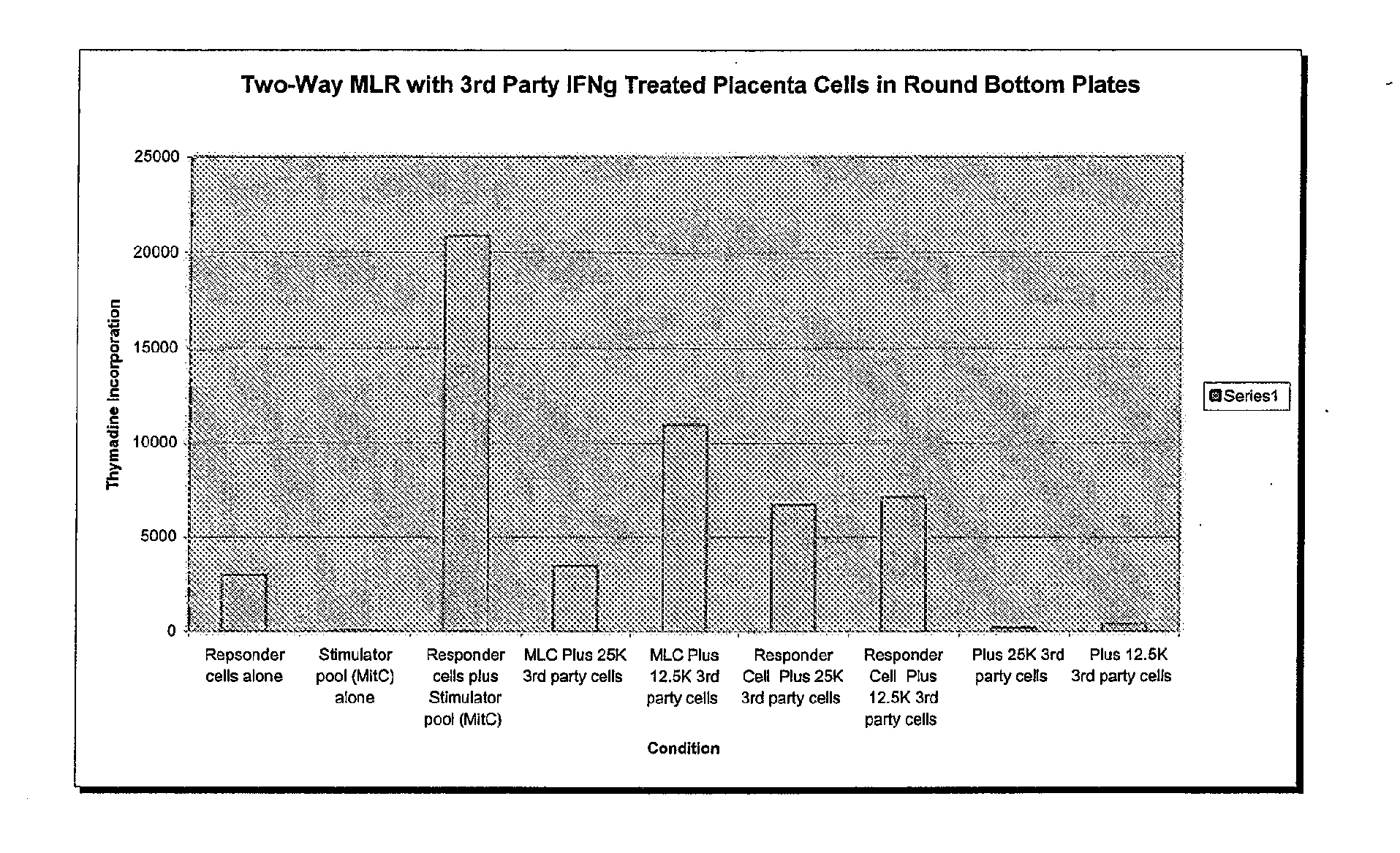
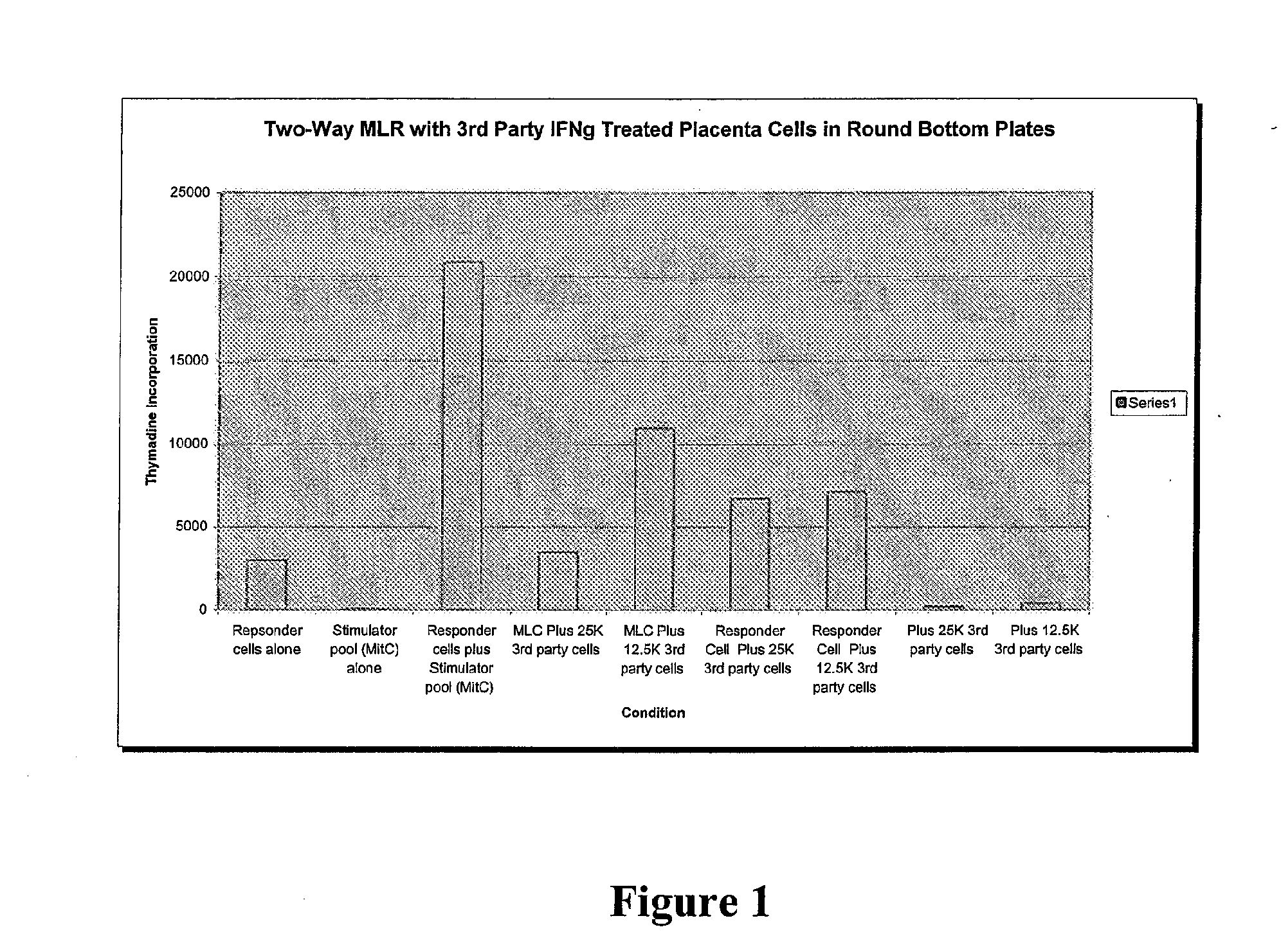
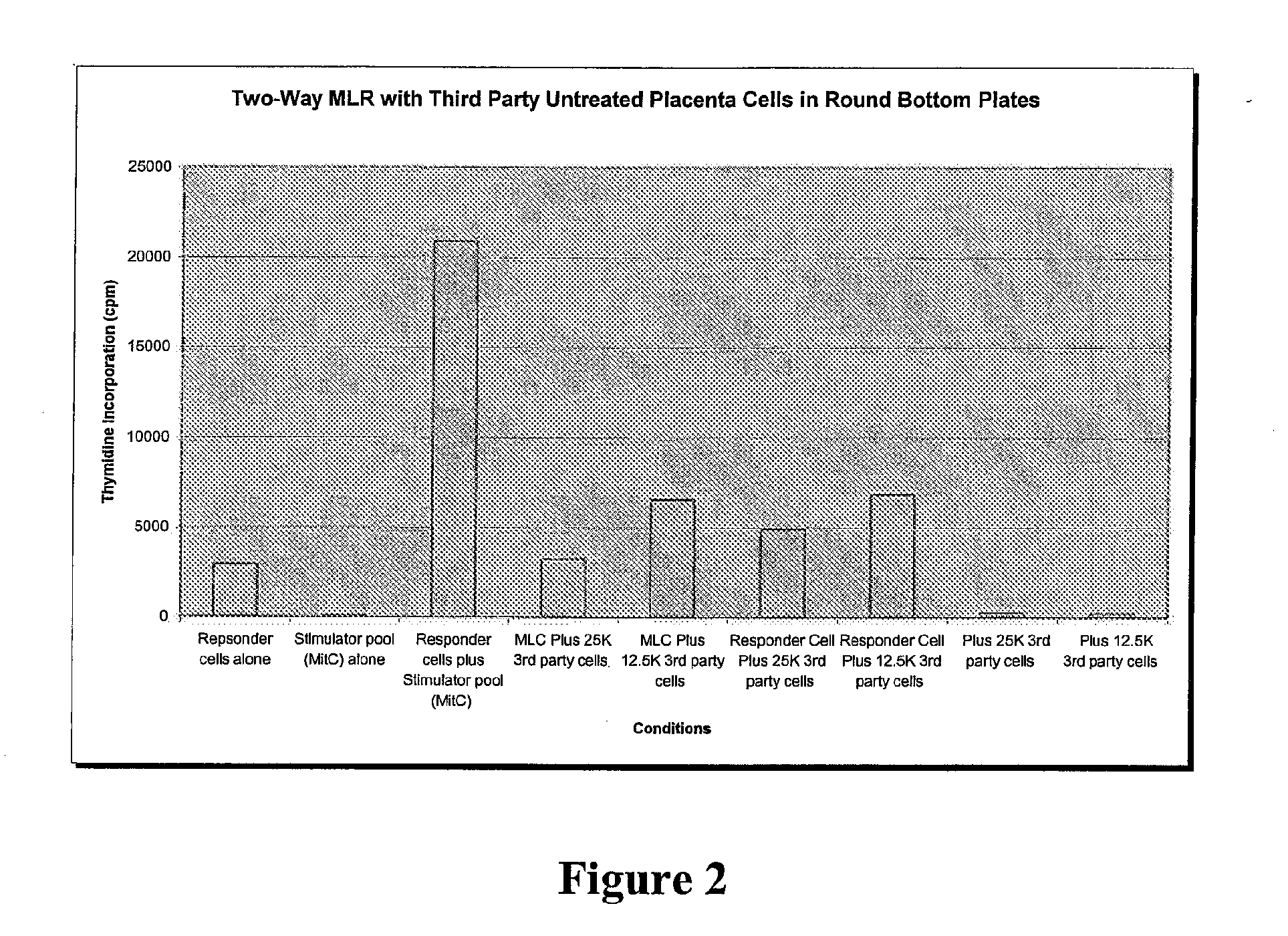
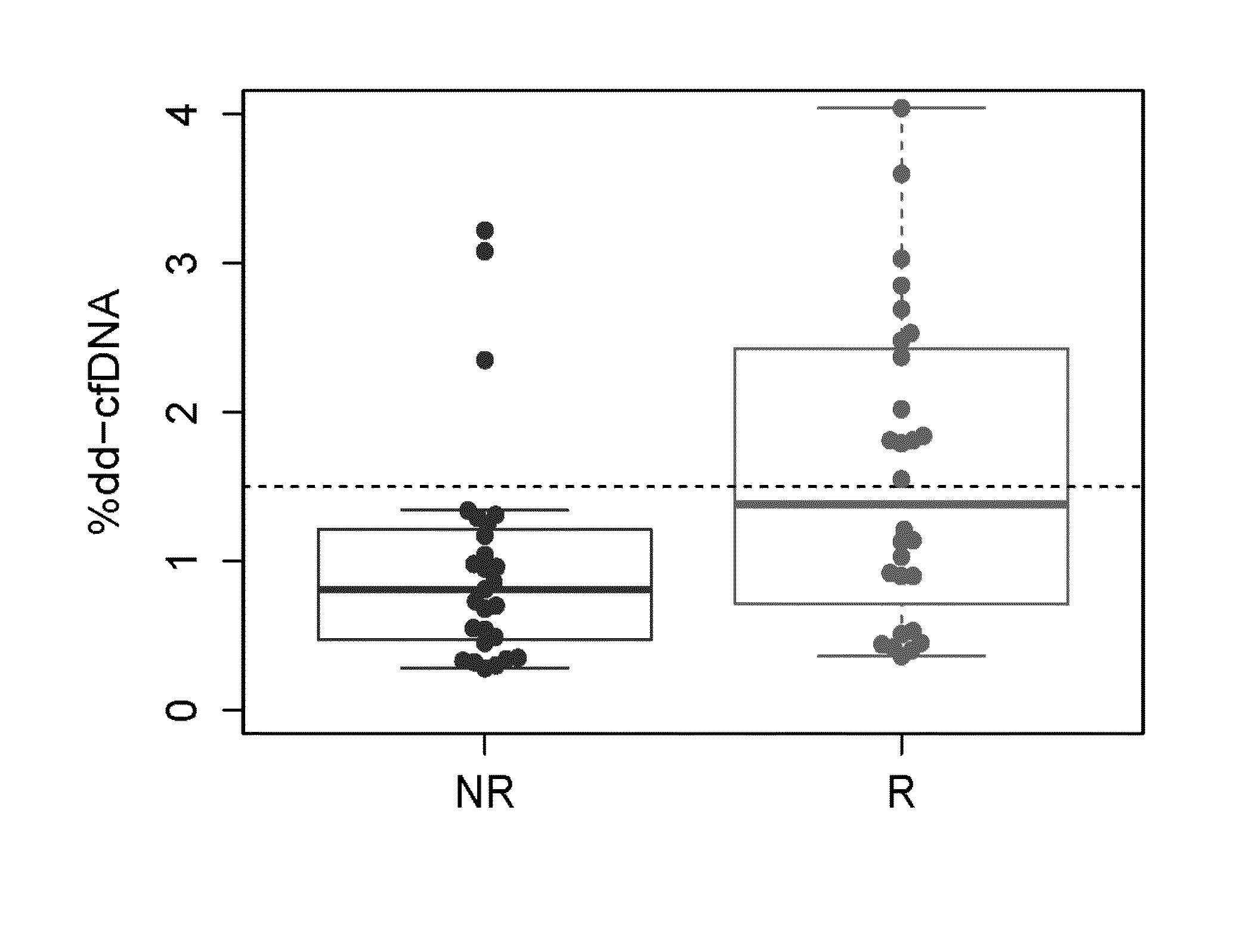
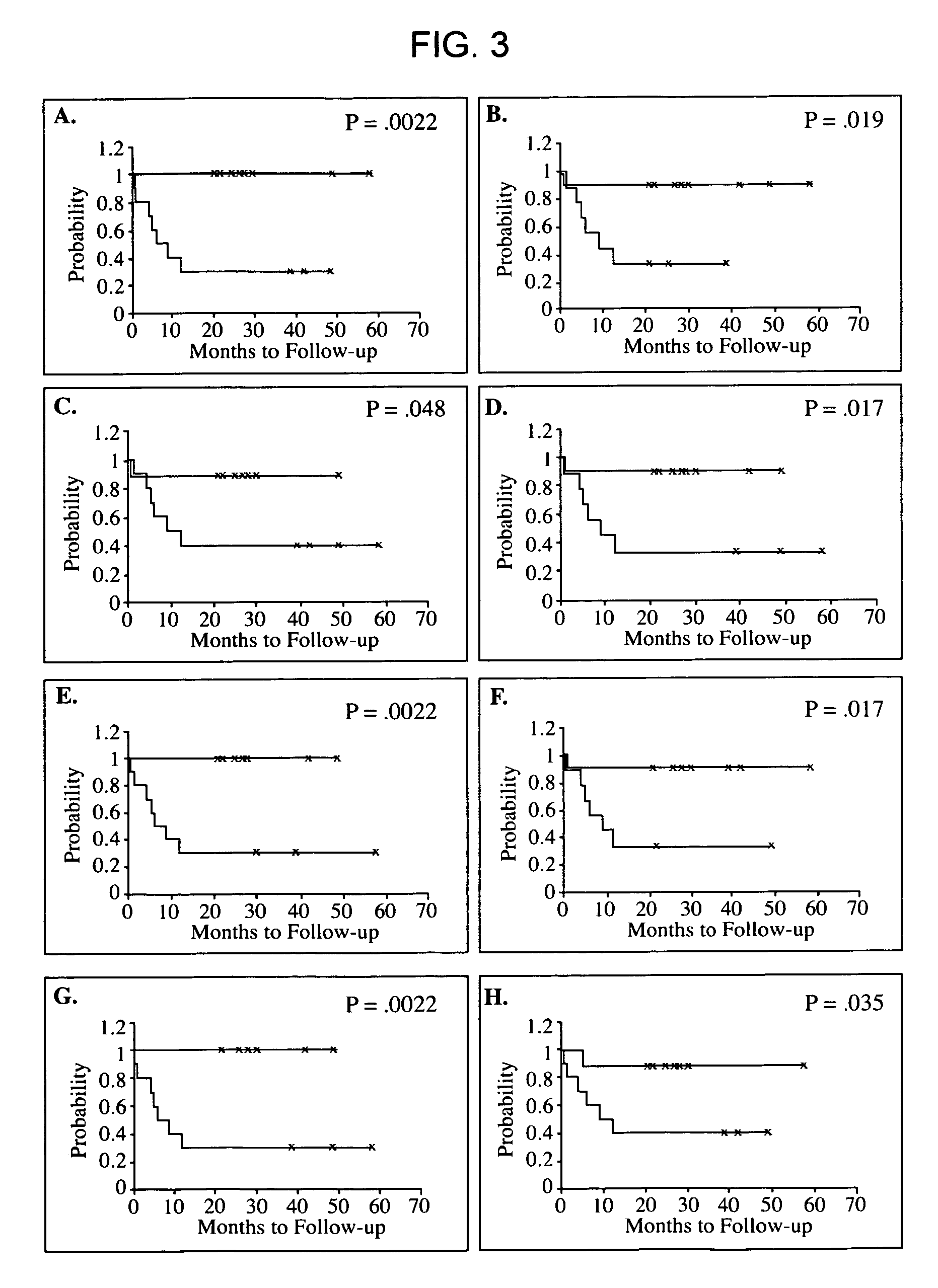
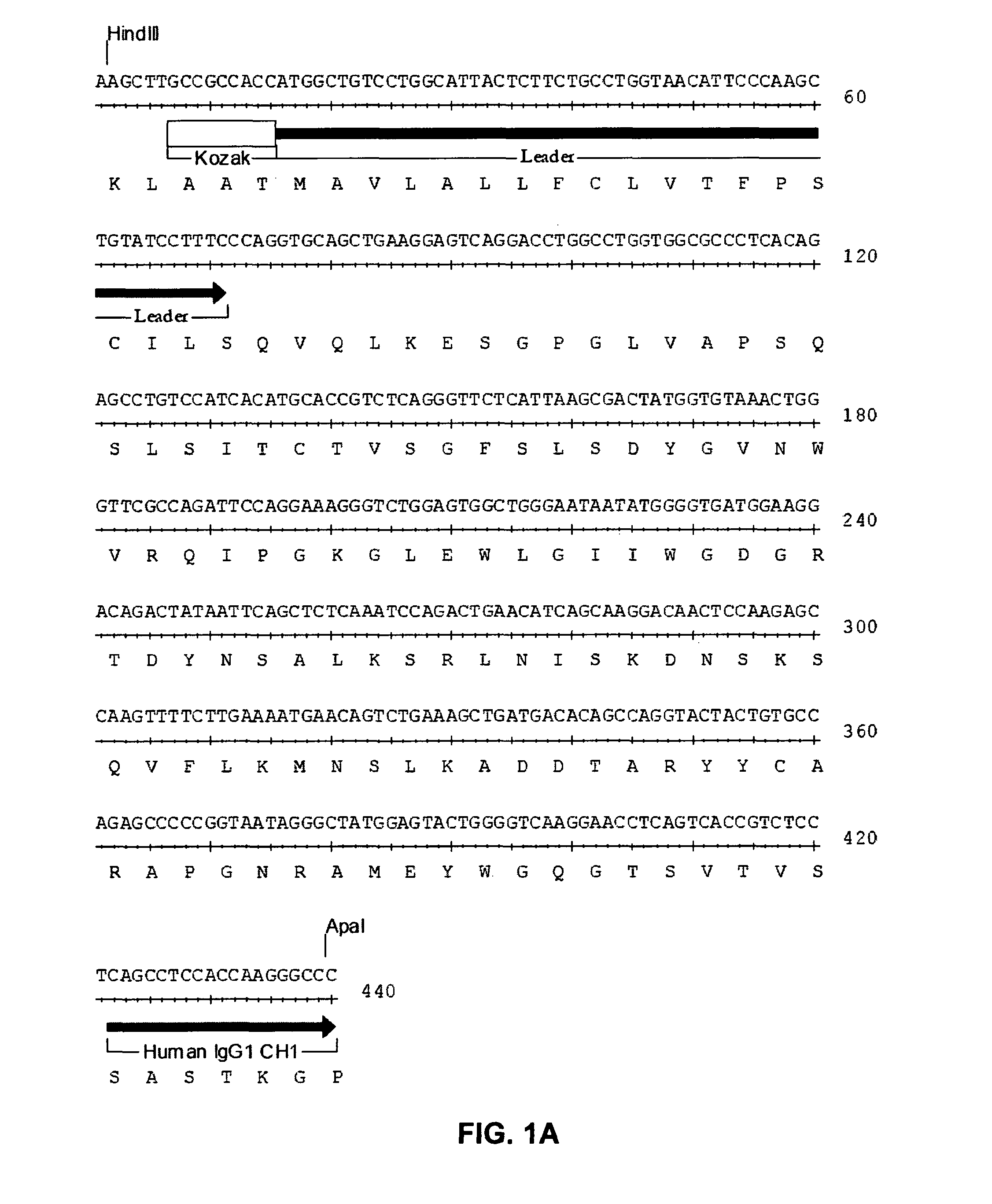
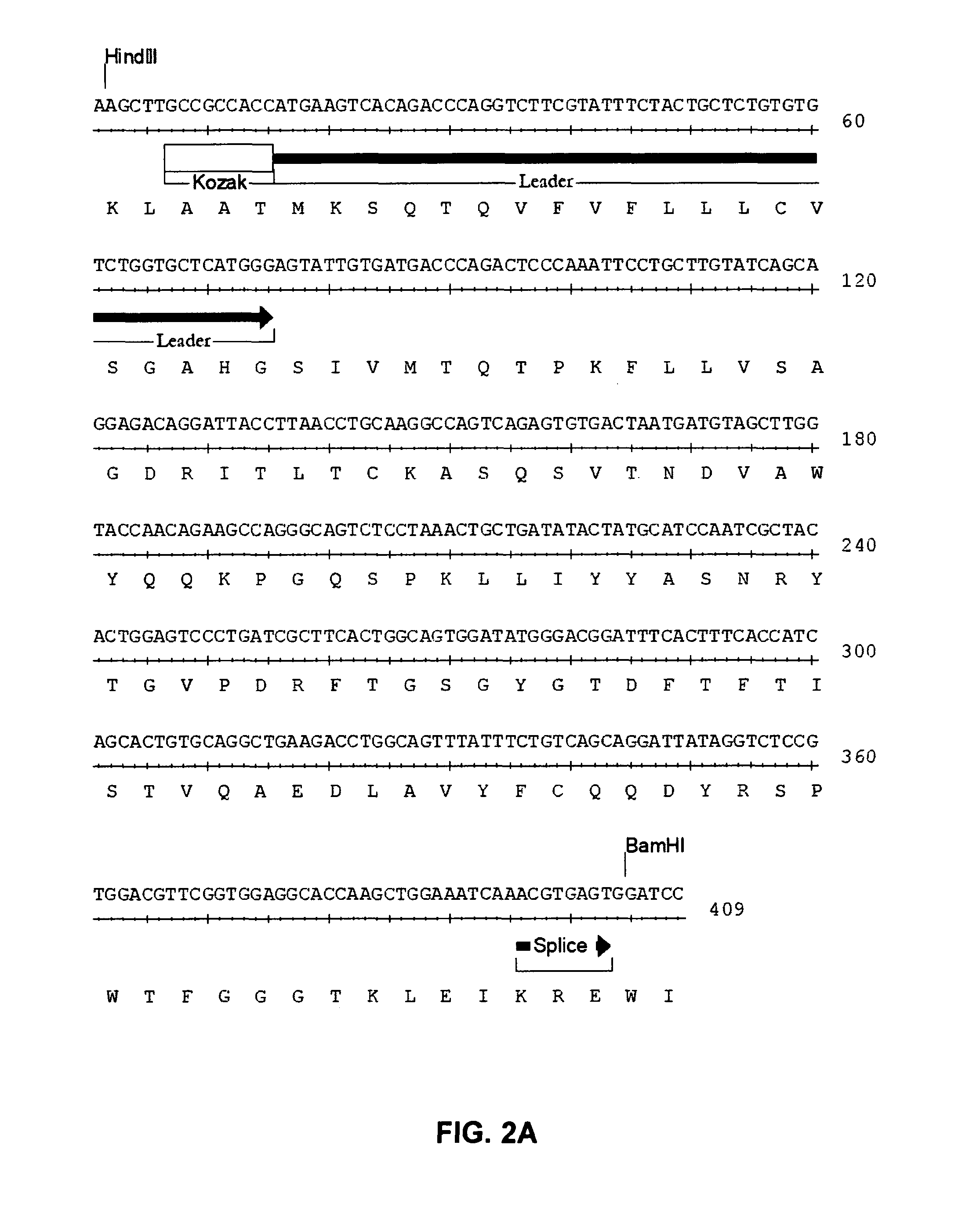
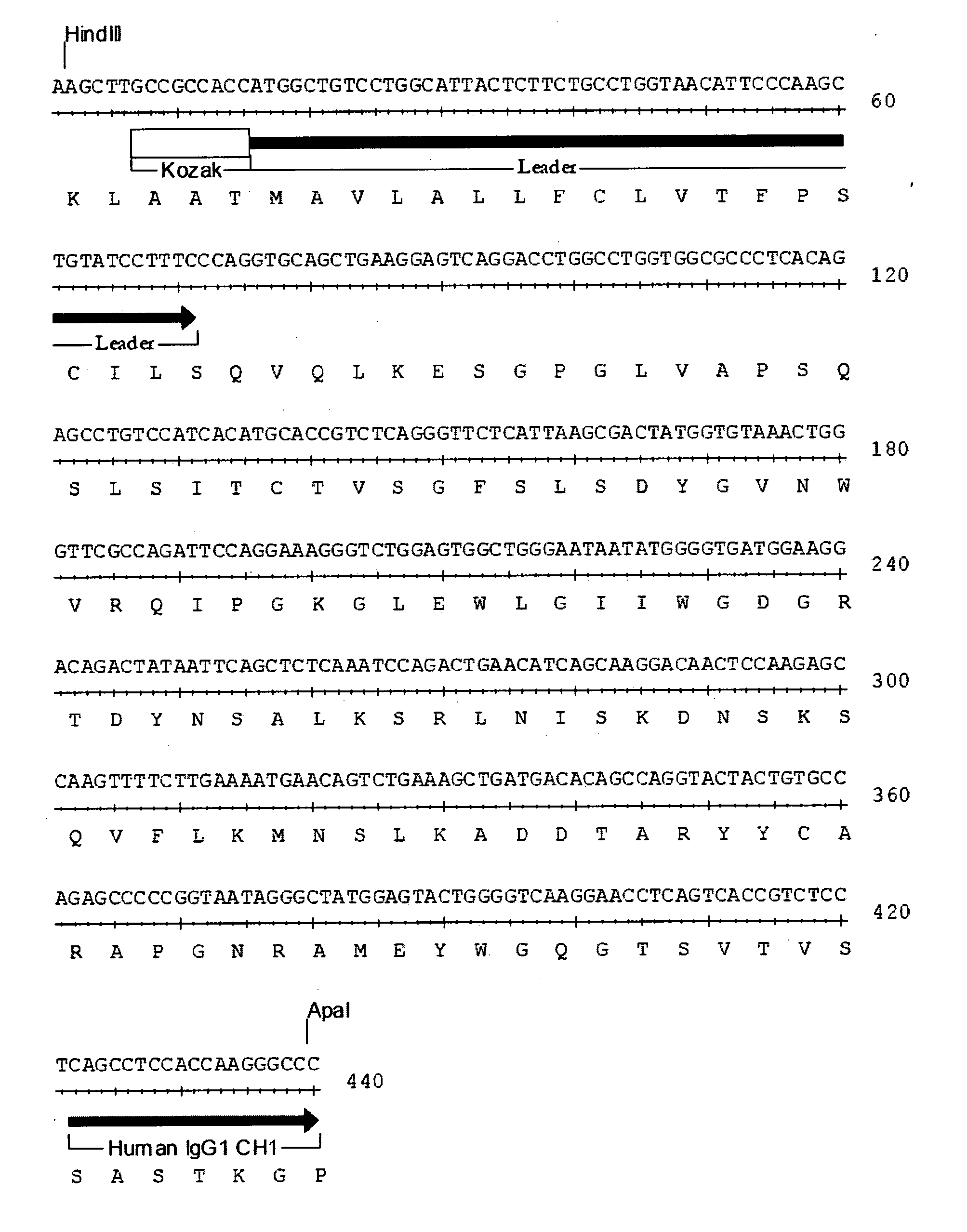
Patents
Literature
116 results about "Transplant recipient" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Compositions and methods for inhibiting adverse immune response in histocompatibility-mismatched transplantation
ActiveUS20070264269A1Effective for adverse immune responseAntipyreticAnalgesicsGraft versus host disease inductionCell based
Cell-based compositions and methods of their use to inhibit an adverse immune response such as graft versus host disease or rejection of transplanted tissue in a transplant recipient that is histocompatibility mismatched to the transplant donor are disclosed. The compositions and methods utilize postpartum-derived cells, such as cells derived from the placenta or umbilicus.
Owner:DEPUY SYNTHES PROD INC
Methods of monitoring immunosuppressive therapies in a transplant recipient
PendingUS20160145682A1Improve the level ofLower Level RequirementsHealth-index calculationMicrobiological testing/measurementMedicineTransplant recipient
The present disclosure relates to methods of monitoring the status of an allograft in a transplant recipient, as well as to methods of monitoring and adjusting immunosuppressive therapies being administered to the transplant recipient.
Owner:XDX
Methods and compositions for evaluating graft survival in a solid organ transplant recipient
ActiveUS20060246485A1Microbiological testing/measurementBiological testingOrgan transplantationProtein level
Methods are provided for evaluating a subject for graft survival, e.g., in terms of predicting graft survival, identifying the presence of a deleterious graft condition, such as CAN and DT, identifying the severity and class of acute rejection, etc, in a subject are provided. In practicing the subject methods, the expression of at least one gene in a sample from the subject, e.g., a blood or biopsy sample, is assayed, e.g., at the nucleic acid and / or protein level, to evaluate the subject. Also provided are compositions, systems and kits that find use in practicing the subject methods. The methods and compositions find use in a variety of applications.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Humanized anti-CD22 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
InactiveUS7829086B2Sugar derivativesImmunoglobulins against animals/humansAntigenImmunoglobulin Heavy Chain Variable Region
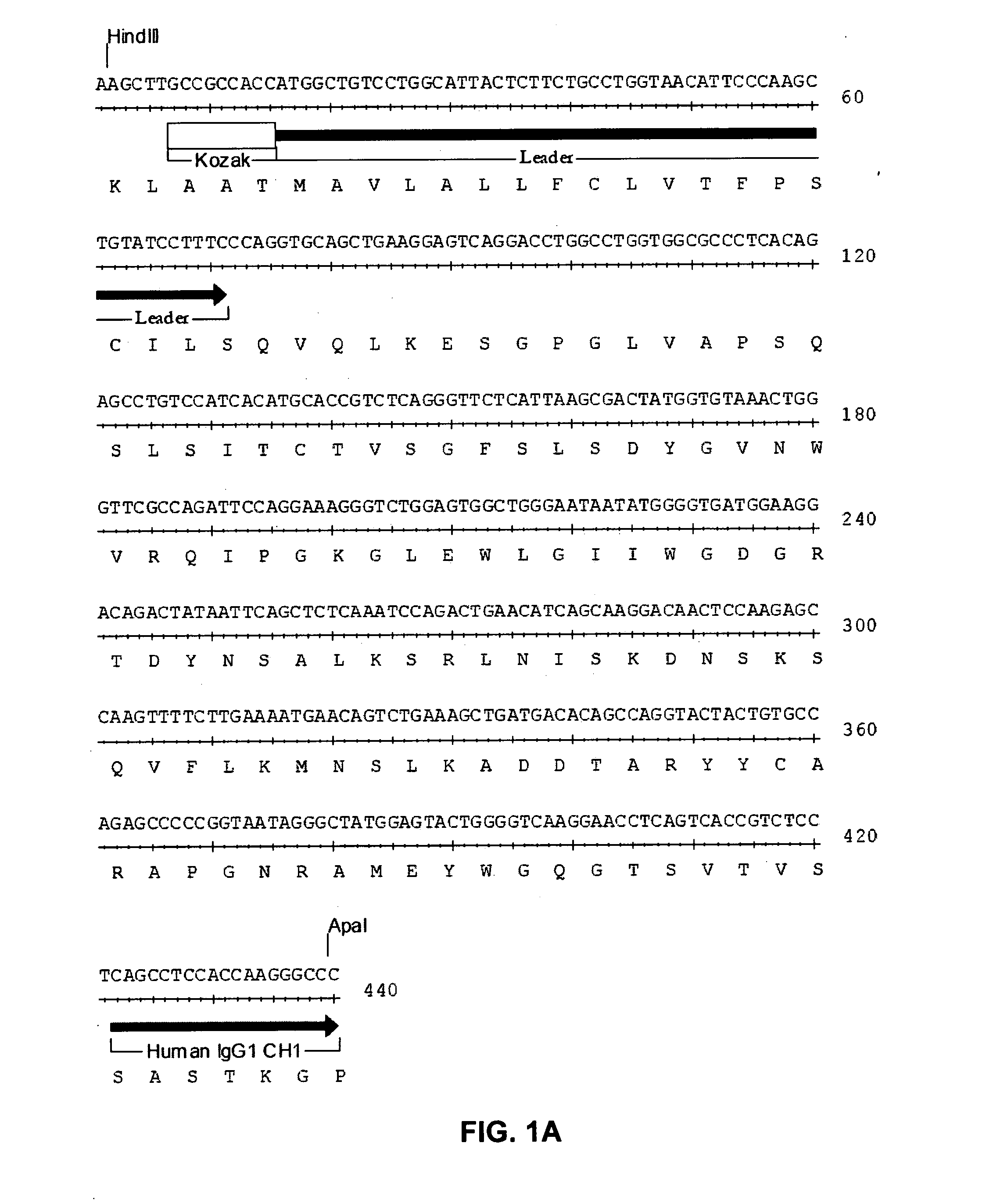
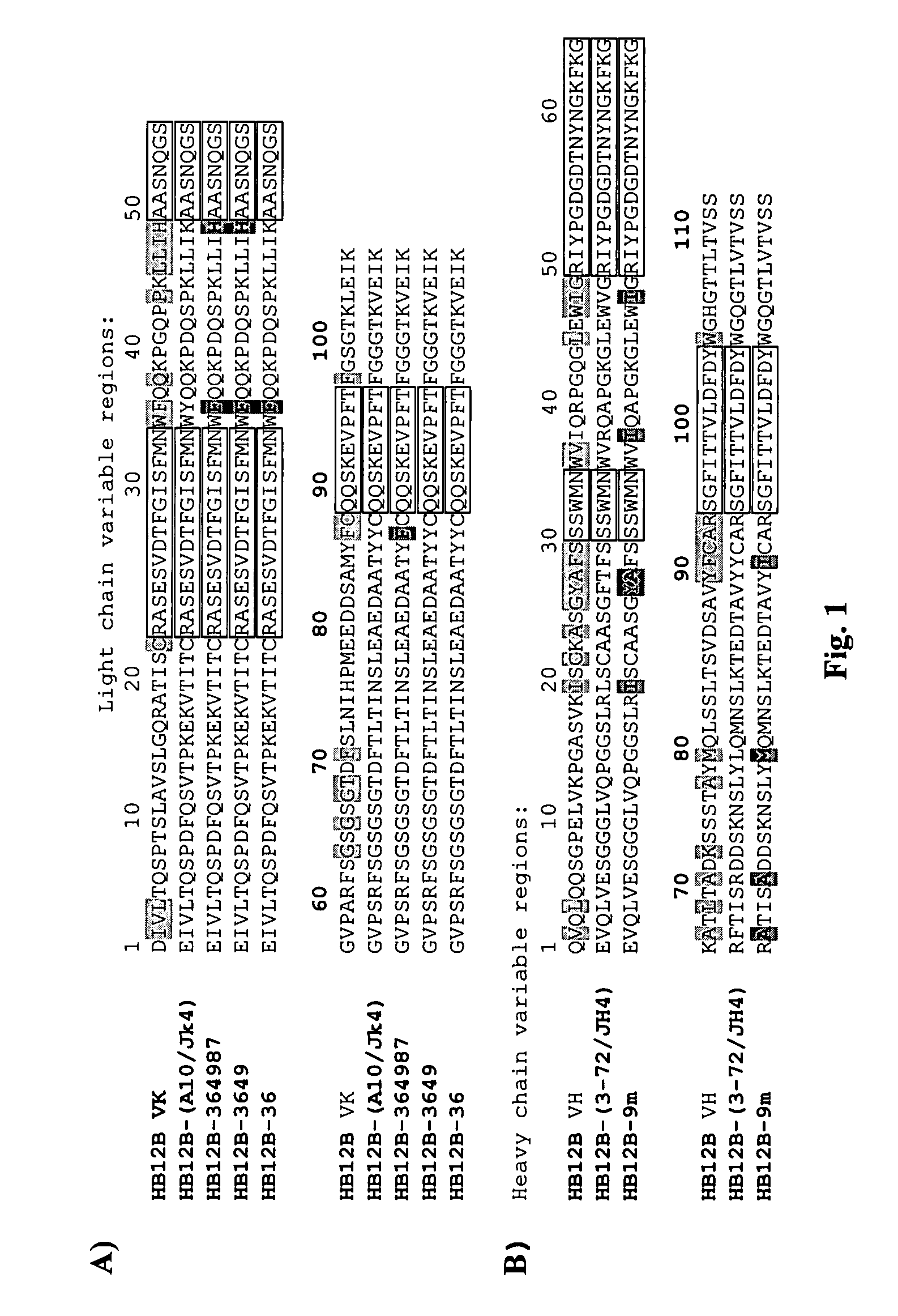
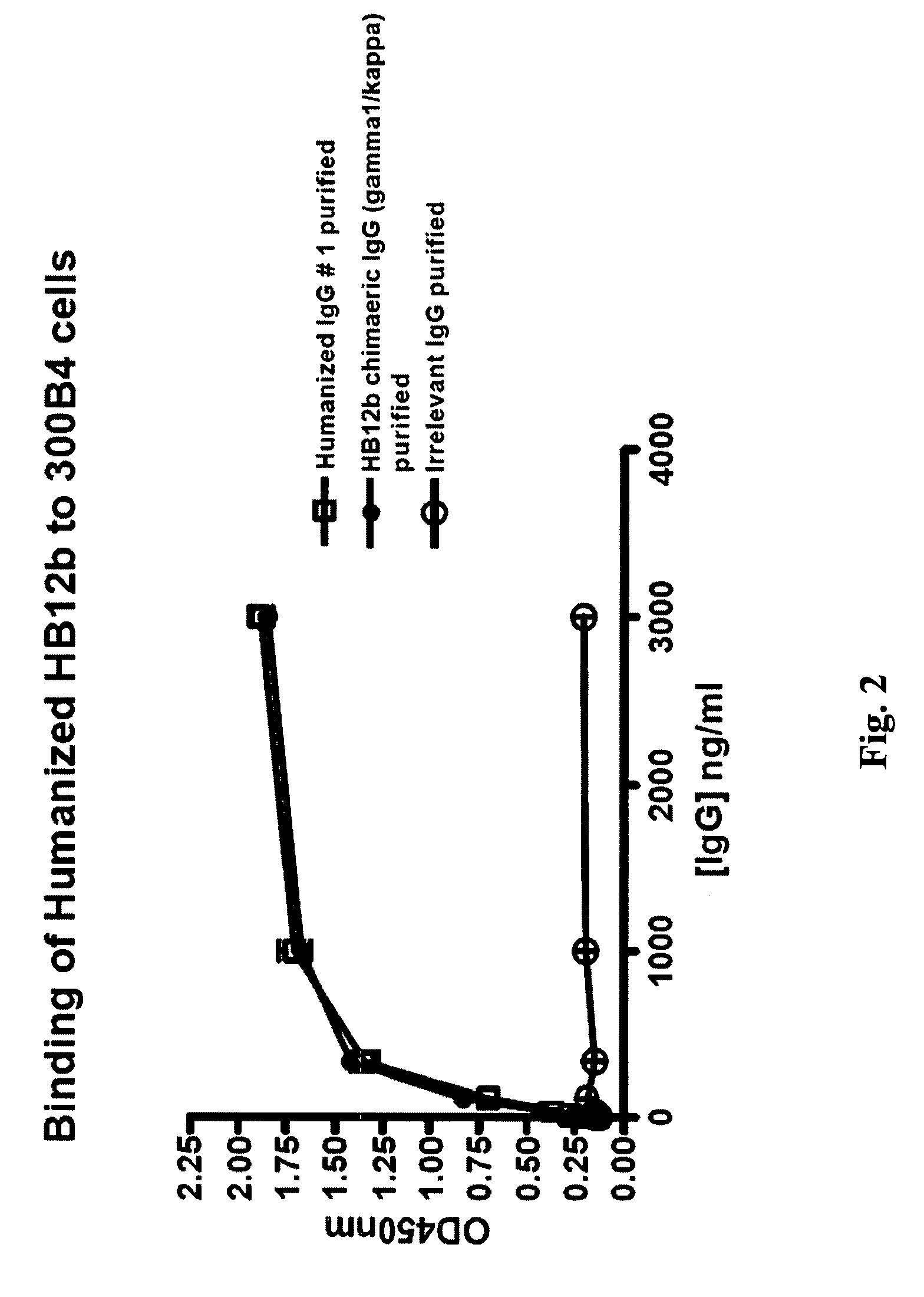
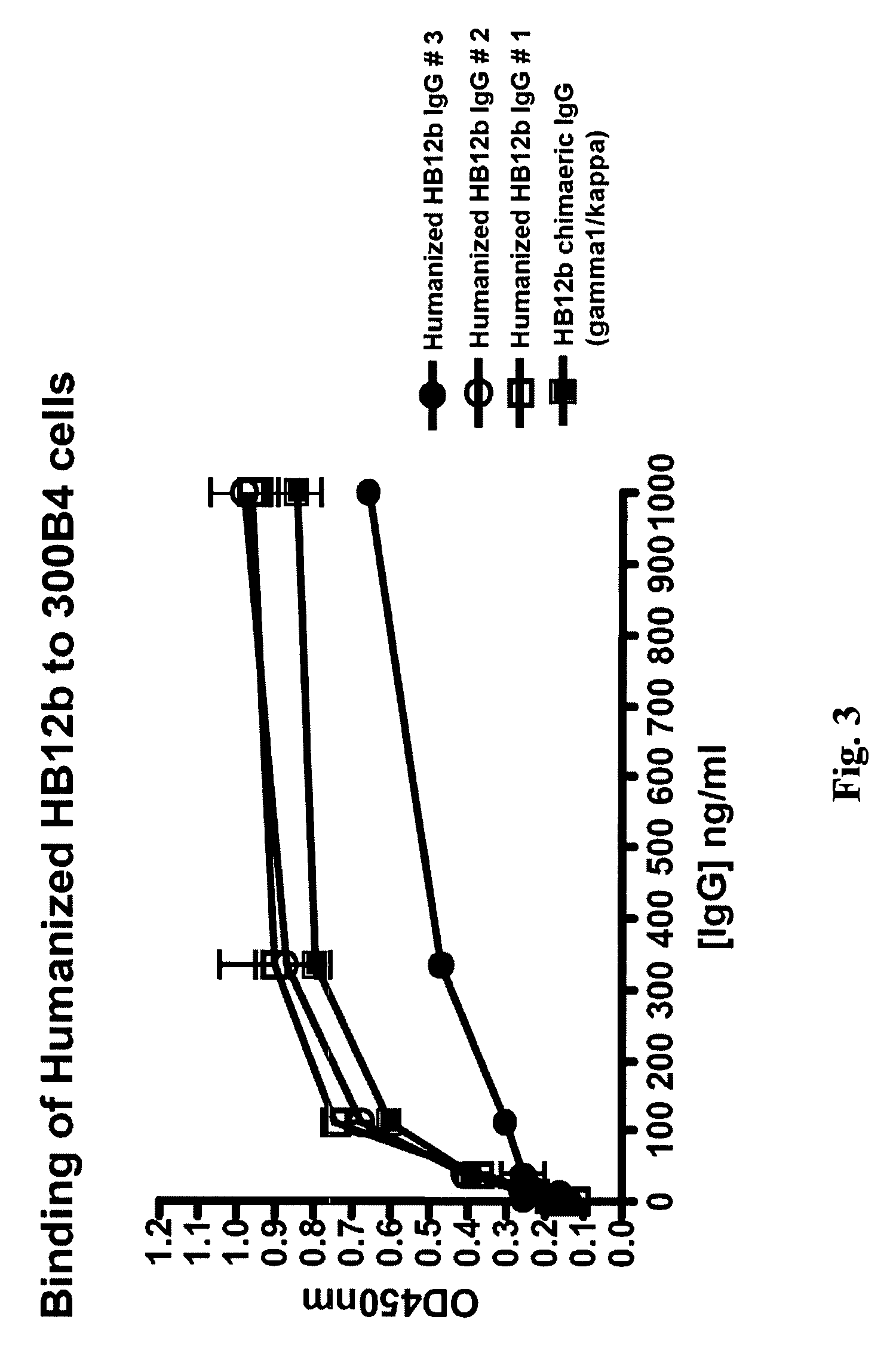
The present invention provides chimeric and humanized versions of anti-CD22 mouse monoclonal antibody, HB22.7. The anti-CD22 antibodies of the invention comprise four human or humanized framework regions of the immunoglobulin heavy chain variable region (“VH”) and four human or humanized framework regions of the immunoglobulin light chain variable region (“VK”). The invention further comprises heavy and / or light chain FW regions that contain one or more backmutations in which a human FW residue is exchanged for the corresponding residue present in the parental mouse heavy or light chain. Human or humanized VH framework regions of antibodies of the invention may comprise one or more of the following residues: a valine (V) at position 24 of framework region 1, a glycine (G) at position 49 of framework region 2, and an asparagine (N) at position 73 of framework region 3, numbered according to Kabat. The invention further relates to pharmaceutical compositions, immunotherapeutic compositions, and methods using therapeutic antibodies that bind to the human CD22 antigen and that preferably mediate human ADCC, CDC, and / or apoptosis for: the treatment of B cell diseases and disorders in human subjects, such as, but not limited to, B cell malignancies, for the treatment and prevention of autoimmune disease, and for the treatment and prevention of graft-versus-host disease (GVHD), humoral rejection, and post-transplantation lymphoproliferative disorder in human transplant recipients.
Owner:MEDIMMUNE LLC +1
Humanized anti-CD22 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
InactiveUS20070258981A1Efficient productionEfficiently depletedSugar derivativesImmunoglobulins against animals/humansAntigenAutoimmune condition
Owner:MEDIMMUNE LLC +1
Purging of cells using viruses
InactiveUS20020037543A1Preventing graft-versus-host diseasesHigh selectivityNervous disorderPeptide/protein ingredientsAutoimmune conditionGraft versus host disease induction
The subject invention relates to viruses that are able to purge (reduce or eliminate) undesirable cells in a mixture of cells. Undesirable cells can include neoplastic cells, cells mediating graft-versus host diseases, and autoimmune cells. The subject invention also relates to the purging of undesirable cells from bone marrow or peripheral blood cell harvests in the treatment of mammals including cancer patients, transplant recipients, and patients with autoimmune disease.
Owner:PRO VIRUS
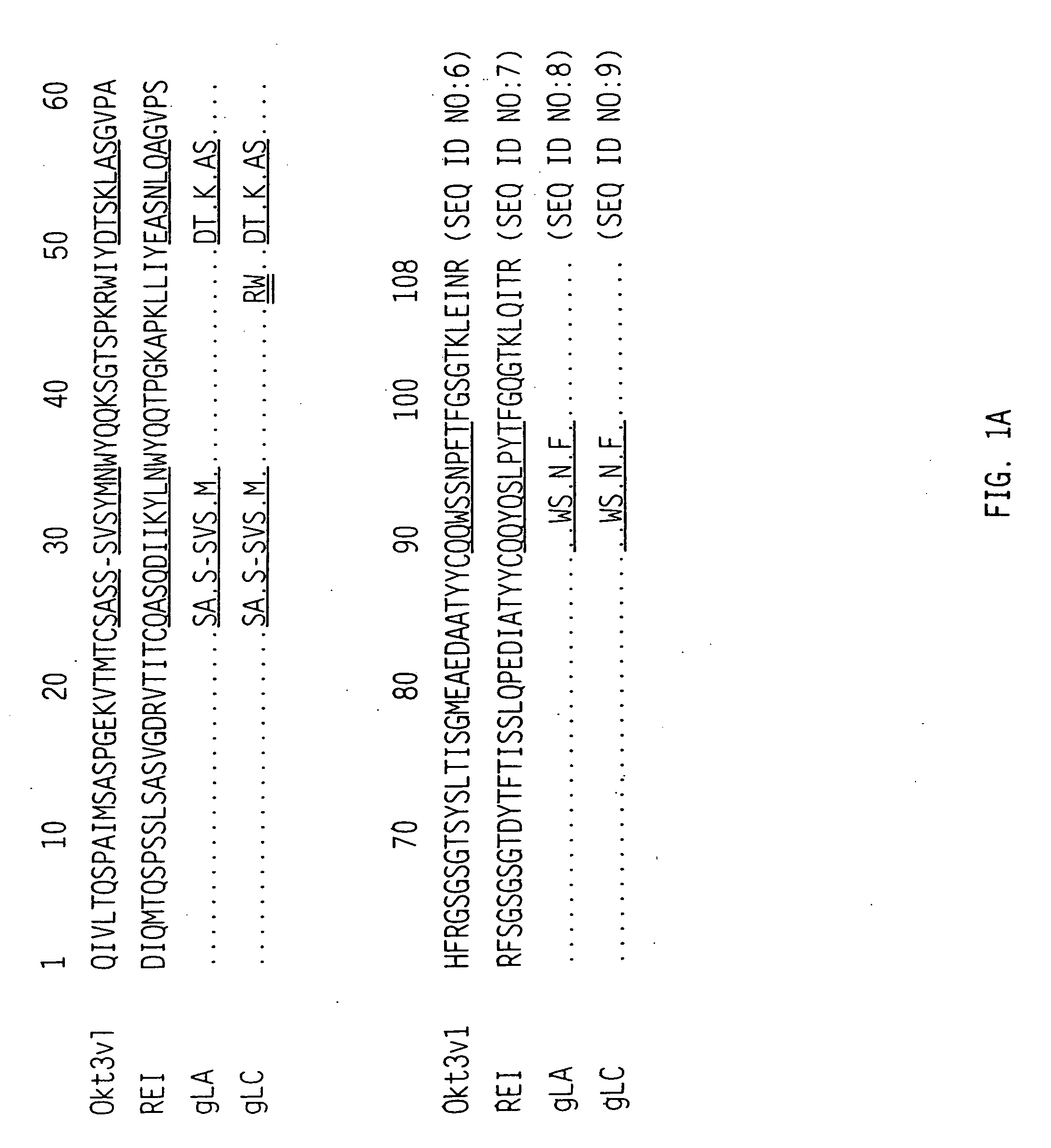
Methods and materials for modulation of the immunosuppressive activity and toxicity of monoclonal antibodies
InactiveUS20060002933A1Eliminate concernsReduced activityPeptide/protein ingredientsAntibody mimetics/scaffoldsSide effectActivation cells
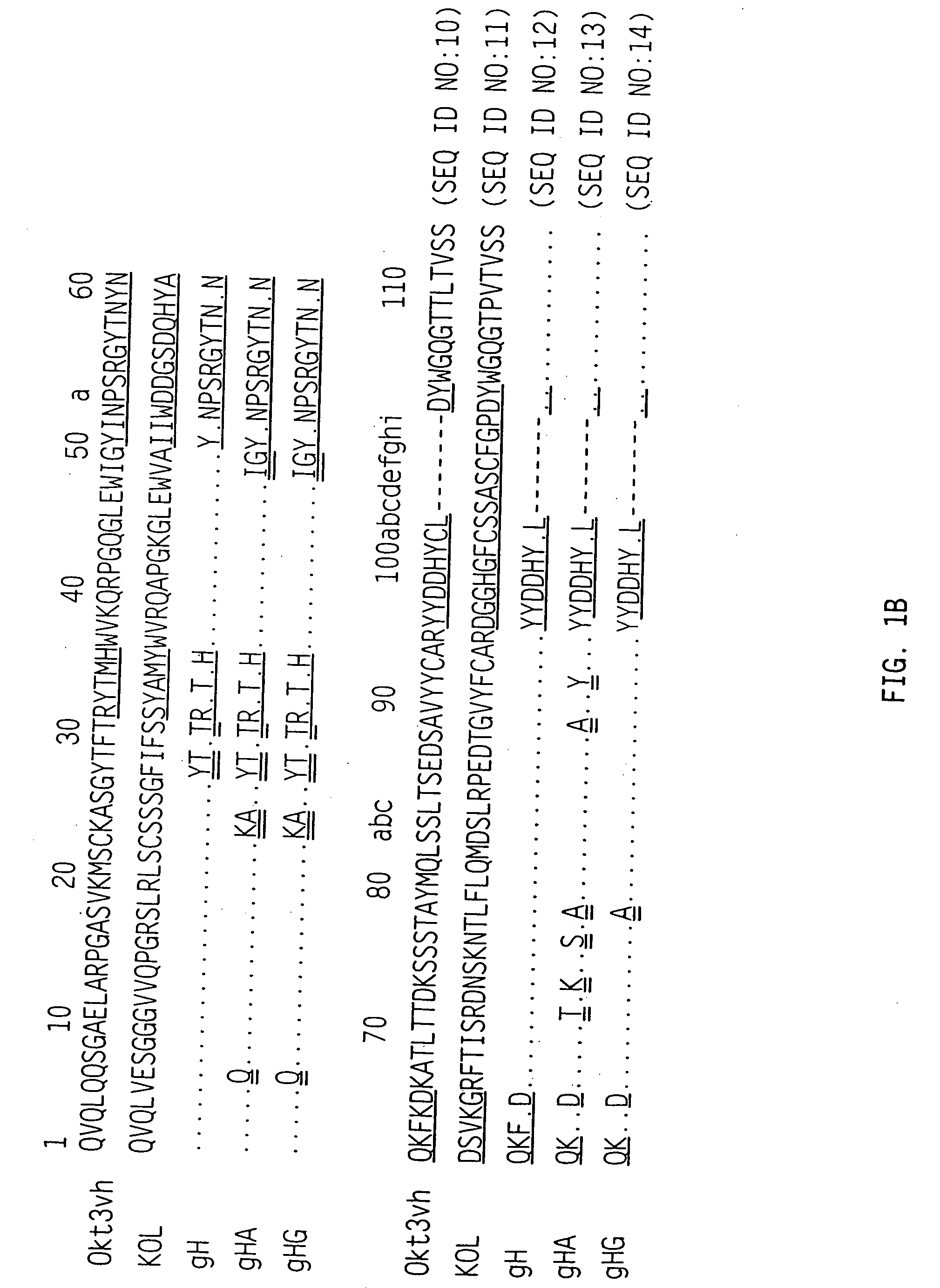
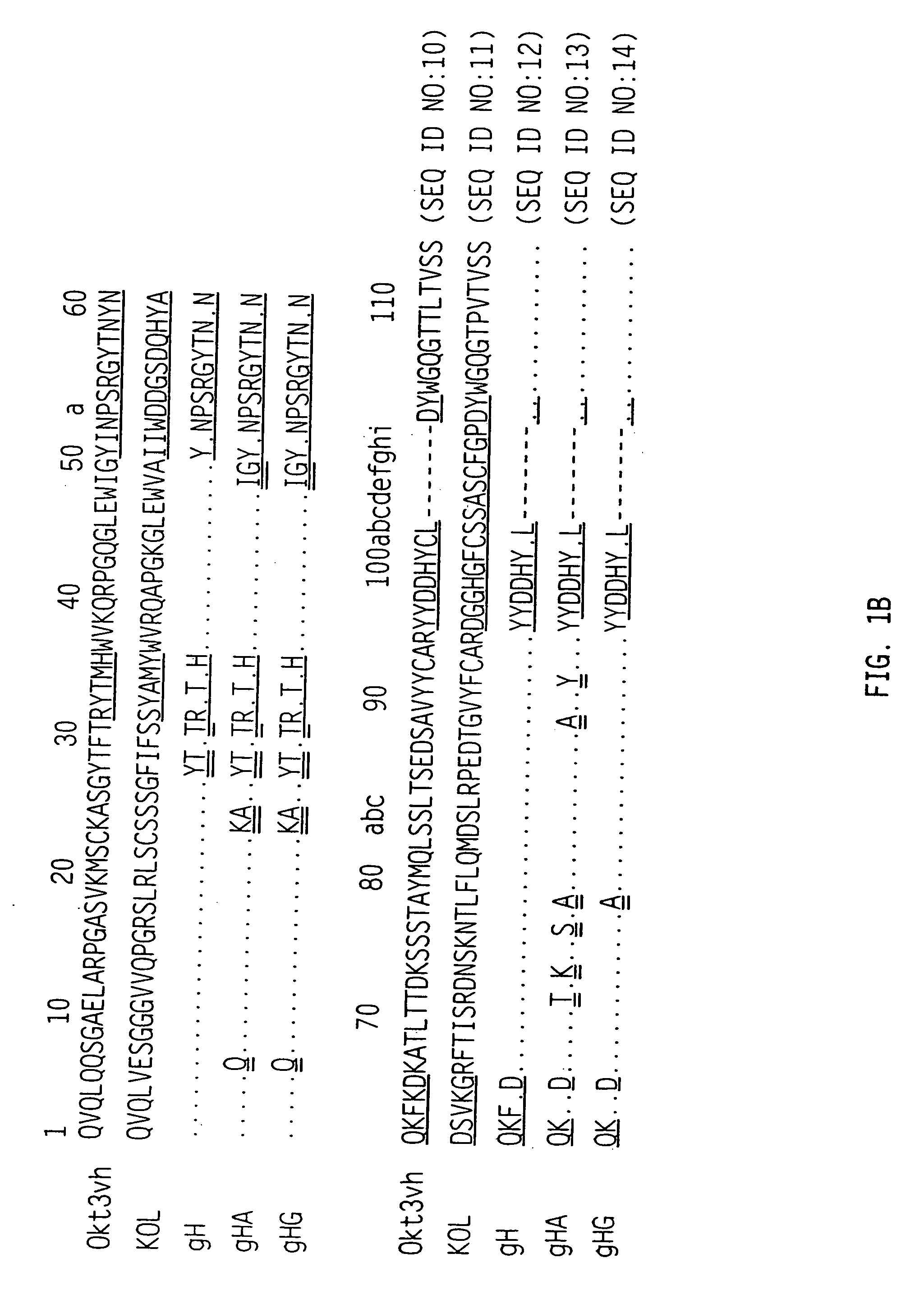
The binding specificity of the murine OKT3 has been transferred into a human antibody framework in order to reduce its immunogenicity. “Humanized” anti-CD3 mAbs, such as gOKT3-5 and gOKT3-7, have been shown to retain, in vitro, all the properties of native OKT3, including T cell activation which has been correlated, in vivo, with the severe side-effects observed in transplant recipients after the first administration of the mAb. Disclosed are modified versions of humanized anti-CD3 mAbs that do not have the property of T cell activation. Further dislosed are methods of using such mAbs.
Owner:BLUESTONE JEFFREY +2
Humanized anti-CD19 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
ActiveUS8323653B2Alleviation and mitigation and decreaseSenses disorderNervous disorderAntigenApoptosis
Owner:VIELA BIO INC
Methods for identifying a preferred liver transplant donor
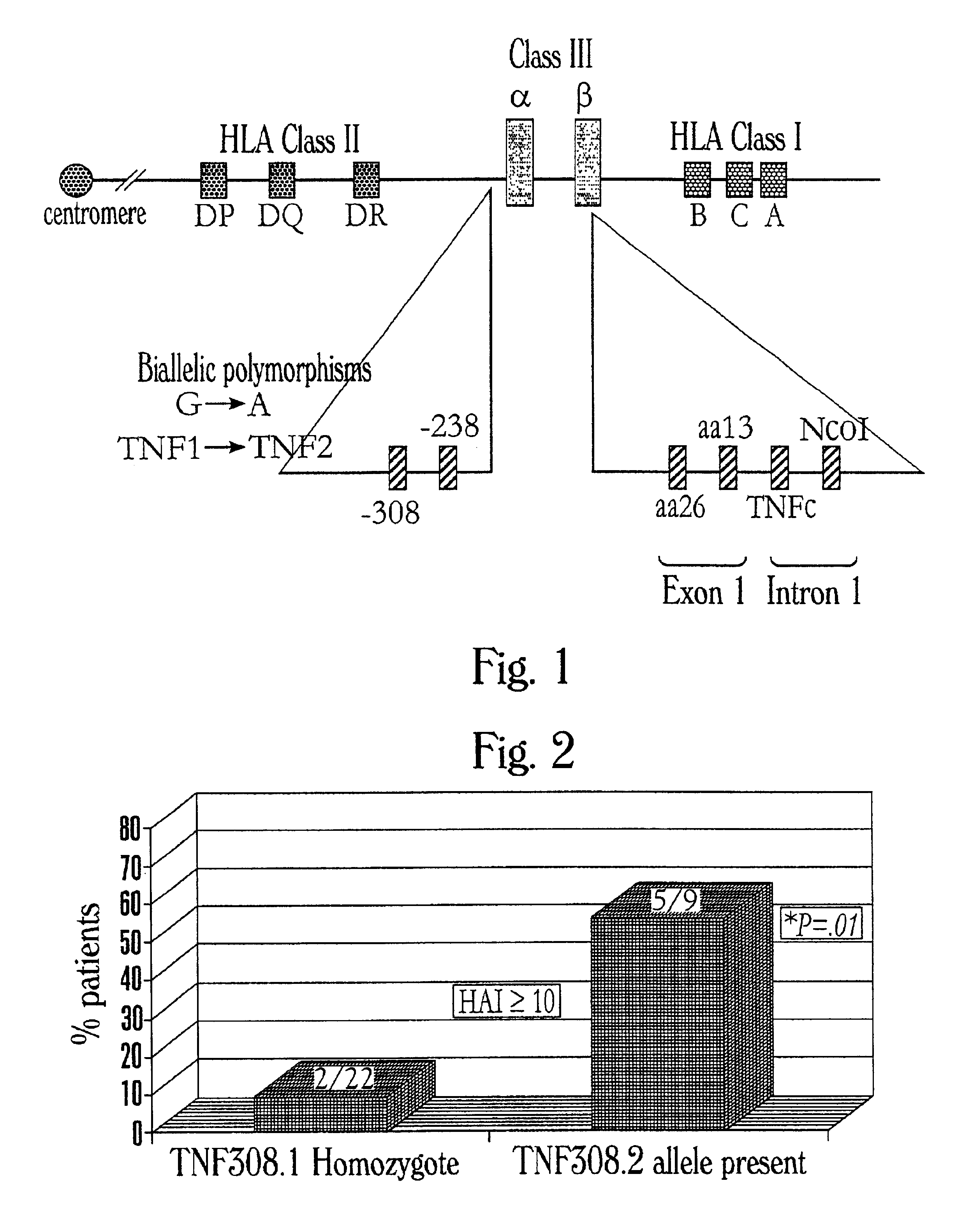
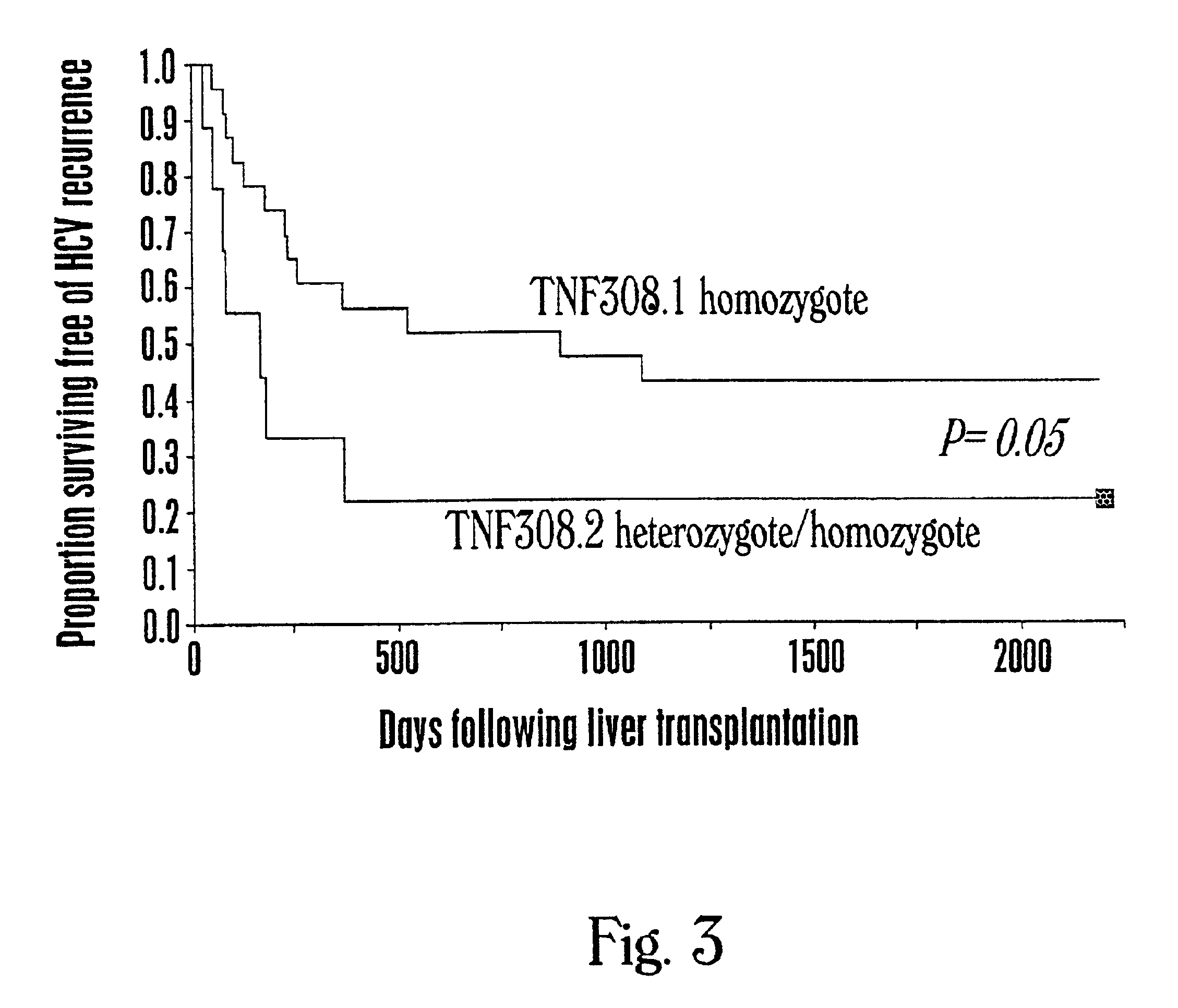
The present invention provides a method of identifying a preferred liver transplant donor. The method includes the step of determining in an individual the presence or absence of a preferred genotype at a polymorphic site, where the preferred genotype is associated with altered activity of a tumor necrosis factor, and wherein the presence of the preferred genotype indicates that the individual is a preferred liver transplant donor. A preferred genotype can be associated with lower activity of a tumor necrosis factor such as TNF-α and can be, for example, TNF308.1. The methods of the invention are useful for identifying a preferred donor liver for transplant into a HCV infected patient. The invention additionally provides a method for selecting a preferred liver for transplantation. The invention further provides a method for limiting the recurrence of HCV infection in a liver transplant recipient.
Owner:UNITED STATES OF AMERICA
Methods of treating lupus using CD4 antibodies
Methods of treating lupus, including systemic lupus erythematosus, cutaneous lupus erythmetosus, and lupus nephritis, are provided. The methods involve administration of a combination of a non-depleting CD4 antibody and another compound used clinically or experimentally to treat lupus. Methods of treating lupus nephritis by administration of a non-depleting CD4 antibody that results in an improvement in renal function and / or a reduction in proteinuria or active urinary sediment are also provided. Methods of treating multiple sclerosis by administration of a non-depleting CD4 antibody, optionally in combination with another compound used clinically or experimentally to treat MS, are described. Methods of treating transplant recipients and subjects with rheumatoid arthritis, asthma, psoriasis, Crohn's disease, ulcerative colitis, and Sjogren's syndrome are also provided.
Owner:GENENTECH INC
Early diagnosis and early warning kit for transplanted kidney rejection reaction and detection method
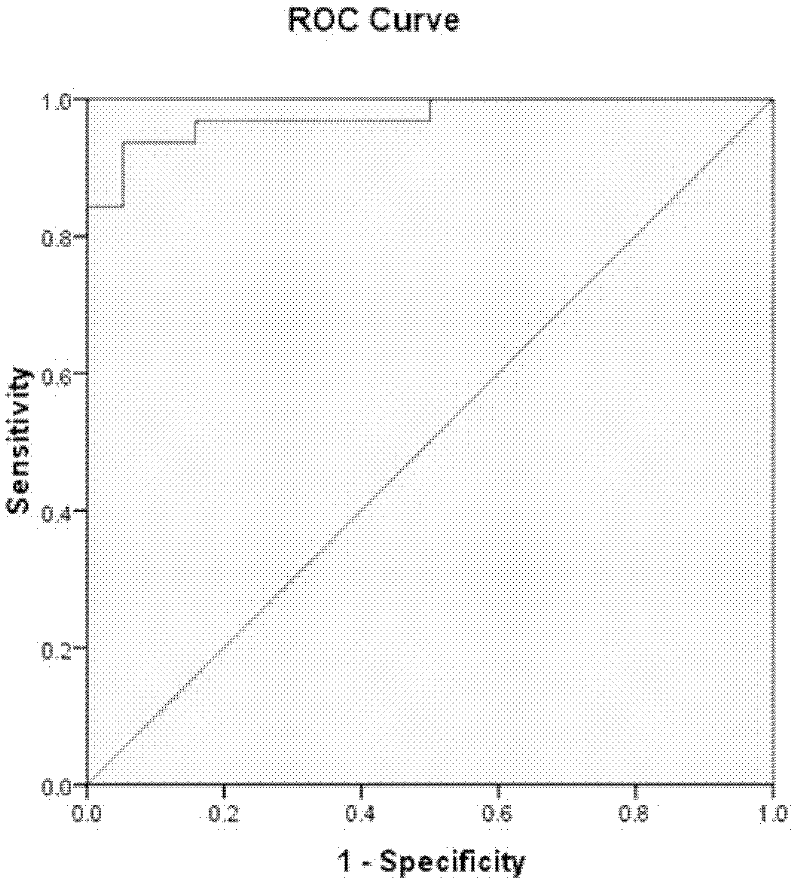
The invention relates to an early diagnosis kit and an early warning kit, in particular to an early diagnosis and early warning kit for transplanted kidney rejection reaction and a use method of the kit, and further to some proteins applied in the early warning of transplanted kidney acute rejection reaction or transplanted kidney rejection reaction. By applying a liquid protein chip (luminex) detection technique, the expression level of 96 cytokines / chemokines and receptors thereof in 266 serum samples from 142 patients having acute transplanted kidney rejection reaction and transplant recipients with stable function of transplanted kidneys are monitored and comprehensively analyzed. An early warning system for the transplanted kidney rejection reaction comprising at least one combined marker and an early diagnosis system for the transplanted kidney rejection reaction comprising at least one factor combined marker are established. The prediction and accuracy rate of the acute rejection achieve 98.6%, and the internal cross validation rates are 97.1% and 97.2%, respectively.
Owner:THE 309TH HOSPITAL OF CHINESE PEOPLES LIBERATION ARMY
Methods for pretreating a subject with extracorporeal photopheresis
The present invention relates to methods for treating a subject predisposed to an autoimmune disease with extracorporeal photopheresis or an effective amount of apoptotic cells before the clinical manifestation of a symptom associated with the autoimmune disease. The present invention alsorelates to methods for treating a subject predisposed to an atopic disease with extracorporeal photopheresis or an effective amount of apoptotic cells before the clinical manitfesation of a symptom associated with the atopic disease. The present invention further relates to methods for treating a transplant donor and / or a transplant recipient, or an implant recipient with extracorporeal photopheresis or an effective amount of apoptotic cells prior to the transplant or implantation procedure.
Owner:NEW ENGLAND MEDICAL CENT HOSPITALS +1
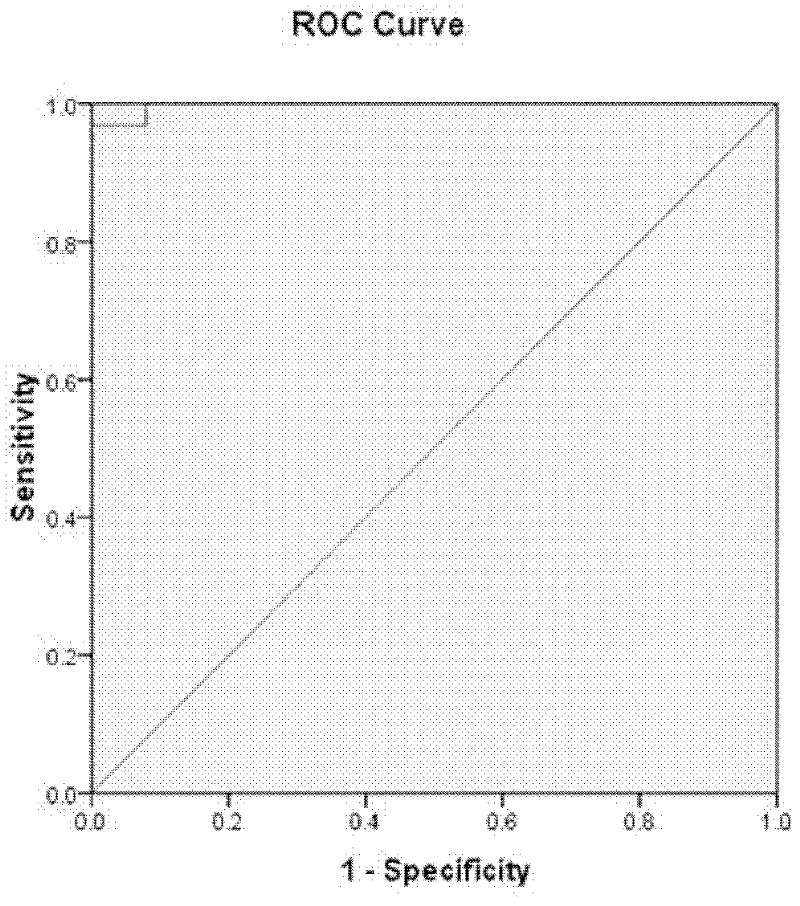
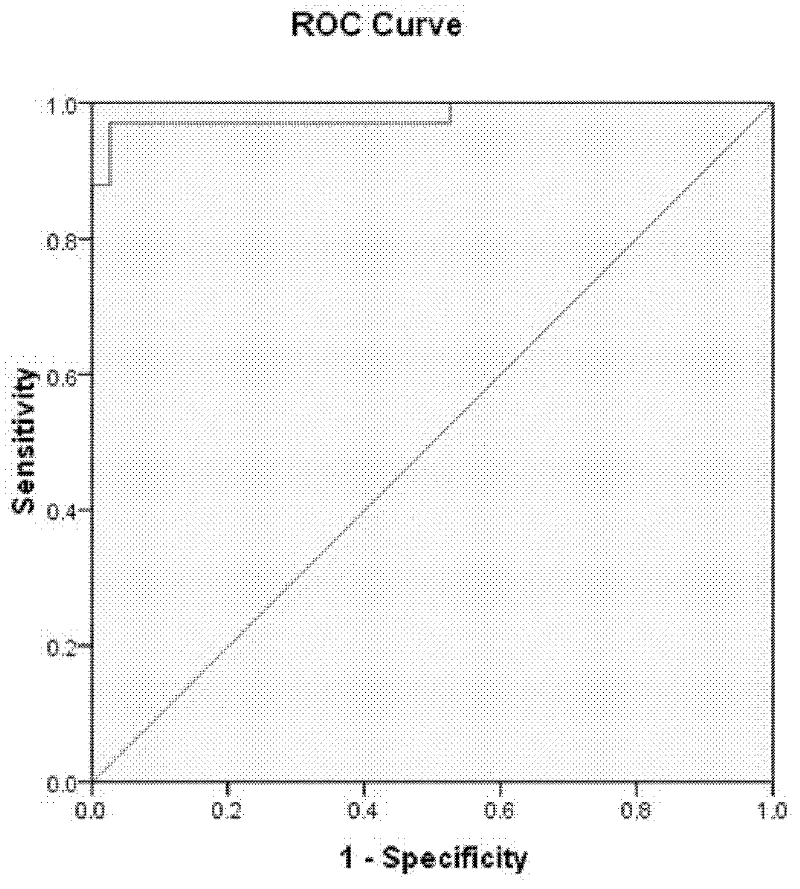
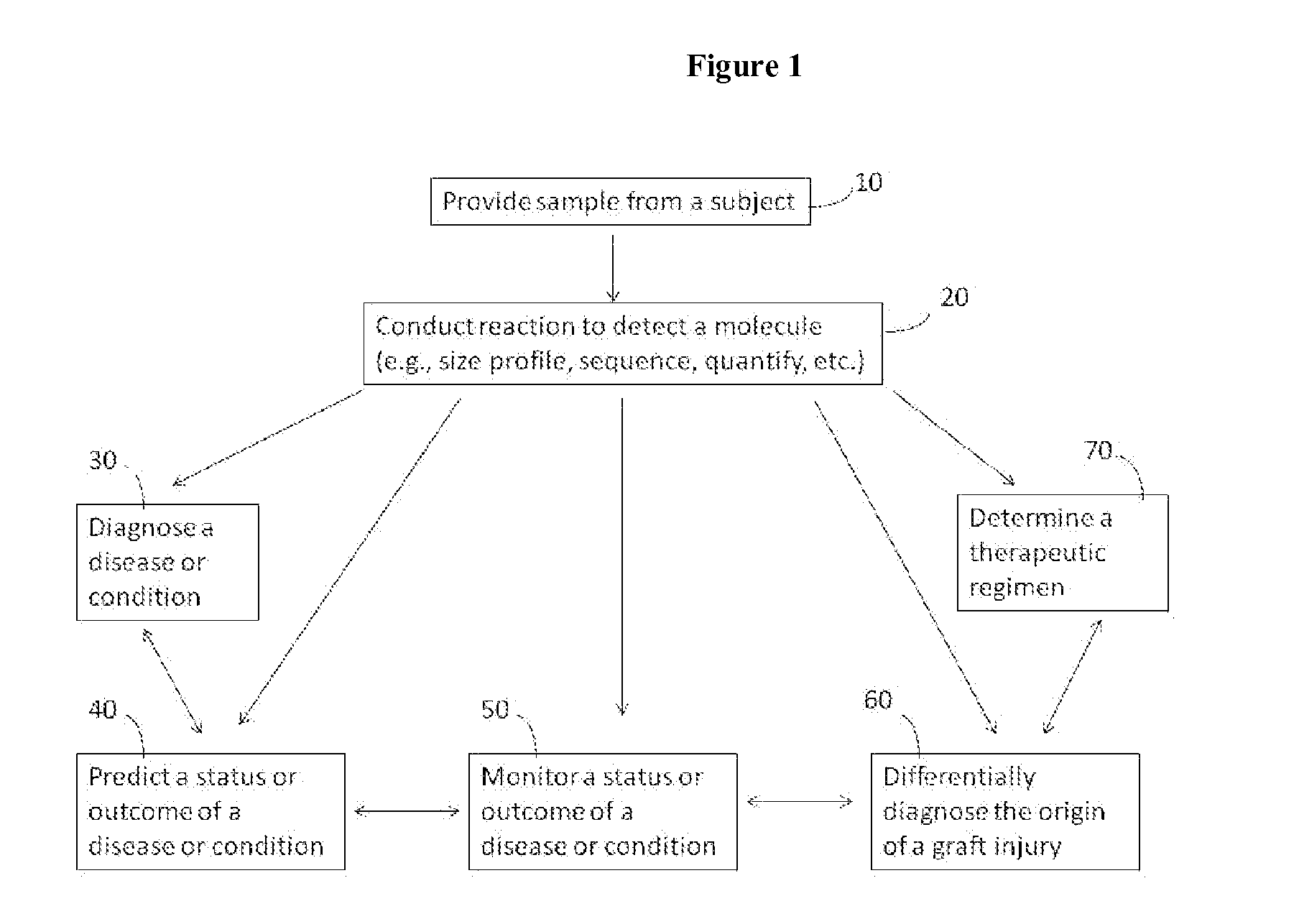
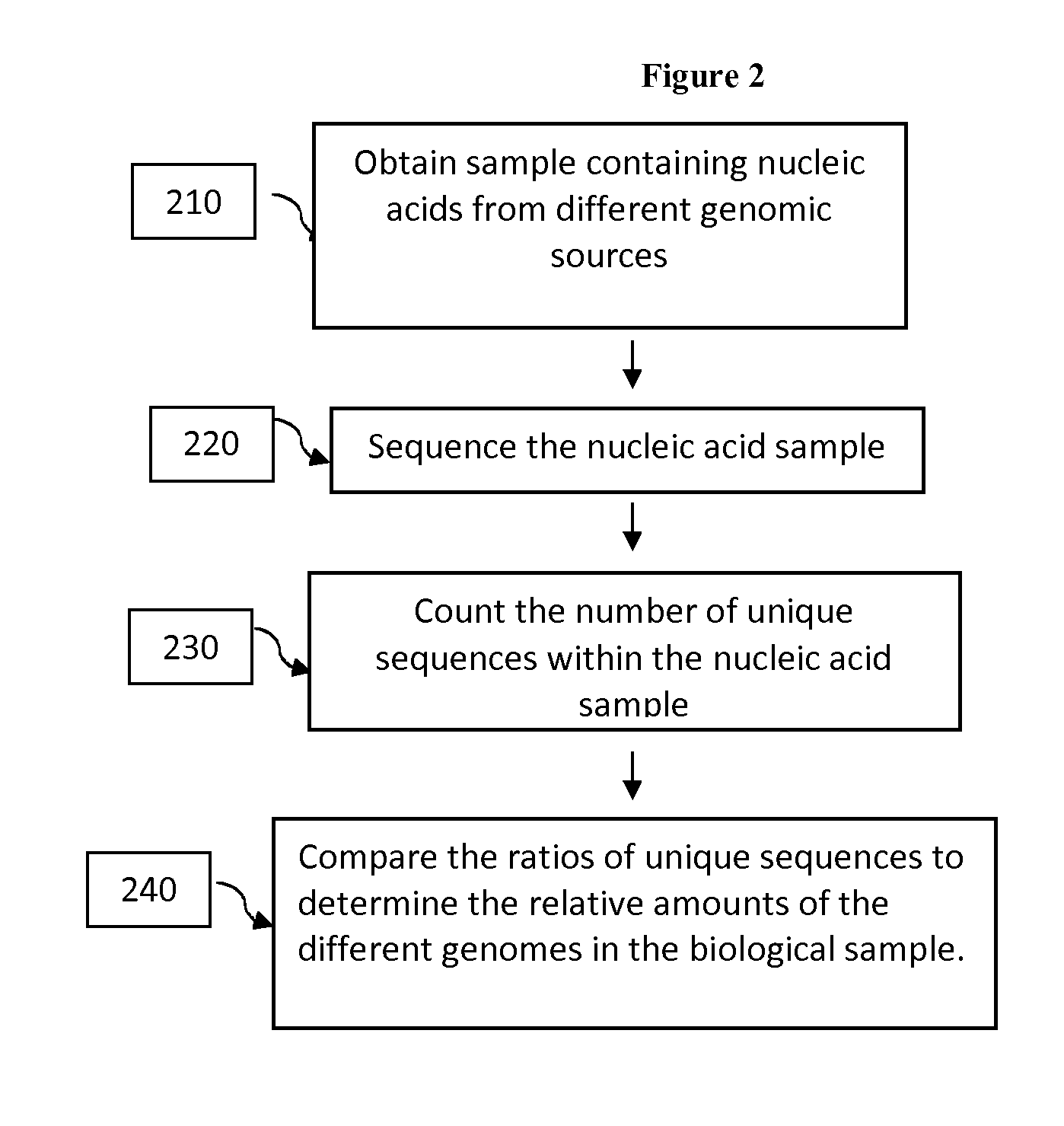
Compositions and methods for analyzing heterogeneous samples
InactiveUS20150211070A1Reducing and stopping therapeutic regimenOrganic active ingredientsGenetic material ingredientsBiologyDisease cause
Methods and compositions for detecting molecules in a heterogeneous sample are disclosed. The methods and compositions disclosed herein may be used for the treatment of a disease or condition characterized by the presence of nucleic acids from at least two different genomic sources. Additionally, the methods and compositions disclosed herein may be used to diagnose, predict, or monitor the status or outcome of a disease or condition characterized by the presence of nucleic acids from at least two different genomic sources. The heterogeneous samples may be from a transplant recipient, a chimeric individual, a subject suffering from a pathogenic infection, or a subject suffering from a different condition such as cancer.
Owner:LINEAGE BIOSCI
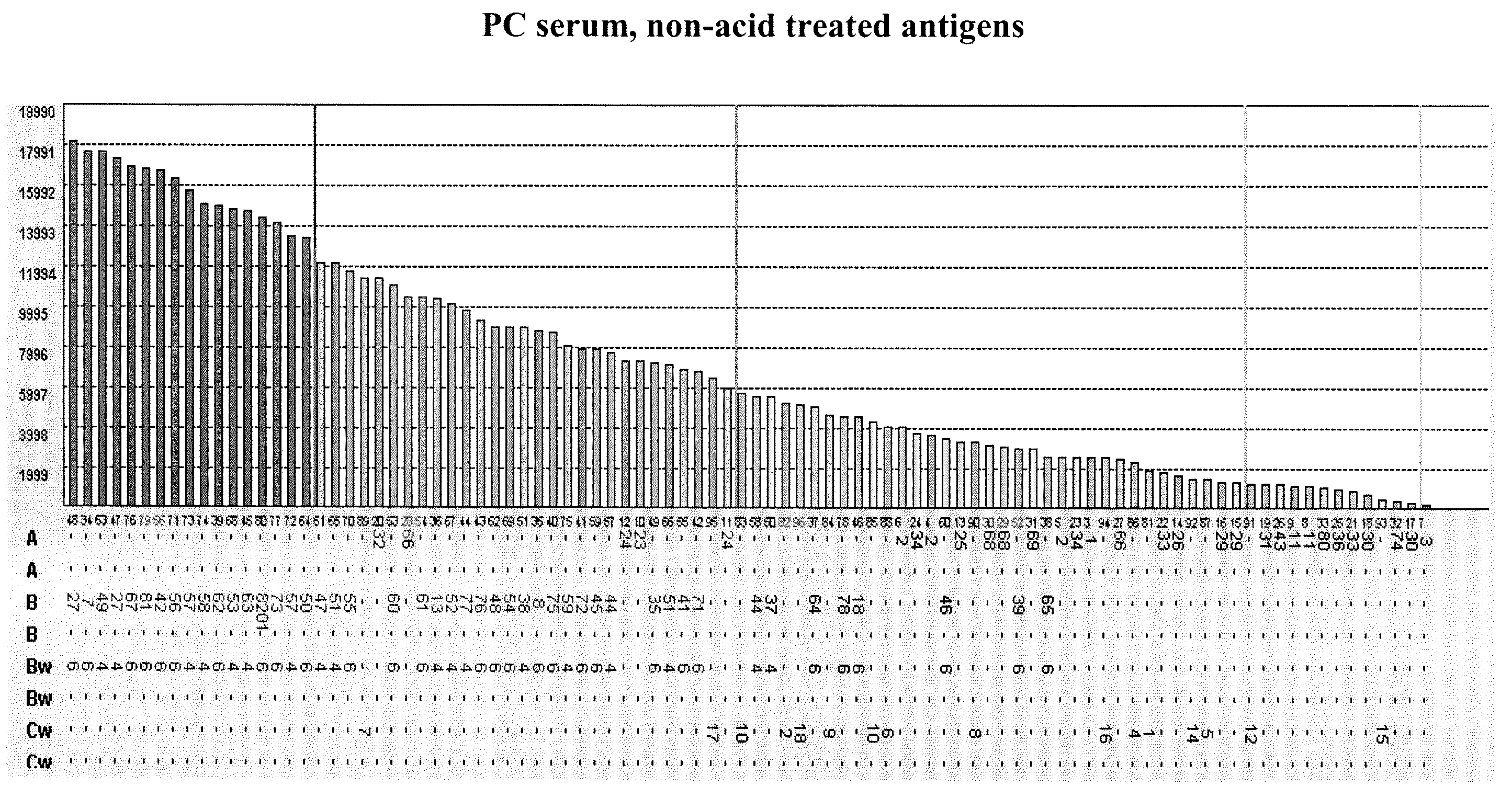
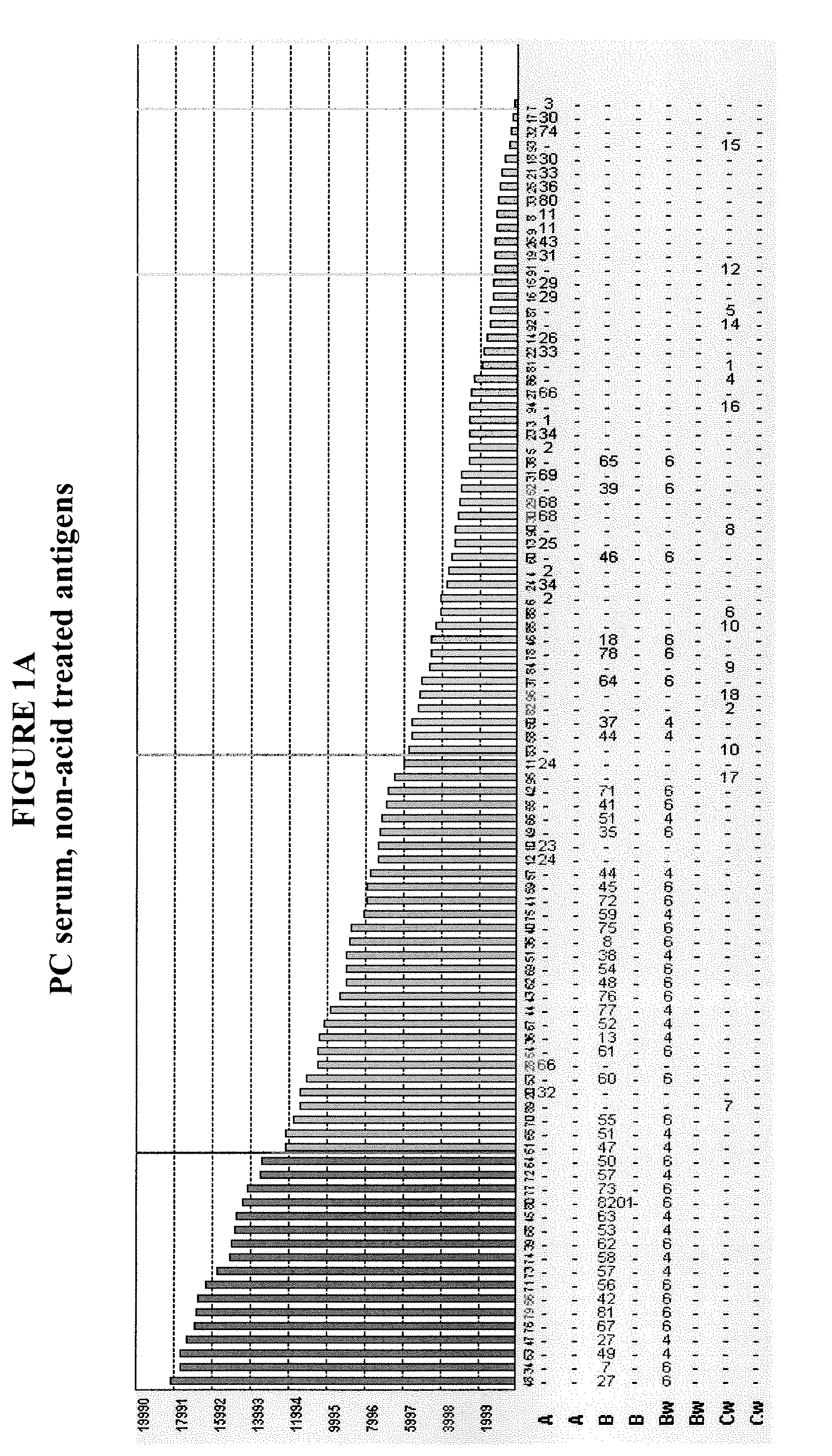
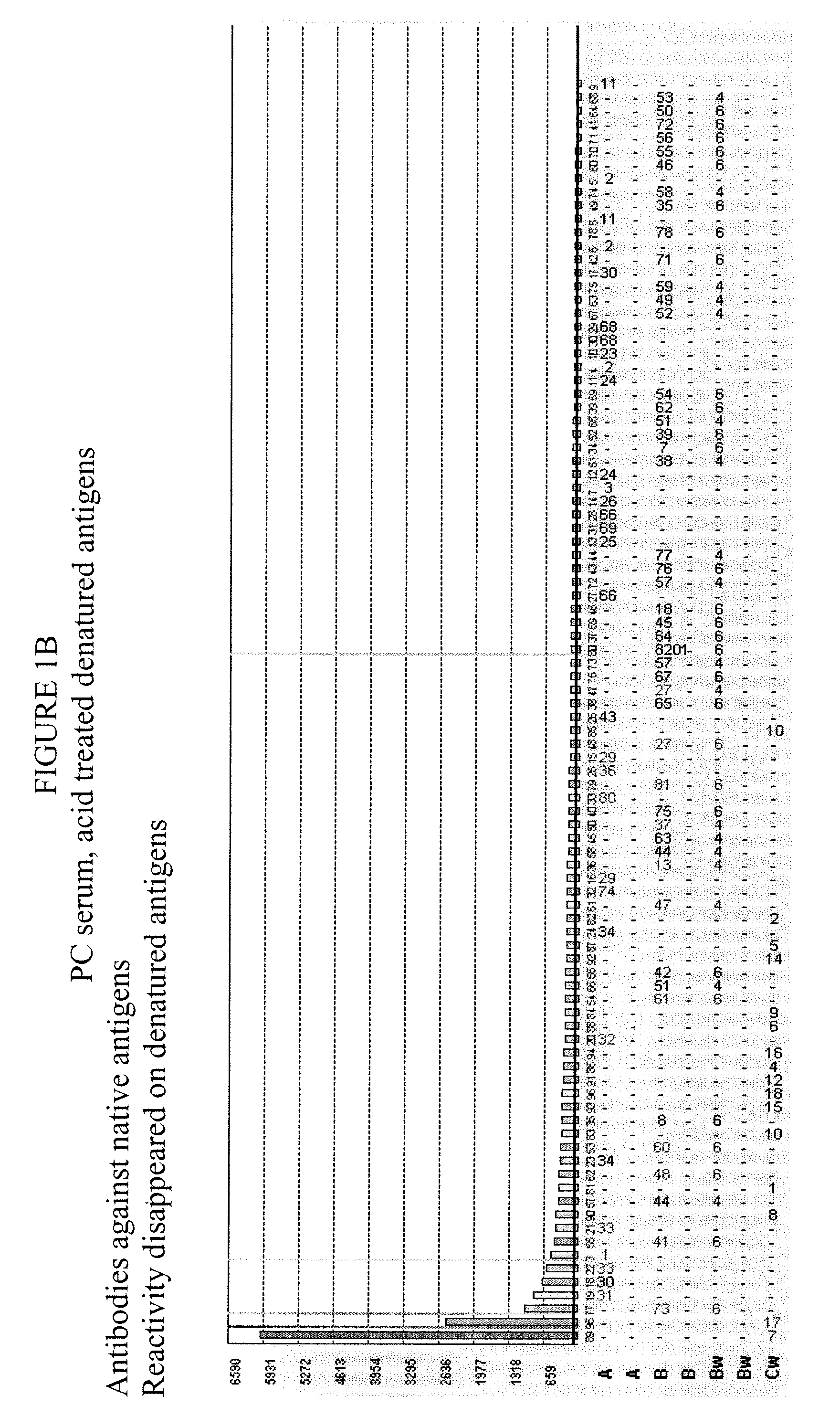
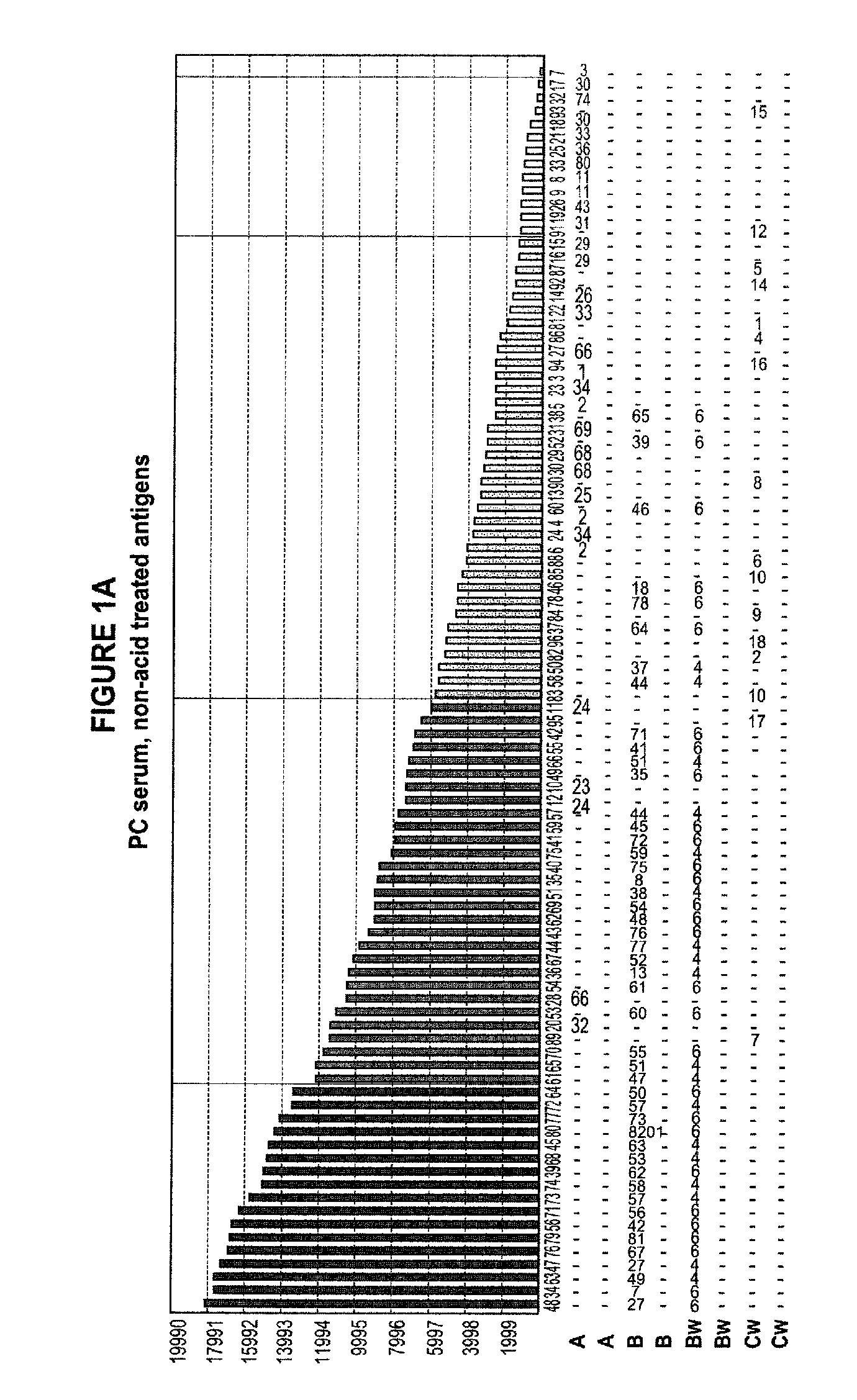
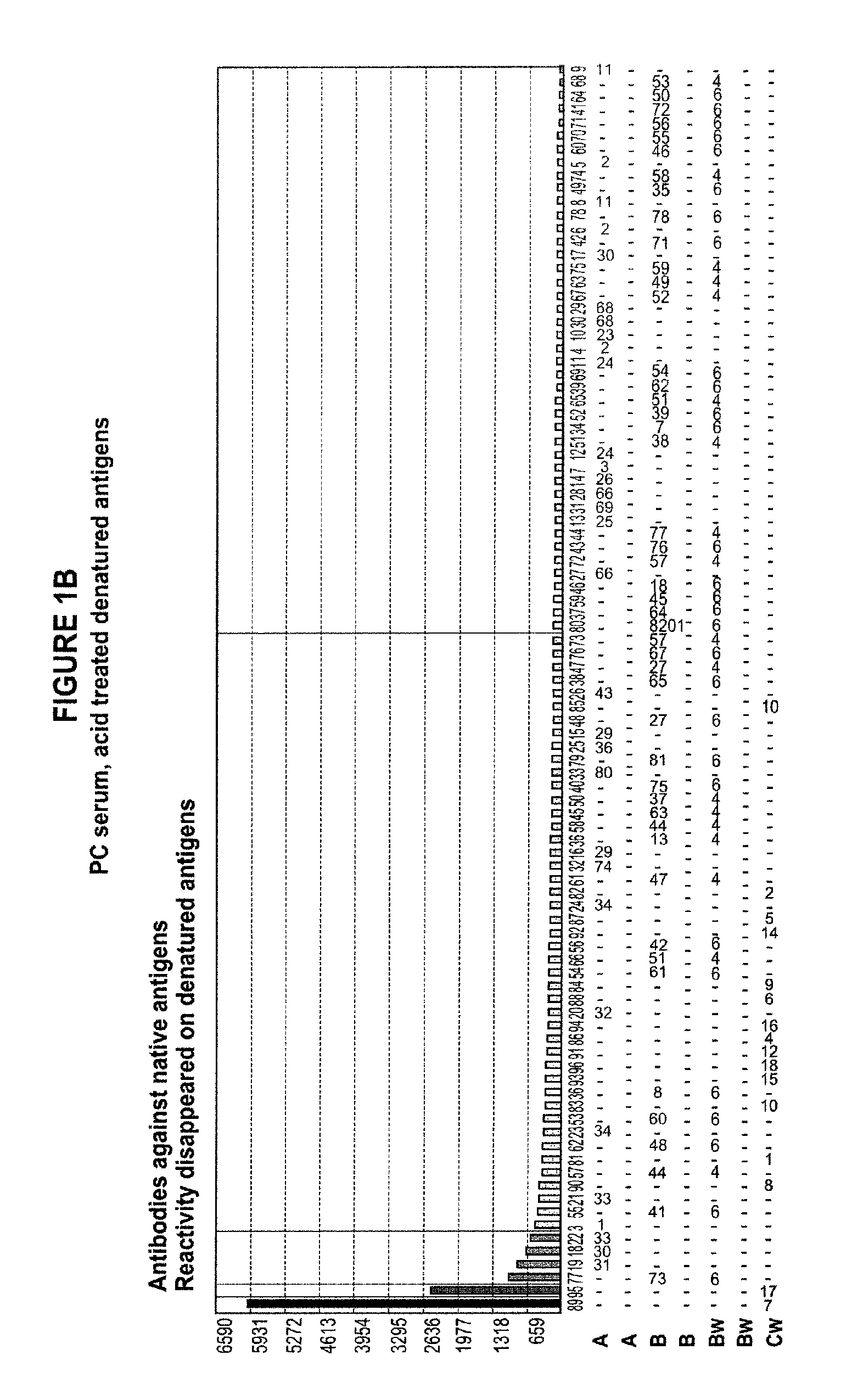
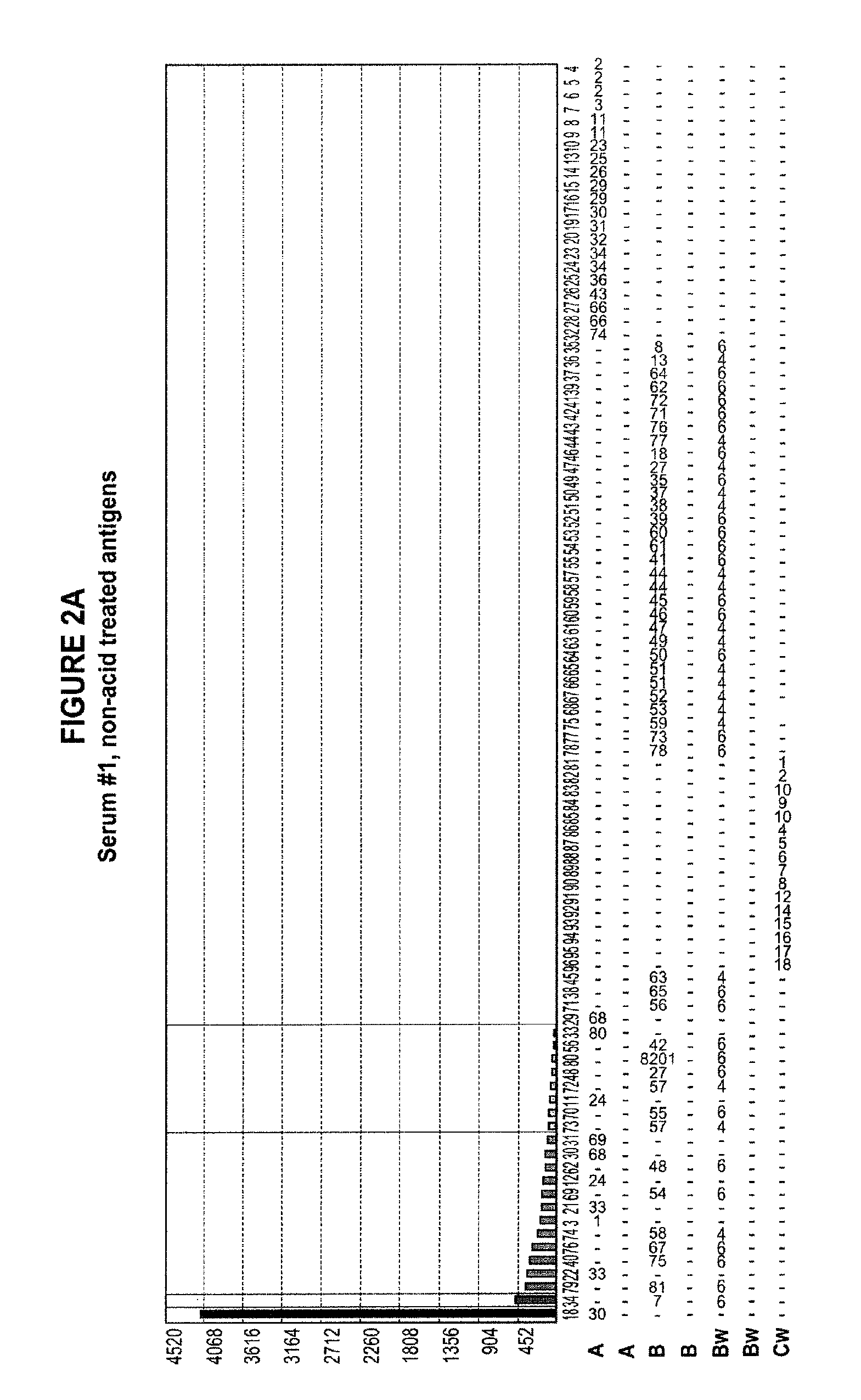
Methods of Detecting Antibodies Specific for Denatured HLA Antigens
ActiveUS20090011437A1Improve responseIncreased riskBioreactor/fermenter combinationsBiological substance pretreatmentsIncreased riskTransplanted Organs
The invention is directed to methods of screening for HLA antibodies comprising detecting antibodies specific for native HLA antigens and denatured HLA antigens. The invention also provides for method of predicting whether a transplant recipient has an increased risk for rejecting the transplanted organ.
Owner:ONE LAMBDA INC
Methods and materials for modulation of the immunosuppressive activity and toxicity of monoclonal antibodies
InactiveUS20060292142A1Reduced activityLow affinityPeptide/protein ingredientsAntibody mimetics/scaffoldsSide effectActivation cells
The binding specificity of the murine OKT3 has been transferred into a human antibody framework in order to reduce its immunogenicity. “Humanized” anti-CD3 mAbs, such as gOKT3-5 and gOKT3-7, have been shown to retain, in vitro, all the properties of native OKT3, including T cell activation which has been correlated, in vivo, with the severe side-effects observed in transplant recipients after the first administration of the mAb. Disclosed are modified versions of humanized anti-CD3 mAbs that do not have the property of T cell activation. Further dislosed are methods of using such mAbs.
Owner:BLUESTONE JEFFREY +2
Specific inhibition of allorejection
InactiveUS20050042217A1Effectively and specifically inhibitedAvoid immune responseNervous disorderVirusesHumoral immune reactionBlood vessel
The present invention provides methods and compositions for specifically inhibiting both cellular and humoral immune responses to alloantigen, thereby finding use in extending the survival of transplant allografts and treating graft versus host disease in transplant recipients.
Owner:ISOGENIS INC
Methods of detecting antibodies specific for denatured HLA antigens
ActiveUS7972804B2Improve responseIncreased riskBioreactor/fermenter combinationsBiological substance pretreatmentsIncreased riskTransplanted Organs
Owner:ONE LAMBDA INC
Methods for treating transplant rejection
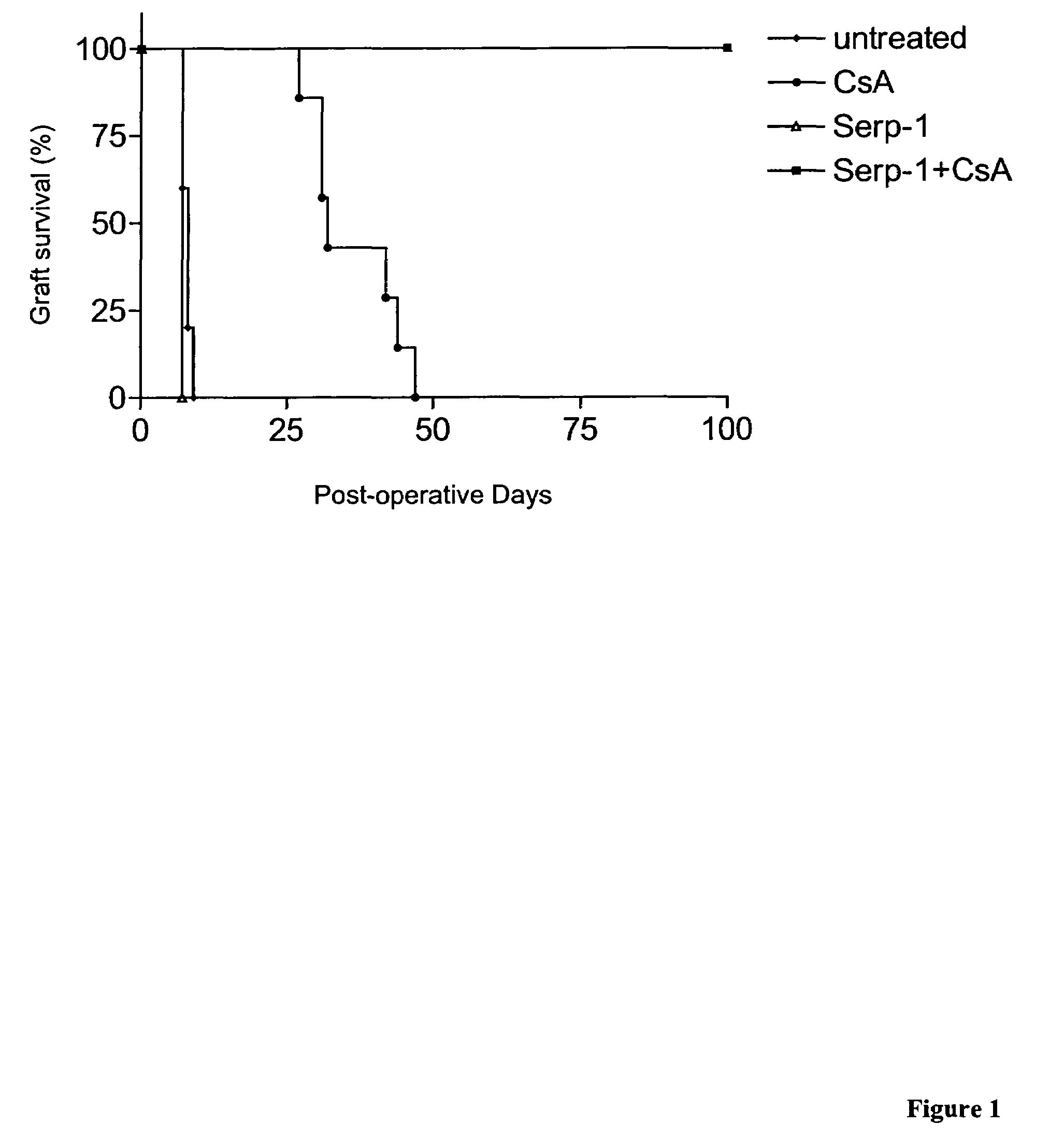
Compositions and methods for treating transplant rejection in a mammalian transplant recipient are provided. The method involves administering a therapeutically effective amount of Serp-1, its analogs, and biologically active fragments thereof in combination with an anti-rejection agent, such as cyclosporin, and a pharmaceutically acceptable carrier to a subject in need of such treatment. The compositions and methods are useful for treating acute and chronic allograft and xenograft transplant rejection in mammals.
Owner:VIRON THERAPEUTICS
Purging of cells using viruses
InactiveUS20040109878A1Preventing graft-versus-host diseasesHigh selectivityBiocideNervous disorderAutoimmune conditionGraft versus host disease induction
The subject invention relates to viruses that are able to purge (reduce or eliminate) undesirable cells in a mixture of cells. Undesirable cells can include neoplastic cells, cells mediating graft-versus host diseases, and autoimmune cells. The subject invention also relates to the purging of undesirable cells from bone marrow or peripheral blood cell harvests in the treatment of mammals including cancer patients, transplant recipients, and patients with autoimmune disease.
Owner:UNIVERSITY OF OTTAWA +1
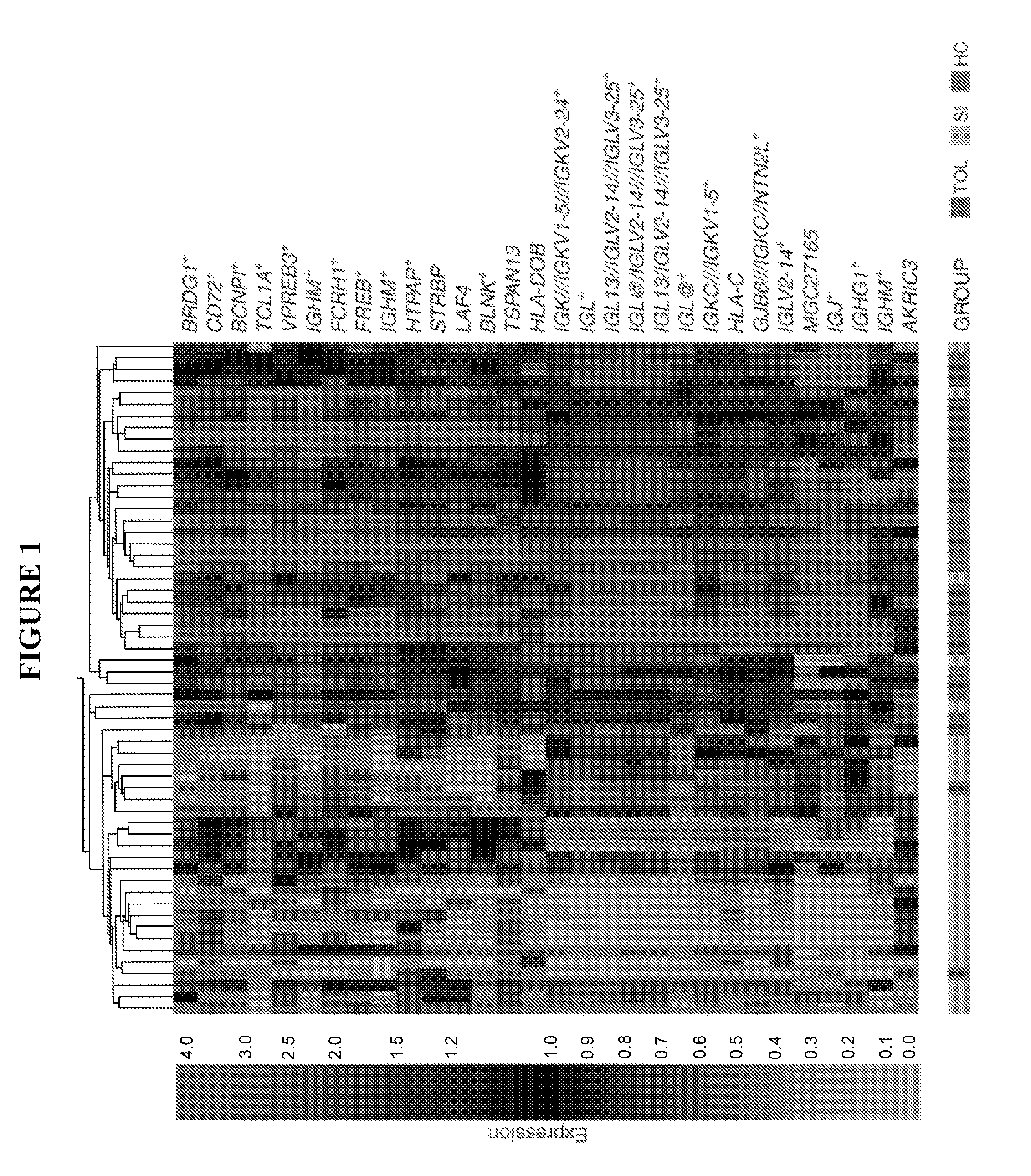
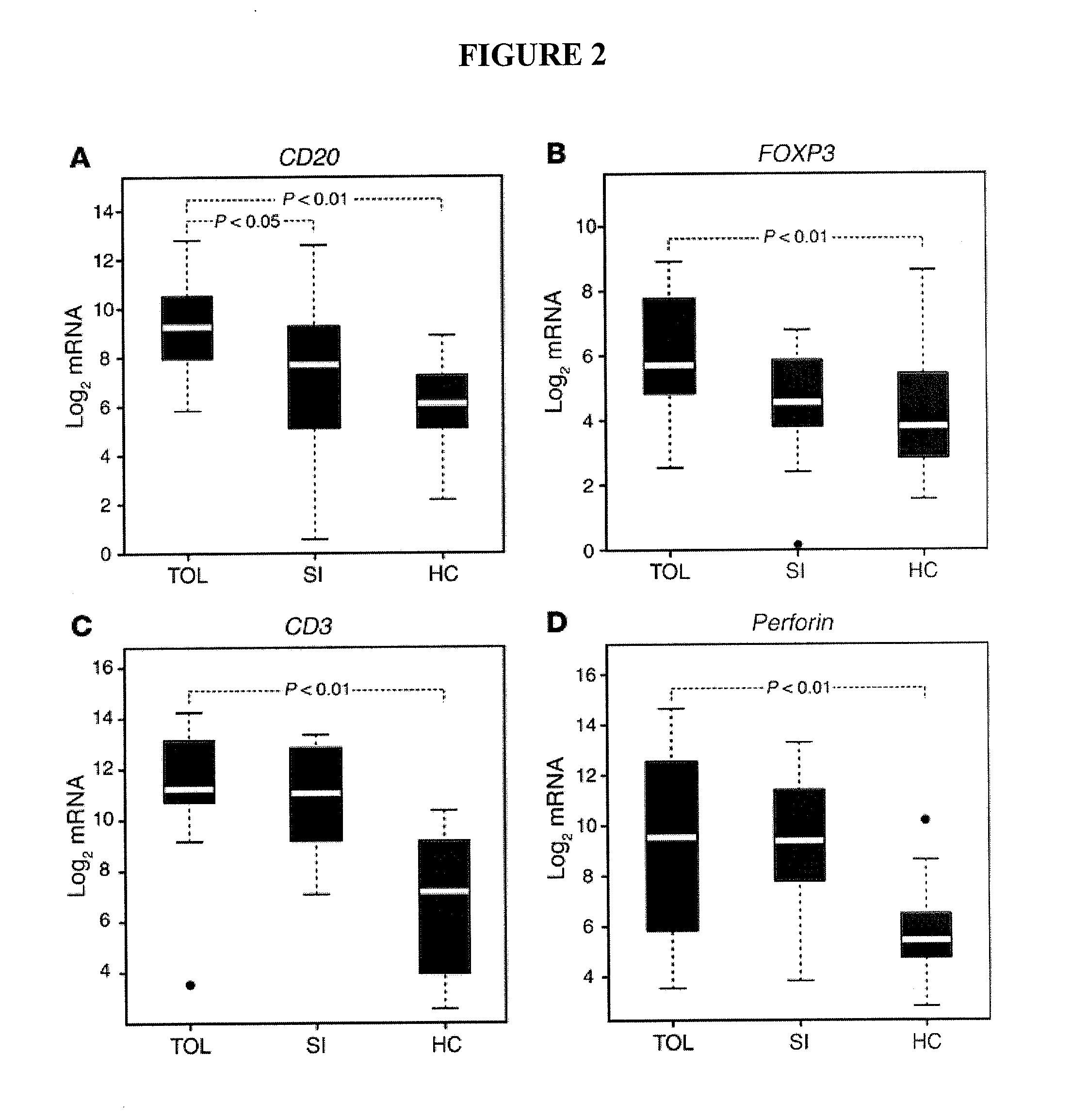
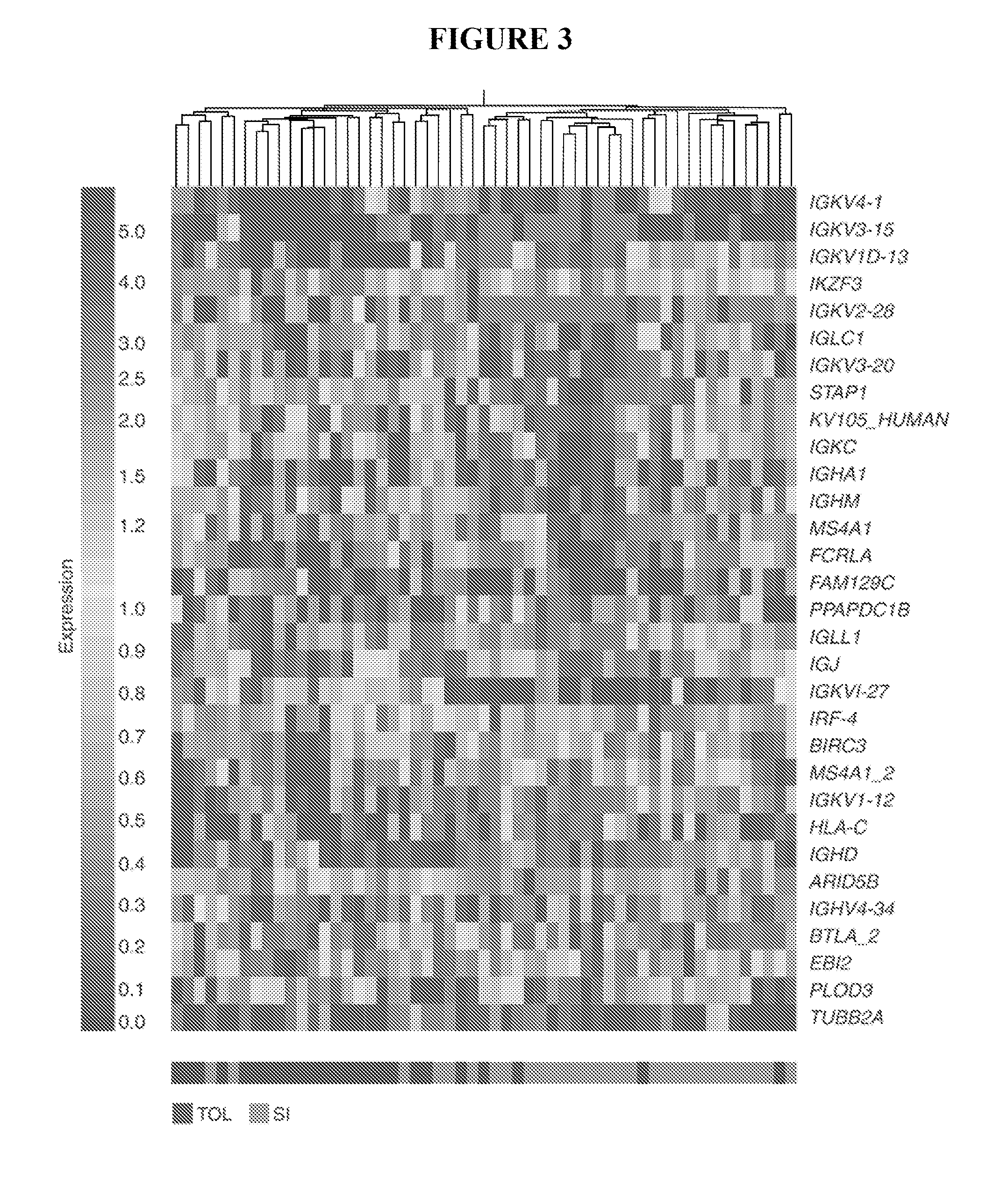
B cell signature associated with tolerance in transplant recipients
InactiveUS20100304996A1Reduce needPeptide librariesMicrobiological testing/measurementTolerabilityKidney
The present invention provides methods for predicting the development of tolerance to a transplant, such as a kidney, using molecular markers that have different expression patterns in tolerant transplant recipients, as compared to non-tolerant or healthy, non-recipient controls.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +2
Use of immunotoxins to induce immune tolerance to pancreatic islet transplantation
InactiveUS7288254B2Sufficient immune toleranceReduce the populationBiocidePeptide/protein ingredientsPancreatic islet transplantationIslet cells
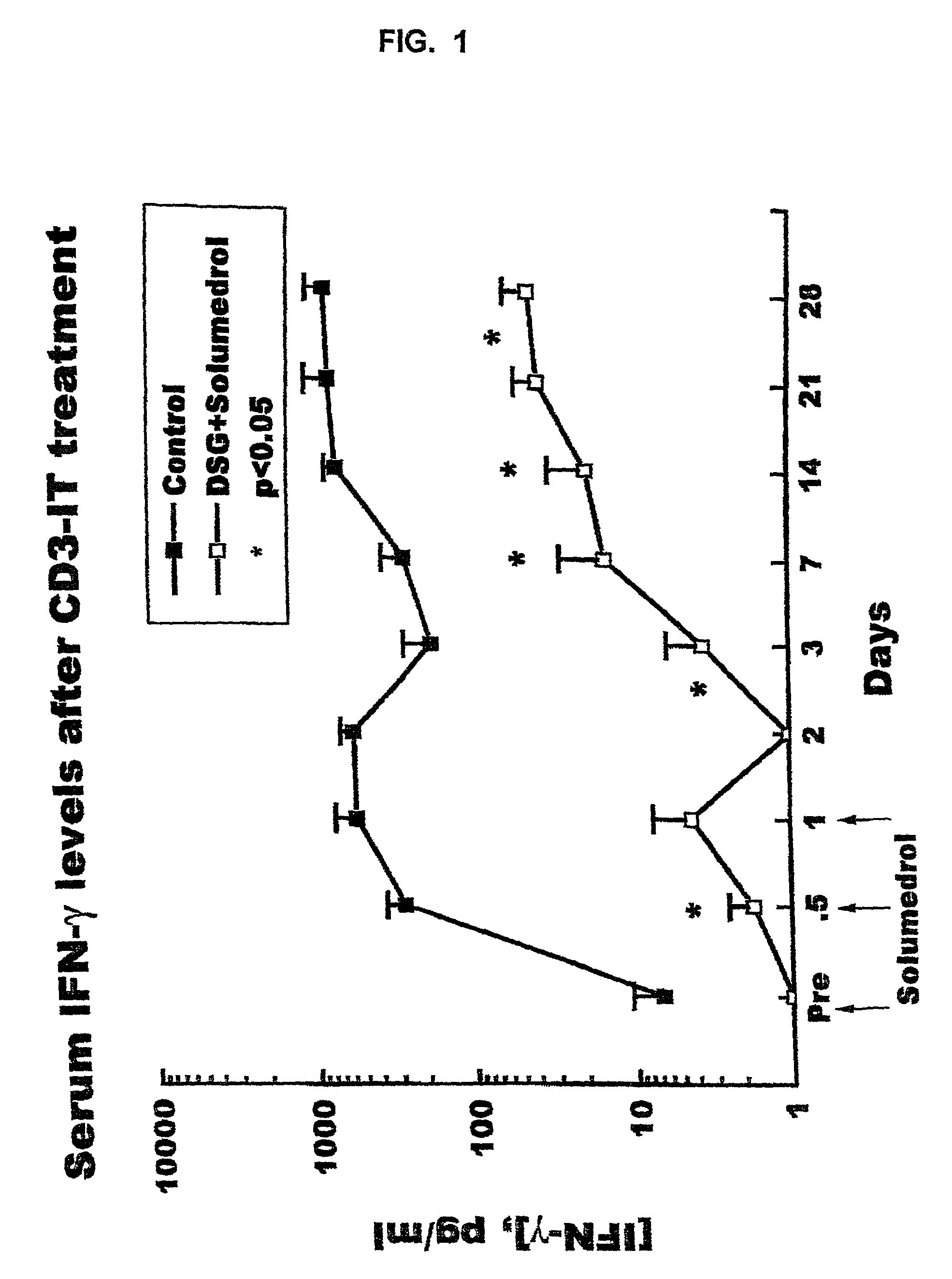
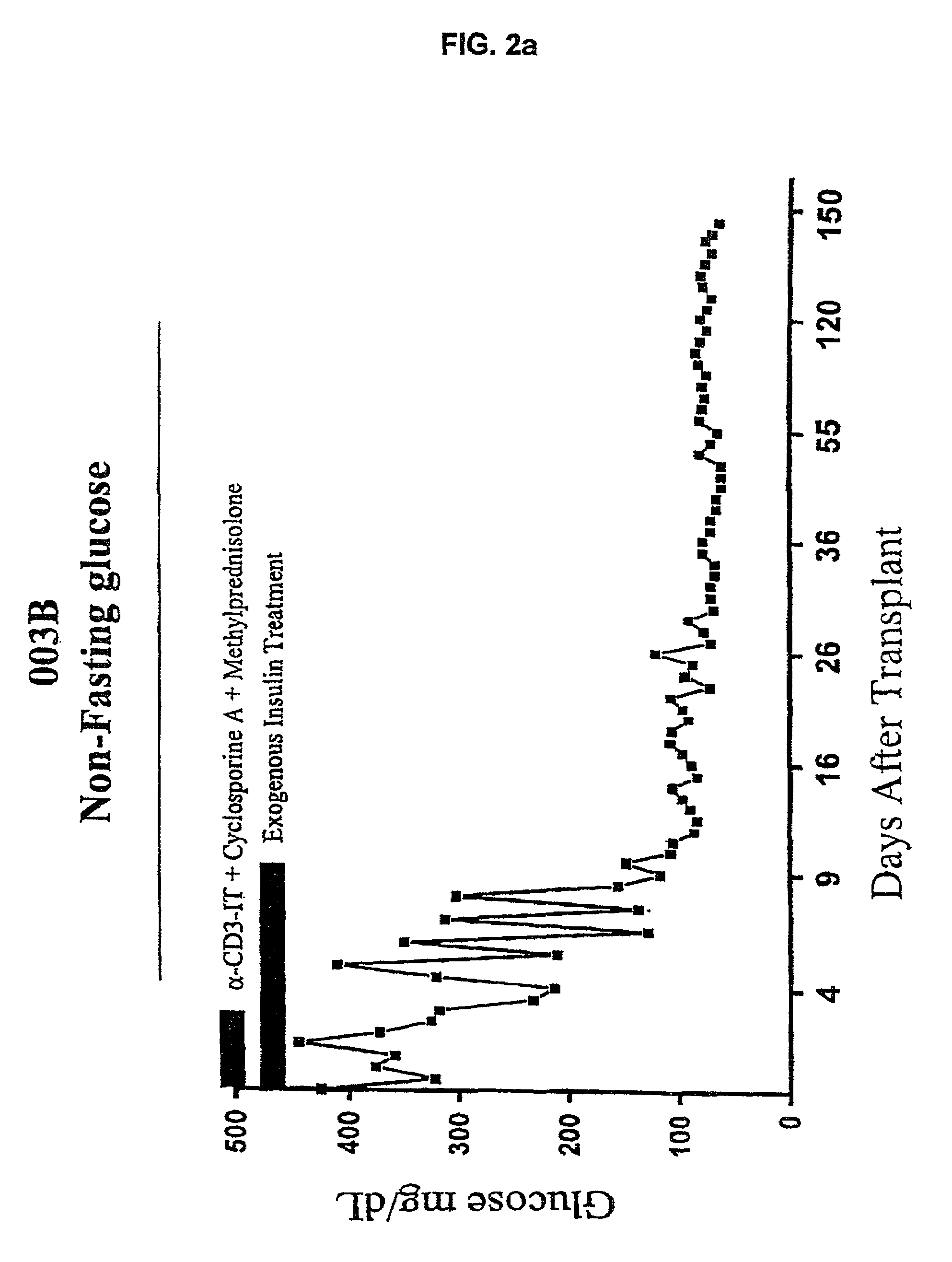
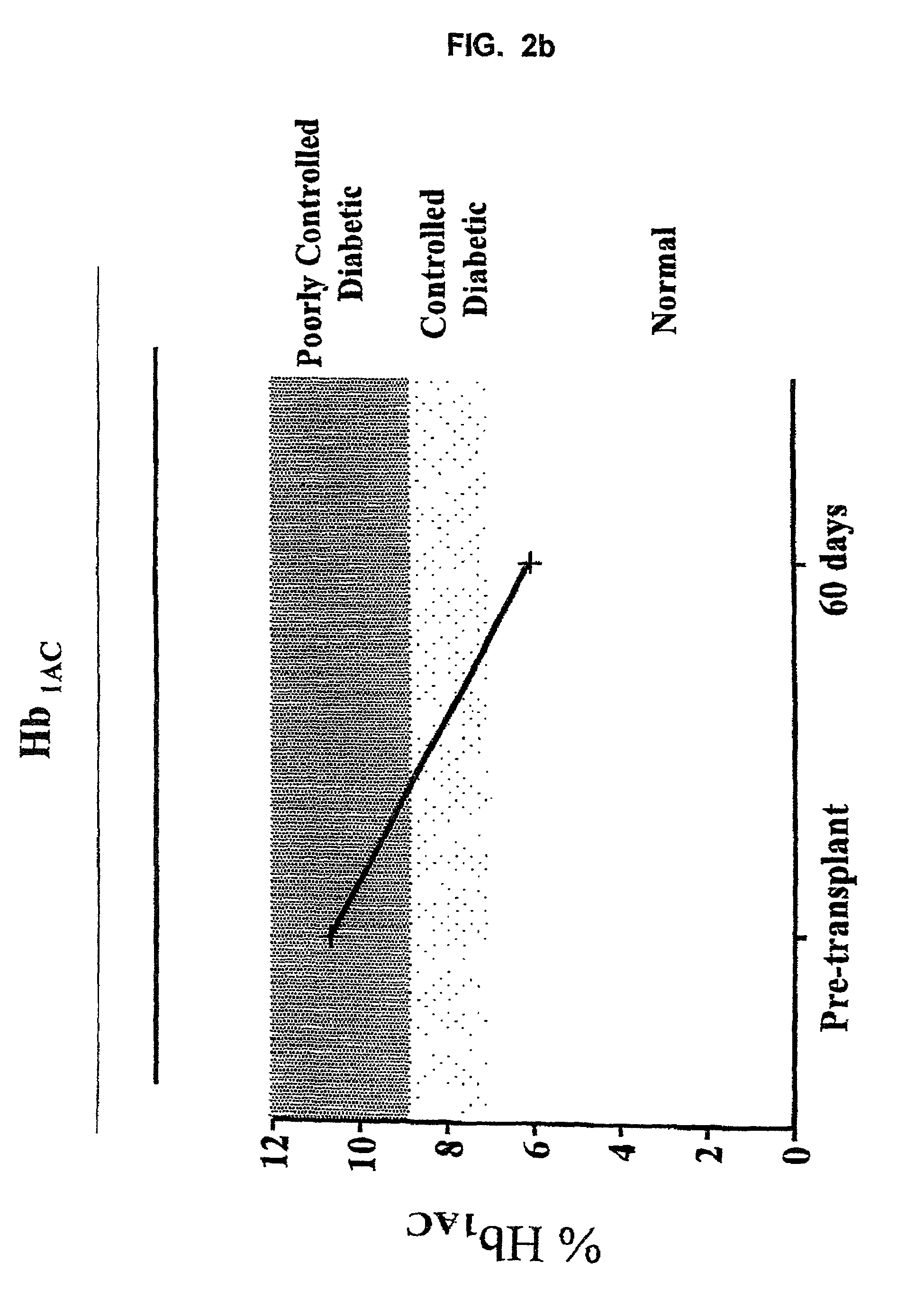
The invention provides a method of treating diabetes in a subject, comprising administering to the diabetic subject an immunotoxin, thereby reducing the subject's T-cell population, and administering to the subject pancreatic islet cells from a donor. The immune tolerance inducing treatment regimen, used optionally with adjunct immunosuppressive agents, prevents pancreatic islet cell rejection while maintaining long term islet cell function following xenogeneic and allogeneic pancreatic islet cell transplantation. Thus, the methods of the present invention provide a means for treating diabetes, wherein the need for exogenous insulin or immunosuppressive agents is decreased or eliminated. Also provided is a method of inhibiting a rejection response of a transplant recipient, comprising administering an immunotoxin during the peritransplant period, thereby transiently reducing the number of T-cell lymphocytes and promoting long-term survival of the transplant.
Owner:UNITED STATES OF AMERICA
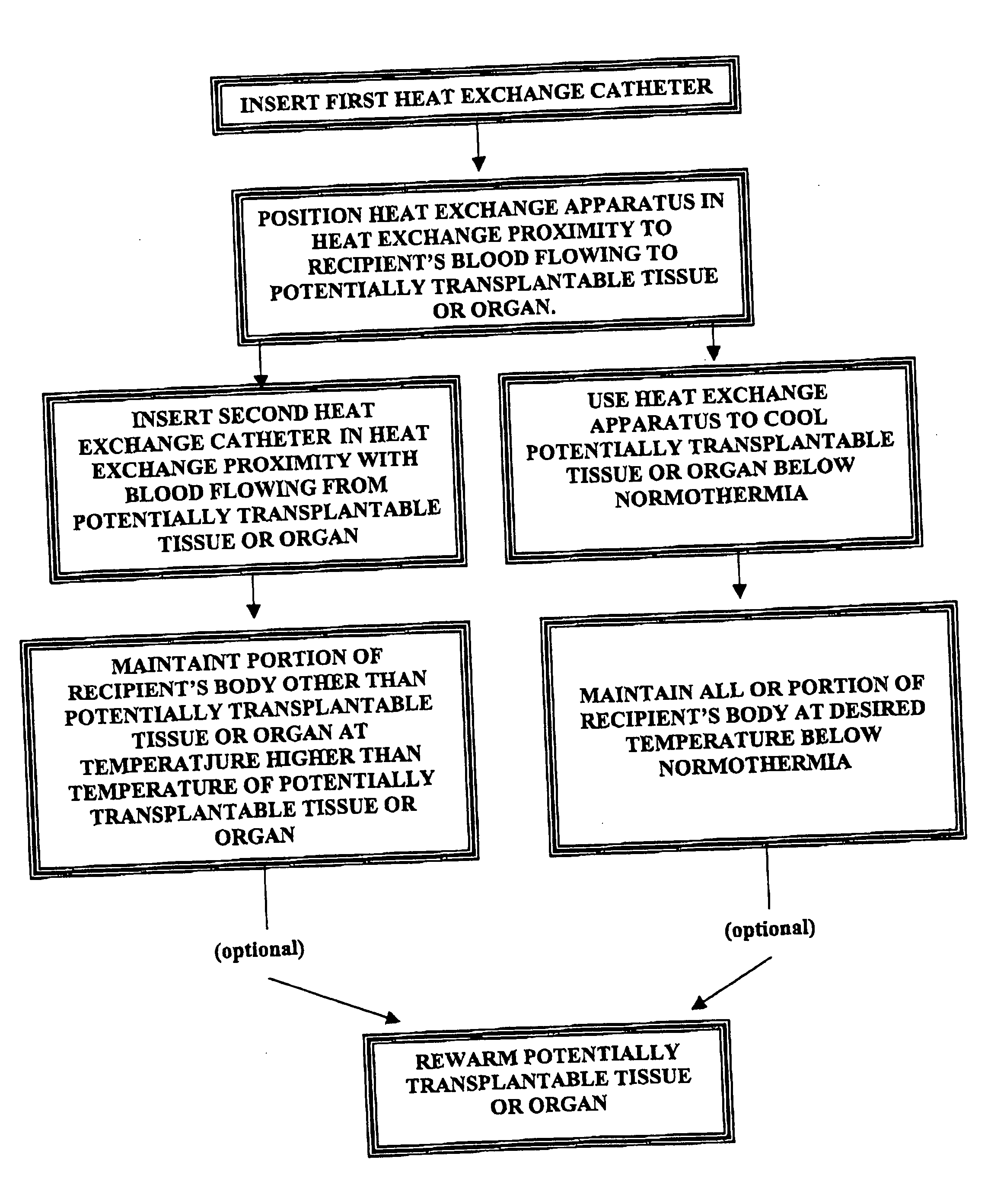
Use of endovascular hypothermia in organ and/or tissue transplantations
InactiveUS20070213793A1Lower potentialDecreasing oxygen demandDiagnosticsSurgerySurgeryTissue transplantation
Methods for (a) preventing hypoxic damage to a potentially transplantable organ or tissue prior to explanation of that organ or tissue from the body of a mammalian transplant donor and (b) preventing rejection of a transplanted organ or tissue in a human or veterinary transplant recipient. The methods comprise placing a heat exchange apparatus in the vasculature of the donor or recipient and using that heat exchange apparatus to cool at least a portion of the body of the donor or recipient to a temperature below normothermia (e.g. below normothermia and sometimes between about 30° C. and about 36° C.).
Owner:ZOLL CIRCULATION
Methods of treating lupus using CD4 antibodies
InactiveCN101443040AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsUlcerative colitisSystemic lupus erythematosus
Methods of treating lupus, including systemic lupus erythematosus, cutaneous lupus erythmetosus, and lupus nephritis, are provided. The methods involve administration of a combination of a non-depleting CD4 antibody and another compound used clinically or experimentally to treat lupus. Methods of treating lupus nephritis by administration of a non-depleting CD4 antibody that results in an improvement in renal function and / or a reduction in proteinuria or active urinary sediment are also provided. Methods of treating multiple sclerosis by administration of a non-depleting CD4 antibody, optionally in combination with another compound used clinically or experimentally to treat MS, are described. Methods of treating transplant recipients and subjects with rheumatoid arthritis, asthma, psoriasis, Crohn's disease, ulcerative colitis, and Sjogren's syndrome are also provided.
Owner:GENENTECH INC
Methods of treating stroke using stem cell-like menstrual blood cells
InactiveUS20110268710A1Maintain potencyImprove proliferative abilityBiocideNervous disorderRisk strokeUmbilical cord
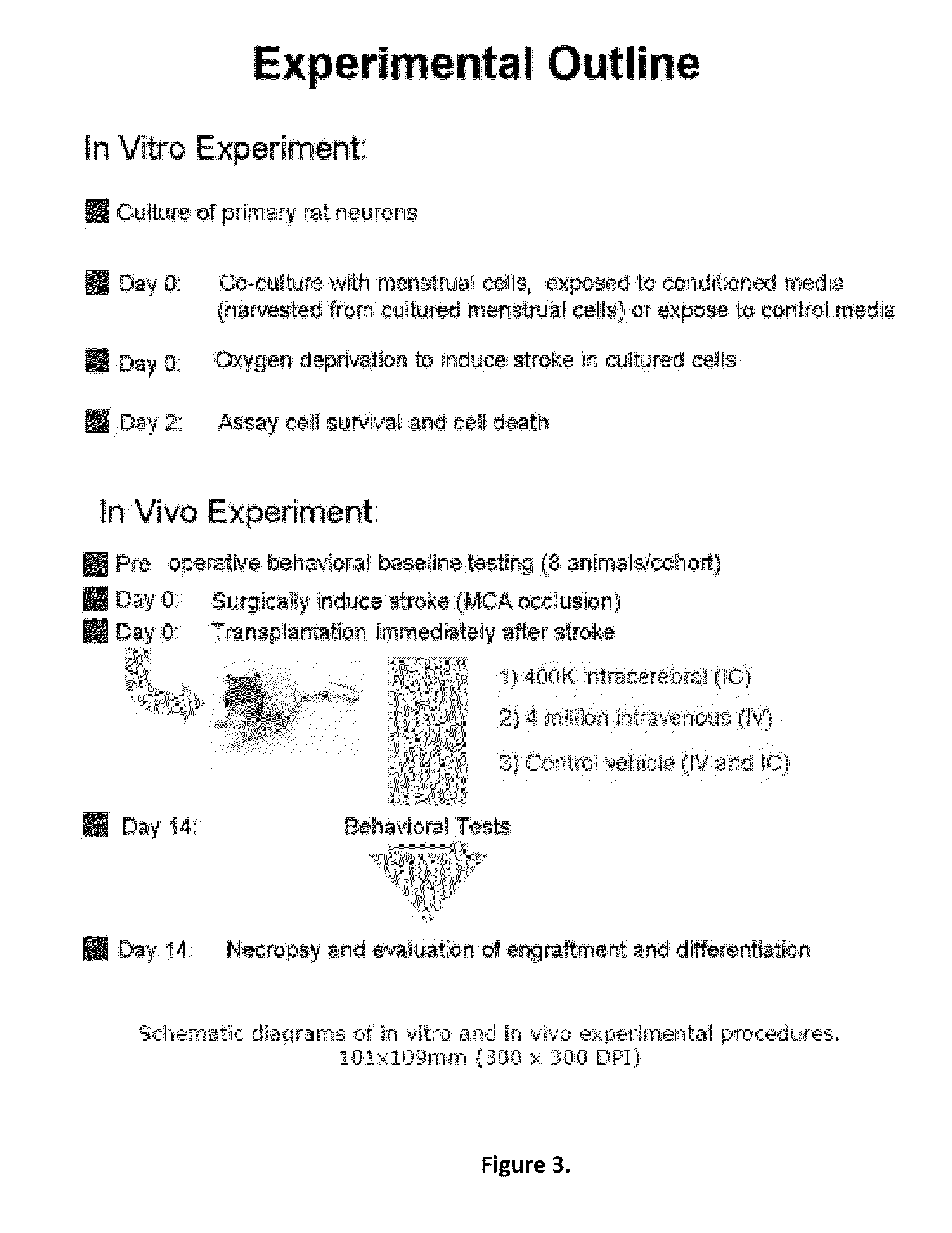
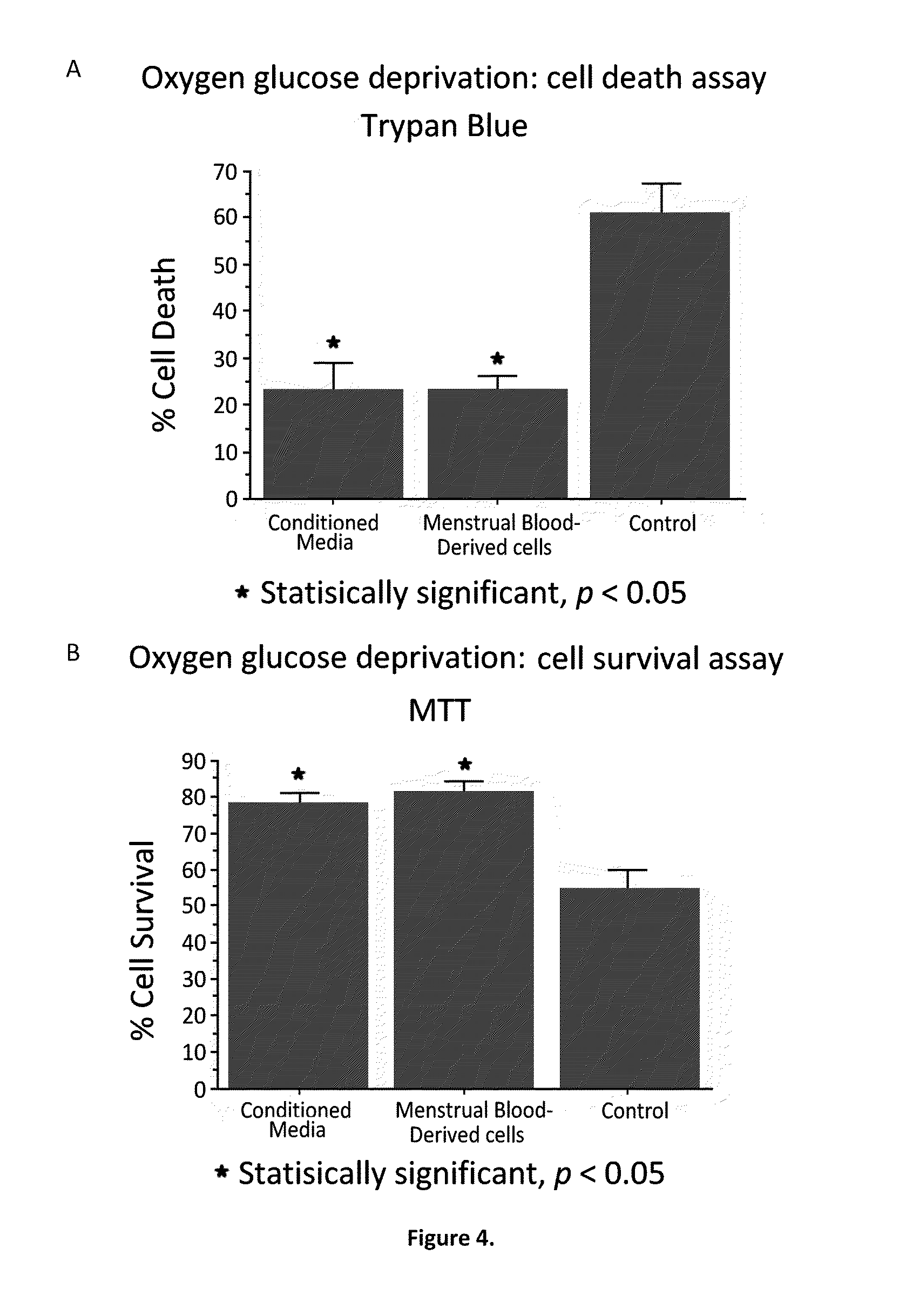
A cell type that is a complete match of the transplant recipient appears as an optimal scenario to open treatment options to a large patient population with minimal complications. The use of autologous bone marrow or umbilical cord blood has been proposed as a good source of stem cells for cell therapy. Menstrual blood is found to be another important source of stem cells. Assays of cultured menstrual blood reveal that they express embryonic like-stem cell phenotypic markers and neuronal phenotypic markers under appropriate conditioned media. Oxygen glucose deprivation stroke models show that OGD-exposed primary rat neurons, co-cultured with menstrual blood-derived stem cells or exposed to the media from cultured menstrual blood, exhibited significantly reduced cell death. Transplantation of menstrual blood-derived stem cells, either intracerebrally or intravenously, after experimentally induced ischemic stroke in adult rats also significantly reduced behavioral and histological impairments compared to vehicle-infused rats.
Owner:CRYO CELL INTERNATIONAL INC +3
Method of removing cell
ActiveUS20060212074A1Good effectUniform removalHeart valvesHeart defibrillatorsBiological bodyHuman body
In the transplant of a living organism tissue, such as a heart valve, taken from an animal, etc. into a human body, a cell removing solution for removing original cells from the living organism tissue is provided with flow approximately equal to the bloodstream of transplant recipient living body, and the living organism tissue is placed in the flow so as to effect immersion of the living organism tissue in the cell removing solution. In the immersion, it is preferred that the living organism tissue placed in the cell removing solution, while being rotated, be irradiated with microwave. As a result, original cells can be removed from the living organism tissue uniformly and reliably, so that the biocompatibility of living organism tissue after transplant can be enhanced.
Owner:WASEDA UNIV +1
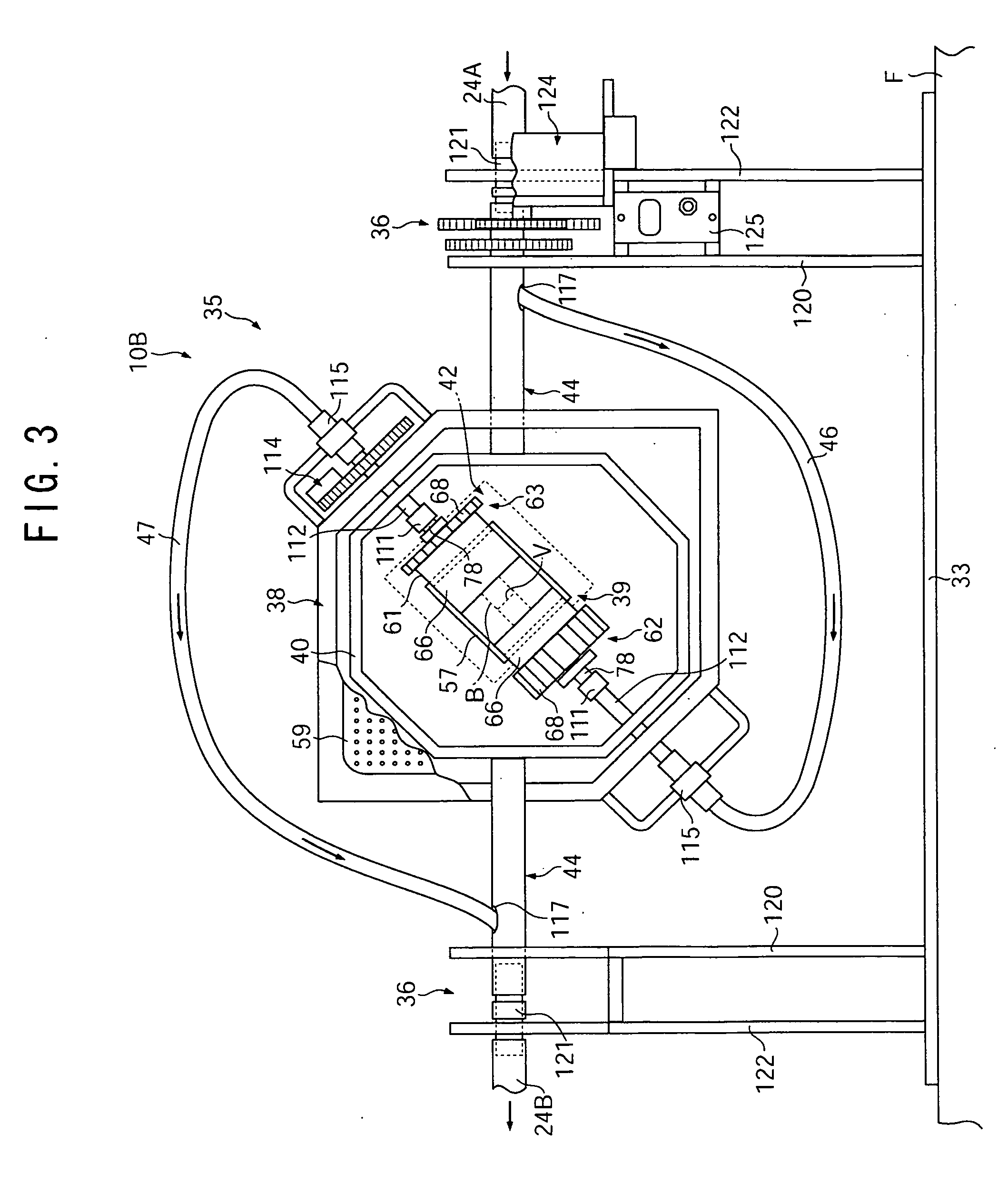
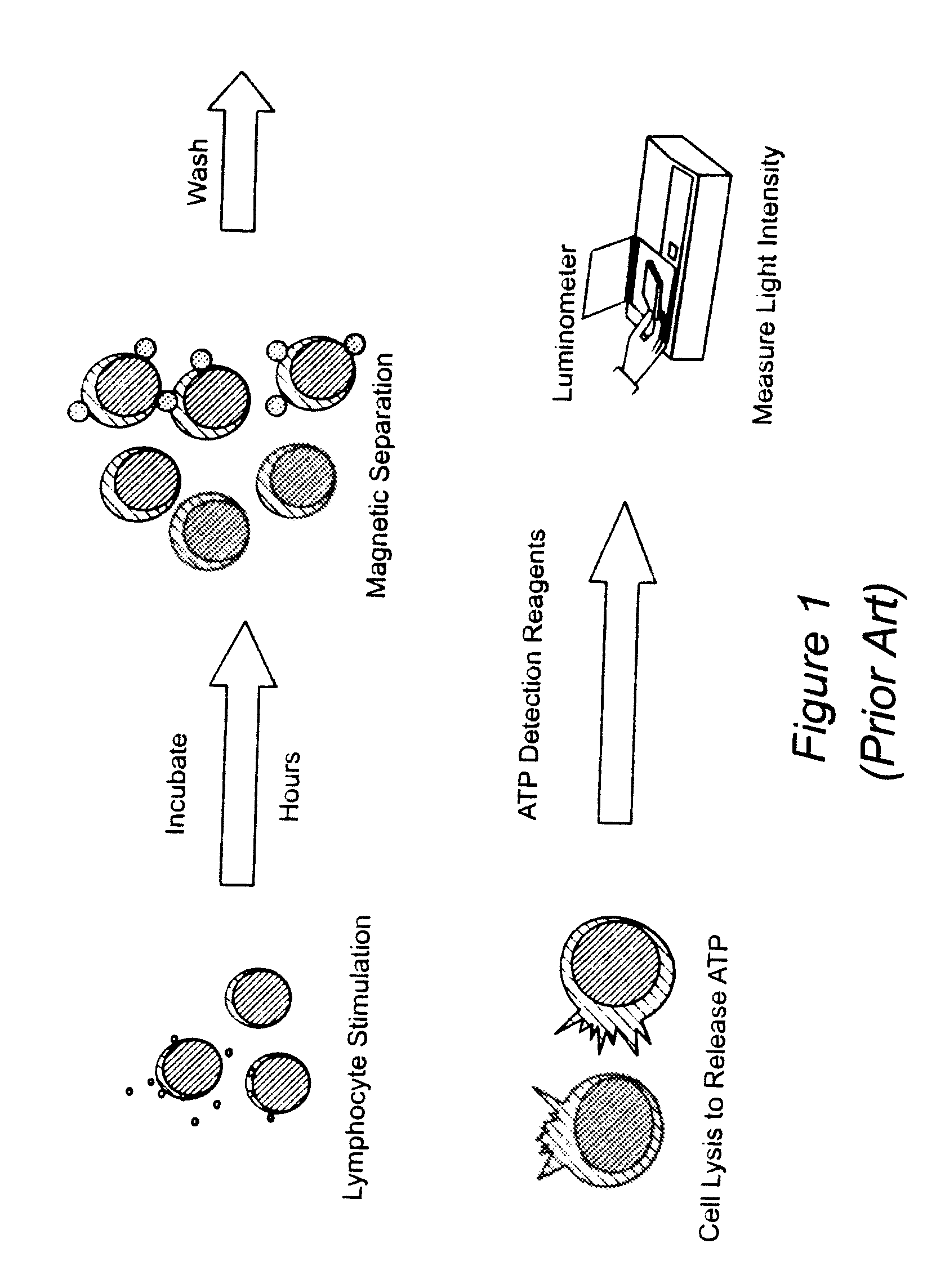
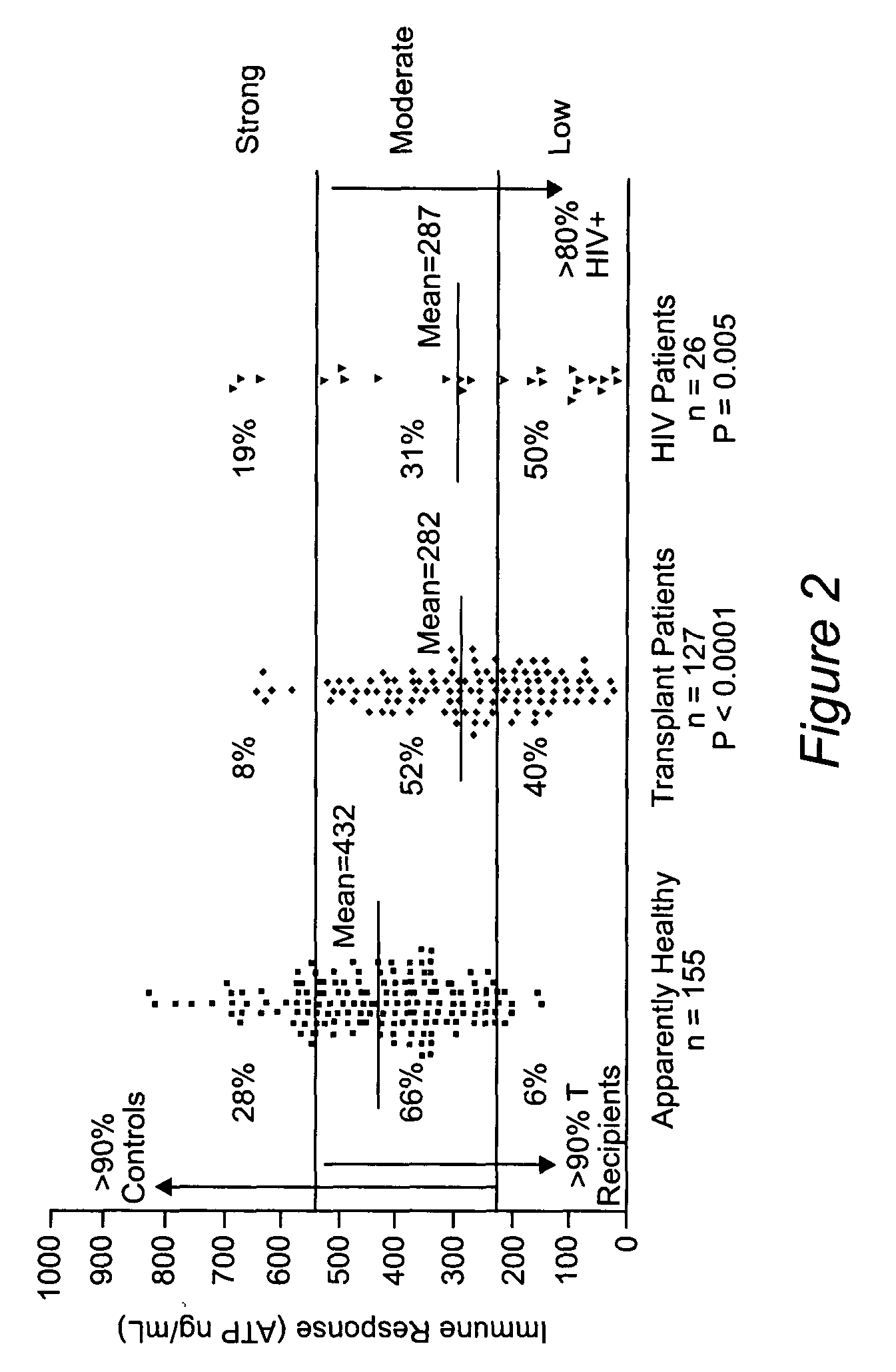
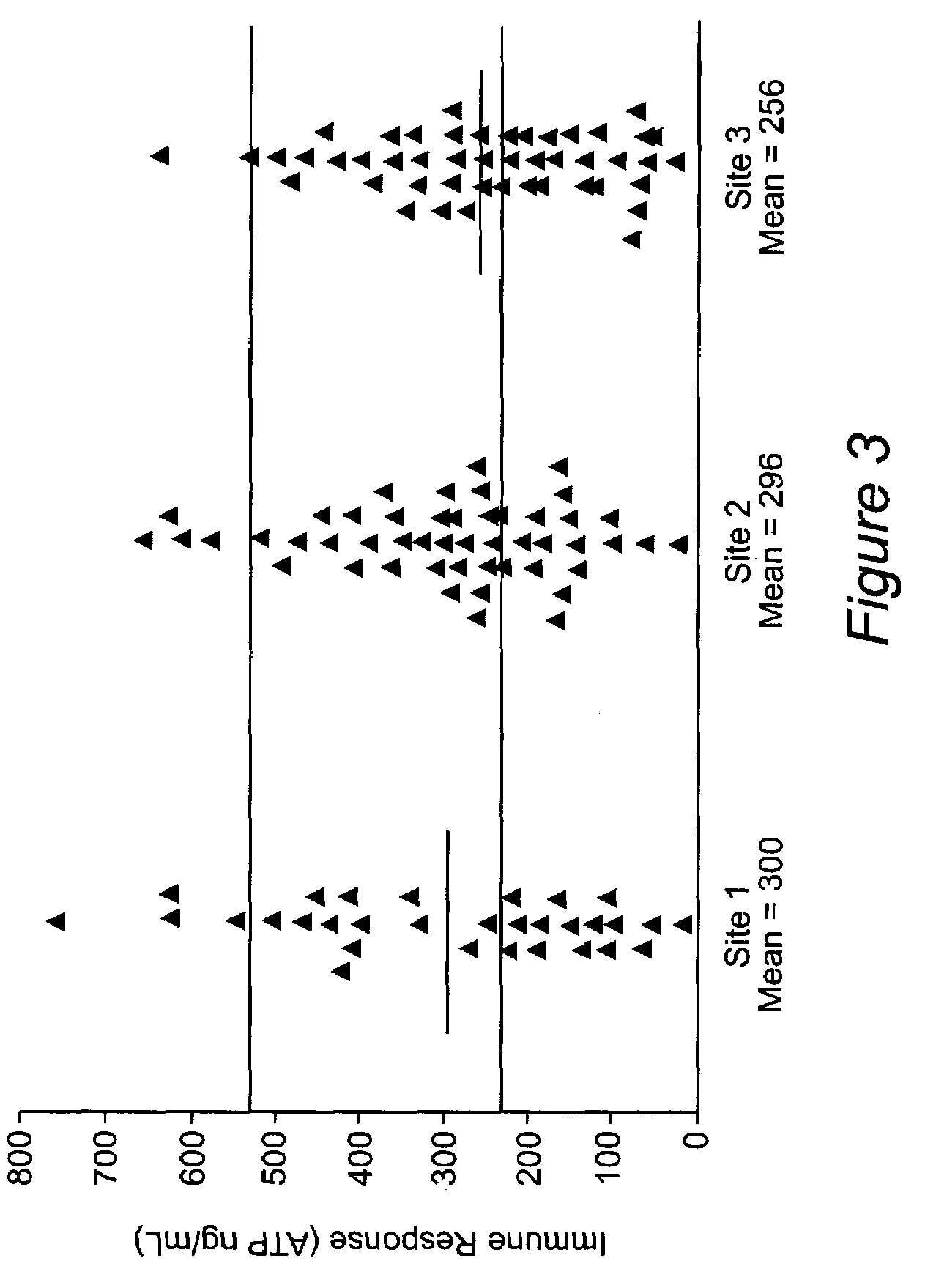
Method for monitoring the immune response and predicting clinical outcomes in transplant recipients
ActiveUS7476514B2Microbiological testing/measurementDisease diagnosisImmunosuppressive drugLymphocyte
Methods for monitoring the immune response and predicting clinical outcomes for patients on immunosuppressive drugs (such as transplant patients) are provided. The methods are based on the measurement of an intracellular metabolic marker in lymphocytes (such as ATP) as an indicator of a patient's immune response.
Owner:EUROFINS VIRACOR INC
Humanized anti-CD22 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
The present invention provides chimeric and humanized versions of anti-CD22 mouse monoclonal antibody, HB22.7. The anti-CD22 antibodies of the invention comprise four human or humanized framework regions of the immunoglobulin heavy chain variable region (“VH”) and four human or humanized framework regions of the immunoglobulin light chain variable region (“VK”). The invention further comprises heavy and / or light chain FW regions that contain one or more backmutations in which a human FW residue is exchanged for the corresponding residue present in the parental mouse heavy or light chain. Human or humanized VH framework regions of antibodies of the invention may comprise one or more of the following residues: a valine (V) at position 24 of framework region 1, a glycine (G) at position 49 of framework region 2, and an asparagine (N) at position 73 of framework region 3, numbered according to Kabat. The invention further relates to pharmaceutical compositions, immunotherapeutic compositions, and methods using therapeutic antibodies that bind to the human CD22 antigen and that preferably mediate human ADCC, CDC, and / or apoptosis for: the treatment of B cell diseases and disorders in human subjects, such as, but not limited to, B cell malignancies, for the treatment and prevention of autoimmune disease, and for the treatment and prevention of graft-versus-host disease (GVHD), humoral rejection, and post-transplantation lymphoproliferative disorder in human transplant recipients.
Owner:AERES BIOMEDICAL
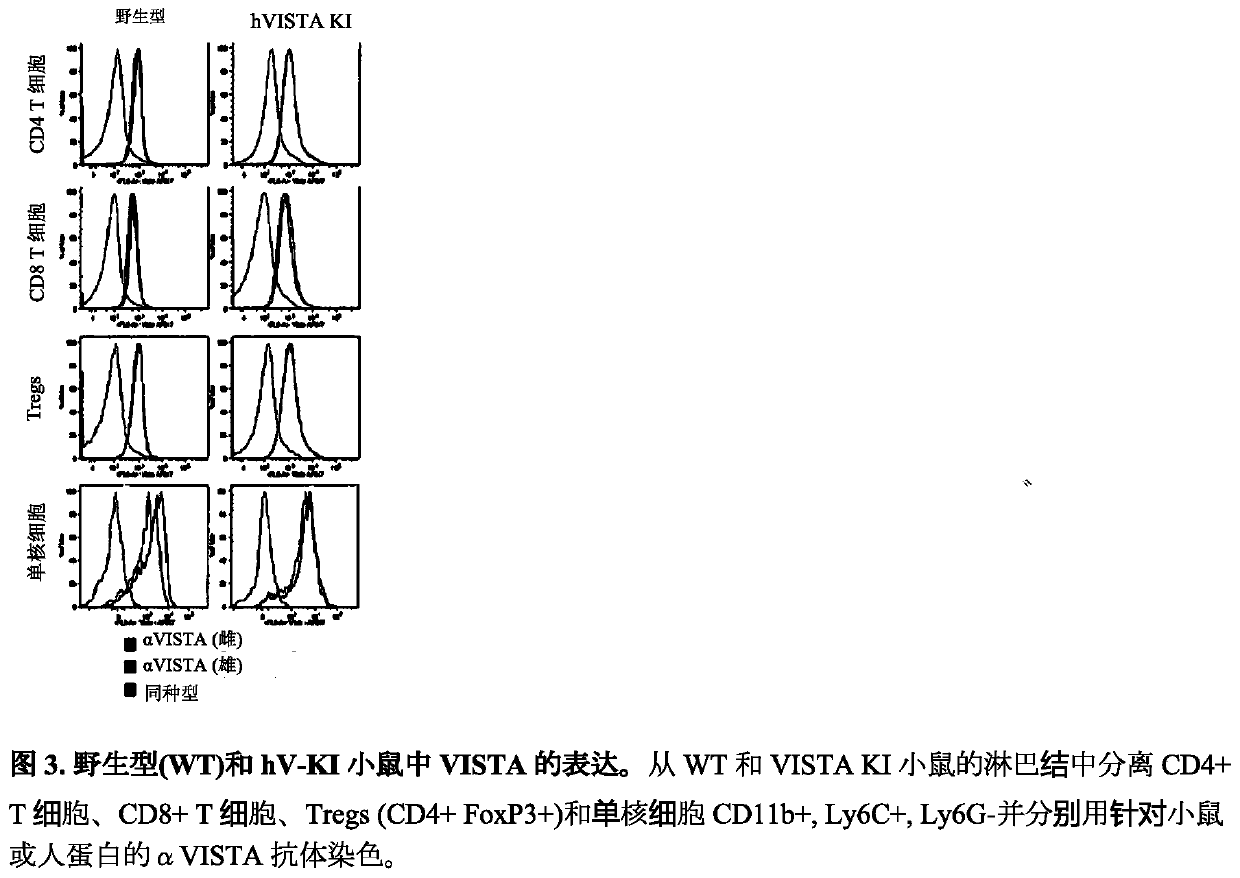
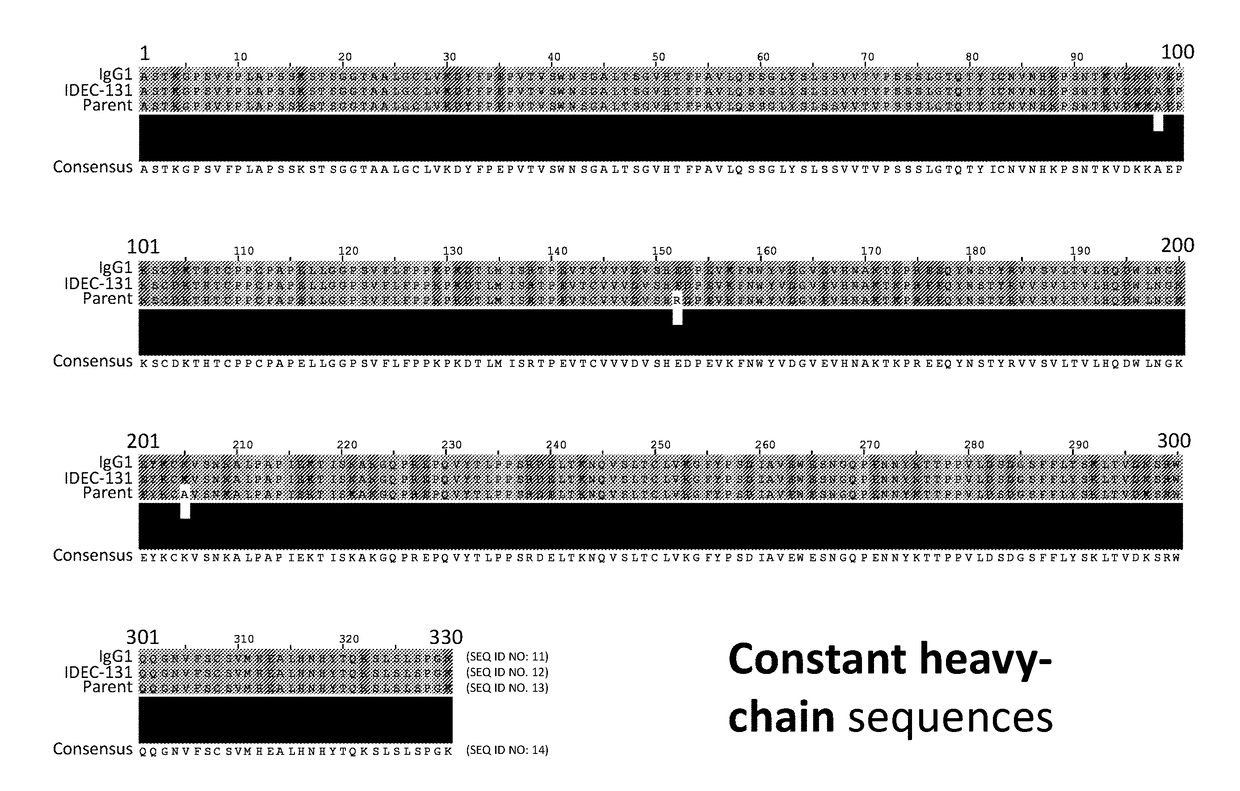
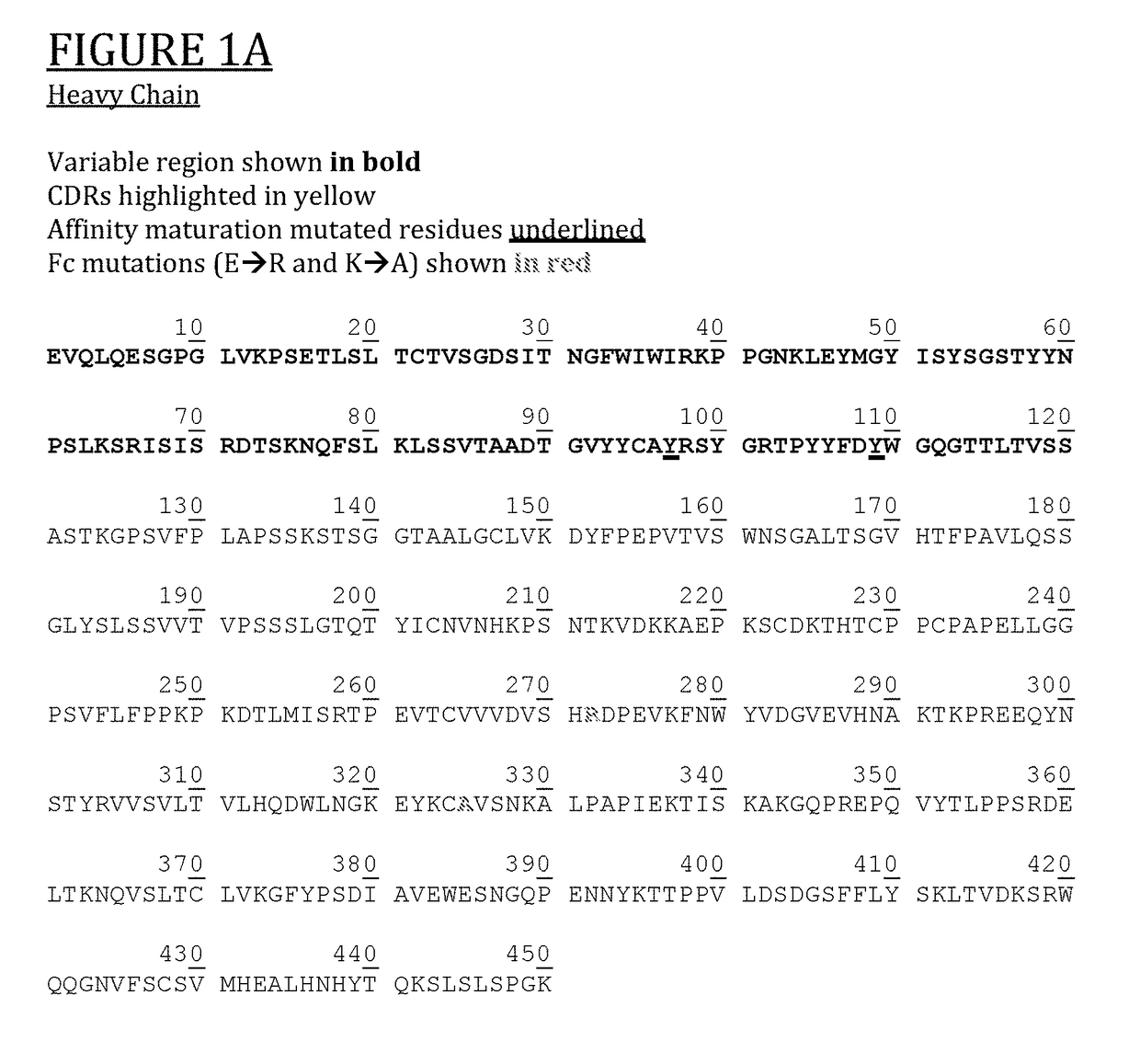
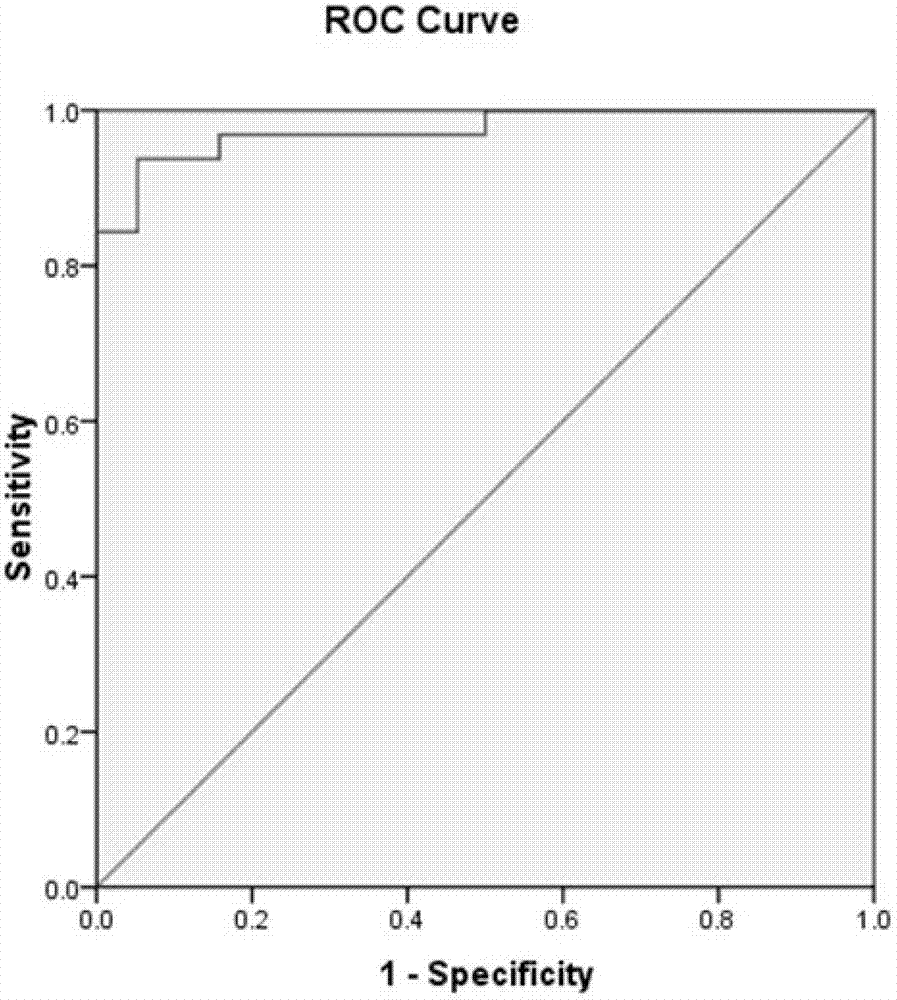
Anti-human vista antibodies and use thereof
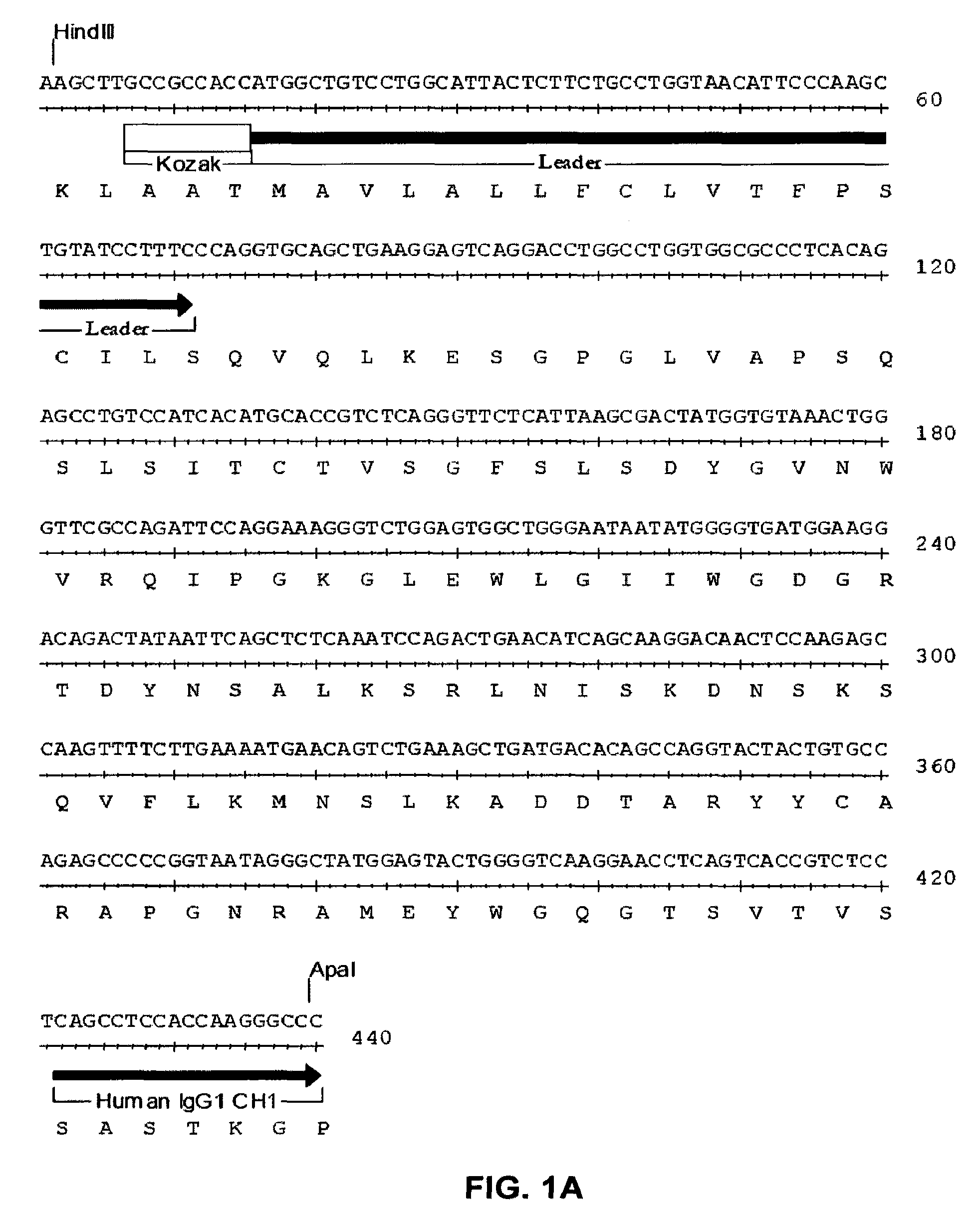
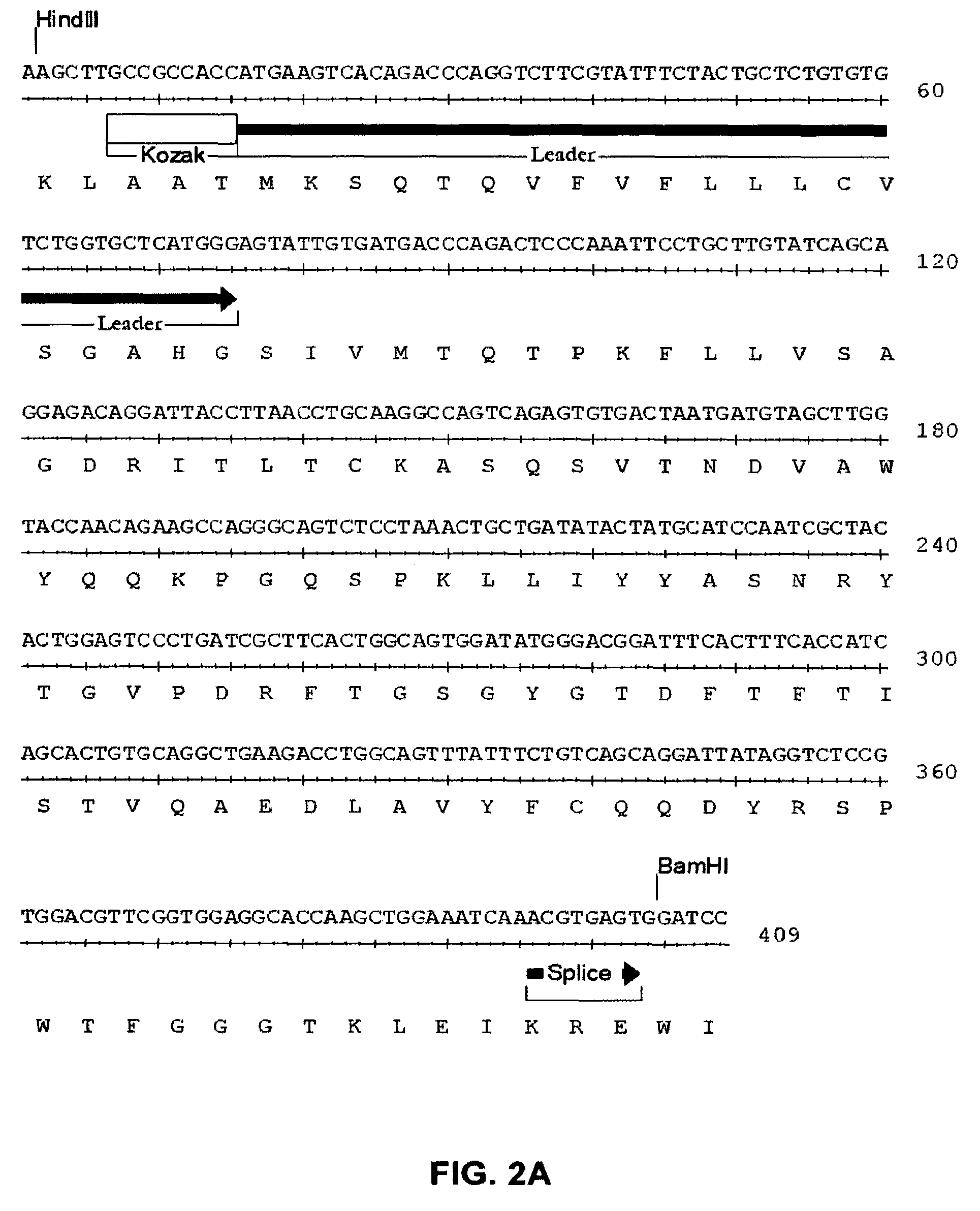
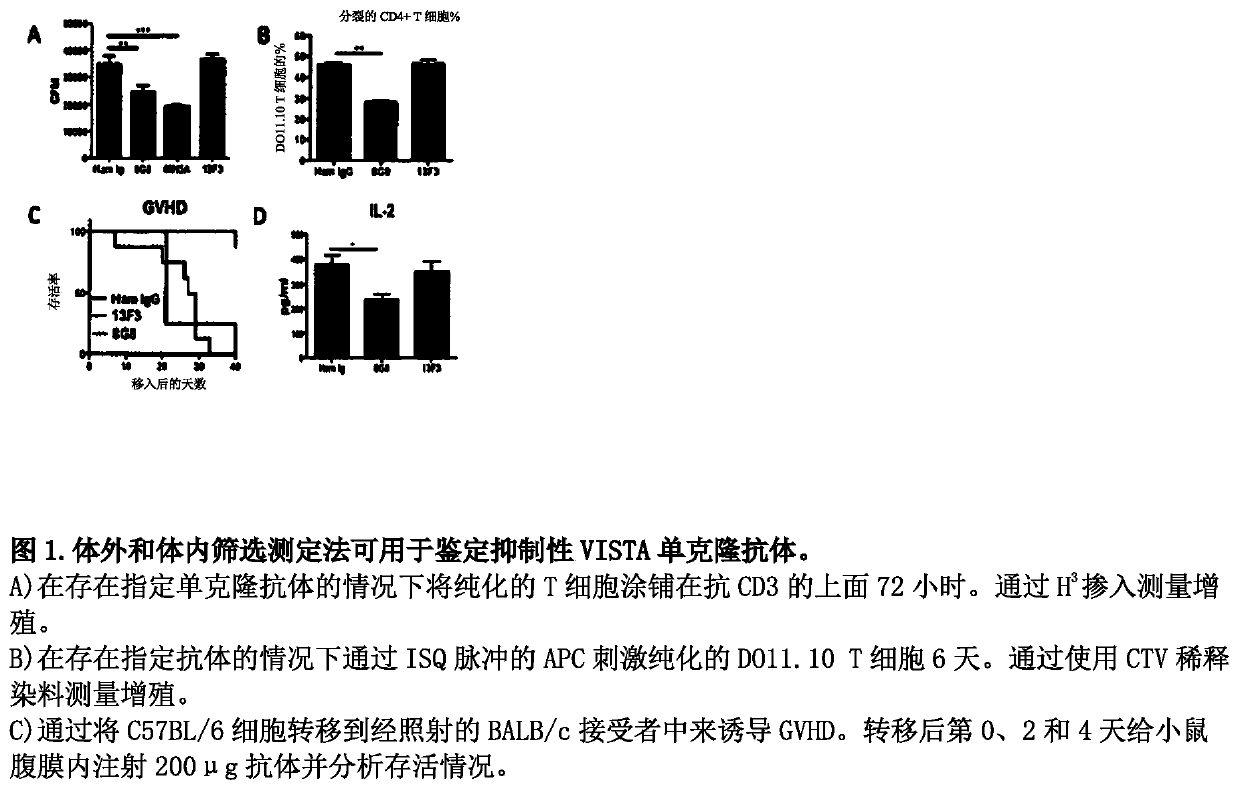
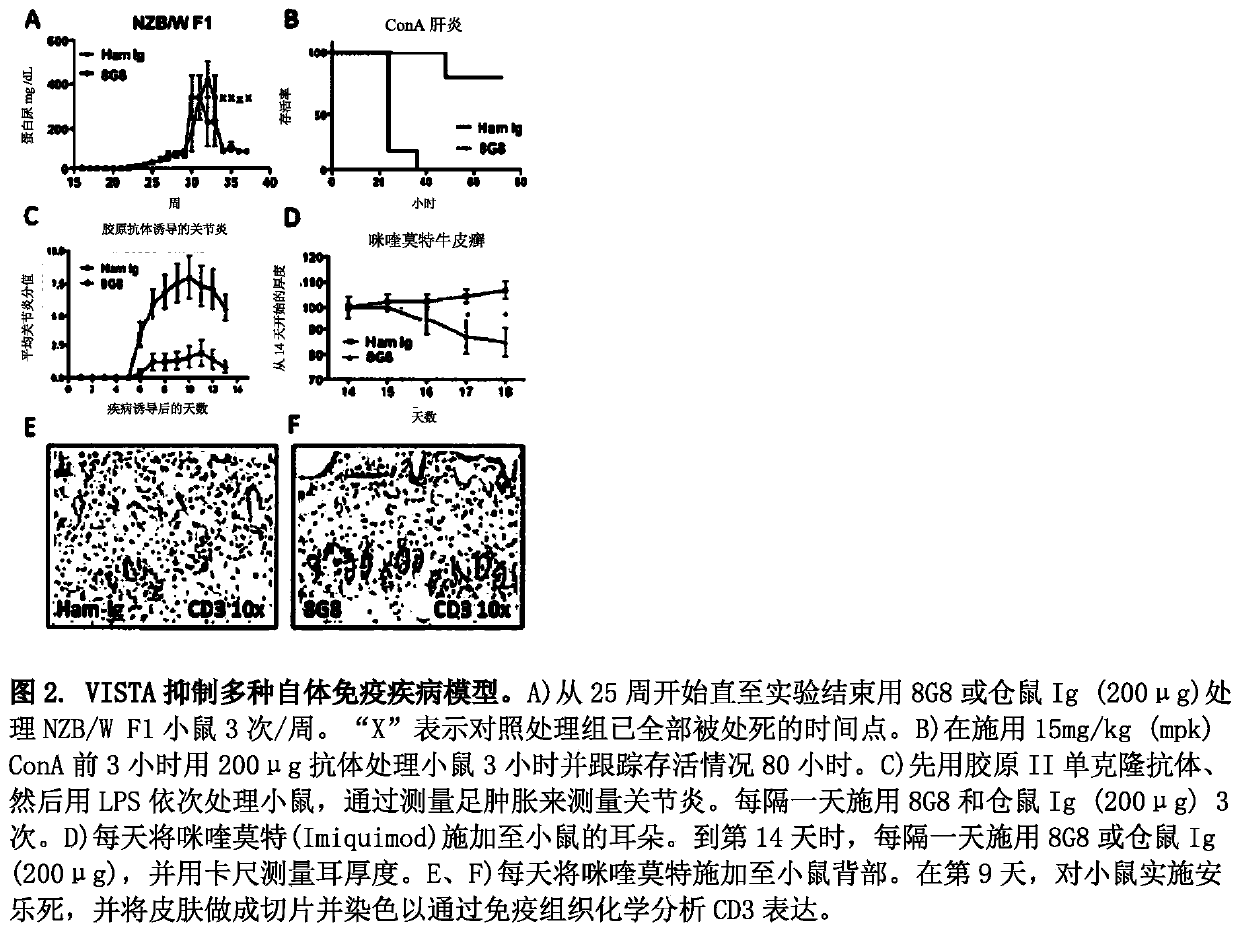
ActiveCN109789201ANervous disorderPeptide/protein ingredientsAntiendomysial antibodiesAntibody fragments
The invention provides agonistic anti-human VISTA antibodies and antibody fragments. These agonist antibodies and antibody fragments may be used to potentiate or enhance or mimic VISTA's suppressive effects on T cell immunity and thereby suppress T cell immunity. These agonist antibodies and antibody fragments are especially useful in the treatment of autoimmunity, allergy, inflammatory conditions, GVHD, sepsis and transplant recipients. Screening assays for identifying these agonists are also provided.
Owner:IMMUNEXT INC +1
Anti-cd154 antibody having improved binding, functional and safety characteristics and use in human immunotherapy
ActiveUS20180193454A1Prolong half-life in vivoImprove efficacyAntibacterial agentsSenses disorderDiseaseHalf-life
Improved anti-CD154 antibodies are provided herein which have improved therapeutic potency, in vivo half-life and ablated FcR binding and / or complement binding / activation. The use of these antibodies for inducing tolerance and treating immune diseases including autoimmunity, inflammation, transplant recipients, fibrosis and allergic disorders is disclosed herein.
Owner:IMMUNEXT INC
Kit for early warning rejection reaction after kidney transplant and use method of kit
The invention relates to an early warning kit, and in particular relates to a kit for early warning transplant kidney rejection reaction and a use method of the kit. A transplant kidney rejection reaction early diagnosis system is built by monitoring and comprehensively analyzing the expression levels of 96 cell factors / chemotactic factors in 266 serums of 142 patients with acute transplant kidney rejection reaction and transplant recipients with stable transplant kidney functions and receptors of the cell factors / chemotactic factors by applying the liquid protein chip luminex detection technology and the acute rejection reaction prediction and accuracy rates of the system are 98.6% and the internal cross validation rates of the system are respectively 97.1% and 97.2%.
Owner:THE 309TH HOSPITAL OF CHINESE PEOPLES LIBERATION ARMY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com