Organ preservative fluid and preparation method thereof
A technology of organ preservation solution and ph value, which is applied in the medical field, can solve the problems of not enough to prevent cell acidification, not suitable for general application, high potassium ion content, etc., to achieve outstanding anti-blood reperfusion injury, prevent ischemia-reperfusion injury, Effect of enhancing SOD activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] formula:
[0043]
[0044] Preparation:
[0045] 1. Concentrated preparation: dissolve potassium citrate, sodium citrate, magnesium sulfate, sodium dihydrogen phosphate, adenosine, arginine, tryptophan, mannitol and sodium hydroxide with 500ml of water for injection, and add Add water for injection to 80% of the total volume, add 0.5 g of medicinal charcoal, stir well, and decarbonize to a clear liquid.
[0046] 2. Dilute formulation: add ligustrazine hydrochloride to the above-mentioned concentrated formulation, add water for injection to the full amount, stir well, and filter the liquid medicine through a 0.45 μm filter membrane.
[0047] 3. Check the pH value and osmotic pressure value of the diluted solution in step 2: the pH value is 7.65, and the osmotic pressure value is 370mOsm / L. Filter the drug solution through a 0.2 μm membrane filter.
[0048] 4. Potting: Put the liquid medicine on the assembly line for potting.
[0049] 5. Sterilization: Sterilize the ...
Embodiment 2
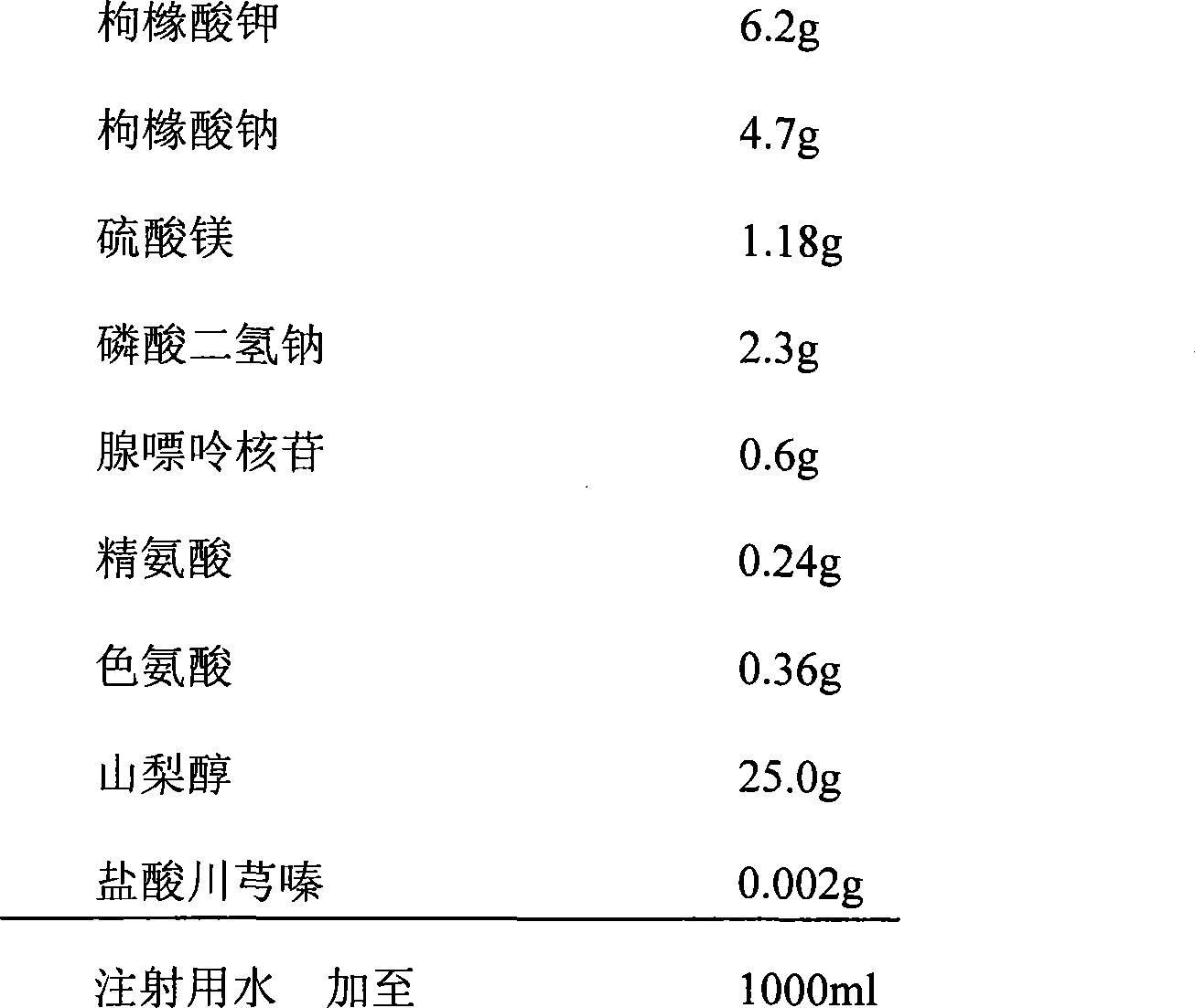
[0051] formula:
[0052]
[0053] Preparation:
[0054] 1. Concentrated preparation: Dissolve potassium citrate, sodium citrate, magnesium sulfate, sodium dihydrogen phosphate, adenosine, arginine, tryptophan, sorbitol and sodium hydroxide with 500ml of water for injection, supplement Add water for injection to 80% of the total volume, add 0.5 g of medicinal charcoal, stir well, and decarbonize to a clear liquid.
[0055] 2. Dilute formulation: add ligustrazine hydrochloride to the above-mentioned concentrated formulation, add water for injection to the full amount, stir well, and filter the liquid medicine through a 0.45 μm filter membrane.
[0056] 3. Check the pH value and osmotic pressure value of the dilute solution in step 2: the pH value is 7.61, and the osmotic pressure value is 366mOsm / L. Filter the drug solution through a 0.2 μm membrane filter.
[0057] 4. Potting: Put the liquid medicine on the assembly line for potting.
[0058] 5. Sterilization: Sterilize ...
Embodiment 3
[0060] formula:
[0061]
[0062] Preparation:
[0063] 1. Concentrated preparation: dissolve potassium citrate, sodium citrate, magnesium sulfate, sodium dihydrogen phosphate, adenosine, arginine, tryptophan, mannitol and sodium hydroxide with 500ml of water for injection, and add Add water for injection to 80% of the total volume, add 0.5 g of medicinal charcoal, stir well, and decarbonize to a clear liquid.
[0064] 2. Dilute formulation: add ligustrazine hydrochloride to the above-mentioned concentrated formulation, add water for injection to the full amount, stir well, and filter the liquid medicine through a 0.45 μm filter membrane.
[0065] 3. Check the pH value and osmotic pressure value of the dilute solution in step 2: the pH value is 7.89, and the osmotic pressure value is 377mOsm / L. Filter the drug solution through a 0.2 μm membrane filter.
[0066] 4. Potting: Put the liquid medicine on the assembly line for potting.
[0067] 5. Sterilization: Sterilize the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com