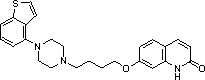
A kind of freeze-dried oral preparation containing ebiprazole and preparation method thereof
An oral preparation, ebiprazole technology, applied in the field of freeze-dried oral preparations containing ebiprazole and its preparation, can solve problems such as blanks, and achieve the goals of avoiding irritation and damage, realizing pregastric absorption, and improving bioavailability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] This embodiment provides three kinds of freeze-dried oral preparations containing ebiprazole, and their prescriptions and contents are shown in Table 1 below:
[0042] Table 1
[0043] prescription
1
2
3
Ebiprazole
0.25mg
2.1mg
4mg
Hydroxypropyl-β-cyclodextrin
5mg
15mg
25mg
gelatin
1mg
5.5mg
10mg
Mannitol
2mg
13mg
25mg
aspartame
0.001mg
0.005mg
0.01mg
Anhydrous citric acid
0.1mg
0.395 mg
0.8mg
[0044] In the above prescription, aspartame and anhydrous citric acid can also be used as flavoring agents alone, and the dosage is 0.001~0.81mg. Preferably, aspartame and anhydrous citric acid are selected as flavoring agents at the same time to adjust the sweetness and sourness of the medicine. The above-mentioned D-mannitol is used as a propping agent for the skeleton support of the lyophilized oral preparation, hydroxypropyl-β-cyclodextrin ...
Embodiment 2
[0047] This embodiment provides a method for preparing the lyophilized oral preparation containing ebiprazole described in Example 1, comprising the following steps:
[0048] A. Measure 400ml of purified water into the container, add the prescribed amount of hydroxypropyl beta cyclopaste and stir until clarified; then add ebiprazole in batches, and continue stirring; after the solution is clarified, add in sequence Gelatin, mannitol, aspartame, and anhydrous citric acid were stirred separately until clarified and diluted to 500ml with purified water to obtain a clarified medicinal solution.
[0049] B. Pour the clarified medicinal solution obtained in step A into a cold aluminum foil mold in a 0.5ml single-dose package, use liquid nitrogen (-80°C) spray technology to freeze quickly, and transfer it to a freeze dryer for freeze-drying. The moisture content is <2%, and the lyophilized oral preparation is obtained.
[0050] The above-mentioned freeze-drying includes four stages:...
Embodiment 3
[0054] In order to make the weight of the lyophilized oral preparation lighter, this embodiment optimizes the prescription in Example 1, because hydroxypropyl-β-cyclodextrin can not only be used as a solubilizing excipient, but also as a proppant, Therefore, the use of D-mannitol was canceled. The prescription of this lyophilized oral preparation is shown in Table 2 below:
[0055] Table 2
[0056] prescription
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com