Stent
a technology of stents and polymers, applied in the field of stents, can solve the problems of stents used alone not being able to effectively prevent restenosis, decomposition or degradation of therapeutic substances (biologically/physiologically active substances) incorporated in polymers, and achieve the effect of stable loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0101] A polymer film having a thickness of 30 .mu.m was prepared by casting in a petri dish from a hexane solution of two-pack silicone elastomer (10 wt % in concentration) incorporated with PTFE fine particles (not larger than 1 .mu.m in particle diameter). The two-pack silicone elastomer is "Q7-4840 Silastic" (from Dow Corning Corporation) consisting of solution-A and solution-B in equal quantities. The amount of the PTFE is 30 wt % on the total amount of the polymer in the silicone elastomer and the PTFE fine particles.
[0102] The thus obtained polymer film was placed at the intermediate part 6 of two chambers 7 and 8 of the apparatus shown in FIG. 5. One chamber 7 of the apparatus was filled with an aqueous solution of simvastatin (SVS) which had been filtered off through a 0.45 .mu.m thick membrane filter made by Advantec. The aqueous solution contains 50 .mu.g / ml conc. of SVS and 2 wt % of surfactant ("Tween 20" from Nikko Chemical Co, . Ltd.). The other chamber 8 was filled w...
example 2
[0103] A polymer film having a thickness of 30 .mu.m was prepared by casting in a petri dish from a hexane solution of two-pack silicone elastomer (10 wt % in consentration) incorporated with sodium chloride fine particles (not larger than 1 .mu.m in particle diameter). The two-pack silicone elastomer is "Q7-4840 Silastic" (from Dow Corning Corporation) consisting of solution-A and solution-B in equal quantities. The amount of sodium chloride is 0.25 wt % on the total amount of the polymer in the silicone elastomer and the sodium chloride fine particles. The thus obtained polymer film was measured for the rate of permeation of SVS in the same way as in Example 1. The results of the test are shown in Table 1.
example 3
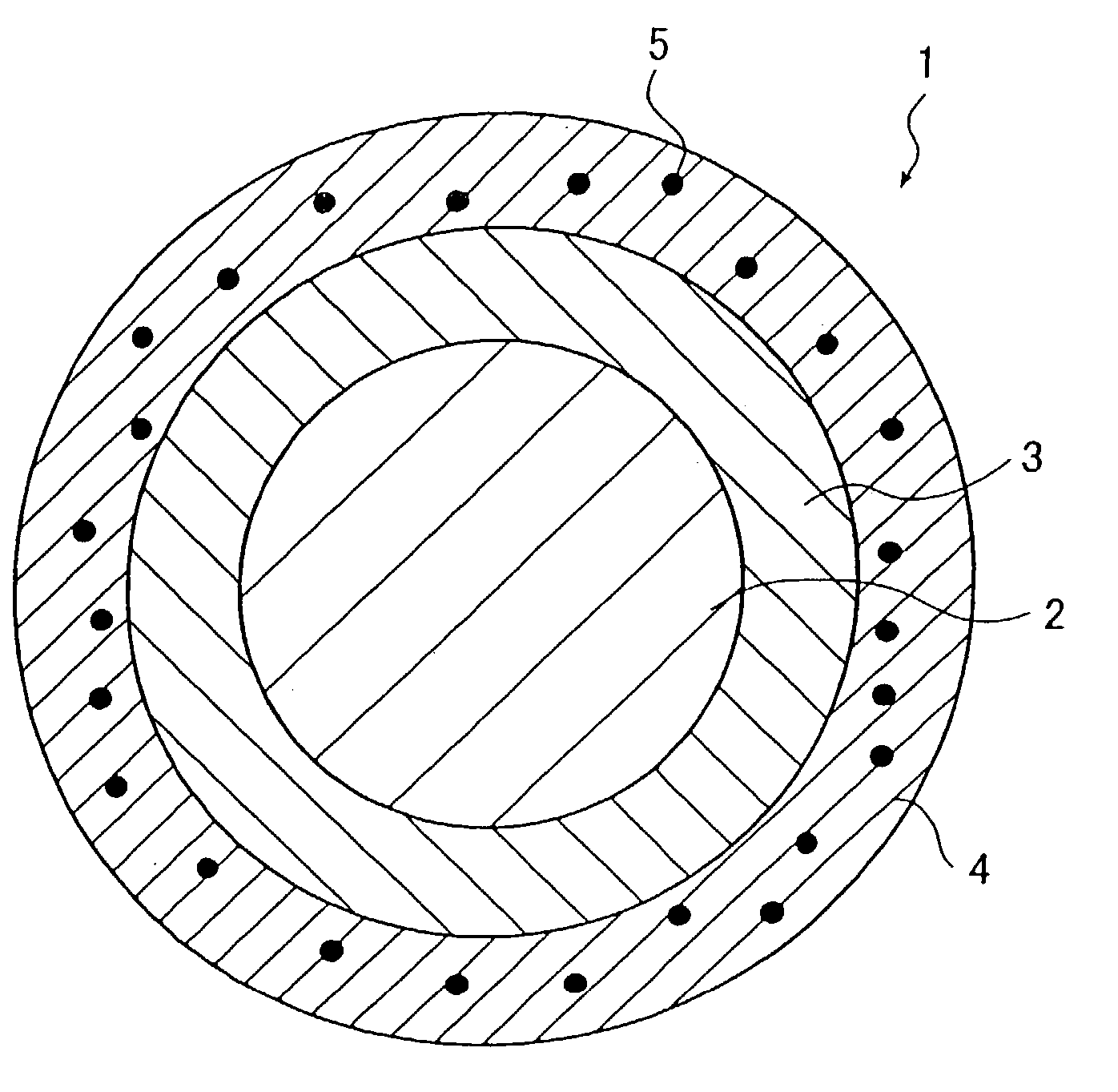
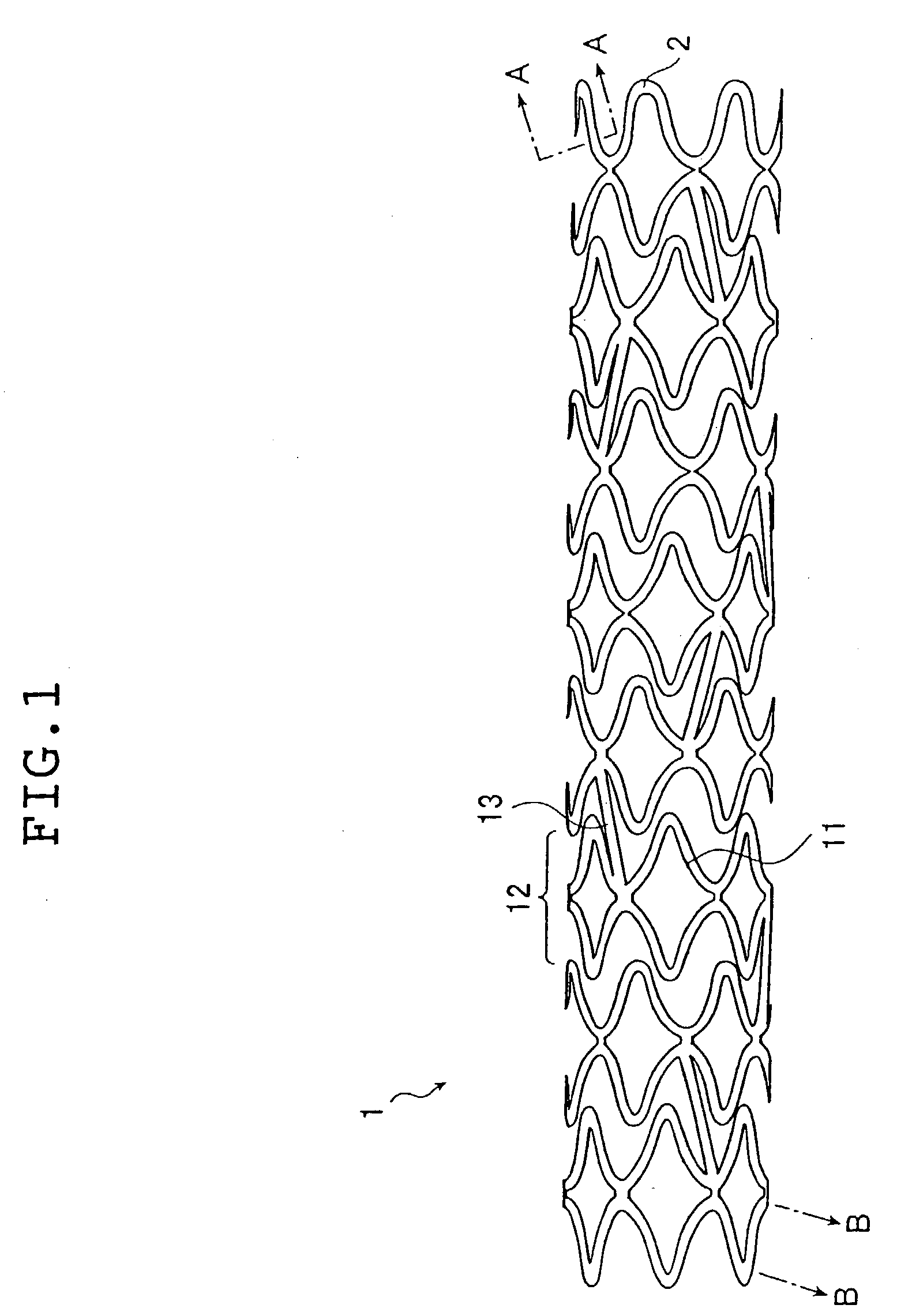
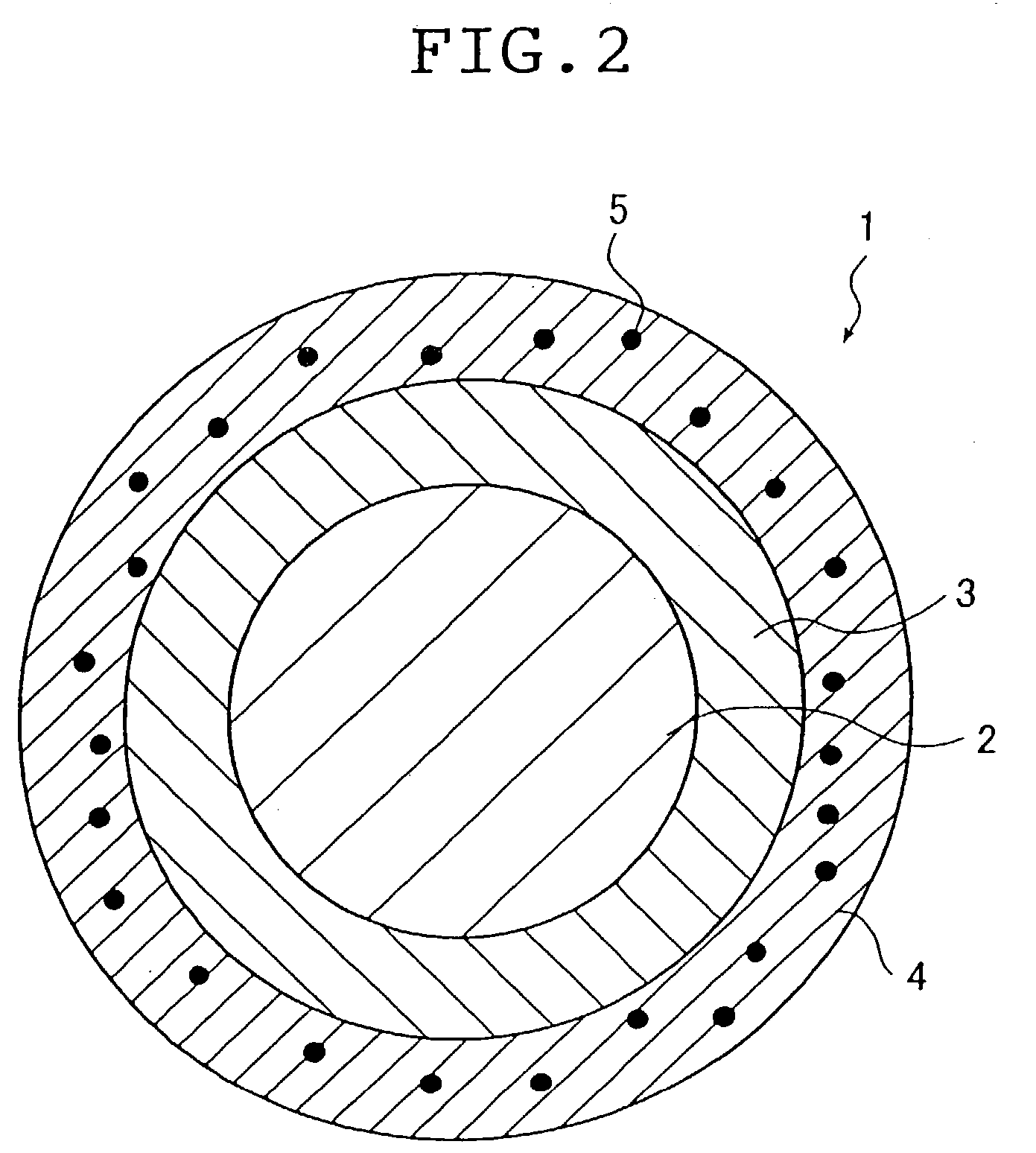
[0107] A stent sample was prepared in the following manner from a stent main body which is in a cylindrical shape (1.8 mm in outside diameter and 15 mm long, having approximately rhombic holes) and is composed of fibrous members (0.1 mm in diameter) of stainless steel (SUS 316L), as shown in FIG. 1. This stent main body was sprayed with a hexane solution of SVS (5 wt % in concentration) by using a hand spray (HP-C made by Iwata Corporation). It was confirmed that the surface of the fibrous members constituting the stent main body was coated with about 200 .mu.g of SVS. Upon complete solvent (hexane) removal by evaporation, there was formed a layer of SVS (biologically / physiologically active substance) on the surface of the fibrous members constituting the stent main body.
[0108] The coated stent main body was sprayed with a hexane solution of two-pack silicone elastomer incorporated (2 wt % in concentration) with PTFE fine particles (not larger than 1 .mu.m in particle diameter). The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com