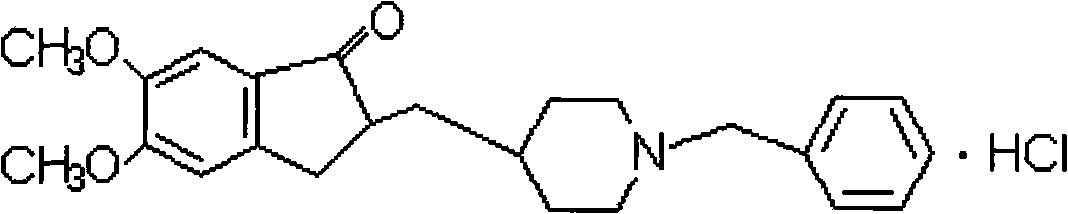
Donepezil hydrochloride orally disintegrating tablet and preparation method thereof
A technology of donepezil hydrochloride and oral disintegrating tablets, which is applied in the directions of pharmaceutical formulations, medical preparations of inactive ingredients, and pill delivery, etc., can solve the problem of poor compliance of the elderly with dementia, delaying the best time for treatment, and inconvenience in use. and other problems, to achieve the effect of alleviating gastrointestinal discomfort, less adverse reactions, and no unpleasant taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The orally disintegrating tablet of donepezil hydrochloride includes main ingredients and auxiliary materials, and is characterized in that it is prepared according to the following weight percentages: main ingredients 2.5%, auxiliary materials 97.5%.
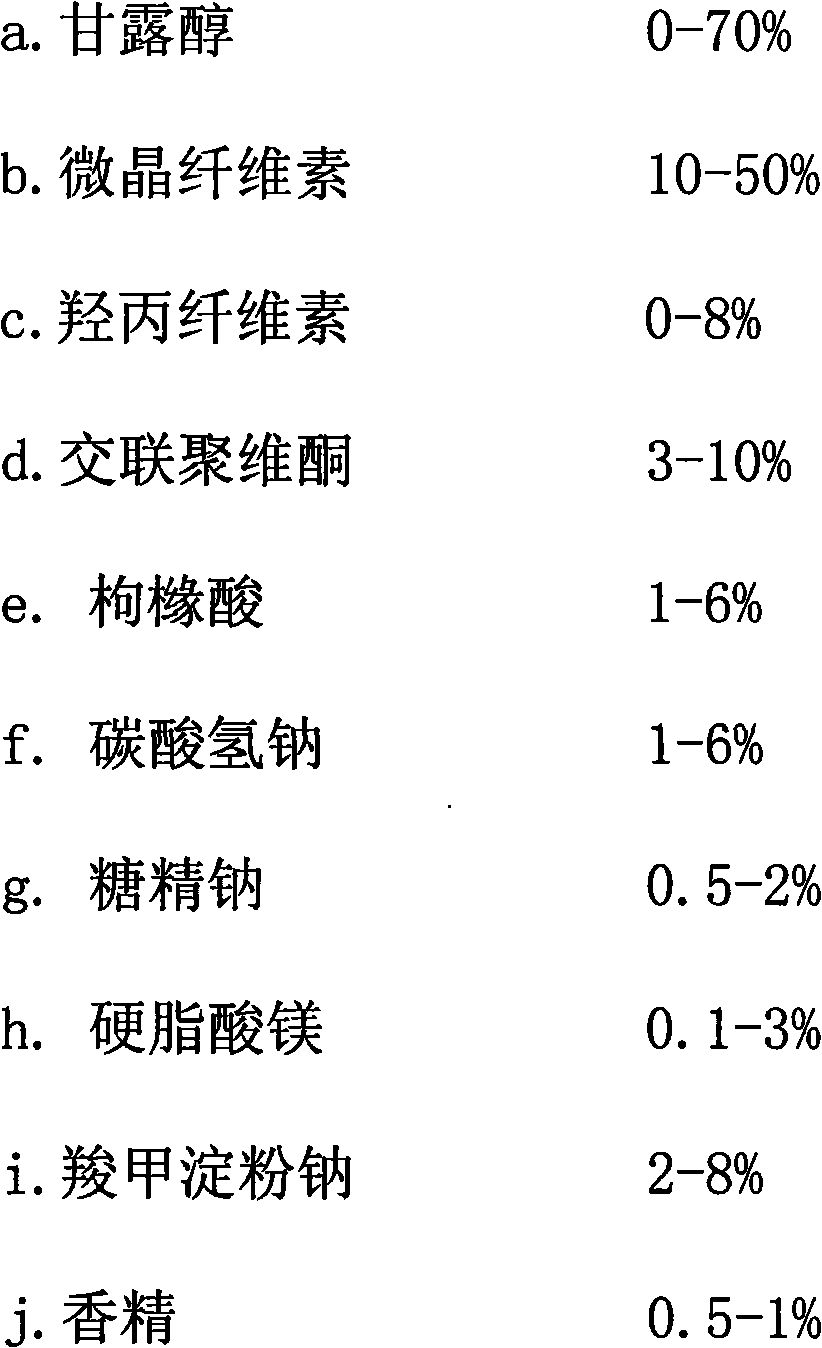
[0032] The orally disintegrating tablet of the present invention is prepared from the following raw materials in weight ratio:
[0033]
[0034]
[0035] Among them, mannitol and microcrystalline cellulose are fillers; hydroxypropyl cellulose, crospovidone and sodium starch glycolate are disintegrants; citric acid and sodium bicarbonate are effervescent agents; magnesium stearate is Lubricants; Sodium saccharin, fruit flavors are flavoring agents.
[0036] The preparation method of donepezil hydrochloride orally disintegrating tablets of the present invention comprises the steps of pulverizing raw materials, weighing and mixing, and tableting, and is characterized in that the specific process steps are as follows: ...
example 2
[0044] The orally disintegrating tablet of donepezil hydrochloride includes main ingredients and auxiliary materials, and is characterized in that it is prepared according to the following weight percentages: main ingredients 2.5%, auxiliary materials 97.5%.
[0045]
[0046] Among them, mannitol and microcrystalline cellulose are fillers; hydroxypropyl cellulose, crospovidone and sodium starch glycolate are disintegrants; citric acid and sodium bicarbonate are effervescent agents; magnesium stearate is Lubricants; Sodium saccharin, fruit flavors are flavoring agents.
[0047] The preparation method of the orally disintegrating cetirizine hydrochloride tablet of the present invention comprises pulverizing raw materials, weighing and mixing, granulating and tabletting, and is characterized in that the specific process steps are as follows:
[0048] Step 1: Grinding donepezil hydrochloride and the above-mentioned various auxiliary materials respectively, then passing through ...
example 3
[0057] The orally disintegrating tablet of donepezil hydrochloride comprises main ingredients and auxiliary materials, and is characterized in that it is formulated according to the following weight percentages: 5% of main ingredients and 95% of auxiliary materials.
[0058]
[0059] Among them, mannitol and microcrystalline cellulose are fillers; hydroxypropyl cellulose, crospovidone and sodium starch glycolate are disintegrants; citric acid and sodium bicarbonate are effervescent agents; magnesium stearate is Lubricants; Sodium saccharin, fruit flavors are flavoring agents.
[0060] The preparation method of the donepezil hydrochloride orally disintegrating tablet of the present invention comprises pulverizing raw materials, weighing and mixing, granulating and tableting, and is characterized in that the specific process steps are as follows:
[0061] Step 1: Grinding donepezil hydrochloride and the above-mentioned various auxiliary materials respectively, then passing th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com