Diclofenac sodium sustained-release tablet and method for preparing same
A technology of diclofenac sodium and sustained-release tablets, which is applied in the field of medicine, can solve the problems of large mucous membrane irritation, difficult drying of particles, and tearing of production personnel, and achieve the effects of reducing irritation, uniform porosity, and reducing hidden dangers in production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
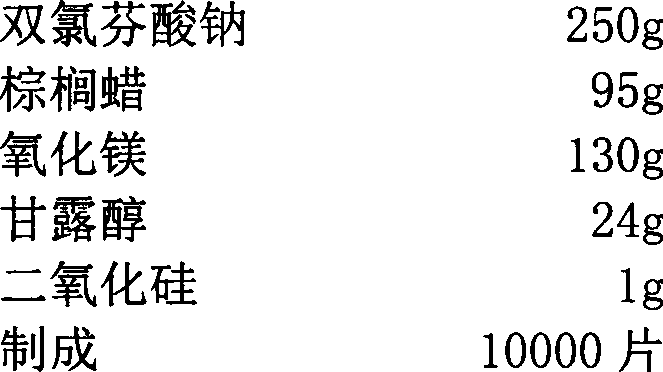
[0024] Components:
[0025]
[0026] Preparation:
[0027] (1) Take the following components by weight:
[0028]
[0029] (2) Get Diclofenac Sodium 250g, Magnesium Oxide 130g, Mannitol 24g to pass through 80 mesh sieves, mix well, get mixed material, set aside;
[0030] (3) Get 71.25g of palm wax, melt it at 90°C, and set aside;
[0031] (4) Get palm wax 23.75g, pulverize, cross 120 mesh sieves, set aside;
[0032] (5) Add the mixed material in (2) above to the melted palm wax in (3), stir evenly, make granules, cool to room temperature, and set aside;
[0033] (6) Take the granules in (5) above, put them in the coating pan, turn on the hot air at 80°C, and rotate at 12r / min, when the surface temperature of the granules reaches 70°C, gradually add the powder of (4) into the coating pan, Keep the surface temperature of the granules at 65°C and rotate for 30 minutes, cool to room temperature, size the granules, add 1g of silicon dioxide, mix well, press into tablets, ...
Embodiment 2
[0036] Components:
[0037]
[0038] Preparation:
[0039] (1) Take the following components by weight:
[0040]
[0041] (2) Get 500g of diclofenac sodium, 255g of calcium dihydrogen phosphate, 50g of talcum powder, and 150g of lactose and pass through a 100-mesh sieve, mix well to obtain a mixture, and set aside;
[0042] (3) Take 240g of glyceryl behenate, melt it at 80°C, and set aside;
[0043] (4) Get cetyl alcohol 80g, pulverize, cross 120 mesh sieves, set aside;
[0044] (5) Add the mixed material in (2) above to the melted glyceryl behenate in (3), stir evenly, make granules, cool to room temperature, and set aside;
[0045] (6) Take the granules in (5) above, put them in the coating pan, turn on the hot air at 55°C, and rotate at 8r / min, and when the surface temperature of the granules reaches 48°C, gradually add the powder of (4) into the coating pan, Keep the surface temperature of the granules at 48°C and rotate for 25 minutes, cool to room temperature, g...
Embodiment 3
[0048] Components:
[0049]
[0050] Preparation:
[0051] (1) Take the following components by weight:
[0052]
[0053] (2) Get 750g of diclofenac sodium, 185g of ethyl cellulose, 100g of calcium sulfate, 60g of microcrystalline cellulose, and 180g of sucrose and pass through a 120-mesh sieve, mix evenly to obtain a mixture, and set aside;
[0054] (3) Take 540 g of cetostearyl alcohol, melt it at 65° C., and set aside;
[0055] (4) Get stearic acid 180g, pulverize, cross 120 mesh sieves, set aside;
[0056] (5) Add the mixed material in (2) above to the melted cetearyl alcohol in (3), stir evenly, make granules, cool to room temperature, and set aside;
[0057] (6) Take the granules in (5) above, put them in the coating pan, turn on the hot air at 58°C, and rotate at 4r / min, and when the surface temperature of the granules reaches 42°C, gradually add the powder of (4) into the coating pan, Keep the surface temperature of the granules at 42°C and rotate for 30 min...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com