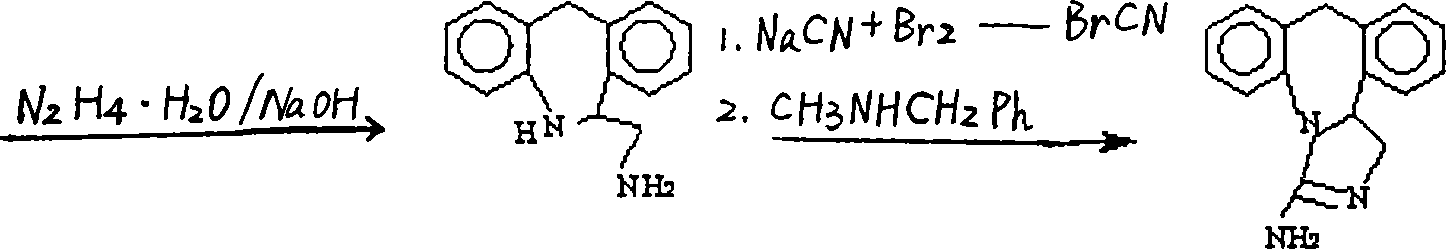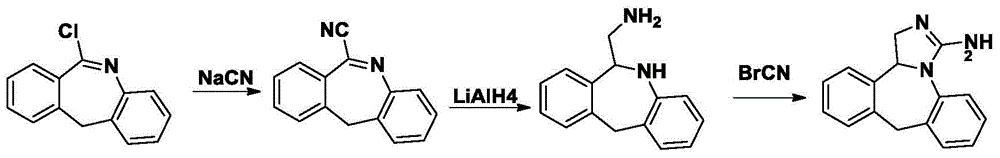Patents
Literature
30 results about "Epinastine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
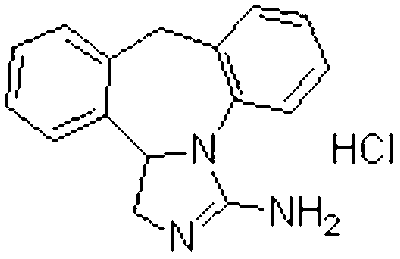
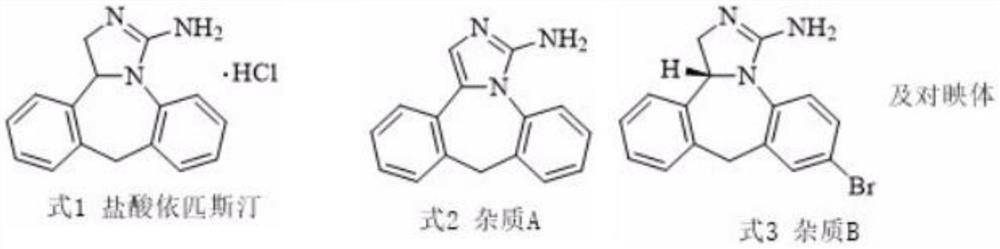
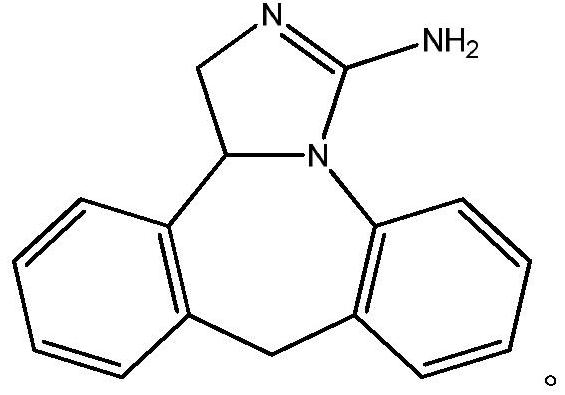
This medication is an antihistamine used to prevent itching of the eyes caused by allergies (allergic conjunctivitis)..
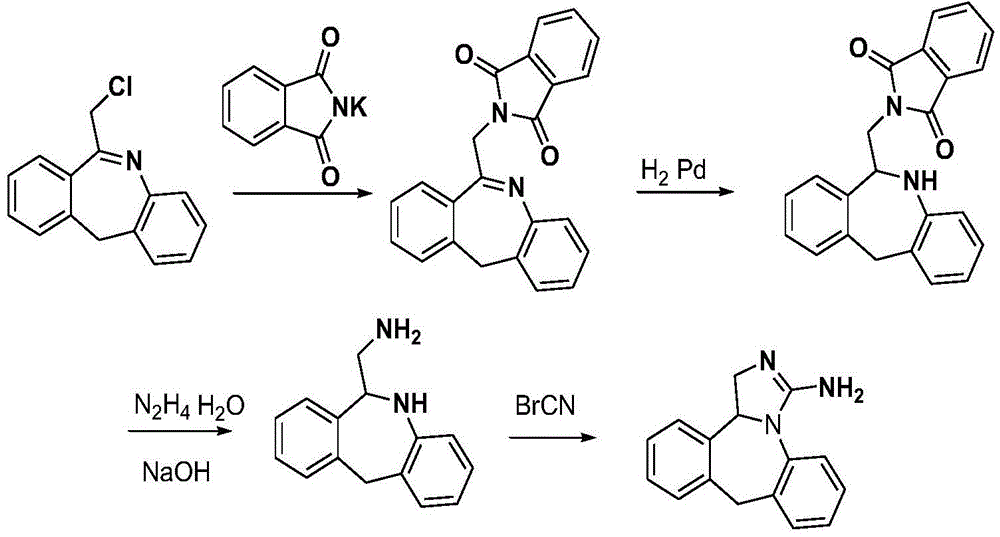
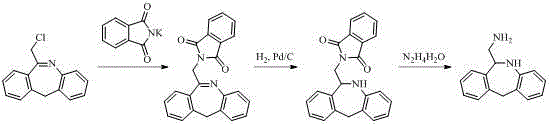
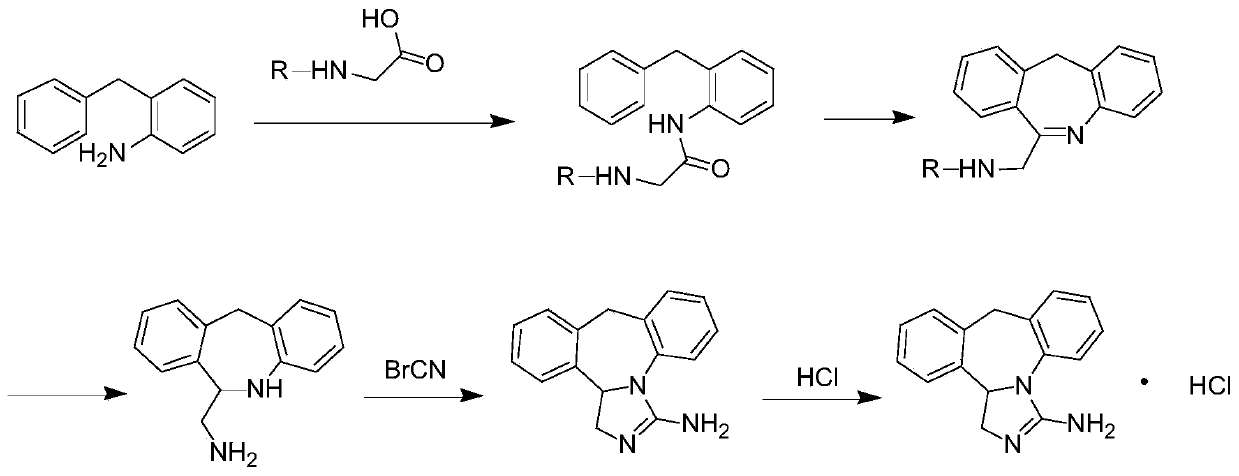
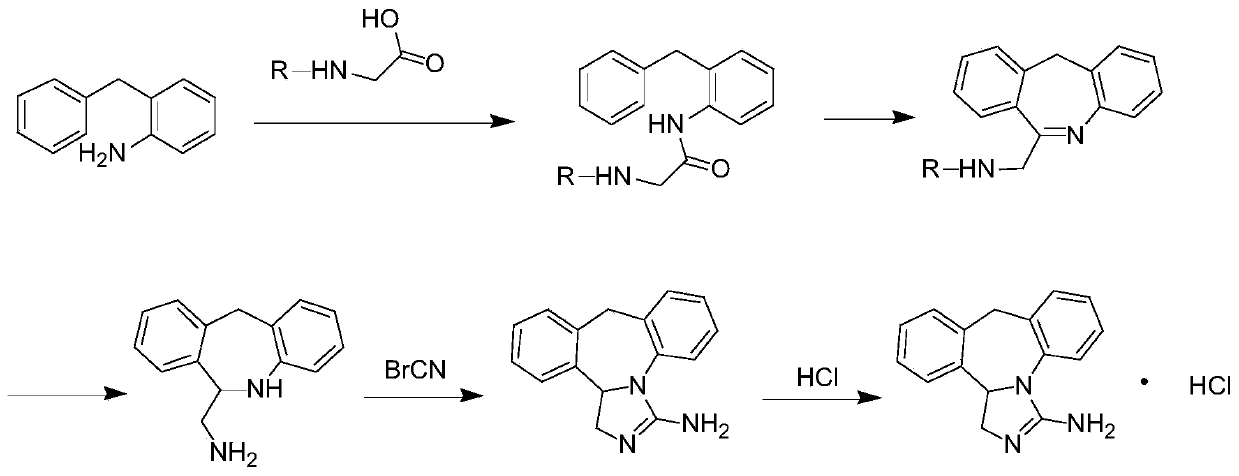
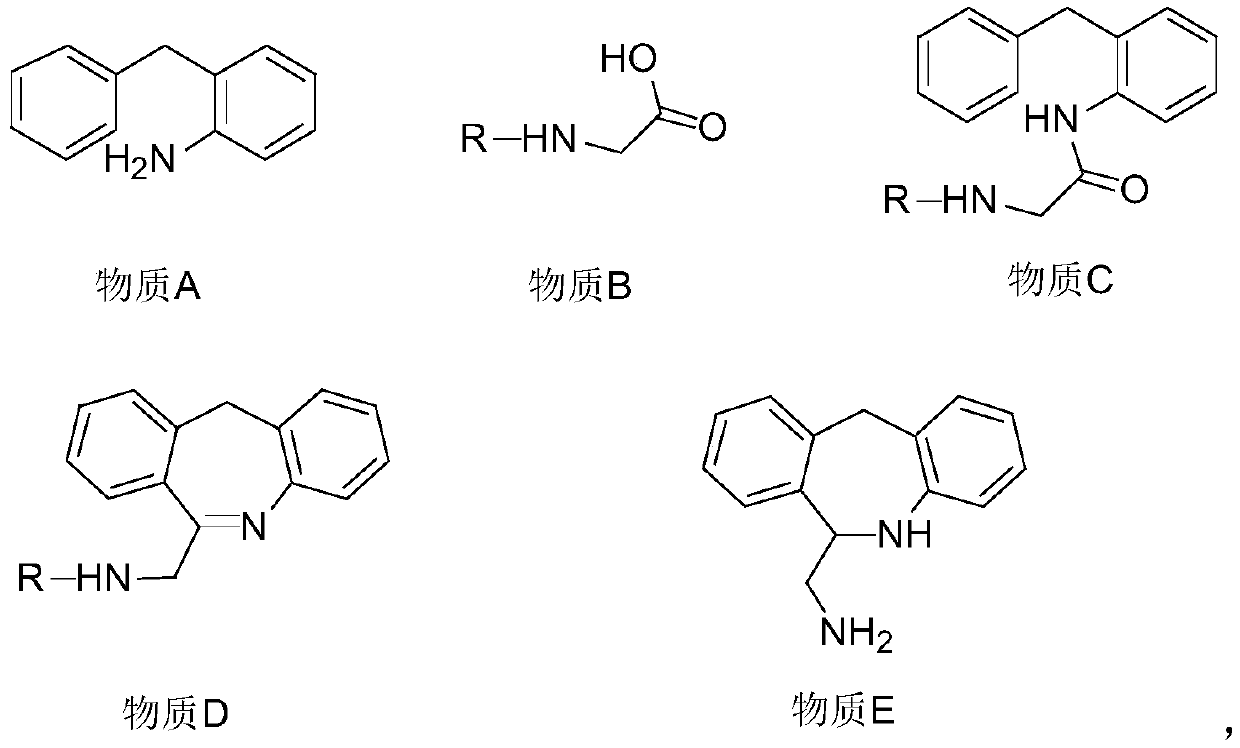
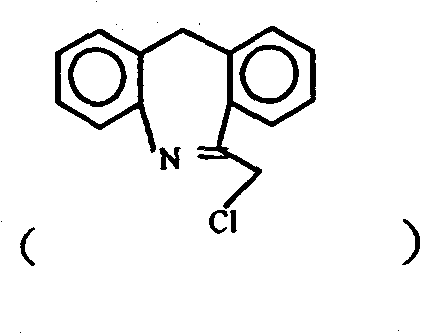
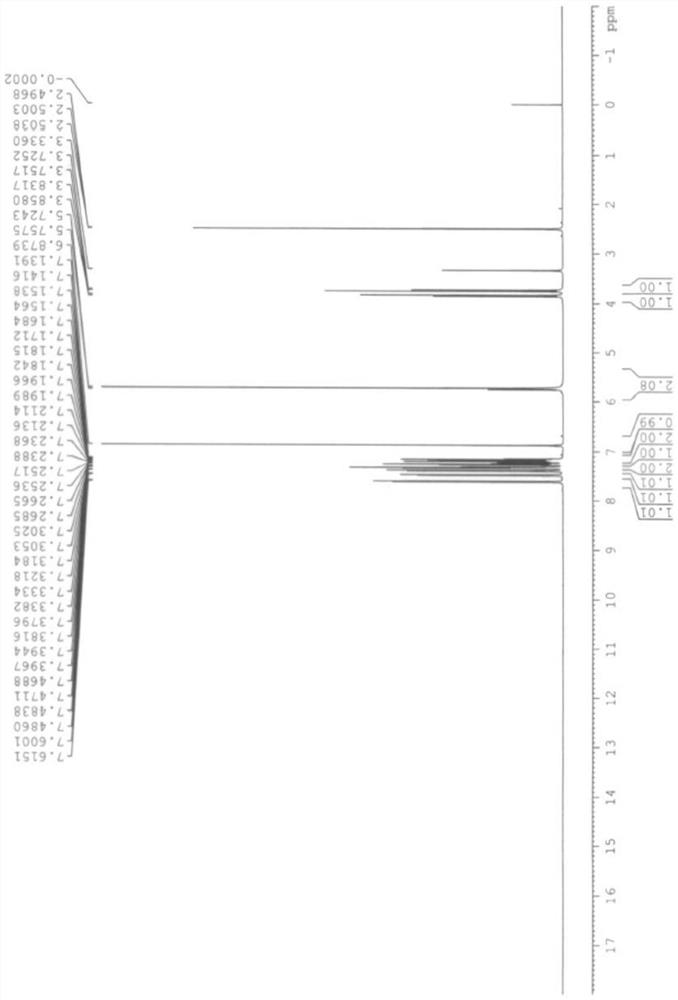
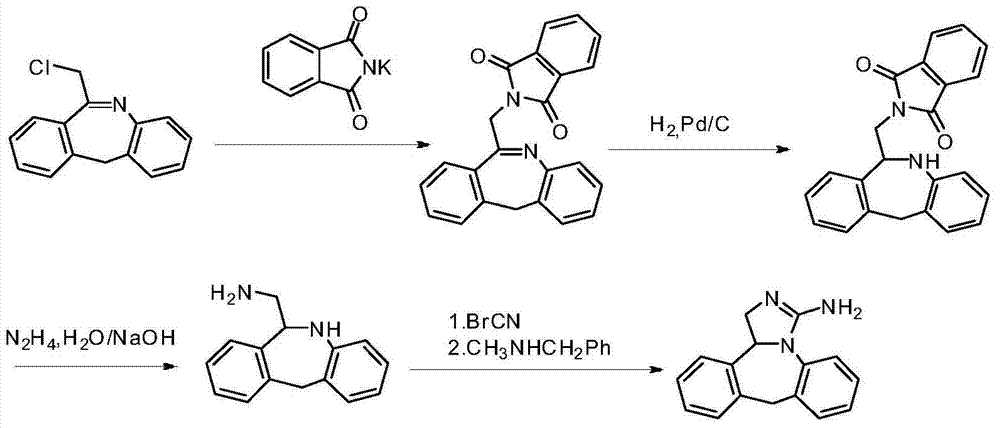
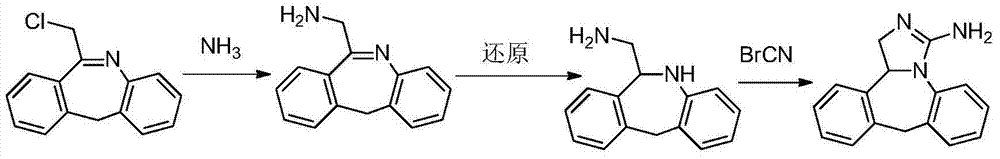
Chemical synthesis method for epinastine
InactiveCN101130544AHigh purityEasy to makeOrganic chemistryImmunological disordersChemical synthesisCyanogen bromide
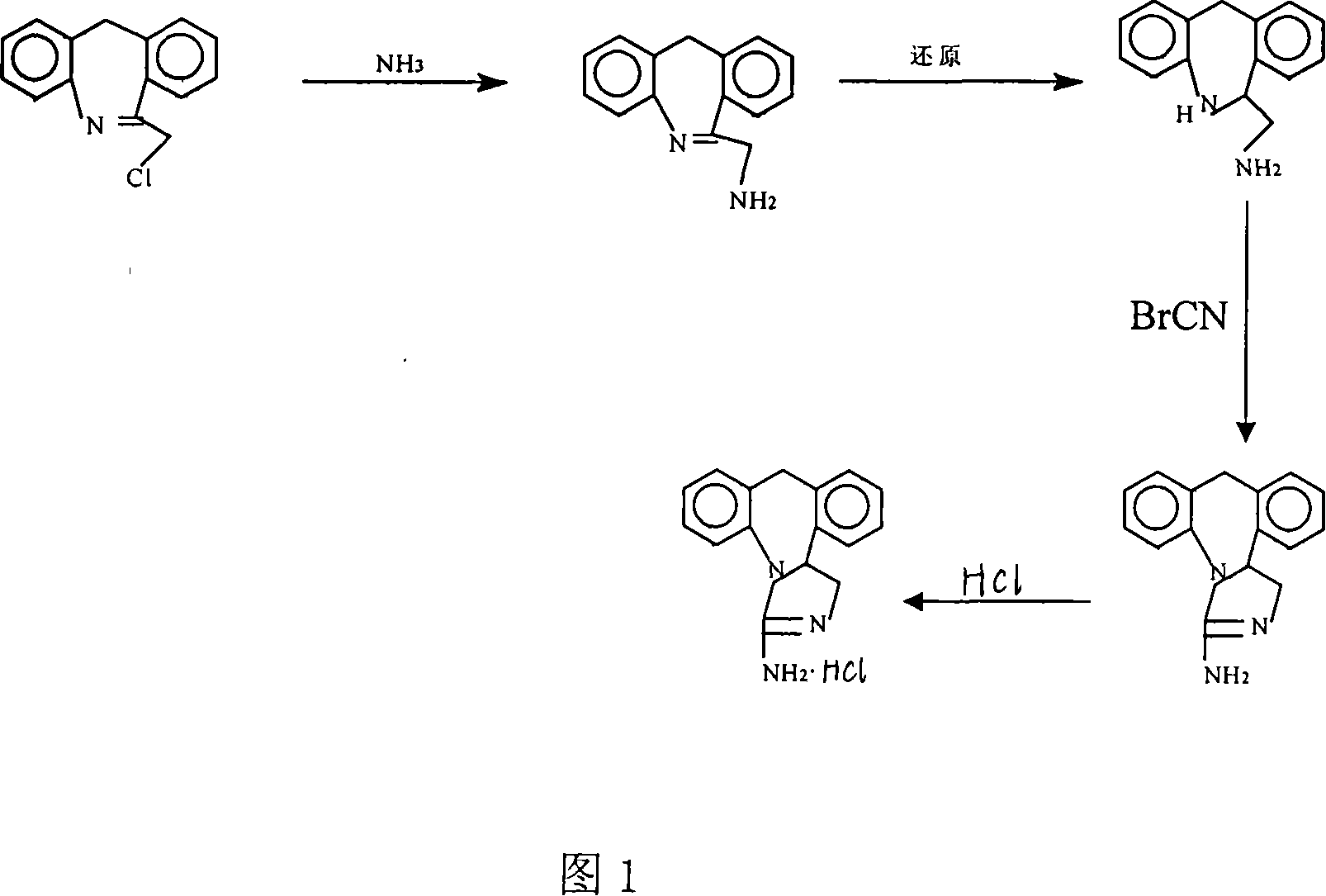
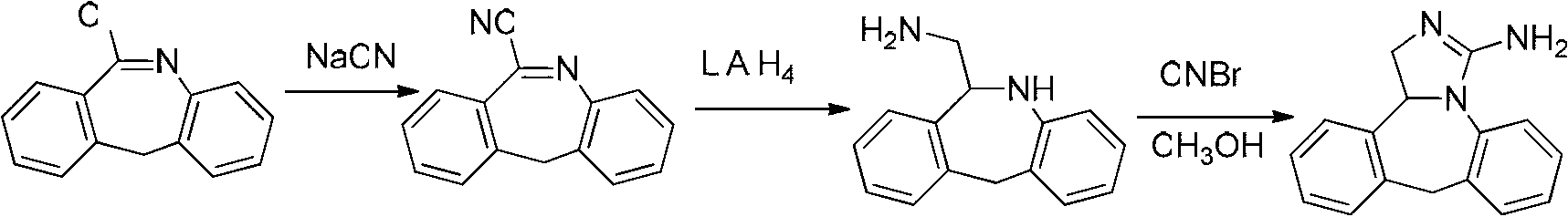
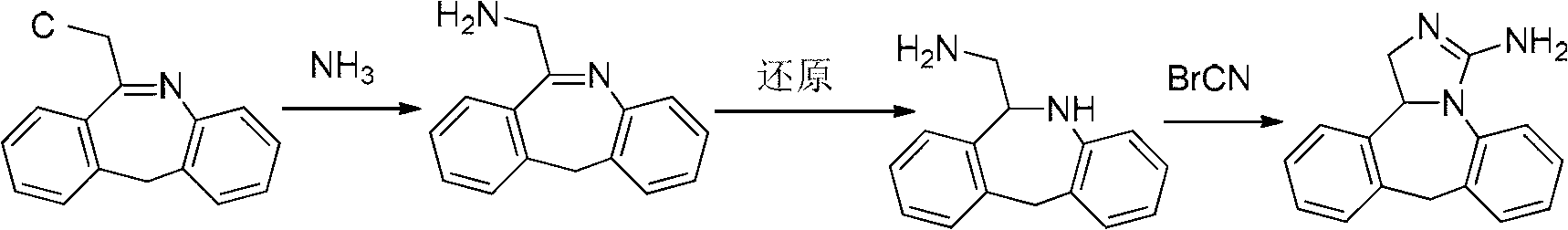
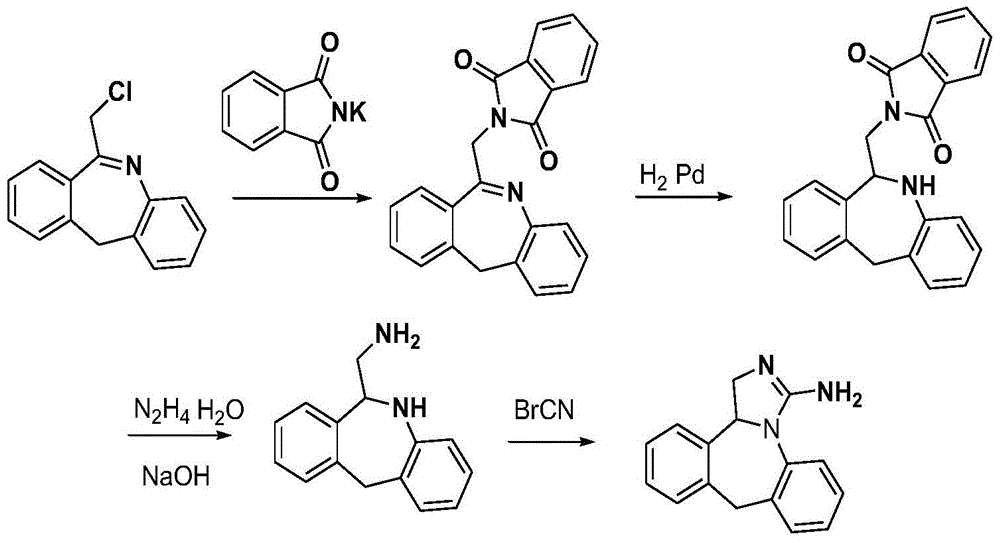
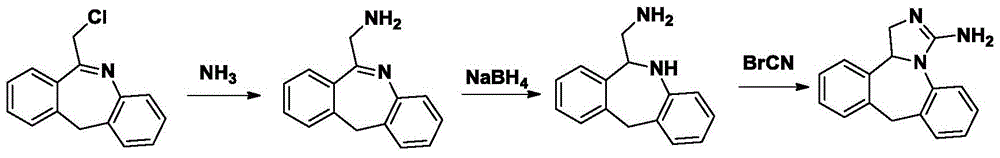
The invention discloses a new chemical synthesizing method of yipisidin, which comprises the following steps: ammonifying 6-chloromethyl-11-dihyrogen-dibenz [b,e] aza to generate 6-aminomethyl-11-dihydrogen-dibenz [b,e] aza; reducing the 6-aminomethyl-11-dihydrogen-dibenz [b,e] aza into 6-aminomethyl-6,11-dihydrogen-5H-dibenz [b,e] aza; generating the product through cyanogen bromide to loop. The invention simplifies the making method with little by-product, which improves the receiving rate by 69% with high purity (HPLC. 99. 0%) for industrial manufacturing.
Owner:HANGZHOU LONGSHAN CHEM CO LTD
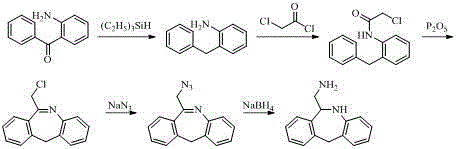
Synthesis method of epinastine
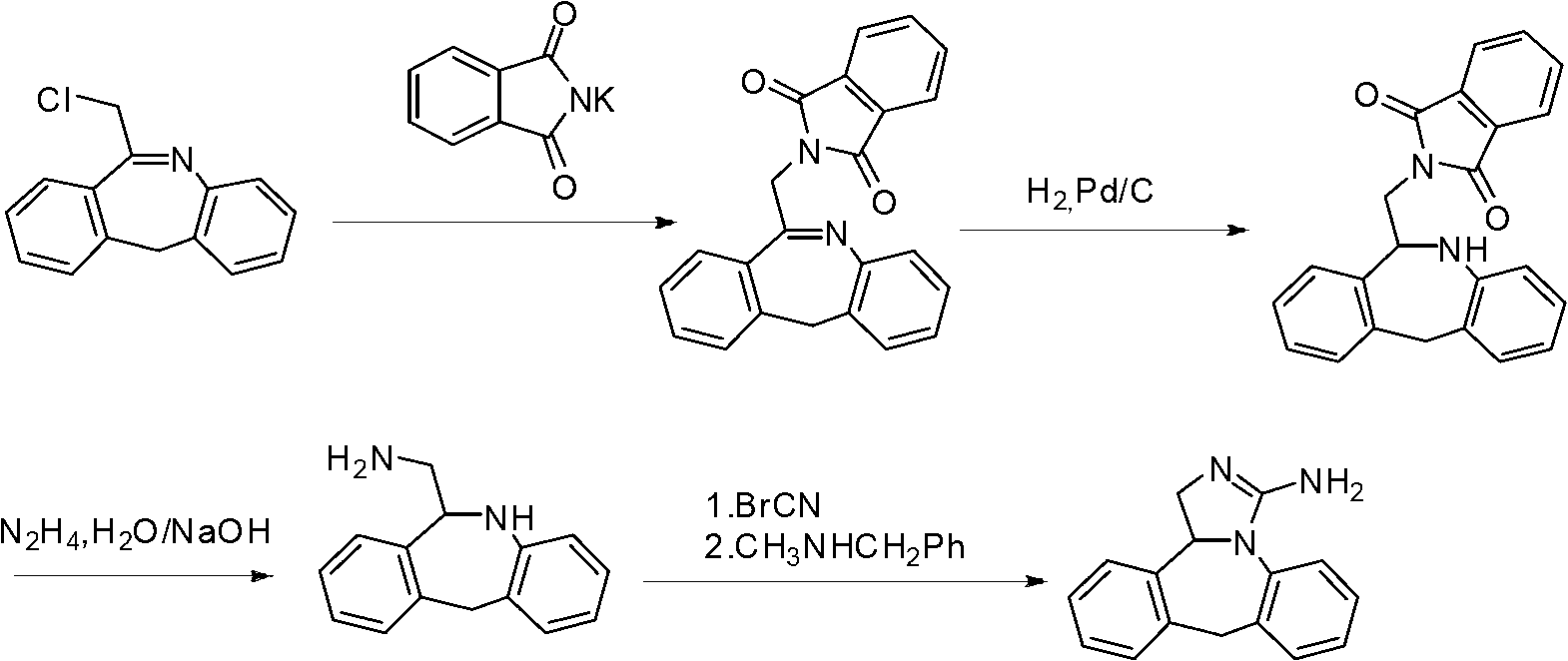
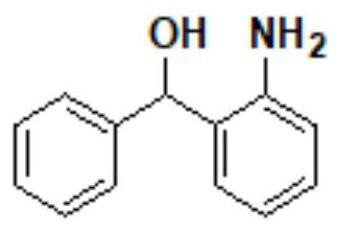
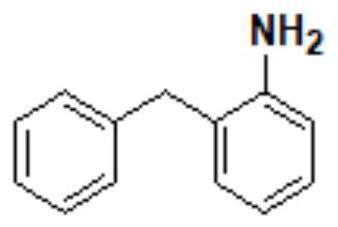
The invention discloses a synthesis method of epinastine. The synthesis method is implemented by taking 2-aminobenzophenone as a raw material and comprises the following steps of: reacting the 2-aminobenzophenone with a silane agent to obtain 2-benzylaniline; then, carrying out acylation reaction on the 2-benzylaniline and 2-chloroacetyl chloride to obtain N-(2-benzyl phenyl)-2-chloroacetamide; carrying out acidamide dehydration and cyclization on the N-(2-benzyl phenyl)-2-chloroacetamide under the action of a dehydrating agent to obtain 6-(chloromethyl)-11H-dibenzo[b,e] azepine; carrying out azidation reaction on the 6-(chloromethyl)-11H-dibenzo[b,e] azepine to obtain 6-(azido-methytbiphenyl)-11H-dibenzo[b,e] azepine; carrying out reduction on the 6-(azido-methytbiphenyl)-11H-dibenzo[b,e] azepine to obtain 6-(aminomethyl)-6,11-dihydro-1H-dibenzo[b,e] azepine; and finally, carrying out cyclization on the 6-(aminomethyl)-6,11-dihydro-1H-dibenzo[b,e] azepine and cyanogen bromide to obtain the epinastine. The synthesis method disclosed by the invention avoids the application of expensive and flammable lithium aluminium hydride and aluminium hydride as well as hypertoxic sodium cyanide, so that the operation is safer in industrial production, and the cost is reduced. The method is simple in process and high in yield, requires mild conditions, and is suitable for industrialized production.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Method for treating allergic rhinitis without adverse effects
The present invention is directed to a method for treating allergic rhinitis without causing an adverse effect of bitter taste. The method comprises administering to a patient an aqueous pharmaceutical formulation comprising 0.1-0.15% (w / v) of epinastine or an acid addition salt thereof, 0.05-0.5% (w / v) of hydroxypropylmethylcellulose to maintain the viscosity between 1.5-10 centipoise, 1-2% (w / v) of propylene glycol, and a buffer to maintain the pH between 5-8, said aqueous epinastine formulation has a tonicity between 200-400 mOsm / kG; the formulation does not contain a sweetening agent. The present invention provides a method for effectively treating allergic rhinitis by delivering a small volume of the epinastine formulation to the nose of a patient using a small volume metered-dose nasal spray pump. The present method does not cause an adverse effect of bitter taste without including sweetening agents in the formulation.
Owner:INSPIRE PHARMA
Percutaneously Absorptive Ophthalmic Preparation Comprising Epinastine
The present invention provides a percutaneously absorptive preparation for preventing or treating allergic eye disease, which comprises epinastine or a salt thereof as an active ingredient. In addition, the present invention provides a method for preventing or treating allergic eye disease, which comprises applying a percutaneously absorptive preparation comprising epinastine or a salt thereof to the skin surface including the skin surface of an eyelid, thereby causing transfer of a therapeutically effective amount of epinastine or a salt thereof from the preparation to an anterior ocular segment through the skin of the eyelid rather than a systemic blood flow. The present preparation can exert a pharmacological effect over a prolonged period by a single application, as compared to conventional preparations such as eye drops.
Owner:SENJU PHARMA CO LTD
Treating rhinitis by topically administering an epinastine solution to the nasal mucous membrane
A method for treating allergic rhinitis, comprising topically administering to the nasal mucus membrane of a host in need of such treatment a solution comprising: epinastine, optionally in the form of its racemate, its enantiomers, or its pharmacologically acceptable acid addition salts, in a pharmacologically acceptable carrier.
Owner:BOEHRINGER INGELHEIM INT GMBH
Stable granular medicine composition containing epinastine or hydrochloride thereof
InactiveCN103251563AStable decompositionPharmaceutical non-active ingredientsGranular deliveryDrug productAllergic reaction
The invention discloses a stable granular medicine composition containing epinastine or hydrochloride thereof. The stable granular medicine composition also comprises a binding agent which has a stabilizing effect on the epinastine or the hydrochloride thereof. Meanwhile, the invention also discloses a preparation method of the stable granular medicine composition. The granular medicine composition disclosed by the invention can also be used as a medicament which urgently needs to be developed in clinic and has an excellent effect on allergic reaction diseases of children.
Owner:BEIJING KEYUAN CHUANGXIN TECH
Composition for treating respiratory and skin diseases, comprising at least one leukotriene antagonist and at least one antihistamine
A pharmaceutical composition useful in the treatment of sneezing, itching runny nose, nasal congestion, redness of the eye, tearing, itching of the ears or palate, shortness of breath, inflammation of the bronchial mucosa, reduced Forced Expiratory Volume In One Second (FEV1), coughs, rash, itchy skin, headaches, and aches and pains associated with seasonal allergic rhinitis, perennial allergic rhinitis, common colds, otitis, sinusitus, allergy, asthma, allergic asthma and / or inflammation, in a mammalian organism in need of such treatment. The composition comprises: i) an effective amount of at least one leukotriene antagonist selected from a) montelukast, b) 1-(((R)- (3-(2-(6,7- difluoro-2- quinolinyl)ethenyl) phenyl)-3-(2- (2-hydroxy-2- propyl)phenyl) thio)methylcyclopropaneacetic acid; c) 1-(((1(R)-3 (3-(2-(2,3- dichlorothieno[3, 2-b]pyridin-5-yl) -(E)-ethenyl)phenyl) -3-(2-(1-hydroxy-1- methylethyl) phenyl)propyl) thio)methyl) cyclopropaneacetic acid; d) pranlukast; or f) [2-[[2-(4-tert -butyl-2-thiazolyl) -5-benzofuranyl] oxymethyl]phenyl] acetic acid; or a pharmaceutically acceptable salt thereof; in admixture with ii) an effective amount of at least one antihistamine which is descarboethoxyloratidine, cetirizine, fexofenadine, ebastine, astemizole, norastemizole, epinastine, efletirizine or a pharmaceutically acceptable salt thereof.
Owner:SCHERING AG
Treating rhinitis by topically administering an epinastine solution to the nasal mucous membrane
A method for treating allergic rhinitis, comprising topically administering to the nasal mucus membrane of a host in need of such treatment a solution comprising: epinastine, optionally in the form of its racemate, its enantiomers, or its pharmacologically acceptable acid addition salts, in a pharmacologically acceptable carrier.
Owner:TRACH VOLKER +1
Novel epinastine nasal drug delivery preparation and preparation method thereof
InactiveCN104173289AImprove complianceImprove conveniencePowder deliveryPharmaceutical non-active ingredientsEngineeringEpinastine
The invention provides a novel epinastine nasal drug delivery preparation and a preparation method thereof. According to the requirements of nose drops and biological characteristics of epinastine, an appropriate protective agent, a thickener and a corrosion remover are added to prepare aqueous solution nose drops. The novel epinastine nasal drug delivery preparation is free of bitter taste and thrill, and is suitable for nasal delivery, convenient to use, and especially suitable for medication on children and the old. The technological process provided by the invention has the advantages of being simple and reliable, and good in repeatability, can be applied to prevention and treatment of anaphylactic (including seasonal or non-seasonal) rhinitis, and has certain application value in clinical.
Owner:广州艾格生物科技有限公司
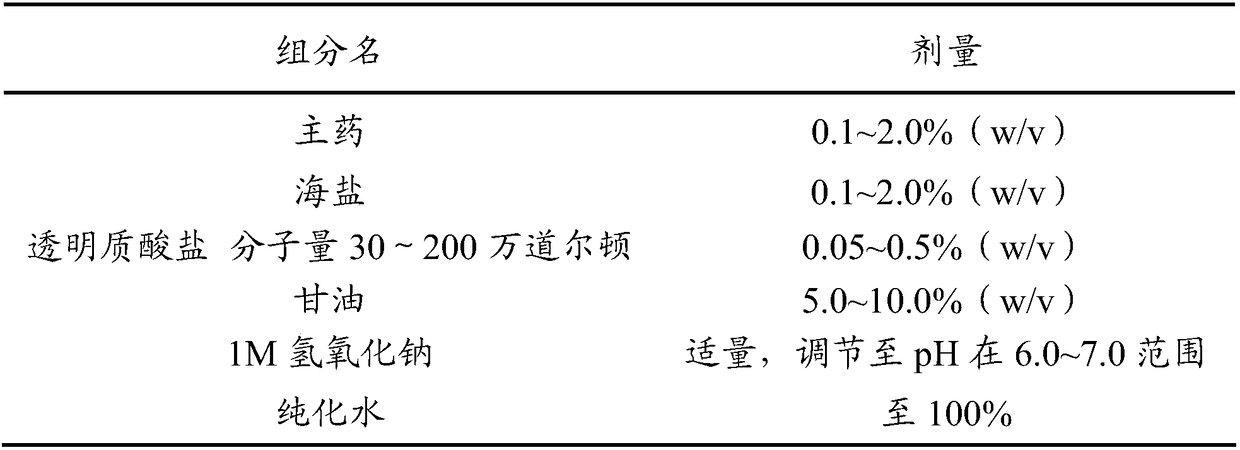
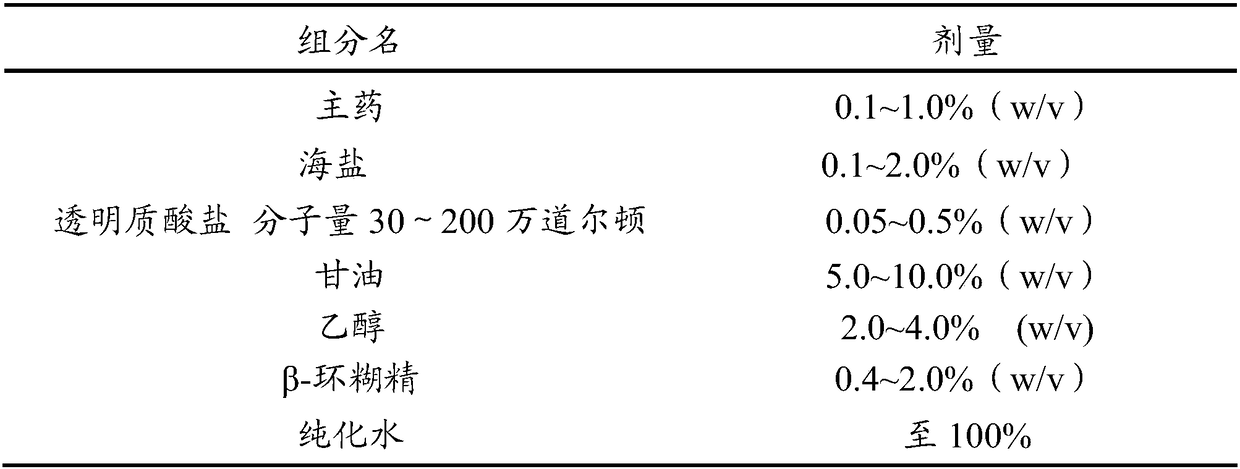
Antiallergic nasal medicine composition with high moisture retention and preparation methods and applications thereof
InactiveCN109276715AUse lessImprove comfortPharmaceutical delivery mechanismPharmaceutical non-active ingredientsIrritationGlycerol
The invention provides an antiallergic nasal medicine composition with high moisture retention and preparation methods and applications thereof. The composition has high moisture retention and no irritation to a nasal mucosa. The composition comprises levocetirizine, azatadine, loratadine, ebastine, setastine, bilastine, clemastine, mizolastine, epinastine and moxifloxacin which are antiallergic medicine as main medicine, wherein the main medicine can be salts or free alkalis, sea salt as an osmotic pressure regulator, and a mixture of sodium hyaluronate and glycerin of different molecular weights as a moisturizing excipient. Plant essential oil can be contained in the composition. Preparations can be an aqueous solution, a cyclodextrin-coated suspension or a nanosuspension. Dosage forms include spray, aerosol and nose drops. Main indications include nasal dryness, runny nose, nasal itching, nasal obstruction and the like due to allergic rhinitis.
Owner:XIAN LIBANG PHARMA TECH
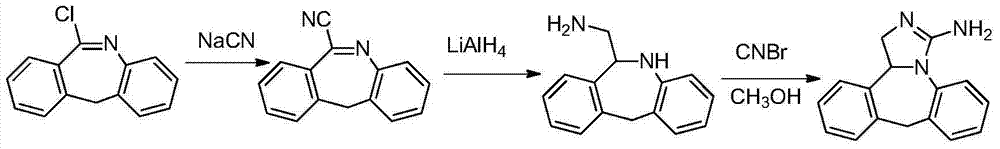
Method for synthesizing epinastine
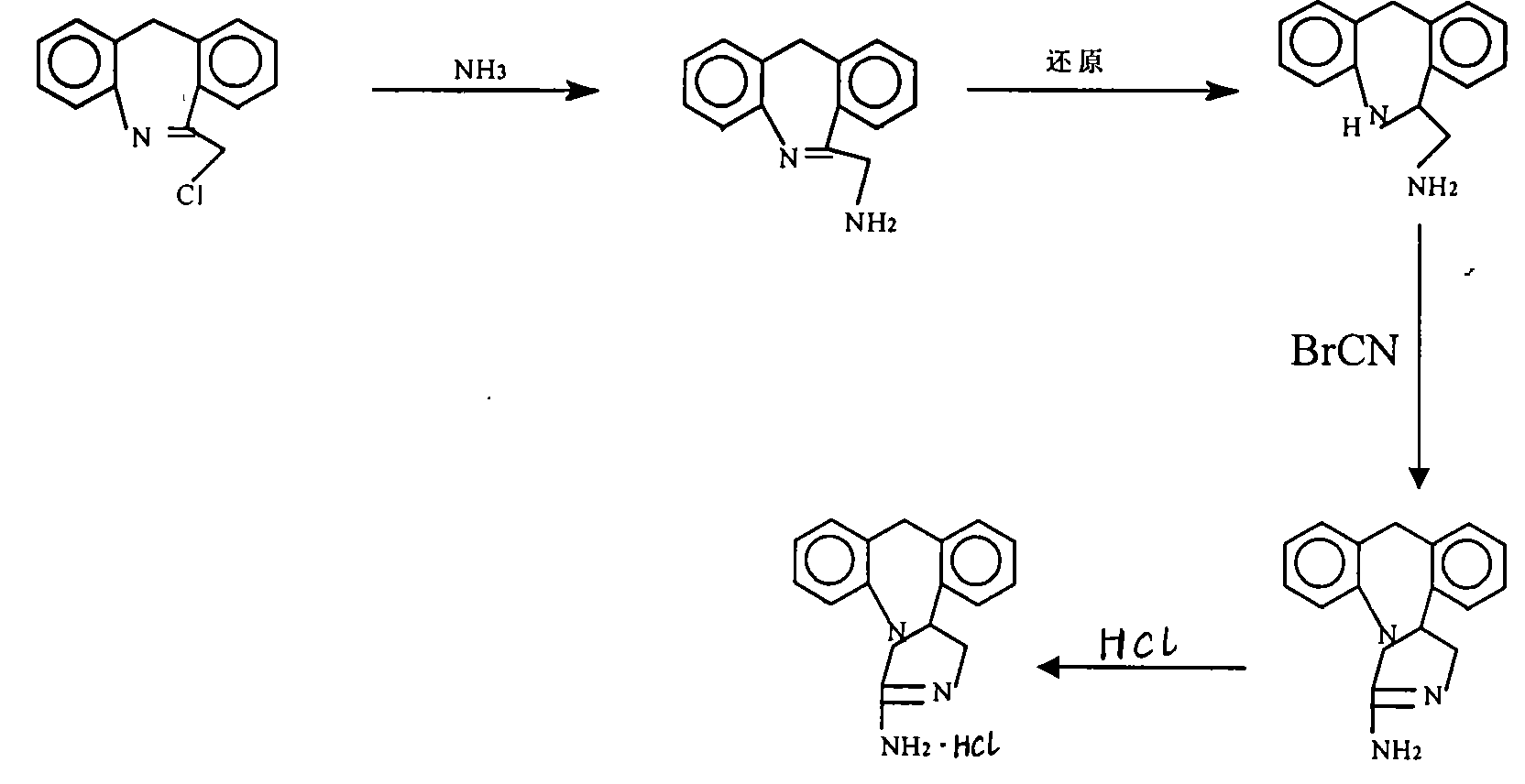
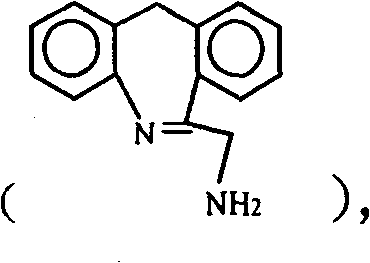
The invention discloses a method for synthesizing epinastine. The method comprises the following steps: (1) reacting 6-halomethylmorphanthridine with hexamine in an organic solvent, thereby obtaining a 6-halomethylmorphanthridine quaternary ammonium salt; (2) dissolving the 6-halomethylmorphanthridine quaternary ammonium salt in the organic solvent to carry out an acid hydrolysis reaction, thereby obtaining 6-halomethylmorphanthridine hydrochloride; (3) reducing the product 6-halomethylmorphanthridine hydrochloride obtained in the step (2) by using a reducing agent, thereby obtaining 6-aminomethyl-6,11-dihydro-5H-dibenzo[b,e]aza-cycloheptatrien; and (4) adding cyanogen bromide to carry out a ring-closure reaction, thereby obtaining the epinastine. According to the synthetic method disclosed by the invention, use of high-price and flammable lithium aluminum hydride or aluminum hydride is avoided, use of virulent sodium cyanide is avoided, and the security risk and production cost are effectively reduced. The method disclosed by the invention is simple in synthetic process, the reaction conditions are mild, the product is high in yield and high in purity, and industrial production is facilitated.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
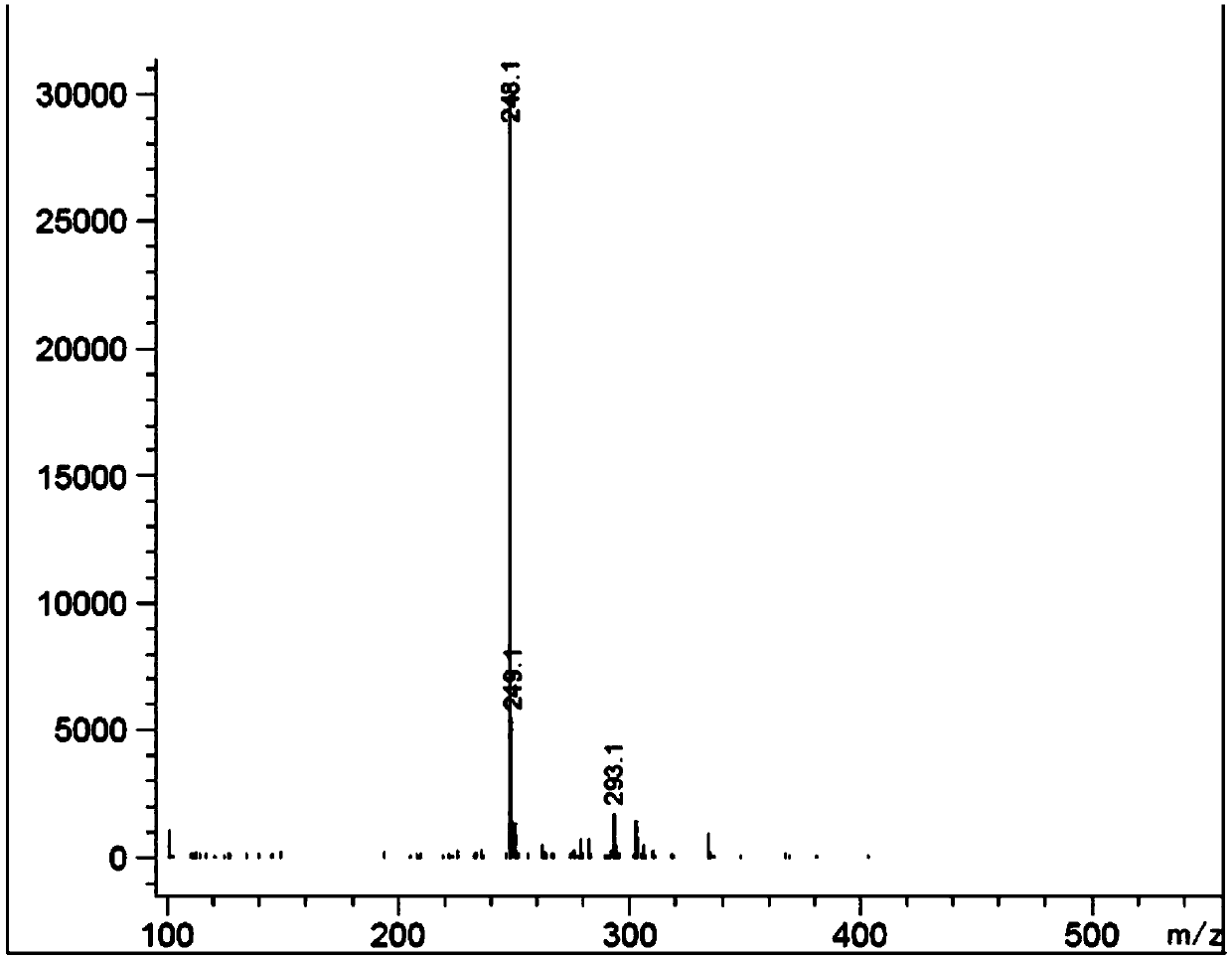
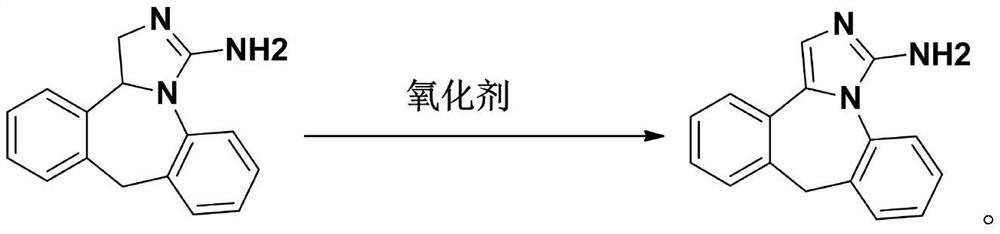
Preparation method of epinastine related substance
The invention discloses a preparation method of an epinastine related substance and relates to the technical field of medicines. The preparation method comprises steps as follows: epinastine is takenas a raw material, an oxidizing agent, a catalyst and a reaction system solvent are added, the mixture is stirred uniformly, an organic solvent is added after the reaction ends, extraction, washing, drying and suction filtration under reduced pressure are sequentially performed, and the organic solvent is recovered. An epinastine impurity A with the purity up to 97% is obtained through oxidation preparation, separation and purification steps, and the method is simple in preparation process, low in cost and high in yield.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
Aqueous formulations of epinastine for treating allergic rhinitis
InactiveUS20080194544A1Relieve symptomsMaintain viscosityBiocideAnimal repellantsObstructive Pulmonary DiseasesLiposome
There is provided homogeneous pharmaceutical compositions for the treatment of, for example, rhinitis, asthma and / or chronic obstructive pulmonary disease comprising a corticosteroid and an antihistamine, a polar lipid liposome and a pharmaceutical-acceptable aqueous carrier.
Owner:INSPIRE PHARMA
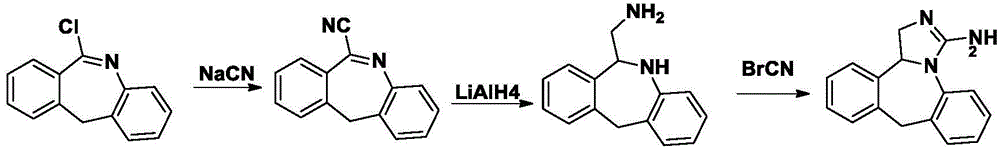
Synthesis method for epinastine intermediate
InactiveCN104693119AHigh purityRaw materials are cheap and easy to getOrganic chemistryBenzeneHydrogen
The invention relates to a synthesis method for an epinastine intermediate 6-aminomethyl-6, 11-dihydro-5H-dibenzo[b, e]azepine. The synthesis method includes: taking 6-cyano-11h-dibenzo[b, e]azepine as the raw material, conducting reduction by a borane tetrahydrofuran complex, then carrying out dilute hydrochloric acid quenching, alkali dissociation and other routine technique aftertreatment so as to obtain the 6-aminomethyl-6, 11-dihydro-5H-dibenzo[b, e]azepine. The synthesis method has the characteristics of cheap and easily available raw materials, no need for pretreatment of the raw materials and solvent needed in the whole process, no need of high pressure reaction equipment, simple reaction operation and mild conditions, and the prepared product has the advantages of high purity, good safety, high yield, and easy industrial production.
Owner:YAOPHARMA CO LTD +1
A kind of synthetic method of epinastine hydrochloride
ActiveCN107141297BHigh yieldHigh purityOrganic chemistryBulk chemical productionCyanogen bromidePhosphoric acid
Owner:HEFEI HUAFANG PHARMA SCI & TECH
Stable solution containing epinastine or hydrochloride of epinastine
InactiveCN103222955AGreat tasteAccurate dosePharmaceutical delivery mechanismPharmaceutical non-active ingredientsPEG 400Drug product
The invention discloses a stable liquid medicine composition containing epinastine or hydrochloride of epinastine. 1000ml of the liquid medicine composition mainly comprises 0.1-10.0g of epinastine or hydrochloride of epinastine, 10-50g of polyethylene pyrrolidone and 5-100ml of polyethylene glycol 400. Meanwhile, the invention discloses a preparation method of the liquid medicine composition, wherein pH of liquid is controlled to be 4.5-6.5. The liquid medicine composition can also be taken as a medicine which urgently needs to be developed in clinic and has an excellent effect on allergic reaction diseases of children.
Owner:BEIJING KEYUAN CHUANGXIN TECH
Epinastine hydrochloride composition
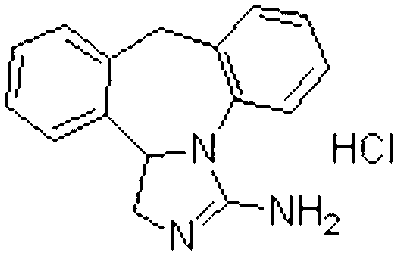
InactiveCN105708840ASolve the disadvantage of extremely bitter tasteImprove complianceGranular deliveryImmunological disordersCelluloseEpinastine hcl
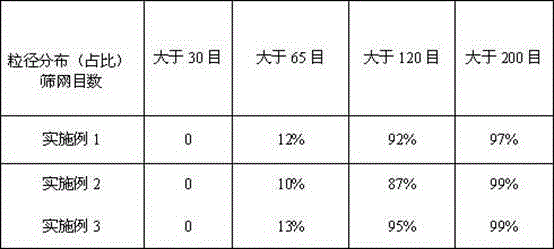
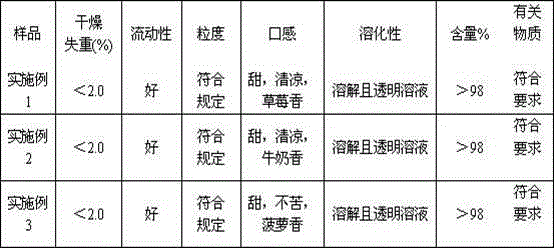
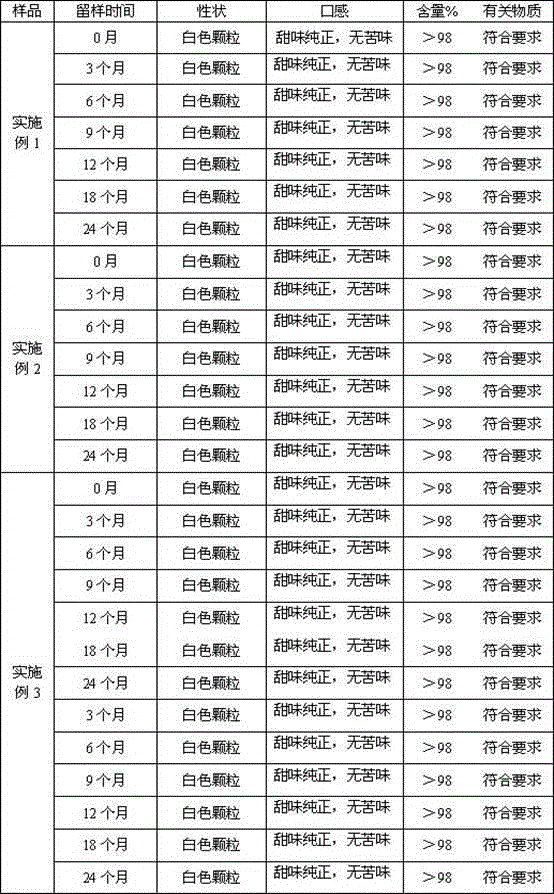
The invention discloses an epinastine hydrochloride composition. In terms of 1000 weight parts, the prescription of the composition involves 15-50 parts by weight of epinastine hydrochloride, 830-950 parts by weight of filler, 25-60 parts by weight of a composite flavoring agent, 0.5-5 parts by weight of hydroxypropyl cellulose, 0.5-5 parts by weight of essence. The invention adopts the composite flavoring agent for taste modifying, also selects the filler with sweet taste, and is supplemented by essence, effectively covers up the disadvantage of extremely bitter taste of epinastine hydrochloride, and improves the clinical medication compliance. The invention effectively solves the problems of large particle size distribution and much particle fine powder in epinastine hydrochloride composition, can obtain stable and uniform particles, and is suitable for industrial production requirements.
Owner:重庆瑞泊莱医药科技有限公司
A kind of preparation method of epinastine related substance
The invention discloses a preparation method of an epinastine related substance and relates to the technical field of medicines. The preparation method comprises steps as follows: epinastine is takenas a raw material, an oxidizing agent, a catalyst and a reaction system solvent are added, the mixture is stirred uniformly, an organic solvent is added after the reaction ends, extraction, washing, drying and suction filtration under reduced pressure are sequentially performed, and the organic solvent is recovered. An epinastine impurity A with the purity up to 97% is obtained through oxidation preparation, separation and purification steps, and the method is simple in preparation process, low in cost and high in yield.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
Method for refining brexpiprazole
The invention belongs to the field of medicinal chemistry, and relates to a refined method of ebiprazole, which involves stirring and crystallizing crude ebiprazole with a mixture of ethers and ketones; collecting by filtration, washing, and vacuum drying to obtain pure ebiprazole The yield of prarazole crystals is more than 90%, and the purity (HPLC) reaches 99.4%, which reduces the impurities to about 0.1%.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Preparation method for epinastine intermediate
InactiveCN105061246AAvoid generatingAvoid introducingOrganic compound preparationCarboxylic acid amides preparationBoiling pointProduct gas
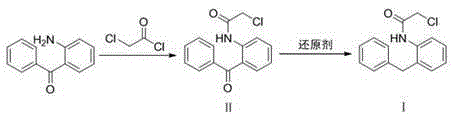
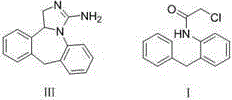
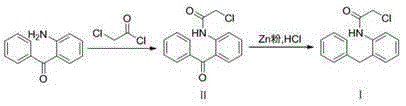
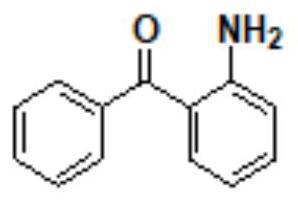
The present invention discloses a new method for preparing an epinastine intermediate N-[2-(phenyl methyl) phenyl]-2-chloroacetamide. The method comprises the following steps of: by taking 2-aminobenzophenone as a starting raw material, firstly performing acylation and then performing reduction;, and introducing HCl gas to carry out selective reduction reaction for reducing carbonyl into methylene in the presence of Zn powder. The preparation method has the advantages of avoiding introduction of a solvent with a high boiling point and a toxic agent; and the method is simple in operation, mild in reaction condition, short in reaction time, low in cost, small in pollution, relatively high in product yield and product purity, and is suitable for industrial mass production.
Owner:UNIV OF JINAN
Pharmaceutical formulations containing combinations of epinastine, pseudoephedrine, and methylephedrine
A pharmaceutical composition comprising: (a) an antihistaminically-effective amount of epinastine or a pharmaceutically acceptable salt thereof; (b) a decongestant-effective amount of pseudoephedrine or a pharmaceutically acceptable salt thereof; (c) methylephedrine or a pharmaceutically acceptable salt thereof, and (d) a pharmaceutically acceptable carrier or excipient. wherein the composition does not comprise Belladonna.
Owner:BOEHRINGER INGELHEIM INT GMBH
A kind of freeze-dried oral preparation containing ebiprazole and preparation method thereof
ActiveCN105078910BDisintegrates quicklyRapid dissolutionOrganic active ingredientsPowder deliveryDissolutionPharmaceutical formulation
Owner:CHENGDU XINJIE HIGH TECH DEV CO LTD
Percutaneously absorptive ophthalmic preparation comprising epinastine
Owner:SENJU PHARMA CO LTD
Synthesis of Epilastine
The invention discloses a method for synthesizing epinastine. The method comprises the following steps: (1) reacting 6-halomethylmorphanthridine with hexamine in an organic solvent, thereby obtaining a 6-halomethylmorphanthridine quaternary ammonium salt; (2) dissolving the 6-halomethylmorphanthridine quaternary ammonium salt in the organic solvent to carry out an acid hydrolysis reaction, thereby obtaining 6-halomethylmorphanthridine hydrochloride; (3) reducing the product 6-halomethylmorphanthridine hydrochloride obtained in the step (2) by using a reducing agent, thereby obtaining 6-aminomethyl-6,11-dihydro-5H-dibenzo[b,e]aza-cycloheptatrien; and (4) adding cyanogen bromide to carry out a ring-closure reaction, thereby obtaining the epinastine. According to the synthetic method disclosed by the invention, use of high-price and flammable lithium aluminum hydride or aluminum hydride is avoided, use of virulent sodium cyanide is avoided, and the security risk and production cost are effectively reduced. The method disclosed by the invention is simple in synthetic process, the reaction conditions are mild, the product is high in yield and high in purity, and industrial production is facilitated.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
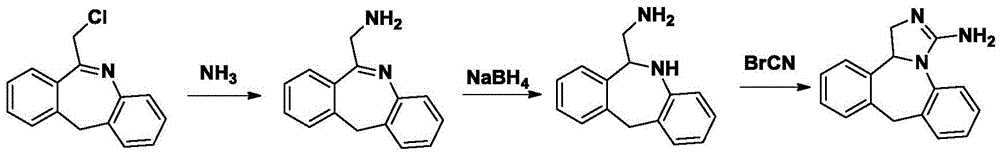
Chemical synthesis method for epinastine
InactiveCN101130544BHigh purityEasy to makeOrganic chemistryImmunological disordersChemical synthesisBenzene
The invention discloses a new chemical synthesizing method of yipisidin, which comprises the following steps: ammonifying 6-chloromethyl-11-dihyrogen-dibenz [b,e] aza to generate 6-aminomethyl-11-dihydrogen-dibenz [b,e] aza; reducing the 6-aminomethyl-11-dihydrogen-dibenz [b,e] aza into 6-aminomethyl-6,11-dihydrogen-5H-dibenz [b,e] aza; generating the product through cyanogen bromide to loop. Theinvention simplifies the making method with little by-product, which improves the receiving rate by 69% with high purity (HPLC. 99. 0%) for industrial manufacturing.
Owner:HANGZHOU LONGSHAN CHEM CO LTD
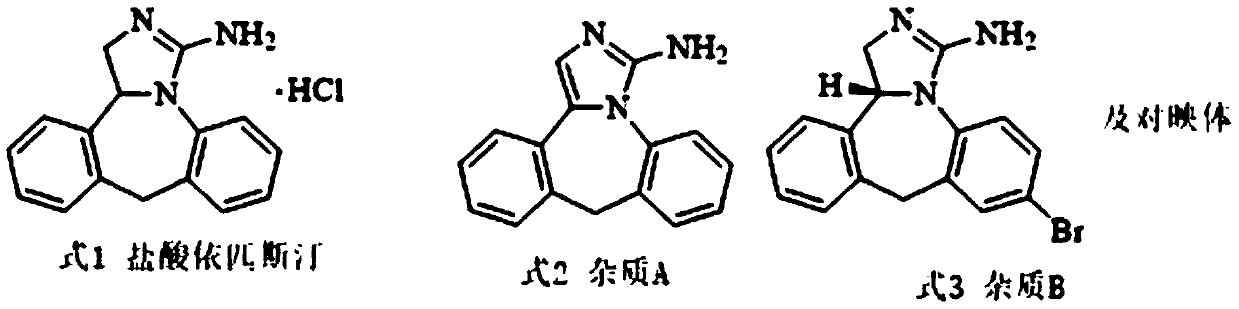
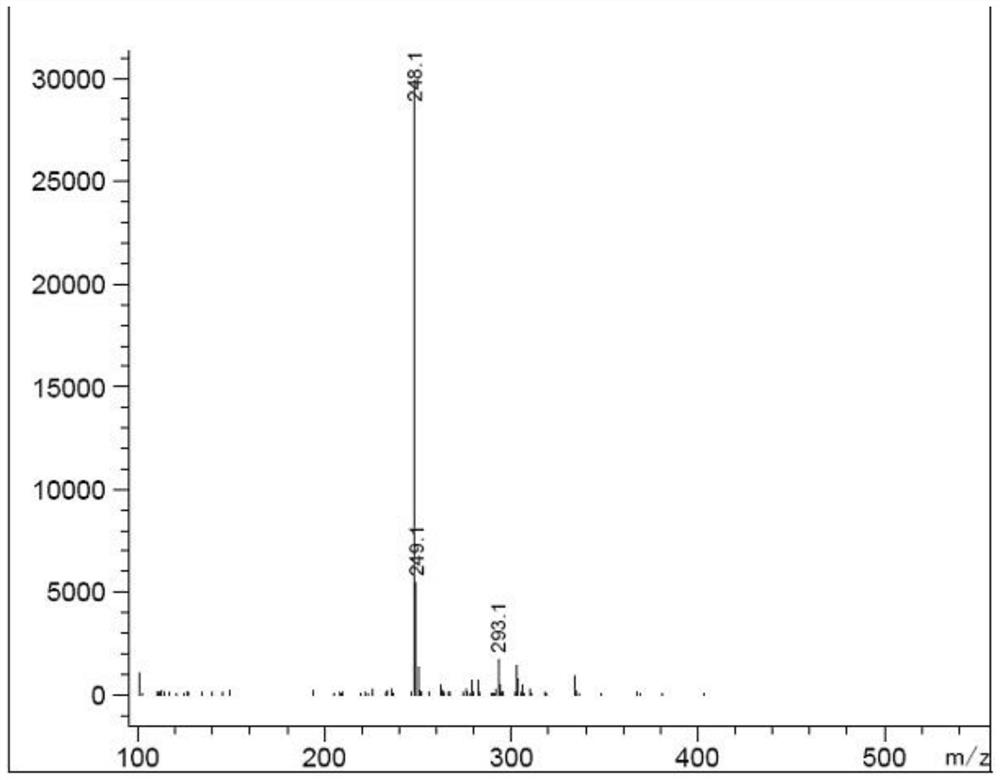
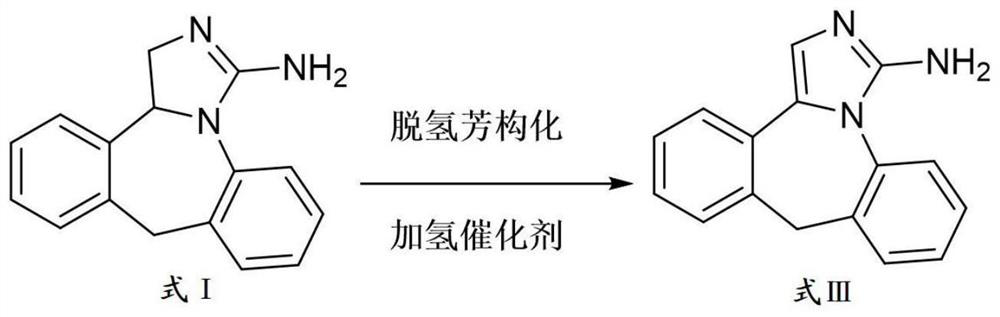
Preparation method of epinastine impurity A
The invention belongs to the technical field of medicines, and particularly relates to a preparation method of an epinastine impurity A. The invention provides a preparation method of an epinastine impurity A. The preparation method comprises the following steps: step 1, mixing epinastine free alkali, a hydrogenation catalyst and a reaction solvent, and carrying out heating reaction to prepare a reactant 1; step 2, filtering the reactant 1, removing the hydrogenation catalyst, and concentrating to obtain a reactant 2; and step 3, mixing the reactant 2 with a pulping solvent, pulping, filtering and drying to obtain an epinastine impurity A. The epinastine free alkali has a structure as shown in a formula I. The invention provides a preparation method of an epinastine impurity A. The technical defects of low purity and low yield of the prepared epinastine impurity A caused by many side reactions or low reaction conversion rate of an existing preparation method of the epinastine impurity A can be effectively overcome.
Owner:广州艾格生物科技有限公司
Synthesis method of epinastine
The invention discloses a synthesis method of epinastine. The synthesis method is implemented by taking 2-aminobenzophenone as a raw material and comprises the following steps of: reacting the 2-aminobenzophenone with a silane agent to obtain 2-benzylaniline; then, carrying out acylation reaction on the 2-benzylaniline and 2-chloroacetyl chloride to obtain N-(2-benzyl phenyl)-2-chloroacetamide; carrying out acidamide dehydration and cyclization on the N-(2-benzyl phenyl)-2-chloroacetamide under the action of a dehydrating agent to obtain 6-(chloromethyl)-11H-dibenzo[b,e] azepine; carrying out azidation reaction on the 6-(chloromethyl)-11H-dibenzo[b,e] azepine to obtain 6-(azido-methytbiphenyl)-11H-dibenzo[b,e] azepine; carrying out reduction on the 6-(azido-methytbiphenyl)-11H-dibenzo[b,e] azepine to obtain 6-(aminomethyl)-6,11-dihydro-1H-dibenzo[b,e] azepine; and finally, carrying out cyclization on the 6-(aminomethyl)-6,11-dihydro-1H-dibenzo[b,e] azepine and cyanogen bromide to obtain the epinastine. The synthesis method disclosed by the invention avoids the application of expensive and flammable lithium aluminium hydride and aluminium hydride as well as hypertoxic sodium cyanide, so that the operation is safer in industrial production, and the cost is reduced. The method is simple in process and high in yield, requires mild conditions, and is suitable for industrialized production.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
A kind of freeze-dried oral preparation containing ebiprazole and preparation method thereof
ActiveCN105106142BDisintegrates quicklyRapid dissolutionOrganic active ingredientsPowder deliveryFreeze-dryingDissolution
The invention provides a freeze-dried oral preparation containing ebiprazole and a preparation method thereof, belonging to the field of pharmaceutical preparations. The freeze-dried oral preparation includes a medicinal amount of ebiprazole and pharmaceutical auxiliary materials. The preparation method prepares raw materials and auxiliary materials into a freeze-dried oral preparation by adopting a freeze-drying method. The present invention fills the blank of ebiprazole in the technical field of freeze-dried preparations by preparing ebiprazole bulk drug into freeze-dried oral preparations, not only provides clinical patients with a variety of drug dosage forms, but also contains ebiprazole The lyophilized oral formulation of azole can disintegrate quickly, so that the ebiprazole can be quickly dissolved and absorbed by the patient, and the bioavailability is improved; at the same time, the lyophilized oral formulation can be disintegrated in the patient's mouth without water, so that Part of the drug can be absorbed through mucosal transport, thus realizing pregastric absorption, avoiding the stimulation and damage of the drug to the stomach, and improving the compliance of clinical patients.
Owner:CHENGDU XINJIE HIGH TECH DEV CO LTD
Synthesis method of epinastine
PendingCN114835712AReduce usageLower recovery costsOrganic compound preparationAmino compound preparationDouble bondSodium azide
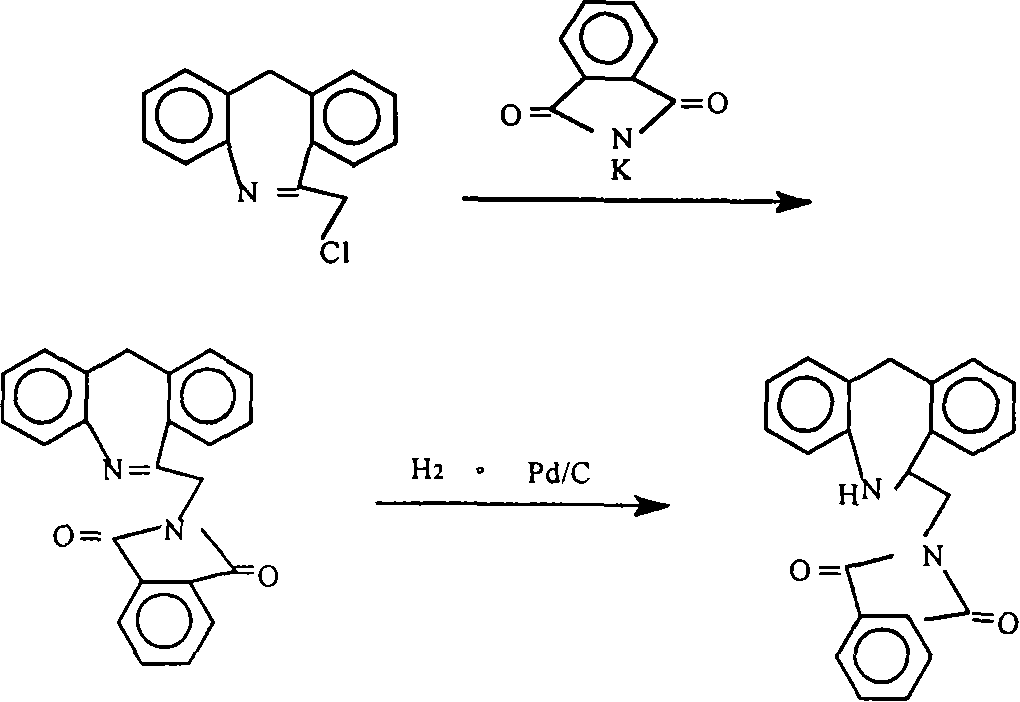
The invention discloses a synthesis method of epinastine, which comprises the following steps: taking cheap and easily available 2-aminobenzophenone as an initial raw material, reducing through hydroboron and lewis acid to obtain 2-benzylaniline, acylating with an acid-binding agent and chloroacetyl chloride to obtain N-[2-(phenylmethyl) phenyl]-2-chloroacetamide, cyclizing with a dehydrating agent to obtain 6-chloromethylmorphanthridine, and purifying to obtain the epinastine. The preparation method comprises the following steps: carrying out a condensation reaction on 2-(4-chlorophenyl)-6, 11-dibenzo-[b, e] azepine under the action of an acid-binding agent to obtain 6-(phthalimidomethyl)-6, 11-dihydro-dibenzo-[b, e] azepine, reducing carbon-carbon double bonds under the action of hydroboron to obtain 6-(phthalimidomethyl)-6, 11-dihydro-5H-dibenzo-[b, e] azepine, and carrying out a condensation reaction on the 6-(phthalimidomethyl)-6, 11-dihydro-5H-dibenzo-[b, e] azepine and the 6-(phthalimidomethyl)-6, 11-dihydro-dibenzo-[b, e] azepine. The preparation method comprises the following steps: firstly, preparing 6-aminomethyl-6, 11-dihydro-5H-dibenzo [b, e] azepine, hydrolyzing to obtain 6-aminomethyl-6, 11-dihydro-5H-dibenzo [b, e] azepine, and finally cyclizing with cyanogen bromide to obtain epinastine. According to the synthesis method disclosed by the invention, the use of an expensive silane reagent is avoided, the synthesis cost is greatly reduced, dangerous chemicals such as sodium azide and lithium aluminum hydride are not used, and the industrial operation is easy.
Owner:重庆恩联生物科技有限公司
Ophthalmic composition for suppressing degradation of soft contact lens
The present invention provides: an ophthalmic composition which has an effect of suppressing the degradation of a soft contact lens, the ophthalmic composition containing a boric acid or a salt thereof and an epinastine or a salt thereof; and a pollen rupture inhibitor containing a specific concentration of a boric acid or a salt thereof and an epinastine or a salt thereof.
Owner:SANTEN PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com