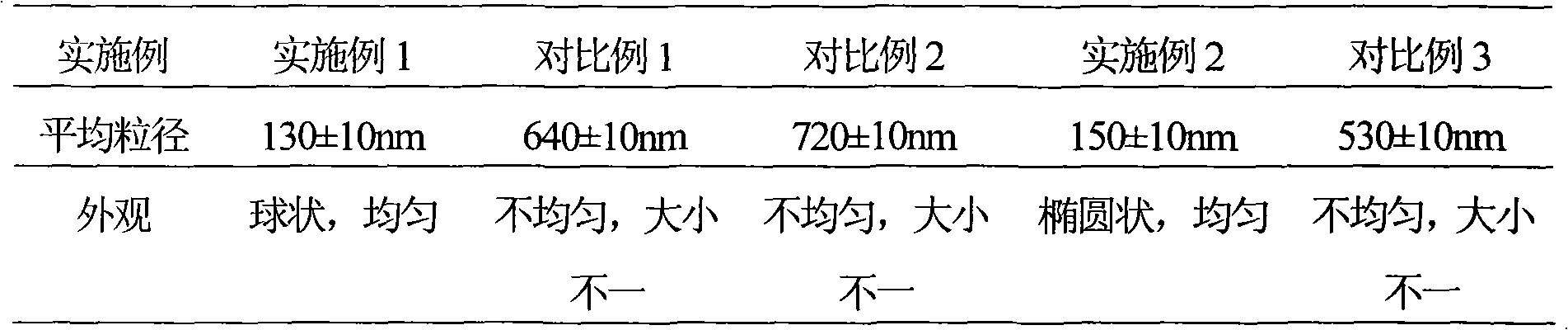
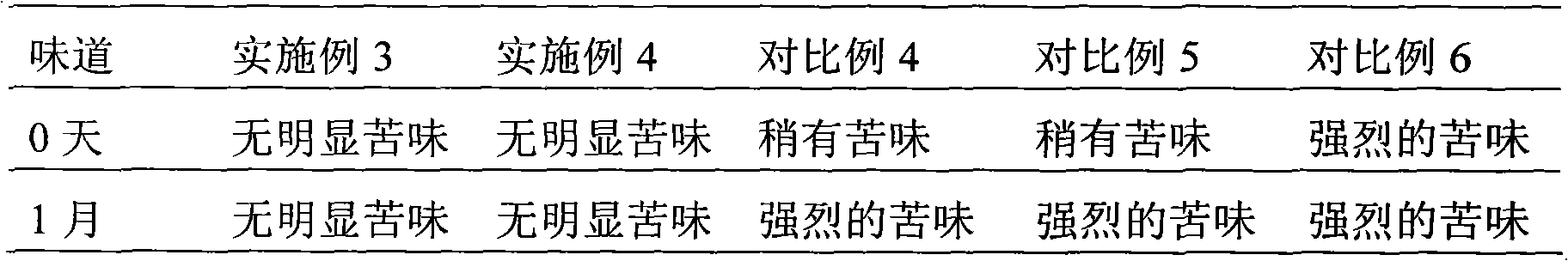
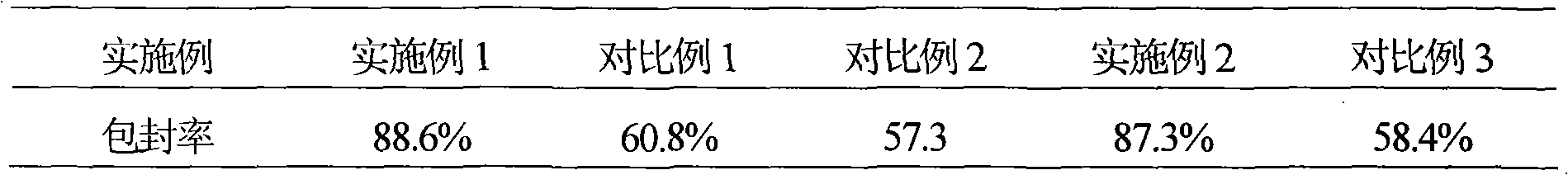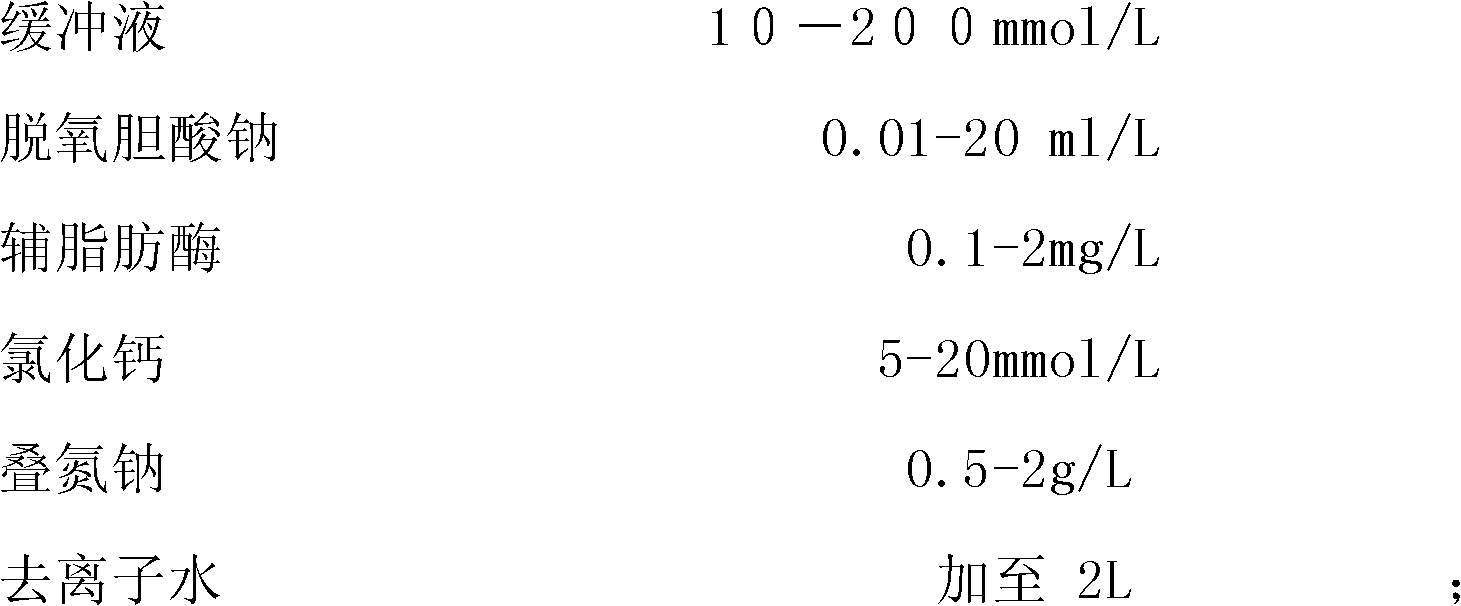Patents
Literature
211 results about "Sodium Deoxycholate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sodium Deoxycholate is the the sodium salt of deoxycholic acid, an ingredient frequently used in mesotherapy injections to break up fat cells, potentially treating cellulite.
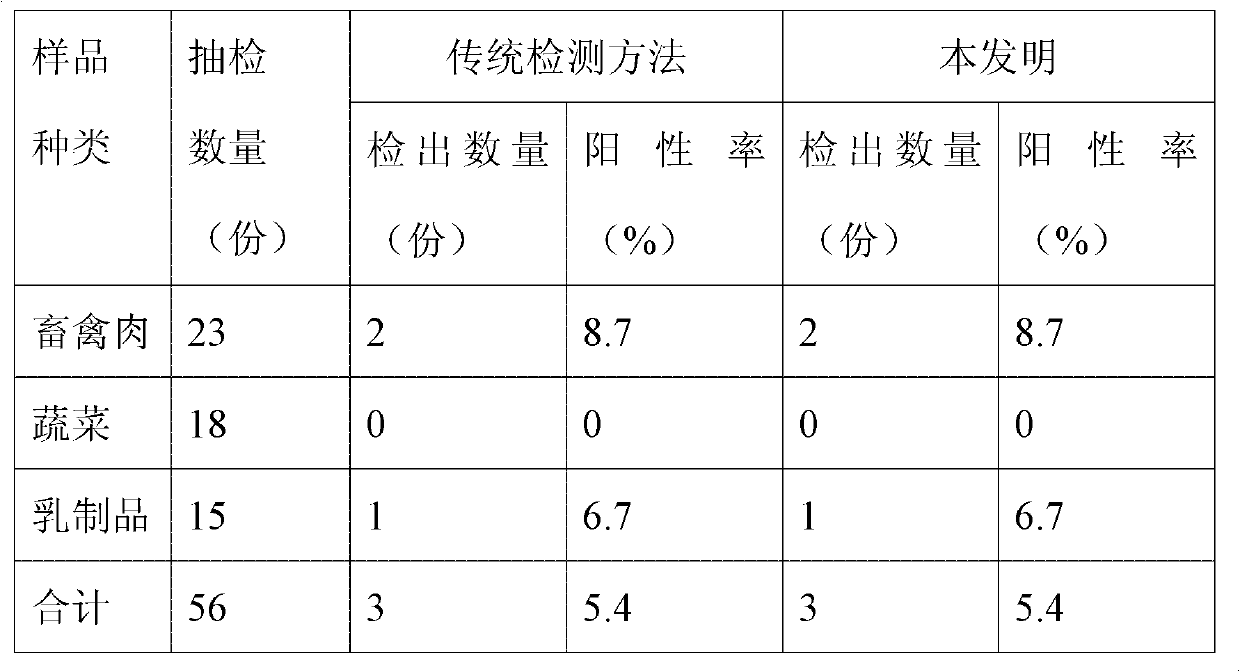
Salmonella characteristic chromogenic liquid nutrient medium, preparation method thereof and rapid detection method of salmonella
ActiveCN102433373AEasy to prepareEase of industrial productionMicrobiological testing/measurementMicroorganism based processesLithium chlorideDipotassium phosphate
The invention relates to the field of safety monitoring of food microorganisms, and discloses a salmonella characteristic chromogenic liquid nutrient medium, a preparation method thereof and a rapid detection method of salmonella. The salmonella characteristic chromogenic liquid nutrient medium comprises the following main components: tryptone, yeast powder, sodium chloride, lithium chloride, sodium deoxycholate, dipotassium phosphate, combined inhibitor, combined accelerator, characteristic enzymolysis substrate and cosolvent. The rapid detection method disclosed by the invention comprises two steps, i.e. pre-enrichment culture and chromogenic identification, and is characterized in that salmonella characteristic enzyme hydrolyzes corresponding substrates to result in that the culture medium is purple, thus rapidly judging the existence of salmonella; and the addition of the accelerator contributes to recovering damaged cells of salmonella and promoting growth of salmonella; and the added inhibitor can selectively inhibit the growth of other competitors so as to reduce the interference on detection by the competitors. The detection method disclosed by the invention has the advantages of short detection period, strong specificity and high accuracy, is simple to operate and is suitable for large-throughput detection of salmonella in food.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
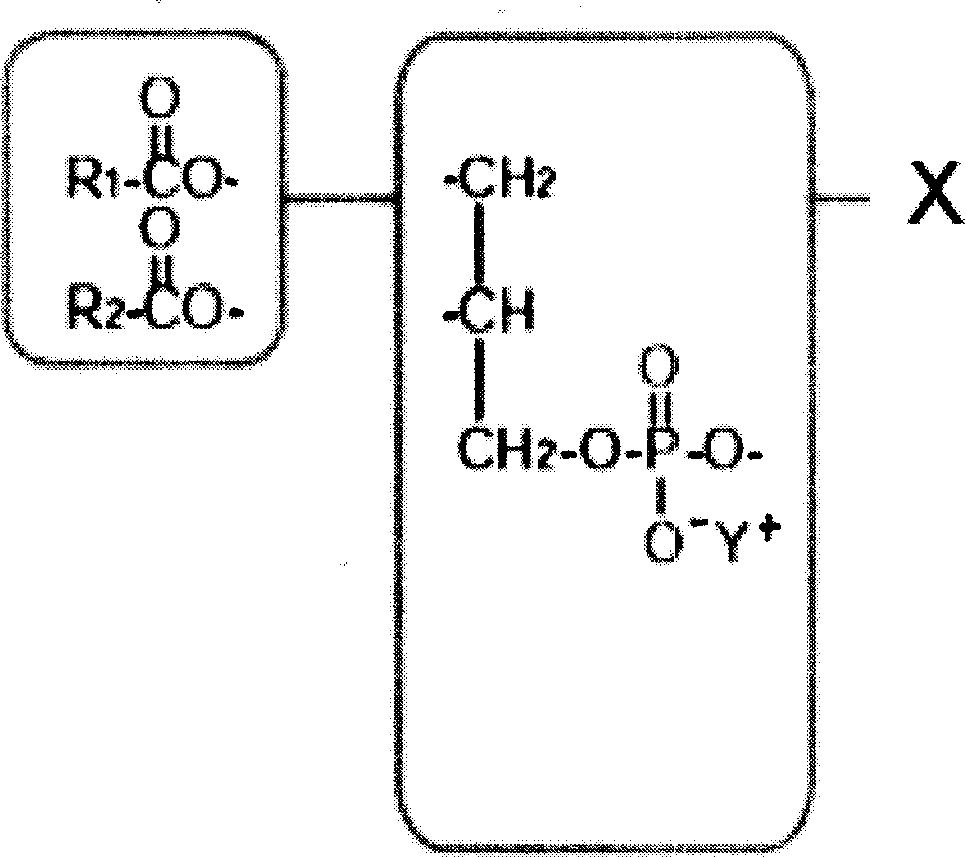
Freeze dried polyene lecithin powder for injection and its prepn
ActiveCN1771914AImprove solubilityImprove drug activityPowder deliveryOrganic active ingredientsPolyene phosphatidylcholineSodium dehydrocholate
The present invention discloses one kind of freeze dried polyene phosphatidylcholine powder for injection and its preparation process. It features that the preparation contains polyene phosphatidylcholine and solubilizer sodium deoxycholate, sodium dehydrocholate or sodium cholate in the weight ratio of 0.5-1.5.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Elastic nano vesicle preparations containing paclitaxel or docetaxel and preparation thereof
InactiveCN101209251AOrganic active ingredientsPharmaceutical non-active ingredientsDocetaxelPhosphate
The invention pertains to the technical field of medicine, which more particularly relates to an elastic nano-vesicle preparation used for transitting the active ingredient paclitaxel (taxol) or docetaxel to penetrate the natural permeability barriers or pores (such as, skin, mucosa, tissues and organs, etc.) and a preparation method thereof. The preparation at least contains the following components with the weight percentages: 3 to 15 percent of phospholipid, 0.3 to 5 percent of sodium cholate or sodium deoxycholate, 0.01 to 5 percent of paclitaxel or docetaxel, 5 to 30 percent of alcohol and the rest of water or phosphate buffer solution. The formed elastic nano-vesicle has lipid double molecular layers, and the diameter of the typical elastic nano-vesicle is less than 200nm. The preparation is used for the treatment of malignant tumors, and the invention can be taken by injection, spray or transdermal drug delivery.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
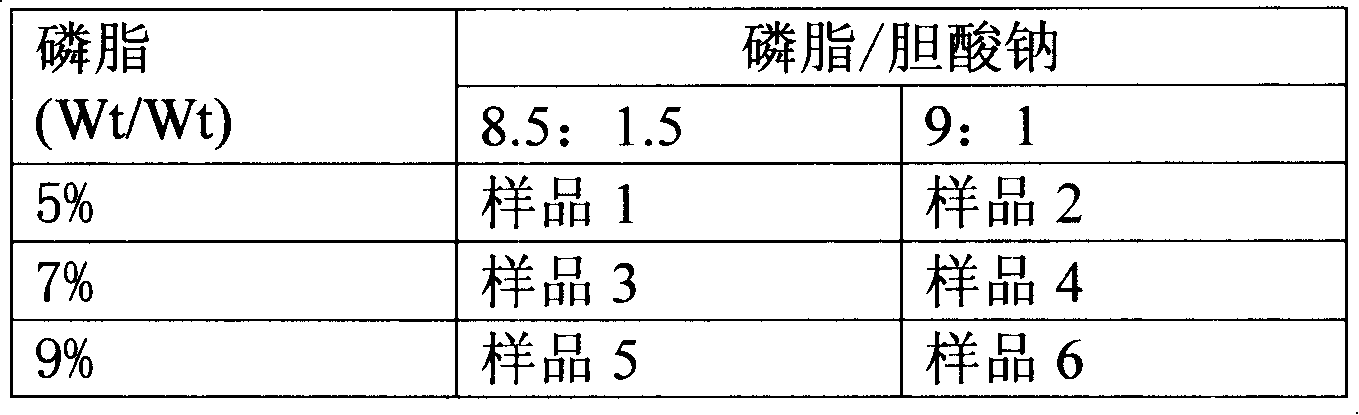
Stable lipase kit
ActiveCN104215632AAvoid stabilityReduce use costMaterial analysis by observing effect on chemical indicatorSolventSodium Taurodeoxycholate
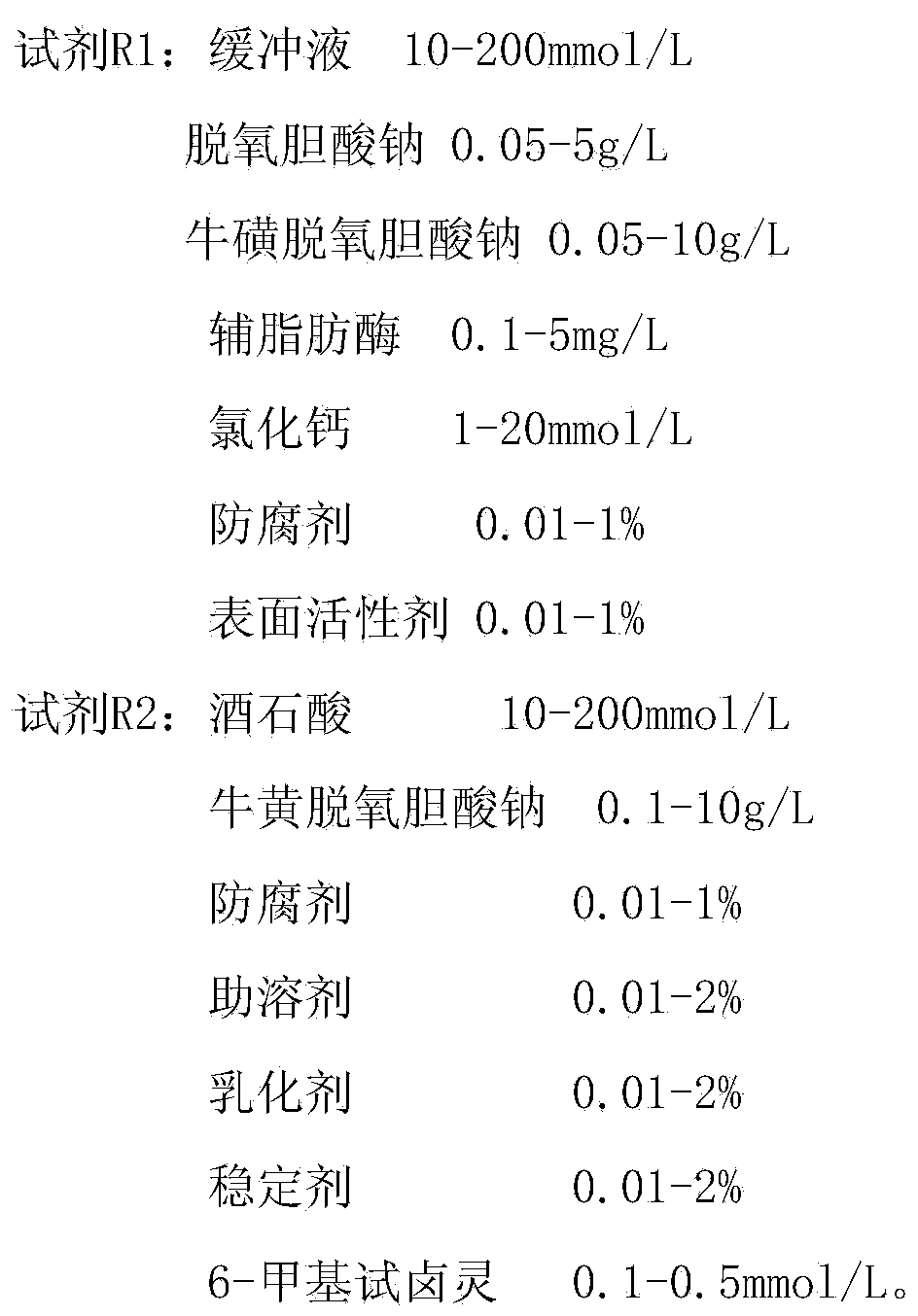
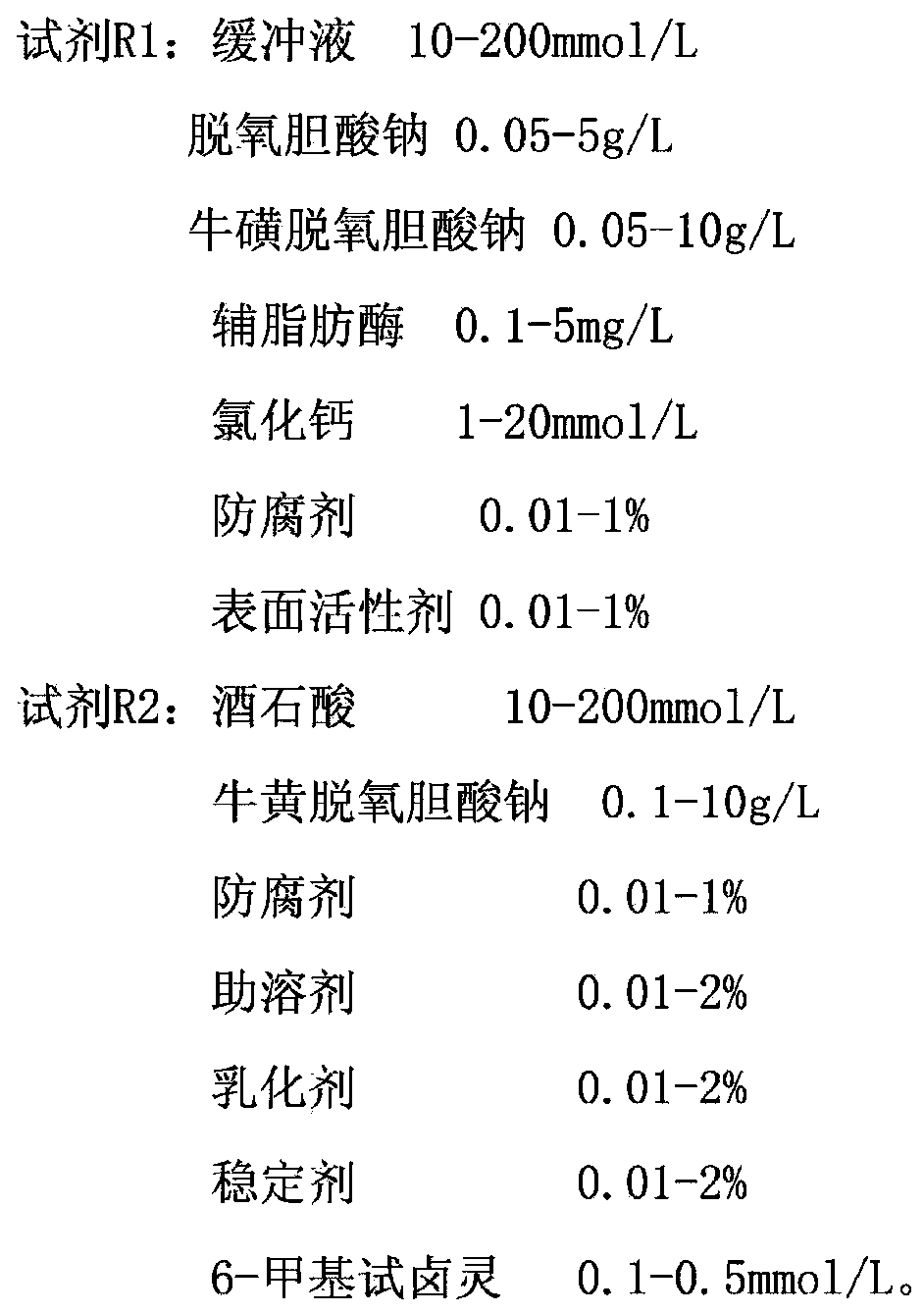
The invention discloses a stable lipase kit which comprises a reagent R1 and a reagent R2, wherein the reagent R1 is prepared from 10-200mmol / L of buffer solution, 0.05-5g / L of sodium deoxycholate, 0.05-10g / L of sodium deoxycholate, 0.1-5mg / L of colipase, 1-20mmol / L of calcium chloride, 0.01-1% of preservative and 0.01-1% of surfactant; the reagent R2 is prepared from 10-200mmol / L of tartaric acid, 0.1-10g / L of taurodeoxycholic acid sodium salt, 0.01-1% of preservative, 0.01-2% of cosolvent, 0.01-2% of emulsifying agent, 0.01-2% of stabilizer and 0.1-0.5mmol / L of 6-methyl resorufin. The kit is high in sensitivity, small in error, stable in quality and convenient to store and has a strong function of protecting a substrate so as to improve the stability of the reagents to obtain a good detection effect. Thus, the kit has a high clinical application value.
Owner:NINGBO RUI BIO TECH
Compound biological ink and preparation method thereof
InactiveCN109054496AGood 3D printable propertiesProtectiveAdditive manufacturing apparatusInksIonic strengthHydrogen
The invention discloses compound biological ink. Preparation raw materials of the compound biological ink comprise acellular dermis matrix hydrogel, a crosslinking curing agent and a bioactive molecule; the invention further discloses a preparation method of the compound biological ink; the preparation method comprises the following steps: (1) preparing the acellular dermis matrix hydrogel: afterremoving target tissues or fat tissues and connective tissues of organs, repeatedly treating with a 1 to 5 percent trion x-100 water solution and a 1.5 to 7 percent sodium deoxycholate water solutionfor a plurality of times in sequence, so as to obtain acellular tissues; freezing and drying the acellular tissues; then crushing and treating through pepsin to obtain a sticky gel solution; then regulating the pH (Potential of Hydrogen) and ion strength at 2 to 8 DEG C; raising the temperature to 33 to 39 DEG C to obtain the acellular dermis matrix hydrogel; (2) adding the crosslinking curing agent, an active factor and a short peptide, and compounding at 1 to 8 DEG C to obtain the compound biological ink. The compound biological ink prepared by the method disclosed by the invention has goodbioactivity and a 3D (Three Dimensional) printable property.
Owner:THE FIRST AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
Polyene phosphatidyl choline lyophilized powdered injection
The invention discloses a polyene phosphatidyl choline freeze dried injection for the treatment of hepatic diseases, which comprises polyene phosphatidyl choline as the main ingredients and pharmaceutically acceptable carrying agents, wherein the carrying agent can be auxiliary solvent selected from sodium cholate, sodium deoxycholate, sodium deoxycholate, hydroxypropyl-beta-cyclodextrin, hydroxyethyl-beta-cyclodextrin and / or methyl cyclodextrin. The injection has higher stability.
Owner:GUANGDONG XIANQIANG PHARMA
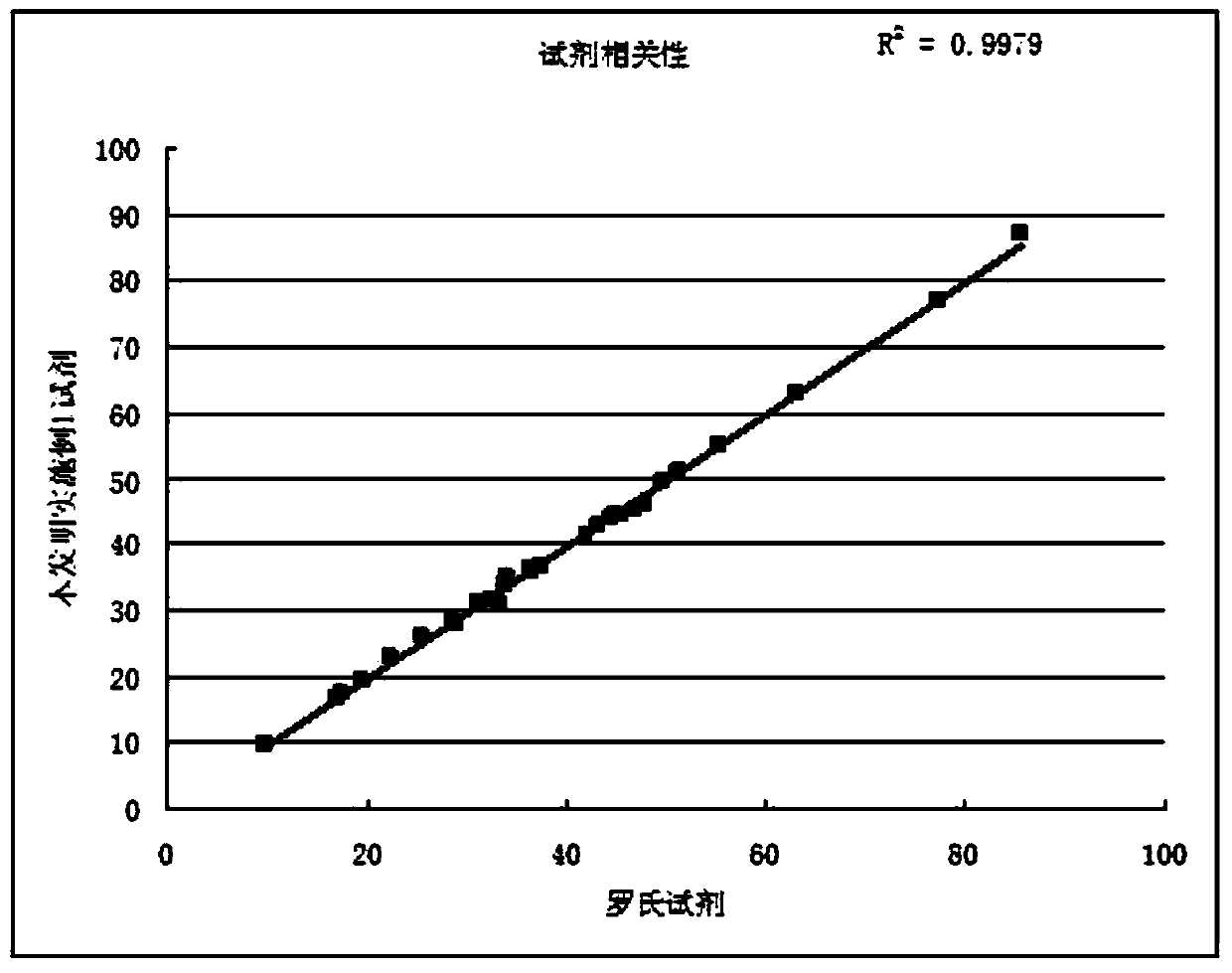
Preparation method of micro-emulsion kit
ActiveCN102621138AImprove stabilityImprove the problem of large difference between batchesMaterial analysis by observing effect on chemical indicatorSodium azideStabilizing Agents
The invention relates to a reagent and particularly relates to a method for preparing a stable micro-emulsion kit. The kit comprises a reagent R1 and a reagent R2, wherein the reagent R1 comprises a TAPS buffered solution, Colipase, sodium deoxycholate, sodium chloride, calcium chloride, taurodeoxycholic acid and sodium azide; and the pH value of the reagent R1 is 8.0. The reagent R2 comprises a tartaric acid buffered solution, sodium cholate, taurodeoxycholic acid, a preservative, sodium chloride and a stabilizing agent; and the pH value of the reagent R2 is 3.5. Compared with the prior art, the improved emulsifying method can be used for solving the problems of poor stability and large batch difference of the reagent on the basis that the reagent does not influence other performances.
Owner:AILEX TECH GRP CO LTD +1
Loratadine-ambroxol pharmaceutical composite and liposome solid preparation thereof
InactiveCN101627998AImprove solubilityImprove stabilityOrganic active ingredientsPharmaceutical product form changeYolkMedicine
The invention relates to a loratadine-ambroxol pharmaceutical composite and a liposome solid preparation thereof and a preparation method thereof; the liposome comprises the following components according to the parts by weight percent: 1 part of loratadine, 5 parts of ambroxol hydrochloride, 3-30 parts of yolk lecithin, 1-14 of cholesterol, 1.2-10 parts of sodium deoxycholate and 3-18 parts of poloxamer 188.
Owner:HAINAN YONGTIAN PHARMA INST
Kit for detecting lipase by enzyme method and preparation method
InactiveCN103173518AEasy to detectImprove protectionMicrobiological testing/measurementSodium azideMercaptoacetic acid
The invention provides a kit for detecting lipase by an enzyme method, and is composed of a reagent 1 and a reagent 2. The reagent 1 is composed of 10-200mmol / L of 2L buffer, 0.01-20ml / L of sodium deoxycholate, 0.1-2mg / L of colipase, 5-20mmol / L of calcium chloride, 0.5-2g / L of sodium azide, and the balance of deionized water to 2L; and the reagent 2 comprises the following components by weight: 10-200mmol / L of 1L tartrate, 0.1-10mmol / L of bezoar sodium deoxycholate, 10-200mmol / L of mannitol, 0.01-0.5ml / L of proclin300, 10-200mmol / L of mercaptoacetic acid, 0.1-0.5mmol / L of 6-methyl resorufin, and the balance of deionized water to 1L. The kit has strong protection effect on a substrate, and the stability of the reagent is increased, the kit has good detection effect, and has large clinical application value.
Owner:SHANGHAI FOSUN PHARMA (GROUP) CO LTD +1
Itraconazole liposome and preparation process thereof
InactiveCN1969830AHighly toxicStrong side effectsOrganic active ingredientsAntimycoticsMANNITOL/SORBITOLPhosphate
The invention discloses an eosin liposome and making method, which comprises the following parts: 18-22mg eosin, 505-509mg lecithin, 90-95mg cholesterol, 73-77mg sodium deoxycholate, 505-509mg mannitol, 505-509mg lactose and 18-22mL phosphate buffer with pH value at 5.5-6.5. the making method comprises the following steps: (1) adding eosin, sodium deoxycholate, lecithin and cholesterol into chloroform; (2) decompressing; evaporating chloroform; (3) adding lactose and mannitol into phosphate buffer; (4) emulsifying phosphate buffer without fat film; (5) freezing; drying to obtain the product.
Owner:HARBIN MEDICAL UNIVERSITY
Peanut oligopeptide-coated nano liposome as well as preparation method and application thereof
InactiveCN105902996AFor maximum encapsulationBest working environmentHydrolysed protein ingredientsAntinoxious agentsCholesterolEntrapment
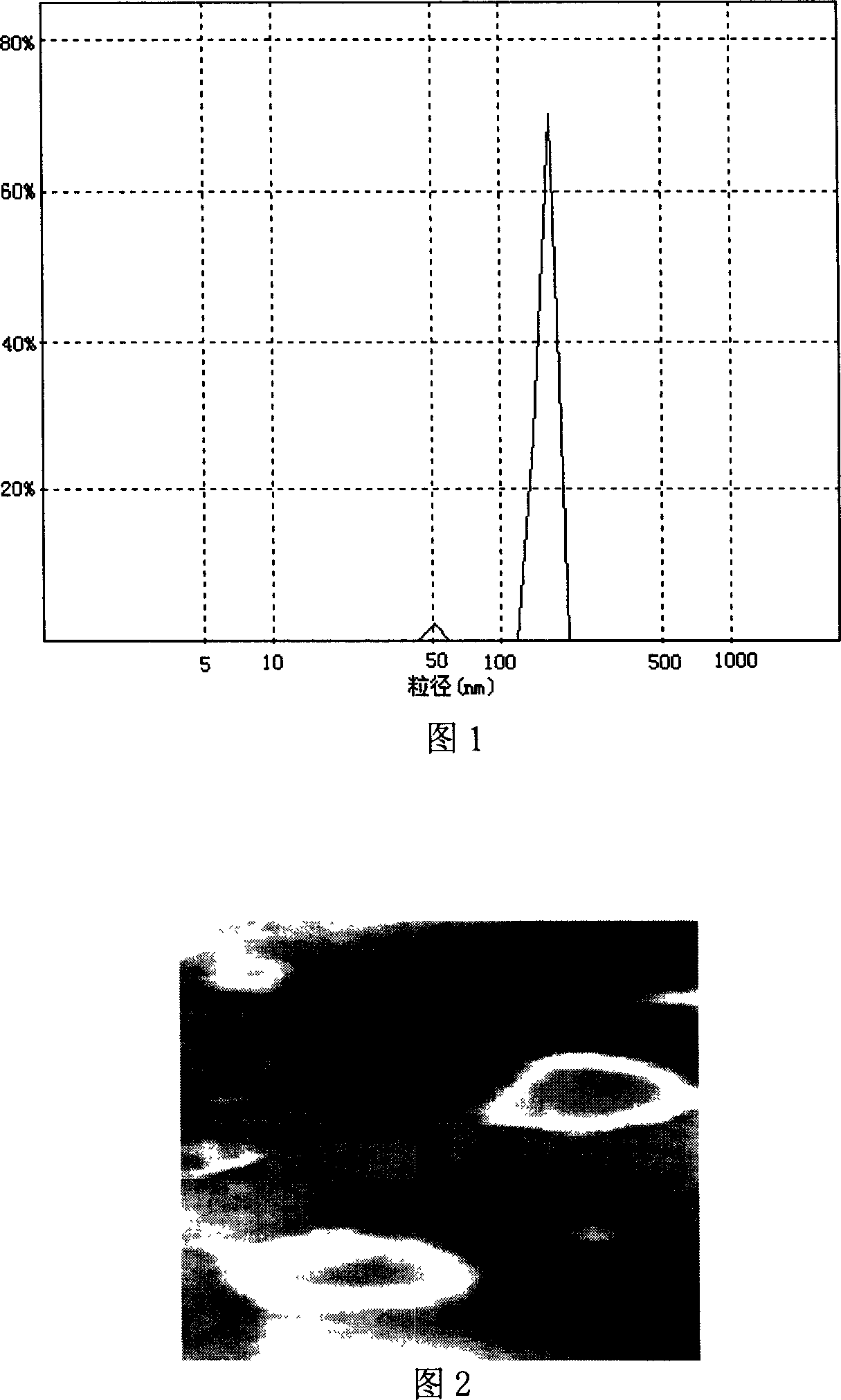
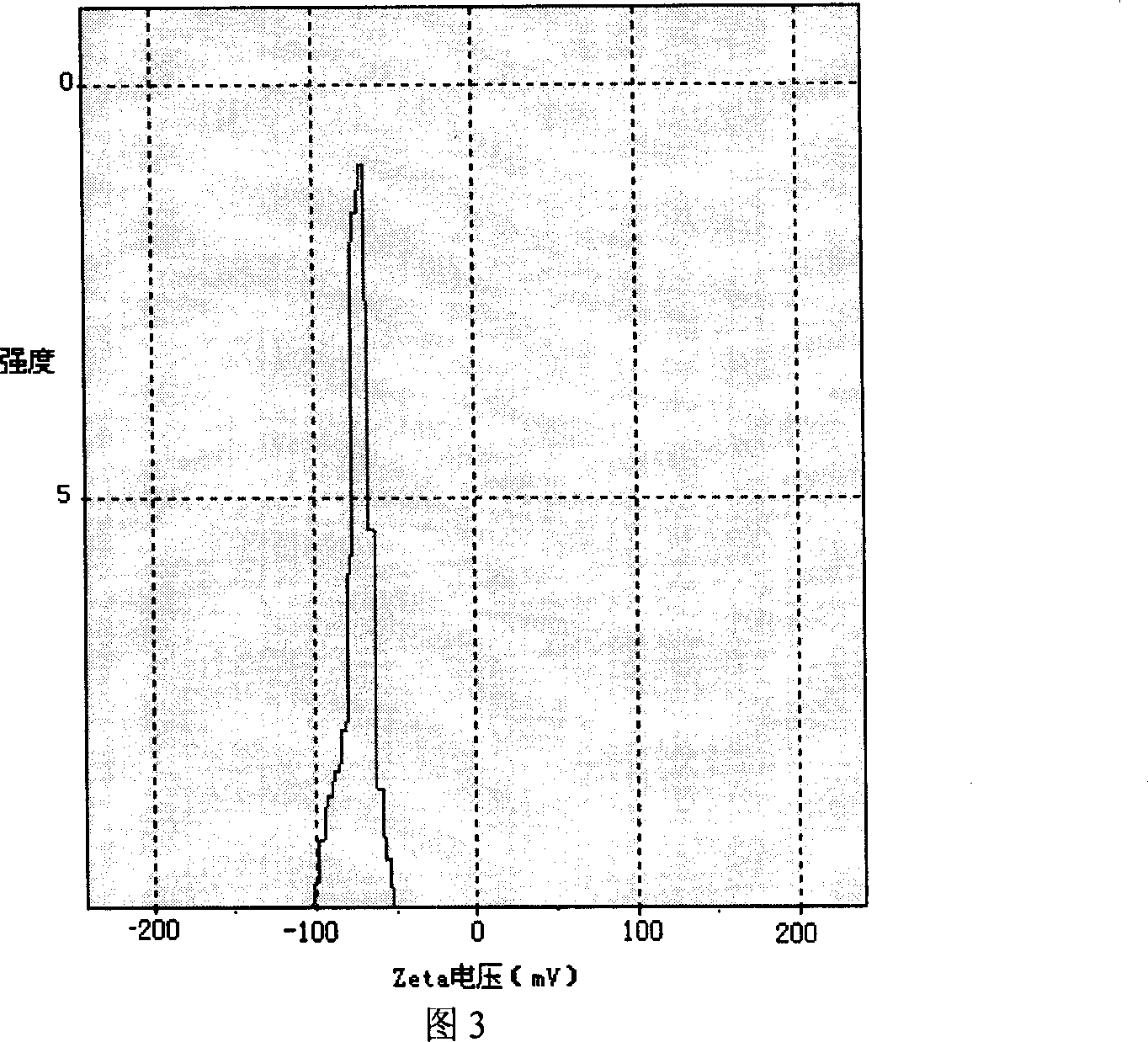
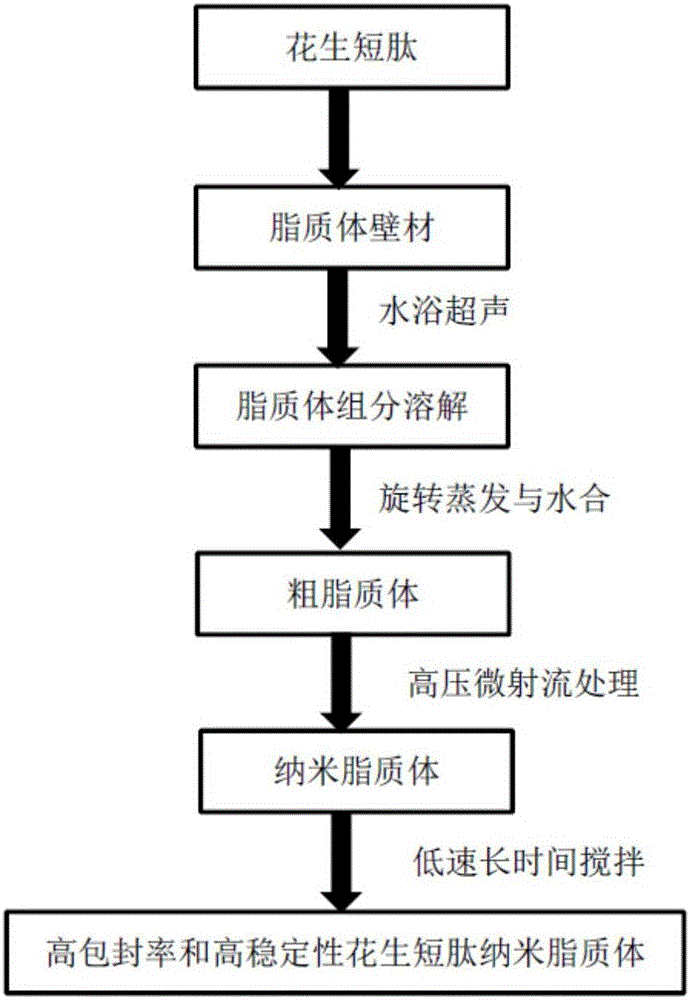
The invention discloses a peanut oligopeptide-coated nano liposome as well as a preparation method and application thereof. The raw materials of the nano liposome comprise lecithin, peanut oligopeptide, cholesterol and sodium deoxycholate; the nano liposome is prepared by combining film dispersion with Ostwald curing. The entrapment efficiency of the nano liposome reaches 82.21%, the particle diameter is 89.70nm, and the nano liposome has good acid-base environment stability, in-vitro slow-release property and digestive stability.
Owner:INST OF AGRO FOOD SCI & TECH CHINESE ACADEMY OF AGRI SCI
Dendrite silver phosphate visible light catalyst and preparation method thereof
InactiveCN102698782AImprove photocatalytic performancePromote degradationPhysical/chemical process catalystsWater/sewage treatment by irradiationSilver phosphateDendrite
The invention provides a dendritic silver phosphate visible light catalyst and a preparation method thereof. The appearance of silver phosphate is adjusted with sodium deoxycholate, so as to form the dendritic silver phosphate which has an efficient visible light catalytic performance and is suitable for degrading organic pollutants under visible light. The invention has the advantages that the preparation process is simple; the condition is mild; and the dendritic silver phosphate visible light catalyst is favorable for application to mass production practice.
Owner:WUHAN UNIV OF TECH
Culture medium for culturing Chlamydomonas reinhardtii by urea plant wastewater and culture method of Chlamydomonas reinhardtii
ActiveCN103966102AReduce outputIncrease productionUnicellular algaeMicroorganism based processesChlamydomonas reinhardtiiArginine
The invention discloses a method for culturing Chlamydomonas reinhardtii by urea plant wastewater. According to the method, urea plant wastewater can be treated in low cost, Chlamydomonas reinhardtii with important economic value can also be obtained, the waste is changed into treasures, harm is turned into a good and many things are achieved at one stroke. A traditional method for culturing Chlamydomonas reinhardtii comprises the step of culturing Chlamydomonas reinhardtii in an open concrete runway pool by virtue of a TAP culture medium, and has the disadvantages of low growth speed, low output, great investment, easy pollution and low efficiency. According to the invention, Chlamydomonas reinhardtii is cultured in a household mineral water bucket to achieve the advantages of low cost, small possibility of pollution, convenience in cleaning, reutilization and convenience in operation; since nutritional ingredients, such as urea plant wastewater, yeast extract powder, sodium deoxycholate, sorbitol, mannitol, sodium thioglycolate, soil extract liquid, sheep manure leachate, chicken manure leachate, L-arginine, potassium dihydrogen phosphate, yeast extract, and diatomite powder are added into the culture medium, and an organic fertilizer is used in combination with an inorganic fertilizer, nutrition is more comprehensive and balanced, the growth rate and yield of Chlamydomonas reinhardtii are substantially increased and the yield is increased by 280%.
Owner:江西赣兴气体有限公司
Epi-doxorubicine liposome and its preparing method
InactiveCN1554354AQuality improvementHigh encapsulation efficiencyOrganic active ingredientsAntineoplastic agentsSide effectCholesterol
The present invention relates to the field of pharmaceutical technology, and is especially epirubicin liposome and its preparation process. The epirubicin liposome of the present invention consists of epirubicin, phosphatide, cholesterol, sodium deoxycholate, etc.; has stable quality and less toxic side effect; and is suitable for clinical application.
Owner:CHINA PHARM UNIV
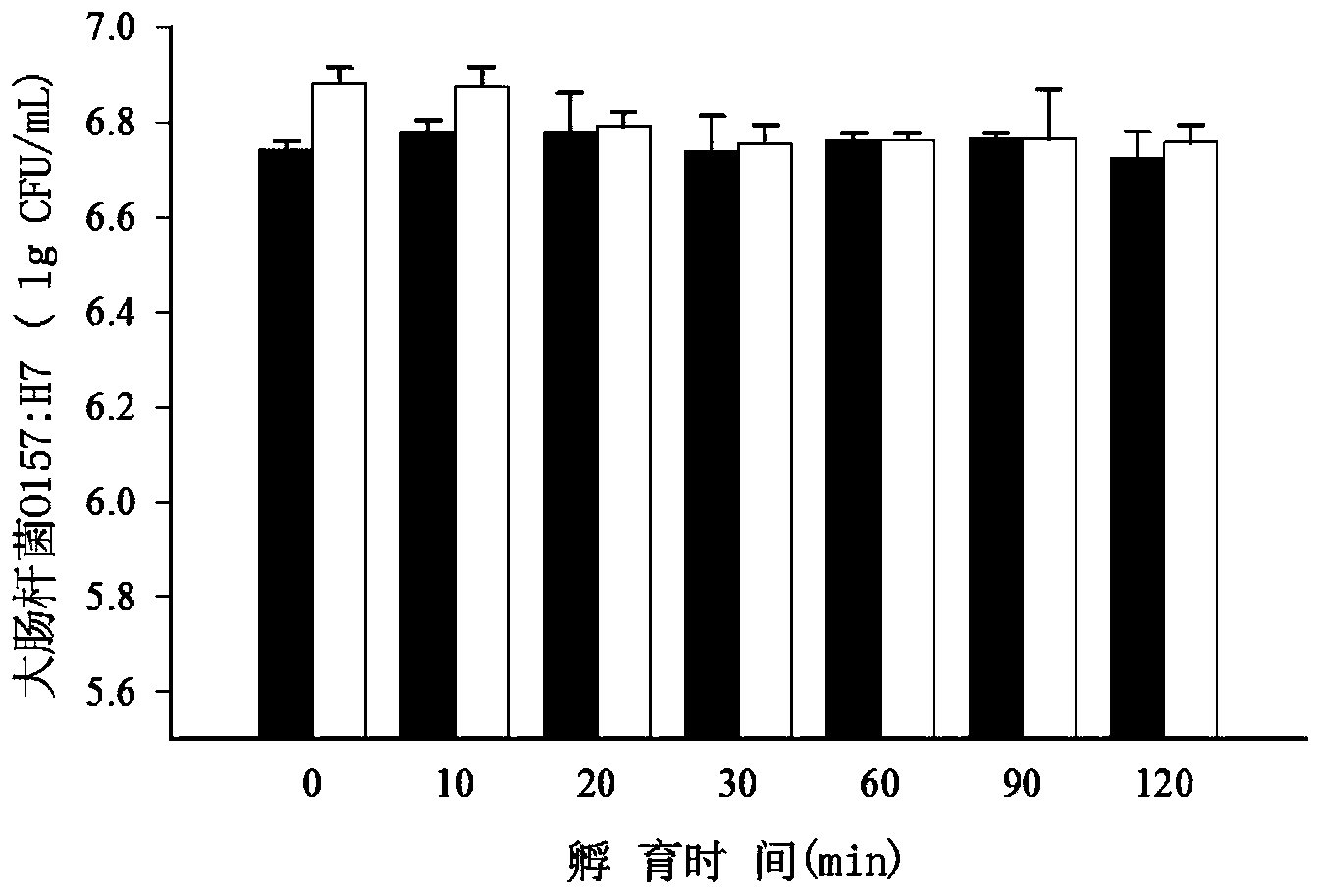
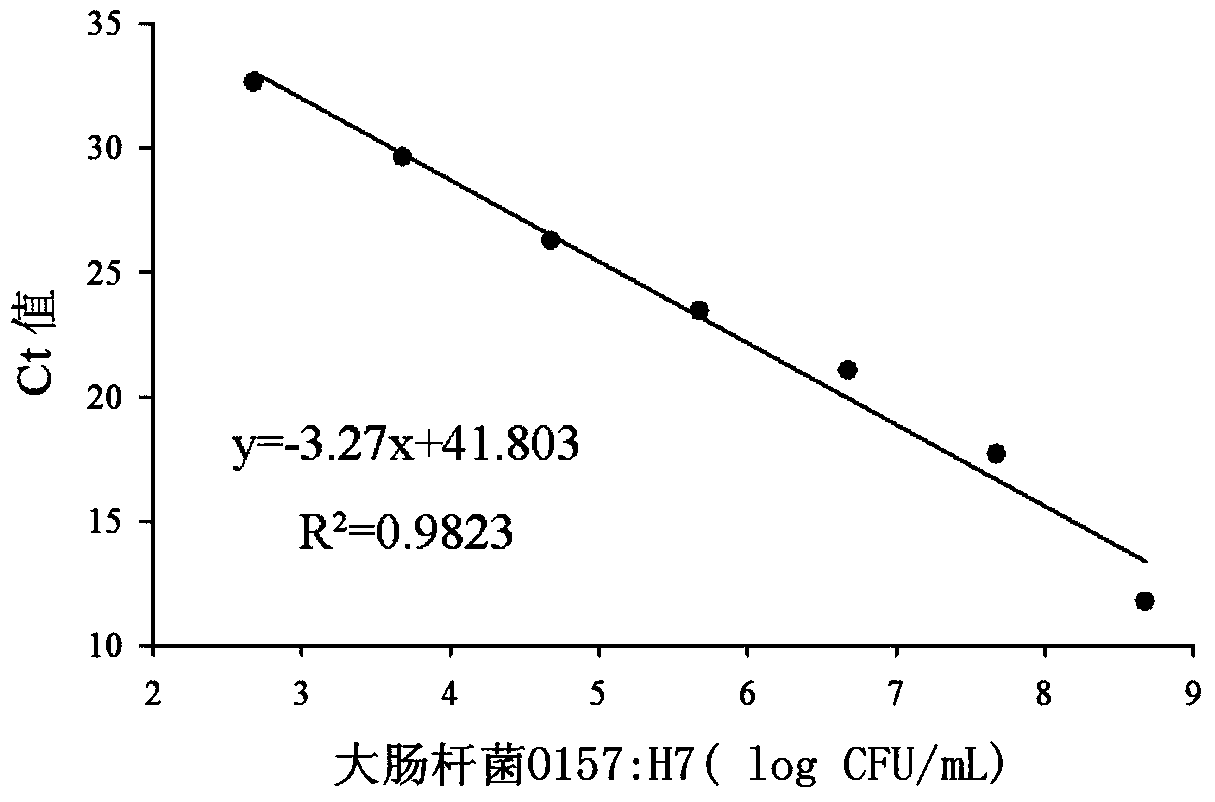
Quantitative detection method for escherichia coli O157:H7 live bacteria in food
InactiveCN103509860AHigh sensitivityOvercoming the inability to distinguish dead bacteriaMicrobiological testing/measurementFluorescence/phosphorescenceEscherichia coliPropidium monoazide
The invention discloses a fluorescence quantitative PCR detection method for escherichia coli O157:H7. aA pair of specific primers amplified with a 20 bp section is designed aiming at the flic of escherichia coli O157:H7. The detection method comprises the following steps: firstly, separating escherichia coli O157:H7 from an actual sample by adopting a magnetic bead enriching method, then eliminating the disturbance brought by dead bacteria in the food sample by the treatment of sodium deoxycholate (SD) and propidium monoazide (PMA), and finally amplifying the live bacteria through a PCR amplification method. The detection method has the advantages of high sensitivity, accurate quantitation, strong specificity, short detection time, simple operation process, and no disturbance caused by the residual DNA or dead bacteria in the food sample.
Owner:WUXI ZODOLABS BIOTECH
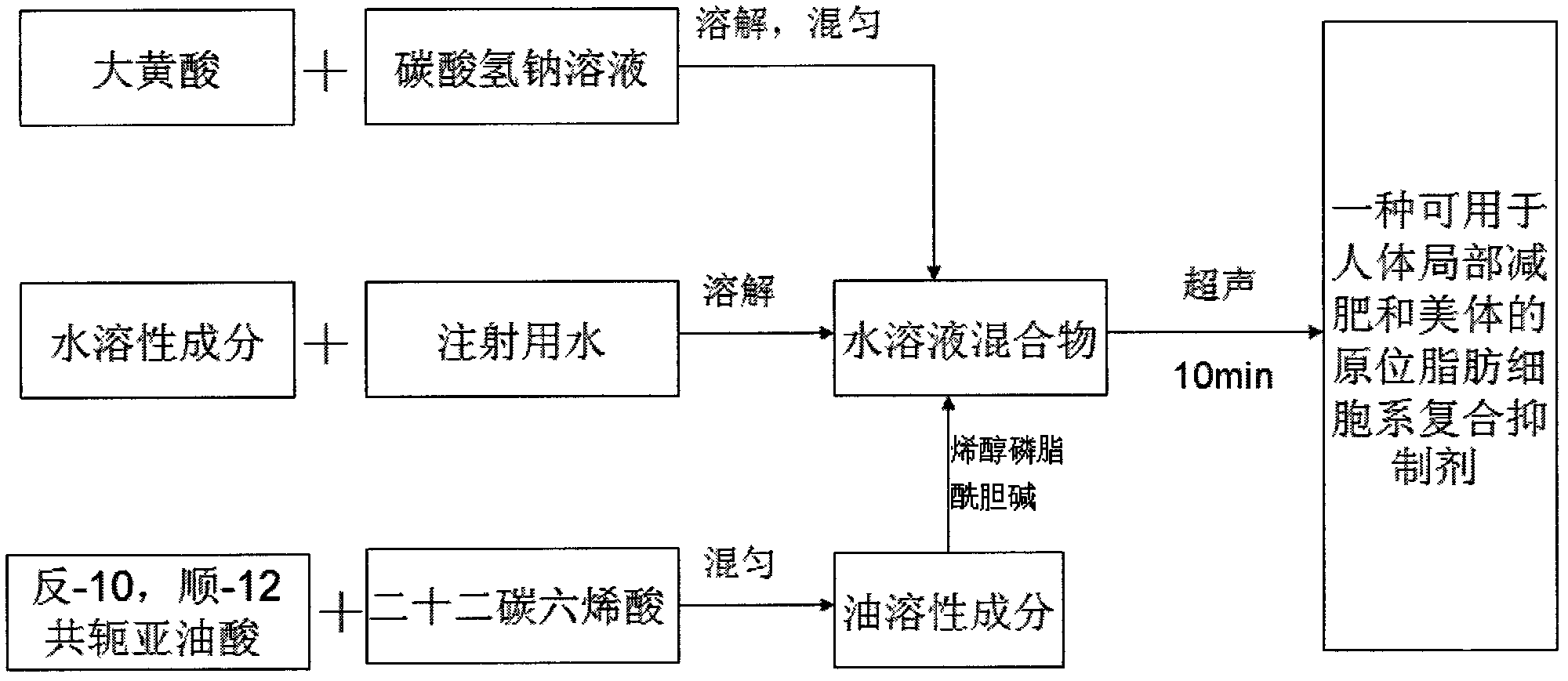
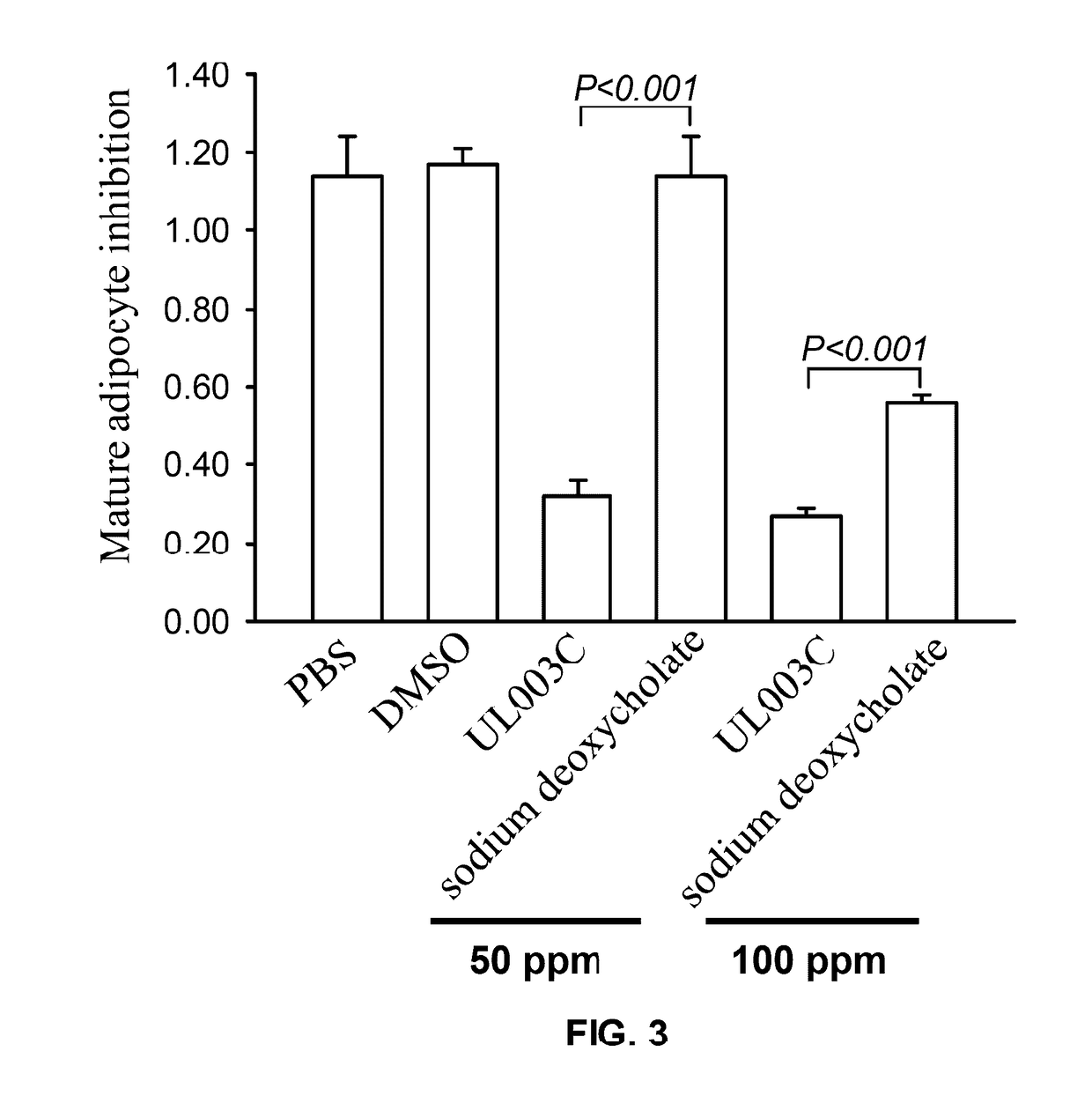
In-situ adipocyte composite inhibitor for local weight loss and body care of human bodies and preparation method thereof
ActiveCN102580087AProtects and sustains biological activityPromote proliferationHydroxy compound active ingredientsMetabolism disorderSodium bicarbonateHuman body
The invention discloses an in-situ adipocyte composite inhibitor for the local weight loss and body care of human bodies and a preparation method thereof. The compound (composite inhibitor) comprises the following bulk drugs: anti-NPY monoclonal antibodies, alpha-aminoethanesulfonic acid, mannitol, enol phosphatidylcholine, sodium deoxycholate, trans-10, cis-12conjugated linoleic acid, docosahexenoic acid, berberine, emodin, sodium bicarbonate and injection water. The compound of the invention can synchronously inhibit the propagation and the differentiation of preadipocytes, accelerate and promote the decomposition and the conversion of fats in mature adipocytes, effectively reduce the quantity of in-situ and newborn mature adipocytes, regulate the expression, the synthesis and the secretion of relevant adipogenic genes and adipocyte growth factors from the gene level and the like, and control the propagation and the fat synthesis of the adipocytes at the source; and, additionally, the absorption utilization rate of the compound is improved through local administration, so the compound has the rapid and efficient weight loss effect.
Owner:董萍 +1
Drug carrier of apigenin and preparation method
ActiveCN107661295AImprove solubilityImproves antioxidant activityOrganic active ingredientsInorganic non-active ingredientsApigeninSucrose distearate
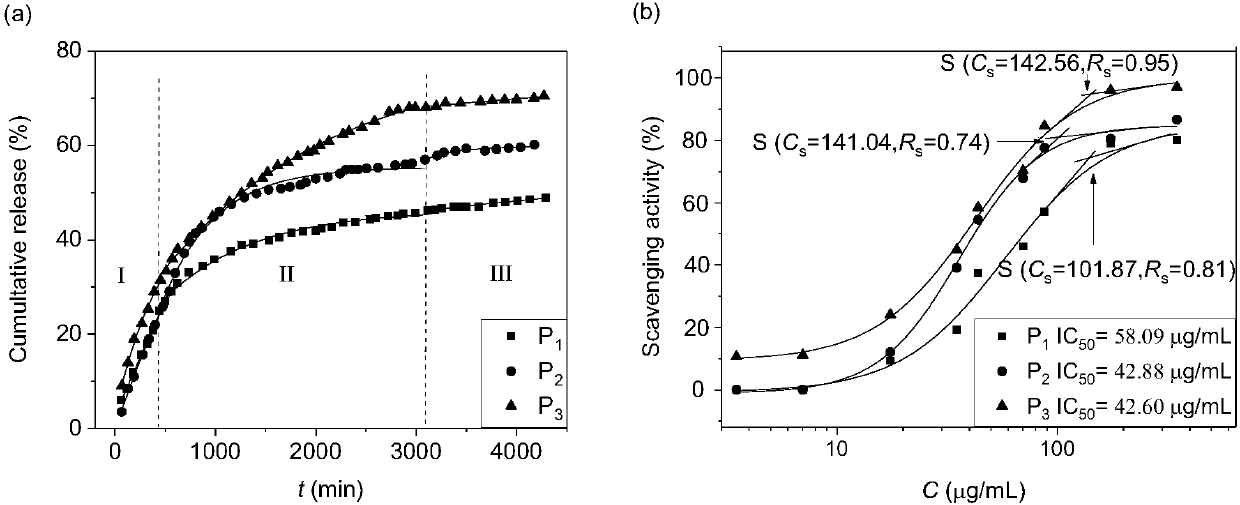
The invention discloses a drug carrier of apigenin and a preparation method. The drug carrier is micro-emulsion prepared by mixing sodium deoxycholate, sucrose stearate, isopropyl alcohol, ethyl acetate and a sodium chloride water solution. The drug carrier disclosed by the invention takes the ethyl acetate as an oil phase and the apigenin has highest anti-oxidization activity and maximum accumulative releasing rate.
Owner:海生健康科技(青岛)有限公司
Plant extract composition for reducing topical fat and promoting weight loss as well as applications thereof
ActiveUS20170157195A1Inhibit adipocyte growthPromote adipocyte apoptosisHydroxy compound active ingredientsPharmaceutical delivery mechanismApoptosisPain responses
Disclosed in the present invention is the composition for reducing local fat and body weight, and pharmaceuticals and use thereof. The composition contains resveratrol and curcumin extract at a weight ration from 1:30 to 10:1. The composition and pharmaceuticals thereof of the invention can inhibit the growth of fat cells, cause planned apoptosis of fat cells, achieve the effects of reducing fat cells, and reducing local fat deposition and body weight without causing inflammations or necrosis of surrounding cells or tissues and inflammations or pain reactions of surrounding tissues, thereby avoiding the problems of tissue damage and inflammatory pains caused by liposuction or low invasion fat-dissolving apparatus used in the prior art and the problems such as surrounding tissue inflammations and necrosis infections triggered by cell disruption and necrosis caused by components of a fat-dissolving injection, phosphatidylcholine or sodium deoxycholate.
Owner:CALIWAY BIOPHARM
Amlodipine besylate liposome tablet
InactiveCN101862302BHigh dissolution rateImprove stabilityPill deliveryCholesterolAmlodipine besilate
Owner:HAINAN LINGKANG PHARMA CO LTD
Lipidosome composition containing Cu and Zn-rhSOD for removing eye wrinkles effectively
ActiveCN103417394APromote infiltrationPrevent agingCosmetic preparationsToilet preparationsWrinkle skinFreeze-drying
The invention relates to the technical field of cosmetics, in particular to a lipidosome composition containing Cu and Zn-rhSOD for removing eye wrinkles effectively. The lipidosome composition comprises a freeze-dried excipient, a skin penetration enhancer, an antioxidant, and a flexible liposome coating Cu, Zn-rhSOD and EGF, and is characterized in that the flexible liposome comprises the following components in parts by weight: 80 to 95 parts of lecithin, 5 to 20 parts of sodium deoxycholate, 2 to 5 parts of cholesterol, 0.01 to 0.05 part of Cu and Zn-rhSOD, 0.01 to 0.03 part of EGF, and 5 to 10 parts of an active protective agent. The lipidosome composition provided by the invention can effectively remove or prevent eye wrinkles, and ensure that skin is relatively fine, smooth, tender, white and resilient, thereby meeting the daily skin care requirements of whitening and wrinkle removing of eye skin of people.
Owner:上海玉华生命科技发展有限公司
Method for preparing molybdenum disulfide-doped graphene fibers
InactiveCN104746180AEasy to prepareLow equipment requirementsArtifical filament manufactureFiberAlcohol
The invention relates to a method for preparing molybdenum disulfide-doped graphene fibers. The method comprises the following steps: dispersing graphite oxide and molybdenum disulfide in water, and performing ultrasonic treatment, thereby obtaining molybdenum disulfide and graphite oxide dilute dispersion; adding sodium deoxycholate, and stirring, thereby obtaining sodium deoxycholate / molybdenum disulfide-graphene oxide gel; extruding the sodium deoxycholate / molybdenum disulfide-graphene oxide gel in anhydrous ethyl alcohol for washing by using an injector, and sucking the anhydrous ethyl alcohol, thereby obtaining graphene oxide fibers after the anhydrous ethyl alcohol is completely evaporated; and adding hydroiodic acid for reacting, washing, and drying, thereby obtaining the molybdenum disulfide-doped graphene fibers. The method disclosed by the invention is simple in process and is easy for industrial production, the prepared molybdenum disulfide-doped graphene fibers are high in conductivity, and have high flexibility and have huge application prospects in the fields of energy storage devices, photovoltaic devices and sensors.
Owner:DONGHUA UNIV
Megestrol acetate nanosuspension and preparation method and application thereof
InactiveCN105769763AIncrease dissolution rateImprove oral bioavailabilityOrganic active ingredientsSolution deliveryMegestrol acetateMethyl cellulose
The invention discloses a megestrol acetate nanosuspension.The megestrol acetate nanosuspension is prepared by mixing megestrol acetate and a stabilizer solution and then grinding the mixture through a nanometer grinder.The stabilizer is a mixture obtained by mixing cellulose derivatives and anionic surfactants according to the mass ratio of 10: (1-2); the cellulose derivatives are hydroxypropyl methyl cellulose and hydroxyl propyl cellulose, and the mass ratio of hydroxypropyl methyl cellulose to hydroxyl propyl cellulose is 10: (1-10); the anionic surfactants are lauryl sodium sulfate and sodium deoxycholate, and the mass ratio of lauryl sodium sulfate to sodium deoxycholate is 10: (1-5).The invention further discloses a preparation method and application of the megestrol acetate nanosuspension.According to the megestrol acetate nanosuspension, the average grain diameter of megestrol acetate is below 1,000 nm, the dissolution rate of megestrol acetate is remarkably increased, and then the oral bioavailability of megestrol acetate is improved.
Owner:西安远大德天药业股份有限公司
Acellular matrix hydrogel as well as preparation method and application thereof
InactiveCN112089890AImprove survival efficiencyNo species involvedAerosol deliveryOintment deliveryAcellular scaffoldUmbilical cord tissue
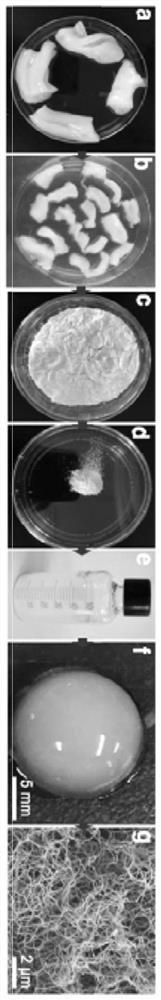
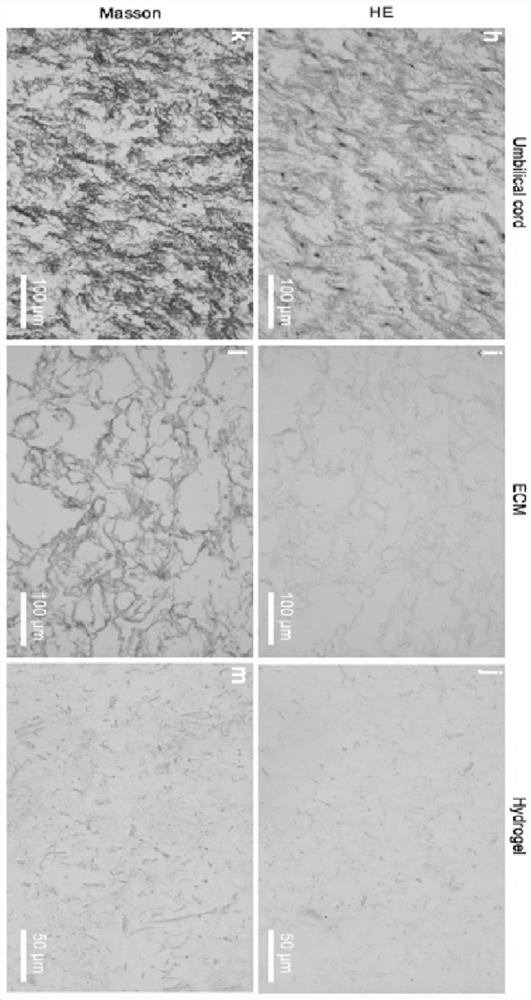
The invention discloses a preparation method of acellular matrix hydrogel. The preparation method comprises the following steps: obtaining a tissue block; transferring the tissue block into a solutioncontaining 0.02% of pancreatin and 0.05% of ethylenediamine tetraacetic acid, and carrying out stirring for 2-3 h; transferring the tissue block into a solution containing 3% of Triton X, and carrying out stirring for 2-3 h; transferring the tissue block into a solution containing 4% of sodium deoxycholate, and performing stirring for 2-3 h; moving the tissue block into deionized water to be soaked for 12-18 h to obtain a decellularized scaffold; transferring the decellularized scaffold into 75% alcohol to be soaked for 30 min, transferring the decellularized scaffold into a solution containing 0.1% peracetic acid, and carrying out stirring for 2-3 h; freeze-drying the decellularized scaffold, grinding the decellularized scaffold into matrix powder, transferring the matrix powder into a solution containing pepsase hydrochloride, and performing stirring for digestion for 2-3 d to obtain pre-gel; and diluting the pre-gel to a preset concentration, and performing standing to obtain the acellular matrix hydrogel. The acellular matrix hydrogel is efficiently extracted from umbilical cord tissue, and the integrity of the internal structure of the acellular matrix is reserved to the maximum extent.
Owner:GUANGDONG UNISUN BIOTECHNOLOGY CO LTD
Preparation method of graphene fiber
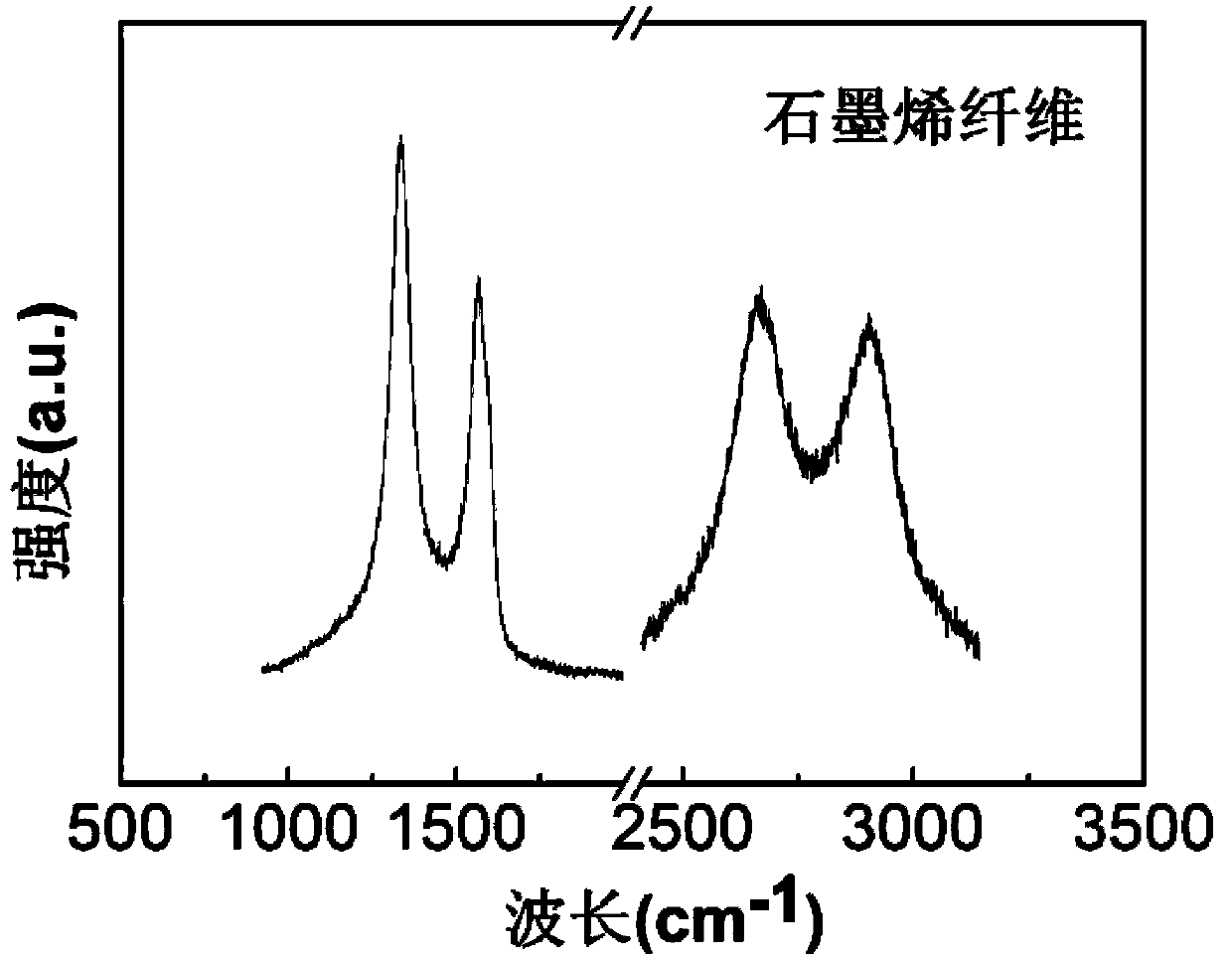
ActiveCN103388197AImprove conductivityImprove flexibilityArtificial filament chemical after-treatmentFiberRoom temperature
The invention relates to a preparation method of a graphene fiber. The preparation method comprises following steps: dispersing oxidized graphite in ultra-pure water at a room temperature, then subjecting the solution to an ultrasonic treatment to obtain oxidized graphene dispersion, adding sodium deoxycholate in the dispersion, stirring to obtain sodium deoxycholate / oxidized graphene hydrogel; wherein the mass ratio of oxidized graphite to sodium deoxycholate to ultra-pure water is 0.1-0.4:1-4:4-10; injecting the sodium deoxycholate / oxidized graphene hydrogel in absolute ethanol to wash by a injector, then removing the absolute ethanol, naturally drying the absolute ethanol to obtain oxidized graphene fiber, then adding hydroiodic acid to have reactions for 2 to 3 hours, washing, drying so as to obtain the graphene fiber. The preparation method has the advantages of simple technology and easy application to industrial production, the prepared graphene fiber has good conductivity and high flexibility, and has vast application prospects in the fields of energy storage devices, photovoltaic devices and sensors.
Owner:DONGHUA UNIV
Nystatin flexible liposome as well as gel and preparation method of nystatin flexible liposome
InactiveCN102600079AImprove flexibilityImprove transmittanceOrganic active ingredientsAntimycoticsNystatinumPharmaceutical drug
The invention provides a nystatin flexible liposome and external preparation gel and simultaneously discloses a preparation method of the nystatin flexible liposome. Through the nanometer liposome technology, nystatin is covered and sealed in the liposome, the solubility of the nystatin is enhanced, the liposome recipe is improved, sodium cholate or sodium deoxycholate is added, the flexible liposome is prepared, the liposome pereutaoeous permeation is enhanced, in addition, the nystatin stability can be improved, a nystatin cutaneous penetration path is developed, and the treatment effect is realized in aspects of skin superficial layer or deep infection and systemic fungal infection. The nystatin flexible liposome is prepared into the gel, the detention time of the nystatin flexible liposome on the skin is prolonged, the use is simple and convenient, the cost is low, in addition, the compliance of users is improved, the toxicity is reduced, medicine storage bases can be formed on the skin, medicine is continuously released, and the medicine acting time is prolonged.
Owner:JILIN UNIV
Composite mesenchymal stem cell functional collagen scaffold and applications thereof
The invention discloses a composite mesenchymal stem cell functional collagen scaffold and applications thereof. According to the present invention, isolated animal fascia is soaked sequentially withacetone, a sodium deoxycholate solution, a Triton X-100 solution, a pepsin aqueous solution and a papain solution, and free-drying is performed to obtain the composite mesenchymal stem cell functionalcollagen scaffold; the composite mesenchymal stem cell functional collagen scaffold is used for acute spinal cord injury and old spinal cord injury, and the comprehensive evaluation results from various aspects show that the composite mesenchymal stem cell functional collagen scaffold can substantially promote the movement function recovery, nerve regeneration, axon myelination and synapse formation of acute and old spinal cord injury dogs; and the functional biomaterial can provide great significance in the repair of spinal cord injury, and can provide theoretical support for the applicationof functional biomaterials in the future.
Owner:BEIJING ZKZKTECH CO LTD
Proteome sample processing kit box and processing method, and application
The invention discloses a proteome sample processing kit box and processing method and an application of the processing method. The kit box comprises a tri(2-carbonyl ethyl) phosphorus hydrochloride,2,2-dichloro acetamide, sodium deoxycholate and a buffer solution. The application of the processing method provided by the invention is as follows: before a protein sample is processed, the tri(2-carbonyl ethyl) phosphorus hydrochloride, 2,2-dichloro acetamide, sodium deoxycholate and buffer solution in the kit box are prepared into treating fluid, wherein splitting and whole protein degenerationin cells are performed on cell / tissue samples via the sodium deoxycholate, the tri(2-carbonyl ethyl) phosphorus hydrochloride is used as a reducing agent, the 2,2-dichloro acetamide is used as an alkylate reagent, and splitting and reductive alkylation of the proteome sample can be completed by several minutes in the treating fluid. That is to say, according to the kit box, processing method andapplication, the splitting and reductive alkylation are completed in one step, so that the proteome sample treating process is simple, and the time is saved.
Owner:北京谷海天目生物医学科技有限公司
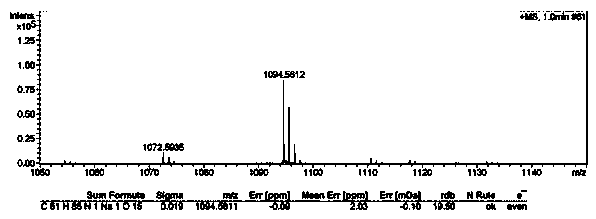
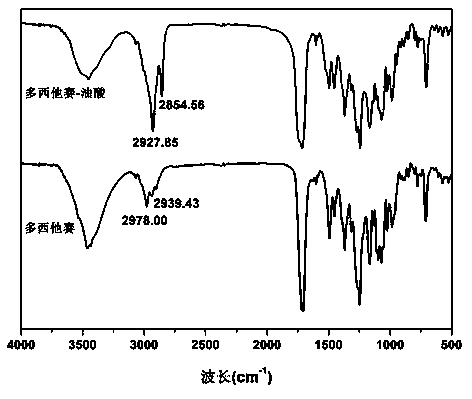
Docetaxel-oleic acid prodrug as well as nanostructure lipid carrier and application thereof
ActiveCN105541762AImprove oral bioavailabilityImprove stabilityOrganic active ingredientsOrganic chemistry methodsLipid formationDocetaxel
The invention relates to preparation of a docetaxel-oleic acid prodrug and application of a nanostructure lipid carrier based on a core-match mechanism to drug delivery. The prodrug takes docetaxel as a parent drug and oleic acid as an aliphatic chain and is linked by a fat key. The nanostructure lipid carrier based on the core-match mechanism takes glycerol monostearate as solid lipid, the oleic acid as liquid lipid, Poloxamer 188 or sodium deoxycholate as a surface active agent and PLV2000 or PLV5000 as a modified material and encapsulates the docetaxel-oleic acid prodrug. The drug loading capacity of the nanostructure lipid carrier prepared is about 23 percent, and the drug loading capacity is obviously improved compared with that (5 percent) of the nanostructure lipid carrier encapsulating the docetaxel; the nanostructure lipid carrier is better in colloidal stability and more excellent in slow controlled release effect. A pharmacokinetic experiment can prove that the nanostructure lipid carrier based on the core-match mechanism can obviously improve the oral bioavailability of the docetaxel, and the docetaxel-oleic acid prodrug is good in stability, high in safety, suitable for oral administration and wider in market application prospect.
Owner:SHENYANG PHARMA UNIVERSITY
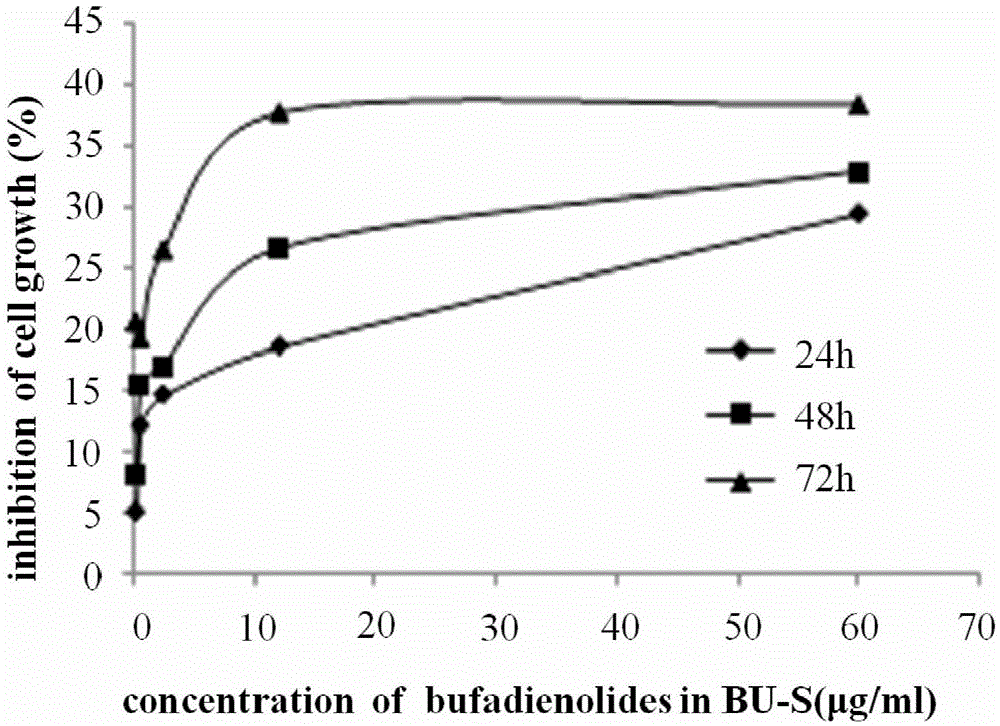
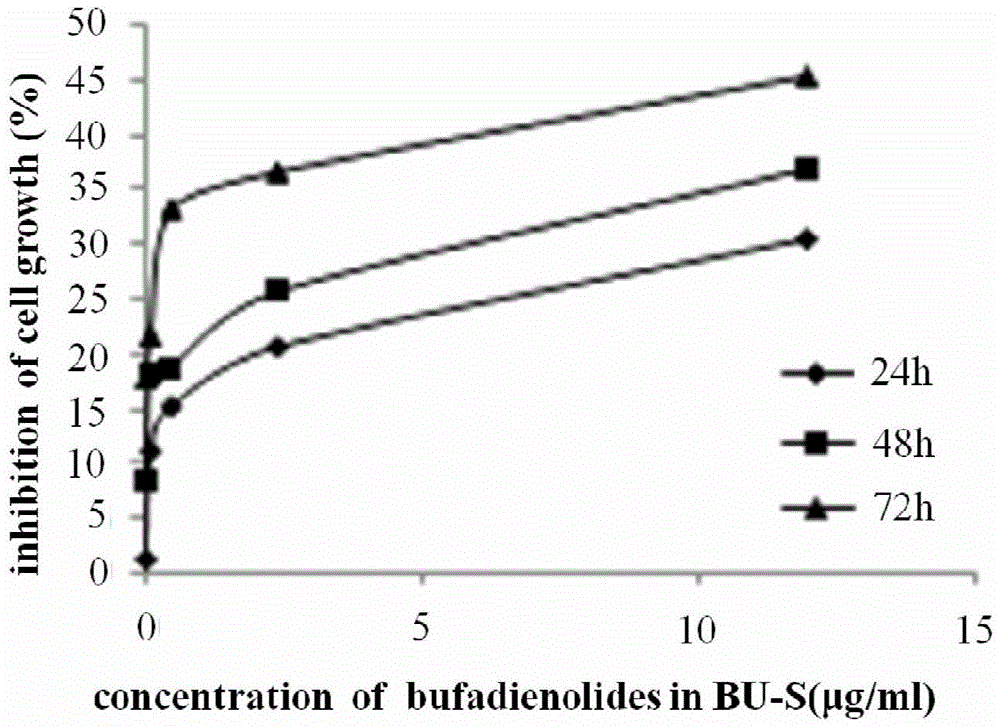
Anti-glioma drugs based on Venenum Bufonis extract and preparation method thereof
InactiveCN105477020AIncrease apoptosis rateGrowth inhibitionAmphibian material medical ingredientsPowder deliveryGlycerolMedium-chain triglyceride
The invention relates to antitumor drugs and discloses anti-glioma drugs based on Venenum Bufonis extract and a preparation method thereof, the drugs being a bufotalin submicron emulsion and a bufotalin nanoparticle preparation, wherein the bufotalin submicron emulsion comprises Venenum Bufonis extract, medium chain triglyceride, a lecithin, poloxamer 188, glycerol, sodium oleate and injection water; the bufotalin nanoparticle preparation comprises Venenum Bufonis extract, glycerol monostearate, medium-chain fatty acid glyceride, oleic acid, a lecithin, poloxamer 188, sodium deoxycholate and injection water. Both the two preparations can combine with endothelial cells in cerebral blood capillaries, enabling the drugs to be transmitted into the brain through membranes or by means of adsorption, medication, endocytosis and transferring, cerebral targeting of the drugs is improved, and further increase of bufotalin in the brain is facilitated, and glioma resistance is achieved by inducing apoptosis and excessive autophagy of cells.
Owner:赵婷
Purification method for split influenza virus vaccine
ActiveCN103721251AGuaranteed uniformityHigh removal rateAntiviralsAntibody medical ingredientsHemagglutininPurification methods
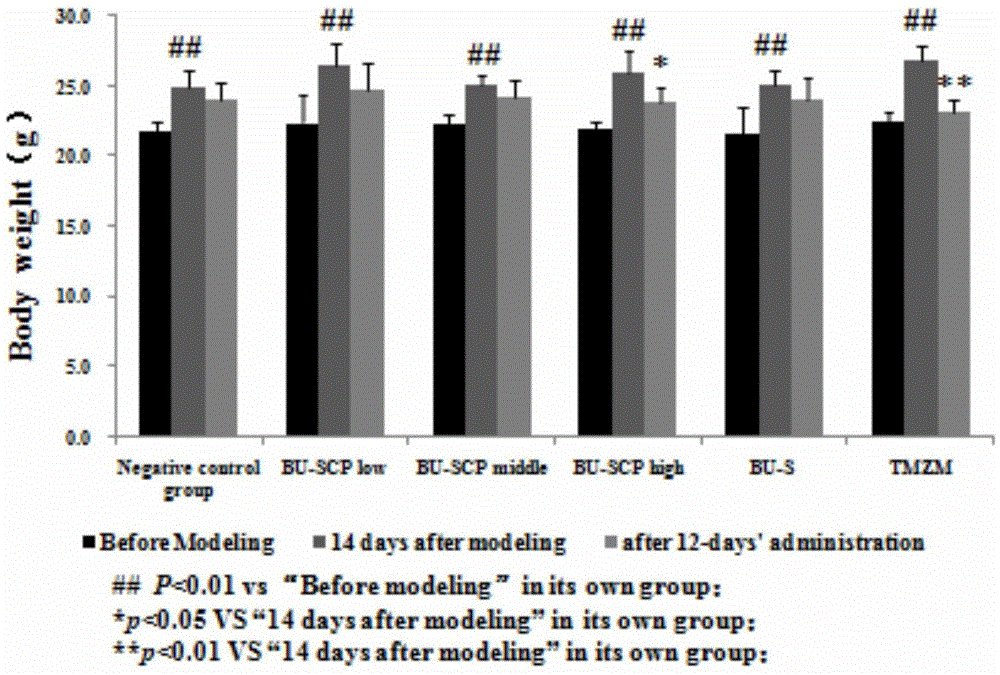
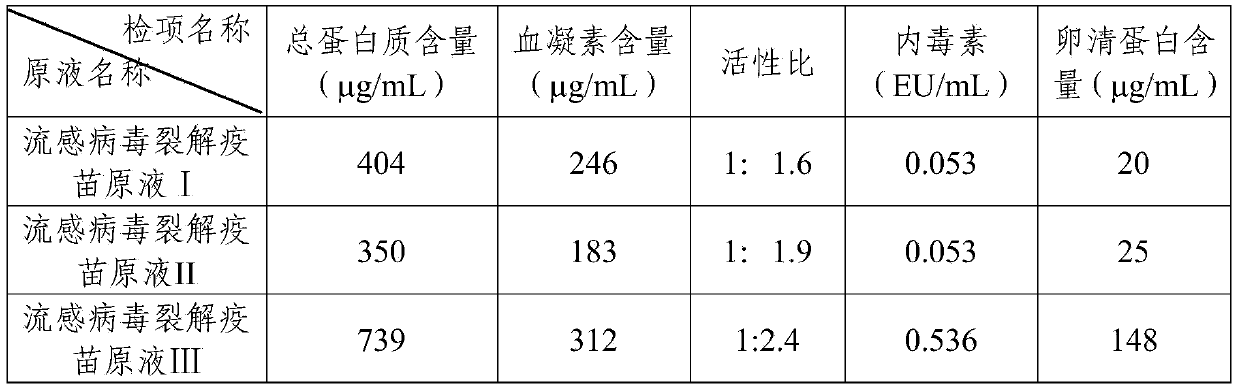
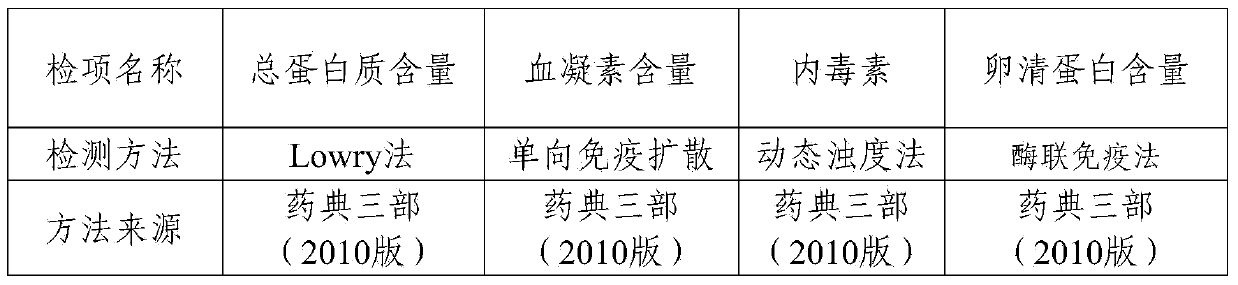
The invention provides a purification method for split influenza virus vaccine. An influenza virus strain is inoculated onto a chick embryo and cultured to obtain a virus solution; the virus solution is sequentially subjected to inactivation and ultrafiltration concentration to obtain an ultrafiltrate; the obtained ultrafiltrate is subjected to cane sugar density gradient centrifugation by using a KII continuous flow centrifuge to obtain an ultra centrifugal solution; the ultra centrifugal solution is subjected to ultrafiltration dialysis for cane sugar removal and molecular sieve gel chromatography to obtain a virus purification solution; the obtained virus purification solution is subjected to virus split by using a split agent TritonX-100 and sodium deoxycholate; after the split is over, the split agent is removed via ultrafiltration dialysis; impure protein is centrifugally removed from the obtained split solution; supernatant is collected, filtered and sterilized so as to obtain the purified influenza virus vaccine primary solution. The purification method is simple and convenient to operate, high in centrifugation capacity and suitable for large-scale production; by adopting a dual-split agent and a centrifugal process, the finished product is greatly improved in activity (hemagglutinin content / protein total content), and approaches the activity ratio of subunit vaccine, as a result, the high-grade split influenza virus vaccine product is obtained.
Owner:SINOVAC BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com