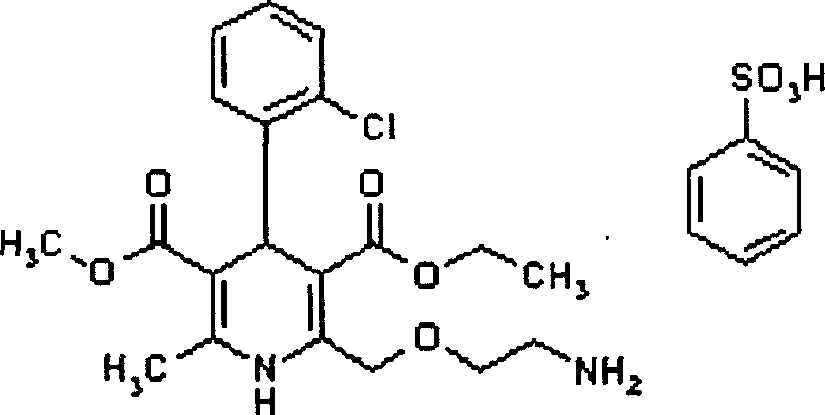
Orally disintegrating tablet of amlodipine besylate and its preparation process
A technology of amlodipine besylate and orally disintegrating tablets, which is applied in the field of amlodipine besylate orally disintegrating tablets and its preparation, can solve problems such as bad smell, easy retention in the mouth, difficulty in swallowing, etc., and achieve no gravel Sensation and unpleasant taste, simple preparation process, good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The amlodipine besylate orally disintegrating tablet comprises main ingredients and auxiliary materials, and is characterized in that it is prepared according to the following weight percentages: the main ingredient is 5.8%, and auxiliary materials are 94.2%.
[0042] The orally disintegrating tablet of the present invention is prepared from the following raw materials in weight ratio:
[0043] Amlodipine Besylate 5.8g
[0044] Lactose 44.0g
[0045] Mannitol 17.0g
[0046] Microcrystalline Cellulose 21.8g
[0047] Low-substituted hydroxypropyl cellulose 2.4g
[0048] Cross-linked polyvinylpyrrolidone 5.0g
[0049] Sodium bicarbonate 1.0g
[0050] Citric acid 1.0g
[0051] Aspartame 0.5g
[0052] Mint essence 0.5g
[0053] Magnesium Stearate 1.0g
[0054] A total of 1000 tablets were produced
[0055] Among them, lactose and mannitol are fillers; low-substituted hydroxypropyl cellulose, microcrystalline cellulose and cross-linked polyvinylpyrrolidone are disin...
Embodiment 2
[0065] The amlodipine besylate orally disintegrating tablet comprises main ingredients and auxiliary materials, and is characterized in that it is prepared according to the following weight percentages: the main ingredient is 5.8%, and auxiliary materials are 94.2%.
[0066] The orally disintegrating tablet of the present invention is prepared from the following raw materials in weight ratio:
[0067] Amlodipine Besylate 5.8g
[0068] Mannitol 60.0g
[0069] Microcrystalline Cellulose 21.6g
[0070] Low-substituted hydroxypropyl cellulose 2.4g
[0071] Cross-linked polyvinylpyrrolidone 5.0g
[0072] Sodium bicarbonate 1.0g
[0073] Citric acid 1.0g
[0074] Aspartame 0.5g
[0075] Mint essence 0.25g
[0076] Cherry essence 0.75g
[0077] Magnesium Stearate 1.0g
[0078] A total of 1000 tablets were produced
[0079] Among them, lactose and mannitol are fillers. Low-substituted hydroxypropyl cellulose, microcrystalline cellulose and cross-linked polyvinylpyrrolidone ...
Embodiment 3
[0091] The amlodipine besylate orally disintegrating tablet comprises main ingredients and auxiliary materials, and is characterized in that it is formulated according to the following weight percentages: 10% of main ingredients and 90% of auxiliary materials.
[0092] The orally disintegrating tablet of the present invention is prepared from the following raw materials in weight ratio:
[0093] Amlodipine Besylate 10.0g
[0094] Lactose 40.0g
[0095] Mannitol 20.0g
[0096] Microcrystalline Cellulose 20.0g
[0097] Low-substituted hydroxypropyl cellulose 4.0g
[0098] Cross-linked polyvinylpyrrolidone 5.0g
[0099] Sodium bicarbonate 1.0g
[0100] Citric acid 1.0g
[0101] Aspartame 0.5g
[0102] Mint essence 0.25g
[0103] Cherry essence 0.75g
[0104] Magnesium Stearate 1.0g
[0105] A total of 1000 tablets were produced
[0106] Among them, lactose and mannitol are fillers. Low-substituted hydroxypropyl cellulose, microcrystalline cellulose and cross-linked po...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com