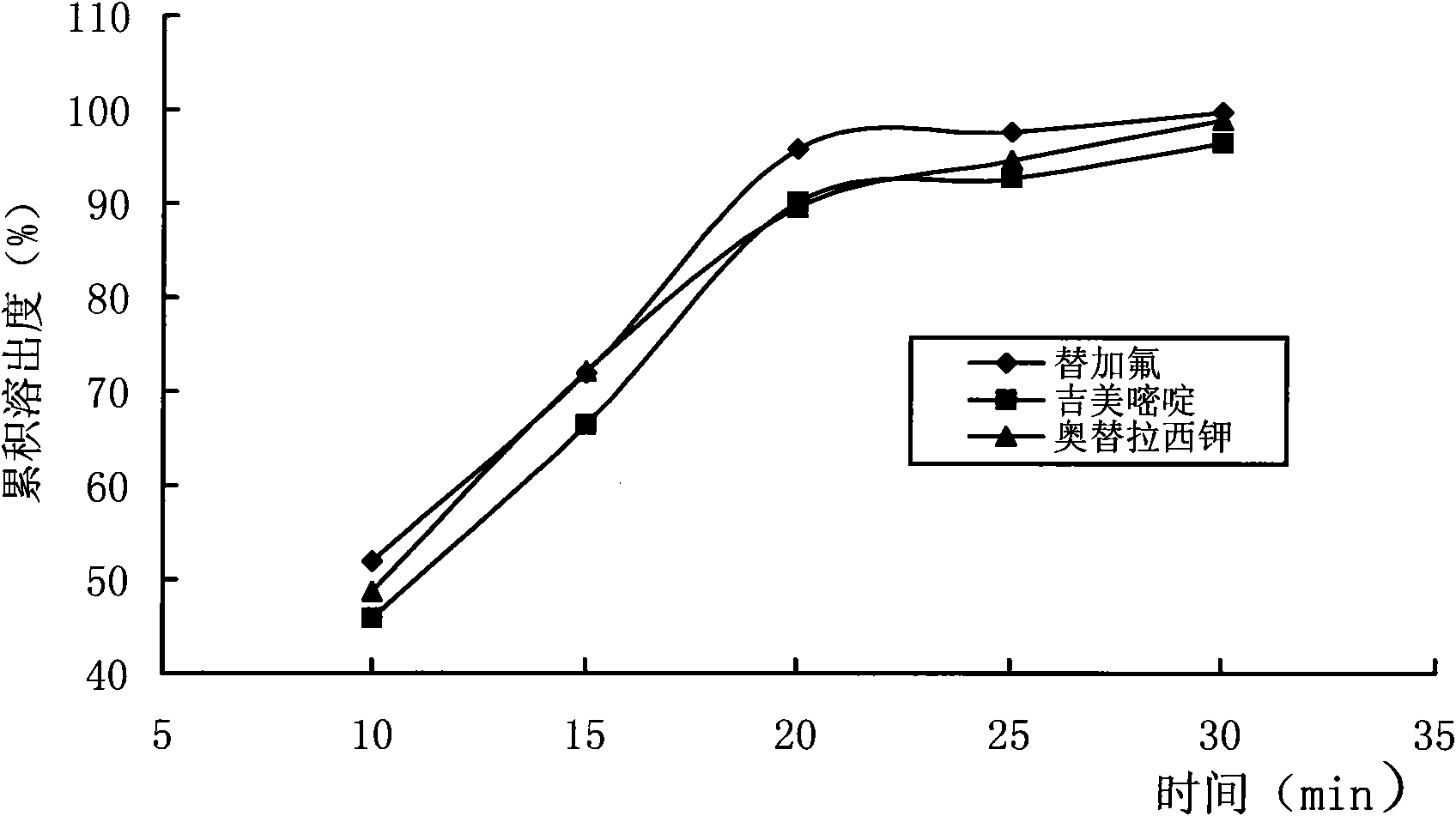
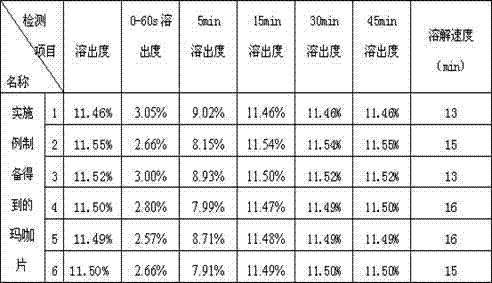
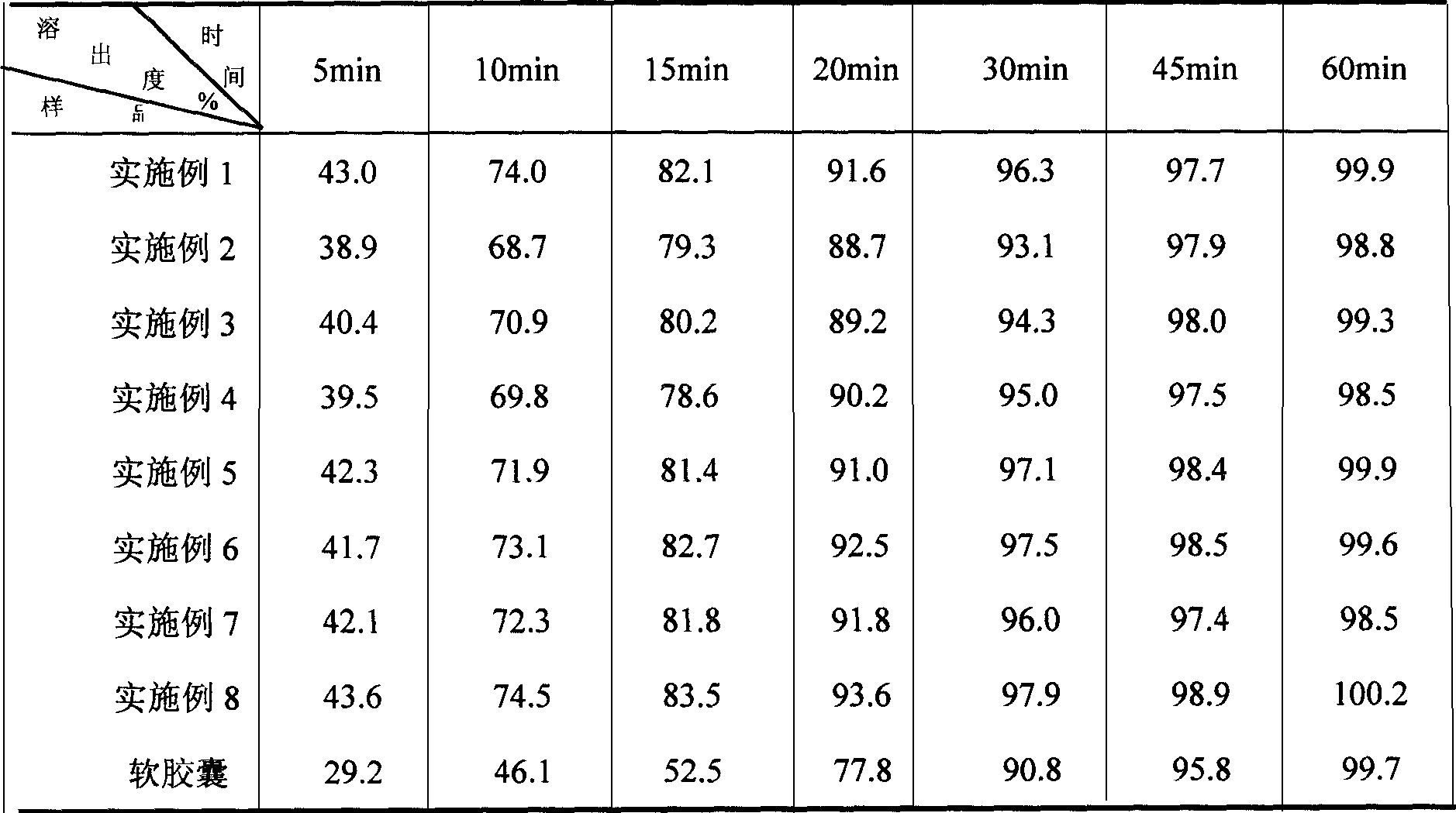
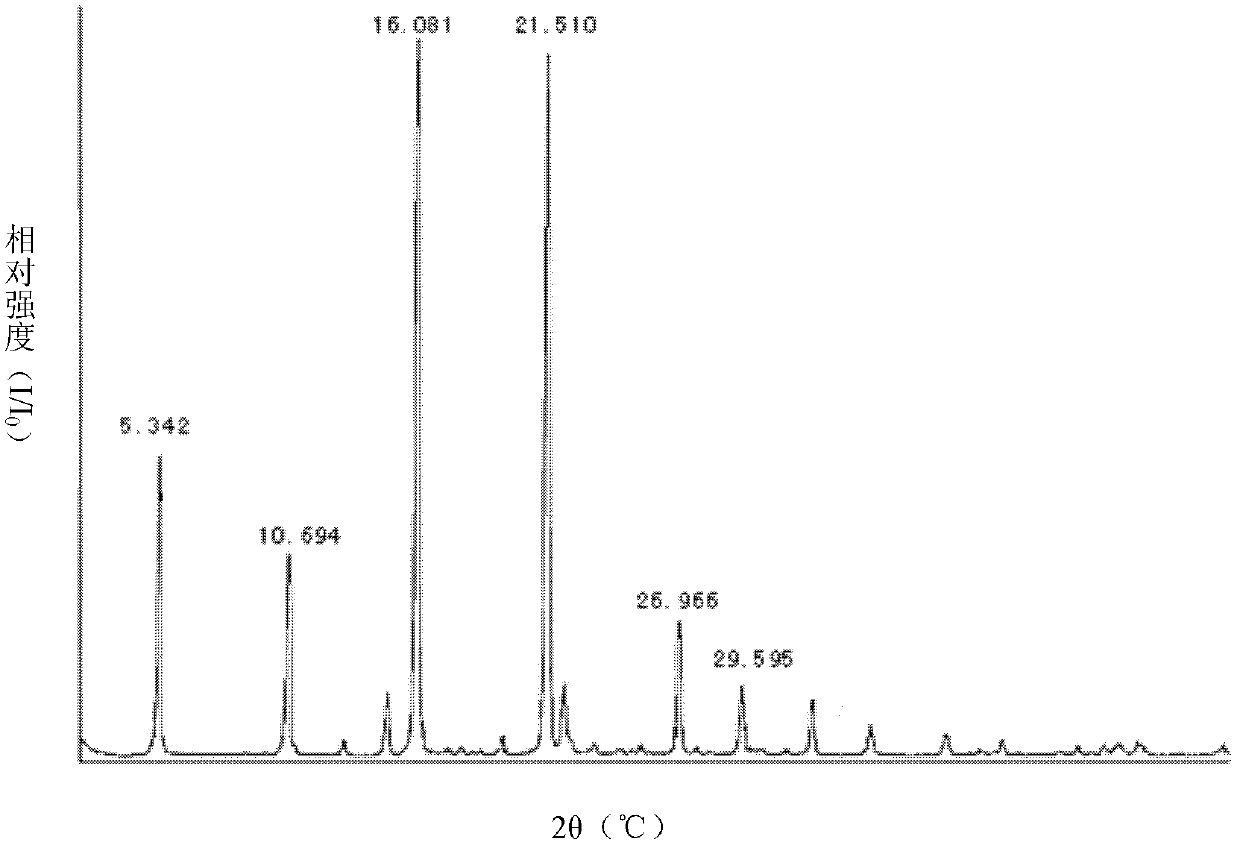
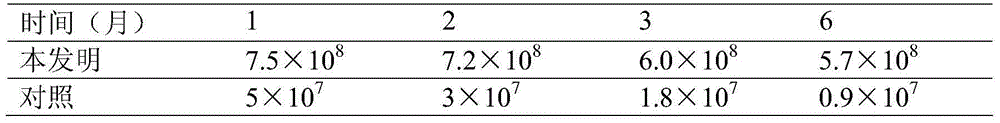Patents
Literature
791results about How to "Rapid dissolution" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nanoparticulate formulations of docetaxel and analogues thereof
InactiveUS20060188566A1Rapid dissolutionLow injection volumeBiocideOrganic active ingredientsDocetaxelDocetaxel-PNP
Owner:ELAN PHRMA INT LTD
Liquid compound biological fertilizer with long shelf life as well as preparation method and application thereof
ActiveCN103553828AAdjust pHSimple production processFertilizer mixturesBacillus megateriumBacillus thuringiensis
The invention relates to a liquid compound biological fertilizer with long shelf life as well as a preparation method and application thereof. The liquid compound biological bacterial fertilizer comprises the following components in parts by weight: 2-5 parts of microbial thalli, 80-130 parts of compound nutrients, 10-30 parts of thalli protective agents and 0.5-3.0 parts of additives, wherein the microbial thalli is metered in terms of the wet weight of deposition obtained after fermentation liquor is centrifugalized, the microbial thalli include bacillus thuringiensis, bacillus megaterium, bacillus mucilaginosus and bacillus amyloliquefaciens, and the total viable count achieves 5*10<8>-5*10<9> / mL. The liquid compound biological bacterial fertilizer disclosed by the invention can be used as a biological pesticide, can be directly applied to the spraying of plant surfaces or applied to seed soaking, root irrigation, and the like, has the effects of insect resistance, phosphate solubilization, potassium solubilization, organic matter solubilization, pest resistance, and the like and is long in shelf life.
Owner:天津北洋百川生物技术有限公司
Solid dispersion and tablets comprising abiraterone acetate, and preparation methods thereof
ActiveCN103070828AAdvantages and Notable ImprovementsGood water solubilityOrganic active ingredientsPowder deliverySolubilityMedicine
The invention relates to a solid dispersion and tablets comprising abiraterone acetate, and preparation methods thereof. The solid dispersion is prepared through the steps that: abiraterone acetate and povidone with a weight ratio of 1:0.5-4 are dissolved in chloroform; and reduced-pressure drying is carried out, such that the solid dispersion is obtained. The tablets comprises 1 part of the abiraterone acetate solid dispersion, 2-8 parts of a filling agent, 0.2-0.8 parts of a disintegrating agent, and 0.05-0.1 parts of a lubricant. According to the invention, a micronization technology and a solid dispersion technology are creatively combined, such that abiraterone acetate water solubility is greatly improved. Therefore, abiraterone acetate can be rapidly dissolved in gastrointestinal tract body fluids.
Owner:SHANDONG NEWTIME PHARMA
TS-1 granules and preparation method thereof
ActiveCN101843621AAvoid interactionImprove stabilityPharmaceutical non-active ingredientsGranular deliverySolubilityPotassium oxonate
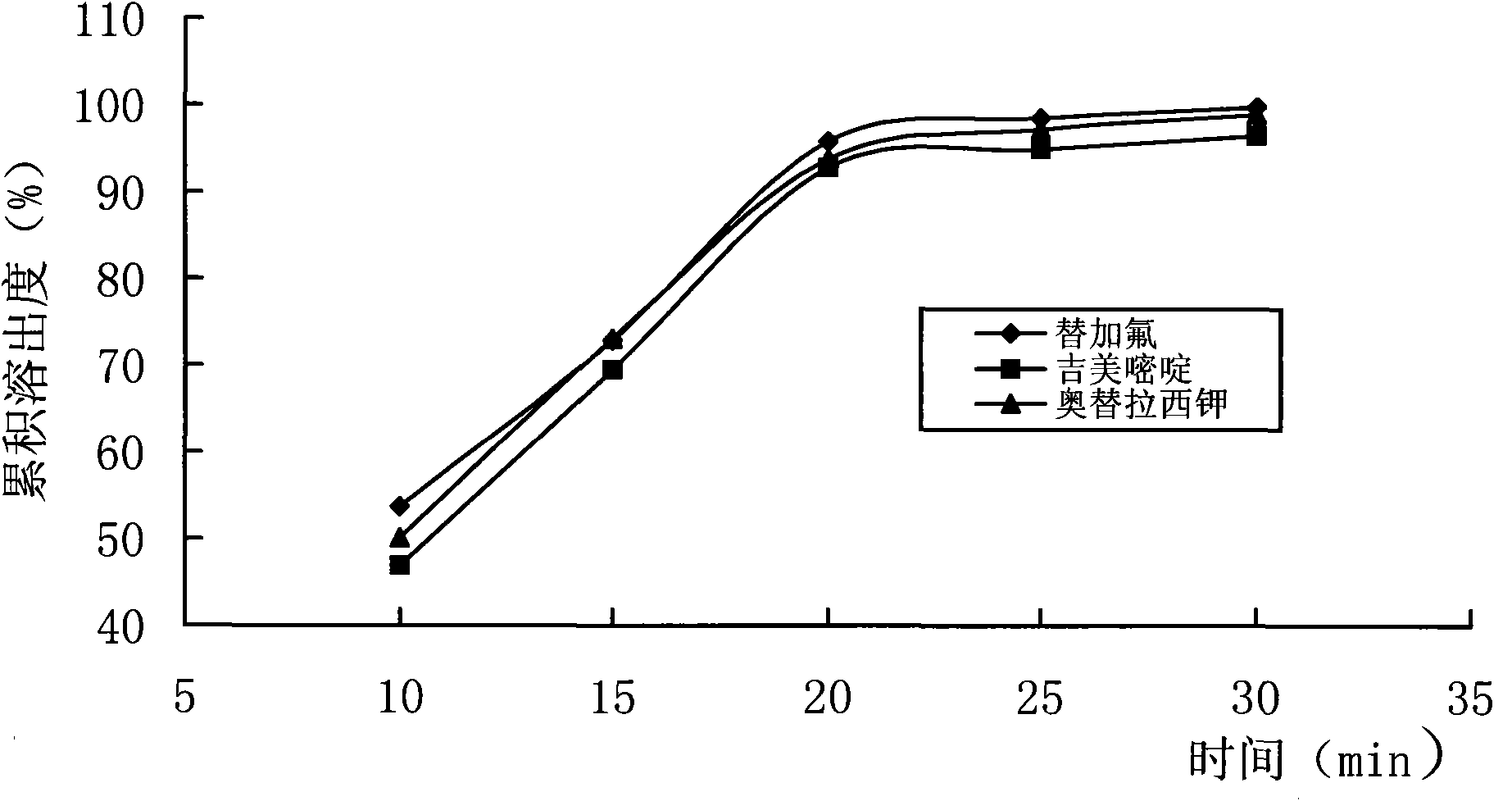
The invention belongs to the technical field of medicaments, and relates to TS-1 granules for treating advanced gastric cancer and a preparation method thereof. The method comprises the following steps of: preparing active medicaments tegafur, gimeracil and potassium oxonate into a cyclodextrin inclusion compound; and sieving the inclusion compound, uniformly mixing the sieved inclusion compound and pharmaceutically acceptable auxiliary materials and performing wet granulation or dry granulation to prepare the granules. The granules have the advantages of increasing the water solubility and stability of the medicaments, covering the bitter taste of the tegafur, prolonging the action time, improving the medication compliance of patients with cancer, greatly improving the bioavailability of the medicaments and reducing the toxic or side effect of the medicaments.
Owner:鲁南新时代生物技术有限公司
Maca tablet and preparation method thereof
InactiveCN102823798AImprove bioavailabilityRapid dissolutionFood preparationMagnesium stearateStearic acid
The invention provides a maca tablet and a preparation method thereof and relates to the technical field of health care products. The maca tablet is prepared by the following raw materials by weight: 581.84 g of maca powder, 56.48 g of microcrystalline cellulose, 30.4 g of lactose, 78.56 g of povidone, 15.12 g of magnesium stearate and 37.6 g of starch; and the average relative molecular weight of the povidone is 2000. The preparation method of the maca tablet comprises the following steps of: uniformly mixing all the raw materials, spraying 90 percent of ethanol solution to moisten, palletizing, drying, carrying out intermediate inspection, granulating and tabletting; and spraying 456.5 g of ethanol solution on every 800 g of raw materials. The maca tablet has the advantages that the effective components are quickly dissolved out, the biological availability is high, and the medical effects of effective component maca can be better realized, and the cost for drug consumption is reduced. The maca tablet has the effects of relieving the physical fatigue, improving the sexual function, improving the fertility, adjusting the internal secretion and the like, and particularly relieving the physical fatigue and the improving the sexual function.
Owner:SHANDONG YIBAO BIOLOGICS
Bean curd fruit glycocide softcapsule
InactiveCN1596903ALarge specific surface areaRapid dissolutionOrganic active ingredientsNervous disorderMedicineSolvent
Owner:KUNMING ZIJIAN BIOTECH
Butylphthalide dripping pill and preparing method
ActiveCN1943571AFast oral onsetPromote absorptionOrganic active ingredientsDispersion deliveryButylphthalideHypromellose
The invention relates to a drip pills of butylbenzene phthalein and its preparation method and it is characterized by comprising butylbenzene phthalein, base material, dispersing agent and coating materials. PEG4000, PEG6000, PEG20000 and poloxamer are selected as base materials. Dispersing agent is from lightweight aerosol and crospovidone. And coating materials are from hypromellose, hydroxypropyl cellulose, ethyl cellulose and Eudragit E30D.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Celecoxib-containing solid dispersion and preparation method thereof
InactiveCN103371976AImprove liquidityEasy to shapeOrganic active ingredientsPowder deliveryVitrificationPolyethylene glycol
The invention provides a celecoxib-containing solid dispersion and a preparation method thereof. The celecoxib-containing solid dispersion comprises celecoxib and copovidone. The celecoxib-containing solid dispersion provided by the invention has the advantages of high rigidity, good friability and moderate glass temperature, can be suitable for large-scale industrialized production and overcomes the defects of low glass temperature, soft material, easy melting and bonding, difficulty in crushing and the like of a celecoxib solid dispersion which takes polyethylene glycol as a carrier.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Secnidazole tablet and its prepn process
InactiveCN1973838AHigh dose of main drugPiece weight smallAntibacterial agentsOrganic active ingredientsCarboxymethyl celluloseMagnesium stearate
The secnidazole tablet contains secnidazole 50-90 wt%, microcrystalline cellulose, starch, pre-gelatinized starch and / or lactose 0.5-40 wt%, cross-linked sodium carboxymethyl cellulose and / or cross-linked polyvinyl pyrrolidone 0.5-9 wt%, polyvinyl pyrrolidone or hydroxypropylmethyl cellulose 0.1-2.5 wt%, magnesium stearate 0.5-1 wt%, and talcum powder and / or fine silica gel powder 0.5-1 wt%. The preparation process of the secnidazole tablet includes the steps of mixing the said materials, palletizing and tabletting. The secnidazole tablet has high effective component content, easy swallowing, no bitter taste and fast leaching.
Owner:湖北科益药业股份有限公司
Tuble vegetable function fertilizer
InactiveCN106699450AAvoid deformitiesVigorousSuperphosphatesMagnesium fertilisersPhosphatePhosphoric acid
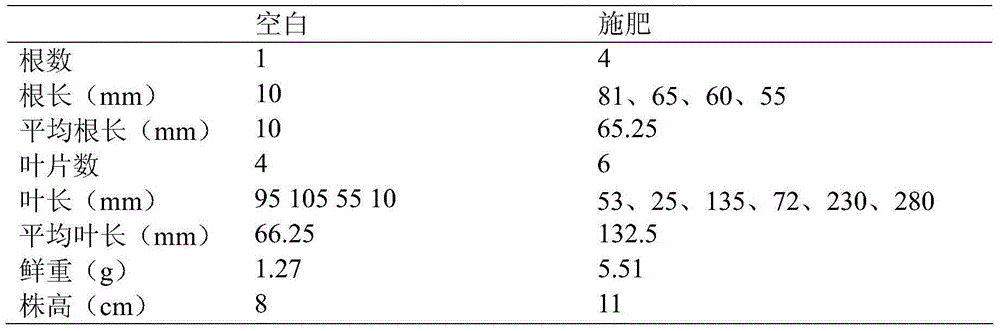
Provided is a tuble vegetable function fertilizer. The tuble vegetable function fertilizer is prepared from, by weight, 25-35 parts of urea, 1-5 parts of ammonium sulfate, 20-35 parts of monoammonium phosphate, 35-45 parts of potassium sulfate, 1-5 parts of organic carbon, 1-5 parts of potassum fulvic acid, 0.5-1 part of calcium superphosphate, 0.1-0.5 part of borax, 0.5-1 part of magnesium sulfate, 1-5 parts of mixed fungicide, 0.1-0.5 part of synergist and 1-5 parts of other additives. A preparation method comprises the steps that the pH value is adjusted for a fermentation waste solution, microelement is added after heating for a complexation reaction, and organic carbon is obtained; the urea, ammonium sulfate, monoammonium phosphate, potassium sulfate, calcium superphosphate, borax, magnesium sulfate, synergist and other additives are prepared into particles; organic carbon and potassum fulvic acid wrap the outer layers of the particles, and finally the tuble vegetable function fertilizer can be obtained by adding the mixed fungicide. According to the tuble vegetable function fertilizer, comprehensiveness of tuble vegetable growth nutrients is effectively guaranteed, soil insect damage is reduced, malformation of tuble crops can be prevented, and the yield is increased.
Owner:SHENZHEN BATIAN ECOTYPIC ENG
Chinese medicinal composition granules and preparation method thereof
ActiveCN101850066AGood effectSafe to takeAntinoxious agentsGranular deliveryDiseaseSalvia miltiorrhiza
The invention relates to Chinese medicinal composition granules and a preparation method thereof. The Chinese medicinal composition granules comprise the following ruptured powder in part by weight: 3 to 18 parts of American ginseng ruptured powder, 1 to 24 parts of pseudo-ginseng ruptured powder, 6 to 36 parts of dendrobium ruptured powder, and 9 to 45 parts of root of red-rooted salvia ruptured powder, wherein the granularity D90 of the ruptured powder is between 5 and 75 mu m. The invention also provides a method for preparing the Chinese medicinal composition granules. The method comprises the following steps of: uniformly mixing the American ginseng ruptured powder, pseudo-ginseng ruptured powder, dendrobium ruptured powder, and root of red-rooted salvia ruptured powder of which the D90 is between 5 and 75 mu m; preparing a soft material by adopting aqueous ethanol at the concentration of over 20 vol percent; and after granulating by using a granulator with 10 to 30 meshes, drying and finishing the granules to obtain the Chinese medicinal composition granules. The Chinese medicinal composition granules are applied to preventing and regulating human cardiac-cerebral vascular system diseases and sub-health state such as weak immunity and fatigability, have the obvious advantages of high medical effect, high quality uniformity, convenient carrying and administration, safety, reliability, and the like, and can meet the requirement on modern fast-paced lifestyle.
Owner:ZHONGSHAN ZHONGZHI PHARMA GRP +1
Preparation process of fish scale collagen protein
ActiveCN103540635ASmall molecular weightTake advantage ofPeptide preparation methodsFermentationWaste materialImpurity
The invention provides a preparation process of a fish scale collagen protein. With the preparation process, the collagen protein can be extracted from fish scales, so that the fish scales are changed into things of value from waste material and thorough utilization of resources is realized. The preparation process comprises the following steps of pretreating, decalcifying, neutralizing and removing impurity, washing by using water, sol, solid-liquid separating and filtering, hydrolyzing, filtering and separating the collagen protein, filtering and separating the macromolecular protein, desalting, dehydrating, drying and the like to obtain collagen protein powder. The collagen protein powder prepared by the preparation process has the molecular weight of less than 5000 Daltons and can be absorbed by the human body easily.
Owner:SHISHI HAIXING FOOD +1
Tacrolimus solid dispersion and its preparing method
InactiveCN1820759ARapid dissolutionImprove bioavailabilityOrganic active ingredientsCapsule deliverySolubilityFreeze-drying
The present invention belongs to the field of chemical medicine preparing technology, and is especially solid Tacrolimus dispersion as one kind of immunodepressant and its preparation process. The present invention adopts Tacrolimus as active medicine component, and prepares the solid Tacrolimus dispersion through adding carrier material, solvent process, solvent-fusing process and freeze drying process, and the solid Tacrolimus dispersion is further prepared into capasule. The present invention has raised medicine solubility, 5-500 times raised solubility in water compared with Tacrolimus, raised leaching speed and increased absorption of the medicine inside body.
Owner:FUDAN UNIV
Ticagrelor solid dispersion and preparation method thereof
ActiveCN104434805ASolve insolubleEvenly dispersedOrganic active ingredientsPowder deliveryPolyvinylpyrrolidoneChemistry
A ticagrelor solid dispersion and preparation method thereof; the solid dispersion is prepared via dispersing the ticagrelor into a carrier material, the carrier material comprising one or more of polyvinylpyrrolidone, copovidone and crospovidone.
Owner:SUNCADIA PHARM CO LTD +1
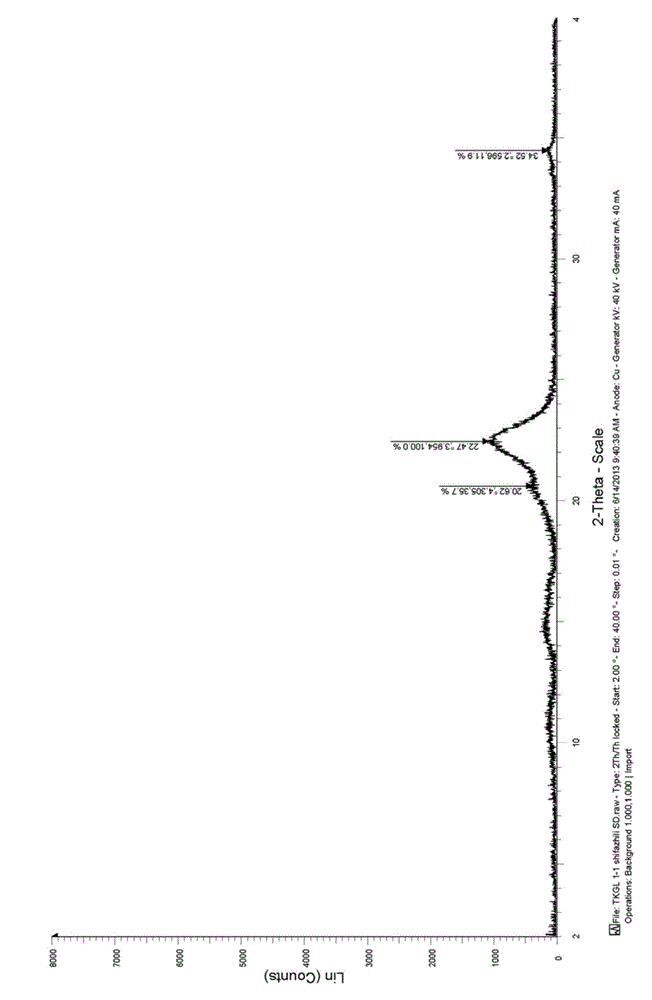
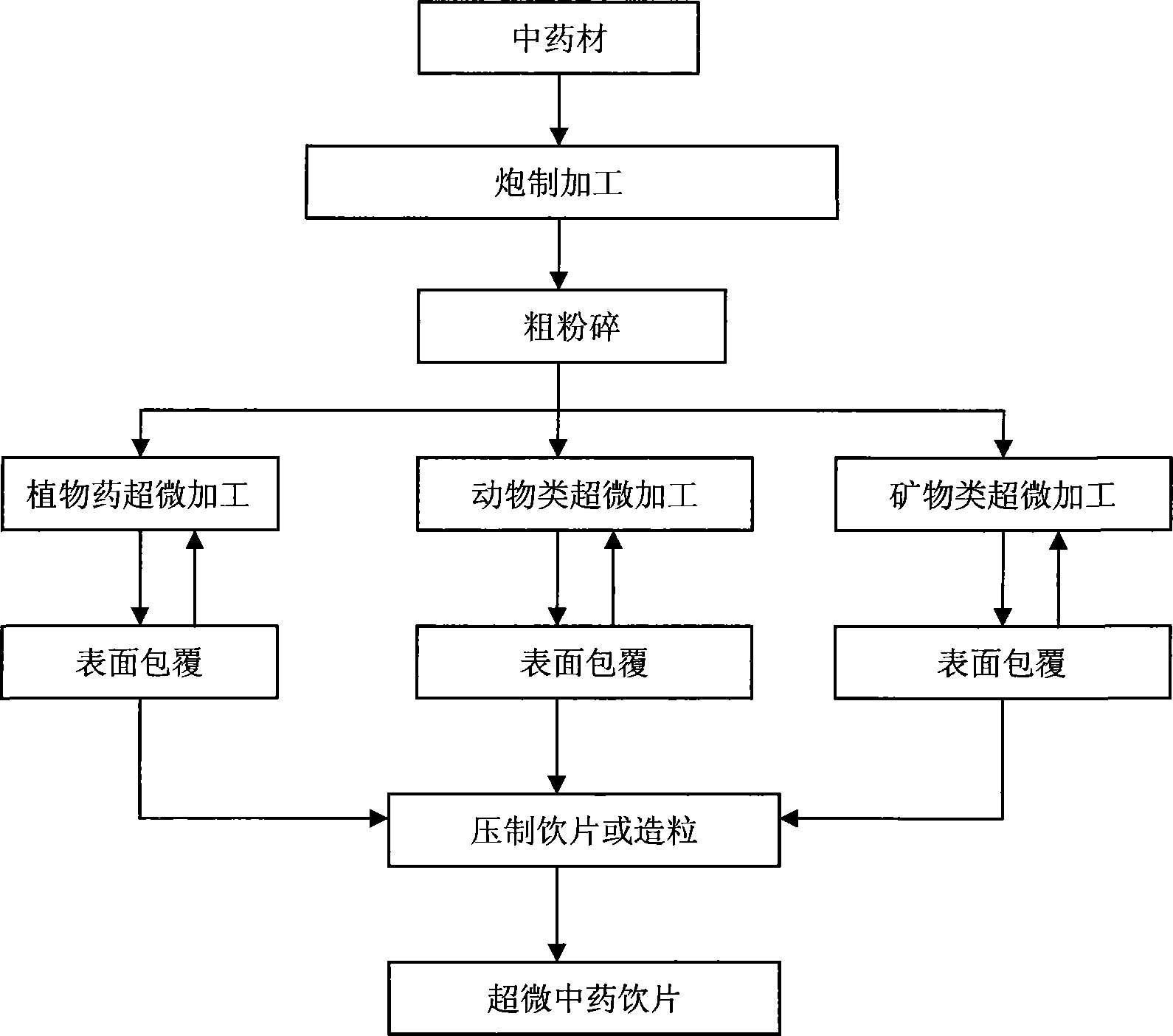
Method for preparing surface cladding super-micro traditional Chinese medicine material crude slice
ActiveCN101143154AEasy to useRapid dissolutionGranular deliveryMacromolecular non-active ingredientsDissolutionTraditional medicine
The invention discloses a preparation method of a decoction piece of superfine traditional Chinese medicine, the surface of which is coated, including the steps of processing, coarse crushing, superfine crushing, tablet forming and granulizing. When the step of the superfine crushing is executed, a surface coating agent is synchronously introduced, and the surface coating agent forms a protective layer at the surface of the traditional Chinese medicine. The method of the invention can retain the effective components of the raw materials of the traditional Chinese medicine and improve the fluidity of the superfine traditional Chinese medicine powder during the processes of traditional Chinese medicine crushing, tablet forming and storing. The decoction piece of superfine traditional Chinese medicine, which is prepared by the invention, can be conveniently used; the dissolution is fast; the biological utilization degree is high; the batch production can be realized.
Owner:GUANGZHOU XIANGXUE PHARMA CO LTD
Pharmaceutical composition for preventing and treating avian coccidiosis and its preparing method
InactiveCN1907358AGood hemostasisGood curative effectAntiparasitic agentsPlant ingredientsCoccidiosisBiotechnology
The invention relates to a pharmaceutical composition for the prevention and treatment of avian coccidiosis and process for preparation, wherein the composition comprises the following raw materials (by weight ratio): dichroa root 10-40 parts, bupleurum root 7-20 parts, flavescent sophora root 15-30 parts, sweet wormwood 7-20 parts, carbonized sanguisorba root 7-20 parts, cogongrass rhizome 7-20 parts. The preparing process comprises steps of ultramicro disintegrating, passing through 100-300 mesh sieves, proportioning and mixing homogeneously.
Owner:TIANJIN SHENGJI GRP CO LTD
Curcumin nanosuspension and preparation method thereof
InactiveCN102961368AInhibit metabolismImprove bioavailabilityNervous disorderAntipyreticSolubilityBioavailability
The invention belongs to the technical field of medicine, and discloses curcumin nanosuspension and a preparation method of the curcumin nanosuspension. As curcumin is poor in stability, small in solubility and low in oral bioavailability, popularization and use of the curcumin are restricted. The curcumin nanosuspension disclosed by the invention is characterized by containing the curcumin, piperine, and zein, and the mass ratio of the curcumin to the piperine to the zein is 10 to (8-9) to (1-2). The preparation method of the curcumin nanosuspension disclosed by the invention is mild in condition, simple and controllable, and the curcumin achieves high oral bioavailability.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
Compound preparation used for joint care and preparation method thereof
ActiveCN101648008AGood treatment effectGood prevention effectPeptide/protein ingredientsSulfur/selenium/tellurium active ingredientsPhosphateArthritis
The invention discloses a compound preparation used for joint care and a preparation method thereof. The compound preparation comprises active ingredients and auxiliary materials, wherein the active ingredients comprise glucosamine, chondroitin sulfate, dimethyl sulfone and collagen, and the glucosamine is glucosamine sulfate or glucosamine hydrochloride or glucosamine phosphate or mixture of theglucosamine sulfate and the glucosamine hydrochloride and the glucosamine phosphate; and the auxiliary materials comprise excipient, disintegrant, lubricant, bonding agent and wetting agent. The invention has better treatment and prevention functions on arthritis and joint injury; the prepared tablet has good disintegration property, rapid digestion, quick effect taking, strengthened stability andhigh absorption and utilization ratio; and the consumption of the raw materials and the auxiliary materials achieves the optimal proportion.
Owner:盛锦合生物科技江苏有限公司
Sustained-release pesticide fertilizer granula and production method thereof
InactiveCN109320340AFast fertilizerImprove fertilizer efficiencyLayered/coated fertilisersFertilizer mixturesCyclodextrinMesoporous silica
The invention relates to the technical field of pesticide preparations and specifically relates to a sustained-release pesticide fertilizer granula and a production method thereof. The pesticide fertilizer granula is prepared from a sustained-release coating layer and an inner effective matter, wherein the sustained-release coating layer is prepared from talcum powder, neutral bentonite, hydroxymethyl cellulose and chitosan which are mixed according to the mass ratio of 8 to 5 to 2 to 1; the effective matter is prepared from sustained-release medicine, compound fertilizer, a wetting expandingagent and a filling agent; the sustained-release medicine is a coated systematic type pharmaceutical preparation; the systematic type pharmaceutical preparation and a mesoporous AcF micro powder carrier are mixed and then coated; a coating material is prepared from the following ingredients of polyglycolic acid, talcum powder, mesoporous silica, cyclodextrin, liquid paraffin, polyvinyl alcohol, calcium dodecyl benzene sulfonate and deionized water. The pesticide fertilizer granula is treated by a special coating technology, has the characteristics of quick and stable fertilizer efficiency andslow and durable insect killing pesticide effect and is suitable for synchronous pesticide fertilizer application of flowers and nursery stock plants.
Owner:JIXI NONGHUA BIOTECH
Zolmitriptan quick-release formulation
ActiveCN1634043ADisintegrates quicklyRapid dissolutionOrganic active ingredientsNervous disorderAdjuvantMedicine
The invention provides a Zolmitriptan quick-release formulation which can further improve the dissolving degree of the main medicament, greatly increase the absorbing velocity of the medicament in stomach and intestine, thus making the curative effect exert completely. Based on the physics and chemistry nature of the Zolmitriptan, adjuvant having specific disintegration and dissolving boosting actions for grease solving Zolmitriptan is selected from a plurality of medicinal findings through experiment.
Owner:LUNAN PHARMA GROUP CORPORATION
Orlistat tablets and preparation method thereof
ActiveCN101791296AGood chemical stabilityImprove physical stabilityOrganic active ingredientsMetabolism disorderOrlistatPharmacy
The invention belongs to the technical field of the medicament and in particular relates to tablets comprising orlistat and a preparation method thereof. Orlistat and cyclodextrin which are subjected to enclosing and auxiliary materials acceptable for pharmacy are pressed into the tablets. The tablets solve the sticking problem of carrying out pressing on the orlistat by the conventional process, obviously improve the chemical and physical stability of the orlistat, cover the unpleasant taste of the orlistat, improve the administration compliance of the dysphagia patients, have good dissolution and improve the curative effect of the orlistat.
Owner:LUNAN PHARMA GROUP CORPORATION
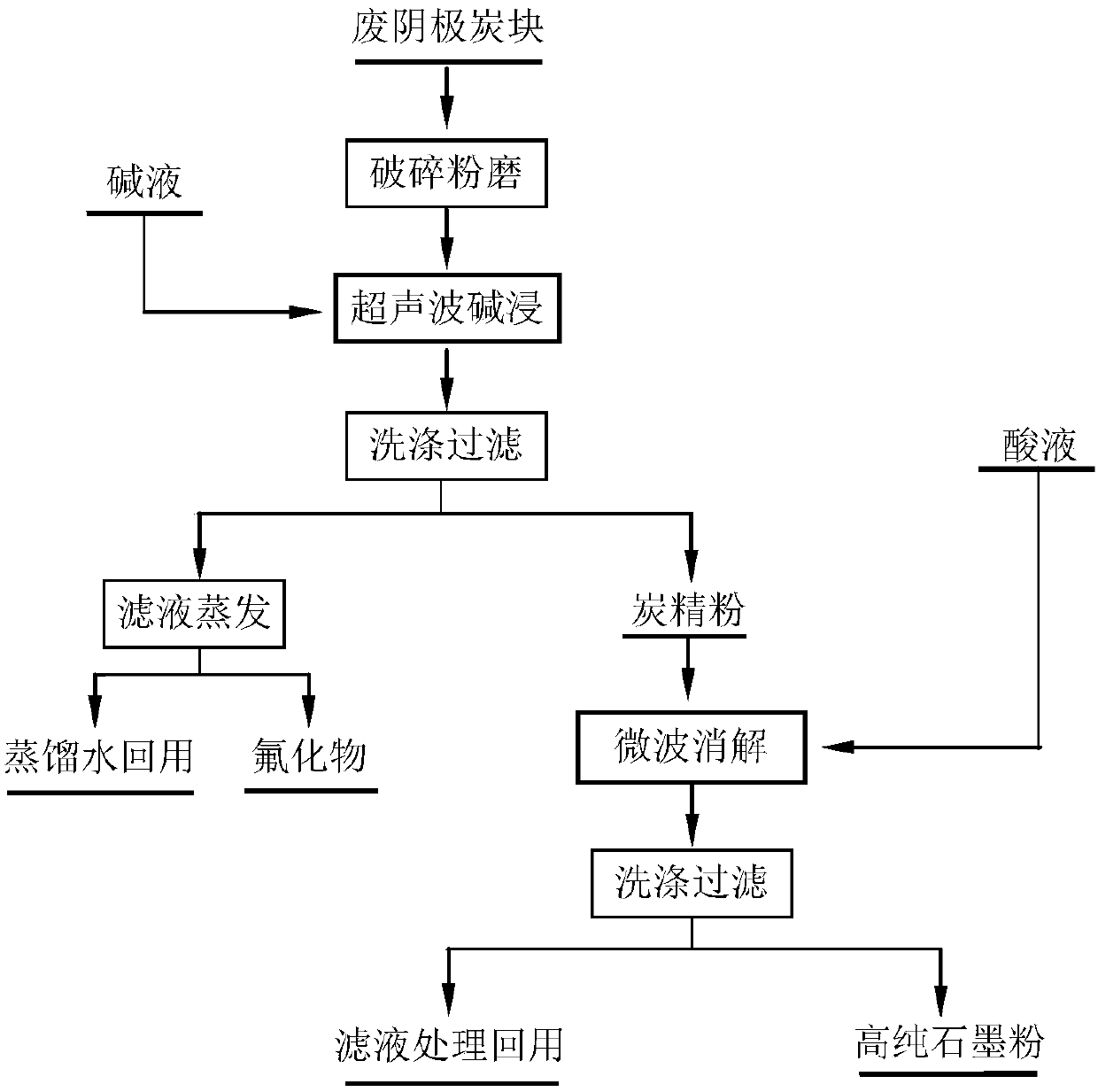
Method for performing ultrasonic alkaline leaching and microwave digestion combined treatment on electrolytic aluminum waste cathode carbon block
InactiveCN107902649AAchieving processing powerImplement resourcesFluoride preparationCarbon compoundsResource utilizationEconomic benefits
The invention relates to a method for performing ultrasonic alkaline leaching and microwave digestion combined treatment on an electrolytic aluminum waste cathode carbon block, and belongs to the technical field of comprehensive utilization of aluminum electrolysis solid waste resources. The method comprises three processes of crushing and grinding, performing ultrasonic alkaline leaching and performing microwave digestion, the ultrasonic alkaline leaching and the microwave digestion are subjected to combined utilization and are synergistic, a carbon material and fluoride are separated and recycled in an efficient and environment-friendly mode, environmental harm is reduced, remarkable environment-protecting benefit and economic benefit are achieved, harmless treatment and resource utilization of the electrolytic aluminum waste cathode carbon block are realized, and the method is a low-energy-consumption, high-efficiency and high-additional-value process technology for performing harmless treatment and resource utilization on the electrolytic aluminum waste cathode carbon block.
Owner:CHONGQING YUANDA CATALYST MFG
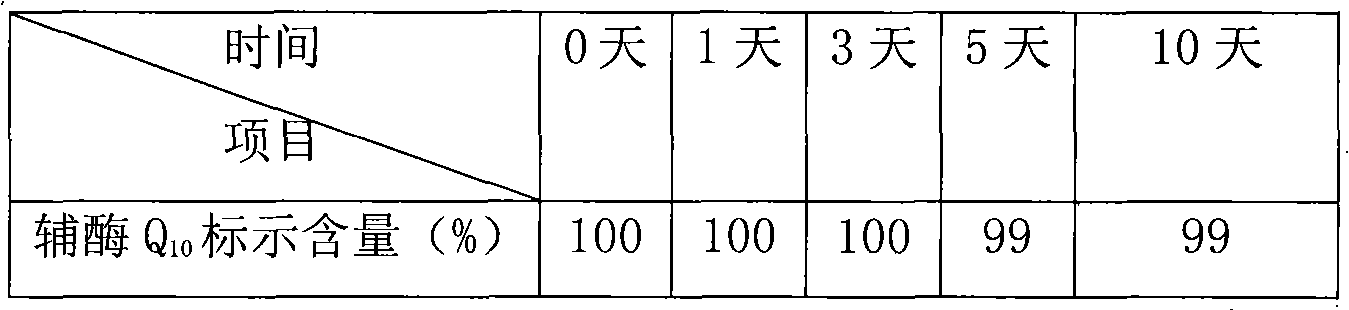
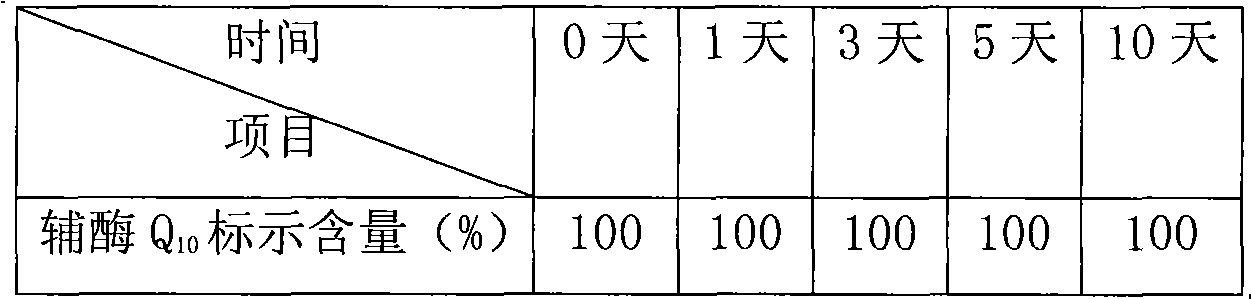
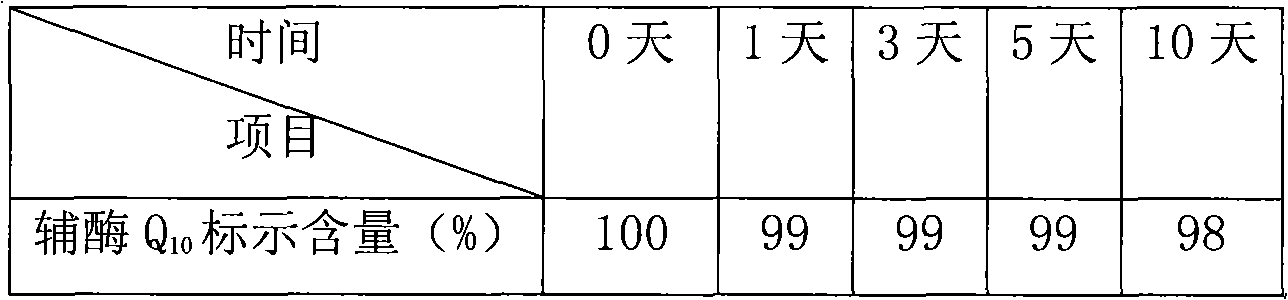
Coenzyme Q10 composite soft capsule and preparation method thereof
ActiveCN101897440AGuaranteed qualityAnti-Aging ProtectionFood preparationWater bathsAdditive ingredient
The invention relates to a coenzyme Q10 composite soft capsule and a preparation method thereof, belonging to the technical field of health-care foods. The coenzyme Q10 composite soft capsule comprises a capsule shell and capsule content, wherein the capsule content comprises the following raw materials in proportions by weight: coenzyme Q10: zinc lactate: sodium selenite: vitamin E: corn oil =1:(0.8-1.6):(0.001-0.005):(1.5-3.5):(15-25); and the soft capsule shell comprises the following ingredients of gelatin, glycerol and pure water. The preparation method comprises the following steps of: preparing the capsule materials: heating the gelatin, the glycerol and the water in water bath to 50-95 DEG C for dissolving according to the proportions of (0.5-2):(0.2-0.8):(0.5-1.5) , and preserving the temperature at 45-80 DEG C; taking the zinc lactate and the sodium selenite according to the formula amount, evenly mixing in an equivalent progressive-increase way, then evenly mixing with the coenzyme Q10, and carrying out ultramicro crushing; putting the natural vitamin E and the corn oil in and stirring in 500-2000rpm to obtain mixed oil, adding fine mixed powder and evenly stirring; and preparing the coenzyme Q10 composite soft capsule by using an auto-rotating capsule rolling machine. the invention has the advantage of greatly increasing the stability of the coenzyme Q10.
Owner:BEIJING DAWN AEROSPACE BIO TECH
Tablet containing everolimus, and preparation method thereof
ActiveCN103585122AImprove hydrophilicityImprove securityOrganic active ingredientsPill deliveryEverolimusBody fluid
The invention relates to a tablet containing everolimus, and a preparation method thereof. The tablet is prepared by tabletting of everolimus solid dispersion and a pharmaceutically acceptable auxiliary material, wherein the pharmaceutically acceptable auxiliary material comprises a filling material, a disintegrating agent and a lubricant. The everolimus solid dispersion is made of everolimus using a specific carrier material, so that hydrophilicity of everolimus is increased greatly, it is beneficial for rapid dissolving of everolimus in gastrointestinal tract body fluids, and medicine safety is improved.
Owner:SHANDONG NEWTIME PHARMA
Sodium-potassium citrate chewing tablet and preparation method thereof
ActiveCN102429887AStable and controllable qualityCool tasteOrganic active ingredientsMetabolism disorderCelluloseMagnesium stearate
The invention provide a sodium-potassium citrate chewing tablet, of which the prescription is composed of the following components by mass percent: 33.0-33.1% of potassium citrate, 27.8-27.9% of sodium citrate, 28-32% of filling agent, 0.05-0.3% of adhesive, 1-1.5% of lubricant, 3-5% of flavoring agent, 0.1-0.3% of aromatizer and 3-4% of moistureproof film coating agent, wherein the filling agentis mannitol or a mixture of mannitol and sorbitol or xylitol; the adhesive is hydroxypropyl methylcellulose or polyvidone K30; the lubricant is magnesium stearate or a mixture of magnesium stearate and micropowder silica gel; the flavoring agent is citric acid or a mixture of citric acid and sodium saccharin, aspartame or steviosin; and the aromatizer is pharmaceutically acceptable essence. The invention also provides a preparation method of the sodium-potassium citrate chewing tablet. The preparation method is simple and convenient to operate, low in cost and suitable for industrial production. The obtained tablet has stable and controllable quality, fresh and cool mouthfeel, sourness and sweetness in taste, mint fragrance or fruit fragrance, smooth and beautiful surface, uniform color, moderate hardness and rapid dissolution, and has good application prospects in treatment of gout and hyperuricemia as well as improvement of children and adult in vivo acidosis symptom and other aspects.
Owner:SOUTHWEST UNIV
Solid oral preparation containing telmisartan and preparation method thereof
ActiveCN102114015ARapid dissolutionQuality improvementOrganic active ingredientsPill deliverySURFACTANT BLENDReagent
The invention provides a solid oral preparation without a surfactant. The solid oral preparation comprises the following components in percentage by weight: 5-25% of telmisartan, an alkaline reagent and the balance of pharmaceutically acceptable vectors, wherein the weight of the alkaline reagent is equal to 7-20% of that of telmisartan. The invention also provides a method for preparing the solid oral preparation. The preparation prepared by the method can be quickly and sufficiently released in the psychophysical pH range of gastrointestinal tracts; and compared with the prior art, the preparation method has the advantages that the process is simpler, the quality of the soli oral preparation is more stable, the cost is greatly saved, and the efficiency is improved.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Rivaroxaban-containing tablets
ActiveCN104721156AAdvantages and Notable ImprovementsSignificant progressOrganic active ingredientsPill deliverySucroseFiller Excipient
The present invention discloses rivaroxaban-containing tablets, which are prepared from a raw material containing micronized rivaroxaban and an auxiliary material containing a water soluble filler, wherein a weight ratio of the rivaroxaban to the water soluble filler is 1:0.5-50, and the water-soluble filler is one or a plurality of materials selected from lactose, mannitol, sorbitol and sucrose. According to the present invention, the hydrophilicity of the rivaroxaban is significantly improved, the rapid dissolution the rivaroxaban in the gastrointestinal tract body fluid is easily achieved, the preparation process is simple, the operation is convenient, and the method is suitable for industrial large-scale production.
Owner:SHANDONG NEWTIME PHARMA
Eucommia bark fermented health care vinegar and preparation method thereof
The invention discloses eucommia bark fermented health care vinegar and a preparation method thereof. The preparation method comprises the following steps: (1) preparing a basic fermentation solution: crushing ponkan pulp, extracting juice, conducting coarse filtration, conveying the obtained juice into a cloudy juice tank, adding a folium cortex eucommiae extracting solution, regulating a sugar content and conducting pasteurization, so that the basic fermentation solution is prepared; (2) conducting primary fermentation: inoculating the basic solution with an activated wine-activating yeast, and conducting fermentation at 20-28 DEG C for 5-10 days, so that eucommia bark-ponkan fruit wine is prepared, conducting filtration, and conveying the fruit wine into an acetic acid fermentation tank; and (3) conducting secondary fermentation: adding 8-12% of domesticated acetic acid bacterium liquid to the eucommia bark-ponkan fruit wine; and conducting aerating agitation for 2-3 times every day at 26-33 DEG C for 3-4 days, 1min per time, so that the fruit wine gets into full contact with oxygen, and subsequently, the health care vinegar is obtained. According to the health care vinegar and the preparation method thereof disclosed by the invention, by effectively integrating such technologies as vacuum pulsation enhanced mass transfer, biological fermentation, enzymatic hydrolysis and the like, an optimum effect of preparing the eucommia bark fermented health care vinegar from the folium cortex eucommiae and the ponkan juice is achieved; and the health care vinegar is strong in market competitiveness and broad in application prospect.
Owner:XIANGXI AUTONOMOUS PREFECTURE BIANCHENG VINEGAR IND TECH
Pioglitazone hydrochloride sustained-release dropping pill and preparation method thereof
InactiveCN101269040AIncrease surface areaHas a wetting effectOrganic active ingredientsMetabolism disorderPharmaceutical formulationBlood drug concentration
The invention discloses to a drug compound for treating diabetes and particularly relates to a drug compound oral pharmaceutical formulation adopting pioglitazone as the ingredient. The drug compound aims to supplement the deficiency of the prior oral pharmaceutical formulation used for treating Type-2 Diabetes and provide a drug compound oral pharmaceutical formulation, sustained-release pioglitazone dropping pill which has high bioavailability, controllable release time, long-acting effect, low frequency of drug taking, steady plasma concentration, low cost and absence of contamination during the production. The sustained-release pioglitazone dropping pill adopts pioglitazone as the chemical ingredient and is prepared jointly with the medicinal carriers of hydrophilic frame ingredients and hydrophobic frame ingredients used as the stroma.
Owner:北京博智绿洲医药科技有限公司
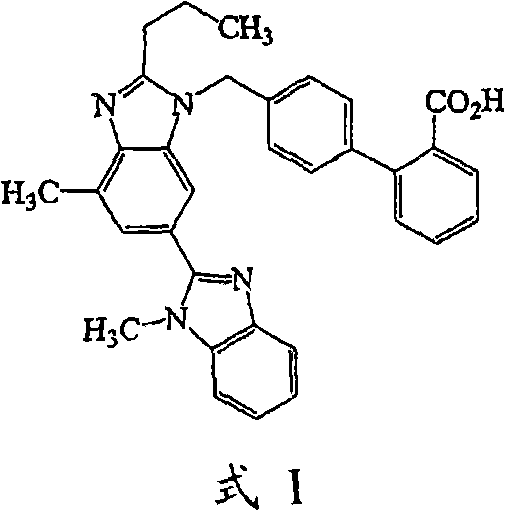
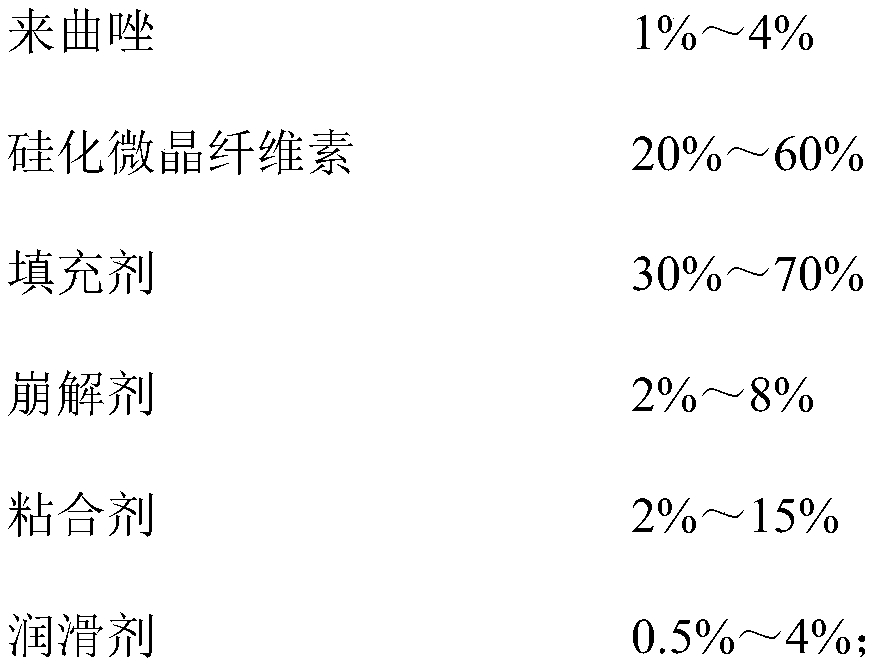
Lelrozol tablet and preparation method thereof
ActiveCN107737112AEvenly dispersedImprove liquidityOrganic active ingredientsPharmaceutical non-active ingredientsMedicineSmallerThan
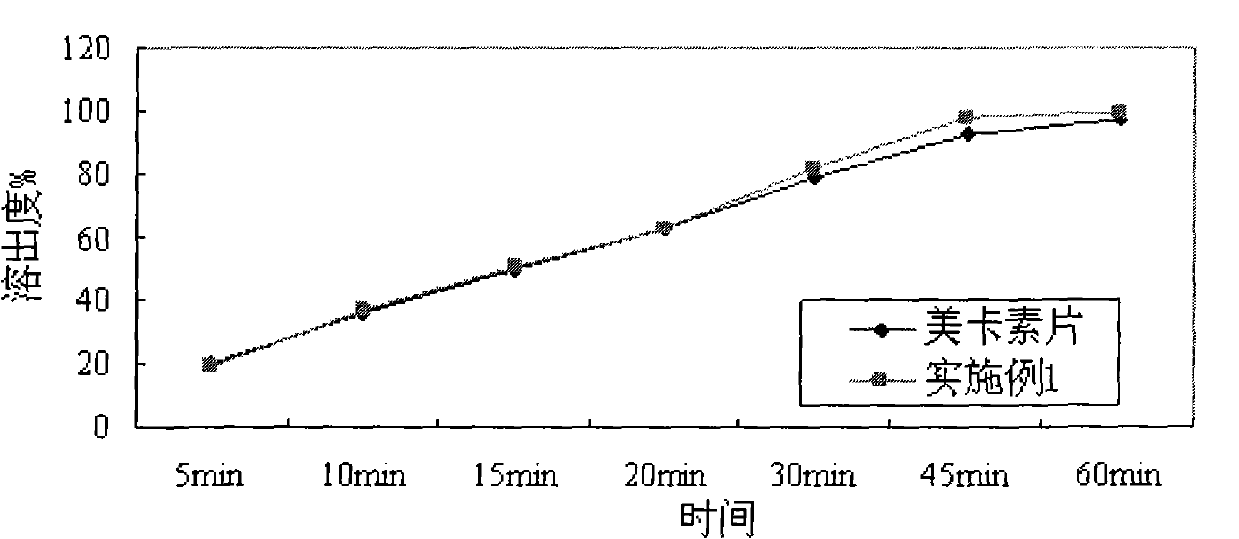
The invention belongs to the technical field of medicine and particularly relates to a lelrozol tablet and a preparation method thereof. The lelrozol tablet is prepared from lelrozol and silicified microcrystalline cellulose, wherein the content of the silicified microcrystalline cellulose accounts for 20 to 60 percent of total weight of the tablets. The particle size D(v, 0.9) of the lelrozol issmaller than or equal to 60mu m, preferably the particle size D(v, 0.9) of the lelrozol is smaller than or equal to 40mu m and more preferably, the particle size D (v, 0.9) of the lelrozol is smallerthan or equal to 8mu m. According to the preparation method of the lelrozol tablet, disclosed by the invention, the silica microcrystalline cellulose is used, so that the trazodone is uniformly dispersed in the mixed powder; in addition, the lelrozol tablet has good fluidity, and the powder can be directly pressured into tablets, so that the technique is simplified, the time is saved and the laborintensity is low; besides, after a crude drug is crushed, the particle size is obviously reduced; by controlling the content of a disintegrating agent, the dissolution rate of the prepared lelrozol tablet is significantly increased, 85 percent or above can be reached in 15 minutes and thereby the lelrozol tablet is rapidly dissolved out.
Owner:HAINAN JINRUI PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com