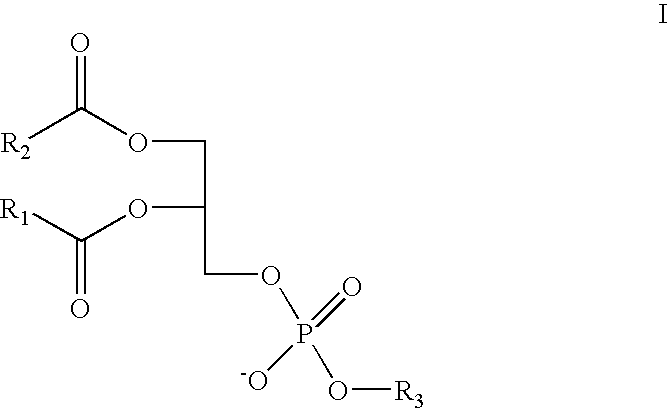
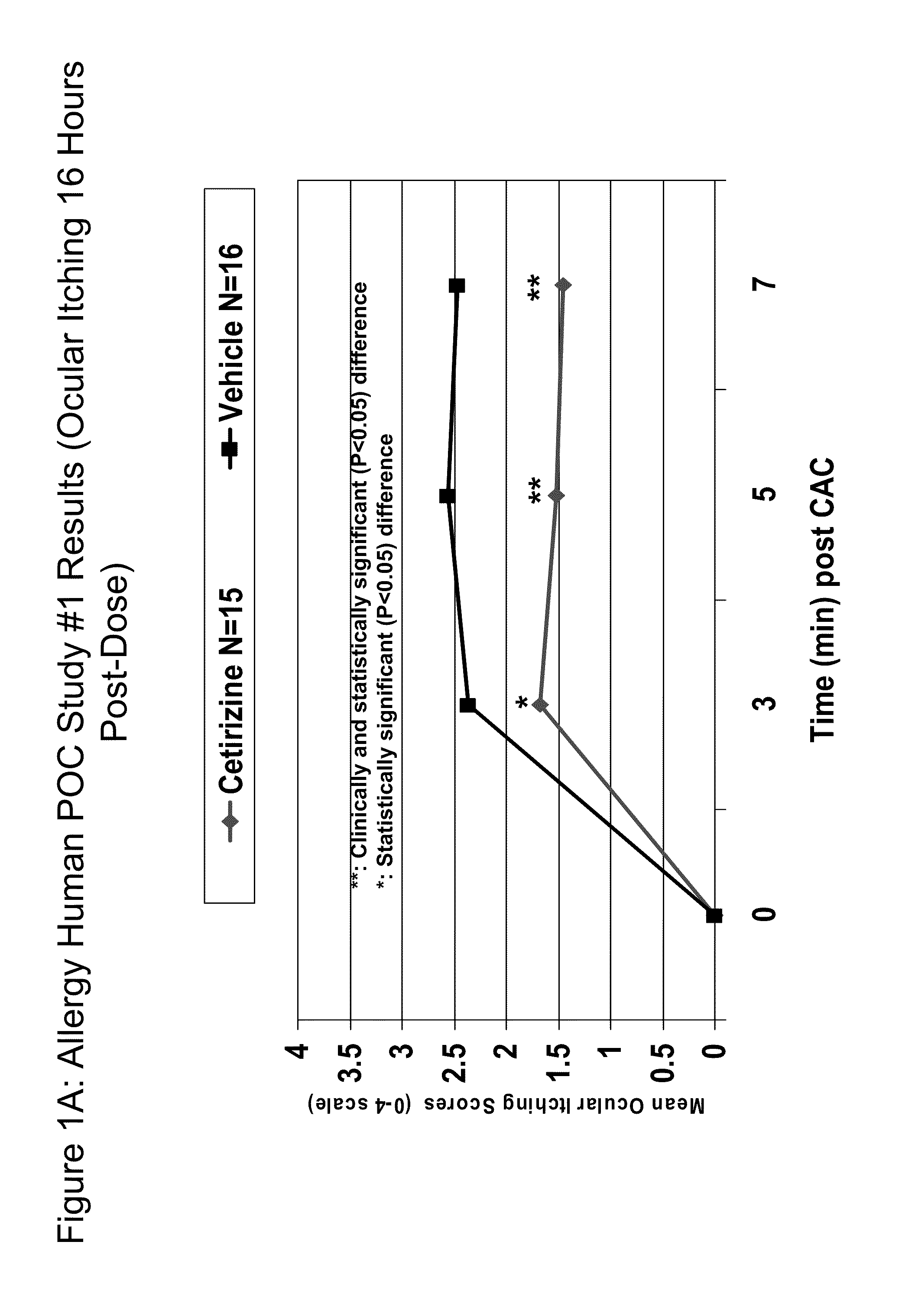
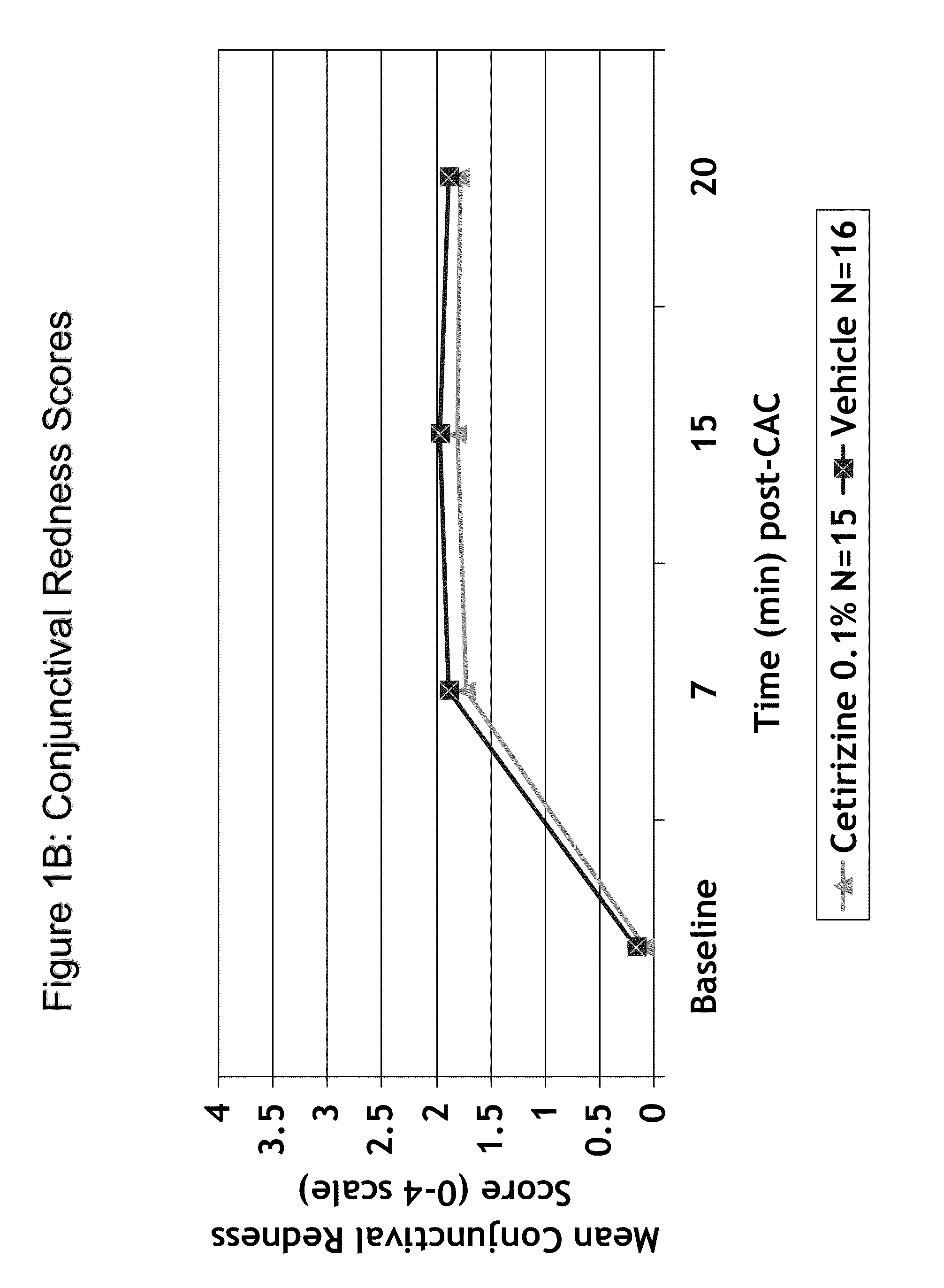
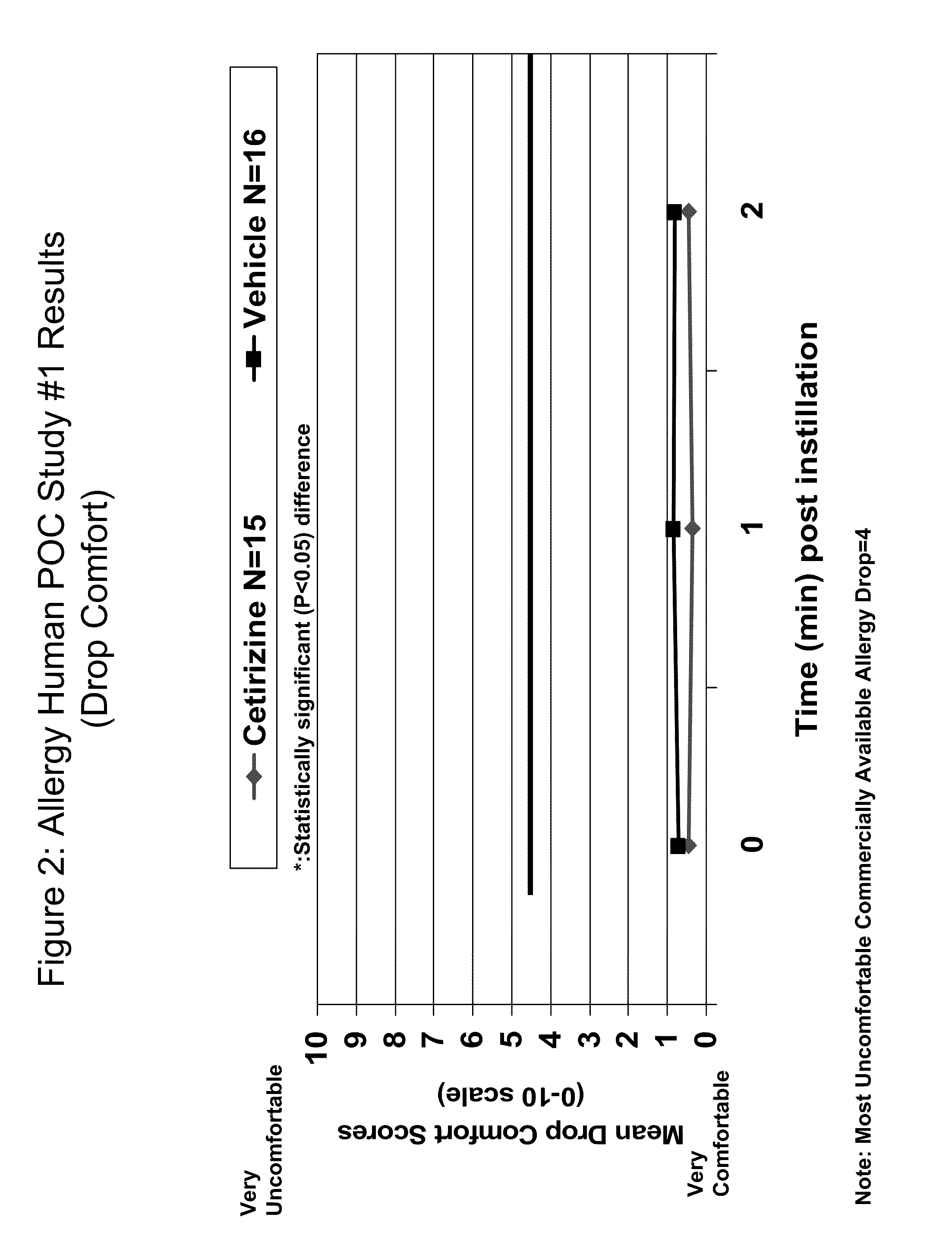
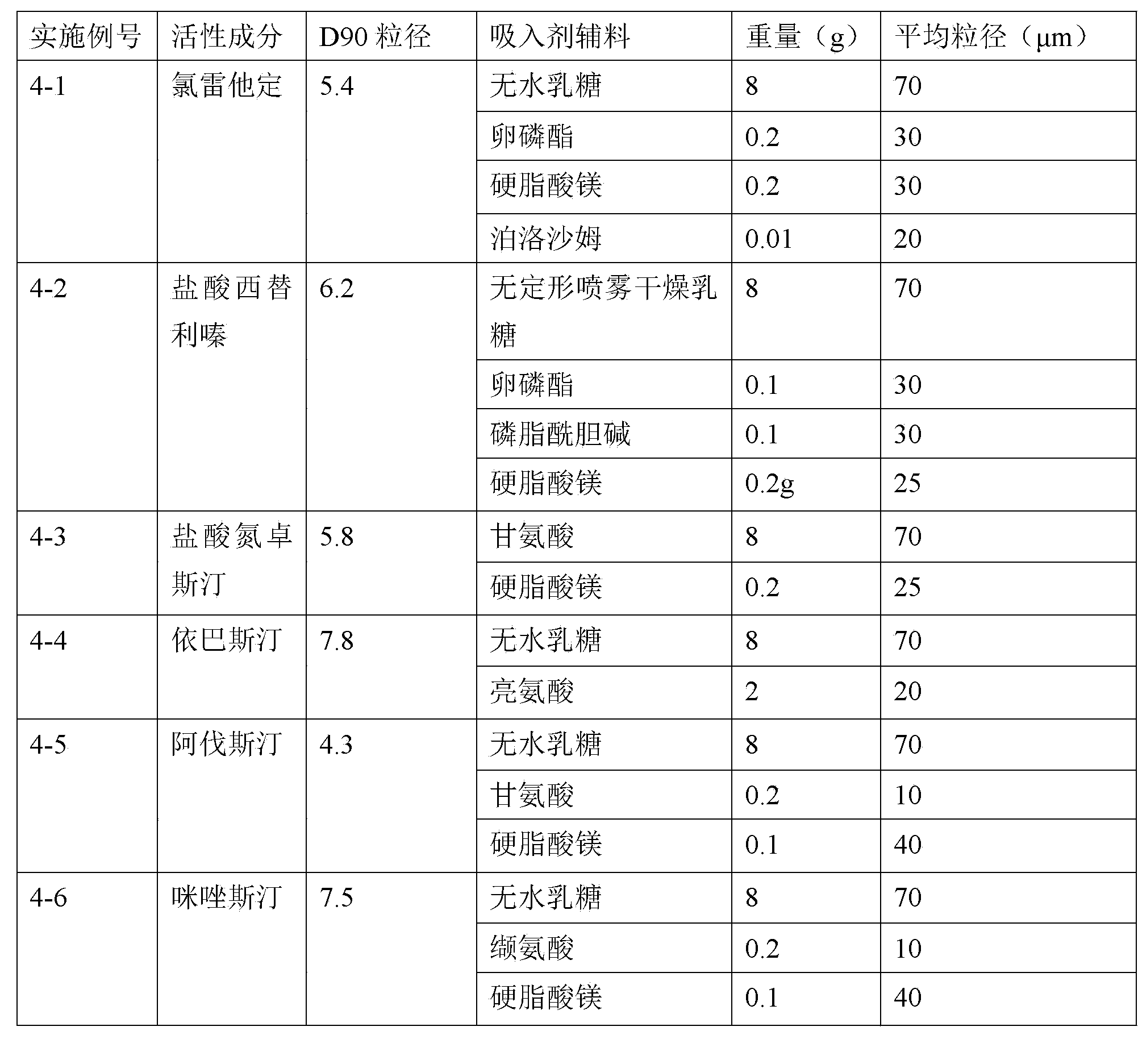
Patents
Literature
83 results about "Cetirizine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cetirizine is an antihistamine used to relieve allergy symptoms such as watery eyes, runny nose, itching eyes/nose, sneezing, hives, and itching.
Combination dosage form comprising cetirizine and pseudoephedrine
A dosage form containing cetirizine as an immediate release component and pseudoephedrine or a pharmaceutically acceptable salt thereof as a controlled release component. A portion of the pseudoephedrine can also be incorporated as an immediate release component. The dosage form is free of alcohols having a molecular weight lower than 100 and reactive derivatives thereof.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Oral pharmaceutical composition
InactiveUS20060198885A1Reduce bitternessOrganic active ingredientsPill deliveryAlkaline earth oxidesCetirizine
An oral pharmaceutical composition having reduced bitterness comprising therapeutically effective amounts of cetirizine or its pharmaceutically acceptable salts, alkaline earth oxide and a pharmaceutically acceptable carrier comprising a disintegrant wherein the composition disintegrates rapidly in the oral cavity.
Owner:SUN PHARMA INDS
Methods and compositions using optically pure (-) cetirizine in combination with leukotriene inhibitors or decongestants
InactiveUS6790849B2Potent antihistaminic activityAvoid it happening againRespiratory disorderOptically-active compound separationDecongestantCetirizine
Methods and pharmaceutical compositions employing (+) cetirizine, (-) cetirizine, or racemic cetirizine, or a pharmaceutically acceptable salt thereof, and a leukotriene inhibitor, or a pharmaceutically acceptable salt thereof, or decongestant for the treatment, management, and / or prevention of inflammation, asthma or symptoms thereof, allergic disorders such as allergic rhinitis, and dermatitis.
Owner:SUNOVION PHARMA INC
Cetirizine molecularly imprinted polymer monolithic column and preparation method thereof
InactiveCN104209104AEasy to makeSimple experimentOther chemical processesSolid sorbent liquid separationTetrafluoroborateGlycol synthesis
The invention relates to a cetirizine molecularly imprinted polymer monolithic column and a preparation method thereof. The cetirizine molecularly imprinted polymer monolithic column is composed of raw materials of, by mass, 1-3% of cetirizine, 3-4% of 4-vinyl pyridine, 20-25% of ethylene gylcol dimethacrylate, 42-45% of mixed solution (eutectic solvent) of ethylene glycol and choline chloride, 4-5% of dimethylformamide, 13-22% of 1-butyl-3-methylimidazolium tetrafluoro borate and 0.5-1% of azobisisobutyronitrile. The method includes the steps of adding ionic liquid and the eutectic solvent of ethylene glycol and choline chloride to a polymerization system, using cobalt ions as a metal ion hub to enhance the effect of imprinting recognition, and preparing molecularly imprinted polymer (MIP) of a successive rod shape in a stainless steel column. The cetirizine molecularly imprinted polymer (MIP) monolithic column obtained through the preparation method has the advantages of good permeability and obvious imprinting effects and can have the model cetirizine imprinting factors up to 31.54, and meanwhile, the method is simple in preparation process and avoids the use of volatile liquid, thereby reducing harmful gas emission to the environment.
Owner:TIANJIN MEDICAL UNIV
Stable medicated chewing gum comprising cyclodextrin inclusion complex
InactiveUS20130022652A1Increase flexibilityOrganic active ingredientsSenses disorderMedicated chewing-gumCyclodextrin
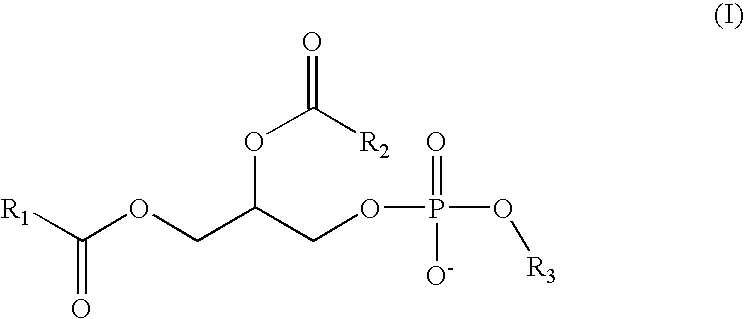
The present invention provides stable medicament-containing chewing gum compositions comprising an inclusion complex comprising cyclodextrin and one or more active compound(s) according to formula I, such as cetirizine, and methods for preparing such chewing gum.
Owner:FERTIN PHARMA AS
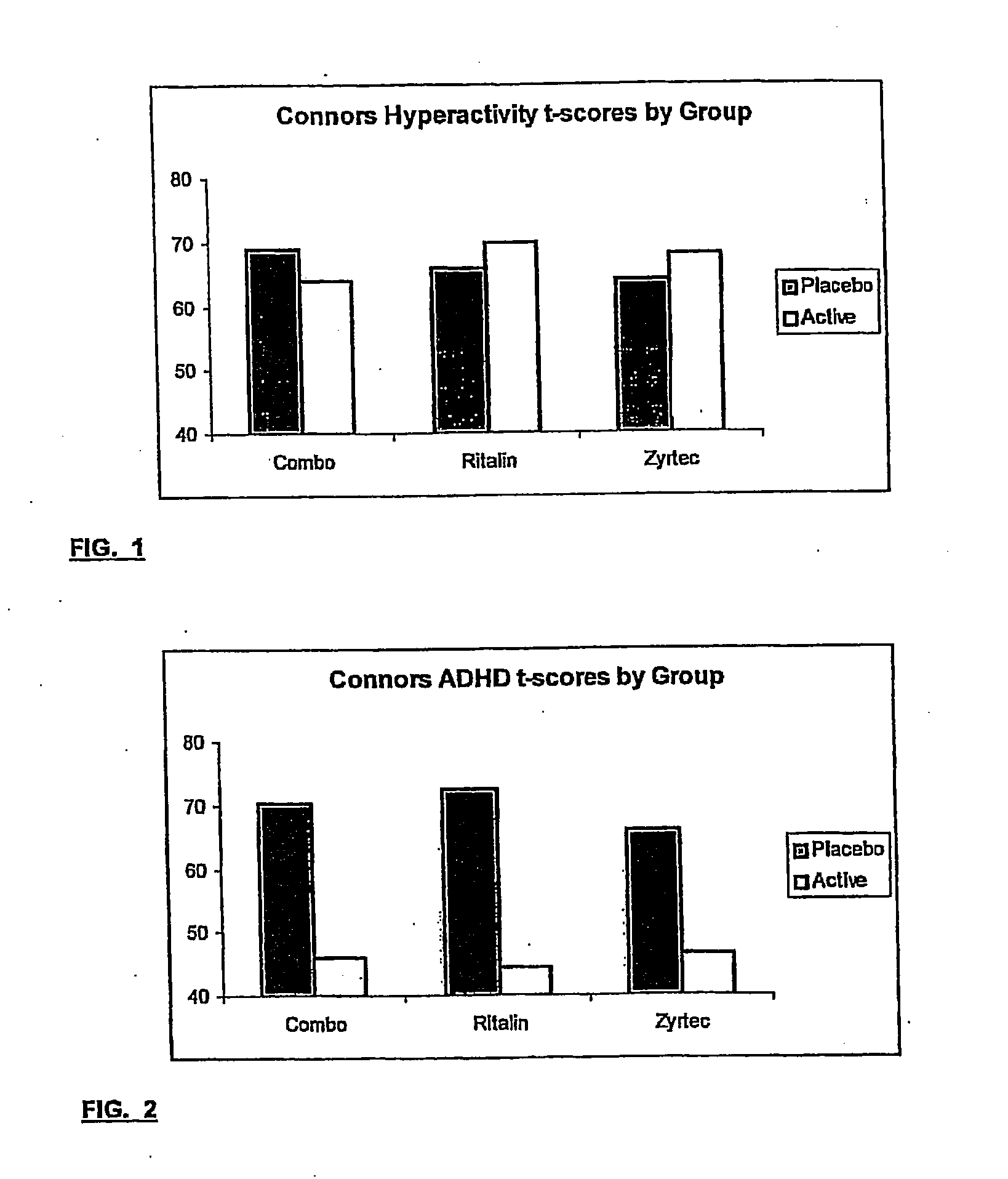
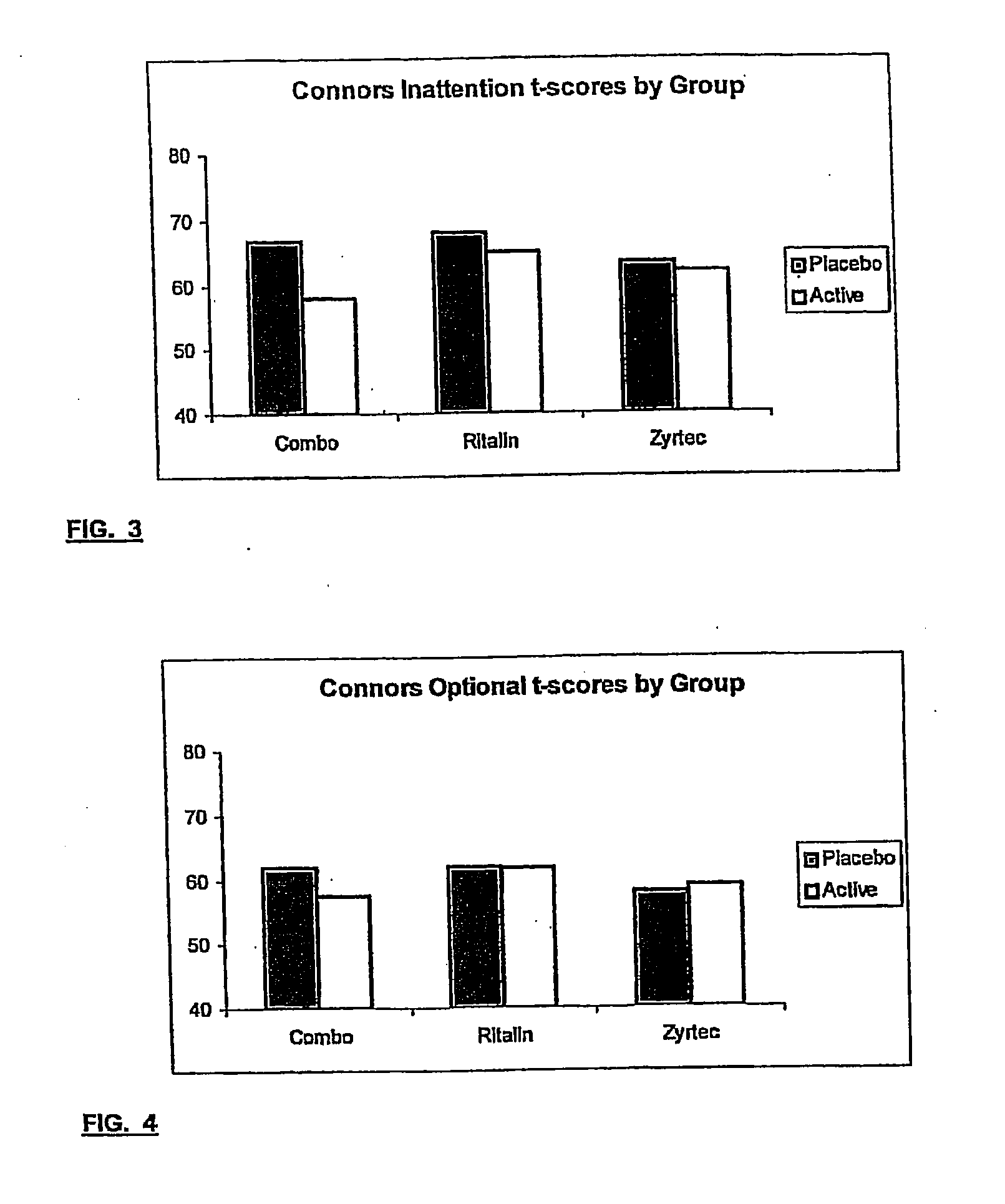
Treatment of behavioral disorders
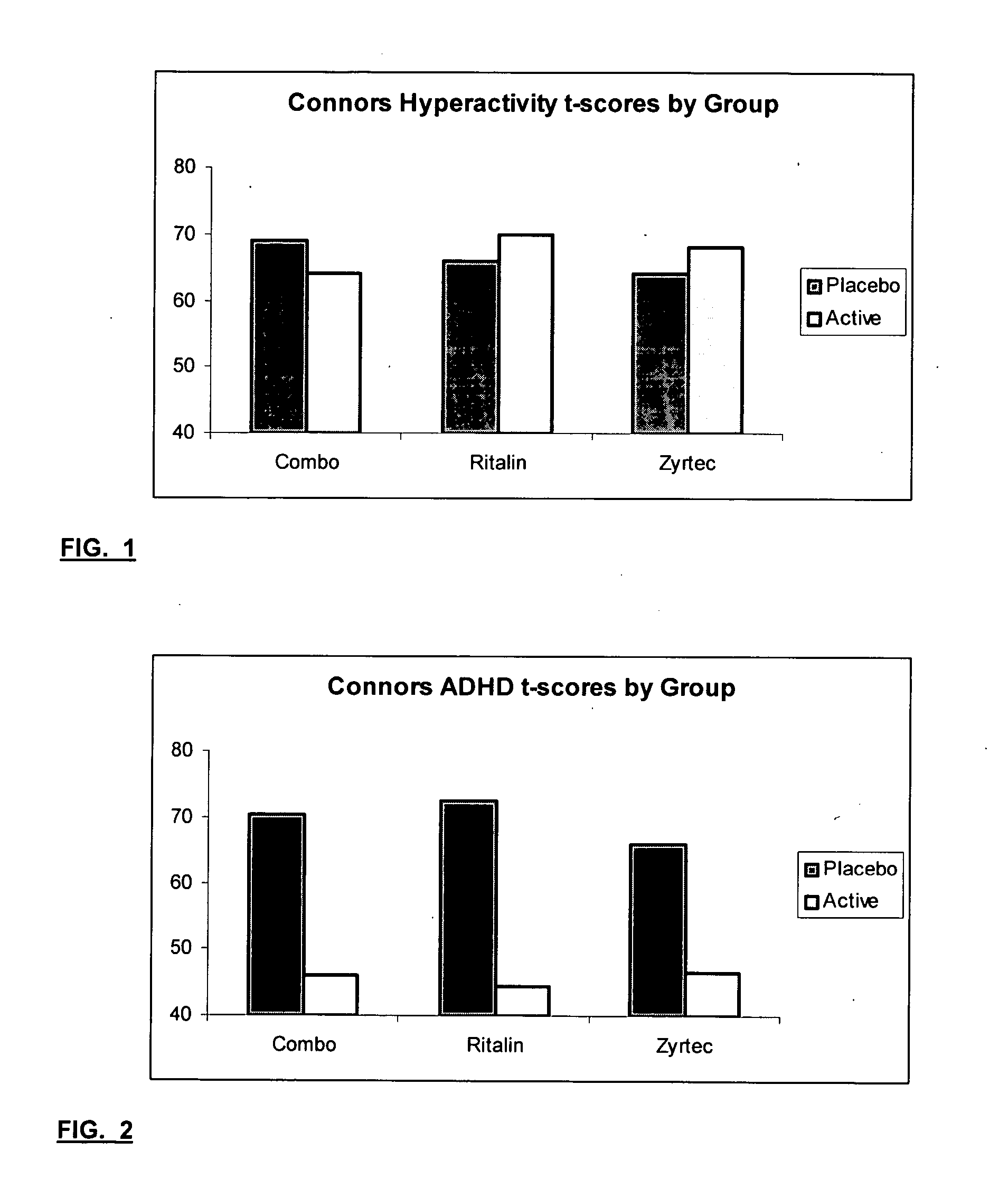
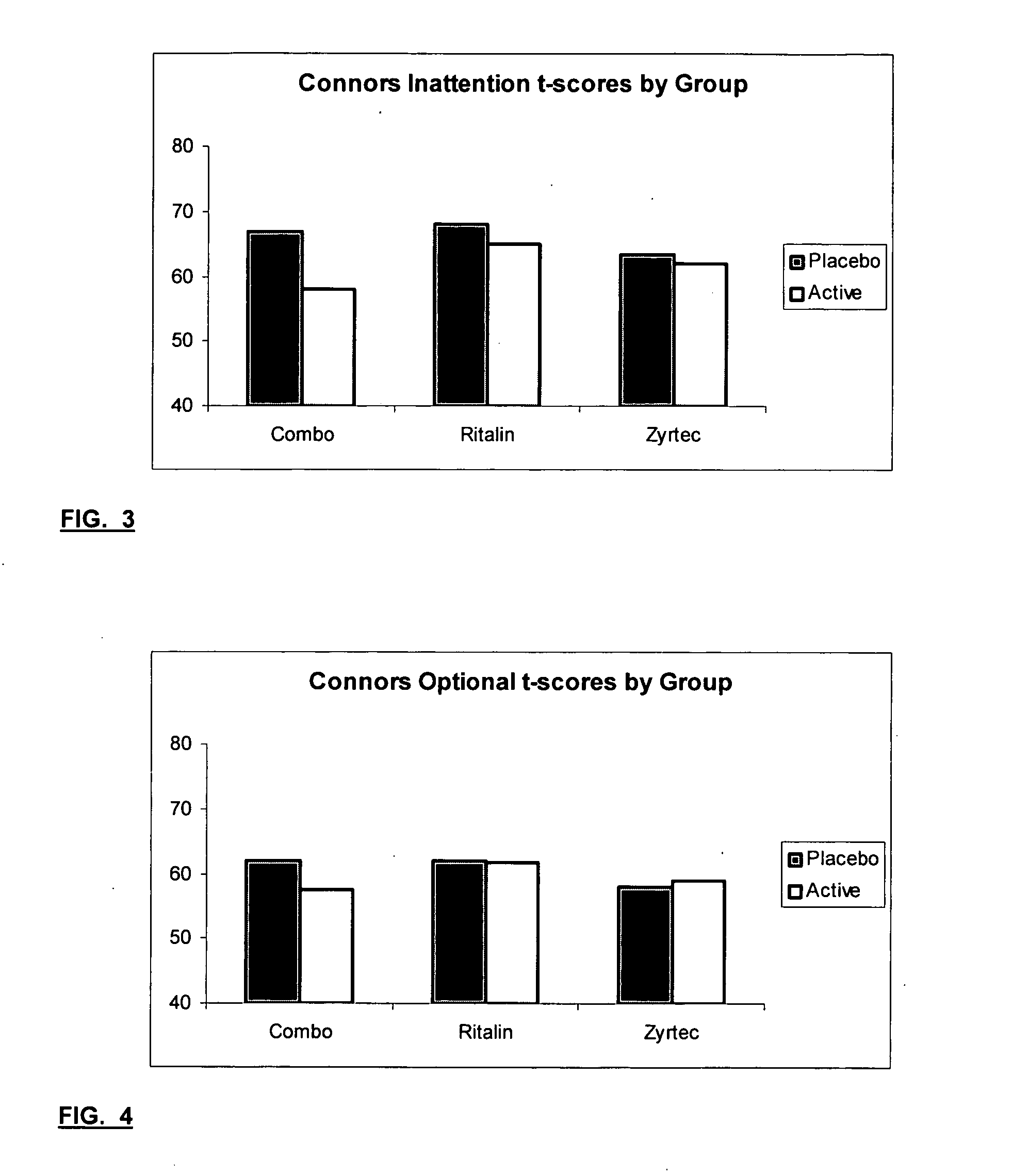
InactiveUS20050192290A1Ameliorate behavioral disorderSufficient amountBiocideNervous disorderTherapeutic ACTHFexofenadine
The present invention relates to a method for treating a behavior disorder comprising the administration of a therapeutically effective amount of antihistamine, such as ceterizine, fexofenadine; loratadine, and desloratadine. The behavioral disorders may include ADHD, anxieity, depression, and autism. The method may include the administration of the antihistamine in combination with a stimulant medication, such as methylphenidate, thereby to achieve a synergistic effect. In any event, the amount of antihistamine and / or stimulant is effective to downregulate neurotrophic factors such as nerve growth factor or CD40. The invention is also directed to a method of preventing the onset of behavior disorders in patients presenting with symptoms of allergic rhinitis.
Owner:MELAMED ISAAC
Cetirizine compositions
InactiveUS20070086974A1Organic active ingredientsIon-exchanger regenerationDiphenylmethylpiperazineOrganic chemistry
Owner:DR REDDYS LAB LTD +1
Method and composition for treating rhinitis
There is provided pharmaceutical compositions for the treatment of rhinitis by, for example, nasal or ocular administration comprising zwitterionic cetirizine, a polar lipid liposome and a pharmaceutical-acceptable aqueous carrier. The compositions are preferably homogeneous in their nature.
Owner:BIOLIPOX AB
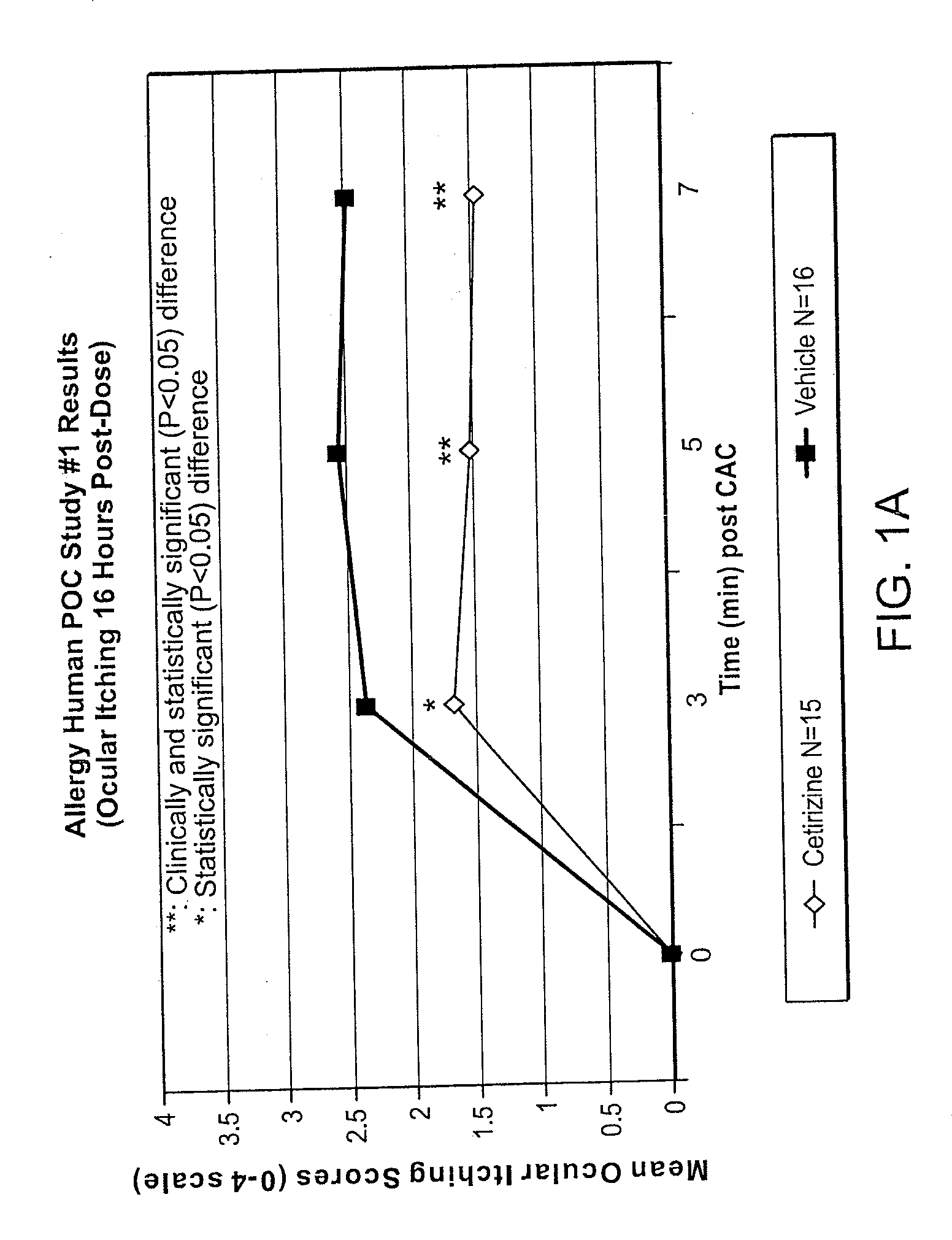
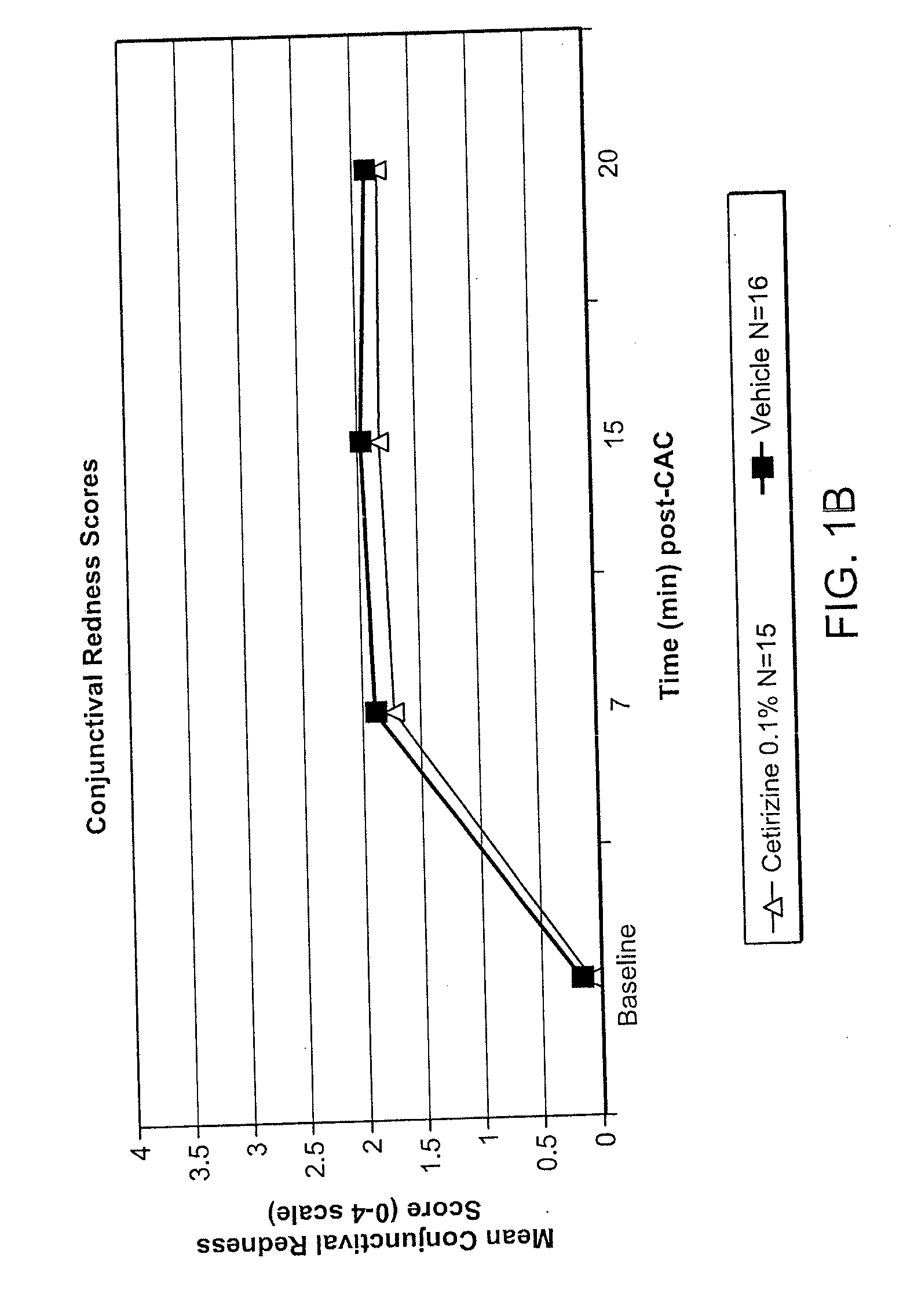
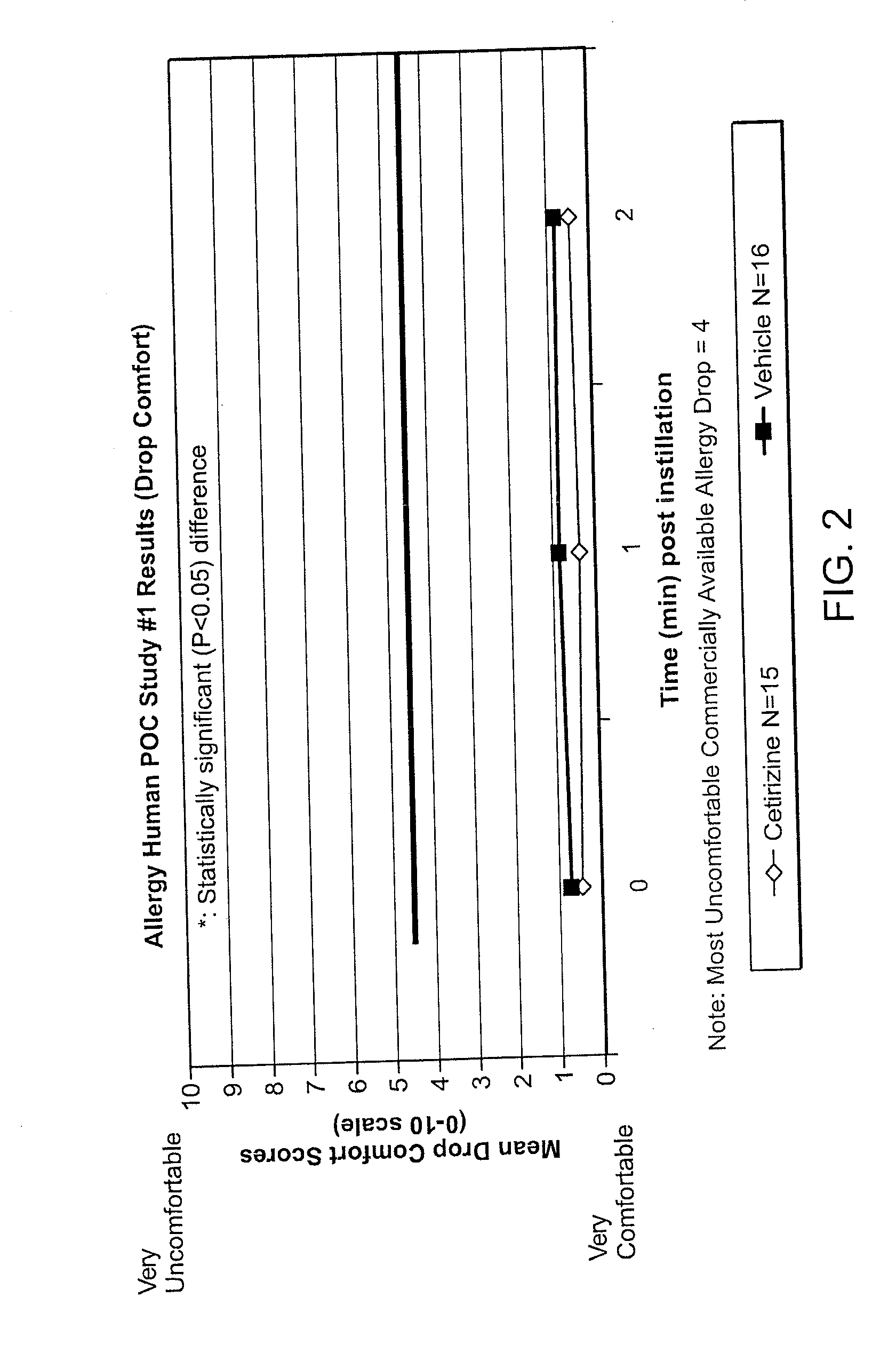
Ophthalmic Formulations of Cetirizine and Methods of Use
ActiveUS20100240625A1Relieve symptomsEffectively masks itchingBiocideOrganic active ingredientsAllergic conjunctivitisCetirizine
The present invention provides stable topical formulations of cetirizine that provide a comfortable formulation when instilled in the eye and is effective in the treatment of allergic conjunctivitis and / or allergic conjunctivitis. The invention further provides methods of treating allergic conjunctivitis and / or allergic rhinoconjunctivitis in a subject in need of such treatment by topical application of the cetirizine formulations of the invention directly to the eye.
Owner:NICOX OPHTHALMICS
Anti-histamine compositions and use thereof
ActiveUS20090048268A1Extended shelf lifeComposition is stableBiocideDispersion deliveryOral medicationMedicine
The present invention provides for a storage stable pharmaceutical liquid solution for oral administration having a pharmaceutically effective amount of an antihistamine and having a purity equal to or greater than about 99% by weight-based HPLC assay, residual solvents of less than about 0.5%, and a total impurity of less than about 0.2%. The storage stable solution preferably contains cetirizine. The present invention further provides a process of preparing the storage stable pharmaceutical liquid solution as well as a method of treating a mammal with a therapeutically effective amount of cetirizine in the stable pharmaceutical liquid solution.
Owner:TARO PHARMA INDS
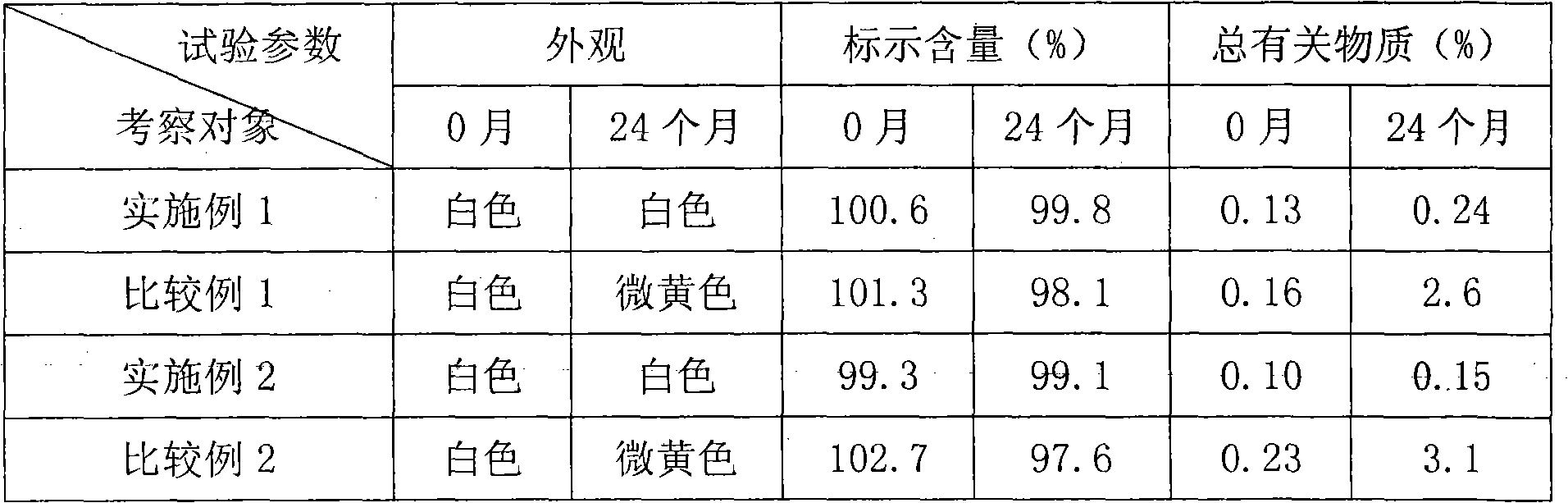
Medicine combination capable of improving cetirizine preparation stability
InactiveCN101491524AImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsMedicineCetirizine
The invention discloses an oral preparation composition prepared by being matched with an alkaline compound taken as a stabilizing agent in cetirizine. The stability of an oral preparation added with the stabilizing agent is greatly improved.
Owner:海南高升医药科技开发股份有限公司
Treatment of Behavioral Disorders
The present invention relates to a method for treating a behavior disorder comprising the administration of a therapeutically effective amount of antihistamine, such as cetirizine, fexofenadine; loratadine, and desloratadine. The behavioral disorders may include ADHD, anxiety, depression, and autism. The method may include the administration of the antihistamine in combination with a stimulant medication, such as methylphenidate, thereby to achieve a synergistic effect. In any event, the amount of antihistamine and / or stimulant is effective to downregulate neurotrophic factors such as nerve growth factor or CD40. The invention is also directed to a method of preventing the onset of behavior disorders in patients presenting with symptoms of allergic rhinitis.
Owner:MELAMED ISAAC
Ophthalmic Formulations Of Cetirizine And Methods Of Use
ActiveUS20110257136A1Alleviate and reduce and systemic exposureStable, comfortable, efficacious and safeBiocideOrganic active ingredientsAllergic conjunctivitisCetirizine
The present invention provides stable topical formulations of cetirizine that provide a comfortable formulation when instilled in the eye and is effective in the treatment of allergic conjunctivitis and / or allergic conjunctivitis. The invention further provides methods of treating allergic conjunctivitis rhinitis, and / or allergic rhinoconjunctivitis in a subject in need of such treatment by topical application of the cetirizine formulations of the invention directly to the eye.
Owner:NICOX OPHTHALMICS
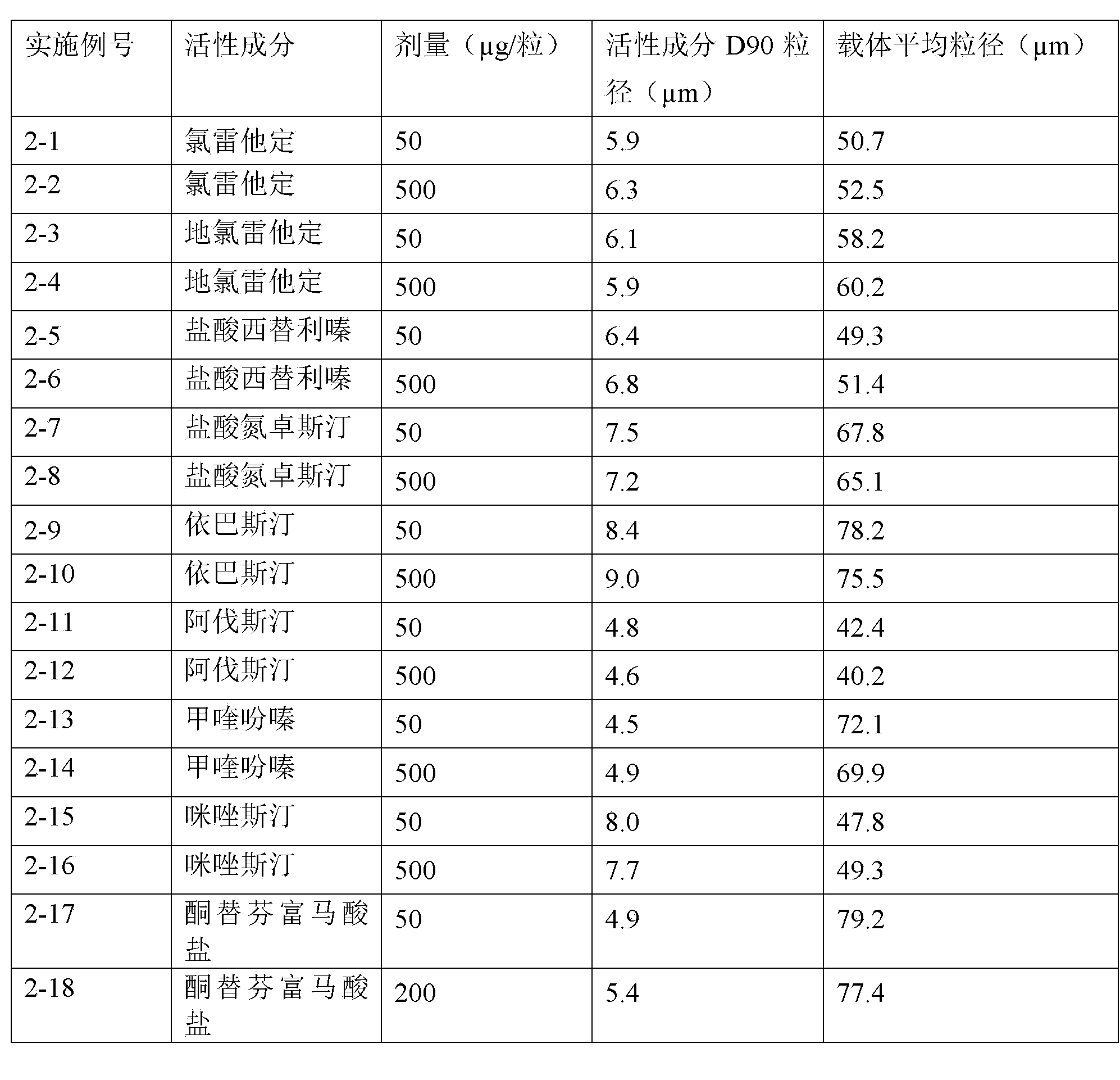
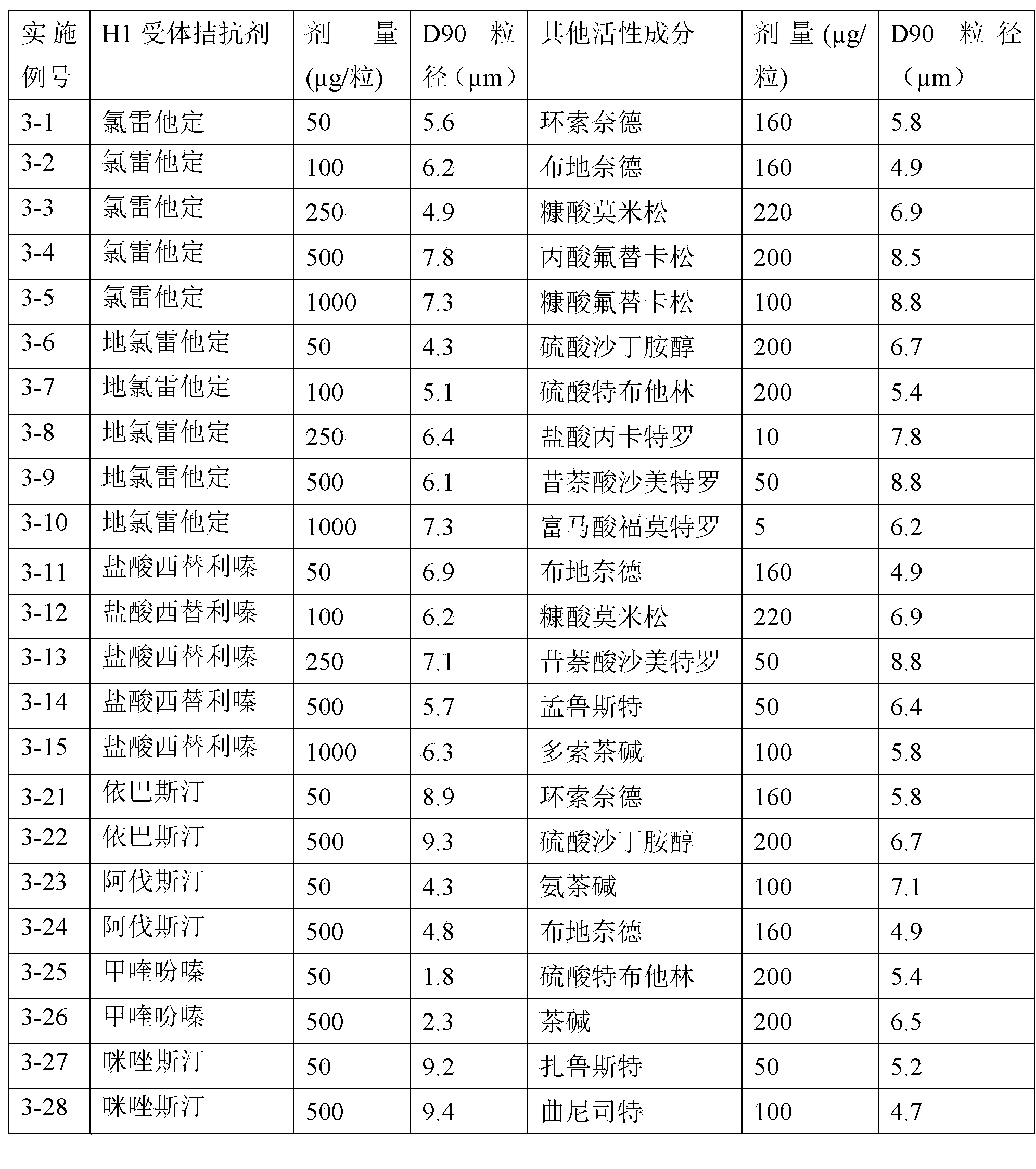
H1-receptor-antagonist-containing inhalation preparation
The invention relates to an H1-receptor-antagonist-containing inhalation preparation which contains an H1 receptor antagonist and one or more pharmaceutical auxiliary materials suitable for inhalation administration. The H1 receptor antagonist is one or more of loratadine, desloratadine, cetirizine, levocetirizine, astemizole, ketotifen, ebastine, fexofenadine, avastin, mequitazine, mizolastine and salts thereof, and preferably one or more of loratadine, desloratadine, cetirizine, levocetirizine, ebastine, mizolastine, avastin, mequitazine, ketotifen and hydrochlorides or fumarates thereof.
Owner:TIANJIN JINYAO GRP
Bis-(6-oxo-m-nitrobenzaldehyde sulfonyl)-beta-cyclodextrin as well as preparation method and application thereof
ActiveCN103965376AGood split effectMaterial analysis by electric/magnetic meansElectrophoretic processesOfloxacinCetirizine
The invention relates to the technical field of preparation and application of a chiral selector, and particularly discloses a bis-(6-oxo-m-nitrobenzaldehyde sulfonyl)-beta-cyclodextrin (beta-CD-N2) as well as a preparation method and an application of the beta-CD-N2 serving as the chiral selector in high-performance capillary electrophoresis, namely the application of the beta-CD-N2 in preparation of a chiral electrophoresis monolithic column for separating chiral materials. The preparation method is characterized in that firstly a synthesis method of the beta-CD-N2 is confirmed, the derivative is characterized by using means such as ultraviolet, infrared, photoelectron spectroscopy, nuclear magnetic resonance, mass spectrometry and elemental analysis, after comprehensive analysis, the molecular formula of the derivative is identified as C54H76O43N2S2, and the derivative is identified as a target derivative and is not reported before. The beta-CD-N2 is used for preparing a chiral HPCE (high-performance capillary electrophoresis) monolithic column and separating the chiral materials such as cetirizine, mexiletine, propafenone, ofloxacin and bupivacaine and can realize baseline separation, and accordingly, a novel method for quantitative determination of HPCE of a multi-chiral material single enantiomer can be established.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Use of Dexibuprofen and Levocetirizine Sustained-release Double-Layer Tablets in the Treatment of Airway Inflammation
InactiveCN102258519ARelieve inflammatory swellingRelieve nasal inflammationOrganic active ingredientsAntipyreticSustained Release TabletMechanism of action
The present invention relates to a use of a dexibuprofen levocetirizine double-layer sustained release tablet in treatment of airway inflammation. The tablet is composed of two layers, wherein one layer is the rapid-release part, composed of dexibuprofen, levocetirizine dihydrochloride and a pharmaceutically acceptable rapid-release additive; the other layer is the sustained-release part, composed of dexibuprofen and a pharmaceutically acceptable sustained-release additive.
Owner:XIAN LIJUN PHARMA CO LTD
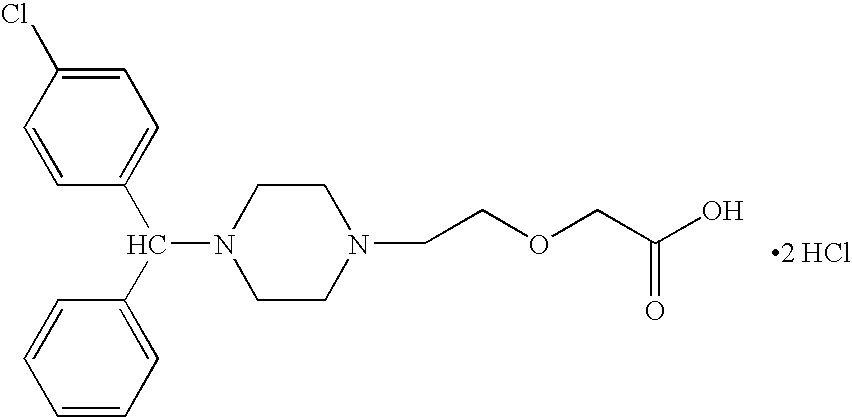
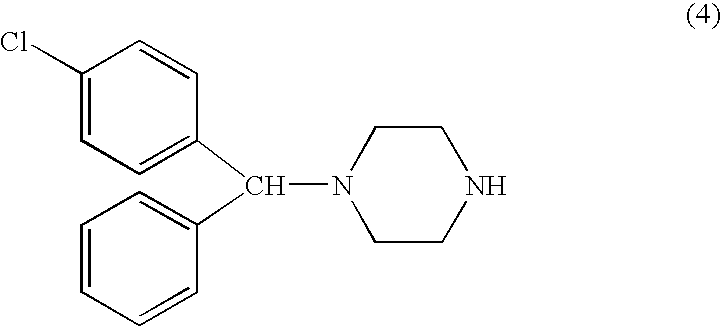
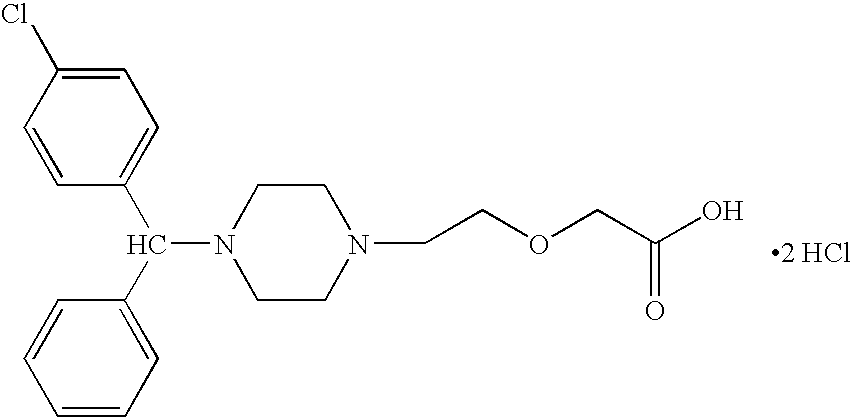
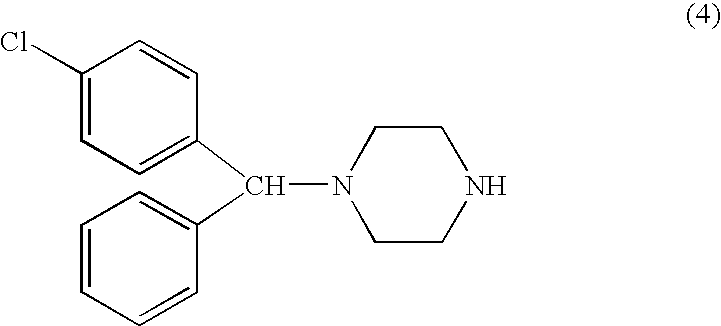
Methods for the manufacture of cetirizine
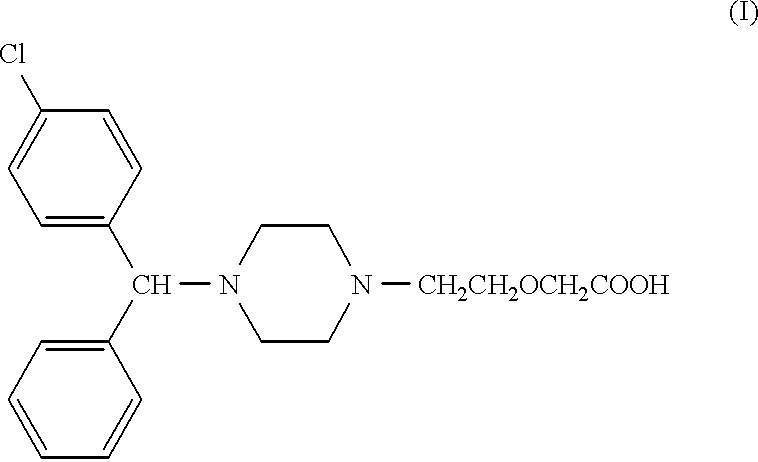
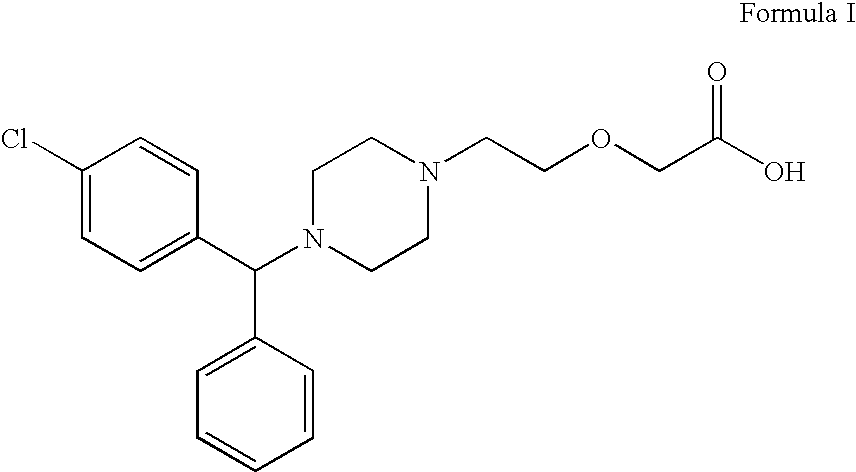
PCT No. PCT / CA97 / 00496 Sec. 371 Date Jan. 20, 1999 Sec. 102(e) Date Jan. 20, 1999 PCT Filed Jul. 11, 1997 PCT Pub. No. WO98 / 02425 PCT Pub. Date Jan. 22, 1998The present invention relates to a process for the preparation of a [2-[4-[4-(chlorophenyl)phenylmethyl]-1-piperazinyl]ethoxy]acetic acid derivative of formula I: and, in particular, for the preparation of cetirizine. The method comprises the oxidation of a primary alcohol of a hydroxyzine. Cetirizine is a non-sedating type histamine H1-receptor antagonist and is used in the treatment of allergic syndromes.
Owner:TAO YONG +2
Composition for treating respiratory and skin diseases, comprising at least one leukotriene antagonist and at least one antihistamine
A pharmaceutical composition useful in the treatment of sneezing, itching runny nose, nasal congestion, redness of the eye, tearing, itching of the ears or palate, shortness of breath, inflammation of the bronchial mucosa, reduced Forced Expiratory Volume In One Second (FEV1), coughs, rash, itchy skin, headaches, and aches and pains associated with seasonal allergic rhinitis, perennial allergic rhinitis, common colds, otitis, sinusitus, allergy, asthma, allergic asthma and / or inflammation, in a mammalian organism in need of such treatment. The composition comprises: i) an effective amount of at least one leukotriene antagonist selected from a) montelukast, b) 1-(((R)- (3-(2-(6,7- difluoro-2- quinolinyl)ethenyl) phenyl)-3-(2- (2-hydroxy-2- propyl)phenyl) thio)methylcyclopropaneacetic acid; c) 1-(((1(R)-3 (3-(2-(2,3- dichlorothieno[3, 2-b]pyridin-5-yl) -(E)-ethenyl)phenyl) -3-(2-(1-hydroxy-1- methylethyl) phenyl)propyl) thio)methyl) cyclopropaneacetic acid; d) pranlukast; or f) [2-[[2-(4-tert -butyl-2-thiazolyl) -5-benzofuranyl] oxymethyl]phenyl] acetic acid; or a pharmaceutically acceptable salt thereof; in admixture with ii) an effective amount of at least one antihistamine which is descarboethoxyloratidine, cetirizine, fexofenadine, ebastine, astemizole, norastemizole, epinastine, efletirizine or a pharmaceutically acceptable salt thereof.
Owner:SCHERING AG
Tablet Containing Cetirizine, Pseudoephedrine, and Naproxen Containing a Barrier Layer
In one aspect, the present invention features a tablet including: (i) a first drug layer including naproxen; (ii) a second drug layer including a decongestant (e.g., pseudoephedrine) wherein said second drug layer is a sustained release layer adapted to deliver a therapeutically effective amount of pseudoephedrine for a period of at least twelve hours; and (iii) a barrier layer that does not include naproxen, wherein the barrier layer is in contact with the first drug layer; and (iv) a third drug layer including cetirizine, wherein the third drug layer is in contact with the barrier layer and is not in contact with the first drug layer.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Chewable Bilayer Tablet Formulation
InactiveUS20090269393A1Effect andOrganic active ingredientsImmunological disordersPolyolCyclodextrin
Disclosed herein is a tablet formulation of an objectionable tasting drug cetirizine or its pharmaceutically acceptable salt in a form of chewable bilayer tablet, wherein the formulation comprises said cetirizine, a combination of water-insoluble and water-soluble polymer in a ratio of about 1:0.5 to about 1:5 and a low molecular weight polyol, wherein the molar ratio of the low molecular weight polyol to cetirizine is more than 10, and the inactive formulation layer comprises beta-cyclodextrin and other pharmaceutically acceptable excipients. Further, the present invention provides a process for preparing the formulation.
Owner:JUBILANT ORGANOSYS LTD
Tablet Containing Coated Particles of Cetirizine, Pseudoephedrine, and/or Naproxen
In one aspect, the present invention features a tablet including a first drug layer and a second drug layer, wherein: (i) the first drug layer includes first drug particles including naproxen and third drug particles including cetirizine, where the first drug particles and / or the third drug particles are coated with an immediate release coating; and (ii) the second drug layer including pseudoephedrine, wherein said second drug layer is a sustained release layer adapted to deliver a therapeutically effective amount of pseudoephedrine for a period of at least twelve hours.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Method and composition for treating rhinitis
InactiveUS20050255154A1Protection is in progressProtects the nasal mucosaOrganic active ingredientsNanotechNasal cavityMedicine
A pharmaceutical composition for the treatment of rhinitis by nasal or ocular administration comprises zwitterionic cetirizine, polar lipid liposome, a pharmaceutical acceptable aqueous carrier and, optionally, a pharmaceutically acceptable buffer capable of providing a pH of from pH 4.0 to pH 8.0, with the proviso that, if the polar lipid comprises phospholipid, the amount of phospholipid in the composition from is from 10 mg per mL to 120 mg per mL. Also disclosed are methods for its preparation and methods for treating rhinitis by its nasal or ocular administration.
Owner:BIOLIPOX AB
Compound sustained-release tablet of cetirizine and pseudoephedrine and preparation method thereof
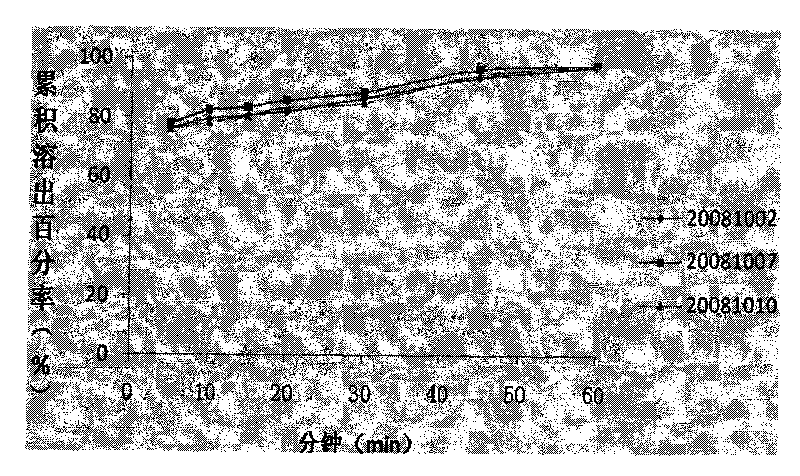
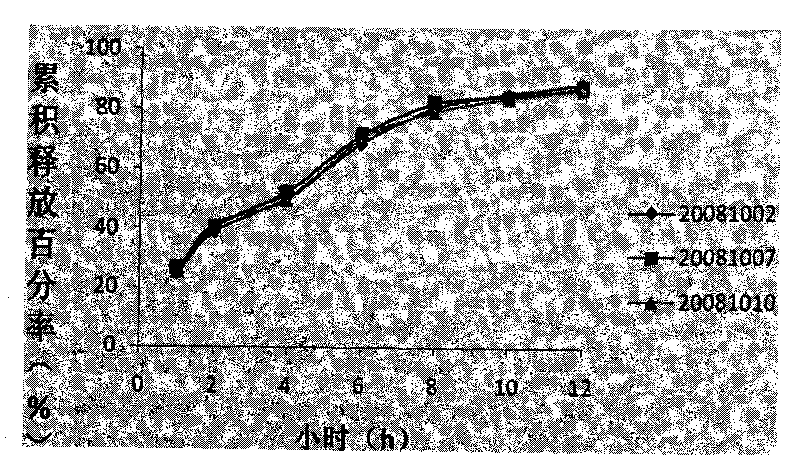
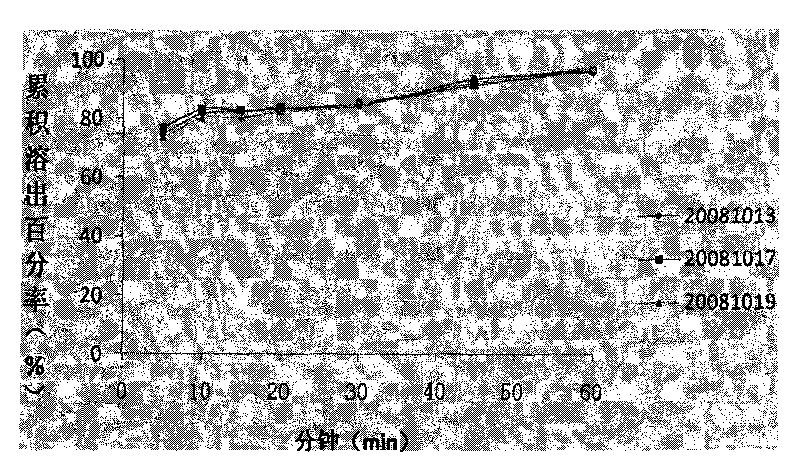
ActiveCN101708178AGood reproducibilitySimple processOrganic active ingredientsRespiratory disorderSustained Release TabletPseudoephedrine
The invention discloses a compound sustained-release tablet of cetirizine and pseudoephedrine and a preparation method thereof. The tablet comprises cetirizine or pharmaceutically acceptable cetirizine salt and pseudoephedrine or pharmaceutically acceptable pseudoephedrine salt. The preparation method comprises the steps of: preparing the pseudoephedrine or the pharmaceutically acceptable pseudoephedrine salt into a sustained-release tablet core; uniformly dispersing the cetirizine or the pharmaceutically acceptable cetirizine salt in a coating solution to coat the surface of the tablet core. Two active materials with different doses are prepared into the compound sustained-release tablet by a coating method. The preparation method solves the problems of the quick release of the cetirizine and the sustained release of the pseudoephedrine, has convenient operation and easy quality control, and is suitable for industrial production. In the tablet, more than 85% of the cetirizine is dissolved within 30 minutes, 90% of the cetirizine is dissolved out within 1 hour, and the pseudoephedrine releases medicaments in a sustained mode within 12 hours or 24 hours. The tablet is taken once or twice a day, and can reduce the administration time, better stabilize the concentration of blood medicaments and reduce adverse effect.
Owner:YANGTZE RIVER PHARM GRP CO LTD +1
Method for detecting related substances in cetirizine hydrochloride sample
The invention provides a method for detecting related substances in a cetirizine hydrochloride sample. The detection method comprises the following steps: using high performance liquid chromatographyto detect the cetirizine hydrochloride sample, wherein the mobile phase of the high performance liquid chromatography comprises a mobile phase A which is an aqueous solution containing 0.05mol / L of sodium dihydrogen phosphate, and of which the pH value is 3.8 and a mobile phase B which is acetonitrile. The detection method of the related substances in the cetirizine hydrochloride sample provided by the invention can simply and quickly detect the related substances in the cetirizine hydrochloride sample and peaks can be separated effectively. The detection method is simple to operate and shorter in analysis time.
Owner:HUMANWELL PURACAP PHARM WUHAN CO LTD
Method for making cetirizine tablets
In one aspect, the present invention features a method of producing a tablet including cetirizine including the steps of: (i) mixing cetirizine, a polyol, and a solvent for the cetirizine to form a cetirizine:polyol complex, wherein the solvent comprises water and an alkalizing agent and has a pH from about 2 to about 7; (ii) isolating particles of the cetirizine:polyol complex from the mixture; and (iii) forming the particles into a tablet.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
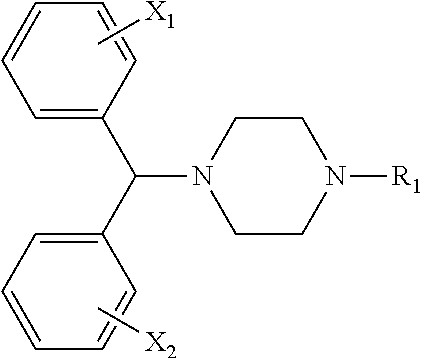
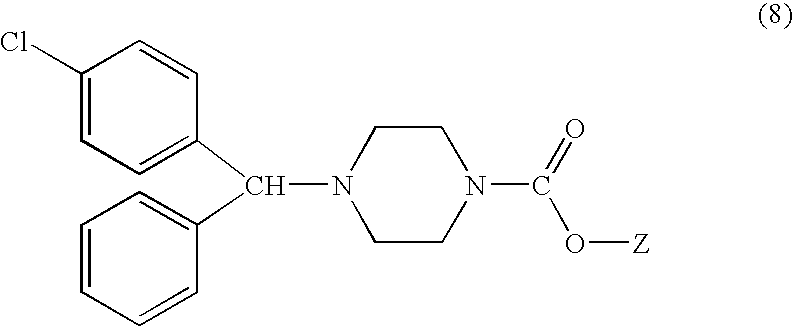
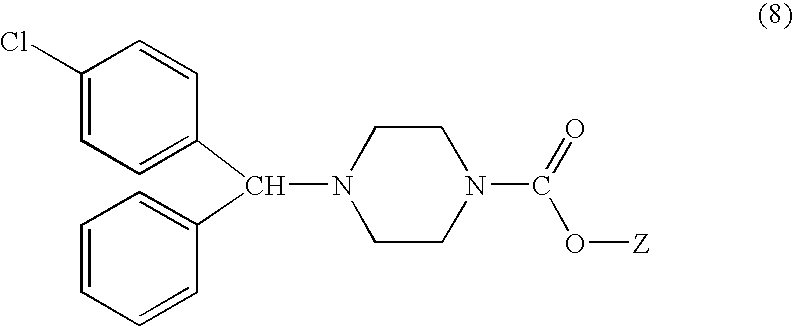
Process for making n-(diphenylmethyl)piperazines
The compound of formula (8), in racemic or single enantiomeric form, is useful in making N-(diphenylmethyl)-piperazines such as cetirizine and levocetrizine.wherein Z is preferably phenyl.
Owner:SYNTHON BV
Method For Making Cetirizine Tablets
In one aspect, the present invention features a method of producing a tablet including cetirizine including the steps of: (i) mixing cetirizine, a polyol, and a solvent for the cetirizine to form a cetirizine:polyol complex, wherein the solvent comprises water and an alkalizing agent and has a pH from about 2 to about 7; (ii) isolating particles of the cetirizine:polyol complex from the mixture; and (iii) forming the particles into a tablet.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Methods and compositions to inhibit dependence on opioids
ActiveUS10675261B2Nervous disorderPharmaceutical delivery mechanismFexofenadineNon steroid anti inflammatory drug
The present invention provides a method of inhibiting dependence to an opioid by a human subject in need thereof. The method comprises administering an effective amount of a pharmaceutical composition to the subject during opioid therapy. The pharmaceutical composition comprises a) a non-steroidal anti-inflammatory drug (NSAID); and b) a co-agent selected from the group consisting of: fexofenadine, ketotifen, desloratadine, cetirizine, salts thereof and combinations thereof.
Owner:SEN JAM PHARMA LLC
Methods and compositions to inhibit tolerance to opioids
ActiveUS11129803B2Nervous disorderAnhydride/acid/halide active ingredientsFexofenadineNon steroid anti inflammatory drug
The present invention provides a method of inhibiting tolerance to an opioid by a human subject in need thereof. The method comprises administering an effective amount of a pharmaceutical composition to the subject during opioid therapy. The pharmaceutical composition comprises a) a non-steroidal anti-inflammatory drug (NSAID); and b) a co-agent selected from the group consisting of: fexofenadine, ketotifen, desloratadine, cetirizine, salts thereof and combinations thereof.
Owner:SEN JAM PHARMA LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com