Acedofenac-paracetamol pharmaceutical composite and liposome solid preparation thereof
A technology of acetaminophen and aceclofenac, applied in the field of medicine, can solve the problems of poor stability, low bioavailability, poor dissolution and the like, and achieve the effects of low cost, simple production process and fast onset of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
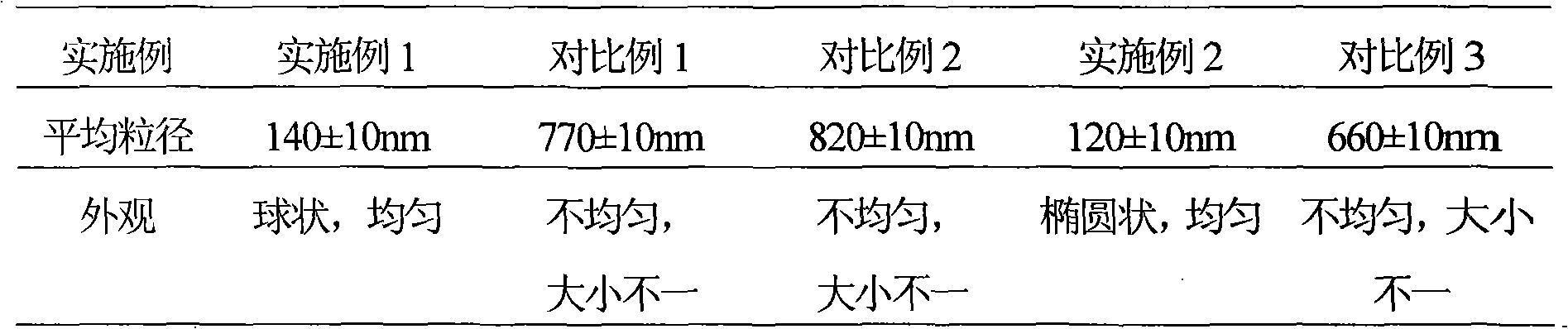
Embodiment 1
[0052] Example 1 Preparation of liposomes of aceclofenac and acetaminophen
[0053] prescription:
[0054] Aceclofenac 100g
[0055] Acetaminophen 500g
[0056] Soy Lecithin 1200g
[0057] Cholesterol 300g
[0058] Sodium deoxycholate 200g
[0059] Poloxamer 188 500g
[0060] Preparation Process
[0061] (1) Dissolve 1200g of soybean lecithin, 300g of cholesterol, 200g of sodium deoxycholate and 500g of poloxamer 188 in 8000ml of isopropanol, mix well, and remove isopropanol under reduced pressure on a rotary film evaporator to prepare Get phospholipid membrane;
[0062] (2) Add 2000ml of phosphoric acid-sodium dihydrogen phosphate buffer solution with pH=4.2, stir to completely hydrate the phospholipid membrane, and then homogenize and emulsify with a tissue masher for 20 minutes at a speed of 12000r / min to prepare a blank liposome suspension Liquid; (100g of aceclofenac is 1 part, 2000ml of buffer solution is 2000g, 2000g / 100g=20 parts)
[0063] (3) Disperse 100g of aceclofenac and 500g ...
Embodiment 2
[0081] Example 2 Preparation of liposomes of aceclofenac and acetaminophen
[0082] prescription:
[0083] Aceclofenac 100g
[0084] Acetaminophen 500g
[0085] Soy Lecithin 2400g
[0086] Cholesterol 900g
[0087] Sodium deoxycholate 600g
[0088] Poloxamer 188 1200g
[0089] Preparation Process
[0090] (1) Dissolve 2400g soybean lecithin, 900g cholesterol, 600g sodium deoxycholate and 1200g poloxamer 188 in 18000ml isopropanol, mix well, and remove isopropanol under reduced pressure on a rotary film evaporator to prepare Get phospholipid membrane;
[0091] (2) Add 5000ml of citric acid-sodium citrate buffer solution with pH=5.6, stir to completely hydrate the phospholipid membrane, and then homogenize and emulsify with a tissue masher for 15min at a rotation speed of 15000r / min to prepare blank liposomes Suspension
[0092] (3) Disperse 100g of aceclofenac and 500g of acetaminophen in 5000ml of water evenly, add them to the blank liposome suspension, keep at 55℃, and then homogenize an...
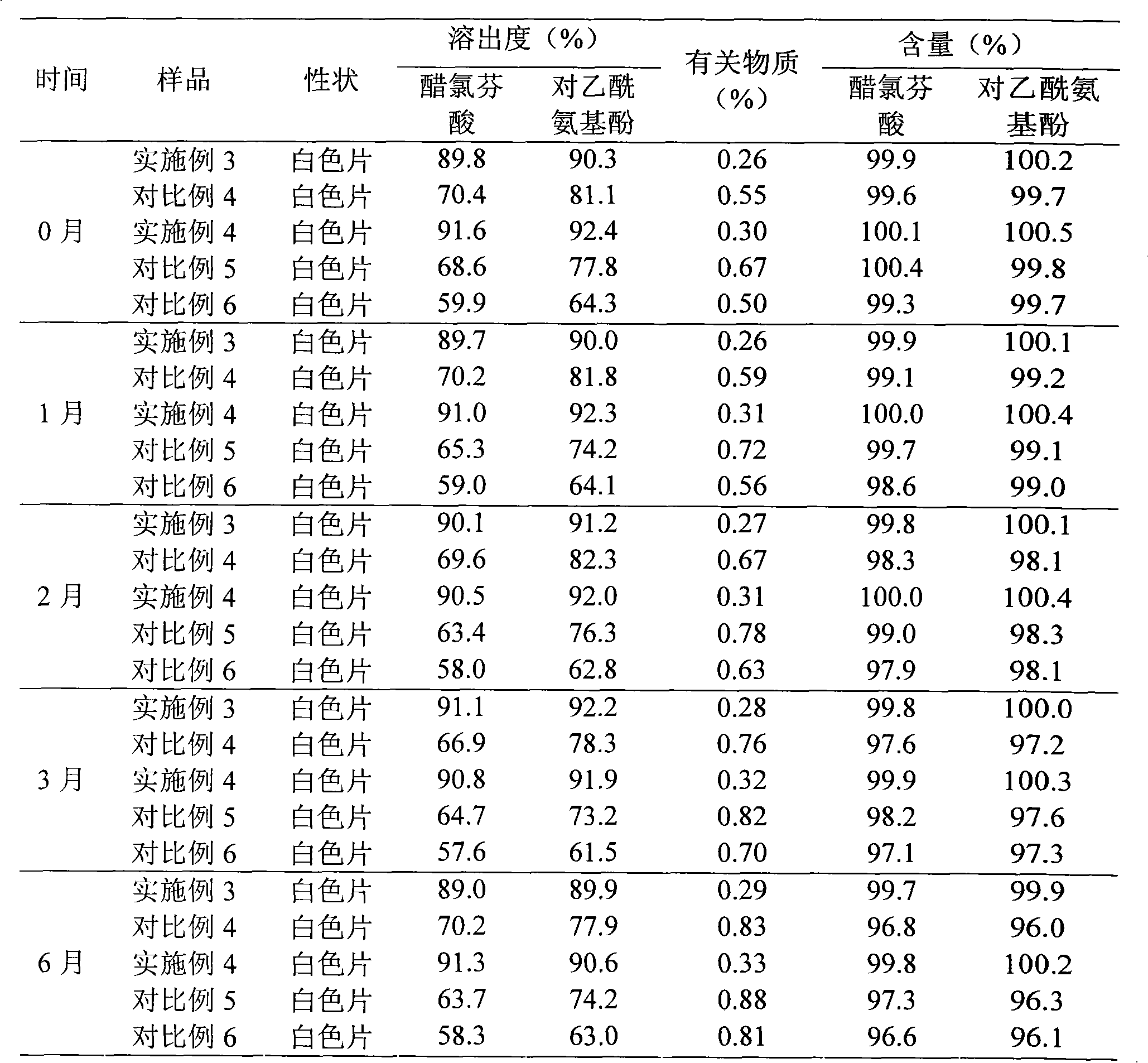
Embodiment 3
[0105] Example 3 Preparation of Aceclofenac and Acetaminophen Tablets
[0106] 600g of liposomes containing aceclofenac and acetaminophen (containing aceclofenac 100g, acetaminophen (from Example 1) phenol 500g)
[0107] Microcrystalline cellulose 76g
[0108] Lactose 50g
[0109] Crospovidone 40g
[0110] Hypromellose 3g
[0111] Colloidal silica 15g
[0112] Talcum powder 20g
[0113] Preparation Process
[0114] (1) The liposomes containing 600g of aceclofenac and acetaminophen prepared in Example 1 were crushed, passed through an 80-mesh sieve, and set aside;
[0115] (2) Weigh 76 g of microcrystalline cellulose, 50 g of lactose, and 40 g of crospovidone, pass through an 80 mesh sieve, mix, and set aside;
[0116] (3) Mix the above raw and auxiliary materials uniformly, add 150ml of 2% hypromellose 50% ethanol solution to make soft material, pass through a 20-mesh sieve to granulate, dry at 60°C, and pass through an 18-mesh sieve for granulation;
[0117] (4) Compress the dried granules ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com