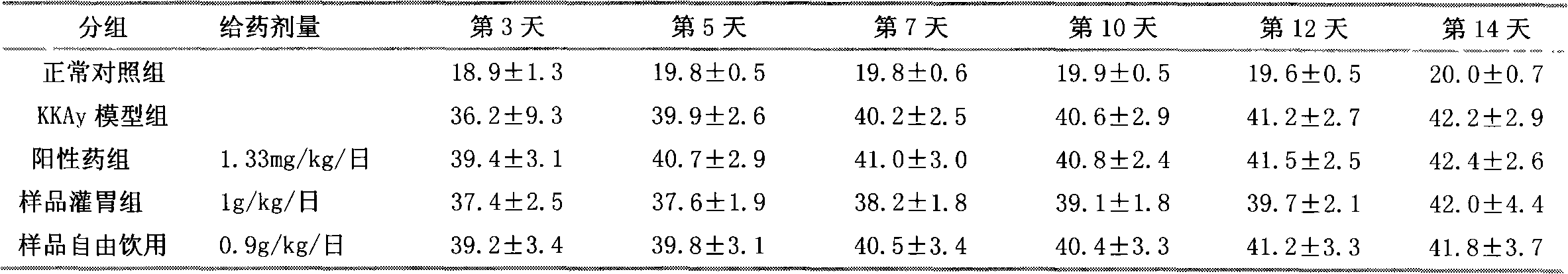
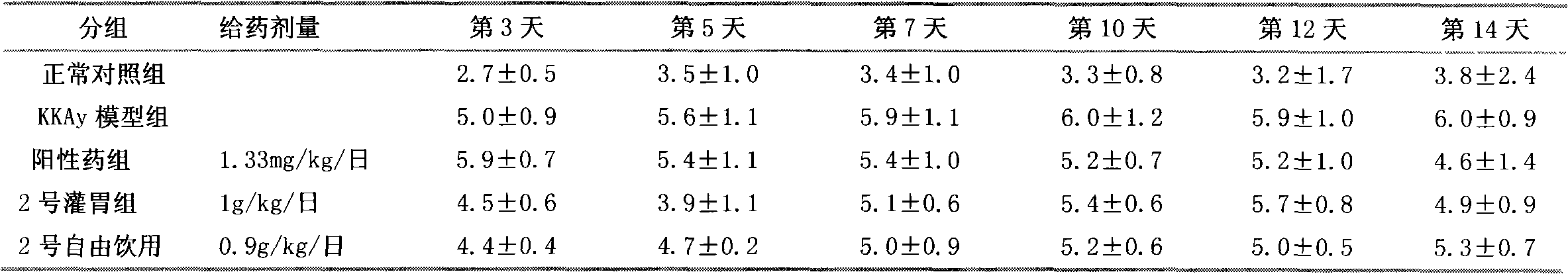
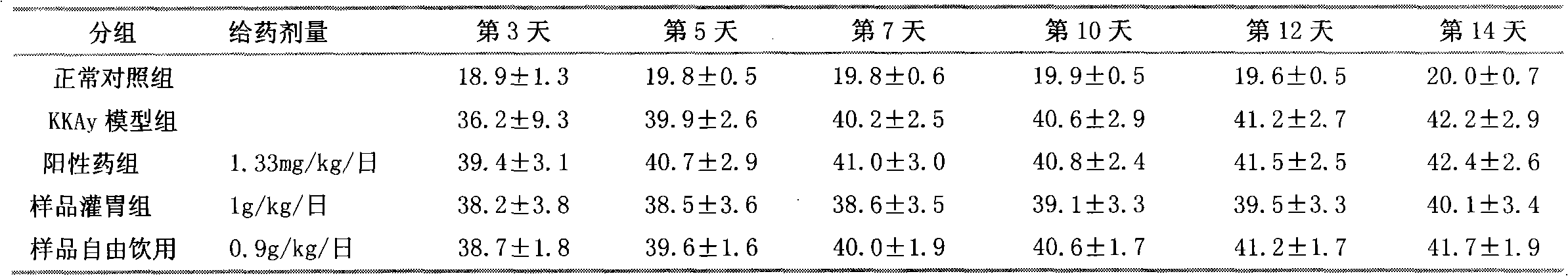
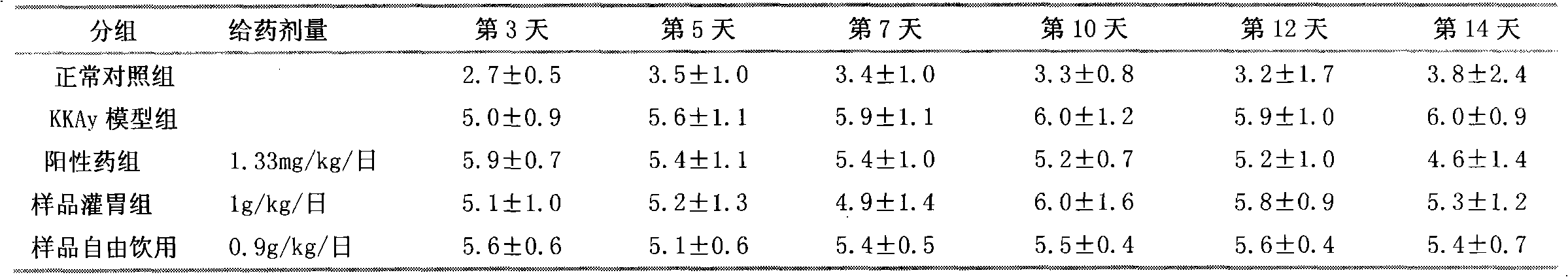
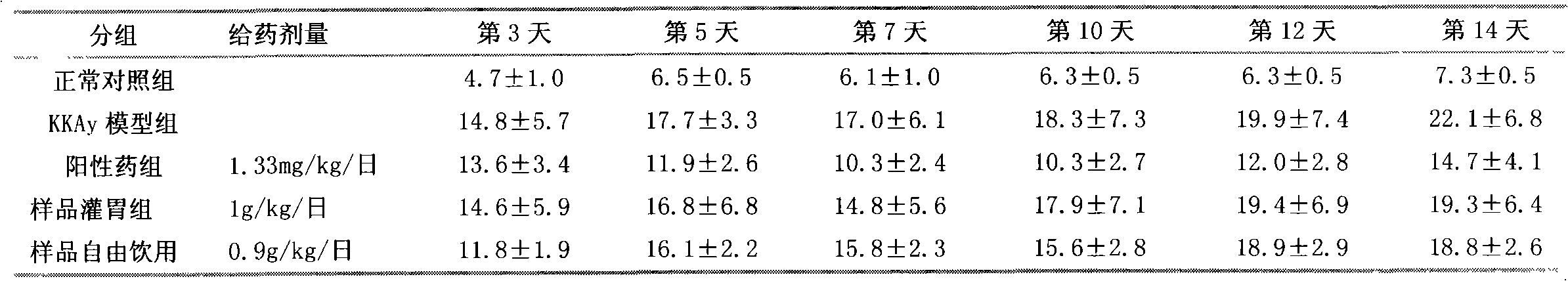
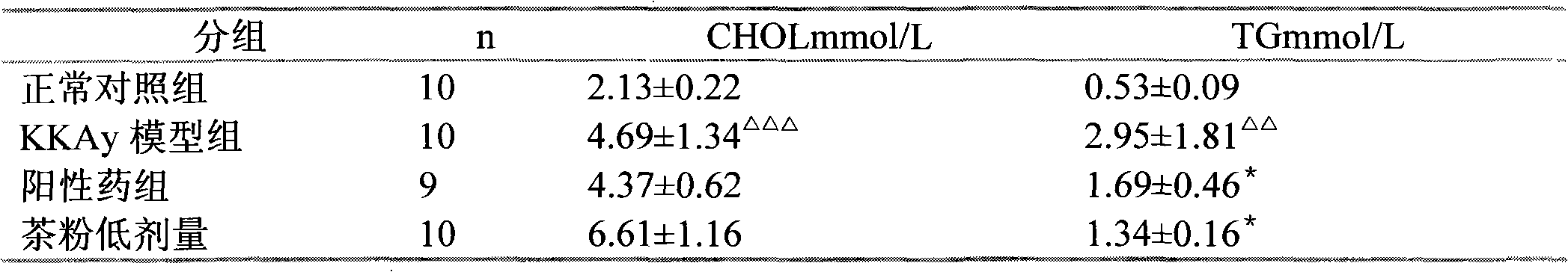
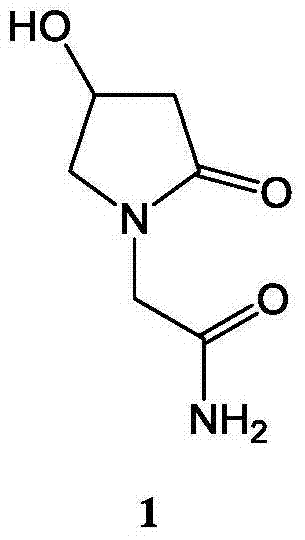
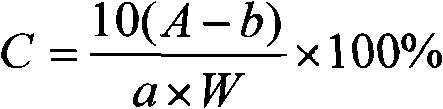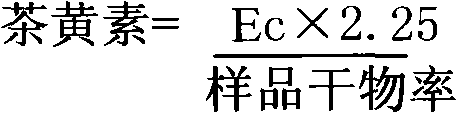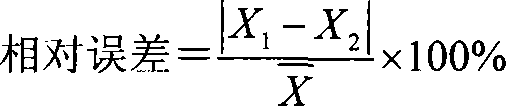Patents
Literature
615results about How to "Stable and controllable quality" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pu'er tea extract and preparation method and application
InactiveCN101961060AEfficient conversionLow costMetabolism disorderTea extractionBiotechnologyMedicine
The invention relates to a Pu'er tea extract, and a preparation method and application thereof. The Pu'er tea extract is high active ingredient content and can reduce blood sugar and blood fat obviously. By the preparation method of the Pu'er tea extract, the active ingredients are completely and effectively converted and dissolved out. The process of the Pu'er tea extract has the advantages of guaranteeing the clarity of the finished products which are dissolved in cold water and reducing investment requirement of refrigerated storage tanks and the difficulty and the work load of filtering operation due to a refrigeration process after concentration, along with low cost, industrialization and stable and controllable quality.
Owner:TIANJIN TASLY GROUP
Pu'er tea extract and preparation method and application thereof
ActiveCN101961425AGuaranteed stabilityGuaranteed operational feasibilityMetabolism disorderPlant ingredientsFlavorCLARITY
The invention relates to a Pu'er tea extract, and a preparation method and application thereof. The Pu'er tea extract is high in active ingredient content and can reduce blood sugar and blood fat obviously. By the preparation method of the Pu'er tea extract, the active ingredients are completely and effectively converted and dissolved out and the original flavor of the Pu'er tea is maintained. The process of the Pu'er tea extract has the advantages of guaranteeing the clarity of the finished products which are dissolved in cold water and hot water and reducing the work load of later centrifugation, along with low cost, industrialization and stable and controllable quality.
Owner:TIANJIN TASLY GROUP
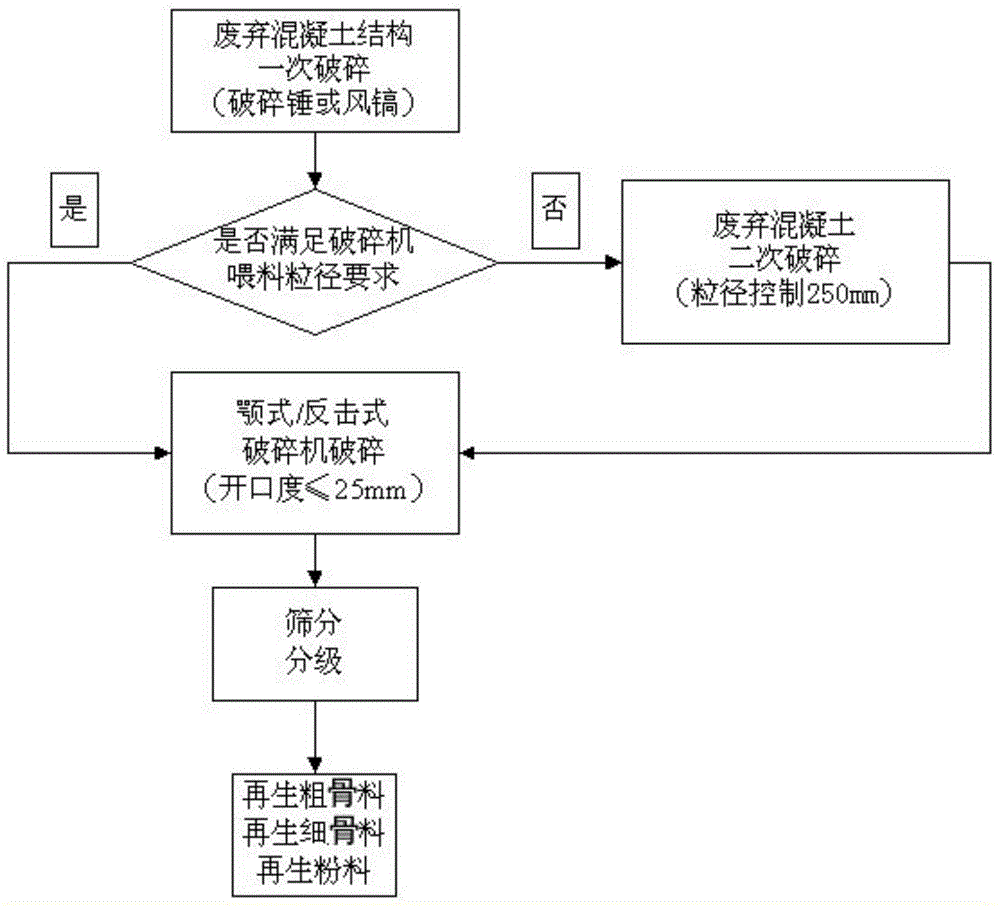
Eco-friendly self-compacting concrete prepared through complete recycling of waste concrete
InactiveCN104788054AWorkability is goodExcellent and workability (liquiditySolid waste managementSuperplasticizerFly ash
The invention discloses eco-friendly self-compacting concrete prepared through complete recycling of waste concrete. The eco-friendly self-compacting concrete comprises components in parts by weight as follows: 100 parts of cement, 10-25 parts of fly ash, 10-25 parts of mineral powder, 20-40 parts of recycled micro-powder, 1-2 parts of a polycarboxylate superplasticizer, 230-380 parts of recycled fine aggregate, 240-400 parts of recycled coarse aggregate and 45-60 parts of water which are mixed. The coarse aggregate only adopts the recycled coarse aggregate, the fine aggregate only adopts the recycled fine aggregate, the recycled micro-powder formed by grinding waste materials produced in production of the recycled aggregate is blended into a mineral admixture, so that complete recycling of the waste concrete is realized, and the eco-friendly self-compacting concrete has a very good eco-friendly effect.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Pu-erh tea extract, preparation method and application
ActiveCN101961061BHigh content of active ingredientsLower blood sugarMetabolism disorderTea extractionOperabilityBULK ACTIVE INGREDIENT
Owner:TIANJIN TASLY GROUP
Puer tea extract, preparation method and application
ActiveCN101961059BEfficient conversionLow costMetabolism disorderTea extractionBiotechnologyPhenolic content in tea
The invention relates to a Puer tea extract, a preparation method and application. The Puer tea extract contains effective ingredients of tea polyphenol, tea Polysaccharide, theabrownine, caffeine and the like the content of which are high, and can obviously lower blood sugar. The preparation method can effectively convert and dissolve out the effective ingredients. The process route can assure clarity after the cold water of a finished product is dissolved, lowers the late centrifugation workload, has low cost and stable and controllable quality and is industrial.
Owner:TIANJIN TASLY GROUP
Oxiracetam capsule and preparation method thereof
ActiveCN103494790AHigh purityLow impurity contentOrganic active ingredientsNervous disorderMedicinal chemistryImpurity
The invention relates to an oral capsule preparation of oxiracetam and a preparation method thereof. The oxiracetam capsule contains 80.0-85.5% by weight of oxiracetam, 9.7-15.2% by weight of xylitol and 4.8% by weight of an emollient, wherein the oxiracetam is in a crystal form. The oxiracetam in the crystal form is a good crystal with excellent mobility. Compared with the crystal form provided in the prior art, the oxiracetam in the crystal form has higher purity, less impurity, better quality stabilizer and better crystal form stability. The quality stability of the disclosed oxiracetam capsule is improved remarkably, the preparation process is simple, and the production cost is lowered.
Owner:CSPC OUYI PHARM CO LTD
Extraction and Refining Technology of Medicinal Components of Scrophulariae Scrophulariae
InactiveCN102274341AHigh extraction rateMeet quality requirementsAntibacterial agentsAntipyreticPhenylpropanoidEthanol precipitation
The invention discloses an extraction and purification process for medicinal components of Scrophulariaceae, namely an extraction and purification process for iridoid and phenylpropanoid components: pulverizing dry roots of Scrophulariaceae, soaking, water extraction, concentration of the extract, and ethanol precipitation , resin adsorption, ethanol elution, concentrated drying and other procedures obtained. The extraction and purification process of the invention has high extraction rate, can reach more than 50%, stable and controllable quality, simple method and low composition, and is suitable for large-scale industrial production, thereby providing a new way for the development and utilization of Scrophulariaceae.
Owner:SHANGHAI UNIV OF T C M
Organ preservative fluid and preparation method thereof
InactiveCN101496512AEffective preservationPrevent ischemia-reperfusion injuryDead animal preservationReperfusion injuryTetramethylpyrazine hydrochloride
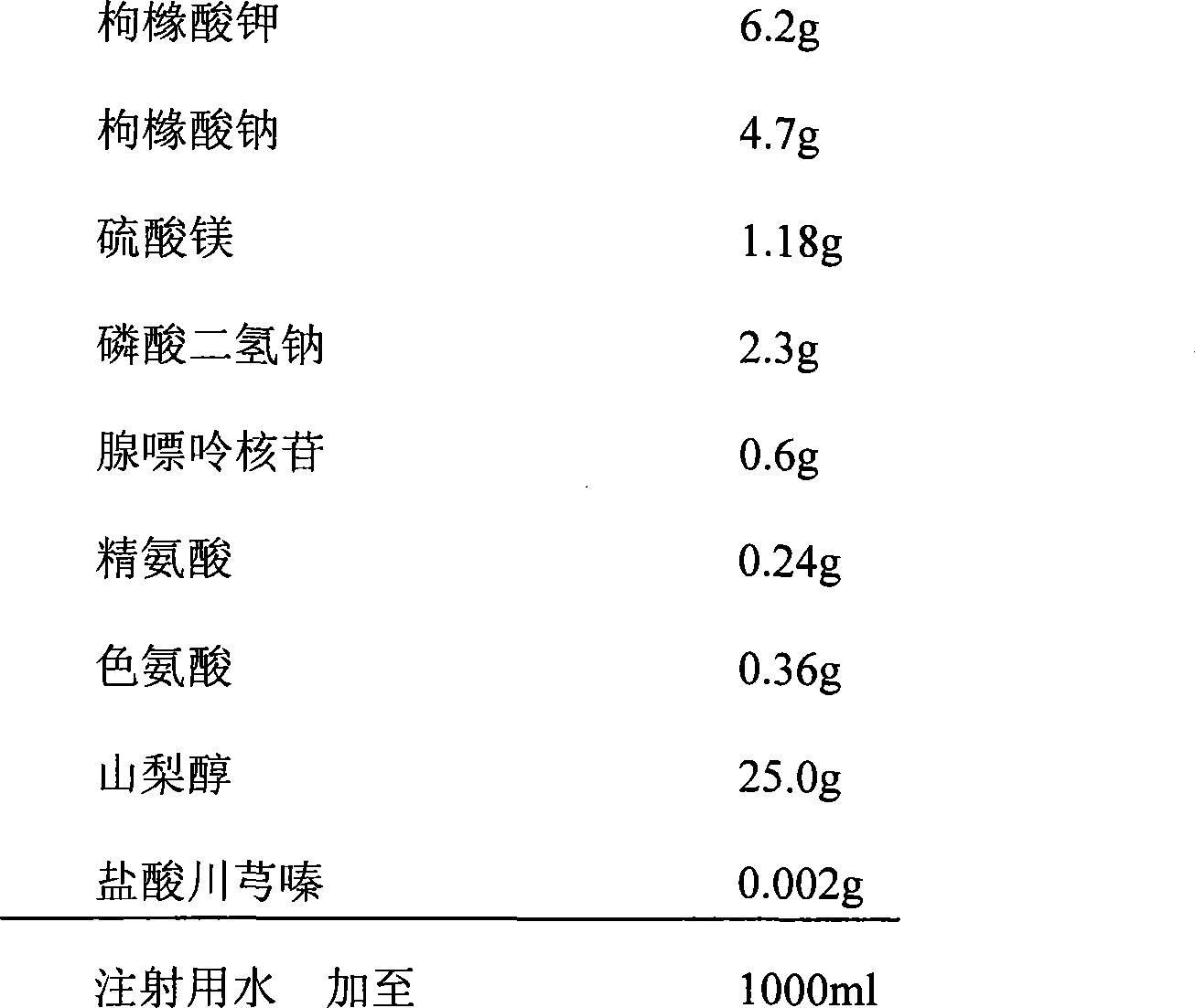
The invention relates to a preservation solution for organs, tissues or cells of human bodies or animals. The organ preservation solution comprises sodium citrate, potassium citrate, magnesium sulfate, sodium dihydrogen phosphate, sodium hydroxide, adenosine, arginine, tryptophan, mannitol, tetramethylpyrazine hydrochloride and other components. The organ preservation solution can be used for cooling, lavaging and preserving the organs of the human bodies or the animals, can effectively preserve human in vitro kidneys for 48 hours and animal in vitro kidneys for 72 hours, can prevent ischemia-reperfusion injury, and has great value for clinical application.
Owner:YANGZIJIANG PHARMA GROUP SHANGHAI HAINI PHARMA
Gemcitabine hydrochloride lyophilized powder injection and preparation method thereof
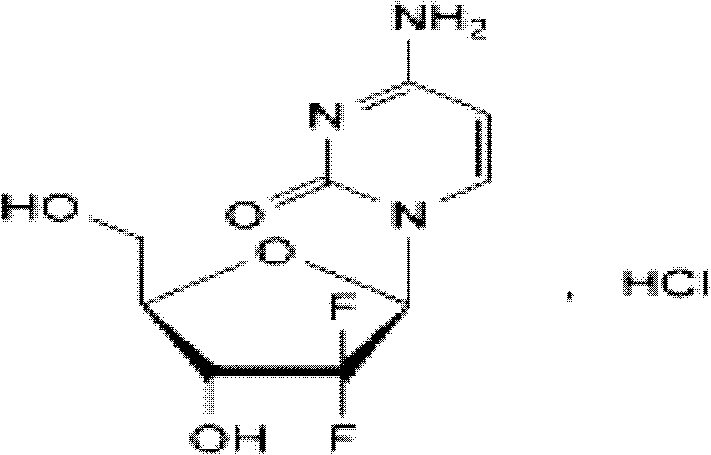
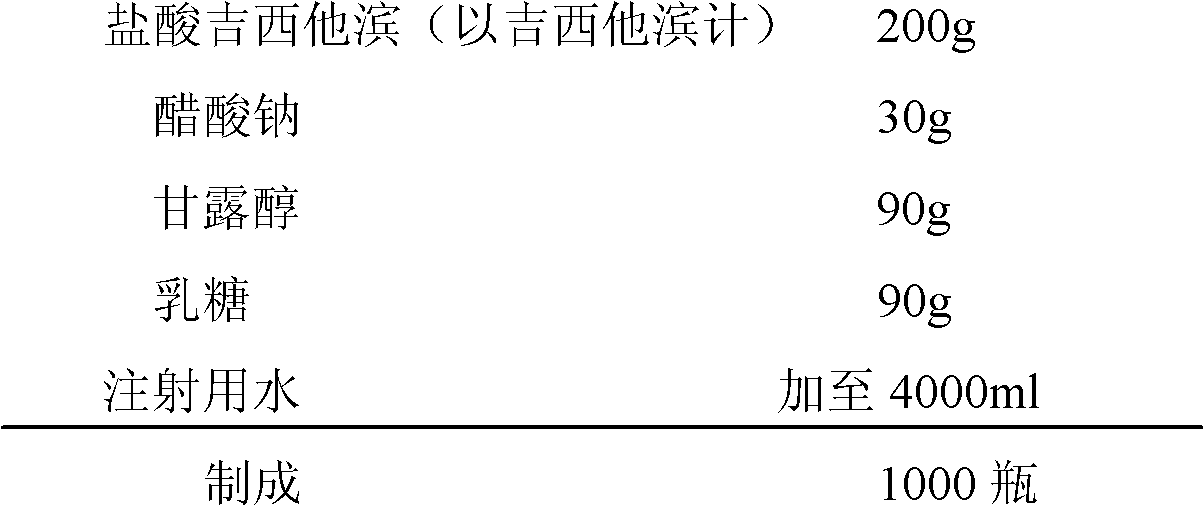
ActiveCN102144981AReduce dosageImprove stabilityPowder deliveryOrganic active ingredientsSodium acetateAdjuvant
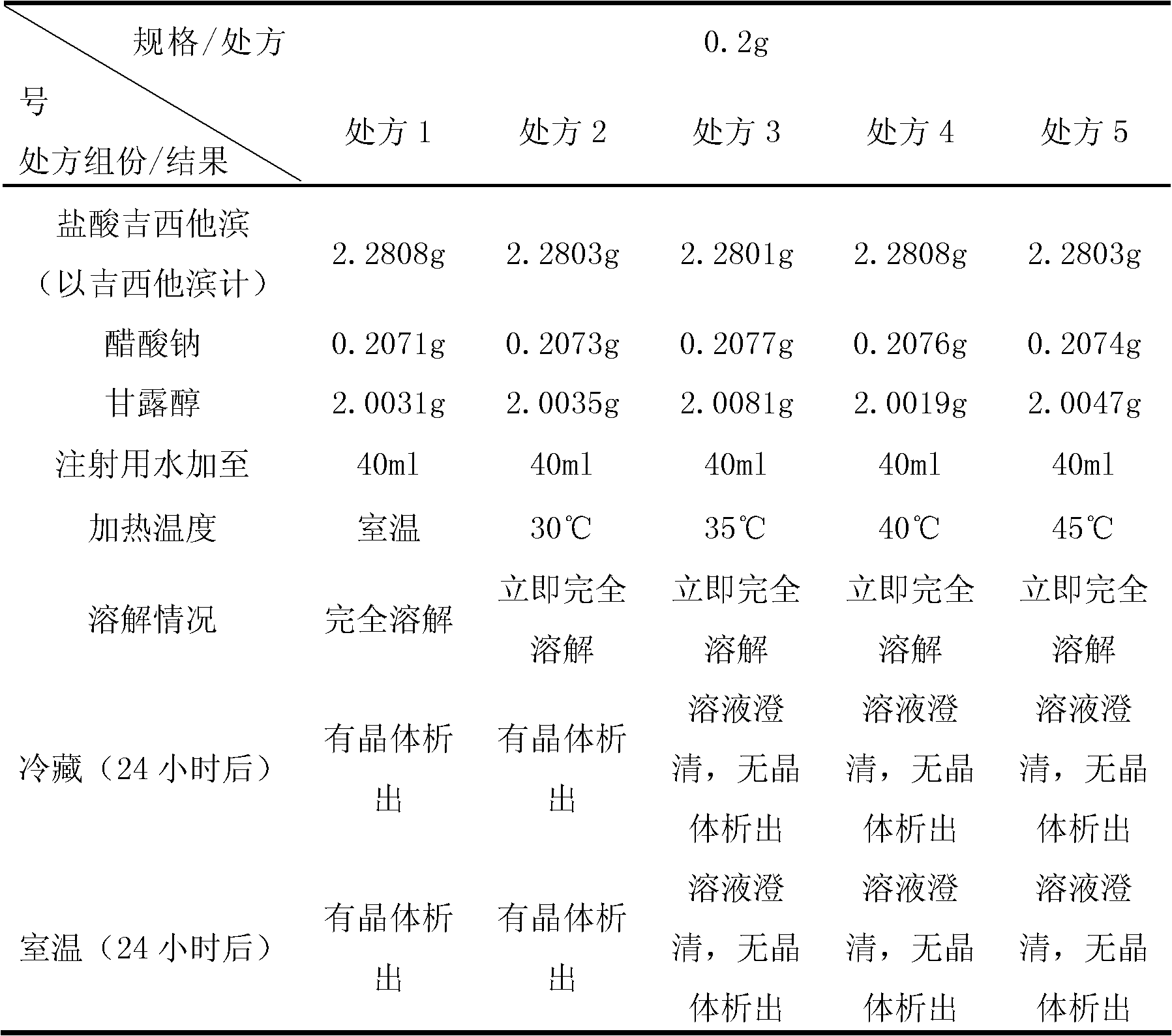
The invention relates to a gemcitabine hydrochloride lyophilized powder injection and a preparation method thereof. The lyophilized powder injection comprises the following components in parts by weight: 20-30 parts of gemcitabine hydrochloride, 5-9 parts of mannitol, and 3-10 parts of sodium acetate. The freeze-drying step includes the following three stages: a pre-freezing stage, a primary drying stage and a secondary drying stage, and the entire freeze-drying time is lower than 20 hours. The gemcitabine hydrochloride lyophilized powder injection provided by the invention has the advantages of less types and amounts of adjuvants, easily-controlled technological parameters, simple process route, short freeze-drying time, convenience in operation, good repeatability, low contents of related substances, and controllable quality; and the redissolved lyophilized powder injection has good clarity and forming performance. The lyophilized powder injection has stable and controllable quality, is easy to realize industrial production, and can generate considerable economic and social benefits.
Owner:HAINAN JINRUI PHARMA CO LTD
Extractive of Pu'er tea and preparation method thereof
ActiveCN101961422APromote conversionPromote dissolutionMetabolism disorderColor/spectral properties measurementsFiltrationCentrifugation
The invention relates to an extractive of the traditional Chinese medicine and preparation thereof, in particular to the extractive of Pu'er tea and the preparation method thereof; wherein the preparation method comprises the technical means such as water extraction, membrane filtration, centrifugation and the like.
Owner:TIANJIN TASLY GROUP
Pantoprazole sodium freeze-dried powder injection and preparation method thereof
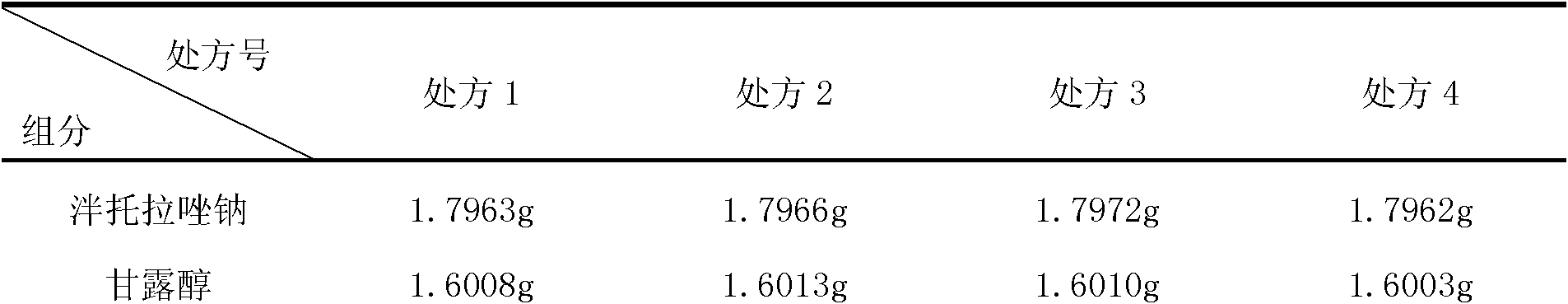
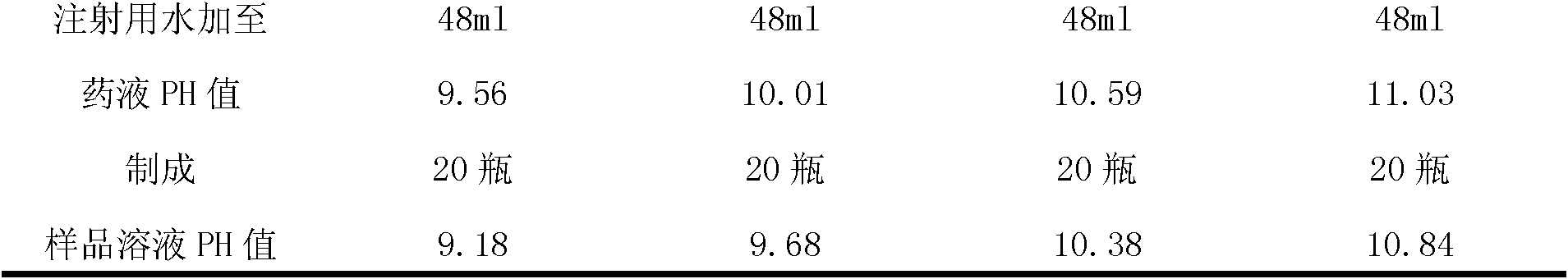
ActiveCN102085190AShorten the secondary drying timeGood lookingOrganic active ingredientsPowder deliveryCLARITYFreeze-drying
The invention relates to a pantoprazole sodium freeze-dried powder injection and a preparation method thereof. The powder injection is prepared from pantoprazole sodium and mannitol, wherein the consumption ratio of the pantoprazole sodium to the mannitol is (1:0.8)-(1:1.6), and the PH value is 10.5-11.0. In the invention, by lowering the pre-freezing temperature, properly lowering the freezing temperature, maintaining the lowered freezing temperature for a proper time, properly shortening two-stage drying time and carrying out other adjustment processes, good appearance and quality of the product can be kept under the condition that the content of the mannitol is low, the processes are reliable and feasible, and the effect is obvious. The prepared product has low content of related substances and has controllable quality, and the freeze-dried product has good clarity and formability after being redissolved.
Owner:HAINAN JINRUI PHARMA CO LTD
Method of controlling the quality of salvia miltiorrhiza raw material fingerprint in the plant medicine for improving hemorheology
ActiveCN101040907AGuarantee normal implementationHigh sensitivityOrganic active ingredientsComponent separationTanshinone IIAColumn temperature
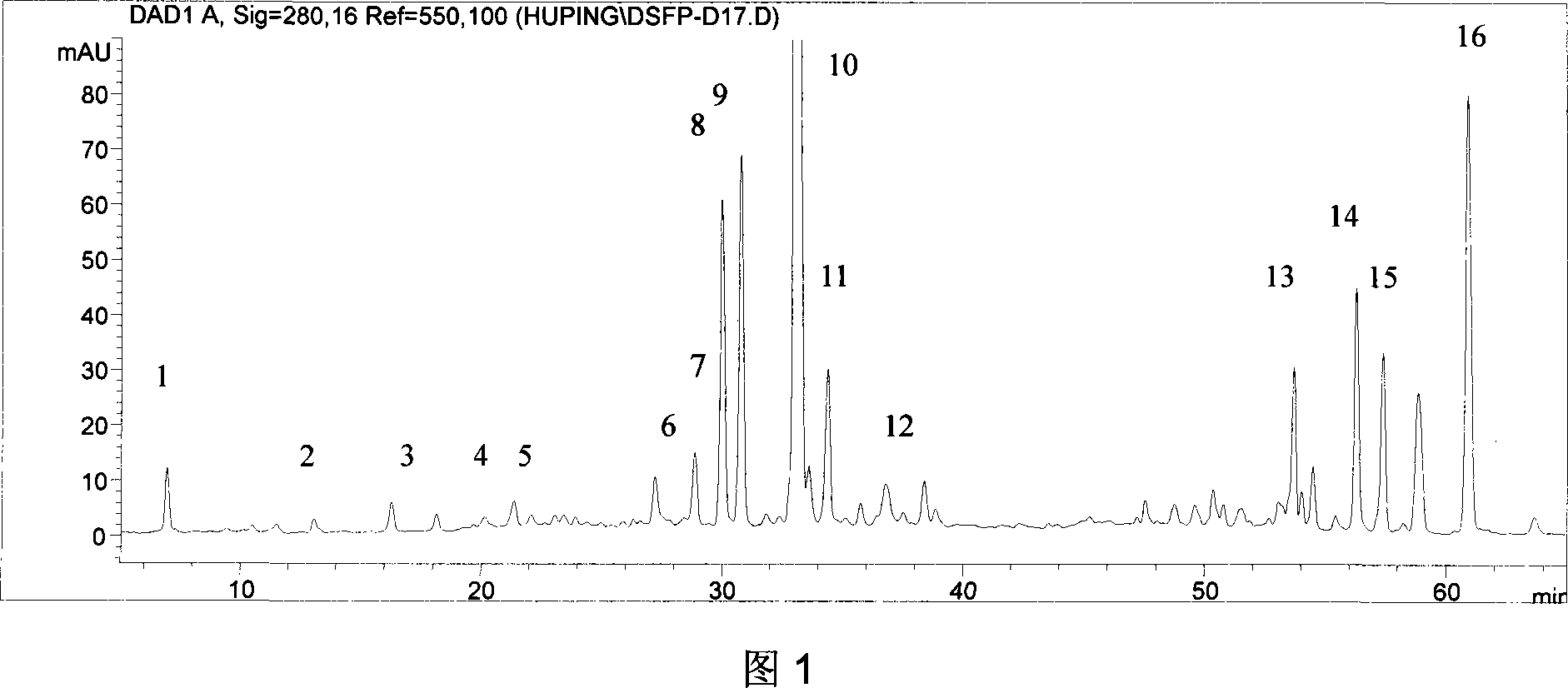
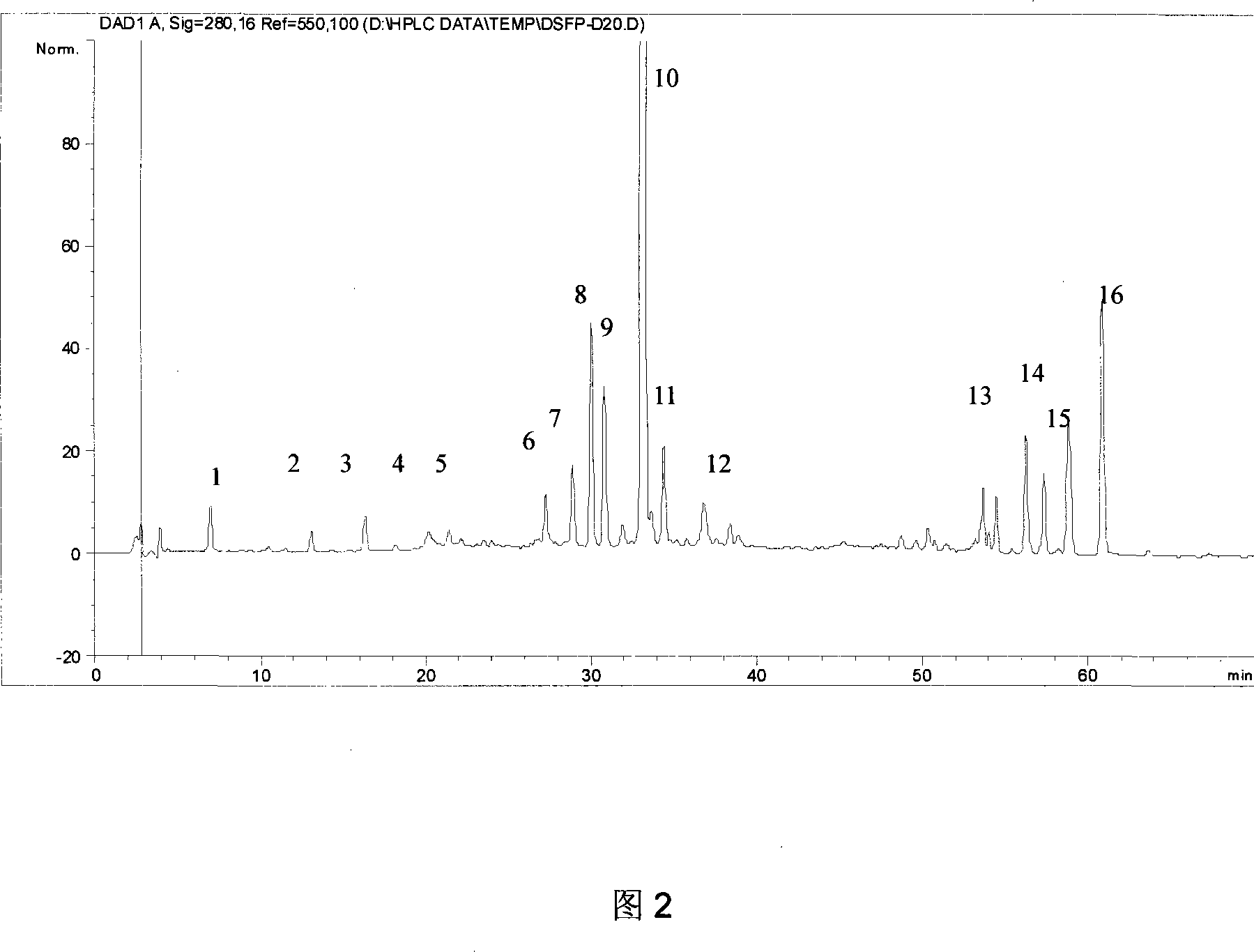
The invention relates to a DanShen fingerprint spectrum quality control method, comprising that (1), adding 1.0g DanShen powder into carbinol to extract via microwave, (2), washing flow phase gradient that the Alltima C18 column is 4.6mm, 250mm, and 5mum, checking wavelength is 280nm, flow speed is 1.0ml / min, the column temperature is 20-40Deg. C, and the sample amount is 10-20ul, (3), building standard fingerprint spectrum that the first peak is DanShen element, the eighth peak is rosmarinic acid, the ninth peak is alkannic acid, the tenth peak is phenolic acid B, the eleventh peak is phenolic acid B isomer, the fourteenth peak is cryptotanshinone and the fourteenth peak is tanshinone IIA, (4), controlling the quality of fingerprint spectrum that the check peak relative holding times are 0.21 of DanShen element, 0.91 of rosmarinic acid, 0.93 of alkannic acid, 1.00 of phenolic acid B, 1.04 of phenolic acid B isomer, 0.92 of cryptotanshinone and 1.00 of tanshinone IIA, (5), the DanShen planting collecting method. The invention can control the quality of materials to assure the stable quality of product.
Owner:ANHUI ZHIHETANG PHARMACY
Ecological nanometer photocatalysis completely recycled concrete
ActiveCN104310891AUnique performanceAchieve full utilizationSolid waste managementWater reducerConstruction aggregate
The invention discloses an ecological nanometer photocatalysis completely recycled concrete. The concrete is prepared from following components in parts by weight: 360-480 parts of cement, 1000-1200 parts of loaded photocatalyst coarse aggregate, 500-600 parts of fine aggregate, 40-90 parts of supplementary cementious material, 4-10 parts of efficient water reducing agent, and 150-180 parts of water. The cement has the advantages that regenerated coarse aggregate, regenerated fine aggregate and regenerated concrete powder produced by waste concrete are used for preparing the concrete, so that full utilization of the waste concrete and zero resource waste are really realized, and the ecological environmental protection effect is obvious; the nanometer photocatalysis component is loaded in the regenerated coarse aggregate for the first time, so that the concrete can decompose vehicle exhaust and improve the atmospheric environment.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Wall-breaking and superfine grinding process and device for better ground traditional Chinese medicine decoction piece
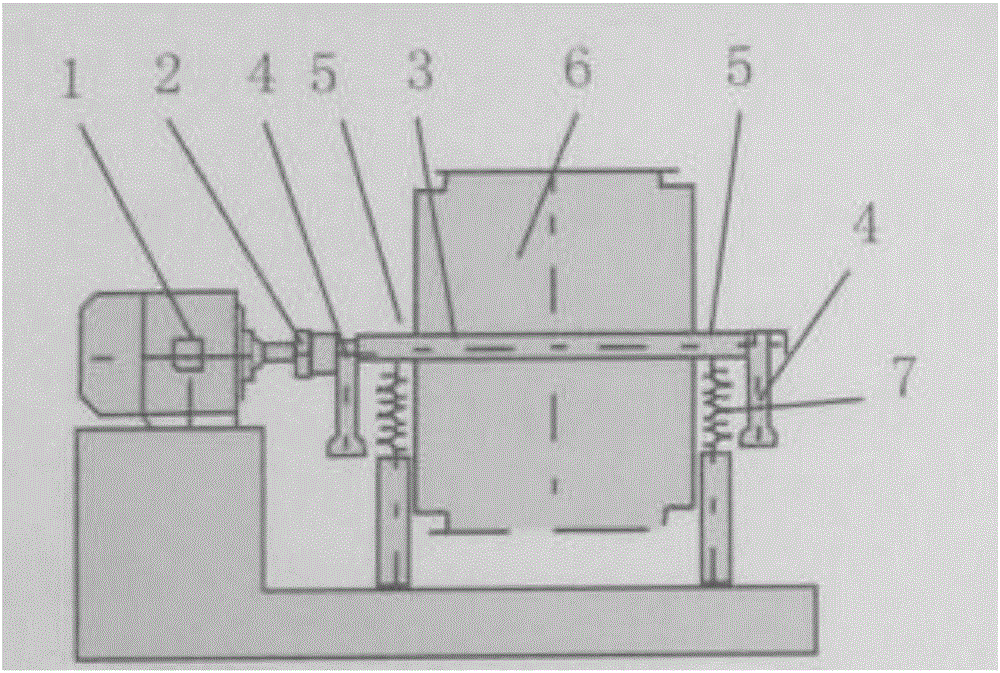
ActiveCN105344428AImprove qualityStable and controllable qualityGrain treatmentsLiquid-crystal displayMedicine
The invention belongs to the field of medicinal material grinding and smashing equipment and method, and particularly relates to a wall-breaking and superfine vibration grinder for a better ground traditional Chinese medicine decoction piece, a traditional Chinese medicine decoction piece preparation method adopting the grinder, and the better ground traditional Chinese medicine decoction piece. The grinder comprises a motor (1), a coupler (2), a rotating spindle (3), a bearing pedestal (4), a drum holder (5), a drum (6), a supporting spring (7), a base, an excitation vibration absorber, a controller, a timer, a liquid crystal display operating panel, a central processing unit and a silencing case. By means of the specific rotating speed and the specific motion trace of the motor of the grinder, the grinder achieves the advantages of energy conservation, high efficiency and smaller sizes of smashed grains.
Owner:SHAANXI NIANQINGBAO PHARMA CO LTD
Process for microwave-freeze-drying dendrobium stem decoction medicine pieces
A process for microwave-freeze-drying dendrobium stem decoction medicine pieces comprises the steps of grading, wherein fresh dendrobium stems are divided into three grades according to the number of stem sections; cleaning, wherein the graded dendrobium stems are cleaned separately and drained off to be reserved; preprocessing, wherein the dendrobium stems which are drained off are placed into a microwave dryer and subjected to microwave processing for 1-3+ / -0.5 minutes; segmented cutting, wherein each cooled dendrobium stem is divided into three segments according to the top, the middle portion and the root portion, and the top and the middle portion of each dendrobium stem are cut into thin sheets with the thickness from one mm to four mm; pre-freezing, wherein the cut dendrobium stem thin sheets are placed into a freezer and pre-frozen at the temperature from minus 10 DEG C to minus 20 DEG C for more than four hours; freeze-drying, wherein the cold trap temperature of a freezer dryer is lowered to be within the range of minus 55 DEG C to minus 65 DEG C for four to six hours, the pre-frozen dendrobium stem thin sheets are placed in the freezer dryer, and after sublimation is over and the temperature of a material disk returns to 20 DEG C, materials can be taken off after the temperature is kept at 20 DEG C for two to three hours, and then the dendrobium stem decoction medicine pieces are obtained.
Owner:CHISHUI TRUSKY TRADITIONAL CHINESE MEDICINE IND DEV
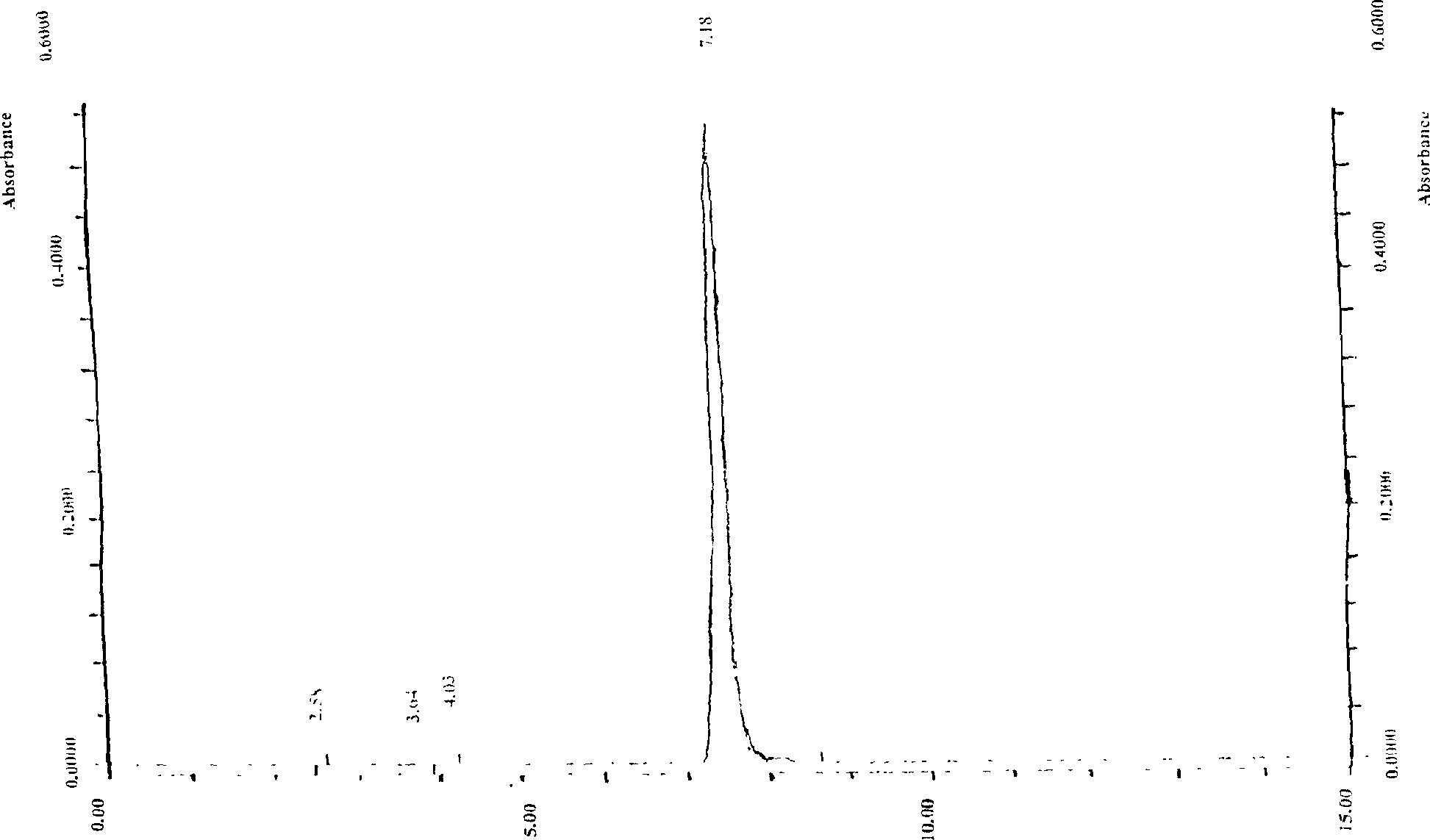
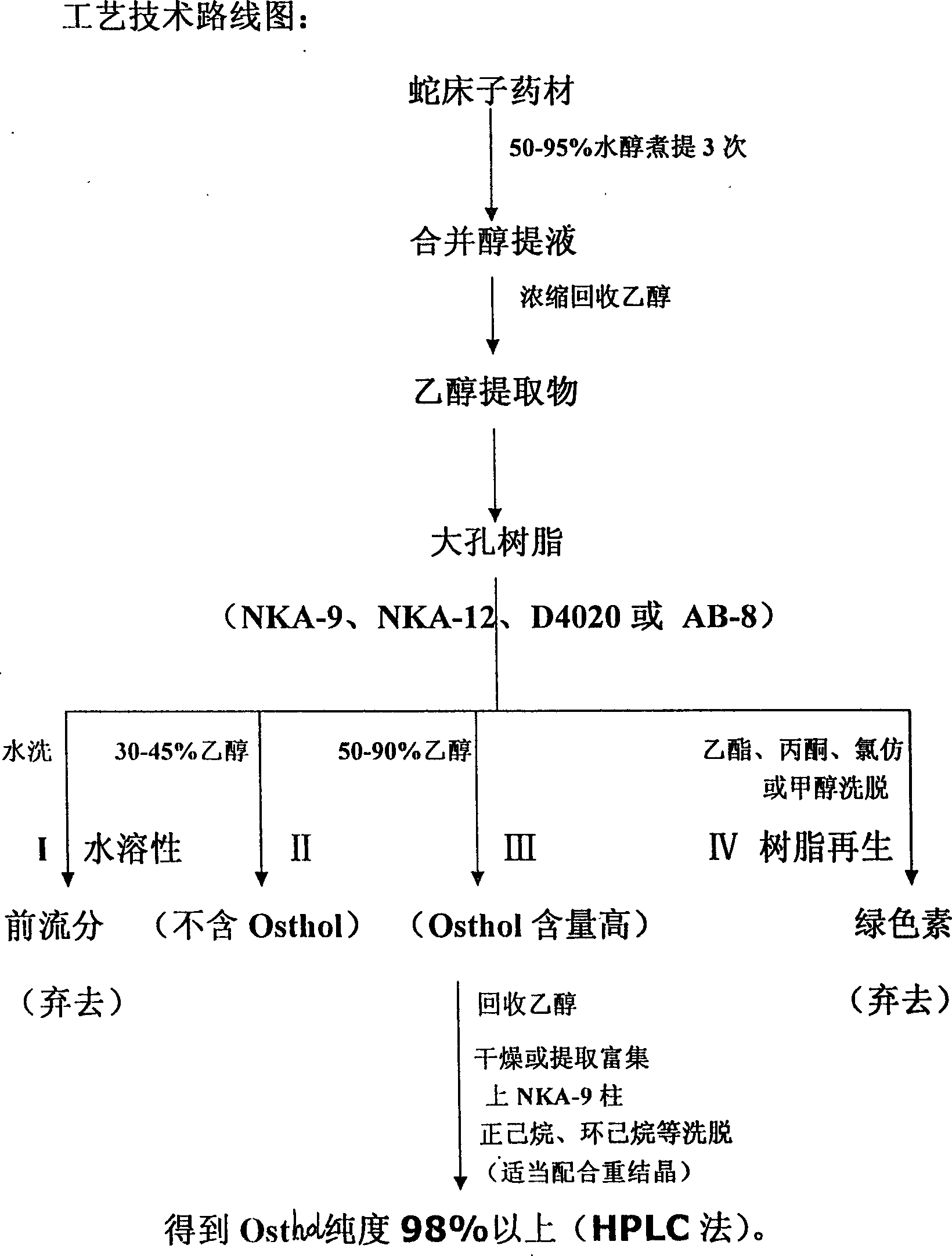
High purity cnidicin and its preparation method and medicinal composition using said compound as active component
InactiveCN1724529AOptimizing the Extraction and Separation ProcessGood reproducibilityOrganic active ingredientsOrganic chemistryStability studyAcute toxicity testing
The invention discloses a high- purity of osthol, structure proof with spectral analysis, method for preparation, stability study, acute toxicity study, the drug compositions containing osthol, and new drug utilities of the natural product. By the technique steps of extracted Chinese pharmacy conidium fruit with alcohol, decolorizing and enriching by large-hole adsorption resin, and separating and purifying, it can prepare osthol with a purity of above 98%. The study expresses that the osthol has a clear utility of anti-cancer, anti-auto lymphoma, and so on.
Owner:INST OF RADIATION MEDICINE CHINESE ACADEMY OF MEDICAL SCI
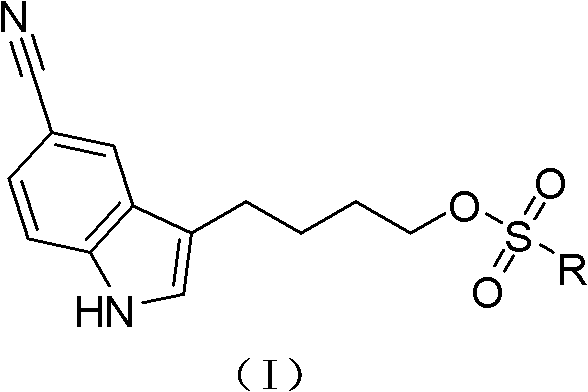
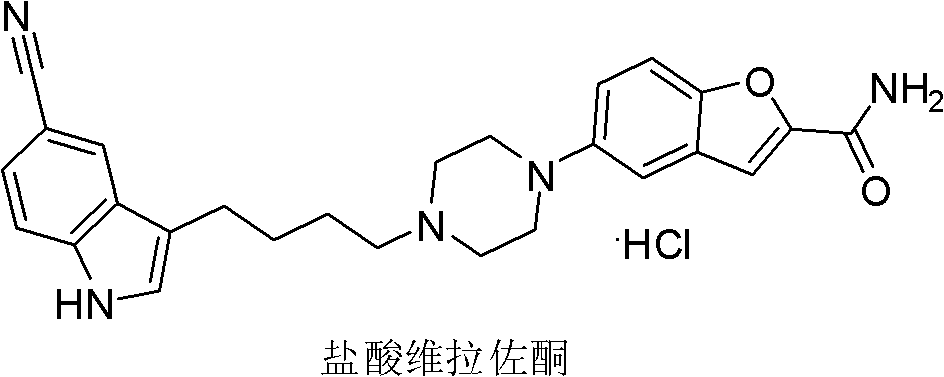
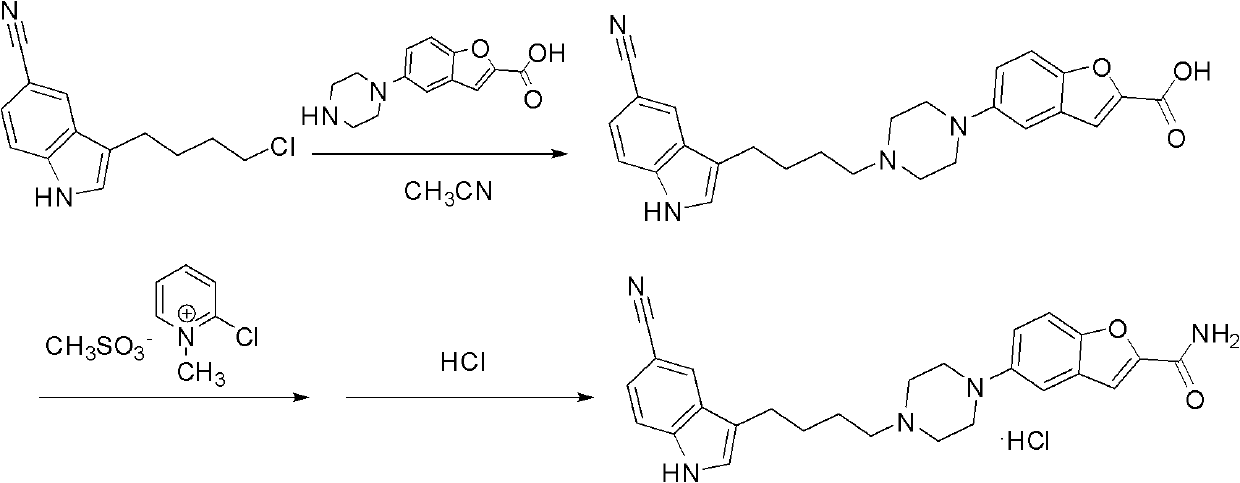
4-(5-cyano-1h-indol-3-yl) butyl substituted sulfonate compounds and their applications
The invention discloses a 4-(5-cyano-1H-indol-3-yl) butyl substituted sulfonate compound and its application. The 4-( 5-cyano-1H-indol-3-yl) butyl substituted sulfonate compounds, as new intermediates, in the preparation of vilazodone and its pharmaceutically acceptable salts, overcome the existing The defects and deficiencies in the preparation method reported in the literature are more suitable for the large-scale industrial preparation of vilazodone hydrochloride, which has obvious creativity, great positive progress effect and practical application value. The general structural formula of the compound shown in formula (I) is as follows:
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Preparing and processing method of prepared aconite slice
ActiveCN103356758AEvenly heatedStable and controllable qualityAntipyreticAnalgesicsBiotechnologyBlood pressure increase
The invention relates to a preparing and processing method of a prepared aconite slice. The preparing and processing method comprises the following steps of: carrying out heat treatment on a raw aconite slice at 102-240 DEG C for 0.25-2 hours; then drying to obtain the prepared aconite slice, wherein the heat treatment is preferably carried out under the damp and hot condition with water vapors at 102-108 DEG C for 0.5-2 hours or carried out under a 140-240 DEG C dry and hot condition for 0.25-1 hour. Experiments indicate that the prepared aconite slice prepared and processed through the method disclosed by the invention can outstandingly reduce the content of a toxic component, namely diester-type alkaloid, and can also retain the dopamine hydrochloride and salsolinol which are the cardiotonic and blood pressure increasing effective components of monoester-type alkaloid and aconite, thereby ensuring the exertion of the recuperating, rescuing, cardiotonic and blood pressure increasing drug effect of the prepared aconite slice.
Owner:SICHUAN ACAD OF CHINESE MEDICINE SCI
Method for preparing donkey-hide gelatin oligopeptide
InactiveCN101294186AImprove bioavailabilityStable and controllable qualityPeptide preparation methodsUnknown materialsUltrafiltrationChemistry
The invention provides a method for preparing donkey hide gelatin oligo-peptide and relates to a process for extracting pharmacodynamic substance from traditional Chinese medicine. The protein in the medicine normally can be absorbed and utilized by an organism only after being decomposed into the oligo-peptide or amino acid by the digestive juice enzyme. The collagen protein, which accounts for 80% of the content of the donkey hide gelatin, is difficult to be digested and absorbed, so that the due drug effect can not be produced. The method adopts the bionic principle and technology, i.e. extracting the pharmacodynamic substance of the oligo-peptide from the donkey hide gelatin by using the biological enzymolysis technology and the ultrafiltration technology. First, pepsin and trypsinase are added to the raw medicine of the donkey hide gelatin for hydrolyzation at a certain pH value and a certain temperature; then, ultrafiltration is conducted by using a 3kD or 5kD ultrafiltration column to obtain an ultrafiltrate, which is then separated and purified by using a macroporous resin column to further obtain a donkey hide gelatin oligo-peptide eluate; finally, the obtained donkey hide gelatin oligo-peptide eluate is decompressed, concentrated at a low temperature and dried to become the finished product of donkey hide gelatin oligo-peptide, which is verified by the pharmacodynamic test, has the efficacies of increasing the blood, enhancing the immunity of the organism, anti-aging, etc., and meanwhile provides the process and raw material for preparing other oral liquids and injections.
Owner:SHANDONG UNIV OF TRADITIONAL CHINESE MEDICINE
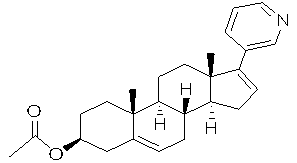
Abiraterone acetate liquid capsule
ActiveCN102961358AHigh dissolution rateSmall toxicityOrganic active ingredientsPharmaceutical non-active ingredientsMonoglycerideOil phase
The invention relates to an abiraterone acetate liquid capsule, comprising a capsule shell and contents, wherein the contents contain the components in percent by weight: 8-25% of abiraterone acetate and 74.8-91.8% of oil phase, wherein the oil phase comprises fatty acid monoglyceride and a solvent in a ratio of 1: 4 to 4: 1 by weight. The capsule disclosed by the invention does not contain a surfactant, as well as is good in dissolution rate, high in safety and simple in preparation process.
Owner:CHONGQING PHARMA RES INST
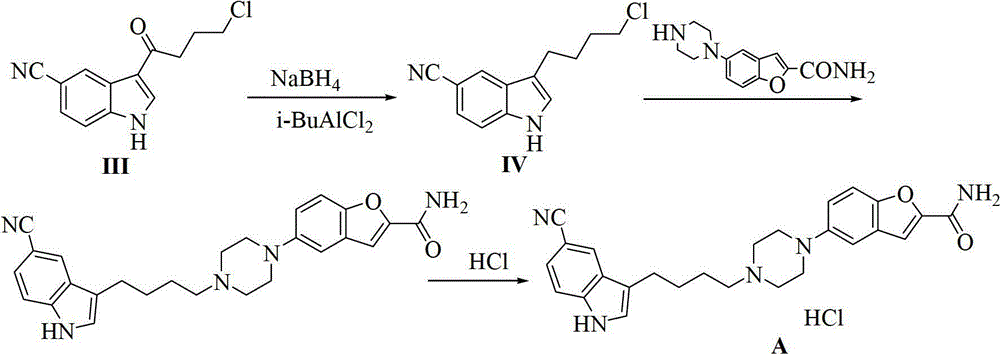
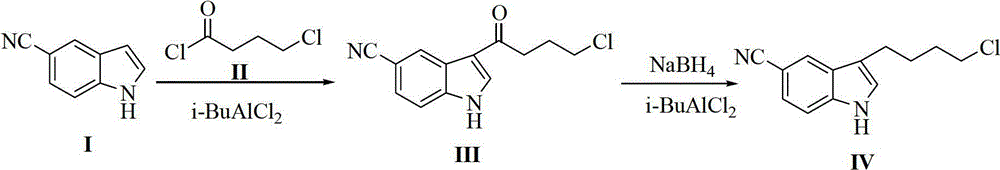
Preparation method of 3-(4-chlorobutyl)-5-cyanoindole
ActiveCN102875440AReduce usageOperational securityOrganic chemistryPotassium borohydrideLewis acid catalysis
The invention discloses a preparation method of 3-(4-chlorobutyl)-5-cyanoindole which is shown as the formula IV. The preparation method comprises the steps as follows: carrying out following carbonyl reduction reaction on a compound III and a hydroboron reducing agent in the solvent under the catalyzing of Lewis acid to obtain the product, wherein Lewis acid is one or more of aluminium trichloride, magnesium chloride, zinc chloride and ferric chloride; and the hydroboron reducing agent is one or more of sodium borohydride, potassium borohydride, lithium borohydride and borane. The preparation method disclosed by the invention is safe in operation, low in requirement on equipment, low in cost, simple in post-processing steps, high in yield of product, high in purity, and is suitable for industrialization.
Owner:CHIRAL QUEST (SUZHOU) CO LTD +1
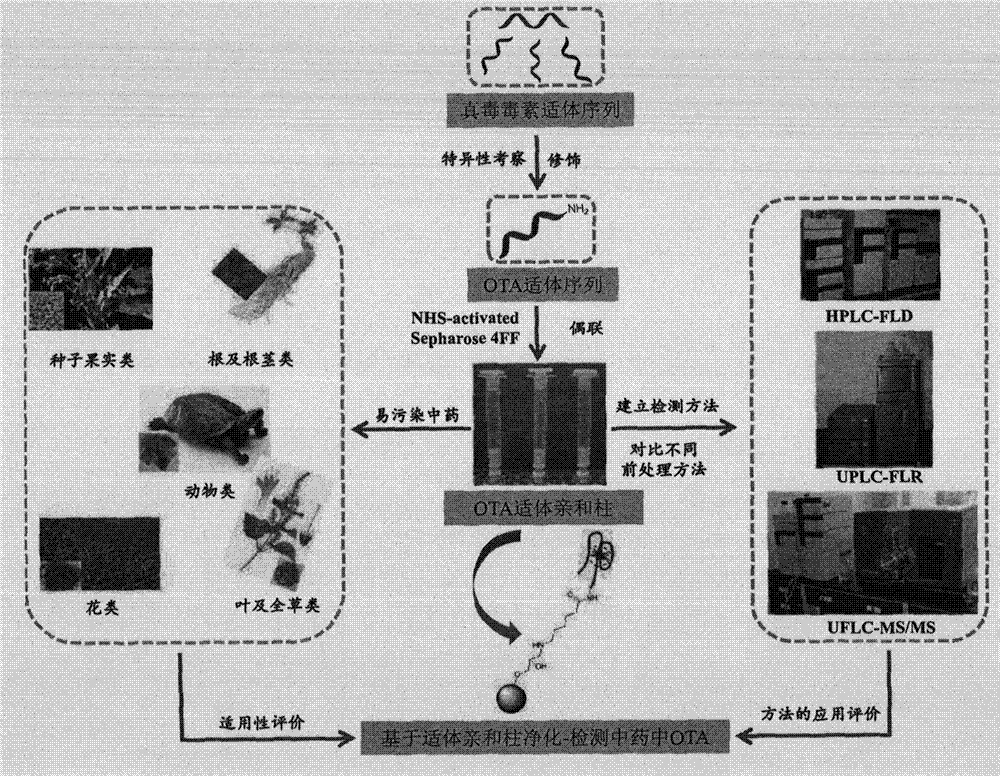
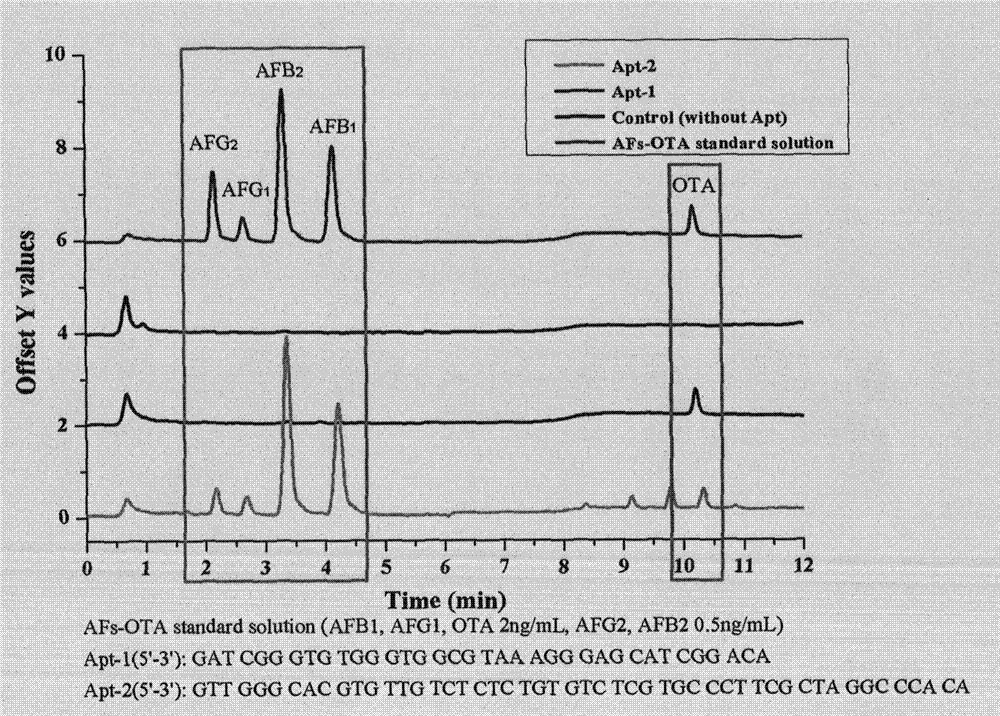
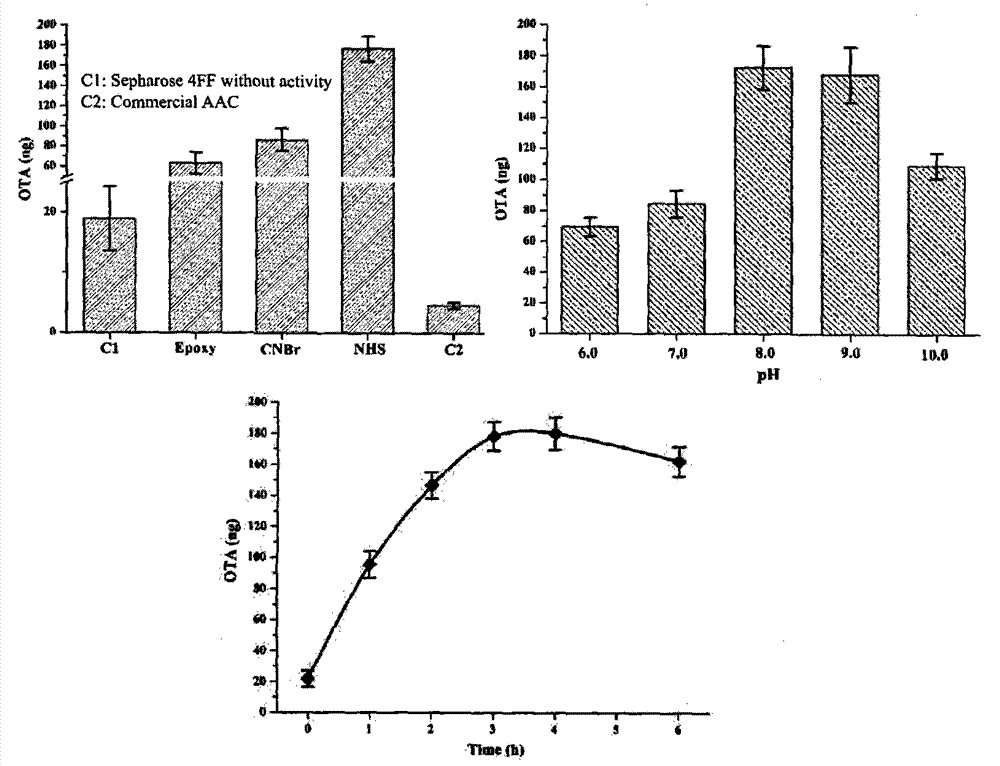
New purification method and testing technology of ochratoxin A in traditional Chinese medicines
ActiveCN104730172AReduce testing costsEasy to prepareOther chemical processesComponent separationAptamerPurification methods
The invention relates to a preparation method of an aptamer affinity column having the advantages of simple operation, fast coupling speed and high coupling efficiency, and the aptamer affinity column is prepared from NHS activated sephrose4FF as a carrier and ochratoxin A specific aptamer as a ligand. After systematical review, the affinity column has good applicability in traditional Chinese medicines, and can achieve the purpose of rapid purification of the ochratoxin A in the traditional Chinese medicines, the established detection method is good in accurate degree and high in sensitivity, and can be used for rapid screening of the ochratoxin A in the traditional Chinese medicines. In addition, the preparation method of the aptamer affinity column is simple, easy, low in production cost, and stable and controllable in quality, can meet the rapid detection of the ochratoxin A in the traditional Chinese medicines, and has broad development prospects.
Owner:INST OF MEDICINAL PLANT DEV CHINESE ACADEMY OF MEDICAL SCI
Oral Chinese medicinal preparation for treating vascular dementia
ActiveCN1657084AShort course of treatmentQuick effectNervous disorderUnknown materialsActive ingredientRhizome
An orally taken Chinese medicine for treating vascular dementia is prepared from fleece flower root, astragalus root, Chinese angelica root, Chuan-xiong rhizome and lucid ligustrum fruit.
Owner:SHANGHAI PHARMA GRP QINGDAO GROWFUL PHARMA CO LTD
Steroid saponin pharmaceutical composition and its preparation method and uses
InactiveCN1754541AQuality improvementEasy to useOrganic active ingredientsPowder deliveryYellow YamMedicine
The invention relates to a steroid glycoside medicinal composition prepared by using yellow yam and Chuanlong yam as the raw material, which comprises (by weight ratio), 5-25 parts of furostanol steroid saponin having a general formula A and / or B, and 1-10 parts of spirostanol steroid saponin 1-10 parts. The invention also provides the process for preparing the medicinal composition and its use.
Owner:CHENGDU DIAO PHARMA GROUP
Compound dendrobium candidum blood-nourishing and liver-softening capsule and preparation method thereof
InactiveCN101703695AKeep active ingredientsSave solventDigestive systemCapsule deliverySalvia miltiorrhizaLycium barbarum fruit
The invention provides a dendrobium candidum blood-nourishing and liver-softening capsule and a preparation method thereof, wherein the dendrobium candidum blood-nourishing and liver-softening capsule takes seven types of traditional Chinese medicines of dendrobium candidum, radix rehmanniae, Chinese angelica, white paeony root, salvia miltiorrhiza bunge, barbary wolfberry fruit and adenophora stricta as raw materials. The main technique comprises the following steps: firstly crushing the radix rehmanniae and the white paeony root into powder for later use, combining and mixing evenly the extracts of the Chinese angelica, the dendrobium candidum, the barbary wolfberry fruit and the like, reducing the pressure under low temperature, drying and filling into a capsule. The invention can nourish the blood and soften the liver, and has the advantages of stable manufacture technique, simple operation, short production cycle and the like.
Owner:浙江森宇实业有限公司
Sodium-potassium citrate chewing tablet and preparation method thereof
ActiveCN102429887AStable and controllable qualityCool tasteOrganic active ingredientsMetabolism disorderCelluloseMagnesium stearate
The invention provide a sodium-potassium citrate chewing tablet, of which the prescription is composed of the following components by mass percent: 33.0-33.1% of potassium citrate, 27.8-27.9% of sodium citrate, 28-32% of filling agent, 0.05-0.3% of adhesive, 1-1.5% of lubricant, 3-5% of flavoring agent, 0.1-0.3% of aromatizer and 3-4% of moistureproof film coating agent, wherein the filling agentis mannitol or a mixture of mannitol and sorbitol or xylitol; the adhesive is hydroxypropyl methylcellulose or polyvidone K30; the lubricant is magnesium stearate or a mixture of magnesium stearate and micropowder silica gel; the flavoring agent is citric acid or a mixture of citric acid and sodium saccharin, aspartame or steviosin; and the aromatizer is pharmaceutically acceptable essence. The invention also provides a preparation method of the sodium-potassium citrate chewing tablet. The preparation method is simple and convenient to operate, low in cost and suitable for industrial production. The obtained tablet has stable and controllable quality, fresh and cool mouthfeel, sourness and sweetness in taste, mint fragrance or fruit fragrance, smooth and beautiful surface, uniform color, moderate hardness and rapid dissolution, and has good application prospects in treatment of gout and hyperuricemia as well as improvement of children and adult in vivo acidosis symptom and other aspects.
Owner:SOUTHWEST UNIV
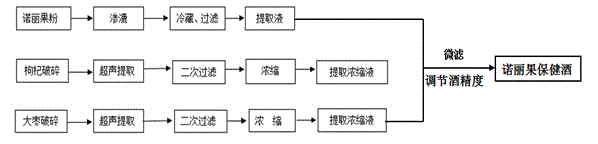
Noni fruit health wine raw material, noni fruit health wine and preparation method thereof
ActiveCN104630005AIncrease health functionGreat tasteDigestive systemAlcoholic beverage preparationSugarLycium chinense
The invention provides a noni fruit health wine raw material which comprises the following components in parts by weight: 300-1000 parts of base white spirits of which the alcoholic strength is greater than or equal to 50 degrees, 30-100 parts of noni fruit powder, 5-20 parts of wolfberry, 2-10 parts of dates and 10-50 parts of rock sugar. The invention further provides noni fruit health wine which comprises the following components in parts by weight: 300-1000 parts of noni fruit percolate, 25-100 parts of wolfberry extracted concentrated liquid, 10-50 parts of date extract concentrated liquid and 10-50 parts of rock sugar. The invention further provides a method for preparing the noni fruit health wine. The preparation method comprises the following steps: respectively extracting noni fruits, dates and wolfberry by using a percolate extraction technique and an ultrasonic extraction technique, further mixing the extracts with rock sugar according to a certain ratio, and adding a certain amount of soft water to reduce the alcoholic strength, thereby obtaining the noni fruit health wine. The noni fruit health wine provided by the invention is not only good in taste, but also good in healthcare function.
Owner:YUNNAN INST OF TROPICAL CROPS
Dutasteride liquid soft capsules
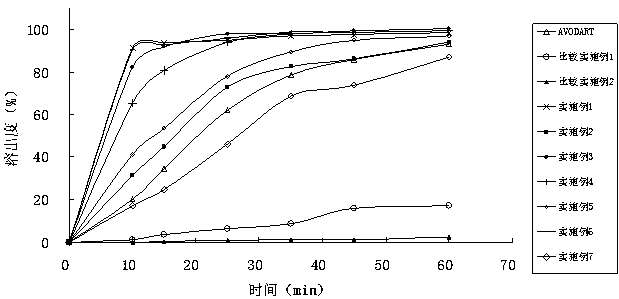
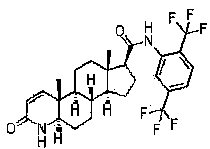
InactiveCN103830201APromote absorption in the bodyImprove the dissolution rate of dutasterideOrganic active ingredientsOrganic non-active ingredientsSoftgelGlycerol
The present invention relates to dutasteride liquid soft capsules and a preparation method thereof. The soft capsules comprise a capsule shell and contents, wherein the contents mainly comprise, by weight, 0.05-0.3% of dutasteride, 19.8-94.8% of medium chain triglyceride, and 5-80% of mono-caprylin glycerate. The soft capsules have characteristics of rapid dissolution, simple preparation process and the like.
Owner:CHONGQING PHARMA RES INST +1
Bamboo-wood straw and manufacturing method
The invention provides a bamboo-wood straw and a manufacturing method. A bamboo-wood straw main body is of a straight-strip tubular structure with a uniform wall thickness, one end of the bamboo-woodstraw main body is cut into a flat cut to serve as a drinking port, the other end of the bamboo-wood straw main body is cut into an inclined cut to serve as a puncturing port, raw materials of the bamboo-wood straw are ripe bamboo or wood which is uniform and consistent in texture, and include sandalwood, beech, oak, phoebe zhennan, ormosia henryi and the like. According to the bamboo-wood straw,a new structure suitable for combination and processing is adopted, requirements for natural formation of the raw materials are small, common materials can be directly adopted, the requirements for selecting materials are small, and the material cost is greatly reduced; the raw materials are environment-friendly, easy to recycle and recyclable, and the bamboo and the wood have the characteristic of environmental protection, and can be conveniently reused in many fields after being used as disposable straws; the processing method is suitable for large-scale production; and the requirements of the raw materials are relatively low, standardized production can be realized, and the quality of products is stable and controllable.
Owner:林仙珠
Extract of preventing and treating coronary heart disease and stenocardia and preparation method and application thereof
ActiveCN102119964APromote circulationDefinite curative effectHydroxy compound active ingredientsPill deliveryCoronary artery diseaseCoronary heart disease
The invention relates to an extract of preventing and treating coronary heart disease and stenocardia and a preparation method and application thereof. The preparation method comprises the following steps of: 1, extracting medicinal materials such as red-rooted salvia root and pseudo-ginseng to obtain an extraction solution; 2, separating the extraction solution by using macroporous resin to obtain an extract; and 3, taking the extract and borneol together serving as medicinal active ingredients to prepare a medicinal preparation.
Owner:TIANJIN TASLY MORDEN TCM RESOURCES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com