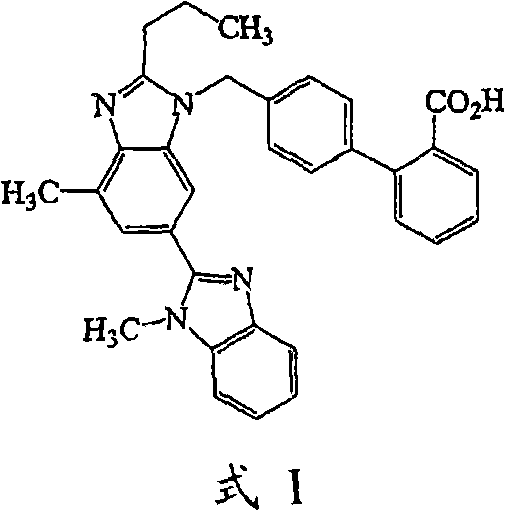
Solid oral preparation containing telmisartan and preparation method thereof
A technology for telmisartan and oral preparations, which is applied in the direction of medical preparations containing active ingredients, pill delivery, pharmaceutical formulations, etc., and can solve the problem of losing time and labor for fluidized bed granulation, prolonging the time of fluidized bed granulation, Increased problems such as explosions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032] 200g of telmisartan, 15.5g of sodium hydroxide and 8.5g of meglumine were dissolved in 200g of water to obtain a homogeneous solution; 60g of hypromellose, 681.8g of mannitol, 180g of corn starch, 36.0g of Mix povidone through a φ2.0mm rotary sieve to obtain a granule mixture; place the granule mixture in a fluidized bed and spray the above homogeneous solution to obtain wet granules; dry the wet granules at 60°C and pass through a φ2.0mm rotary sieve for granulation to obtain Dry granules; the dry granules are mixed with magnesium stearate and compressed into tablets.
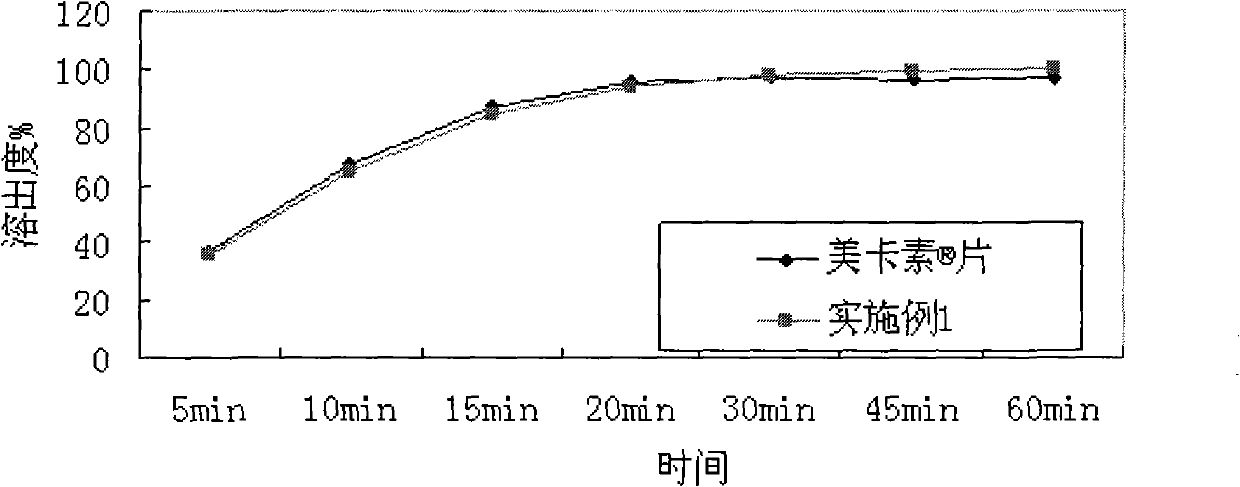
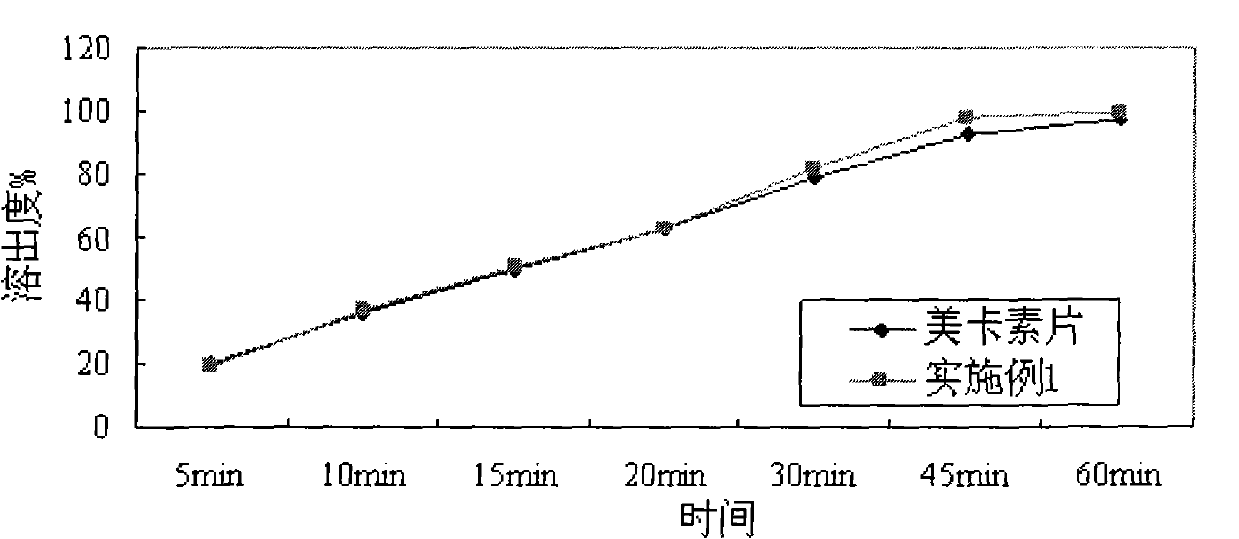
[0033] According to dissolution assay method (Chinese Pharmacopoeia 2005 edition appendix XC second method), with the pH1.0 hydrochloric acid solution of 900ml as dissolution medium, rotating speed is 75rpm, to embodiment 1 sheet and import telmisartan sheet (mecasu , manufacturer: Boehringer Ingelheim, Germany) for dissolution testing, and the results are shown in Table 1 and figure 1 .
...
Embodiment 2
[0042]
[0043] 200g of telmisartan and 14.0g of sodium hydroxide were dissolved in about 400g of water to obtain a homogeneous solution; Sodium is mixed together through a φ2.0mm rotary sieve to obtain a granule mixture; the granule mixture is placed in a fluidized bed, and the above homogeneous solution is sprayed to obtain wet granules; the wet granules are dried at 60°C and passed through a φ2.0mm rotary sieve for granulation to obtain dry Granules; add the above-mentioned magnesium stearate to the dry granules, mix well and compress into tablets.
Embodiment 3
[0045]
[0046] The telmisartan of 200g, 16.0g sodium hydroxide and 24.0g meglumine are jointly dissolved in about 400g water to obtain a homogeneous solution; 2048g mannitol, 1600g microcrystalline cellulose and 40.0g crospovidone Mix with a φ2.0mm rotary sieve to obtain a granule mixture; place the granule mixture in a fluidized bed and spray the above-mentioned homogeneous solution to obtain wet granules; dry the wet granules at 60°C and pass through a φ2.0mm rotary sieve for granulation to obtain dry granules; The granules are added with the above-mentioned magnesium stearate, mixed evenly, and then compressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com