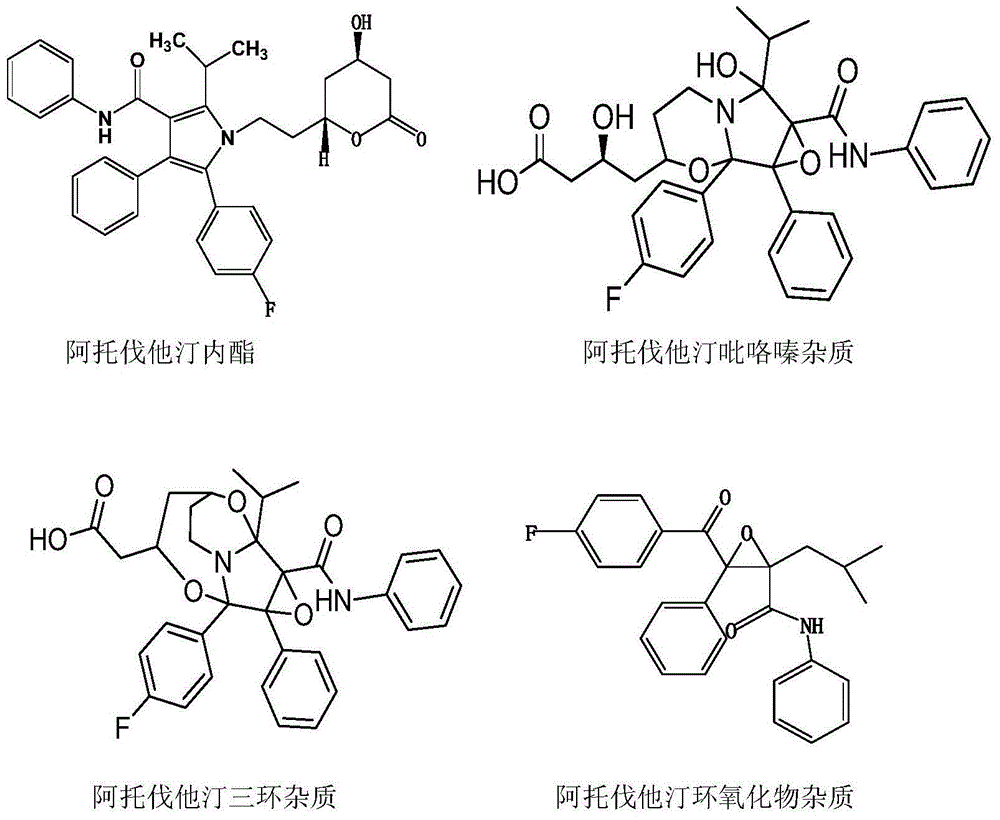
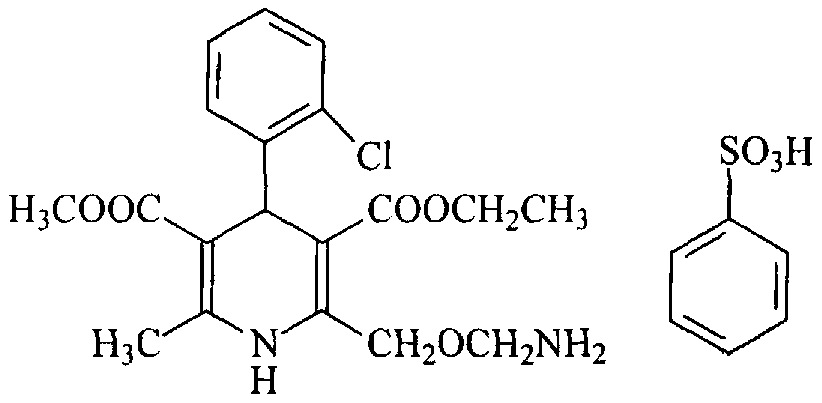
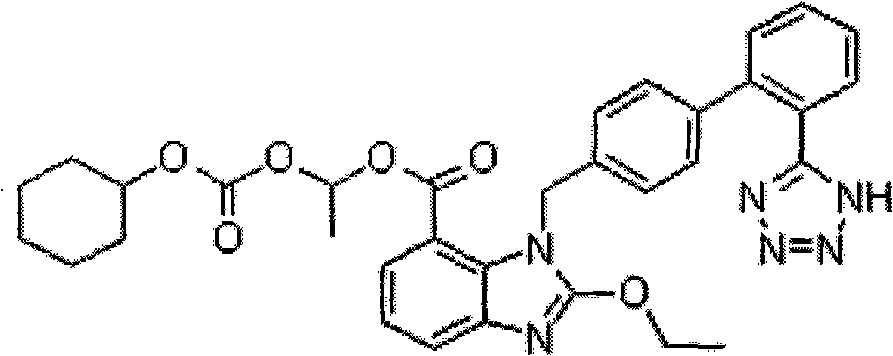
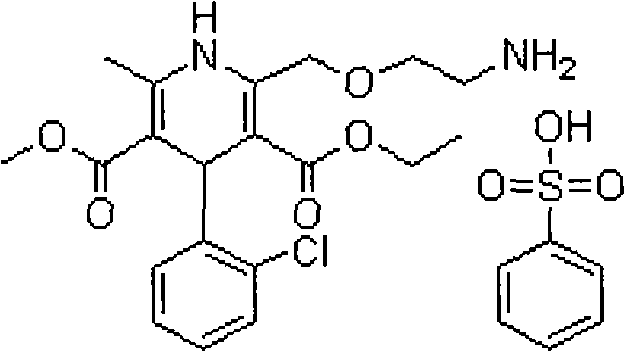
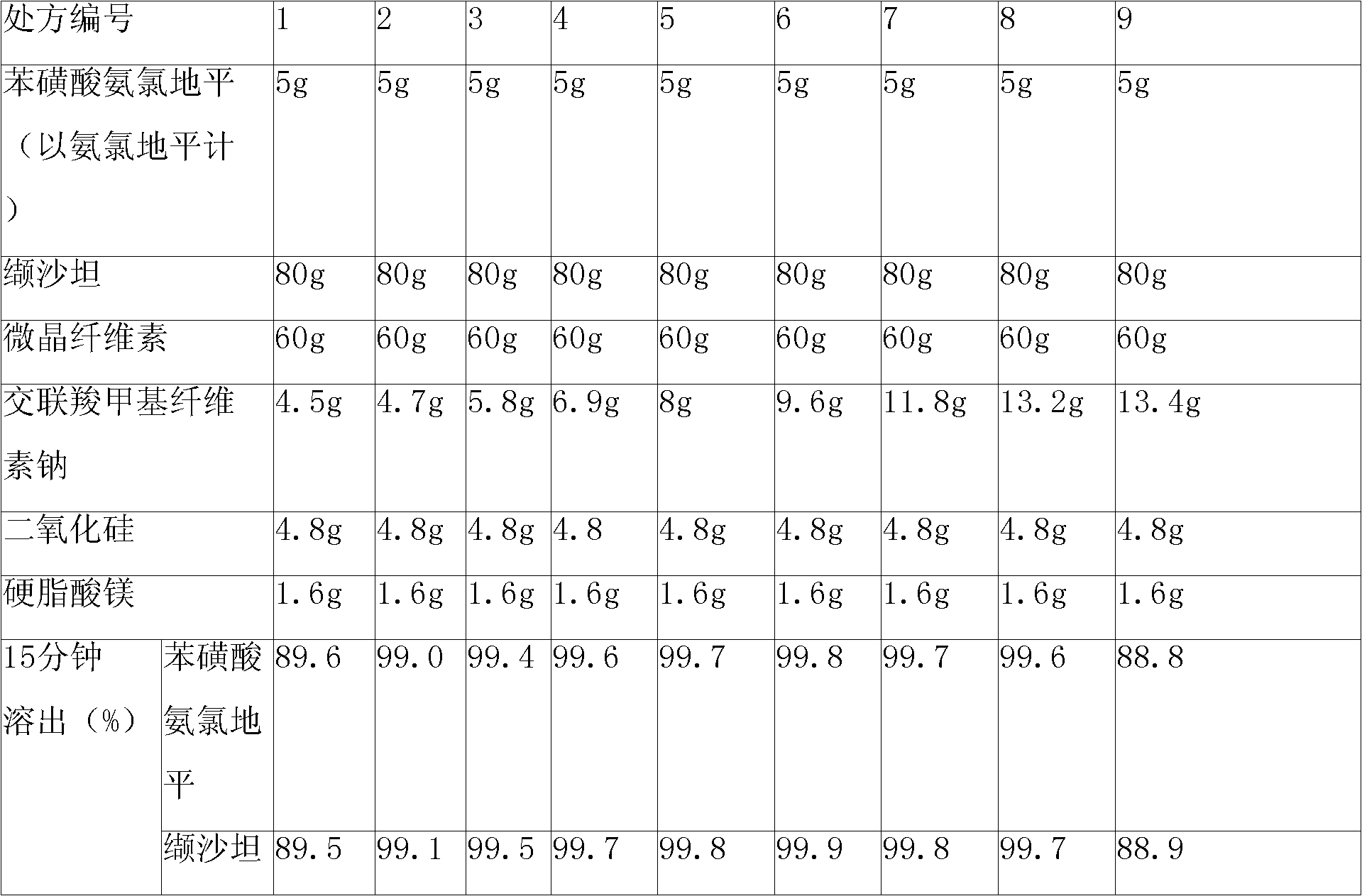Patents
Literature
207 results about "Amlodipine besilate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
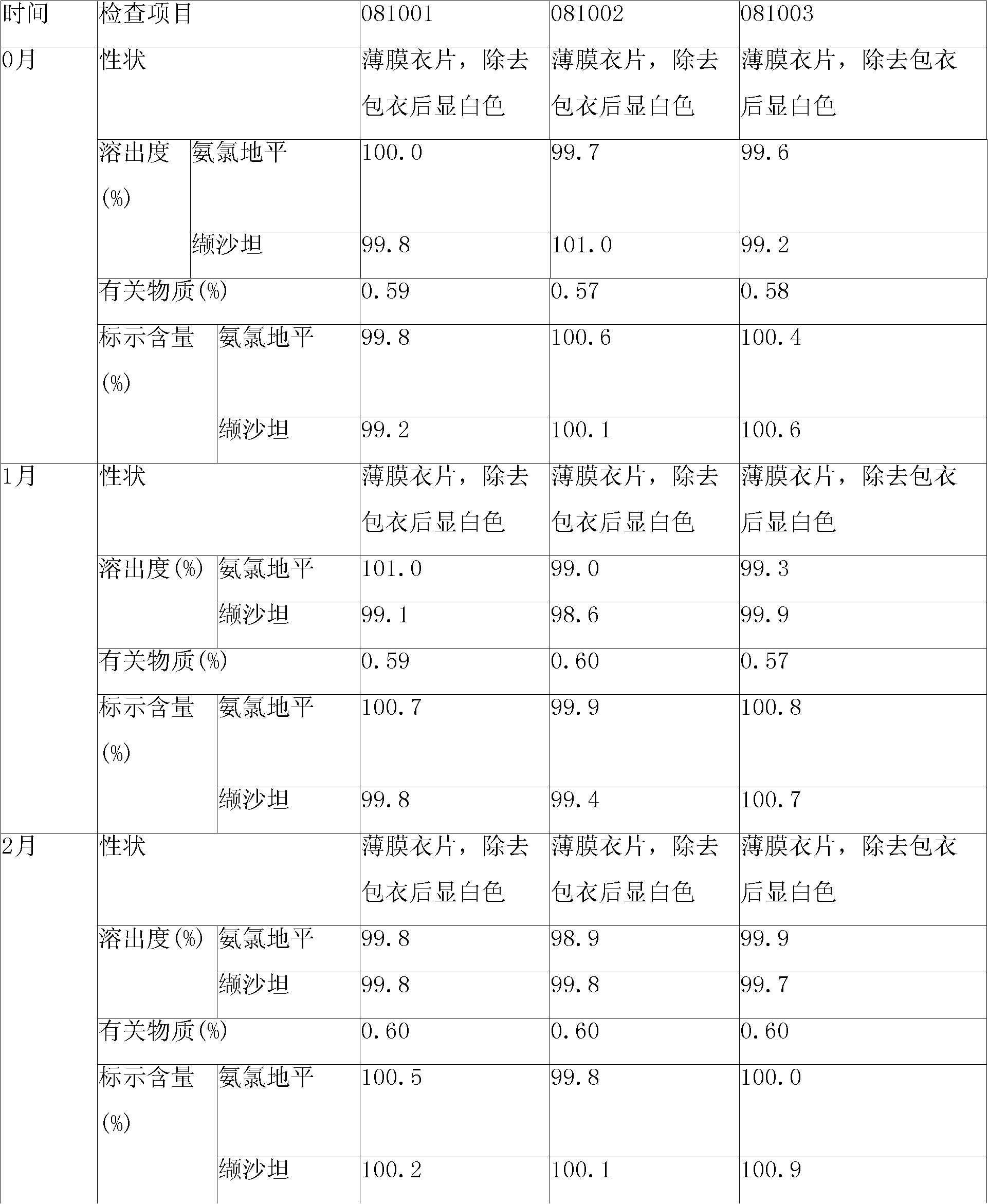
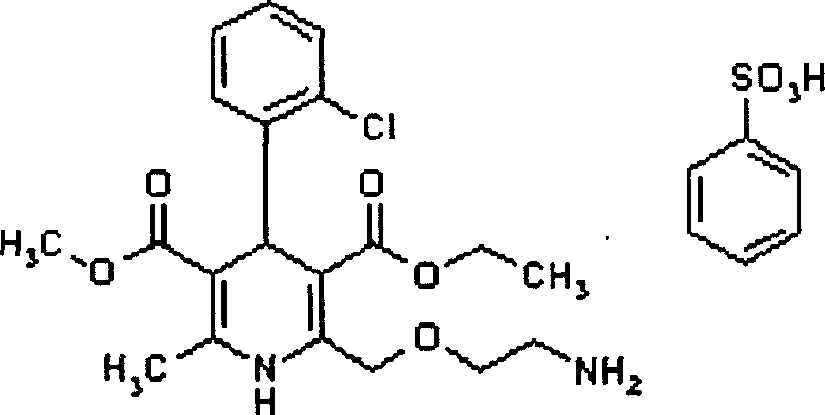
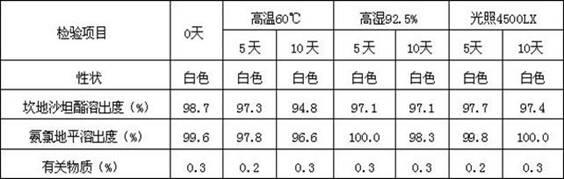
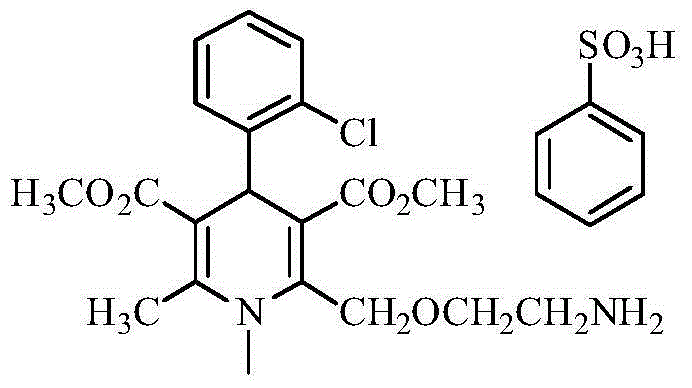
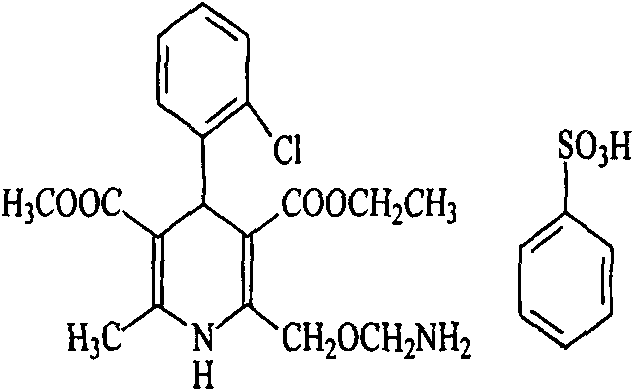
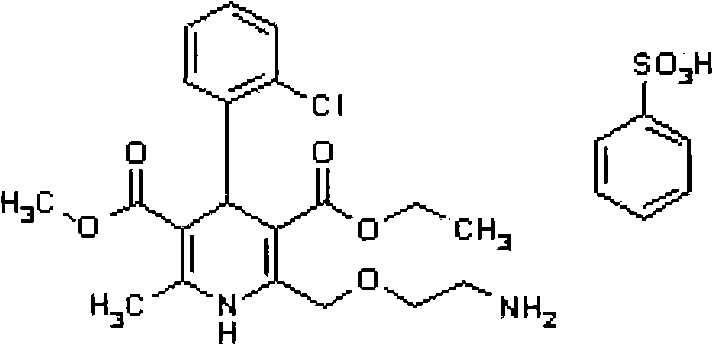
Amlodipine besylate is a white crystalline powder with a molecular weight of 567.1. It is slightly soluble in water and sparingly soluble in ethanol. ... NORVASC (amlodipine besylate) Tablets are formulated as white tablets equivalent to 2.5, 5, and 10 mg of amlodipine for oral administration.
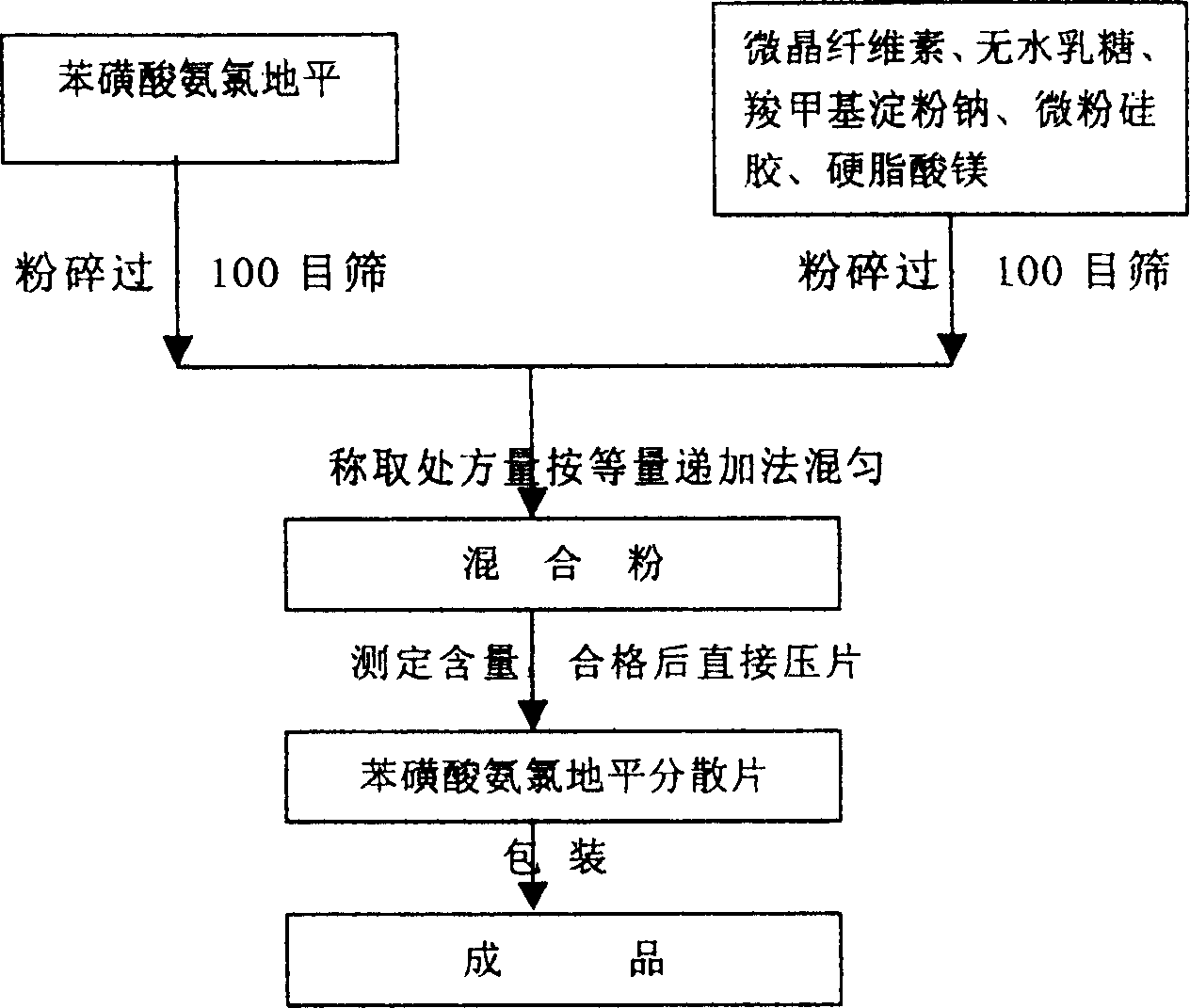
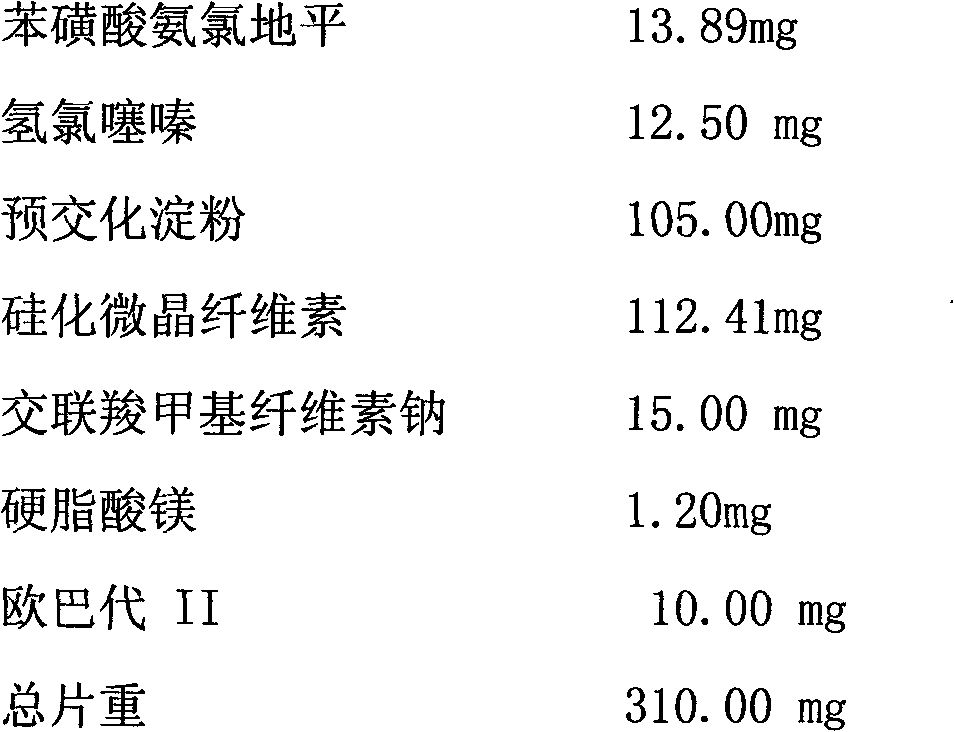
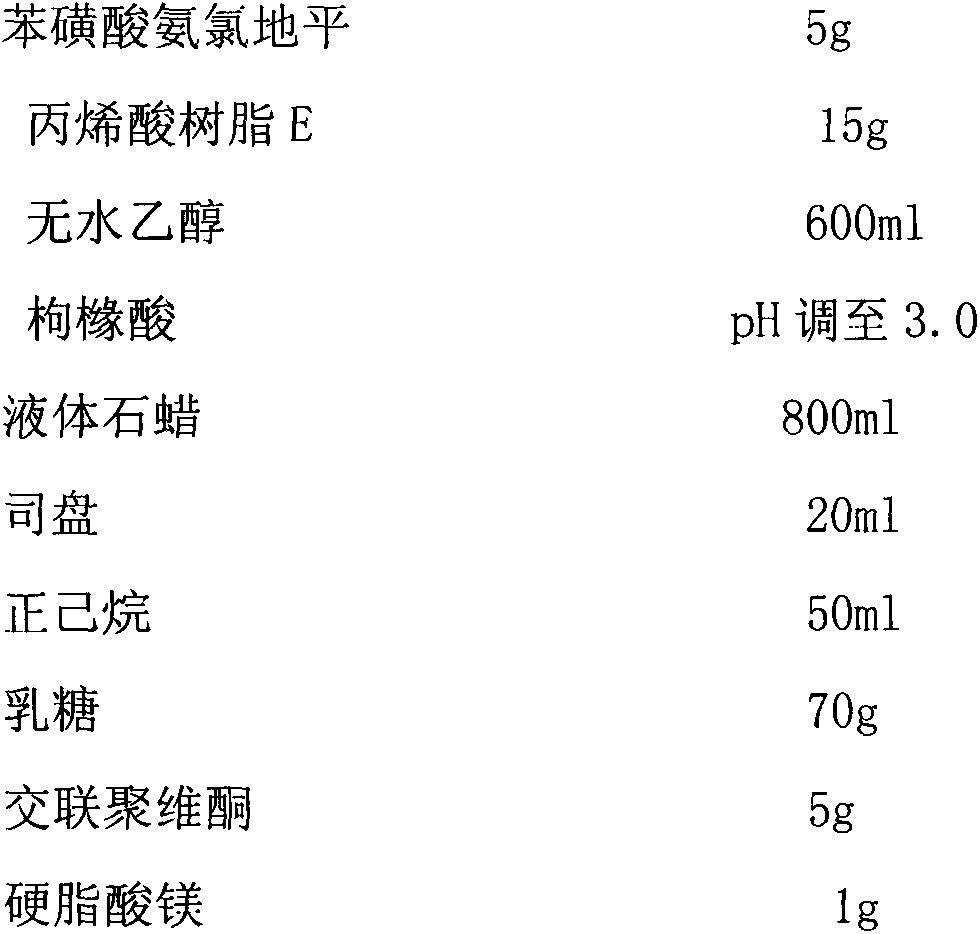
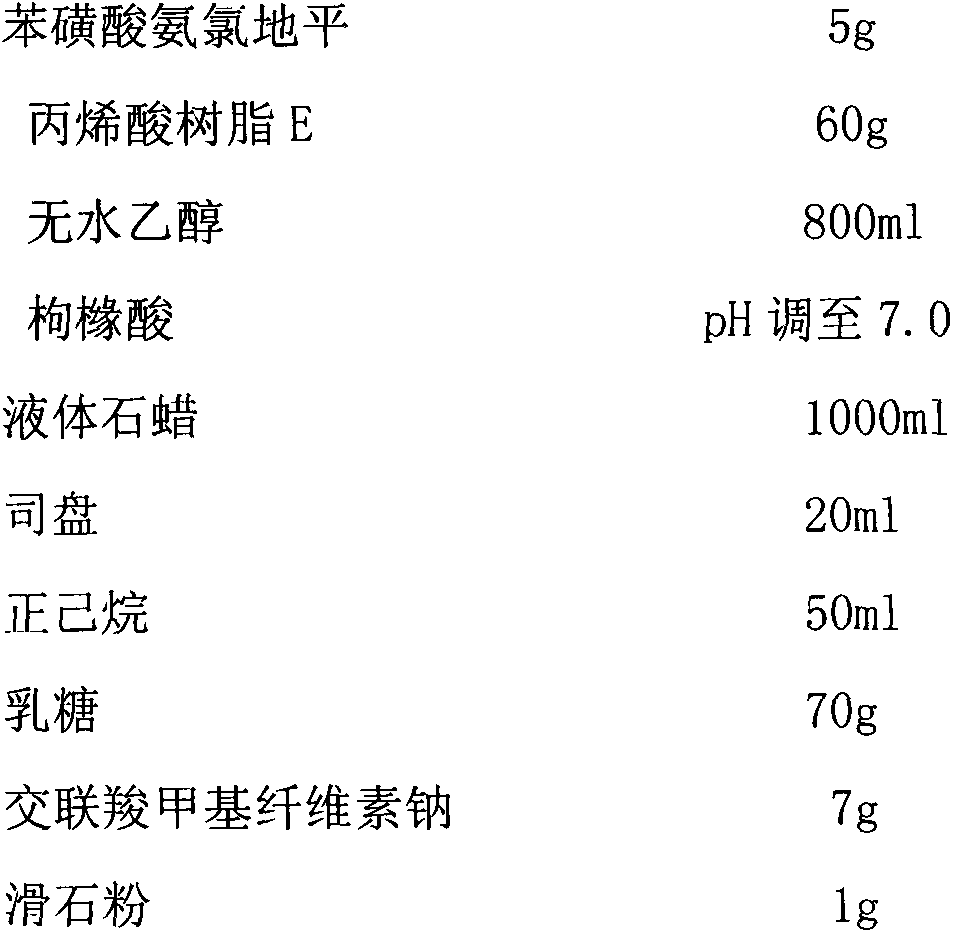
Phenylsulfonic acid amido chloro diping dispersion tablet and its preparation method
InactiveCN1686121AEasy to takeTake fastOrganic active ingredientsPill deliveryCarboxymethyl starchAmlodipine besilate
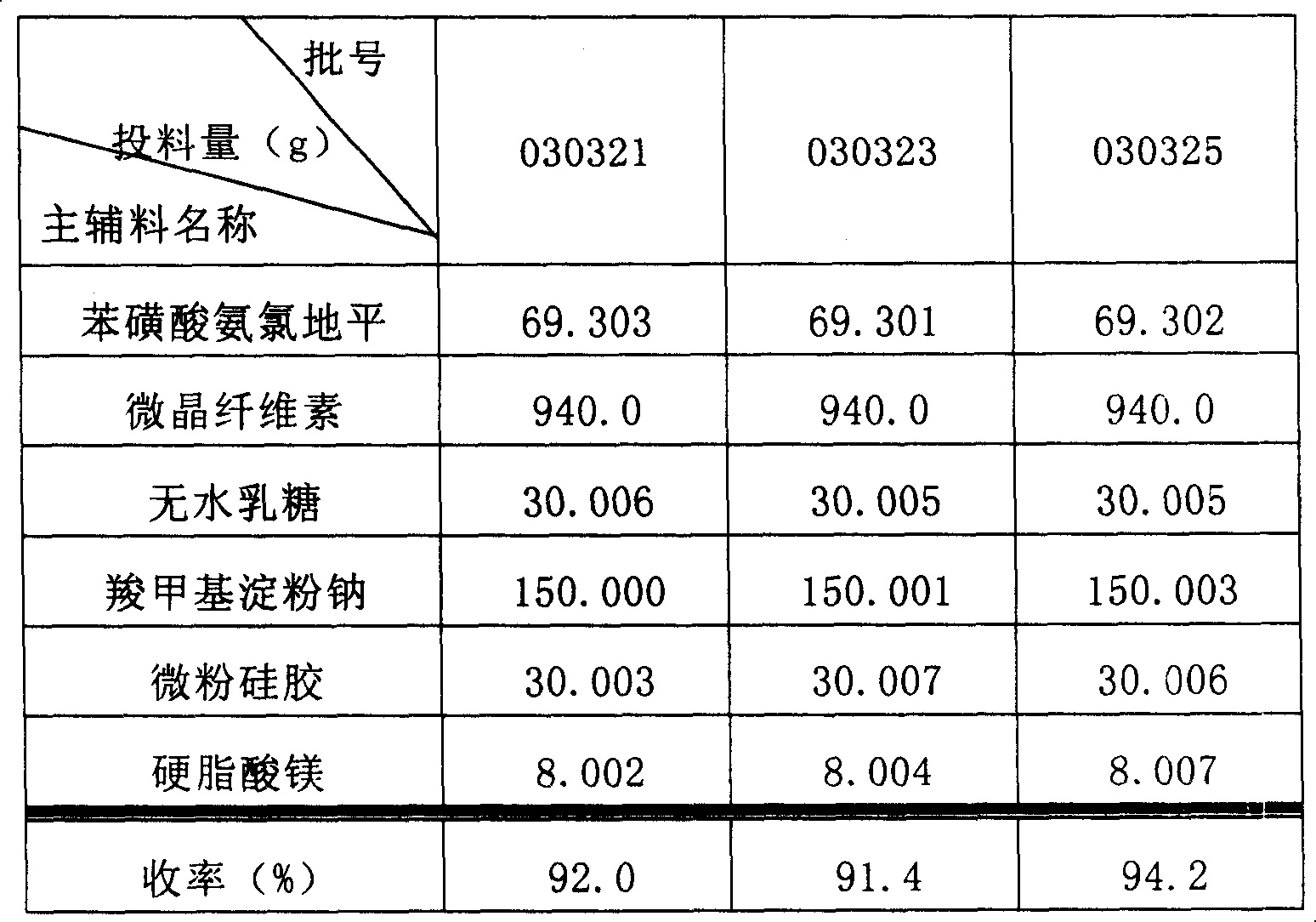
A dispersing table of amlodipine benzosulfonate for treating hypertension is proportionally prepared from amlodipine benzosulfonate, microcrystalline cellulose, anhydrous lactose, carboxymethyl starch sodium, micropowdered silica gel, and magnesium stearate.
Owner:YUNNAN BAIYAO GRP HEALTH PROD CO LTD
Technique of preparing amlodipine besylate tablets
ActiveCN101161241AHigh dissolution rateImprove finenessOrganic active ingredientsPill deliverySolubilityAir volume
A preparation technology of amlodipine besylate is disclosed in the present invention, the technology is with amlodipine besylate, filler, disintegrating agent, lubricant etc. as main components. By using grinding and sieving; reasonably controlling particle water; repeatedly feeling fluidized bed granulating technology parameter, controlling spraying speed, spraying pressure and air quantity, under the situation of non-affecting tablet content and hardness, increasing the solubility of product greatly, thereby promoting the internal quality and curative effect of the product.
Owner:YANGZIJIANG PHARMA GROUP SHANGHAI HAINI PHARMA
Pharmaceutical composition containing Amlodipine besilate and valsartan and preparation method thereof
InactiveCN101647797ALess investmentSimple production processPharmaceutical product form changeDrageesValsartanMedicine
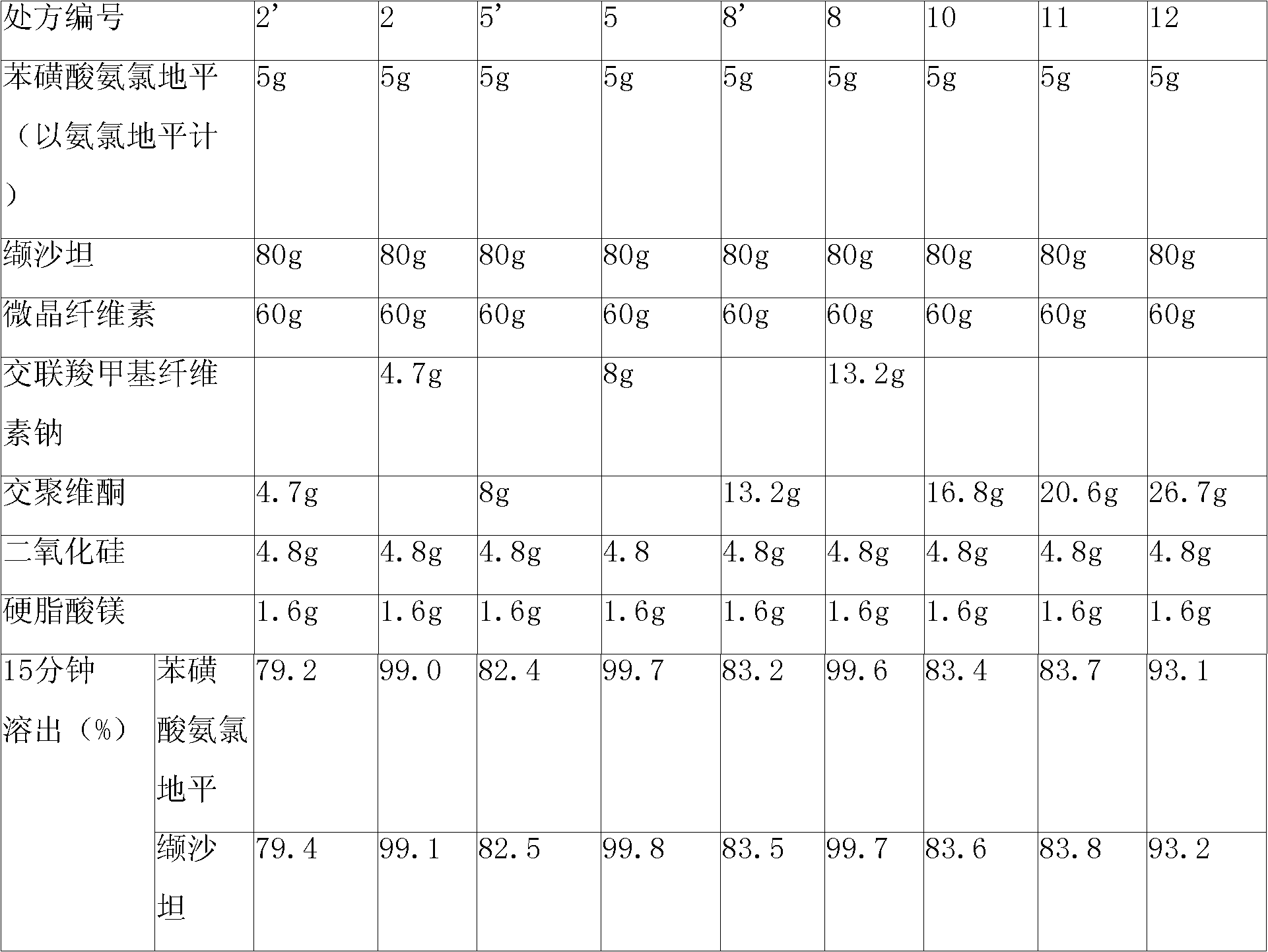
The invention relates to a pharmaceutical composition containing Amlodipine besilate and valsartan and a preparation method thereof. The pharmaceutical composition is prepared from the following ingredients in parts by weight: 5 parts of Amlodipine besilate, 80 parts of valsartan, 60 parts of microcrystalline cellulose, 4.7-13.2 parts of croscarmellose sodium, 4.8 parts of silicon dioxide and 1.6parts of magnesium stearate. The pharmaceutical composition is prepared by using a direct powder compression technique. The invention can reach the dissolution rate of more than 90% by using less disintegrating agent, and has advantages of good stability and faster disintegrating. The preparation method of the invention has simpler production process, reduces the investment of corresponding equipment and plants and saves the production cost; the tablet produced by using direct powder compression technique has faster disintegrating and is helpful to improve the dissolving of pharmaceutical; through detection, the tablet prepared by using the method is dissolved out by more than 90% within 15 min.
Owner:HAINAN JINRUI PHARMA CO LTD
Dispersible tablets containing valsartan and amlodipine besylate and preparation method thereof
InactiveCN101843615AAvoid stabilityAvoid the disadvantages of inconvenient storage and transportationPill deliveryHeterocyclic compound active ingredientsValsartanActive agent
The invention discloses to dispersible tablets containing valsartan and amlodipine besylate and a preparation method thereof, and relates to medicament dispersible tablets and the preparation method thereof, solving the problem of poor stability, slow disintegration and dissolution, low oral bioavailability and high cost existing in the traditional medicine containing valsartan and amlodipine besylate. The dispersible tablets are prepared from valsartan, amlodipine besylate, disintegrant, diluent, adhesive, lubricant, fluidizer, surfactant and flavoring agent. The method comprises the following steps of: weighting and screening materials; mixing the valsartan, disintegrant and diluent to obtain mixed powder A; mixing amlodipine besylate and the mixed powder A to obtain mixed powder B; mixing the adhesive, surfactant and the mixed powder B to prepare soft material; screening, granulating and drying the soft material; mixing and screening and the dried granules with the fluidizer, lubricant, disintegrant and flavoring agent; and finally carrying out size stabilization and tabletting. The medicine prepared by the method has good stability, fast disintegration and dissolution, high oral bioavailability and low cost.
Owner:包丽昕
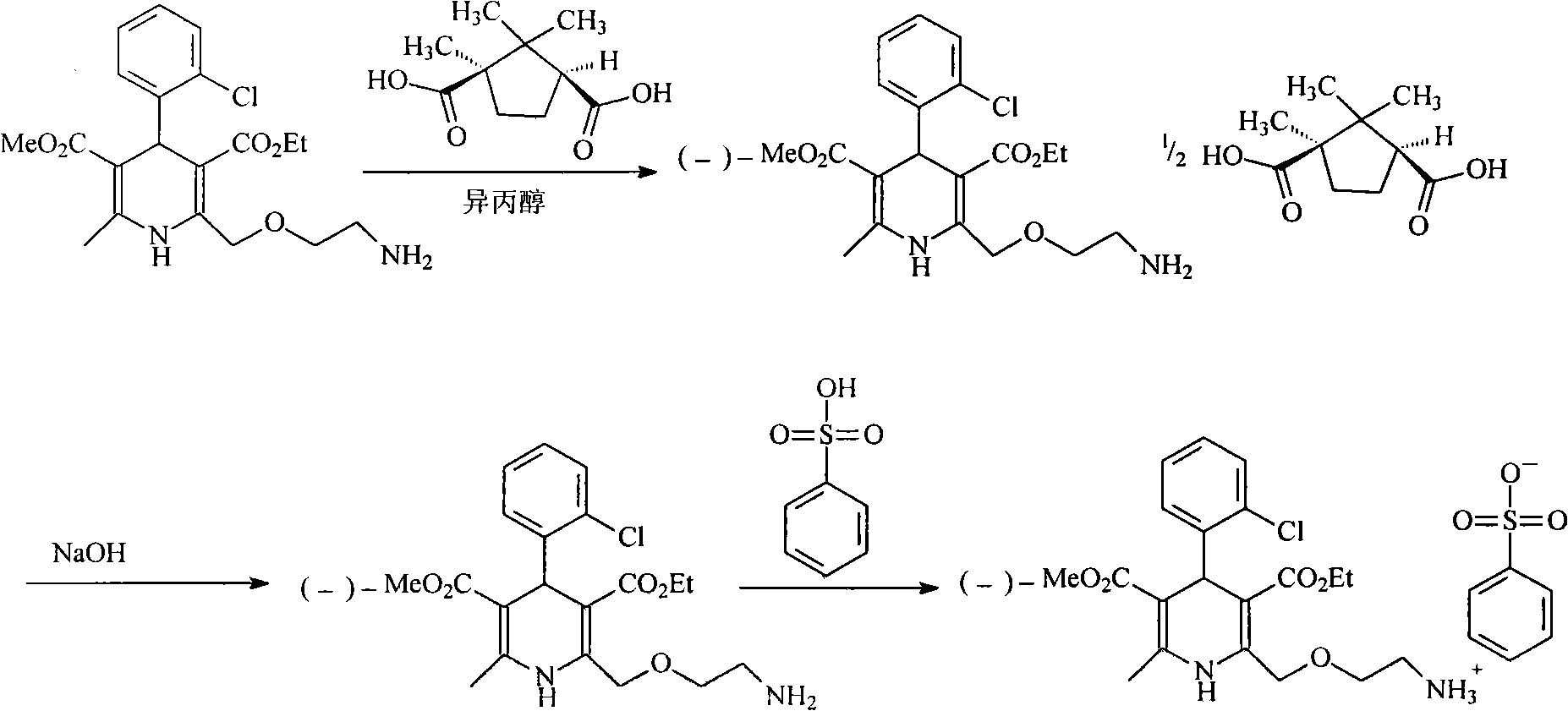
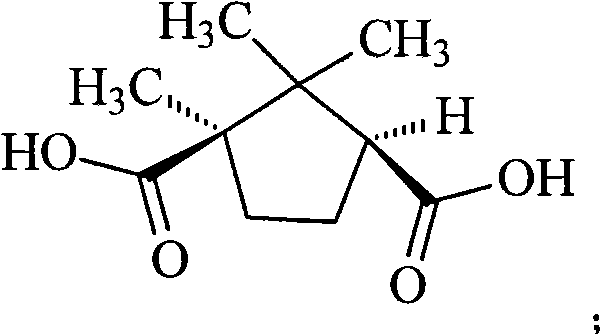
Levamlodipine compound prepared in novel method
InactiveCN101805284AHigh purityNot easy to cause pollutionOrganic chemistryCamphoric acidLevamlodipine
The invention provides a levamlodipine compound prepared in a novel method, which comprises the following steps: producing D-(plus)-levamlodipine camphorate through reaction of amlodipine and D-(plus)-camphanic acid, producing levamlodipine under the effect of sodium hydroxide, and producing the levamlodipine compound through salt forming reaction of the levamlodipine and benzenesulfonic acid. The method adopts a completely novel split reagent D-(plus)-camphanic acid, simplifies reaction procedures and is more suitable for industrial production. Moreover, the method has the advantage of high yield.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Medicament composition containing amlodipine besylate and candesartan cilexetil and medicine box
ActiveCN101371834AGood synergyOrganic active ingredientsCardiovascular disorderCompounding drugsCoronary artery disease
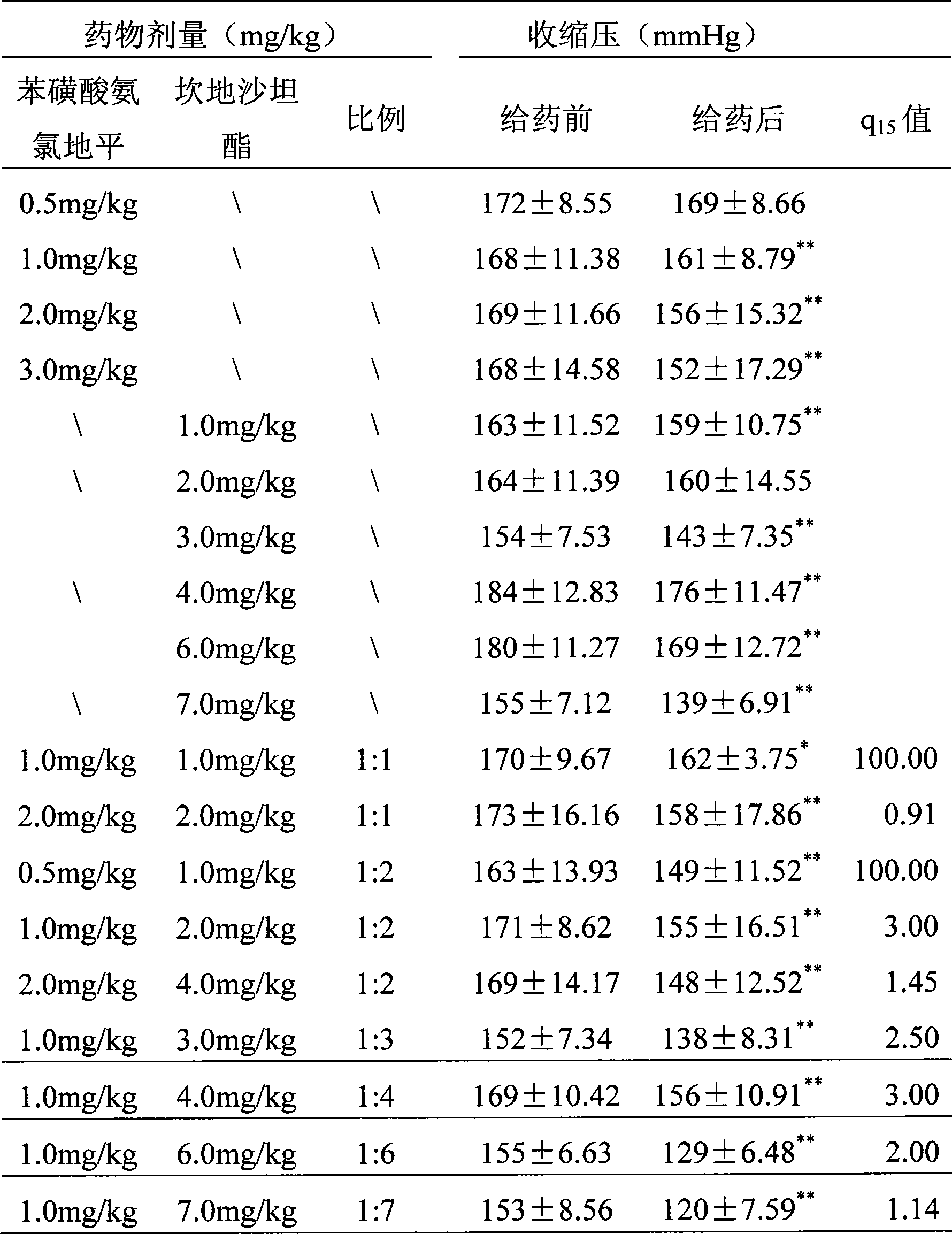
The invention discloses a drug composition containing amlodipine besylate and candesartan cilexetil and a drug box thereof, wherein, the weight ratio of the amlodipine besylate to the candesartan cilexetil is 1:1-6. The compound drug has a better synergistic effect in reducing blood pressure and extending blood vessels, and can be used for curing cardiovascular diseases such as hypertension and coronary heart disease.
Owner:ZHEJIANG YONGNING PHARMA
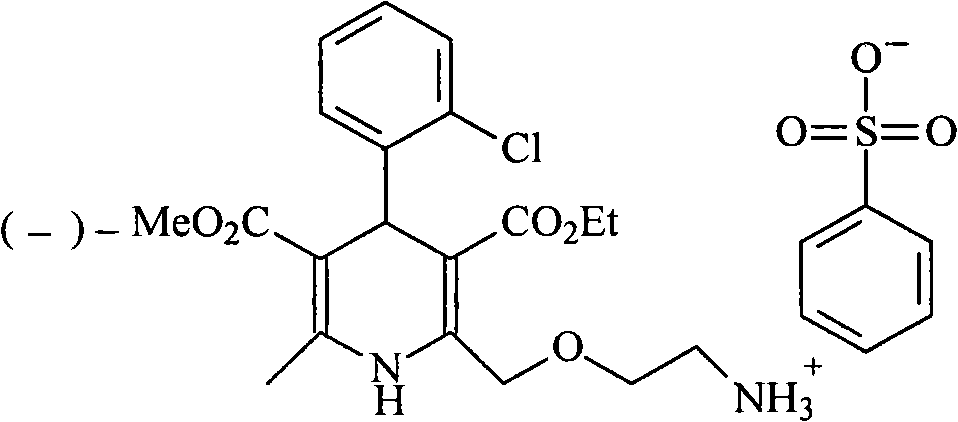
Synthesis of high-purity amlodipine besylate
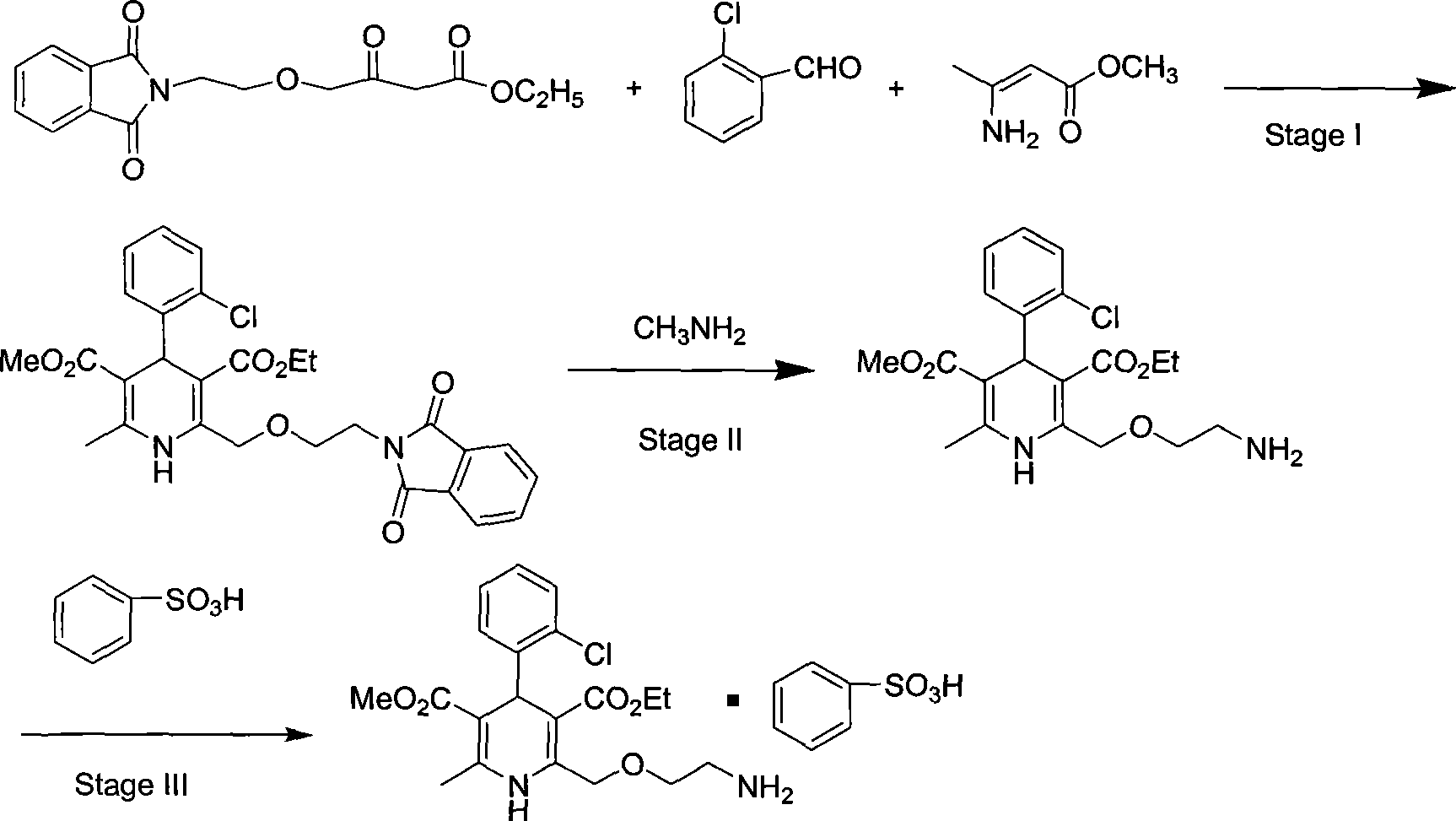
The present invention relates to a synthesis method of high-purity amlodipine besylate. In particular, o-chlorobenzaldehyde, 4-(2-phthalyl imino radical ethoxy) acetoacetic ester and 3-amino methyl cro-tonate are used as raw materials for ring closure in an alcohol solvent, and when the amount of the 3-amino methyl cro-tonate is three times, the intermediate for ring closure can be prepared; the intermediate is refined by toluene / glacial acetic acid, dissolved in methylamine to form a salt in the aqueous solution, and the high-purity amlodipine besylate can be acquired after recrystallization with ethyl alcohol.
Owner:CHINA RESOURCES SAIKE PHARMA
Orally disintegrating tablet of amlodipine besylate and its preparation process
InactiveCN1546024AOvercome the disadvantages of single-actingGood disintegrationOrganic active ingredientsPill deliverySodium bicarbonateOrally disintegrating tablet
The invention discloses an Amlodipine Besylate oral disintegration tablet, wherein the principal drug employs Amlodipine Besylate, the auxiliary materials include lactose, mannitol, crystalline cellulose, low substituted methylcellulose propylene glycol ether, cross bonding polyvinylpyrrolidone, sodium hydrogen carbonate, citric acid, Aspartame, perfume compound, magnesium stearate, The tablet has good treatment action for hypertension and stenocardia.
Owner:CHENGDU SHENGNUO BIOTEC CO LTD
L-amlodipine besilate dripping pill and its preparing method
InactiveCN1899268ASuit one's needsIncrease the dosage form of clinical useOrganic active ingredientsPill deliveryMedicineAmlodipine Maleate
The present invention relates to L-amlodipine besilate dripping pill and its preparation process and belongs to the field of medicine preparing technology. The recipe of the L-amlodipine besilate dripping pill includes L-amlodipine besilate, dripping pill substrate, antioxidant, surfactant, coating material, etc. The preparation process includes the first preparing L-amlodipine besilate into fine powder over 150 mesh, the subsequent melting the substrate, adding fine L-amlodipine besilate powder and supplementary material, mixing to form dispersed liquid, and dropping the dispersed liquid into condensating liquid to form dripping pill with or without coating. Each of the dripping pills contains L-amlodipine besilate in 1.25-15mg and weighs 10-100 mg.
Owner:AOLING BODA MEDICINE SCI & TECH DEV BEIJING
Valsartan amlodipine capsule and preparation method thereof
InactiveCN102028686ASimple production processFast absorptionPharmaceutical non-active ingredientsCapsule deliveryCrospovidonesValsartan
The invention relates to a capsule containing valsartan and amlodipine besylate, which is prepared from high-dose valsartan and amlodipine besylate, disintegrating agent crospovidone, filling agent microcrystalline cellulose and lubricating agent silicondioxide. The problem that the compound preparation contianing a high dose of valsartan and amlodipine besylate is not easy to disintegrate and dissolve is solved by adopting the conventional process and equipment of powder mixing and direct capsule filling without using special equipment and the bilayer tablet process.
Owner:林海平
Compound preparation of valsartan amlodipine tablet (I) and preparation method thereof
ActiveCN103006649AReduce the introductionReduce lossesDrageesCardiovascular disorderValsartanAmlodipine besilate
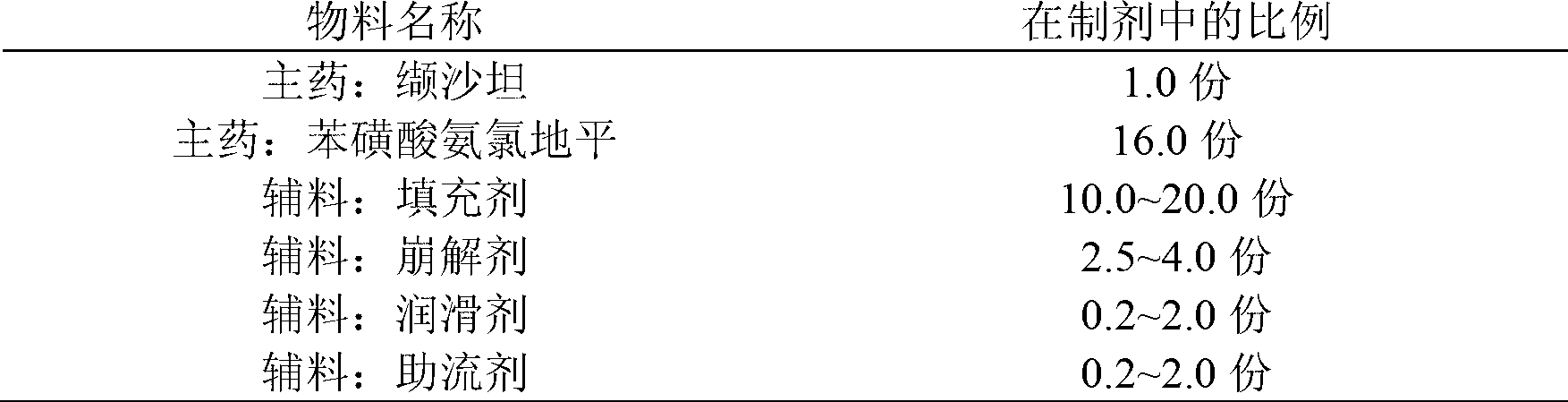
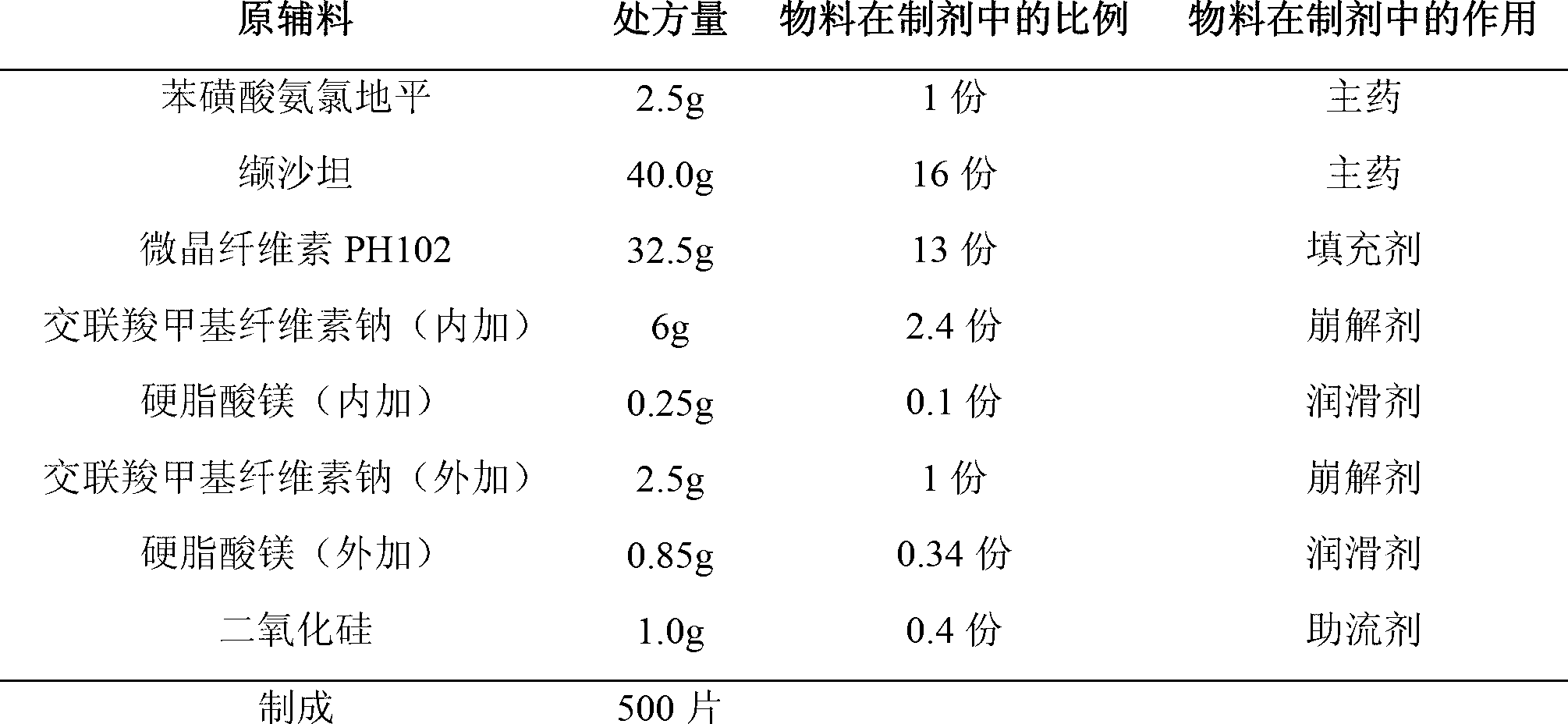
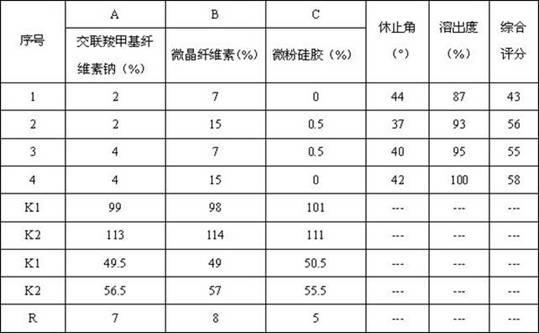
The invention provides a compound preparation of valsartan amlodipine tablet (I) and a preparation method thereof. The compound preparation comprises a tablet, a film coating layer, the tablet comprises main drug and auxiliary materials, the main drug comprises valsartan, benzenesulfonic acid amlodipine, the auxiliary material comprises filler, a disintegrating agent, a lubricant, and glidant, the tablet comprises the valsartan, benzenesulfonic acid amlodipine, the filler, the disintegrating agent, the lubricant and the glidant, and the tablet is obtained by being coated with the film coating layer. According to the method, the defect of unsafety of medicine use caused by increase of relevant material due to wet granulation is overcome, and manufacture cost can be lowered and labor intensity can be relieved due to the dry granulation technology.
Owner:SHIJIAZHUANG HUAXIN PHARMA
Candesartan cilexetil and amlodipine tablet composition and preparation method thereof
ActiveCN102688236AImprove stabilityReduce disorderOrganic active ingredientsPharmaceutical non-active ingredientsCandesartanMedicine
The invention discloses a candesartan cilexetil and amlodipine besylate tablet composition. According to the formula, the tablet composition contains 2 to 4 weight parts of polyethylene glycol (PEG) 6000 serving as a stabilizer; and the active ingredients of the composition, namely the candesartan cilexetil and the amlodipine besylate, have high stability.
Owner:石药集团中诺药业(石家庄)有限公司 +1
Medicament compound preparation formed by mixing olmesartan medoxomil with benzene sulfonic acid amlodipine and hydrochlorothiazide
The invention relates to a medicament compound preparation formed by mixing olmesartan medoxomil with benzene sulfonic acid amlodipine and hydrochlorothiazide. Both the olmesartan medoxomil and amlodipine are unstable compounds, and are difficultly prepared into mixed medicaments with stable active ingredients. According to the invention, the adjuvant of the olmesartan medoxomil and amlodipine compound preparation is regulated, so that the amounts of degradation products and impurities of the olmesartan medoxomil are effectively reduced. The invention provides a method for preparing the fixed-dose compound preparation which takes the olmesartan medoxomil, the amlodipine and hydrochlorothiazide as the active ingredients.
Owner:ZHUHAI EBANG PHARMA
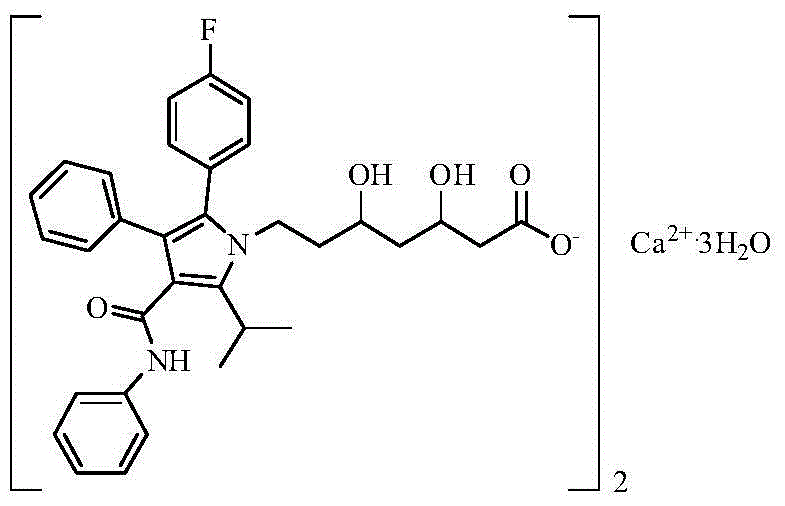
Preparation method of high-stability amlodipine atorvastatin calcium tablet
InactiveCN104644633ASimple controlMetabolism disorderPharmaceutical delivery mechanismAmlodipine besilateLubricant
The invention relates to a preparation method of a high-stability amlodipine atorvastatin calcium tablet. The preparation method of the high-stability amlodipine atorvastatin calcium tablet is characterized in that the amount of peroxide in microcrystalline cellulose is controlled. The preparation method of the high-stability amlodipine atorvastatin calcium tablet comprises the following steps: A, granulating of atorvastatin calcium, concretely comprising the steps of firstly dissolving a surfactant in water, adding a binding agent, stirring and dissolving, secondly mixing atorvastatin calcium, calcium carbonate, other diluents and a disintegrant, thirdly, granulating, and fourthly, drying wet granules obtained in the step three, so that dry atorvastatin calcium granules are prepared; and B, preparing of finished granules, concretely comprising the steps of firstly adding amlodipine besylate, a disintegrant and a flow aid into the dry atorvastatin calcium granules, secondly, uniformly mixing powder obtained in the step one in a mixing machine, thirdly, adding a lubricating agent, and uniformly mixing, and fourthly, pressing the powder into tablets.
Owner:CHINA RESOURCES SAIKE PHARMA
Amlodipine benzenesulfonate tablet and preparation method thereof
ActiveCN103191073AAvoid influenceEvenly dispersedOrganic active ingredientsPill deliveryAlcoholMedicine
The invention discloses an amlodipine benzenesulfonate tablet which is prepared by dissolving amlodipine benzenesulfonate and hydroxypropyl betadex into absolute ethyl alcohol so as to prepare a coating solution, coating blank pellets, subsequently uniformly mixing with pharmaceutically acceptable auxiliaries, and directly tabletting. An inclusion technique and a coating technique are combined, so that the influence of moisture to medicines in the production process of the preparation is avoided, and the tablet is coated and at the same time a hydroxypropyl betadex amlodipine benzenesulfonate clathrate compound is prepared, therefore, the stability is good; and meanwhile, as the clathrate compound is uniformly dispersed on the surface of the pellets, the contact area of dissolved mediums in dissolution measurement is increased, and the dissolution is rapid.
Owner:广东彼迪药业有限公司
Oral tablet containing candesartan cilexetil and benzene sulfonate amlodipine and preparation method for oral tablet
ActiveCN102670603AAvoid stickingSimple processOrganic active ingredientsPill deliveryCandesartanAmlodipine besilate
The invention discloses an oral tablet containing candesartan cilexetil and benzene sulfonate amlodipine. The oral tablet consists of benzene sulfonate amlodipine, candesartan cilexetil and medicinal auxiliary materials, wherein the medicinal auxiliary materials contain a stabilizing agent and other auxiliary materials such as one or more of a filling agent, a binding agent and a lubricating agent. The tablet containing candesartan cilexetil and benzene sulfonate amlodipine is attractive in surface and stable in quality. The invention also provides a preparation method for the oral tablet; and the process is simple, low in cost and suitable for commercial production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Amlodipine benzenesulfonate slow-releasing capsule and its preparing method
InactiveCN1562009ALittle side effectsFully absorbedOrganic active ingredientsPharmaceutical delivery mechanismAcrylic resinCellulose acetate
A slowly-releasing capsule of amlodipine benzosulfonate is prepared from amlodipine benzosulfonate, auxiliaries and slow-releasing material chosen from acrylic resin, hydroxypropylmethyl cellulose, ethylcellulose, etc.
Owner:天津米克莱特生物技术有限公司
Oral tablet containing valsartan, hydrochlorothiazide and amlodipine besylate
ActiveCN102526748AAvoid interactionPromote dissolutionPill deliveryPharmaceutical non-active ingredientsMedicineAmlodipine besilate
The invention discloses an oral tablet containing valsartan, hydrochlorothiazide and amlodipine besylate. The oral tablet consists of valsartan, hydrochlorothiazide, amlodipine besylate and medicinal auxiliary materials, wherein the medicinal auxiliary materials include pre-gelatinized starch. The tablet containing valsartan, hydrochlorothiazide and amlodipine besylate provided by the invention has attractive tablet surface and stable quality, and is dissolved quickly. The invention further provides a preparation method of the oral tablet. The method has a simple process and low cost, and is suitable for industrial mass production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Valsartan and amlodipine tablet and preparation method thereof
ActiveCN107823170AGood disintegration and dissolutionGuaranteed tablet weight variancePharmaceutical non-active ingredientsCoatingsValsartanMedicine
The invention discloses a valsartan and amlodipine tablet, which comprises a tablet core and a coating outside the tablet core. The tablet core comprises the following ingredients (by weight): 8-12 parts of valsartan, 0.8-1 part of amlodipine besylate, 8-12 parts of microcrystalline cellulose, 0.4-0.6 part of a disintegrating agent and 0.1-0.2 part of colloidal silicon dioxide. The coating comprises the following ingredients (by weight): 0.2-0.4 part of a disintegrating agent, 0.1-0.2 part of colloidal silicon dioxide and 0.4-0.6 part of magnesium stearate. The invention also correspondingly provides a preparation method of the valsartan and amlodipine tablet. The valsartan and amlodipine tablet of the invention has good disintegration and dissolution and good dispersion uniformity of active ingredients. In addition, the technology of the invention is simple and easy to operate and is suitable for industrial production.
Owner:HUNAN QIANJIN XIELI PHARMA CO LTD
Amlodipine besylate liposome tablet
InactiveCN101862302BHigh dissolution rateImprove stabilityPill deliveryCholesterolAmlodipine besilate
Owner:HAINAN LINGKANG PHARMA CO LTD
Valsartan amlodipine tablet and preparation method thereof
ActiveCN108785267AImprove solubilityExtended stayPharmaceutical non-active ingredientsCoatingsSolubilityValsartan
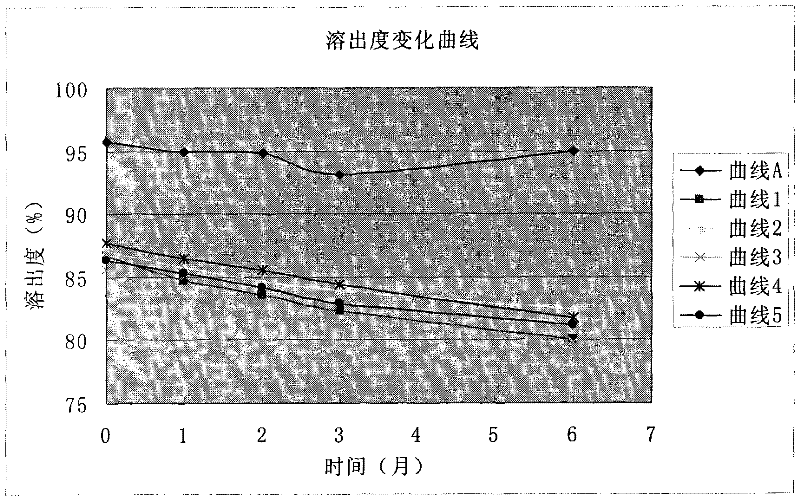
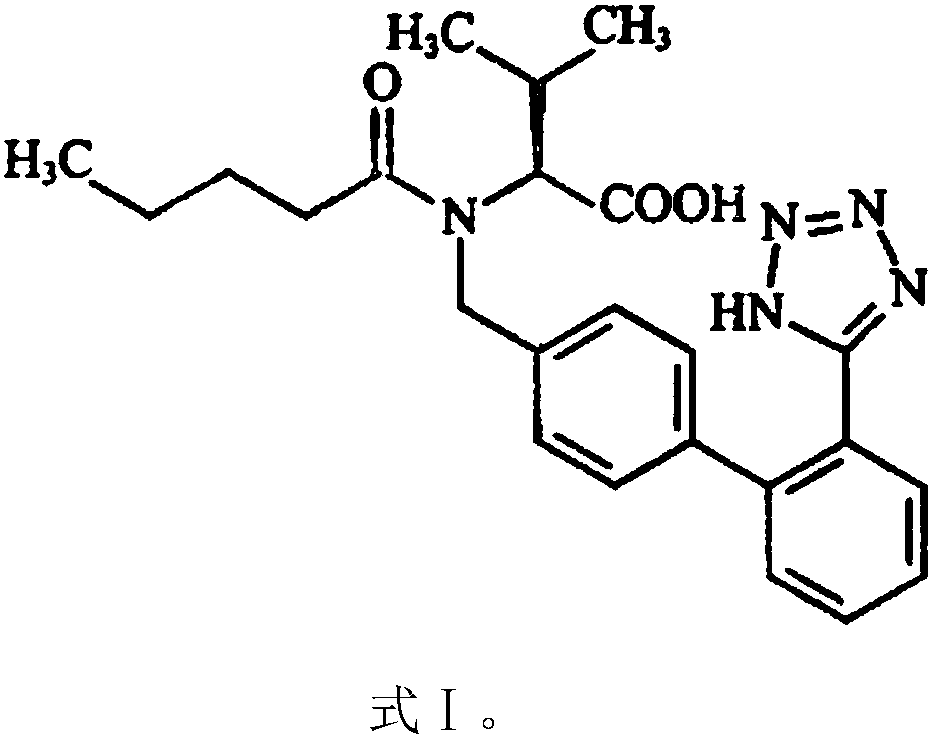
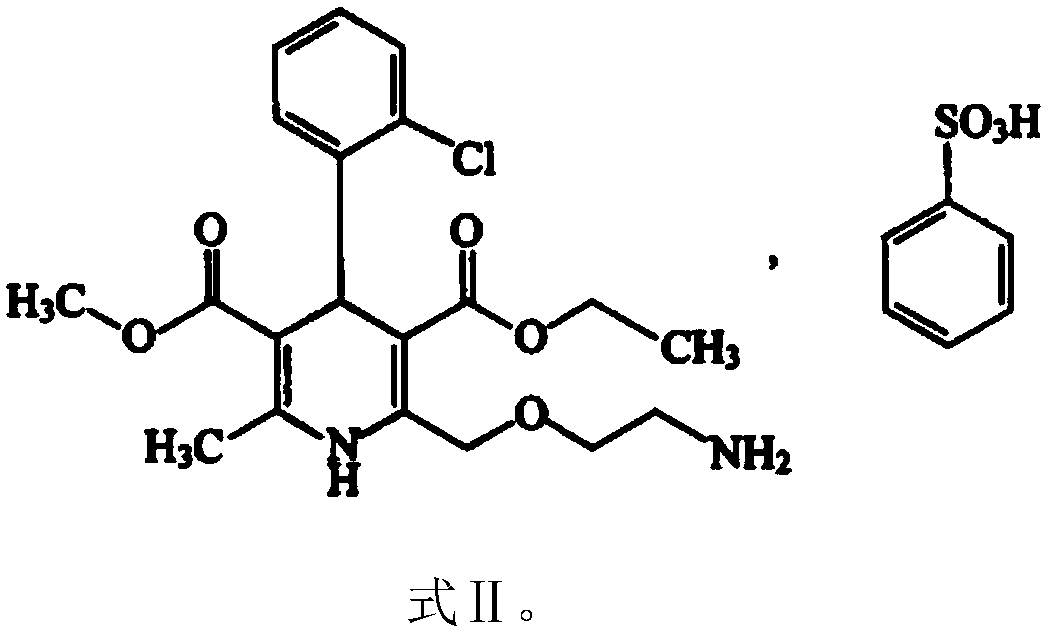
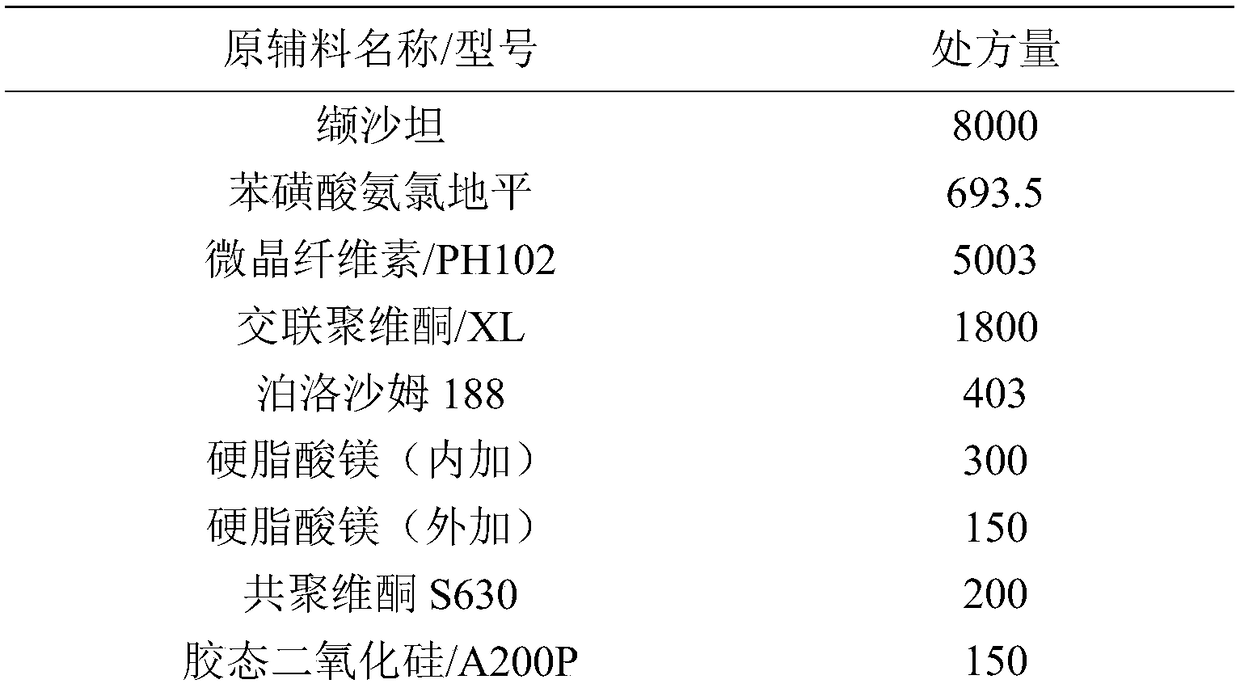
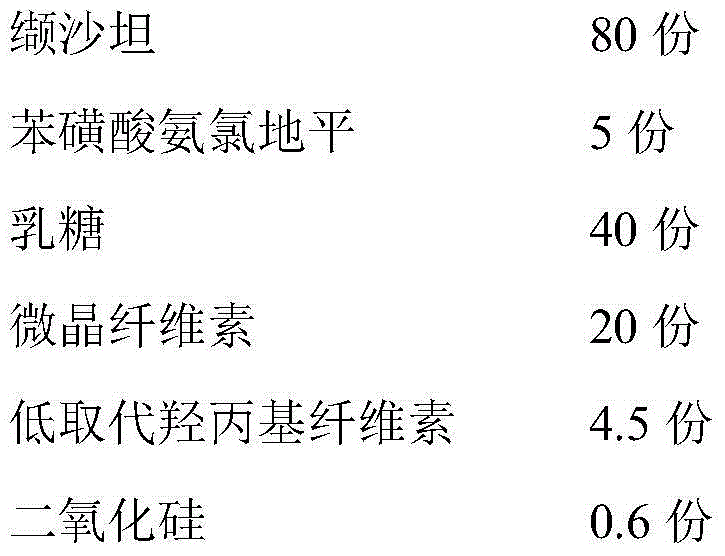
The invention discloses a valsartan amlodipine tablet and a preparation method thereof. The valsartan amlodipine tablet comprises the following substances as raw materials in parts by weight: 80 partsof valsartan, 5 parts of amlodipine besylate (counted by amlodipine), 50-100 parts of microcrystalline cellulose PH102, 8-30 parts of crospovidone XL, 0.1-10 parts of poloxamer 188, 1-5 parts of magnesium stearate, 1- 3 parts of copovidone S630, 1-5 parts of colloidal silicon dioxide and 4-10 parts of a film coating premix (gastric). Through addition of the poloxamer 188 into a medicinal auxiliary material of the valsartan amlodipine tablet, the solubility of the valsartan is improved and the retention time thereof in the gastrointestinal tract is prolonged, so that absorption is improved, and thus the bioavailability of the valsartan amlodipine tablet is improved; through addition of the copovidone S630 into a coating solution, the solubility and the bioavailability of the valsartan canbe also improved, and thus the bioavailability of the valsartan amlodipine tablet is further improved. The preparation method of the valsartan amlodipine tablet is stable, and is suitable for large-scale preparation.
Owner:BEIJING BAIAO PHARMA
Amlodipine besylate oral preparation and preparation method thereof
InactiveCN104055740AImprove stabilityPromote dissolutionOrganic active ingredientsPharmaceutical non-active ingredientsAcrylic resinMicrosphere
An amlodipine besylate oral preparation and a preparation method thereof. Microspheres are prepared from amlodipine besylate and acrylic resin E, and direct tabletting of the microspheres and pharmaceutically acceptable auxiliary materials is carried out to obtain the product.
Owner:QINGDAO UNIV
Preparation method for coating tablets containing telmisartan and amlodipine
InactiveCN102488690AOrganic active ingredientsPharmaceutical non-active ingredientsPolyethylene glycolAmlodipine besilate
The invention relates to a composition of telmisartan and benzene sulfonate amlodipine, comprising an effective dosage of telmisartan and benzene sulfonate amlodipine and selectively comprising one or a plurality of pharmaceutically acceptable additives or no; the composition is characterized in that the telmisartan and the pharmaceutically acceptable additives are used as a plain tablet, the benzene sulfonate amlodipine is dispersed in a coating material, and a coating material containing lactose, or sodium chloride, or polyethylene glycol 1500 or sorbierite is positioned between the plain tablet and the coating material. The invention has the advantages of effectively preventing the alkaline matters contained in the telmisartan plain tablet from degrading the benzene sulfonate amlodipine and quickly dissolving out the drug in an unexpected manner.
Owner:北京汇诚瑞祥医药生物科技有限公司
Valsartan amlodipine tablet composition and preparation method
The invention relates to a valsartan amlodipine tablet composition and a preparation method, an active component of the valsartan amlodipine tablet composition is valsartan and amlodipine besylate tablet , an auxiliary material comprises microcrystalline cellulose, croscarmellose sodium , hydroxypropyl methylcellulose, magnesium stearate and silica; the valsartan amlodipine tablet composition employs a wet method for granulation and tabletting; the preparation method has the advantages of little disintegrating agent , high dissolution rate , simple production technology and low equipment requirement; through detections, the tablet prepared by the valsartan amlodipine tablet composition and the preparation method has good dissolution performance.
Owner:BEIJING RED SUN PHARMA
Amlodipine besylate tablet for treating hypertension and preparation method thereof
InactiveCN106074418AStable industrialized mass productionSimple structureOrganic active ingredientsTransportation and packagingWestern medicineTyrosine
The invention relates to an amlodipine besylate tablet for treating hypertension and a preparation method thereof, and belongs to the technical field of western medicine preparation. The amlodipine besylate tablet comprises 10-20 parts of amlodipine besylate, 20-50 parts of microcrystalline cellulose, 30-70 parts of lactose, 10-20 parts of L-tyrosine, and 20-50 parts of pregelatinized starch. The materials are mixed with a special particle blender. The tablet is stable to release.
Owner:NANJING HUAKUANG INFORMATION CONSULTING CENT
Method for preparing amlodipine benzenesulfonate
ActiveCN1850801ASimple operation processHigh yieldOrganic active ingredientsOrganic chemistryOrganic solventAlcohol
This invention relates to medicine chemical field, concretely relates to benzene monsulfonic acid ammonia chlorine horizon preparation method. The ammonia chlorine horizon is reacted with benzene monsulfonic acid in component organic solvent of ester and alcohol. Its preparation method and operation technique is simple, yield is high, and product quality is easy to control.
Owner:SUZHOU DAWNRAYS PHARM CO LTD
Tablet containing olmesartan medoxomil and amlodipine and preparation method of tablet
ActiveCN103006651AIncrease dissolution rateHigh dissolution rateOrganic active ingredientsPill deliveryOlmesartanMethyl cellulose
The invention discloses a tablet containing olmesartan medoxomil and amlodipine. The tablet is prepared from amlodipine besylate solid dispersion, olmesartan medoxomil and pharmaceutic adjuvants, wherein the amlodipine besylate solid dispersion consists of amlodipine besylate and hydroxypropyl methyl cellulose according to the weight rate of 1: (7-12). The amlodipine besylate in the compound tablets can be rapidly dissolved out and absorbed by organisms; and after orally taken by hypertensive, the olmesartan medoxomil and amlodipine-containing tablet plays the roles of enhancing the synergistic hypotensive effect of two active ingredients and remarkably reducing the adverse drug reaction.
Owner:NANJING CHIA TAI TIANQING PHARMA
Preparation method of amlodipine besylate tablets
ActiveCN103356493AAppropriate disintegration timeImprove liquidityOrganic active ingredientsPharmaceutical non-active ingredientsAmlodipine MaleateAmlodipine besilate
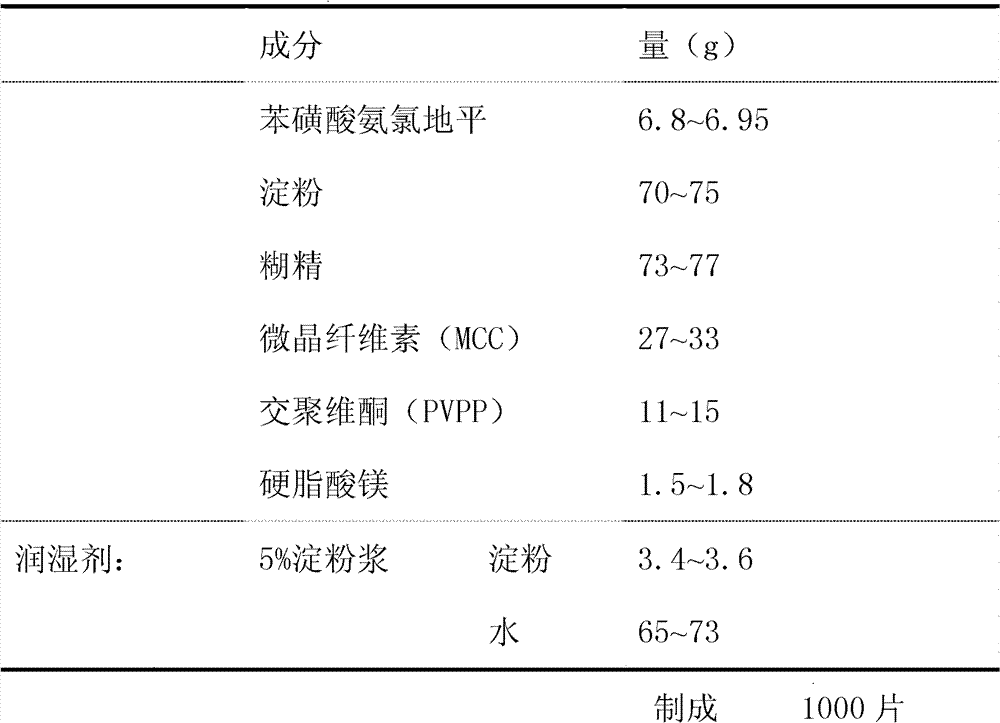
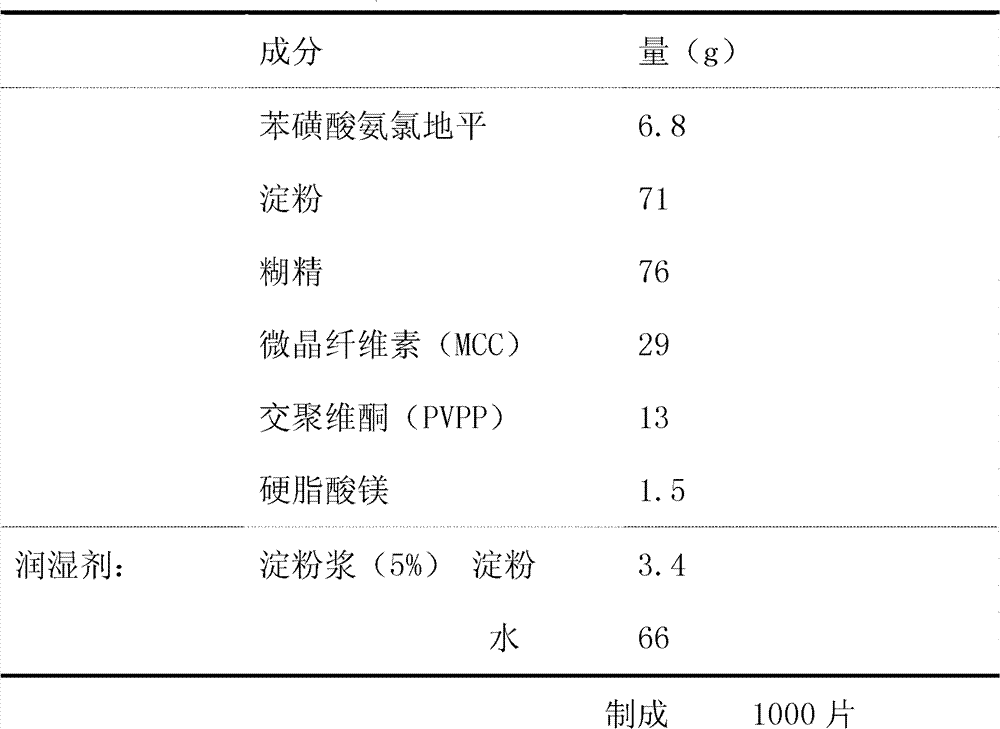
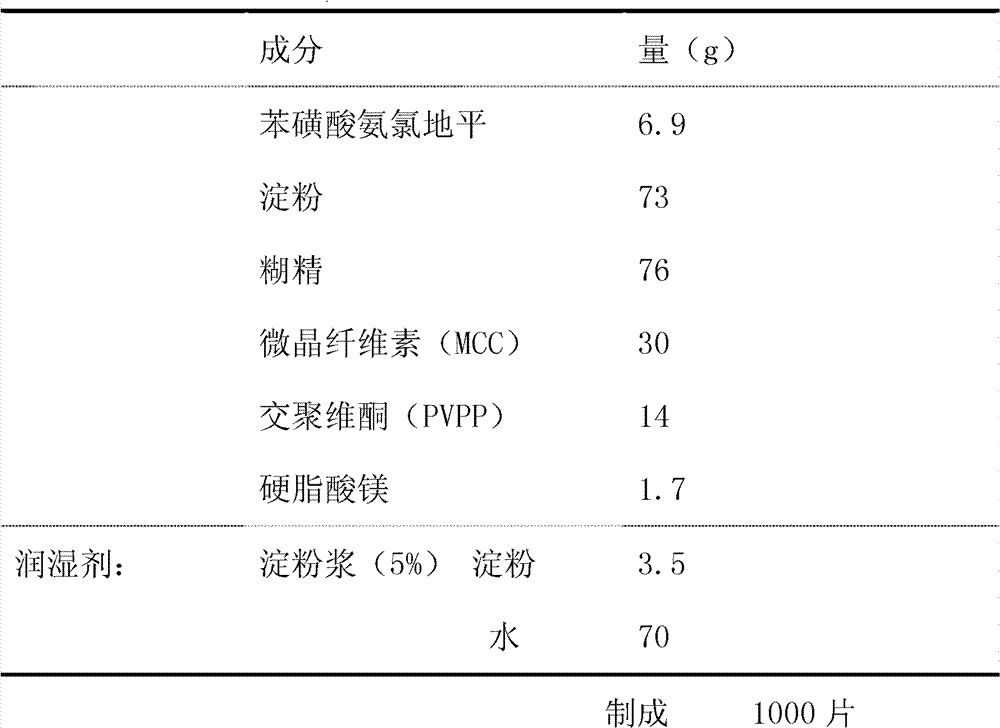
The invention provides a preparation method of amlodipine besylate tablets. The preparation method comprises the following steps of: employing wet granulation and tabletting, filtering each component in a formula through an 80-mesh sieve, and then drying the filtered components at 50 DEG C for future use; evenly mixing amlodipine besylate, microcrystalline cellulose and crospovidone together, forming a soft material from the mixture by using 5% starch slurry, granulating through an 18-mesh sieve, drying at 50 DEG C, and then shaping the granules through the 18-mesh sieve, thereby obtaining granules I; next, evenly mixing starch with dextrin, forming a soft material from the mixture by using 5% starch slurry, granulating through the 18-mesh sieve and drying at 50 DEG C, and then shaping the granules through the 18-mesh sieve, thereby obtaining granules II; finally, mixing the granules I with the granules II, adding magnesium stearate and evenly mixing all the materials, and then tableting the mixture, thus obtaining the amlodipine besylate tablets. According to the invention, the major component amlodipine besylate tablets are white crystalline powder which is good in fluidity; the prepared granules are excellent in both compressibility and fluidity. The disintegration time of the tablets is appropriate, and the content of the tablets, the content uniformity and the dissolution rate all meet the requirements; therefore, the tablets have high clinical application value. The method provided by the invention is simple and suitable for industrial production.
Owner:SHANGHAI SINE PROMOD PHARMA
Valsartan amlodipine pharmaceutical composition and preparation method thereof
InactiveCN104367574APromote dissolutionImprove stabilityPharmaceutical non-active ingredientsCoatingsValsartanMedicine
The invention relates to a valsartan amlodipine pharmaceutical composition and a preparation method thereof. The pharmaceutical composition is composed of the following formula: 5 parts by weight of amlodipine besylate, 80 parts by weight of valsartan, 20-50 parts by weight of lactose, 10-40 parts by weight of microcrystalline cellulose, 3-6 parts by weight of low substituted hydroxypropyl cellulose, 0.3-2 parts by weight of silica and 0.5-5 parts by weight of sodium stearyl fumarate. The pharmaceutical composition is prepared from a direct powder compression process. The invention adopts a small amount of disintegrating agent to reach dissolution of more than 90%, and the composition has the advantages of good stability and fast disintegration. The preparation method provided by the invention has simple production process, reduces the corresponding investment in equipment factory, and saves the production cost. The tablet produced by the direct powder compression has faster disintegration, and helps to improve the dissolution of the drug; and through test, the tablet prepared by the method of the invention has dissolution above 90% in 15 min.
Owner:JIANGXI SHIMEI PHARM CO LTD
Amlodipine besylate-containing pharmaceutical composition
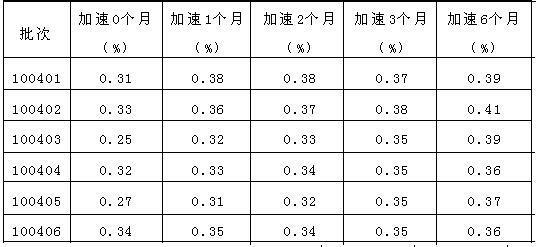
ActiveCN104415036AImprove stabilityPromote dissolutionOrganic active ingredientsPharmaceutical non-active ingredientsDrug contentMedicine
The invention belongs to the medical technicial field, and discloses an amlodipine besylate composition which comprises the following components in parts by weight: 3-6 parts by weight of amlodipine besylate, 1-1.8 parts by weight of a disintegrating agent, 65-70 parts by weight of a filler, 20-25 parts by weight of polyethylene glycol 600, and 0.05-0.1 part by weight of a lubricant. An amlodipine besylate tablet is prepared, the 5 min dissolution rate can reach up to more than 80%, the content of stability testing total impurities is less than 0.30%, the amlodipine besylate tablet having stable main drug content and high bioavailability is prepared, and besides, the preparation process has the advantages of being simple to operate, low in equipment requirement, low in production cost and suitable for industrialized production.
Owner:长春海悦药业股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com