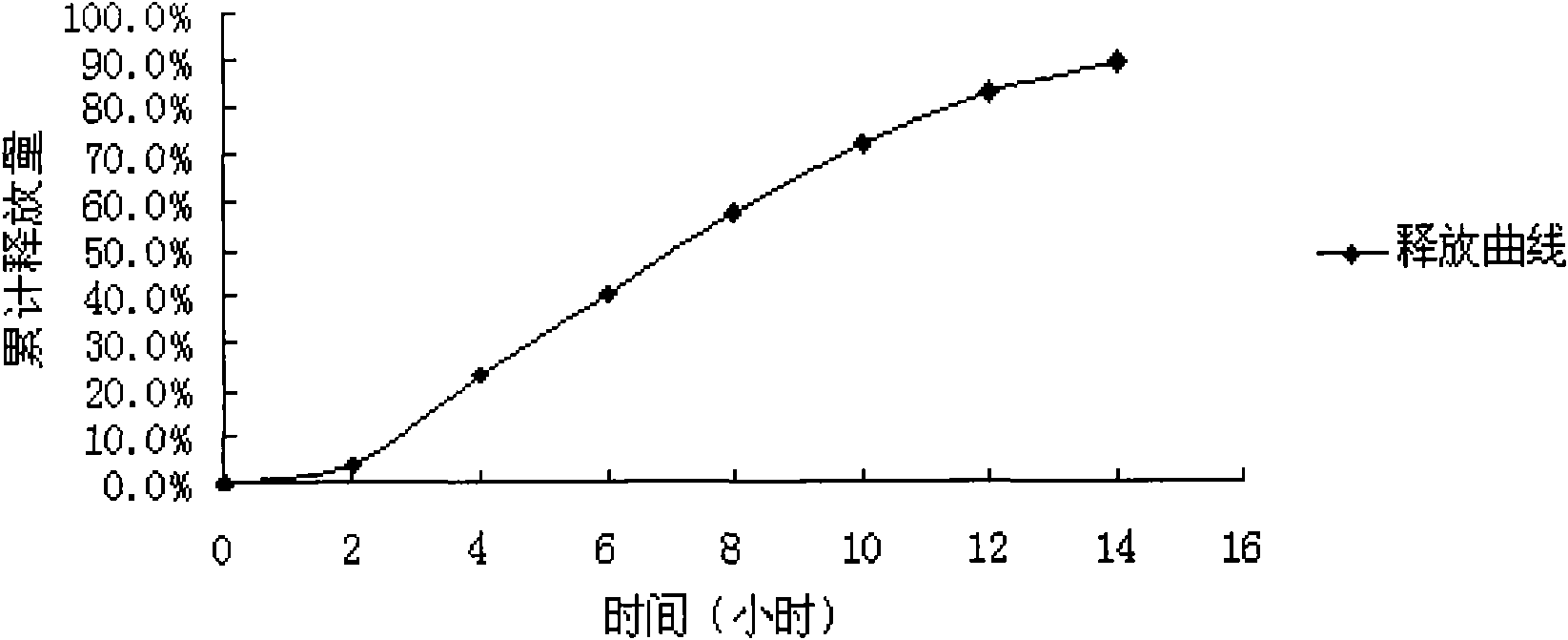
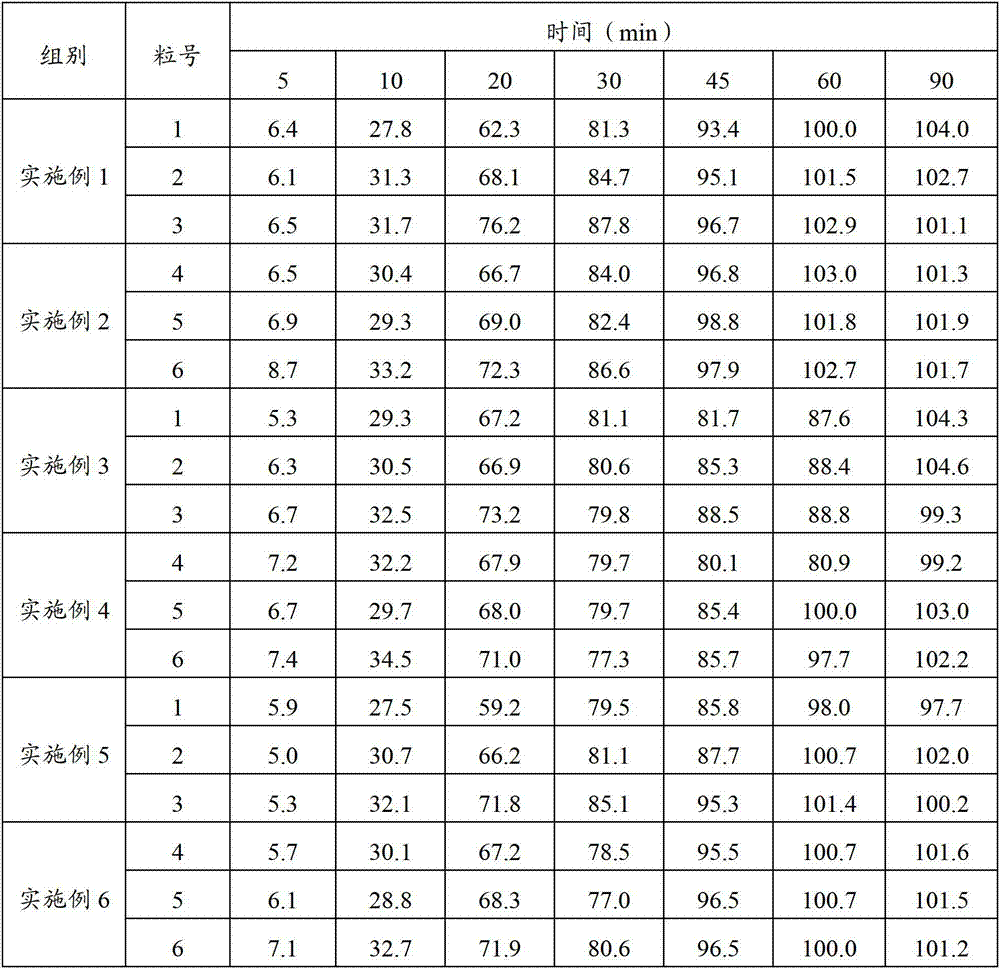
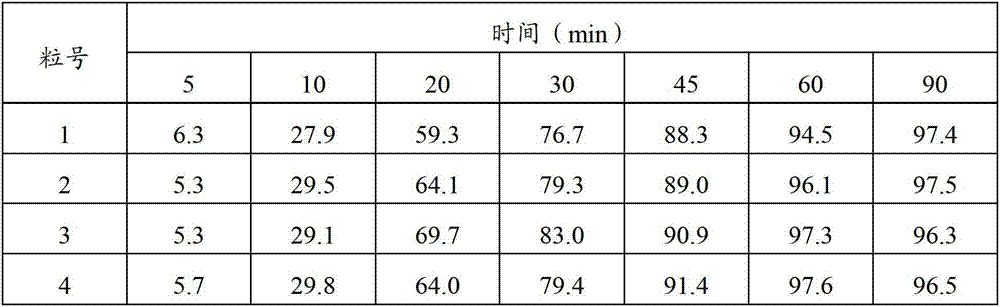
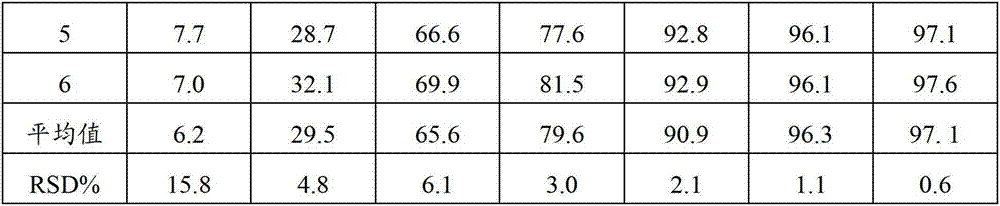
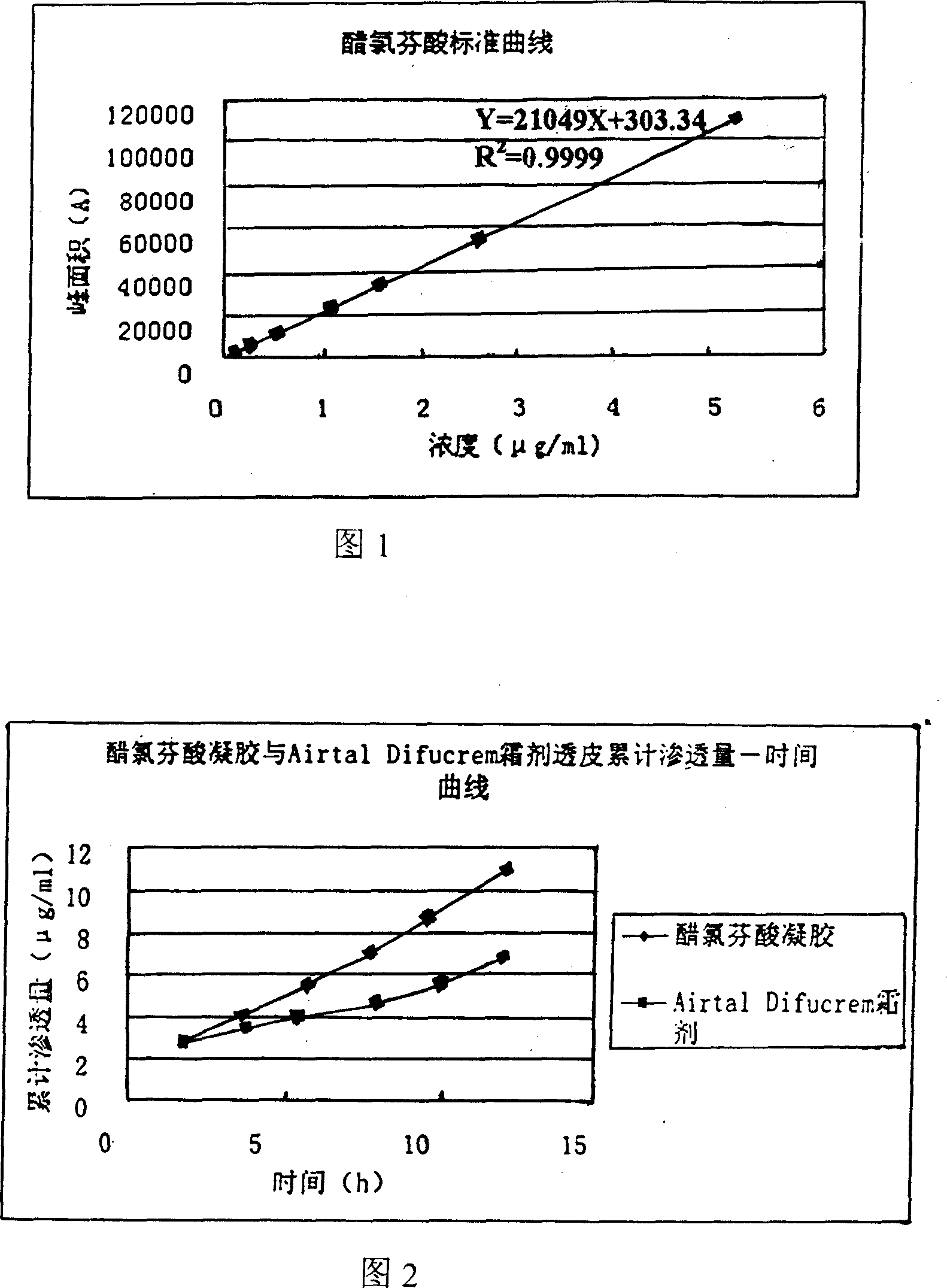
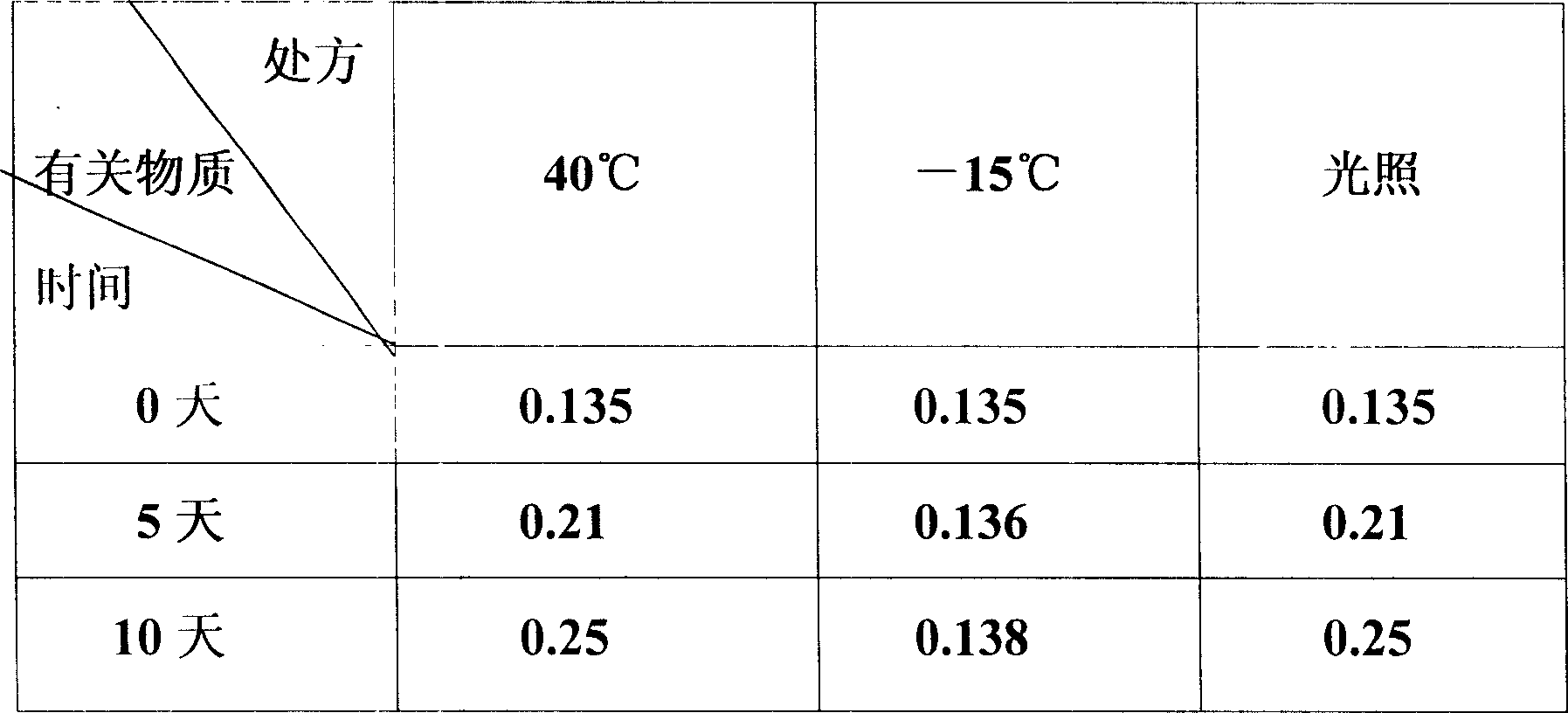
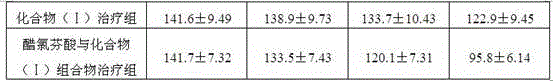
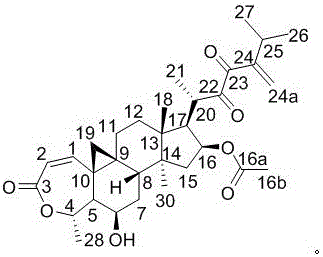
Patents
Literature
49 results about "Aceclofenac" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
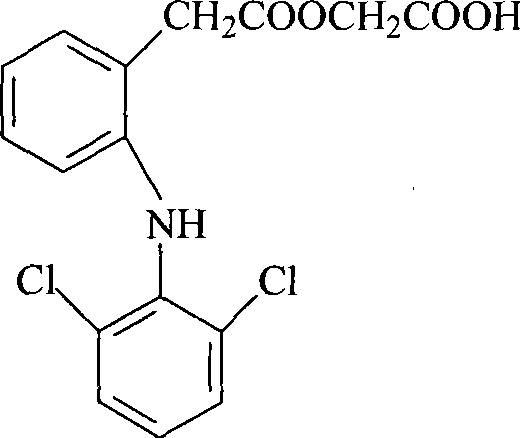
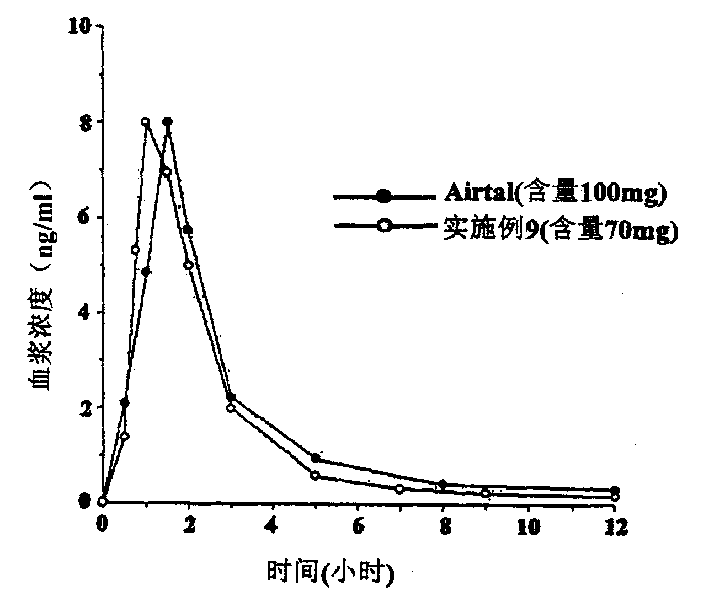
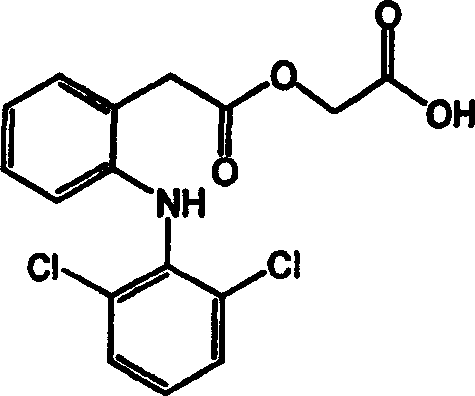
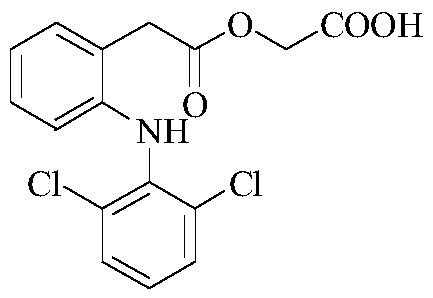
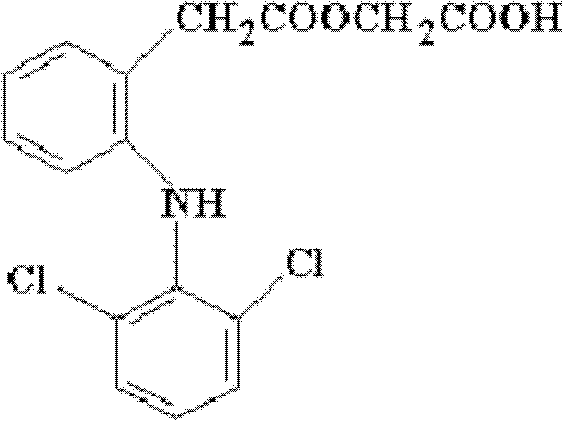
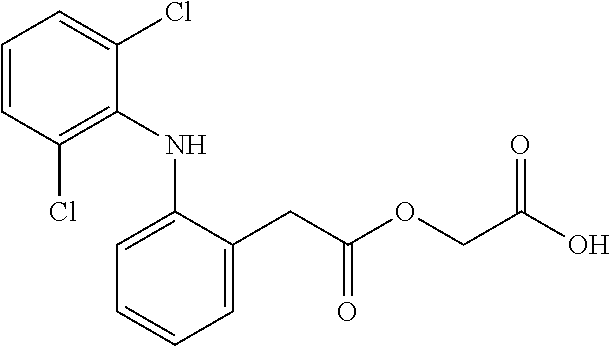
Aceclofenac is a nonsteroidal anti-inflammatory drug (NSAID) analog of diclofenac. It is used for the relief of pain and inflammation in rheumatoid arthritis, osteoarthritis and ankylosing spondylitis.
Improved method for preparing aceclofenac
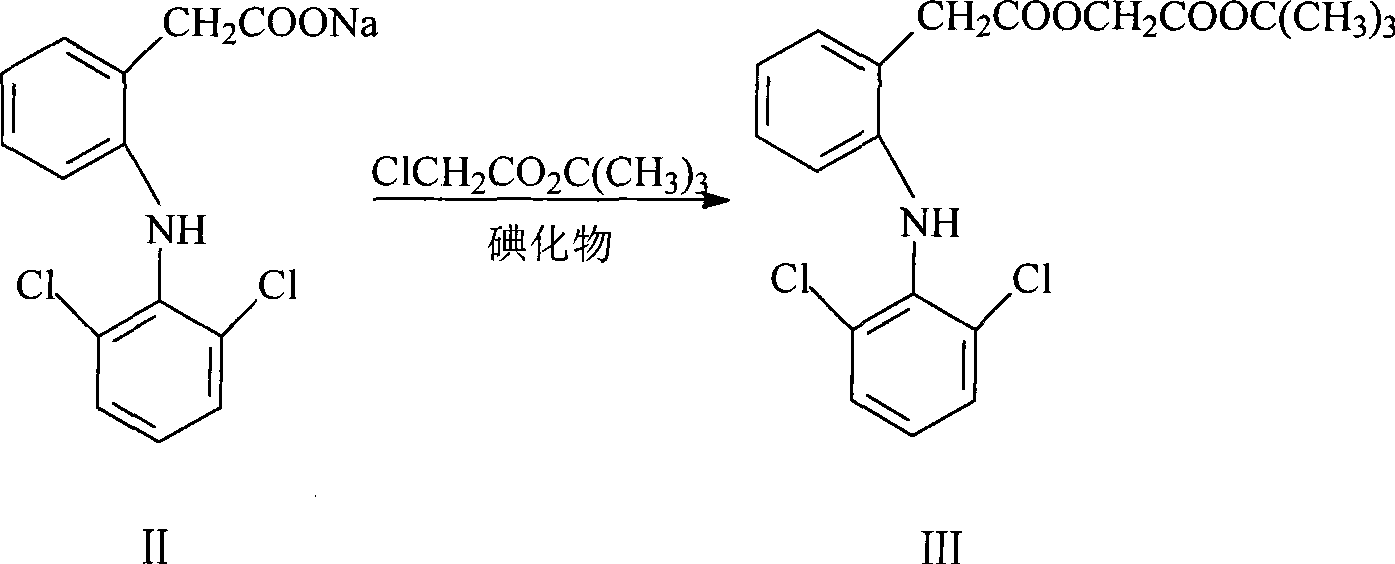
ActiveCN101531607AEasy to recycleShort reaction timeAntipyreticOrganic compound preparationIodideDiclofenac Sodium
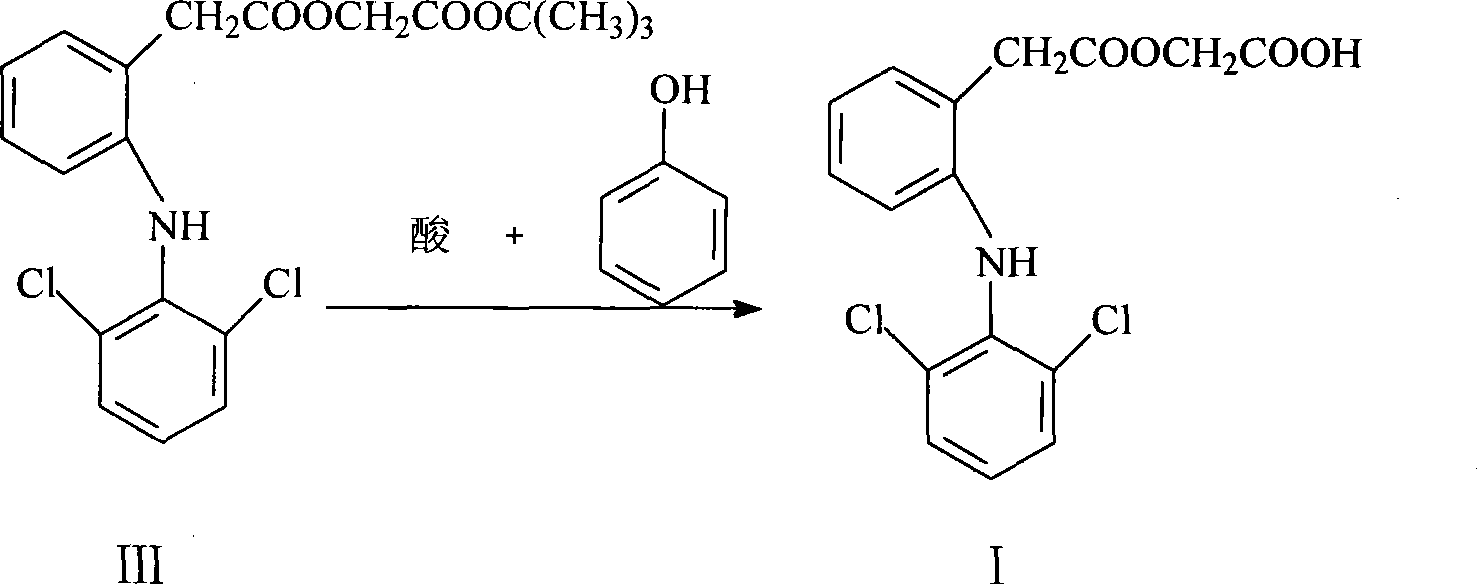
The invention provides an improved method used for preparing aceclofenac. The improved method is characterized in that (1) the method takes diclofenac sodium and tert-butyl chloroacetate as raw materials and iodide as a catalyst, and heats the substances to carry out condensation reaction; (2) the method takes tert-butyl aceclofenac as a raw material to carry out acidolysis reaction under the action of phenol and acid, and obtains aceclofenac crystal after post treatment and fine purification; and the total yield of both steps is above 88 percent and the content is over 99.2 percent (detected by HPLC). The improved method increases the yield and the content, and has short reaction time, simple and convenient operation and mild reaction conditions; and a reaction reagent is easy to recycle, so the improved method reaches the effects of lowering cost and reducing environment pollution.
Owner:LUNAN PHARMA GROUP CORPORATION
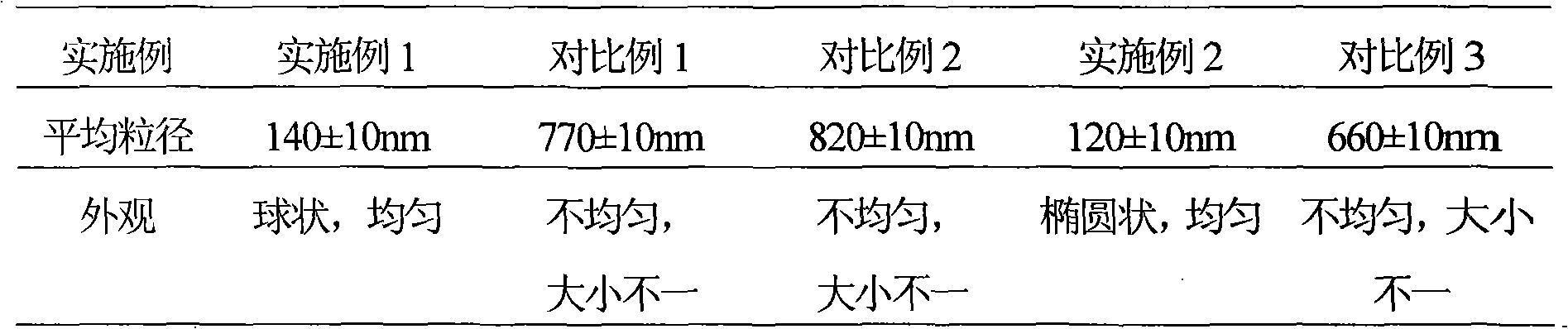
Compositions and preparation methods for bioavailable oral aceclofenac dosage forms
InactiveCN1543359AImprove solubilityIncrease dissolution rateOrganic active ingredientsPowder deliveryWater insolubleAceclofenac
There are provided compositions and preparation methods for bioavailable oral aceclofenac dosage forms. More particularly, the compositions containing water-insoluble aceclofenac, a polymeric base selected from the group consisting of polyvinyl, methyl cellulose, ethyl cellulose, hydroxypropylmethylcellulose, carboxy methyl cellulose, glyceryl monostearate, carbamer, and poloxamer, and surfactant. The compositions of the present invention can be formulated into compressed particles, granules, tablets, capsules or even semisolid preparations, which significantly increase the bioavilablity due to the improvement of dissolution of the drug in gastrointestinal tract, and reduce the manufacturing cost by simple process.
Owner:PHARM TECH RES +2
Aceclofenac preparation method
ActiveCN103086907AHigh selectivityReduce breakageOrganic compound preparationAmino-carboxyl compound preparationHydrogen halideOrganic acid
The invention relates to an aceclofenac preparation method and belongs to the chemical pharmaceutical field. The method comprises a step that acidolysis of aceclofenac tert-butyl ester is carried out in a mixed solution comprising a low-molecular-weight organic acid and hydrogen halide to obtain aceclofenac. The method which allows the acidolysis of aceclofenac tert-butyl ester to be carried out in the mixed solution comprising the low-molecular-weight organic acid and hydrogen halide has the advantages of high selectivity, reduction of the fracture of the ethoxy group in aceclofenac tert-butyl ester to a lowest limit, high acidolysis reaction conversion rate, extremely-low content of diclofenac in obtained aceclofenac, easy aceclofenac refining, and high aceclofenac purity.
Owner:HENAN DONGTAI PHARM
Method for performing headspace gas chromatographic detection on formic acid in aceclofenac bulk pharmaceutical chemicals
InactiveCN102901784AAccurate measurementEasy to measureComponent separationVapor phase chromatographyStrong acids
The invention discloses a method for performing headspace gas chromatographic detection on formic acid in aceclofenac bulk pharmaceutical chemicals. The analysis method comprises the following steps of: (1) performing an esterification reaction on the aceclofenac bulk pharmaceutical chemicals and superfluous methanol solution comprising 5-20 percent of strong acid by volume, or adding methanol first into the aceclofenac bulk pharmaceutical chemicals and then adding strong acid, wherein the strong acid accounts for 5-20 percent of the volume of the mixed strong acid-methanol solution; and (2) after the reaction is finished thoroughly, determining the obtained product which is generated when the formic acid is esterified by using the methanol. According to the method, after the formic acid is esterified by using the methanol, the product is subjected to gas chromatographic detection by using a headspace sampling method, so that a chromatographic response value and the analysis accuracy are greatly improved.
Owner:JIANGSU JIBEIER PHARMA
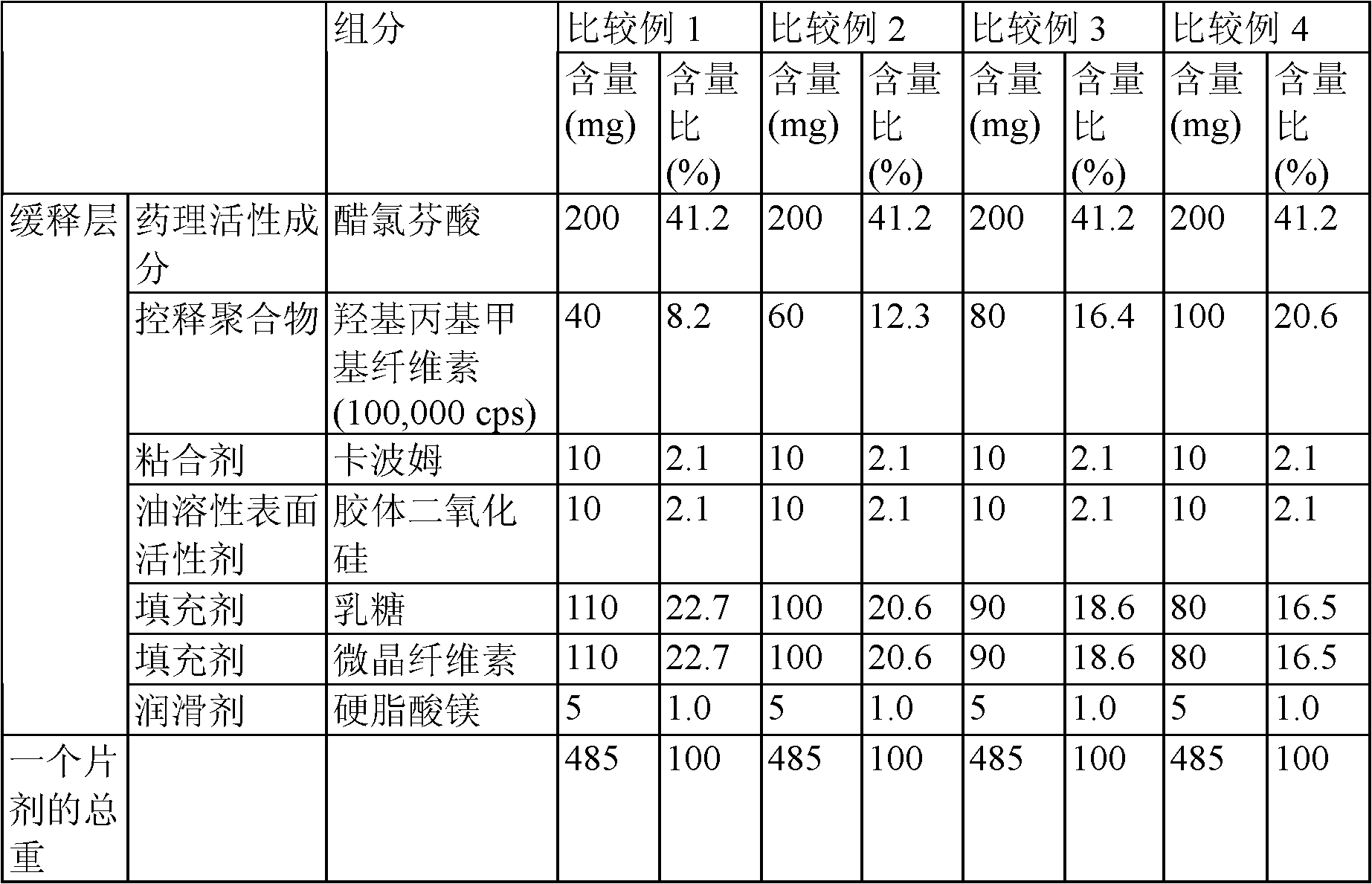
Aceclofenac in extended-released tablets and method of manufacturing the same
ActiveCN101108170AReduce stimulationSmall fluctuations in blood concentrationOrganic active ingredientsAntipyreticSustained Release TabletAdditive ingredient
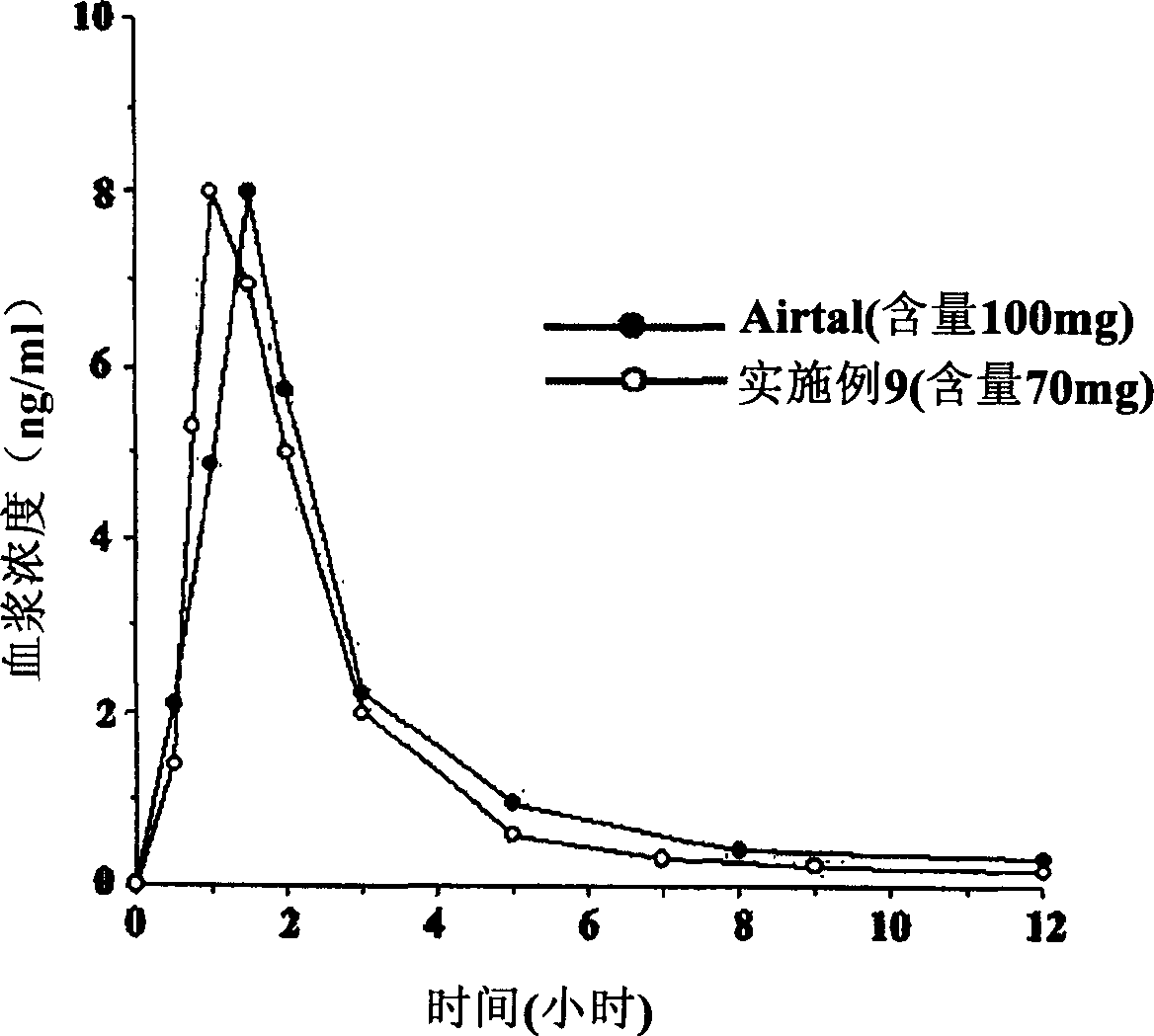
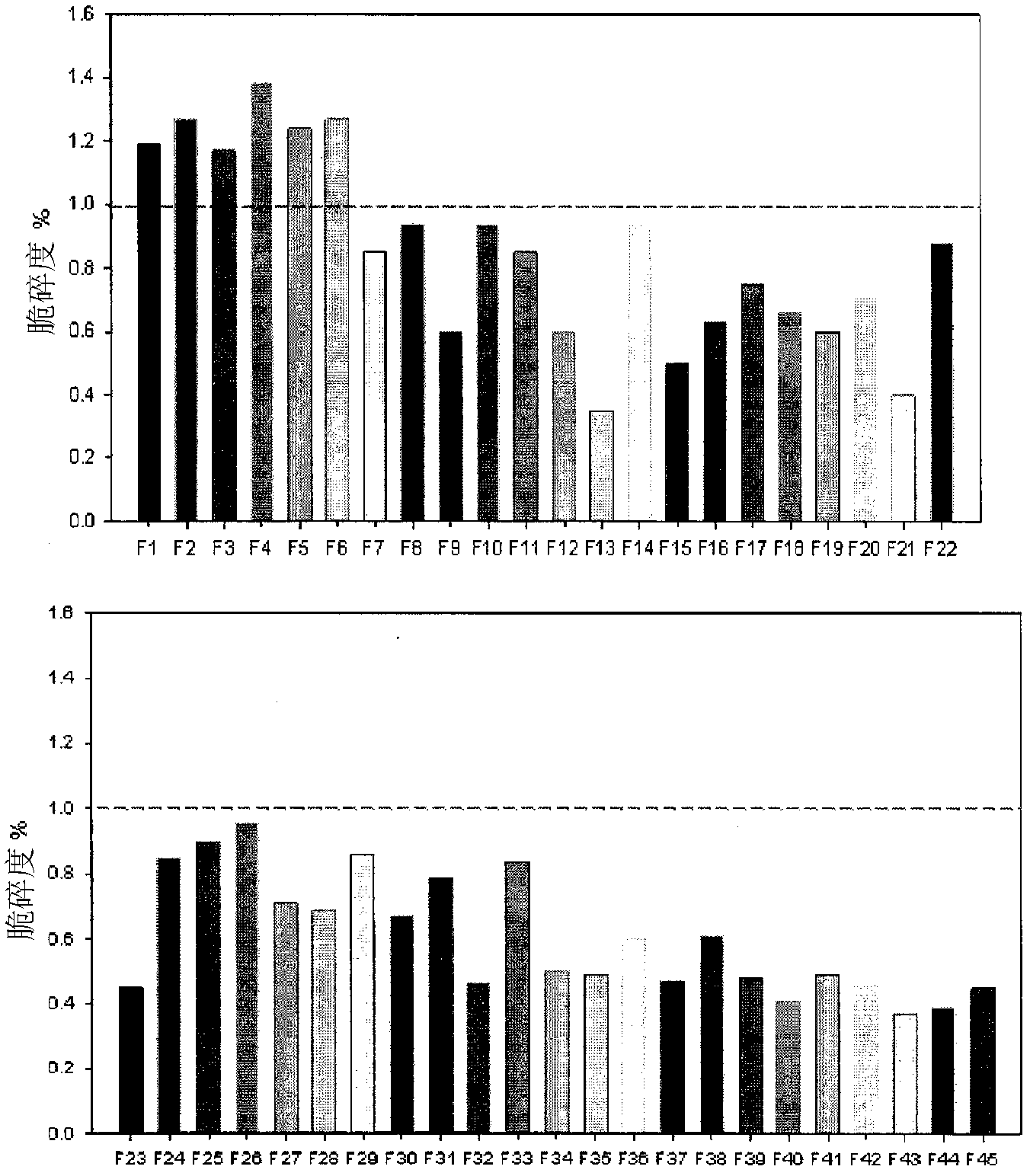
The invention relates to a drug sustained-release preparation and its preparation method, in particular to an aceclofenac sustained-release tablet and its preparation method, which comprises the following ingredients according to the weight percentage: aceclofenac of 60 to 95 per cent, skelecton retarder of 3 to 30 per cent, adhesive of 1 to 10 per cent and lubricant of 0.5 to 15 per cent. The hydroxypropylmethyl cellulose and carboxyvinyl polymer are adopted as optimized skelecton retarder. With such a technical proposal in the invention, the one aceclofenac sustained-release tablet can be taken a day to effectively reduce the fluctuation of blood drug level, prolong the maintenance duration of effective blood drug level and lower down the incitement to gastrointestinal tract. Besides, the invention also provides the preparation method of the aceclofenac sustained-release tablet.
Owner:ZHEJIANG JIANFENG PHARM CO LTD
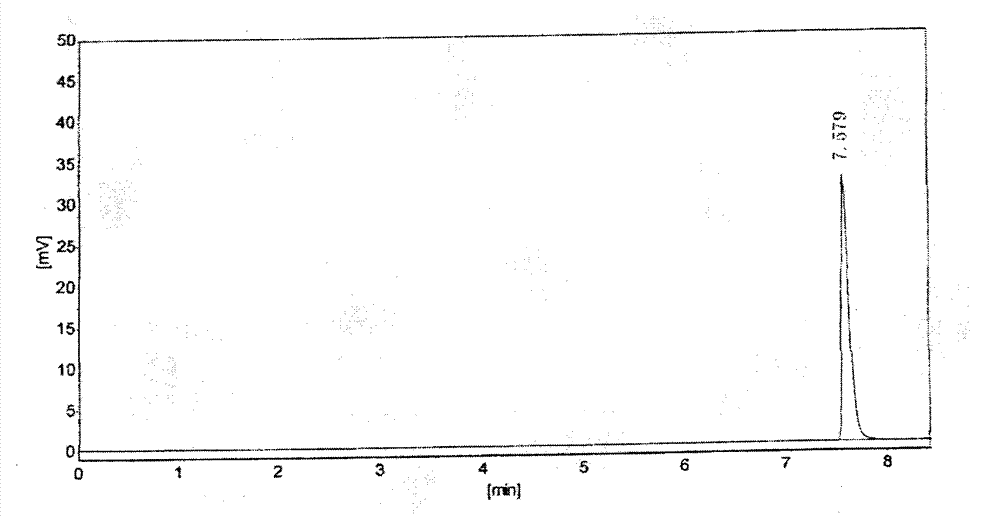
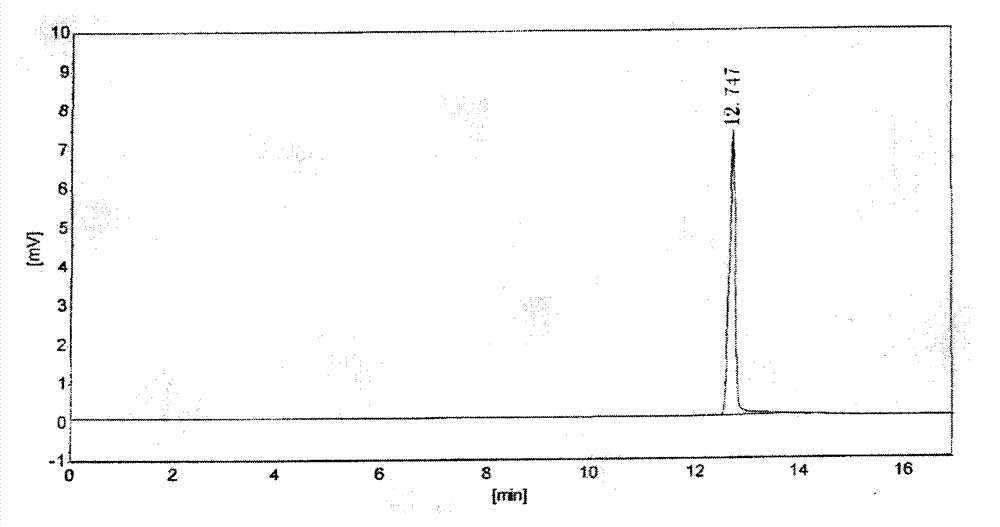
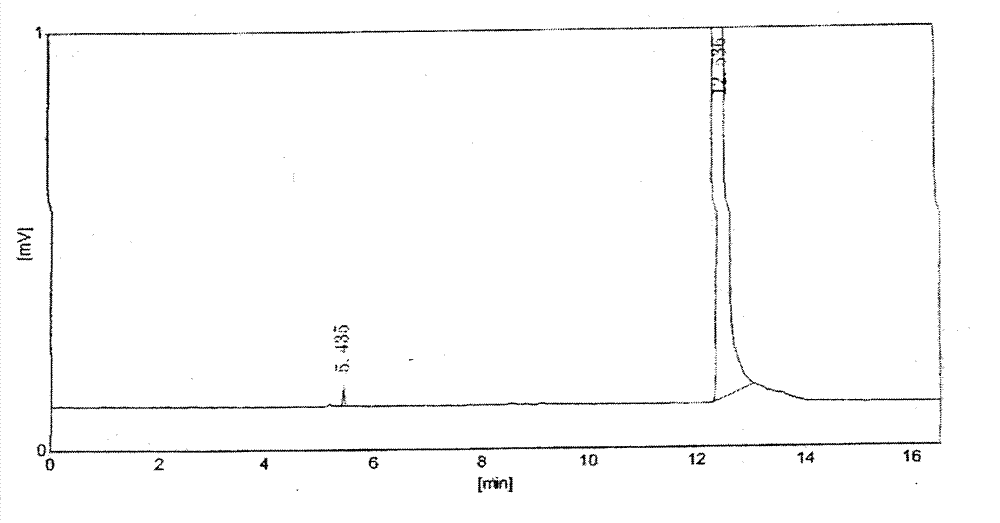
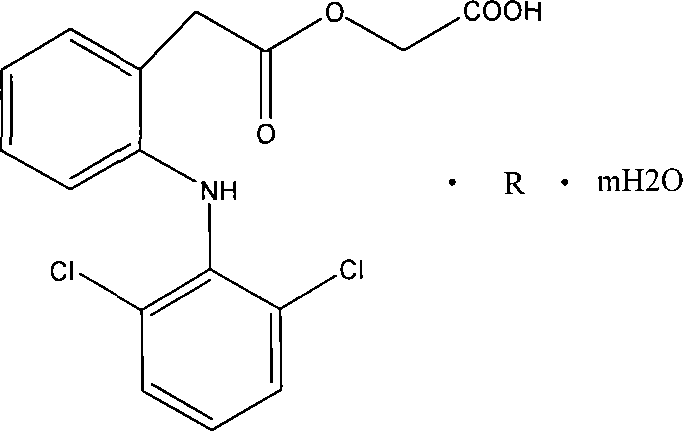
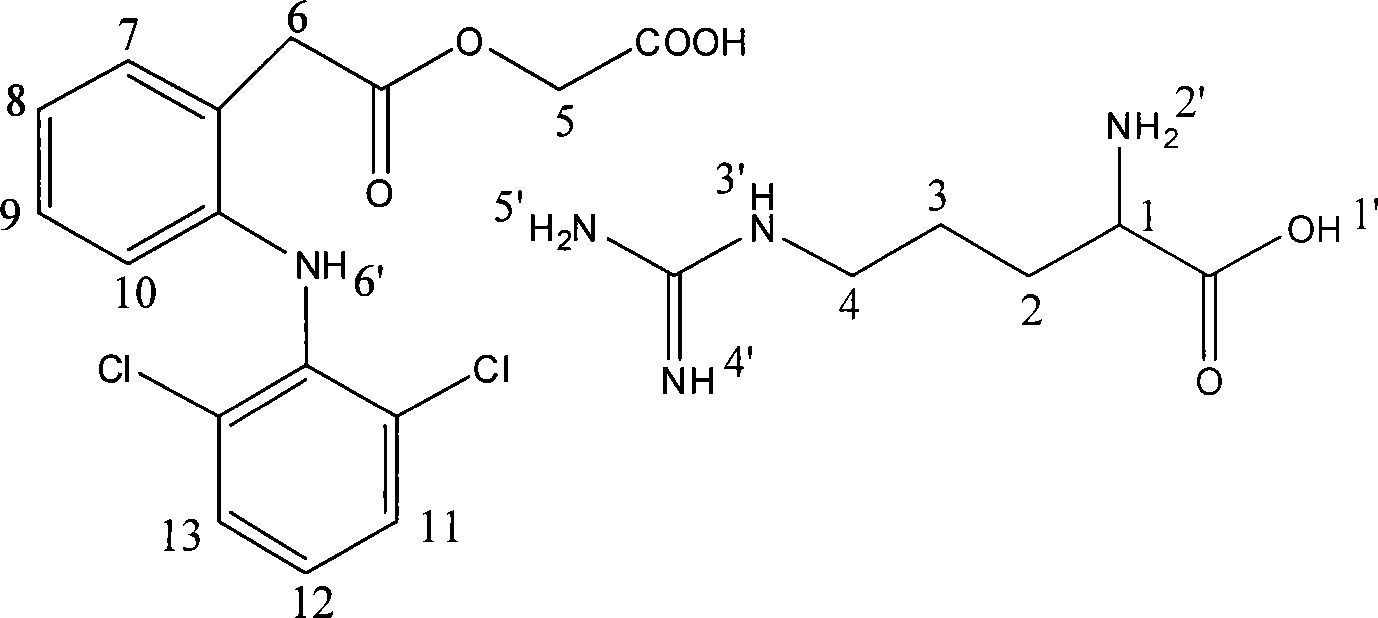
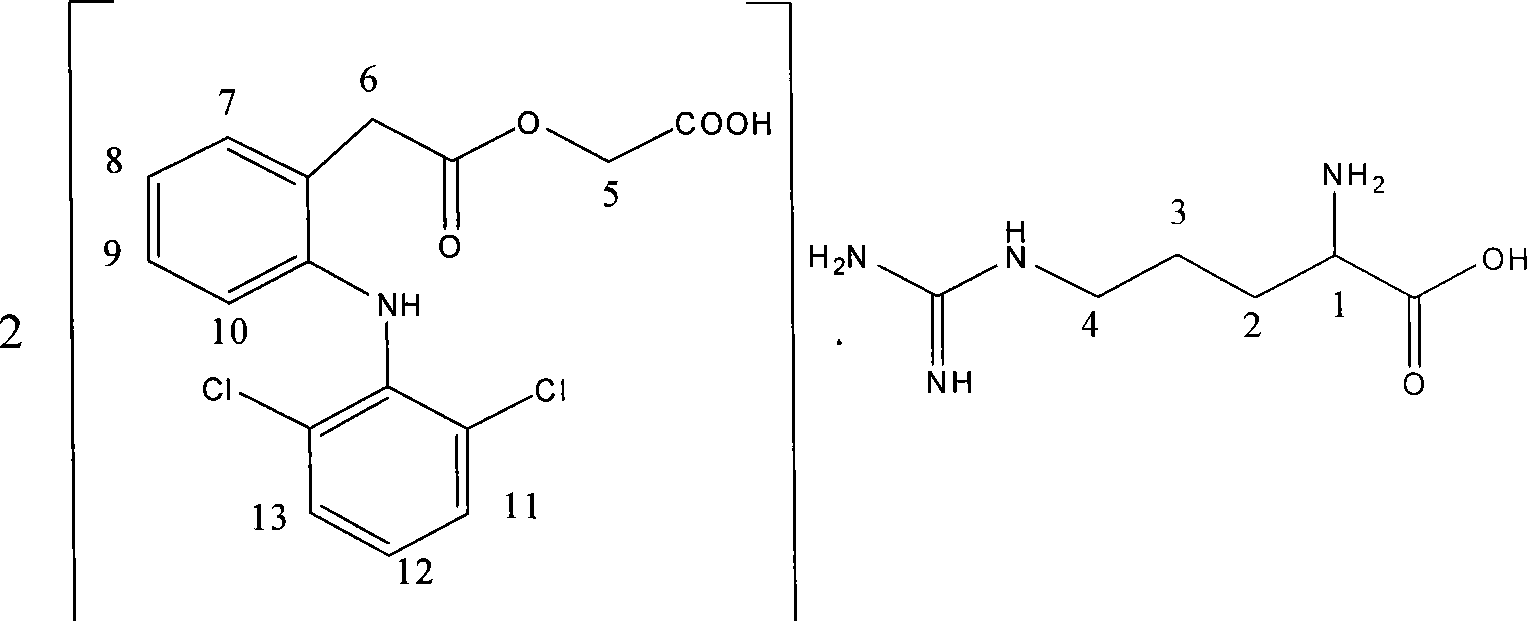
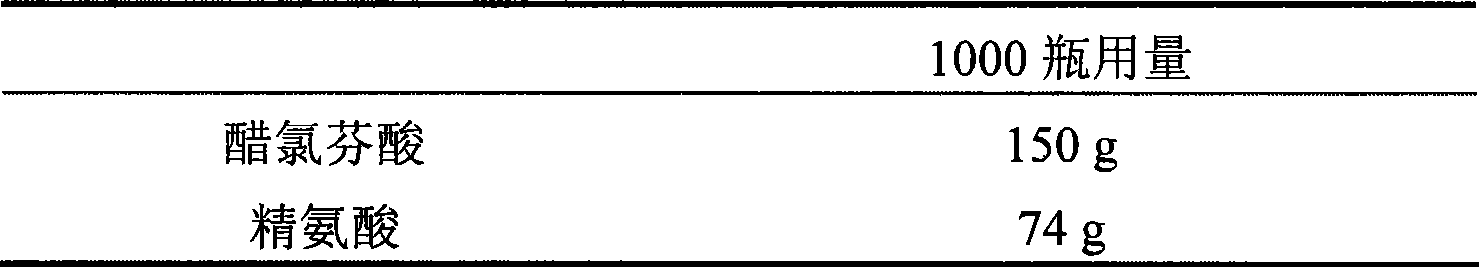
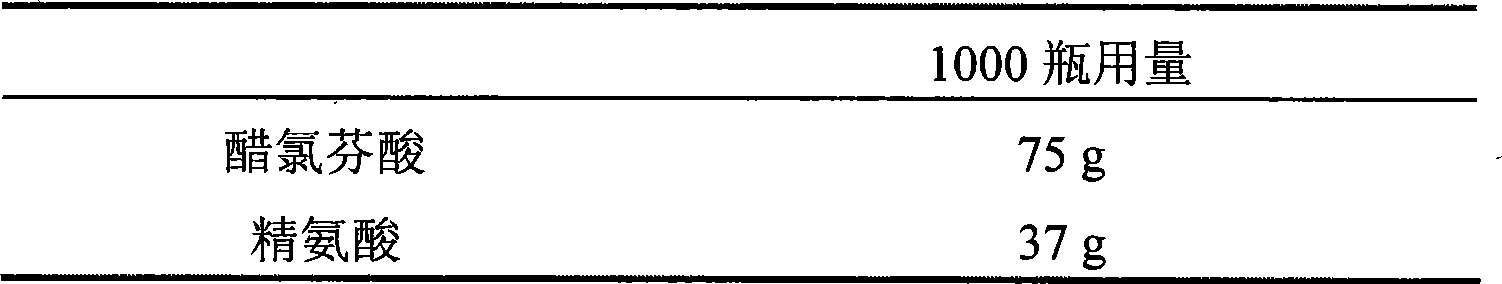
Salt compound formed by aceclofenac and organic base as well as composition and uses thereof
The invention discloses a salt compound formed through the aceclofenac and the organic base and the assemblage and the use. The general formula of the salt compound is AxR mH2O; wherein, A is the aceclofenac (CAS RN: 89796-99-6) and x is 1, 2; R is nitrogen organic base and can be amine and basic amino acid. The amine is most suitable for being selected from the Meglumine and trometamol; the basic amino acid is most suitable for being selected from the Arginine, Lysine, Ornithine, Histidine and Citrulline. The spatial configurations of the amino acids include the DL type, D type, L type and optimized L type; m is 0, 1, 2, 3, 4, 5 and 6. The compound and the assemblage to which the invention relates can be applied to the diseases such as the inflammation and pain, sprain, pull, and other soft tissue injuries and fever, etc., caused by the rheumatoid arthritis, rheumatoid arthritis, osteoarthritis, spondylitis, etc. of the human beings or animals.
Owner:王文菊
Aceclofenac granule
InactiveCN1989957AAppropriate retention timeOrganic active ingredientsAntipyreticDiseasePhenyl acetic acid
The invention belongs to the field of medicine and pharmacology, and is Pharmaceuticals oral granule. The Aceclofenac granule is made by raw material drug aceclofenac, findings and disintegrant. The Aceclofenac granule is phenyl acetic acid non-steroidal anti-inflammatory agent. It has notable anti-inflammatory, analgesic and antipyretic effect. It applies to the treatment of various rheumatoid arthritis, atrophic arthritis, osteoarthritis and spondylitis. It is fit for pain and fever caused by various diseases, and the toxic and side effects is lower than other varieties. Aceclofenac has not granule in prior medicine market; it is innovative formula and benefit for absorbing drug to getting better curative effect and better taste. It is safe, reliable and new drug for treatment of acute, chronic pain and anti-inflammation.
Owner:DISHA PHARMA GROUP BEIJING DISHA BIOTECH
Method for preparing aceclofenac enteric microcapsules
InactiveCN102688221AThe preparation process is stableEase of industrial productionOrganic active ingredientsAntipyreticCellulose acetateAceclofenac
Disclosed is a method for preparing aceclofenac enteric microcapsules, comprising dissolving eudragit II, hydroxypropylmethylcellulose phthalate (HPMCP) or cellulose acetate phthalate in acetone to acquire a solution A; then adding aceclofenac powder into the solution A and fully dissolving the aceclofenac powder to obtain a solution B, and acquiring a primary emulsion by placing the solution B in a flask and stirring; adding span 80 into liquid paraffin and fully stirring; adding the primary emulsion into the well-stirred liquid paraffin, heating up to 75 DEG C gradually while stirring, and acquiring microcapsules by stirring continuously while preserving the temperature; and washing the microcapsules by using n-hexane three times after filtering and drying to acquire the finished microcapsules. According to the invention, the aceclofenac slow-release enteric microcapsules are prepared by using a property that decomposition of a capsule wall material is influenced by pH values. Through controlling the capsule wall material, the following effects can be achieved: drugs are sent directionally to the small intestine and released slowly to gentle the plasma concentration, prolong the action time, and improve the curative effect; the dosing frequency can be reduced and the plasma concentration is maintained in the body; and an adverse reaction in the gastrointestinal tract is reduced effectively and patient compliance is also improved effectively.
Owner:SHAANXI UNIV OF SCI & TECH
Aceclofenac bi-layer osmotic pump controlled release tablets and preparation method thereof
InactiveCN101618027AShort half-lifeImprove complianceOrganic active ingredientsAntipyreticAnkylosing spondylitisAceclofenac
The invention provides an aceclofenac bi-layer osmotic pump controlled release tablet and a preparation method thereof, belongs to the technical field of medicinal preparation. The osmotic pump preparation comprises a tablet core containing aceclofenac and a semipermeable coating membrane which is coated outside the tablet core and provided with orifices, the tablet core comprises a drug layer containing aceclofenac and a boosting layer; wherein, the drug layer comprises the following components: 200mg of aceclofenac, 100mg-300mg of suspension, 10mg-50mg of osmotic stress active substance and0.5 mg-2mg of lubricant; the boosting layer comprises 50mg-150mg of sweller and 5mg-30mg of osmotic stress active substance; the semipermeable coating membrane comprises the following components: 10g-20g of semipermeable high polymer material dissolved in 500ml of acetone and 2g-5g of water soluble pore former dissolved in 20ml of distilled water and the weight of the coating membrane is 5%-10% of the tablet core weight; laser or a power drill is used to drill the orifices on the drug-containing side of the coating tablet. The invention is characterized of less dosing frequency, convenient taking way, long lasting and stable curative effect and can be used to cure pains and inflammations caused by osteoarthritis, rheumatoid arthritis, ankylosing spondylitis and the like.
Owner:SHENYANG PHARMA UNIVERSITY
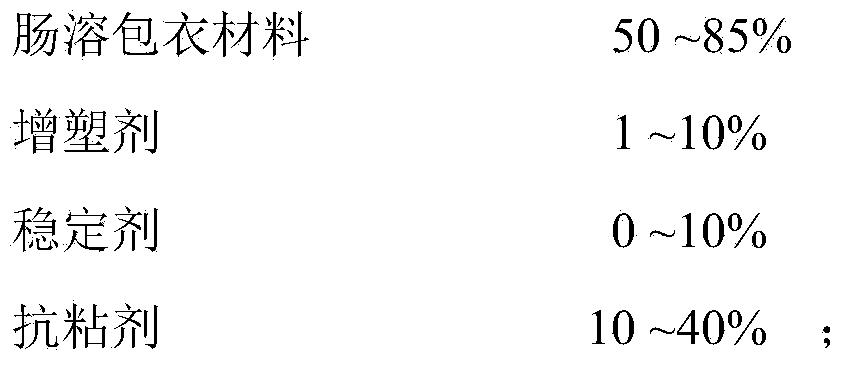
Aceclofenac enteric-coated pellet particle composition and preparation method thereof
ActiveCN102824312AHigh dissolution rateImprove bioavailabilityOrganic active ingredientsAntipyreticAceclofenacDissolution
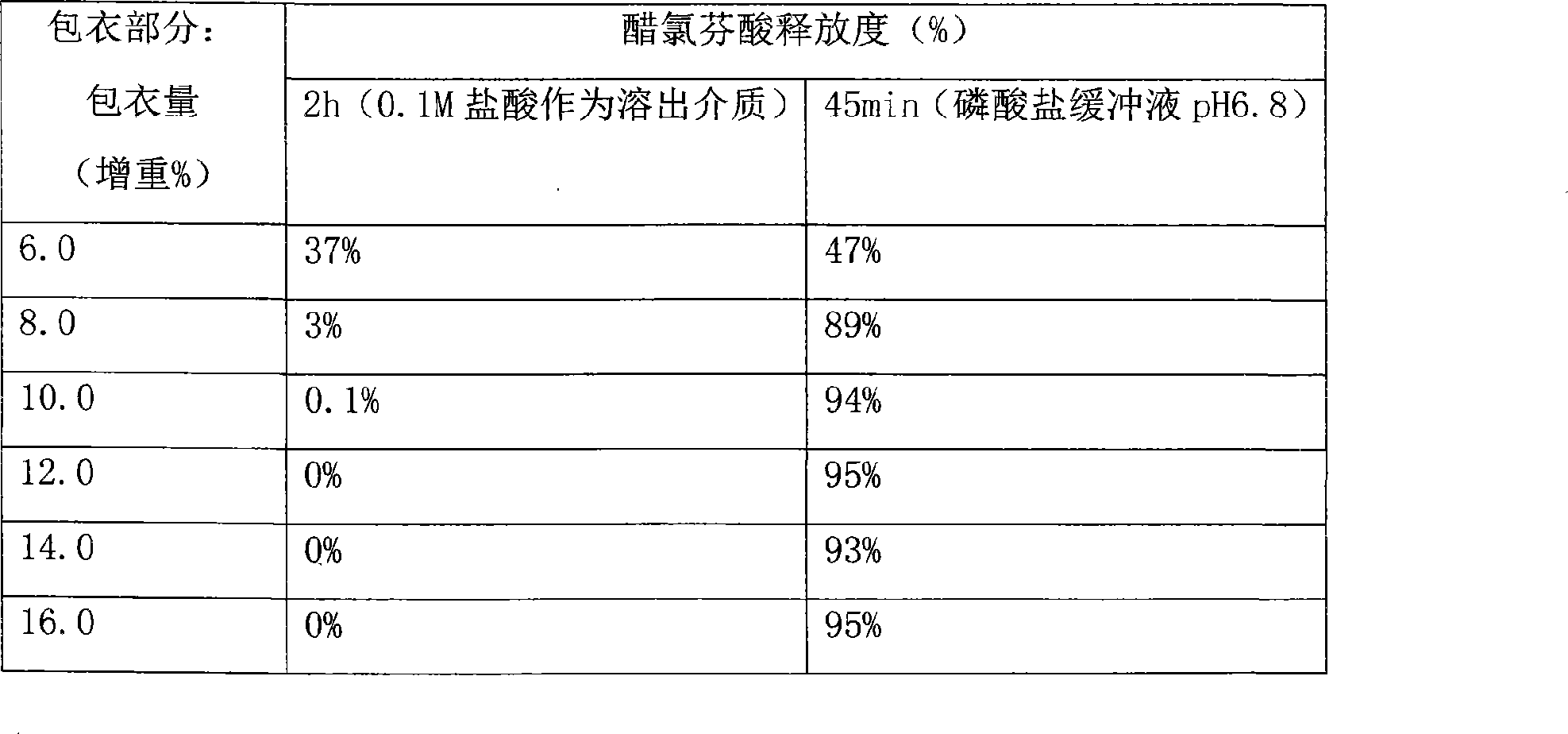
The invention relates to the field of a medicinal preparation, in particular to an aceclofenac enteric-coated pellet particle composition and a preparation method of the aceclofenac enteric-coated pellet particle composition. The aceclofenac enteric-coated pellet particles are higher in dissolution rate due to the fact that the dissolution rate is more than 75% within 45 minutes, the bioavailability is better and reaches up to 92.67%, and the taste is better. The preparation method is convenient to operate, thereby being suitable for industrial production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
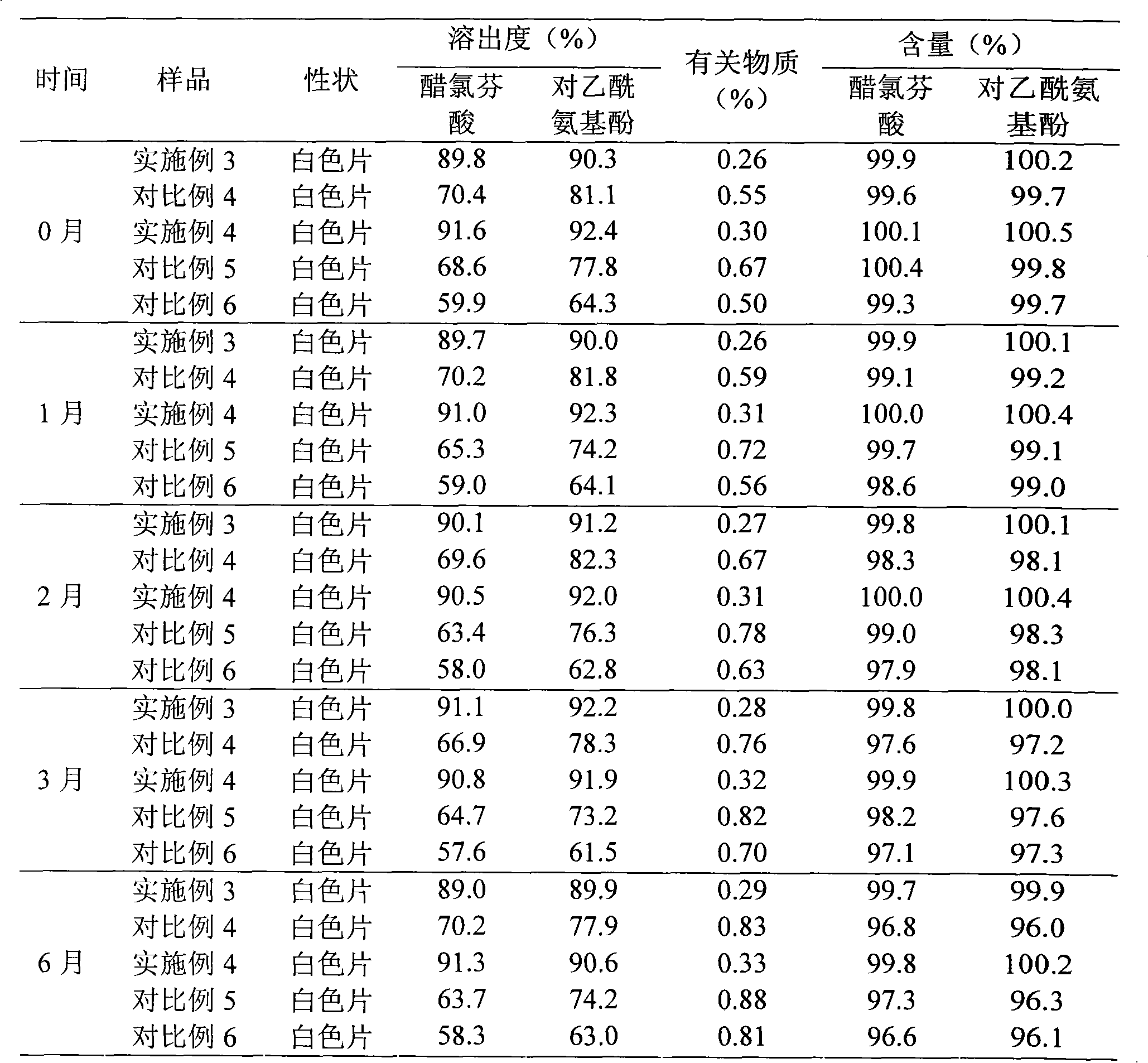
Compound enteric-coated preparation and preparation method thereof
InactiveCN101530406AReduce gastric irritationImprove complianceOrganic active ingredientsAntipyreticAceclofenacEnteric coated
The invention discloses a compound enteric-coated preparation. The aceclofenac in the compound preparation is dissolved by intestines through a pharmacy means and paracetanol is guaranteed to release in stomach, so that stimulus and untoward effect of the aceclofenac in the compound preparation on the stomach are reduced, and the normal absorption of the paracetanol is guaranteed to improve the compliance of a patient.
Owner:YANGTAI PHARMA SHANDONG
Medicinal composition with anti-inflammatory and analgesic effects
The invention which belongs to the technical field of medicines discloses a medicinal composition with anti-inflammatory and analgesic effects. The medicinal composition is characterized in that the medicinal composition comprises aceclofenac, a microcrystalline cellulose, pregelatinized starch, a hydroxypropyl cellulose, a lubricant and polyvinyl acetate phthalate; or the medicinal composition comprises aceclofenac, the microcrystalline cellulose, the pregelatinized starch, the hydroxypropyl cellulose, the lubricant, polyvinyl acetate phthalate and a polyacrylic resin III. The medicinal composition of the invention is determined through a disintegration test, a release degree test and the like, and medicinal preparations prepared from the medicinal composition of the invention have the excellent quality characteristics.
Owner:四川维奥制药有限公司
Preparation method of aceclofenac
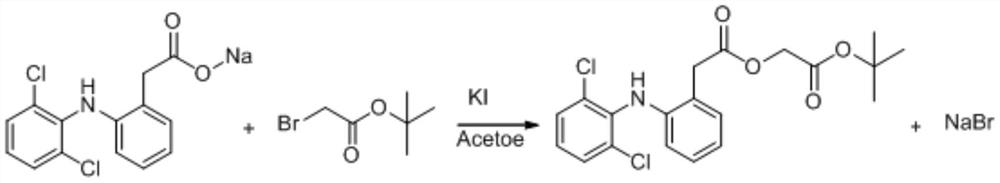
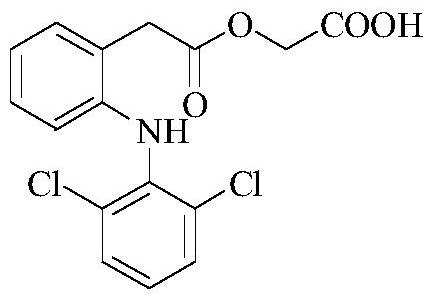
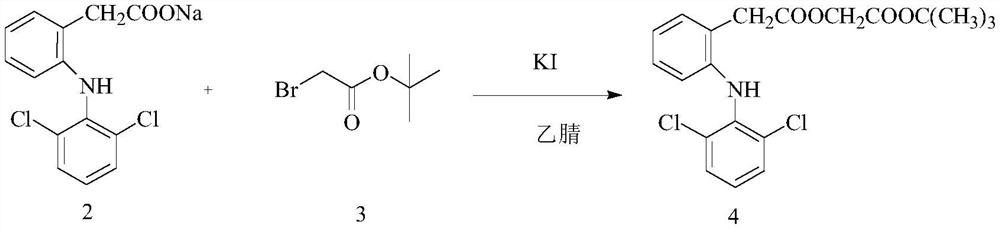
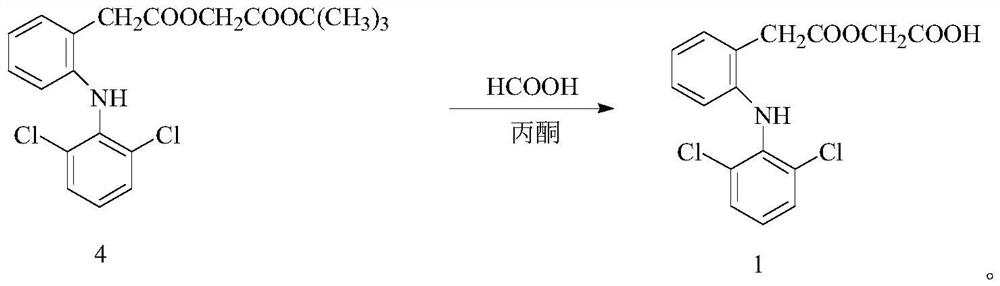
ActiveCN110143891AHigh selectivityReduce breakageOrganic compound preparationAmino-carboxyl compound preparationLower limitAceclofenac
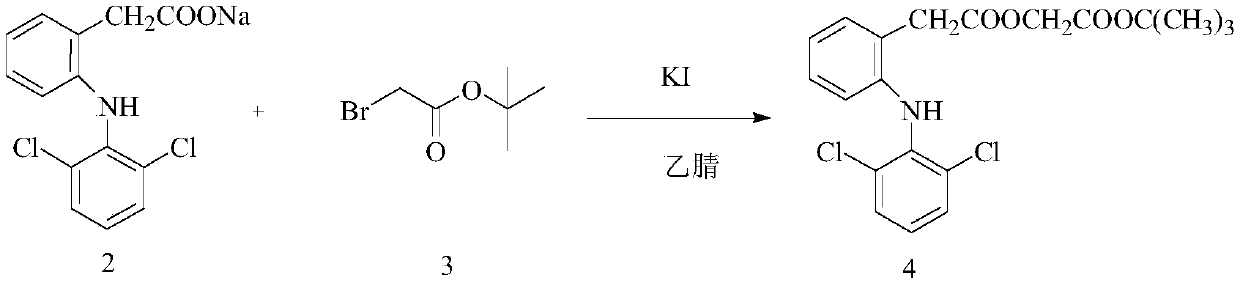
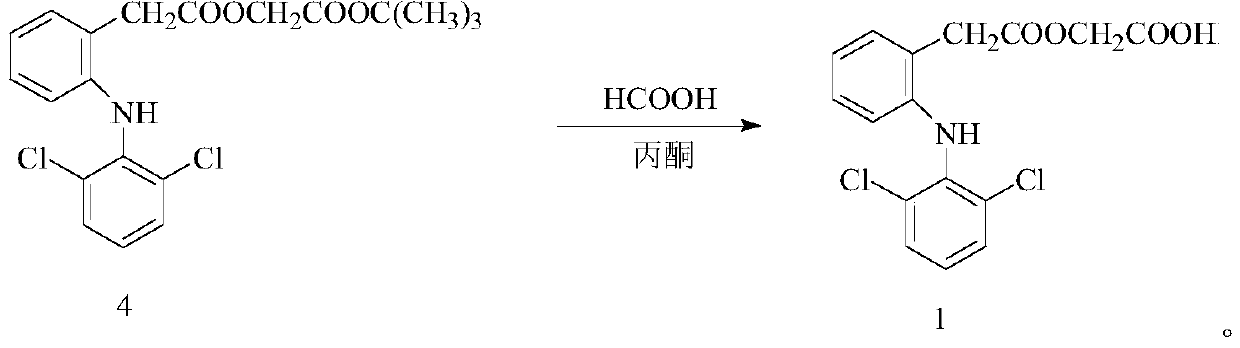
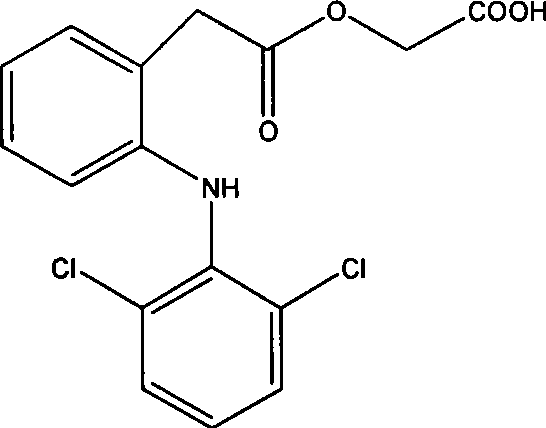
The invention discloses a microwave-assisted preparation method of aceclofenac. The method is as follows: under the microwave condition, a compound 2 and a compound 3 are reacted to form a compound 4under the catalysis of KI; and under the microwave condition, a reaction of the compound 4 is carried out in a mixed solvent of formic acid and acetone. According to the method, the acidolysis of aceclofenac tert-butyl ester is carried out in the mixture of formic acid and acetone under the microwave condition, the acidolysis method has high selectivity, the fracture of the ethoxyl group in aceclofenac tert-butyl ester is reduced to the lowest limit, the conversion rate of the acidolysis reaction is high, the diclofenac content in the product is extremely small, the product is easy to refine,and the purity is high.
Owner:ZHEJIANG UNIV OF TECH
Aceclofenac pharmaceutical composition
InactiveCN101480387AAvoid absorptionIncrease economic burdenOrganic active ingredientsAntipyreticCalcium in biologyCalcium metabolism
The invention discloses an aceclofenac pharmaceutical composition which contains the following raw materials: 10-150 portions of aceclofenac and 2.5-140 portions of organic base solvent. The composition also contains the following raw materials: 0-50 portions of pH modifying agent, 0-500 portions of osmotic pressure regulator, 0-600 portions of stabilizing agent, 0-100 portions of local acetanilide and 2000-10000 portions of water. The invention does also not contain phosphate, does not influence the calcium metabolism and the hormone secretory and increases the absorption of internal calcium and iron; and injection is dissolved by using common injection water or physiological saline without special menstruum.
Owner:王文菊
Aceclofenac enteric-coated pellet capsule and preparation method thereof
ActiveCN103961336AHighlight substantiveSignificant progressOrganic active ingredientsAntipyreticMedicineAceclofenac
The invention relates to an aceclofenac enteric-coated pellet capsule and a preparation method thereof, and belongs to the technical field of a drug preparation. The aceclofenac enteric-coated pellet capsule is prepared from aceclofenac enteric-coated pellets filling in a capsule shell. The aceclofenac enteric-coated pellet capsule is simple in preparation method, low in labor intensity, applicable to industrial production, beneficial to environmental protection, small in gastrointestinal stimulation on a sufferer, high in bioavailability, good in stability and the like.
Owner:PUYANG ZHONGYI PHARMA CO LTD
Aceclofenac slow-release preparation providing an optimum pharmacological clinical effect when administered once a day
ActiveCN102917696ARapid pain reliefRapid anti-inflammatory effectOrganic active ingredientsPill deliveryFast releaseAceclofenac
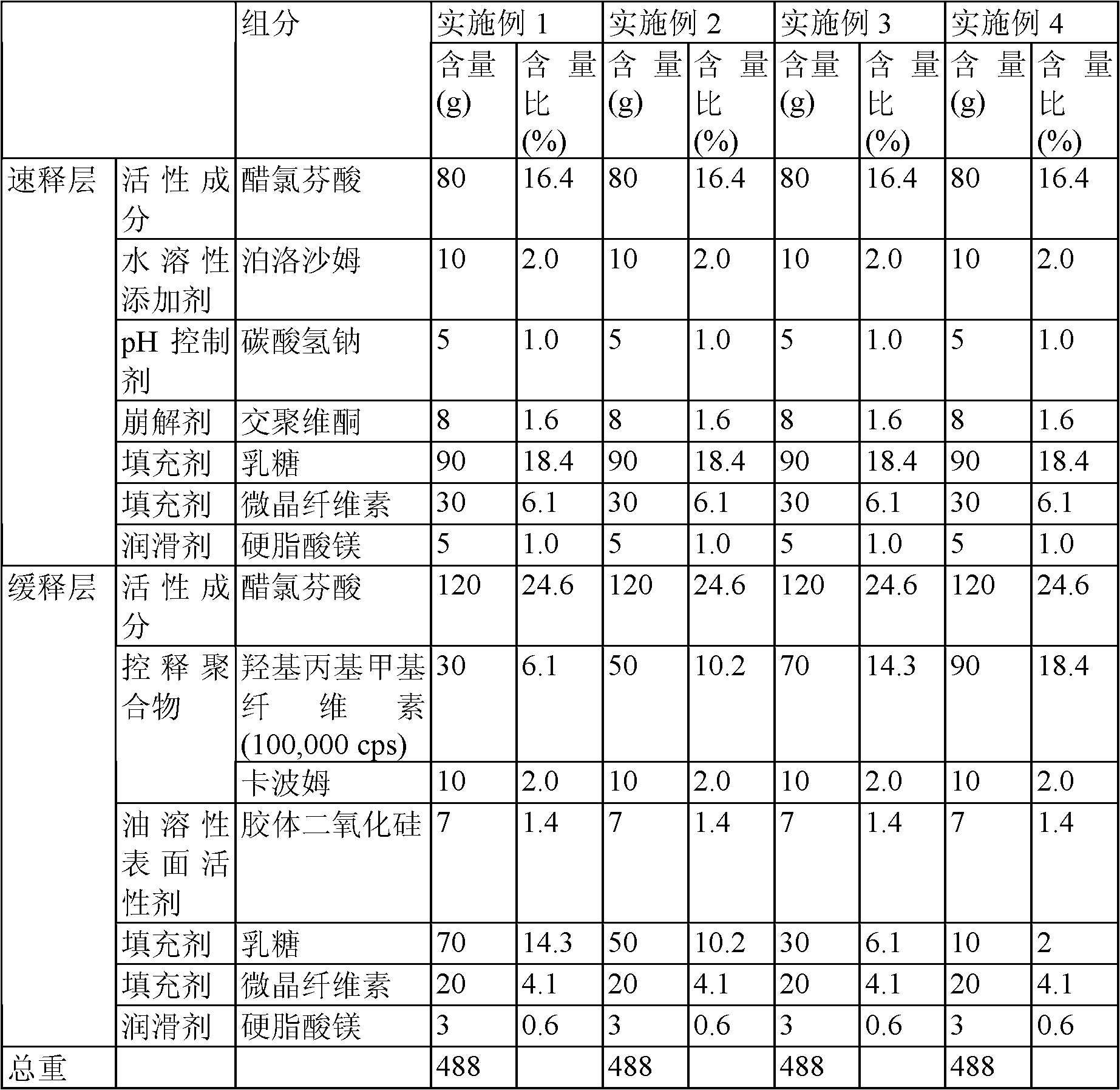
The present invention relates to a controlled-release preparation administered orally once a day that exhibits an optimum pharmacological clinical effect, featuring two-layer tablets, dual tablets and multilayered tablets having a fast-release layer comprising aceclofenac, a water-soluble additive, a non-soluble additive, a solubilizer, a disintegrator and a filler, and a slow-release layer comprising aceclofenac, a slow-release base, a disintegrator, a binder, a filler, a fluidizer, a solubilizer and a lubricant.
Owner:KOREA UNITED PHARMA
Aceclofenac-containing controlled-release oral drug preparations and their manufacturing process
ActiveCN102307575AHigh dissolution rateOrganic active ingredientsSkeletal disorderSodium bicarbonateImmediate release
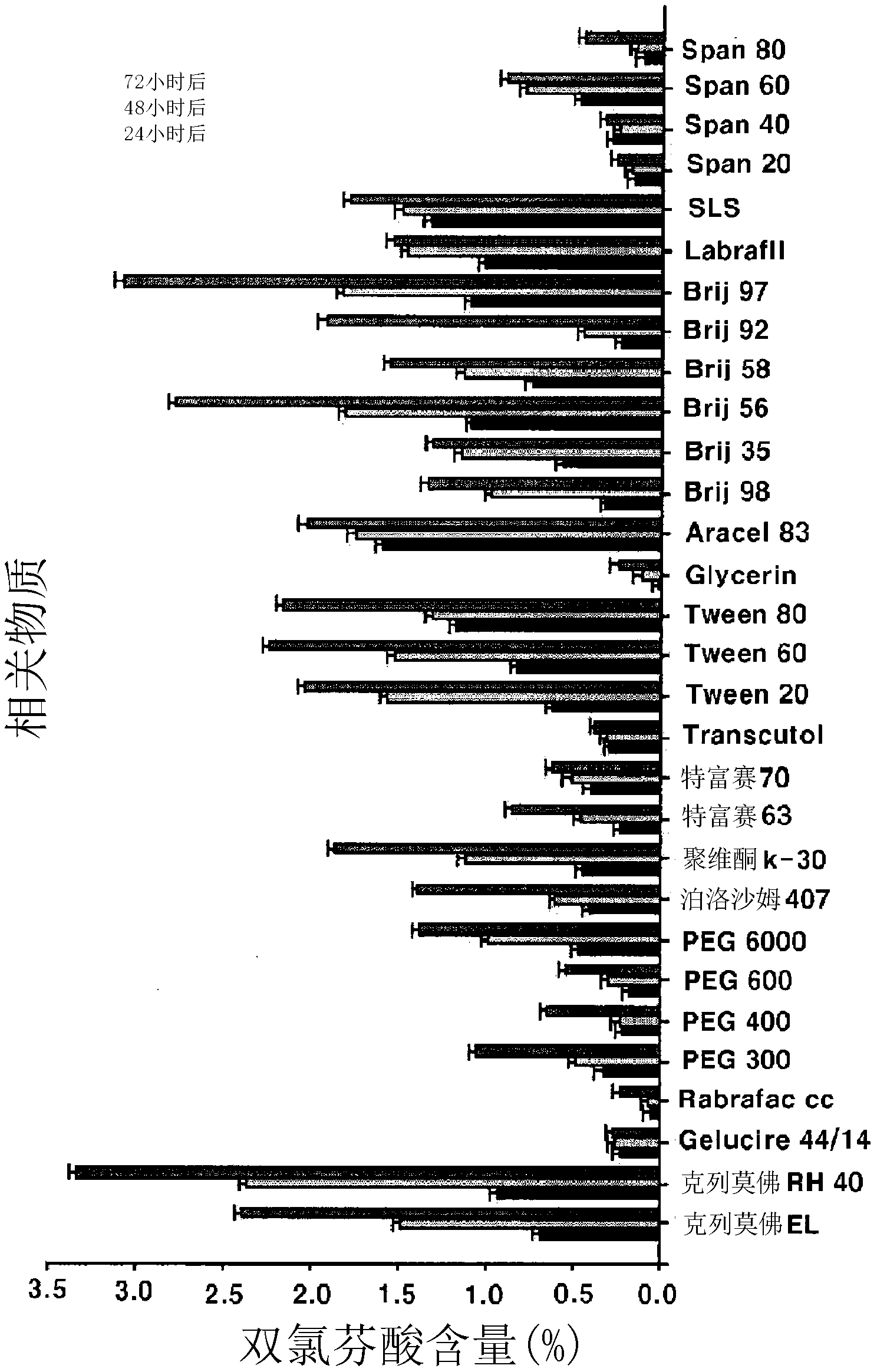
Disclosed herein are single-layer and double-layer tablets, which release aceclofenac in a controlledmanner so as to achieve ideal drug release close to a straight line. Also, the tablets promote drug absorption in the stomach by controlling pH, contain aceclofenac with improved stability and haveboth immediate-release properties and sustained-release properties. Specifically, provided is an aceclofenac sustained-release tablet which is composed of an immediate-release layer containing aceclofenac, a water-soluble additive, a pH-controlling agent, a disintegrant, a filler and a lubricant and of a sustained-release layer containing aceclofenac, a release-controlling polymer, an oil-soluble surfactant, a filler and a lubricant, wherein the pH-controlling agent is sodium hydrogen carbonate, and the release-controlling polymer is a mixture of hydroxypropylmethylcellulose and carbomer.
Owner:KOREA UNITED PHARMA
Skin plaster containing aceclofenac
InactiveCN1682705AImprove permeabilityLess irritatingOrganic active ingredientsAntipyreticSciaticaAceclofenac
The present invention relates to skin plaster, and a kind of local skin plaster containing acelofenac for treating and relieving osteoarthritis, rheumatoid arthritis, ankylosed rachitis and sciatica.
Owner:西安东盛集团有限公司
Method for preparing aceclofenac
InactiveCN101215243AQuality improvementMeet the quality standard requirementsOrganic compound preparationAmino-carboxyl compound preparationOrganic layerAceclofenac
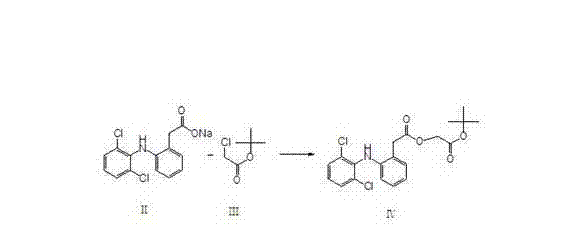
The invention discloses a preparation process of novel aceclofenac. The preparation process of novel aceclofenac which is stated by the invention comprises filling diclofenac and toluene in a reaction bottle, stirring under the condition of outside temperature, dripping triethylamine until the solution is clarified, dripping tert-butyl bromoacetate to react for 3-4 hours when temperature is 50-60 DEG C, dripping 30% NaOH solution to alkalize after reaction, separating layers, scouring organic layer with water, drying with anhydrous sodium sulfate, filtering, steaming out organic solvent, dripping formic acid, reacting for 1 hour with 56-60 DEG C, dripping pure water when temperature is 0-5 DEG C, crystallizing for two hours, filtering and drying to obtain aceclofenac crystal (mp.151-152 DEG C). The process utilizes the advantages that tert-butyl is easy to be removed without the catalysis of palladium-charcoal and hydrogenization reaction, and tert-butyl is removed under the action of formic acid to obtain aceclofenac. The reaction can be conducted under smooth condition, which firstly improves reaction condition, secondly reduces environment pollution, and thirdly increases product yield.
Owner:张宏业
Acedofenac-paracetamol pharmaceutical composite and liposome solid preparation thereof
InactiveCN101627986AImprove stabilityImprove efficacyOrganic active ingredientsSenses disorderCholesterolLiposome
The invention relates to an acedofenac-paracetamol pharmaceutical composite and a liposome solid preparation thereof and a preparation method thereof; the solid preparation comprises the liposome of acedofenac-paracetamol and the excipient which is accepted in pharmacy and the liposome comprises the following components according to the parts by weight percent: 1 part of acedofenac, 5 parts of paracetamol, 5-32 parts of soybean lecithin, 2-17 parts of cholesterol, 2.5-15 parts of sodium deoxycholate and 4-24 parts of poloxamer 188.
Owner:HAINAN YONGTIAN PHARMA INST
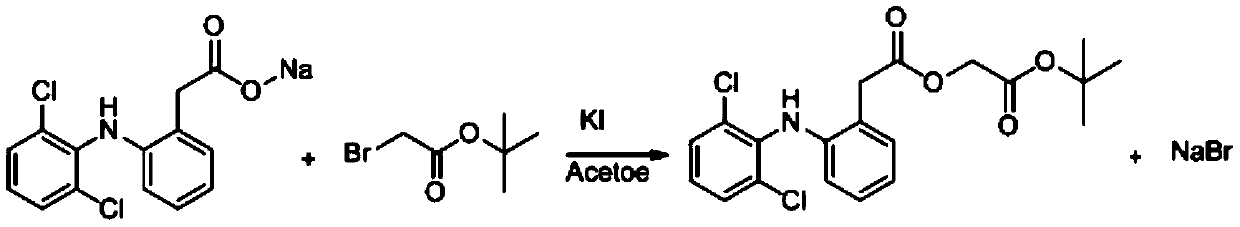
Method for producing aceclofenac tert-butyl ester
ActiveCN110305027AGood removal effectEconomical priceOrganic compound preparationAmino-carboxyl compound preparationOrganic solventIodide
The invention relates to a method for producing aceclofenac tert-butyl ester. The method includes: allowing diclofenac sodium, tert-butyl bromoacetate, catalyst iodide and tetrabutylammonium hydrogensulfate to have reaction in water, slowly cooling, crystallizing, filtering, washing, and drying. The method is mild in reaction conditions, high in yield, capable of reducing waste liquid discharge,capable of avoiding the toxic effect of organic solvents on human bodies and the like.
Owner:NINGBO SMART PHARMA
Pharmaceutical Composition with Antiinflammatory Agents and Production Process
The present invention relates to pharmaceutical compositions including a triphasic release system, which may be delayed release and / or extended release and / or modified release and / or immediate release, of at least three layers for the formation of at least one dosage unit. Each layer includes, as active pharmaceutical ingredients, at least one corticosteroid agent of the betamethasone type and / or the pharmaceutically acceptable salts thereof, at least one non-steroidal anti-inflammatory agent of the Aceclofenac type and / or the pharmaceutically acceptable salts thereof, and at least one pharmaceutically acceptable excipient. Also described is the novel production process. Said triphasic release system generates an enhanced treatment effect to combat inflammation and body pain.
Owner:LAB RAAM DE SAHUAYO S A DE
Externally applied aceclofenac gel prepn and its prepn process
InactiveCN101019850ALess irritatingQuality improvementOrganic active ingredientsAntipyreticGel preparationDisease
The present invention discloses one kind of externally applied aceclofenac gel preparation and its preparation process. The externally applied aceclofenac gel preparation is compounded with aceclofenac 0.5-8 wt%, organic solvent 5-50 wt%, bacteriostat 0.001-10 wt%, pH regulator 0.01-2 wt%, carbomer 0.5-8 wt%, antioxidant 0.01-5 wt% and water for the rest. It is prepared through mixing the said ingredients. It is applied to the joint or other disease focus to result in obvious curative effect.
Owner:夏泽宽 +1
Pharmaceutical composition of aceclofenac and pharmaceutical application thereof
InactiveCN105884858AGood treatment effectOrganic active ingredientsUrinary disorderNatural productTherapeutic effect
The invention discloses a pharmaceutical composition of aceclofenac and a pharmaceutical application thereof. The pharmaceutical composition of aceclofenac, disclosed by the invention, contains aceclofenac and a natural product compound (I) with a novel structure and separated from the dried root of rheum officinale. When the aceclofenac and the natural product compound (I) act separately, the treatment effect on chronic nephritis is relatively low; by combining the aceclofenac and the natural product compound (I), the treatment effect on chronic nephritis is remarkably improved; and the pharmaceutical composition can be developed into a medicine for treating chronic nephritis and has outstanding substantial characteristics and remarkable progress in comparison with the prior art.
Owner:SUZHOU BINUOJIA PHARMA CO LTD
Aceclofenac enteric-coated tablet and preparing method thereof
ActiveCN104940157ASuitable for long term storageSimple preparation processOrganic active ingredientsAntipyreticMedicineIsolation layer
The invention discloses an aceclofenac enteric-coated tablet and a preparing method thereof. The enteric-coated tablet comprises a tablet core, an isolation layer and a coating, wherein the tablet core contains aceclofenac, polyethylene glycol and copovidone. It is found through conventional experiments that the dissolution rate of the aceclofenac enteric-coated tablet is 99% or higher, it is found through acceleration experiments that aceclofenac is suitable for long-time storage, the preparing technology is easy, industrialization large-scale production is easy, and obvious advantages are achieved compared with the prior art.
Owner:LUNAN BETTER PHARMA
Pharmaceutical Composition Comprising Cyclobenzaprine and Aceclofenac in Association
The present invention relates to an association of active ingredients. More specifically: to an association of cyclobenzaprine and aceclofenac. Additionally, the present invention is also related to the use of aceclofenac and cyclobenzaprine, in association for the preparation of a medicine useful in the treatment of painful muscular diseases, as well as to a method of treatment of painful muscular diseases using an association of aceclofenac and cyclobenzaprine.
Owner:BIOLAB SANUS FARMACEUTICA LTD +1
Nonaqueous liquid parenteral aceclofenac formulation
A nonaqueous liquid parenterally deliverable pharmaceutical formulation, and more particularly a nonaqueous liquid parenteral Aceclofenac formulation comprising the selective NSAID Aceclofenac, is disclosed. A process of preparing Aceclofenac formulation, the therapeutic dosage form and storage of dose, and the method of treating a subject having a condition or a disorder wherein treatment with NSAID is indicated, are also disclosed. Diclofenac formed by conversion of Aceclofenac is solubilized by the nonaqueous solubilizer(s), which are substantially inert with respect to such conversion. The composition has Aceclofenac salt stabilizing means for inhibiting precipitation of Aceclofenac. The compositions disclosed in the present invention are stable upon storage at room temperature and at refrigerated temperatures. Compositions disclosed in the present invention, whether ready-to-use or requiring dilution prior to administration, can be prepared by inexpensive processes disclosed herein.
Owner:VENUS REMEDIES LTD
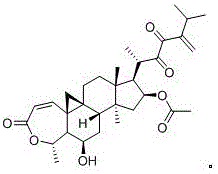
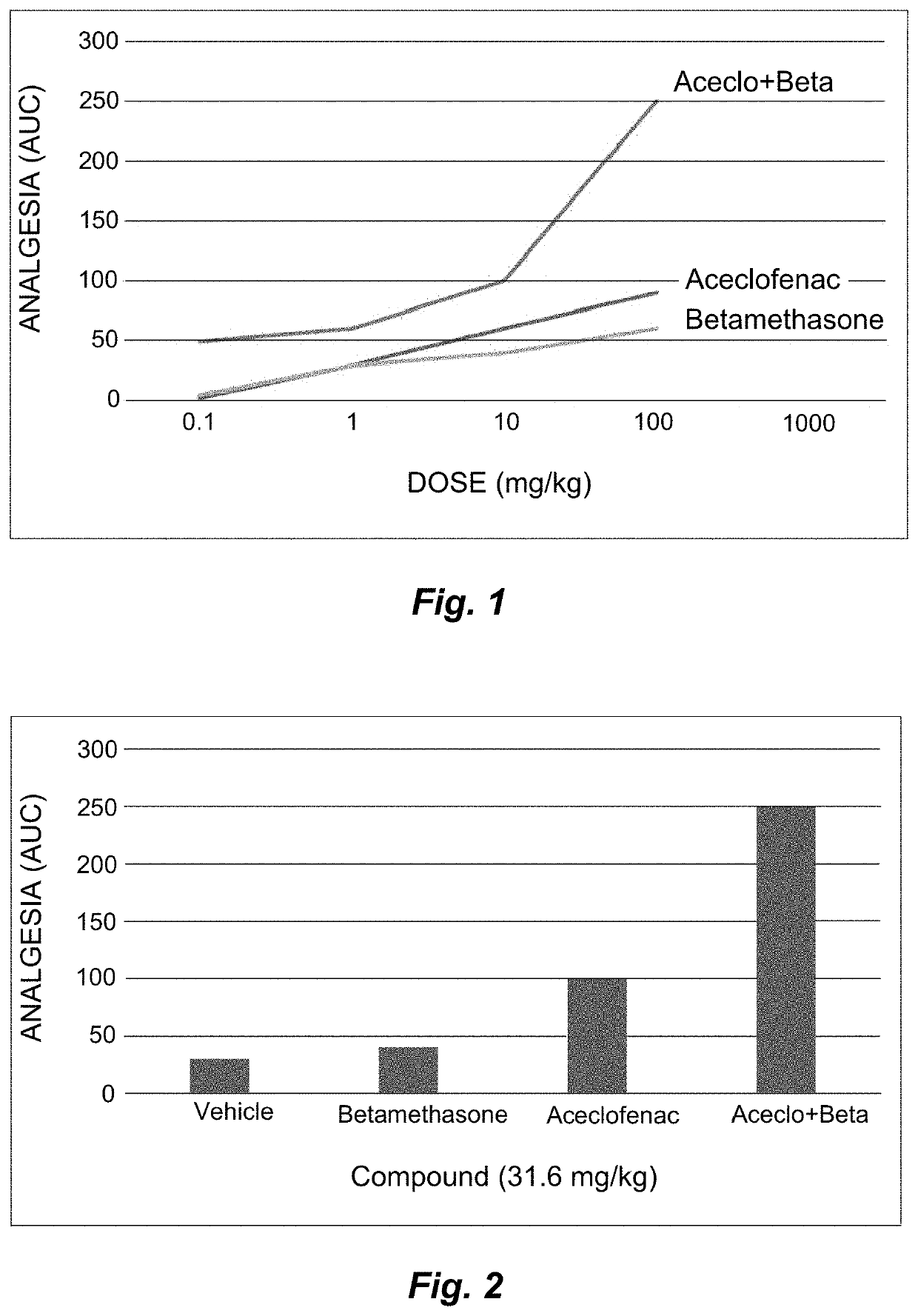
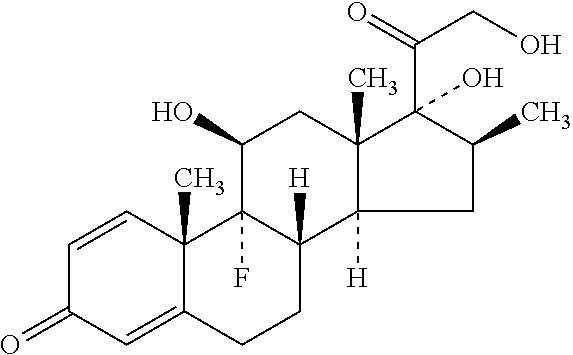
Synergistic pharmaceutical composition comprising aceclofenac and betamethasone for the treatment of pain in localised forms of rheumatic illnesses
PendingUS20210228598A1Improve the quality of lifeConvenient treatmentOrganic active ingredientsAntipyreticDiseasePropanoic acid
This invention is related to a pharmaceutical composition made up by the synergic combination of a nonsteroidal anti-inflammatory analgesic such as Aceclofenac or its pharmaceutically acceptable salts and an anti-inflammatory steroid agent such as the active ingredient Betamethasone or its pharmaceutically acceptable phosphate or dipropionate salts, which are formulated in a single dosing unit for topical, intramuscular or intravenous administration, which is indicated for the treatment of the localized pain of rheumatic diseases.
Owner:AMEZCUA AMEZCUA FEDERICO +1
A kind of method for producing aceclofenac tert-butyl ester
ActiveCN110305027BGood removal effectEconomical priceOrganic compound preparationAmino-carboxyl compound preparationAcetic acidIodide
The invention relates to a method for producing aceclofenac tert-butyl ester. The method comprises: reacting diclofenac sodium, tert-butyl bromoacetate, catalyst iodide and tetrabutylammonium hydrogen sulfate in water, slowly cooling down, crystallizing, filtering, washing and drying. The method has the advantages of mild reaction conditions, high yield, reduced discharge of waste liquid, and avoidance of toxic effects of organic solvents on human body.
Owner:NINGBO SMART PHARMA
A kind of preparation method of aceclofenac
ActiveCN110143891BHigh selectivityReduce breakageOrganic compound preparationAmino-carboxyl compound preparationEthoxidineDiclofenac Acid
A kind of preparation method of aceclofenac under microwave assistance, described method is: under microwave condition, compound 2 and compound 3 are catalyzed by KI, react to generate compound 4; Under microwave condition, compound 4 is in the mixed solvent of formic acid and acetone , the reaction generates compound 1; the present invention adopts the acid hydrolysis of aceclofenac tert-butyl ester in formic acid and acetone mixed solution under microwave conditions, and this acid hydrolysis method has high selectivity, and aceclofenac tert-butyl ester ethoxylated Fracture is reduced to the lowest limit, the conversion rate of acidolysis reaction is high, the content of diclofenac in the product is very small, the product is easy to refine, and has high purity.
Owner:ZHEJIANG UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com