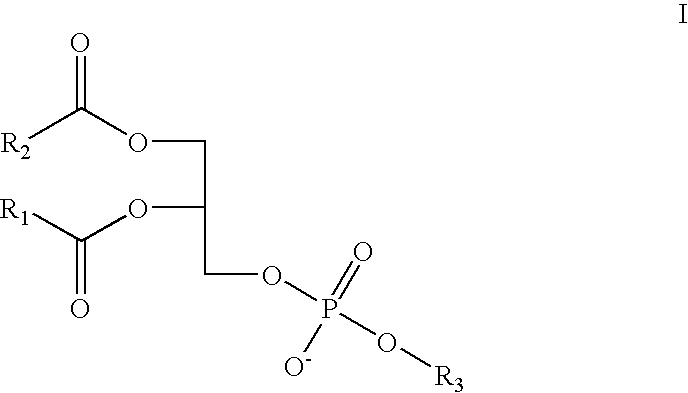
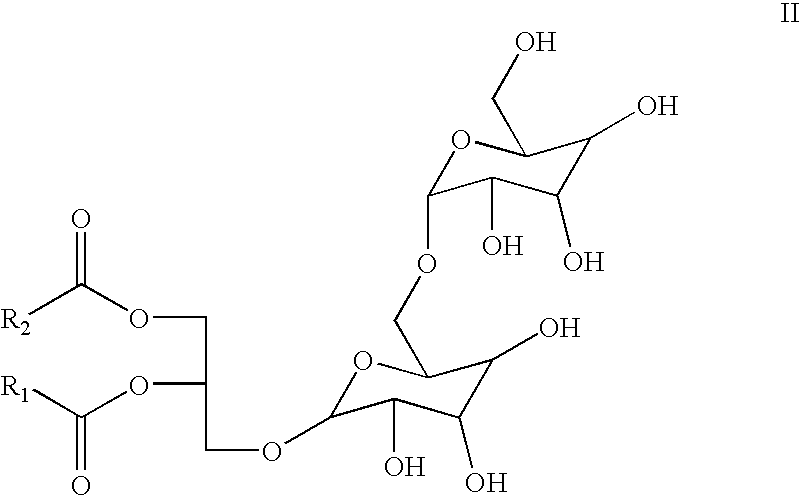
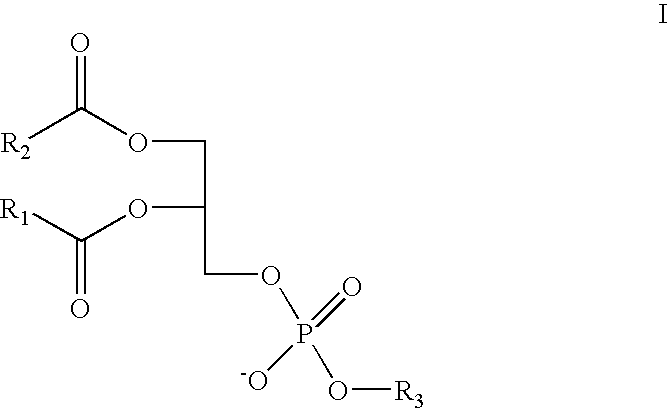
Method and composition for treating rhinitis
a technology for rhinitis and composition, applied in the field of rhinitis treatment, can solve the problems of local irritation, adverse effect of local irritation on treatment efficacy, and side effects of sedation and dry mouth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
[0086]
TABLE 3CompositionCetirizine dinitrate2.22 gPhospholipid (soybean; Lipoid S75; Lipoid GmbH, Germany)7.00 gCitric acid, anhydrous3.84 gSodium hydroxide, solid1.67 gAscorbic acid0.20 gEDTA sodium0.20 gHCl, 1 M and / or NaOH, 1 Mto pH 5.0Water for injectionto 200 mL
[0087] General procedure. For weights and volumes reference is made to Table 3. A buffer solution was prepared by dissolving anhydrous citric acid and solid sodium hydroxide in 160 mL water (80% of the total batch volume) in a 200 mL volumetric flask. The weighed amount of active agent was added and dissolved by stirring with a magnetic stirrer. The phospholipid was separately weighed and added to the cetirizine solution. Stirring was continued until a well dispersed suspension had been formed, the pH of which was adjusted to pH 5.0±0.1 with 1.0 M NaOH and / or 1.0 M HCl. The volume of the preparation was then brought to the final batch volume of 200 mL. The preparation was transferred to a high pressure homogeniser (Ranni...
example 3
[0088] In Table 4, a high pressure homogenation particle size reduction method, as described in Example 2, is compared with high speed homogenisation (Ultra Turrax® T25 homogeniser; Jankel & Kühnke, Germany), as described in Example
[0089] 1. The composition employed was that of Example 1. Particle size distribution was determined by dynamic light scattering (Zetasizer 4, Malvern Instruments, UK) at an angle of 90° and at room temperature, using a ZET5104 sizing cell and auto:CONTIN analysis mode.
TABLE 4Particle size reductionCetirizineZ averageTreatment(mg / mL)mean (nm)High speed homogenisation11.1282High pressure homogenisation at 500 bar11.177High pressure homogenisation at 800 bar11.150High pressure homogenisation at 500 bar0130High pressure homogenisation at 800 bar0121
[0090] The methods used for preparing these exemplary batch compositions were adapted for preparing the following additional examples.
example 4
[0091]
Cetirizine dinitrate 5.6 mgPhospholipid (soybean; Lipoid S75; Lipoid GmbH, Germany).35.0 mgDisodium phosphate dihydrate; Na2HPO4H2O10.7 mgPotassium dihydrogen phosphate; KH2PO4 5.5 mg1 M HCl and / or 1 M NaOHto pH 7.0Water for injectionto 1 mL
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com