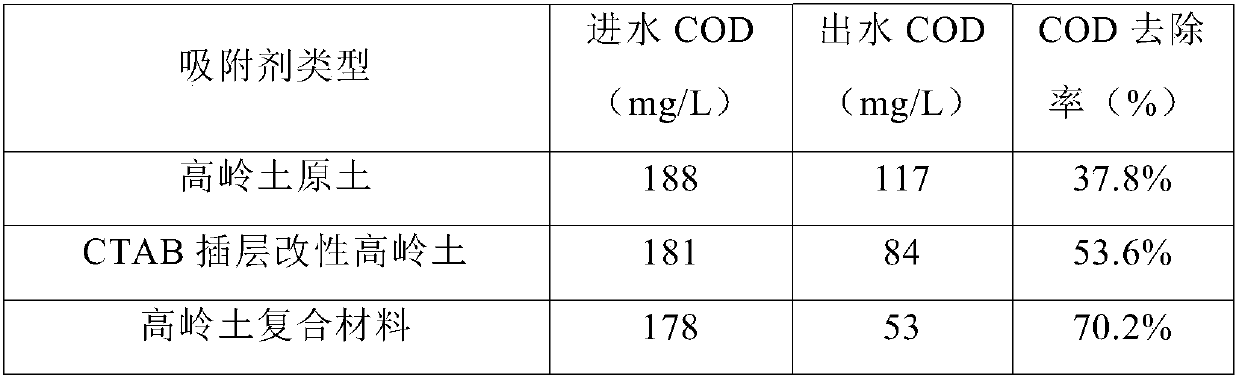
Kaolin composite and preparation method thereof
InactiveCN107694523AImprove adsorption capacityEasy to handleWater/sewage treatment by irradiationOther chemical processesPenicillinWastewater
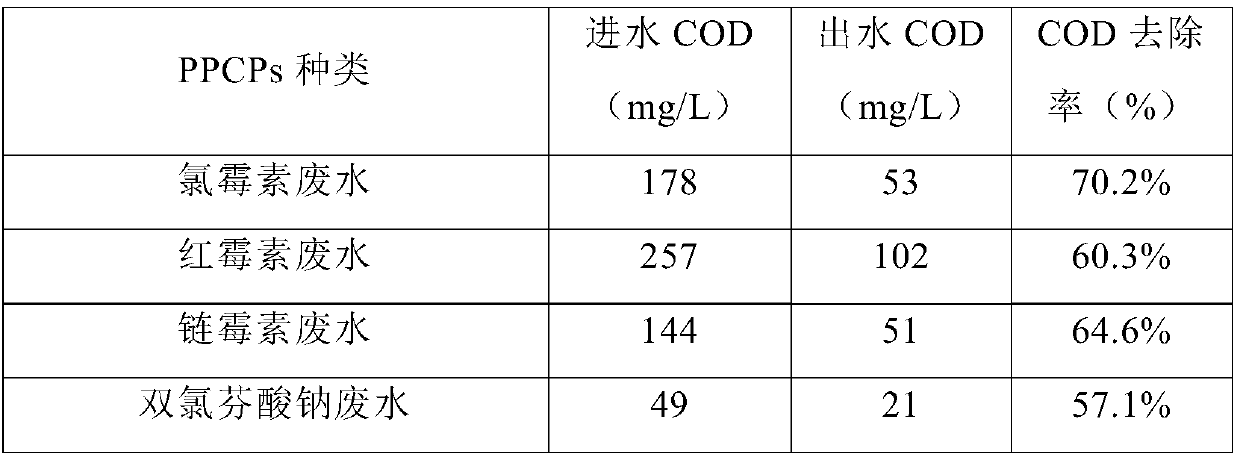
The invention provides a kaolin composite, comprising intercalation modified kaolin and titanium dioxide nanoparticles supported on the same; the intercalation modified kaolin is cetyl trimethyl ammonium bromide intercalation modified kaolin. The invention also provides a preparation method of the kaolin composite. The kaolin composite provided herein is suitable for treating polluting PPCPs (pharmaceutical and personal care products) in wastewater, such as chloramphenicol, penicillin, erythromycin, streptomycin, vancomycin, pipemidic acid and diclofenac sodium, and the treatment effect is good.
Owner:NORTH CHINA UNIV OF WATER RESOURCES & ELECTRIC POWER
Hyperostosis cold compress paste and preparation method thereof
InactiveCN107296852AGuaranteed respiratory metabolismIncrease contact surfaceHydroxy compound active ingredientsSkeletal disorderDihydroxyaluminum aminoacetateIrritation
The invention discloses a hyperostosis cold compress paste which comprises a backing layer, a gel drug layer and an anti-bonding layer, wherein the gel drug layer contains the following raw materials: sodium polyacrylate, carbomer, glycerol, dihydroxyaluminum aminoacetate, tartaric acid, purified water, medical borneol, camphor, diclofenac sodium, menthol, atropa belladonna fluid extract, clove oil, propylene glycol, glycerol, medical azone, diphenhydramine, oleum menthae and capsaicin. The invention also discloses a preparation method for the hyperostosis cold compress paste. The method comprises the following steps: preparing a gel drug and then coating the backing layer with the gel drug by machine so as to form a gel drug layer; covering the gel drug layer with the anti-bonding layer; cutting, puncturing and slicing, thereby acquiring the hyperostosis cold compress paste. No organic solvent is added into the bruise cold compress paste disclosed by the invention; the cold compress paste can be directly coated and does not need to be heated; the technology is simple and no chemical residue exists; the cold compress paste is free from irritability and stimulation, is high in drug loading capacity and moisture retention, has high compatibility with skin, is anti-ageing, has excellent weather fastness and lasting viscidity and can be repeatedly uncovered and pasted.
Owner:四川利佰生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com