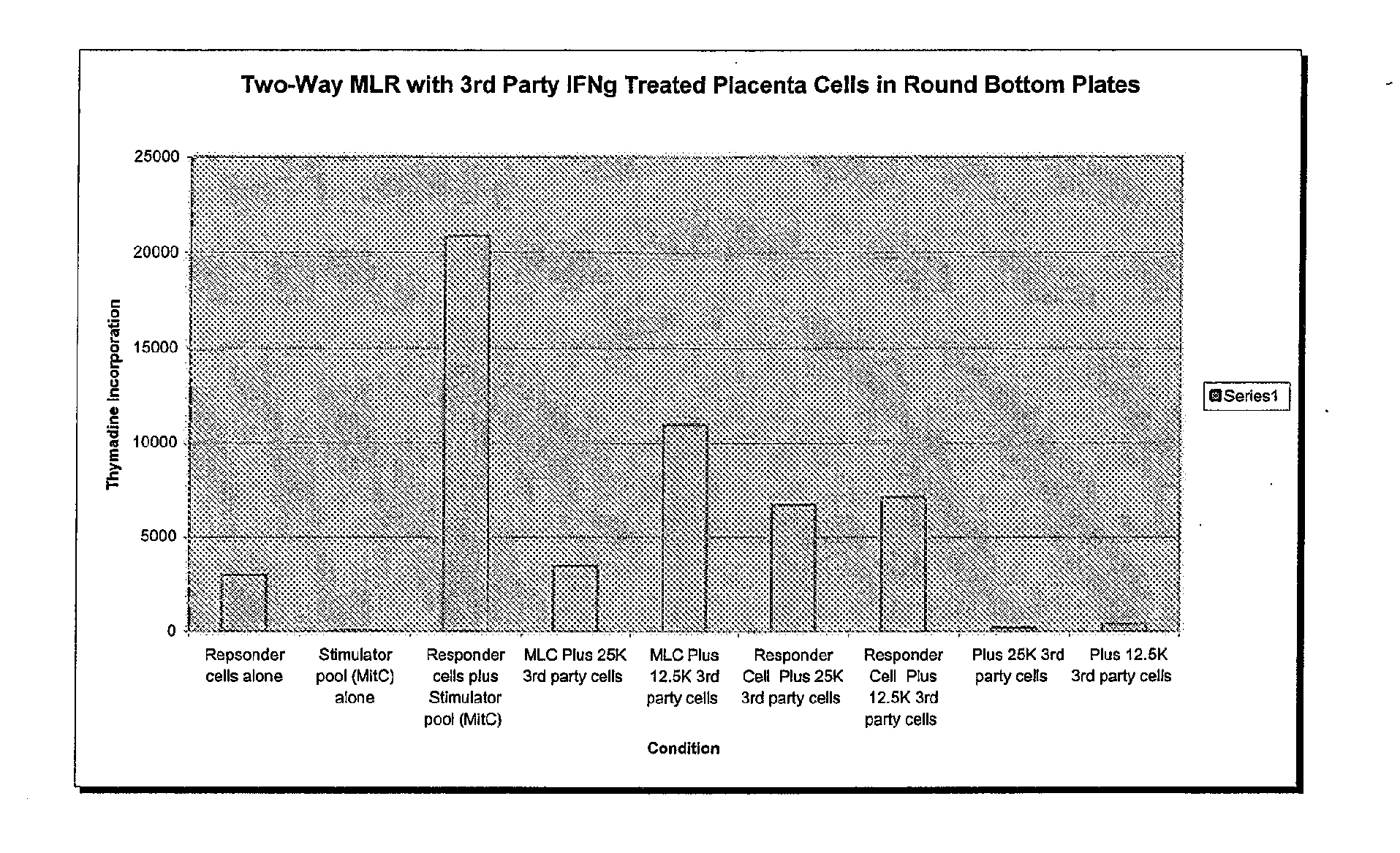
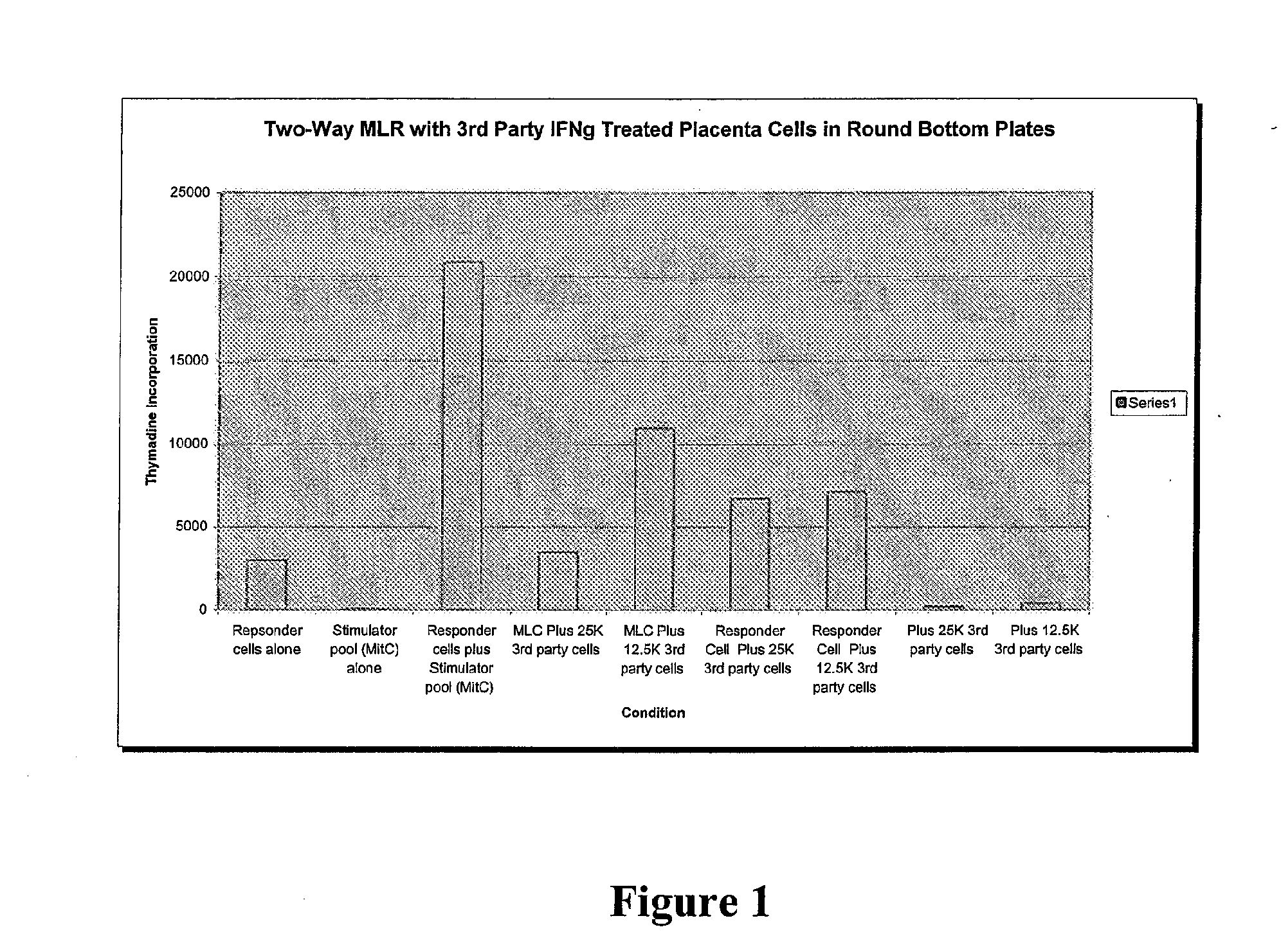
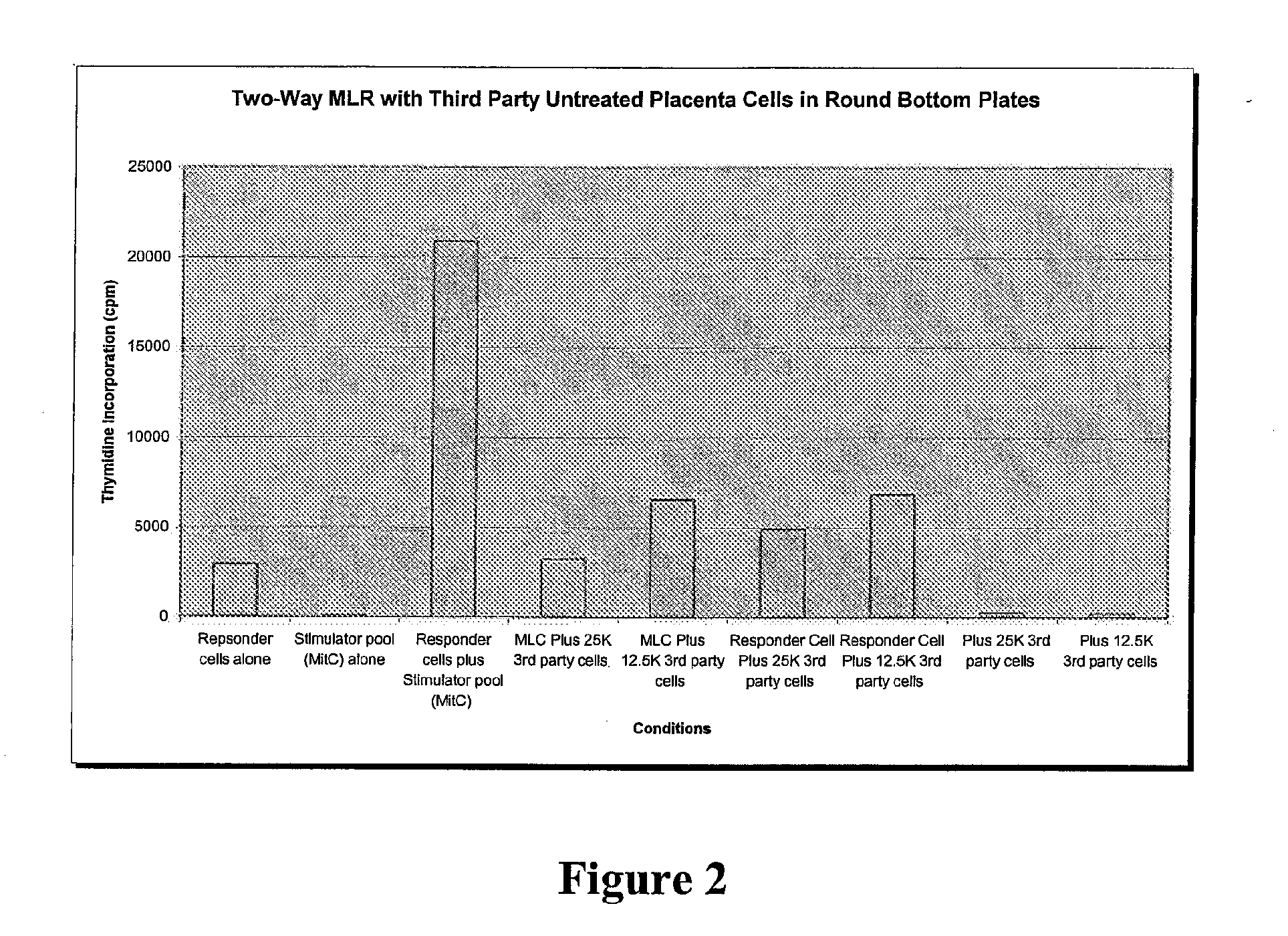
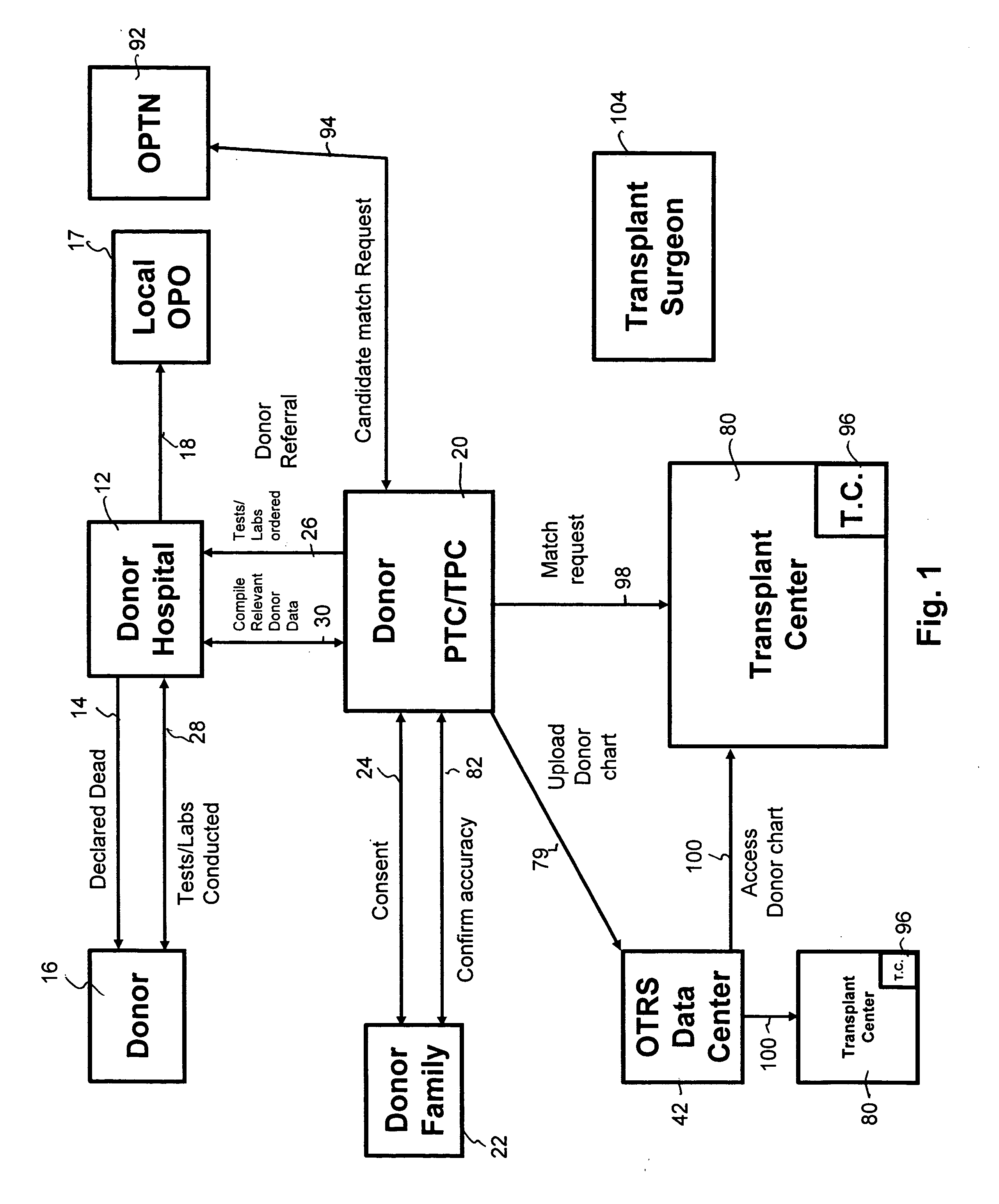
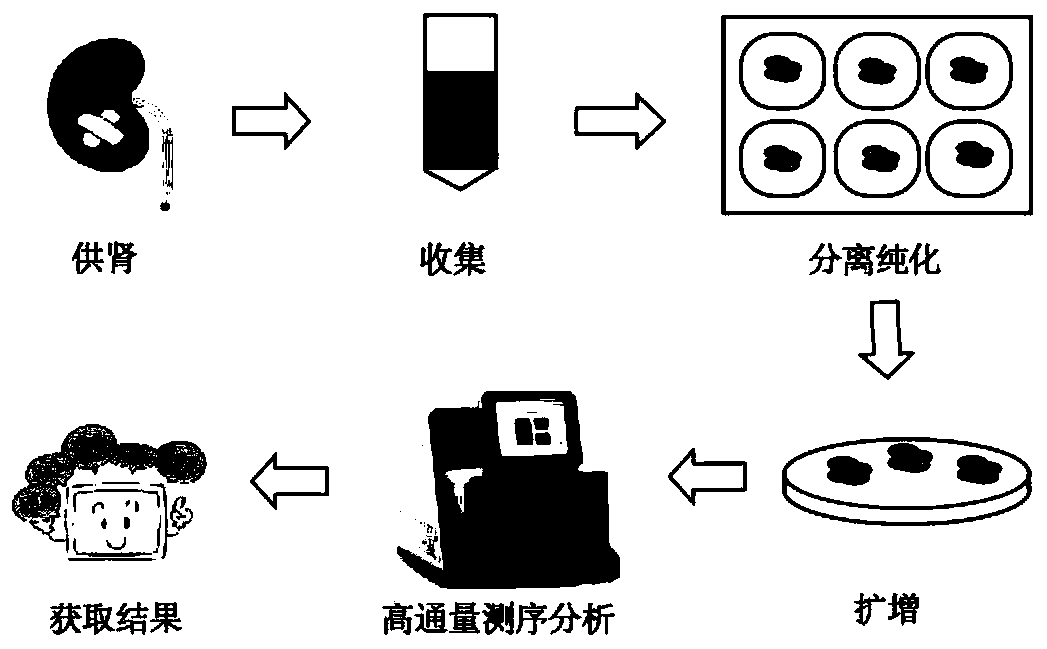
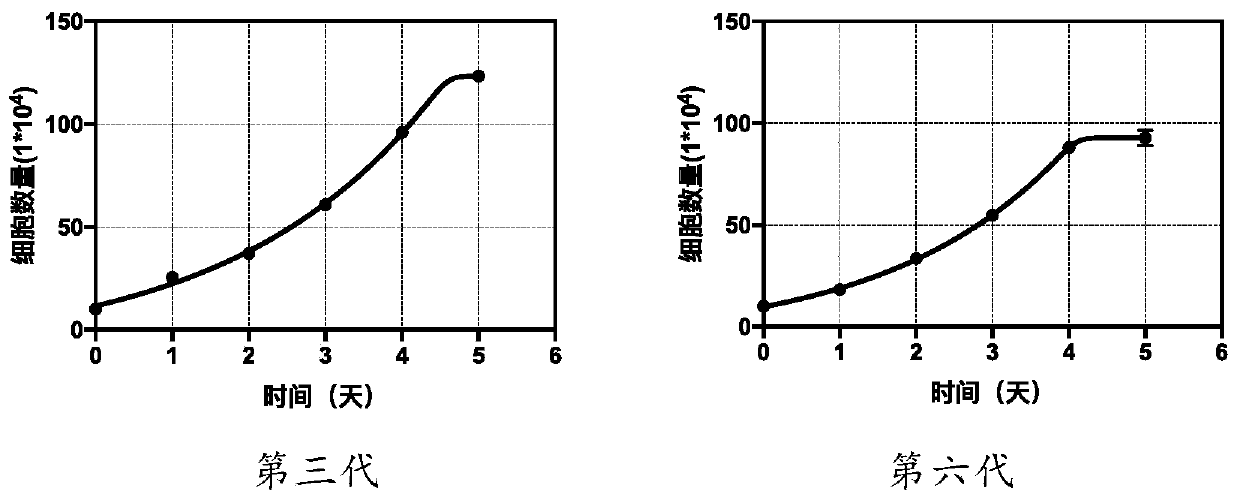
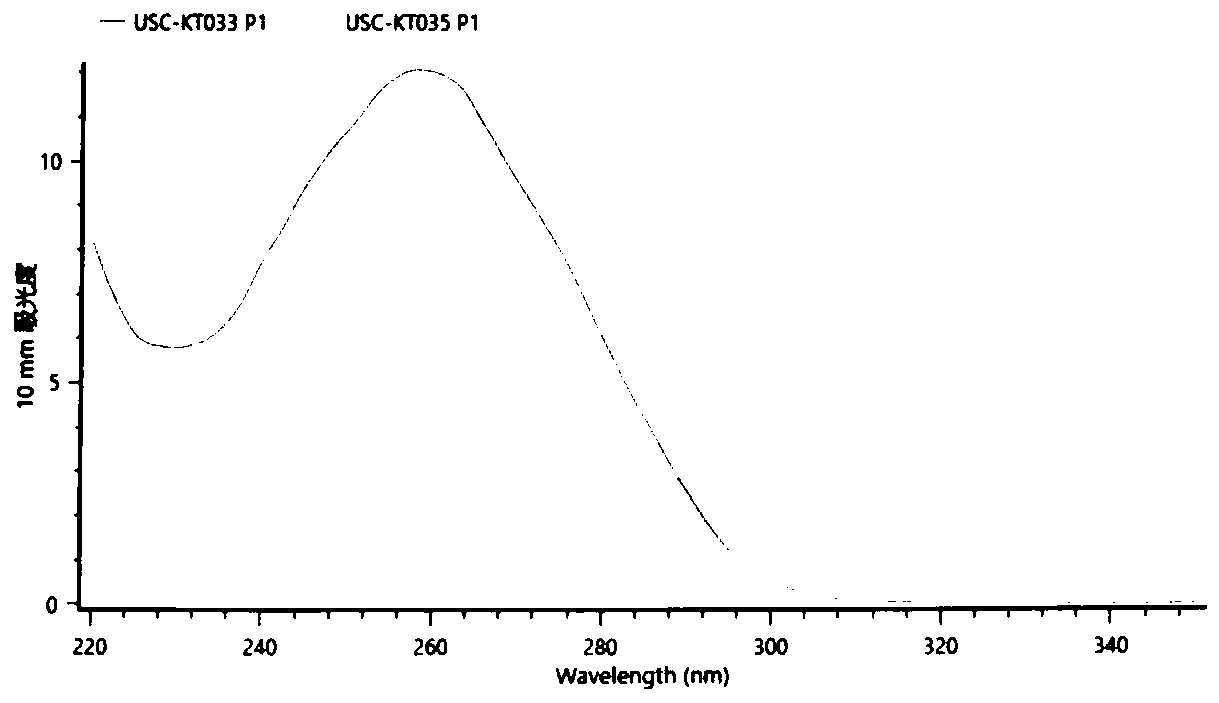
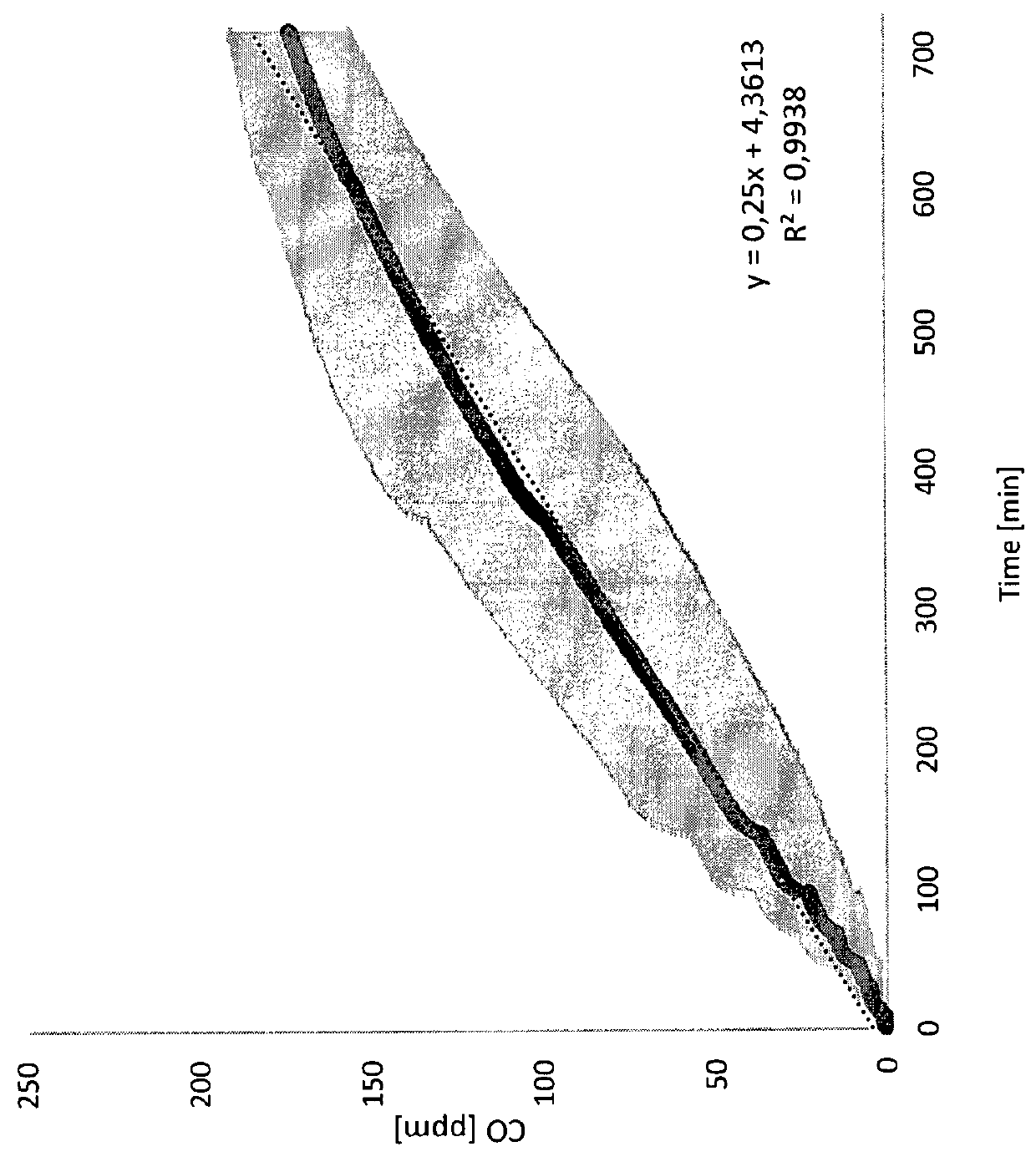
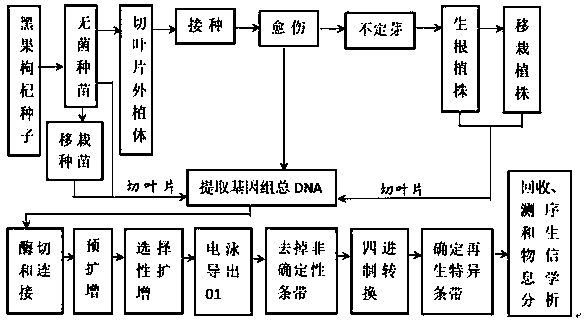
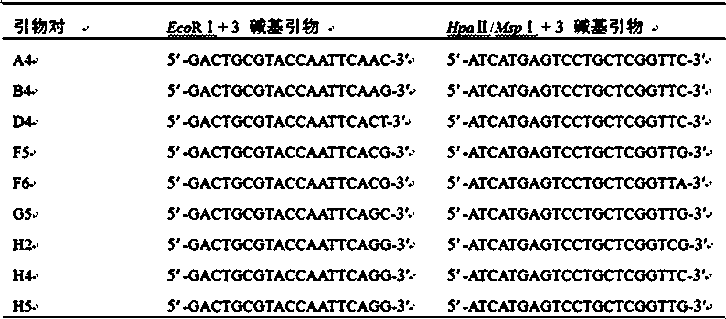
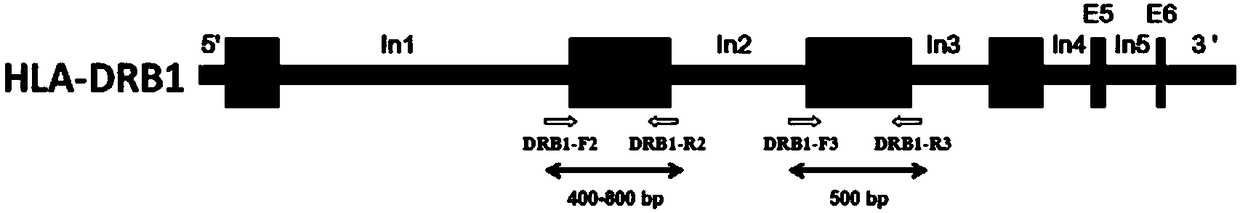
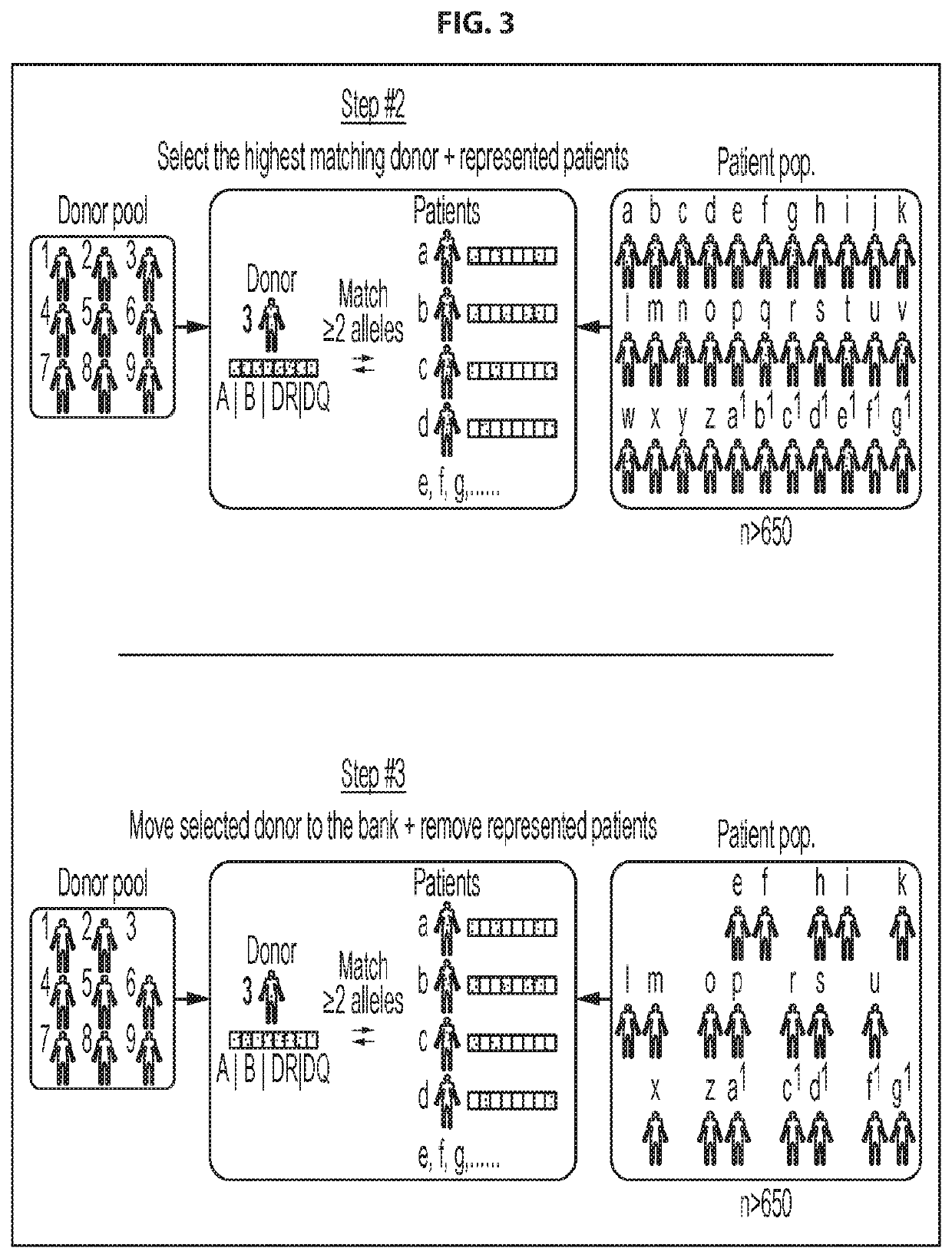
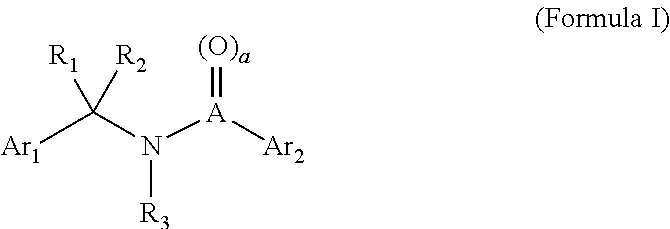Patents
Literature
40 results about "Transplant Donors" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A living-donor transplant is a surgical procedure to remove an organ or portion of an organ from a living person and place it in another person whose organ is no longer functioning properly.
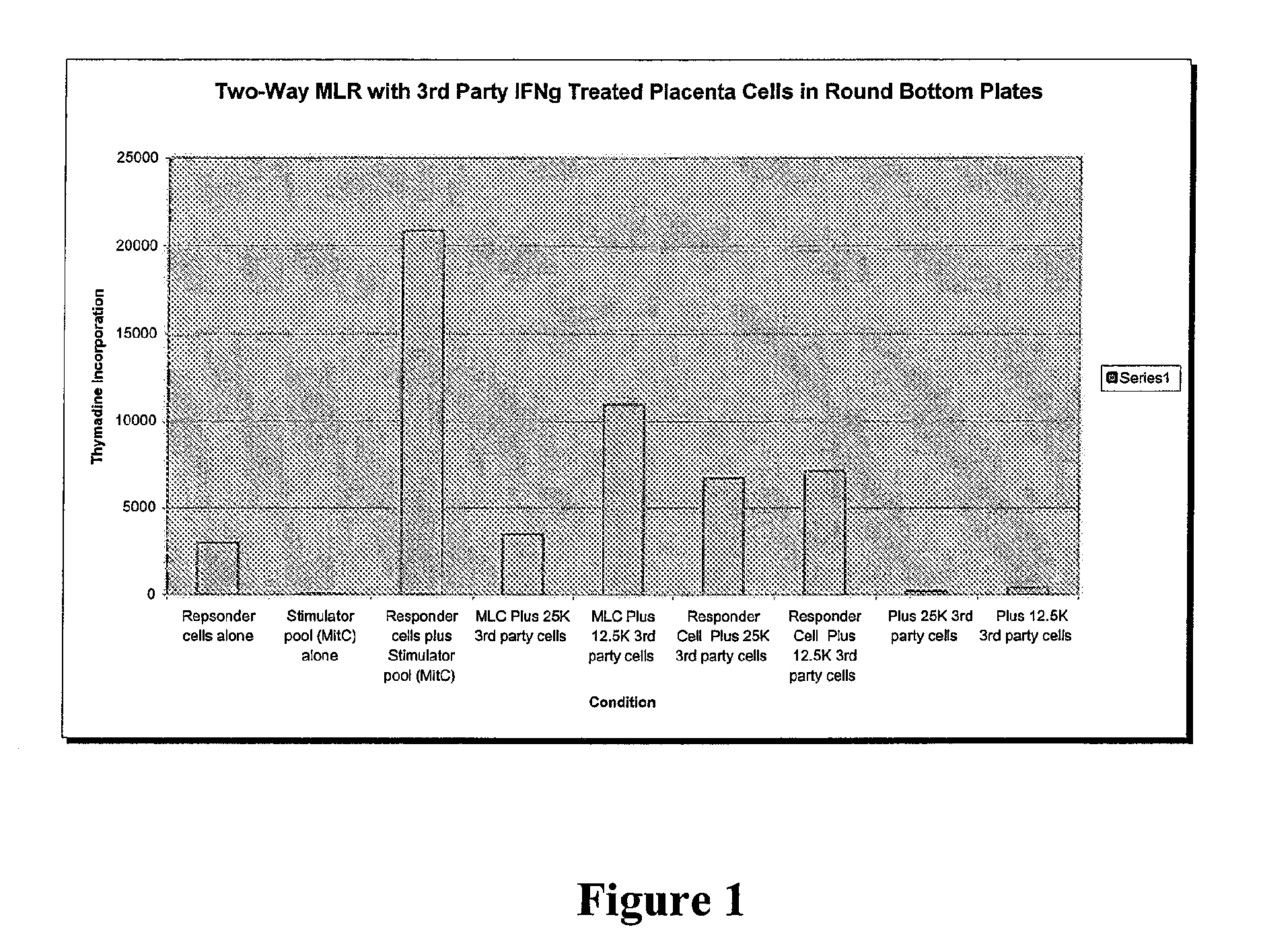
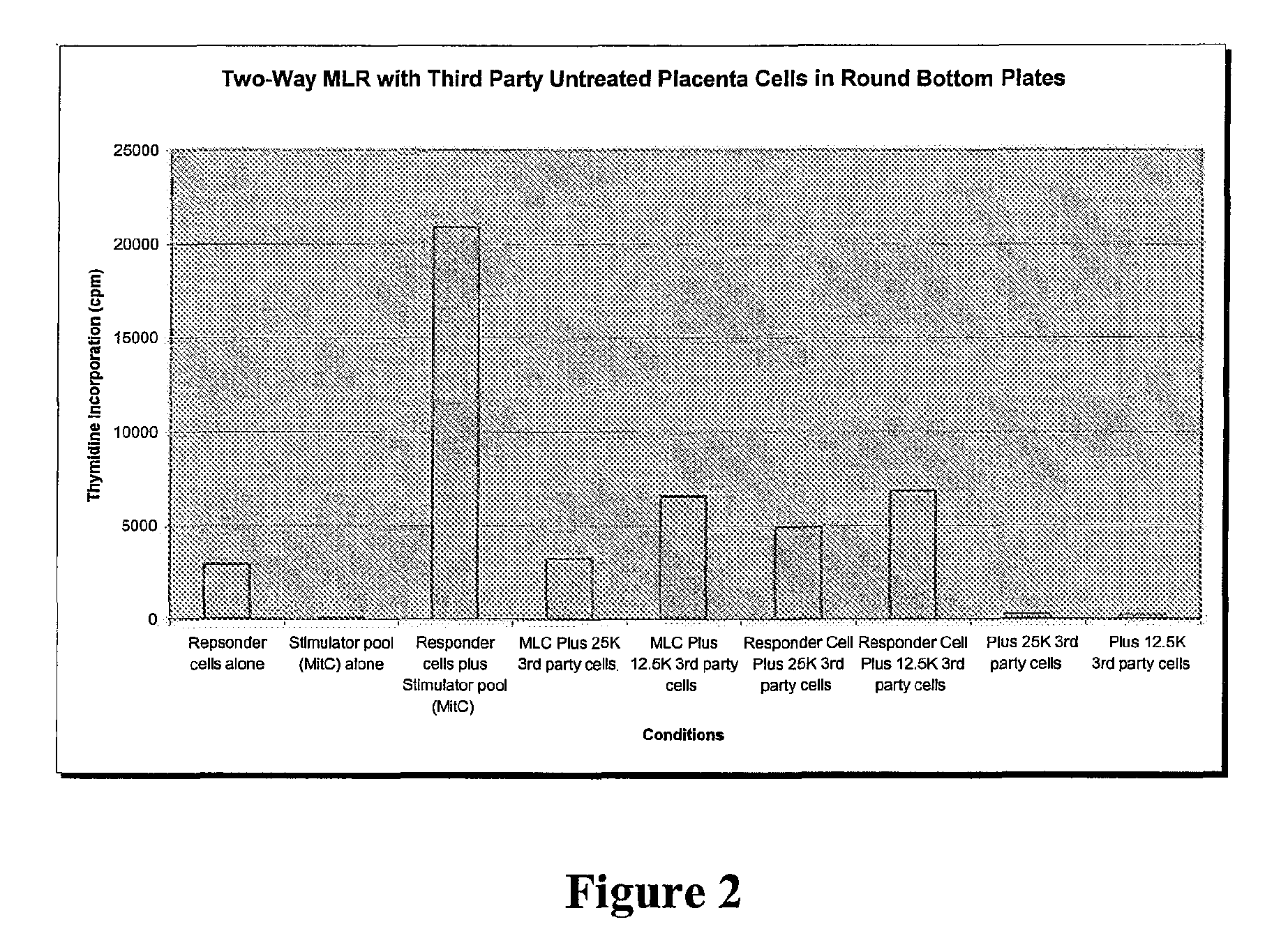
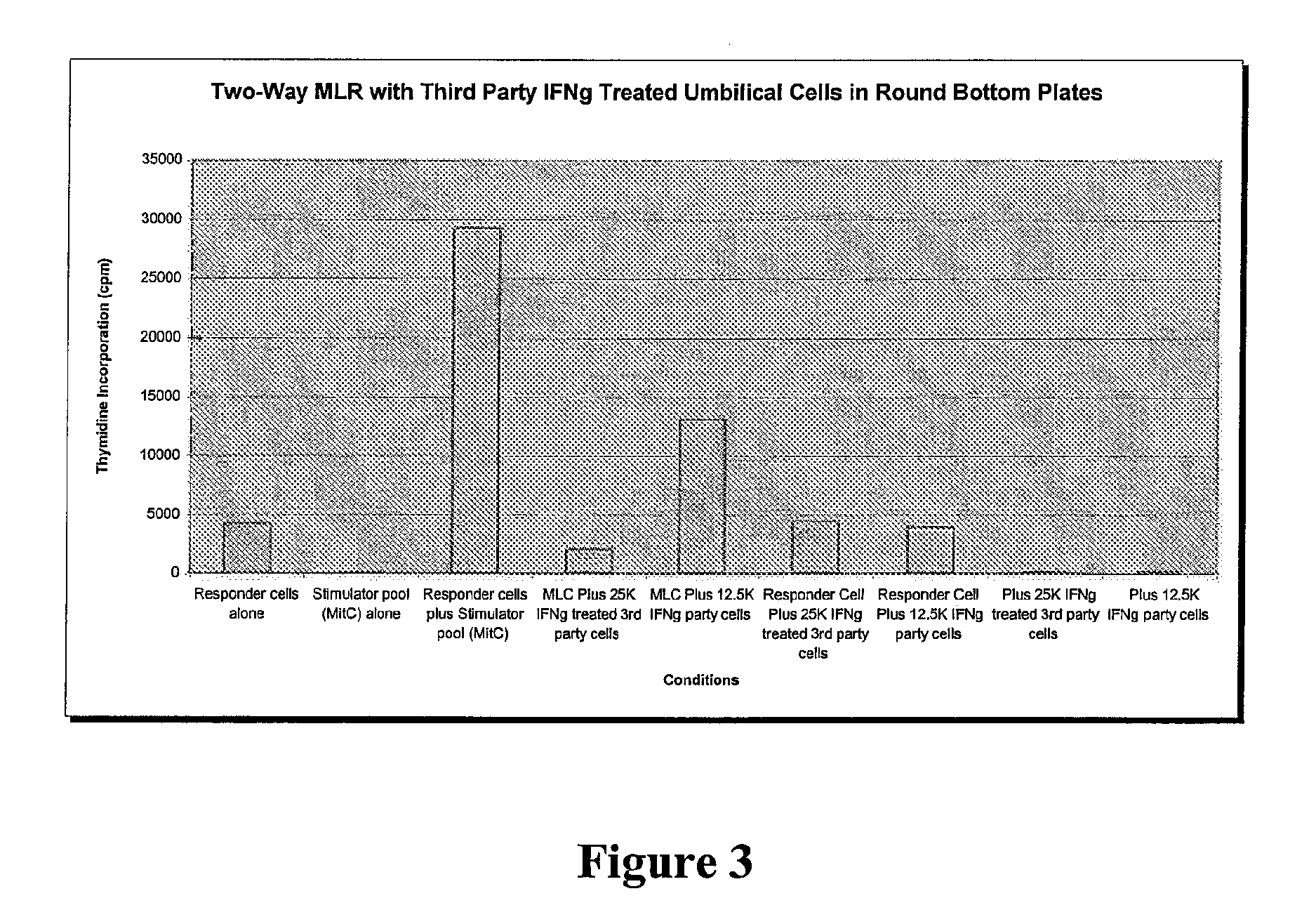
Compositions and methods for inhibiting adverse immune response in histocompatibility-mismatched transplantation
ActiveUS20070264269A1Effective for adverse immune responseAntipyreticAnalgesicsGraft versus host disease inductionCell based
Cell-based compositions and methods of their use to inhibit an adverse immune response such as graft versus host disease or rejection of transplanted tissue in a transplant recipient that is histocompatibility mismatched to the transplant donor are disclosed. The compositions and methods utilize postpartum-derived cells, such as cells derived from the placenta or umbilicus.
Owner:DEPUY SYNTHES PROD INC
Methods for pretreating a subject with extracorporeal photopheresis
The present invention relates to methods for treating a subject predisposed to an autoimmune disease with extracorporeal photopheresis or an effective amount of apoptotic cells before the clinical manifestation of a symptom associated with the autoimmune disease. The present invention alsorelates to methods for treating a subject predisposed to an atopic disease with extracorporeal photopheresis or an effective amount of apoptotic cells before the clinical manitfesation of a symptom associated with the atopic disease. The present invention further relates to methods for treating a transplant donor and / or a transplant recipient, or an implant recipient with extracorporeal photopheresis or an effective amount of apoptotic cells prior to the transplant or implantation procedure.
Owner:NEW ENGLAND MEDICAL CENT HOSPITALS +1
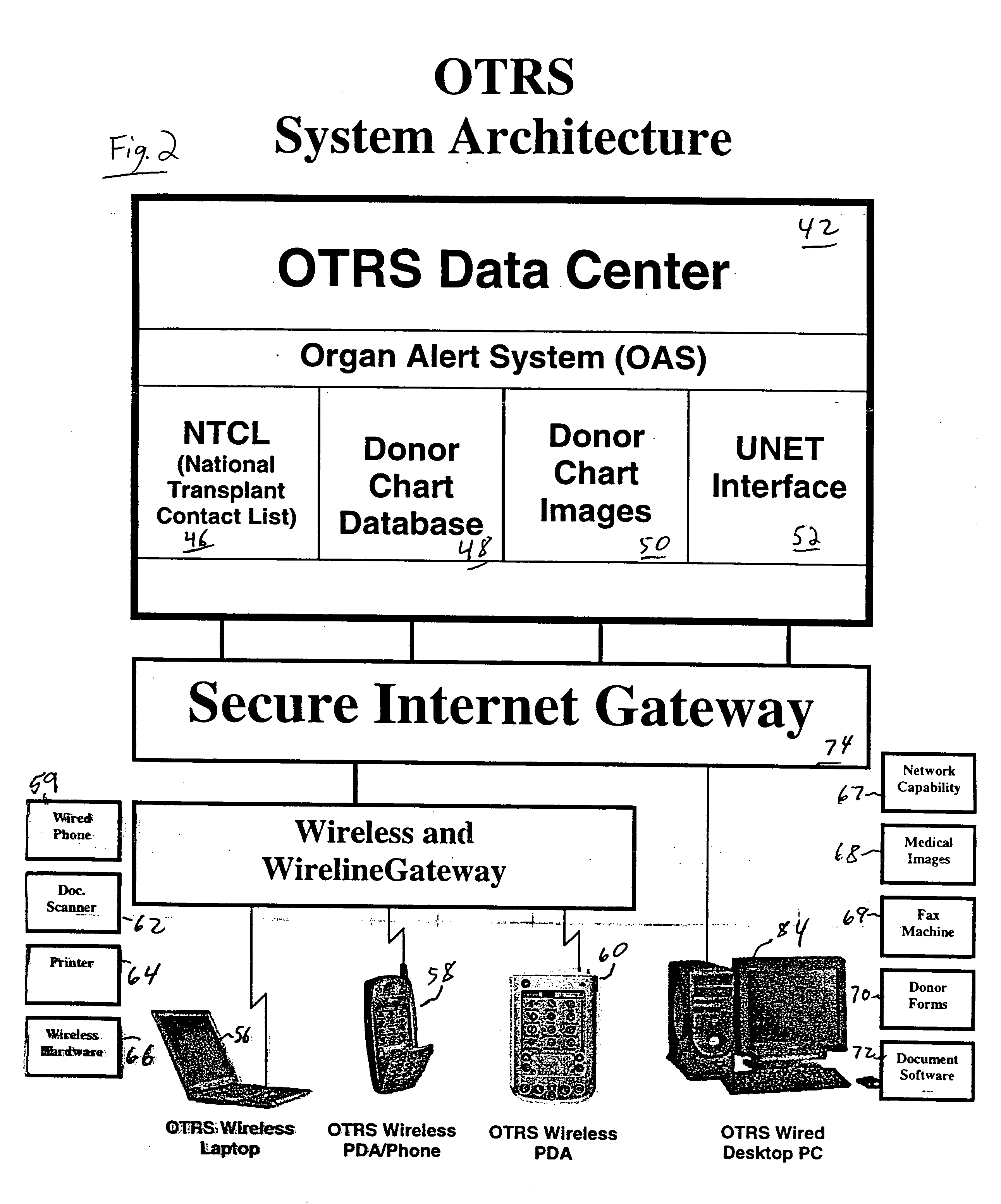
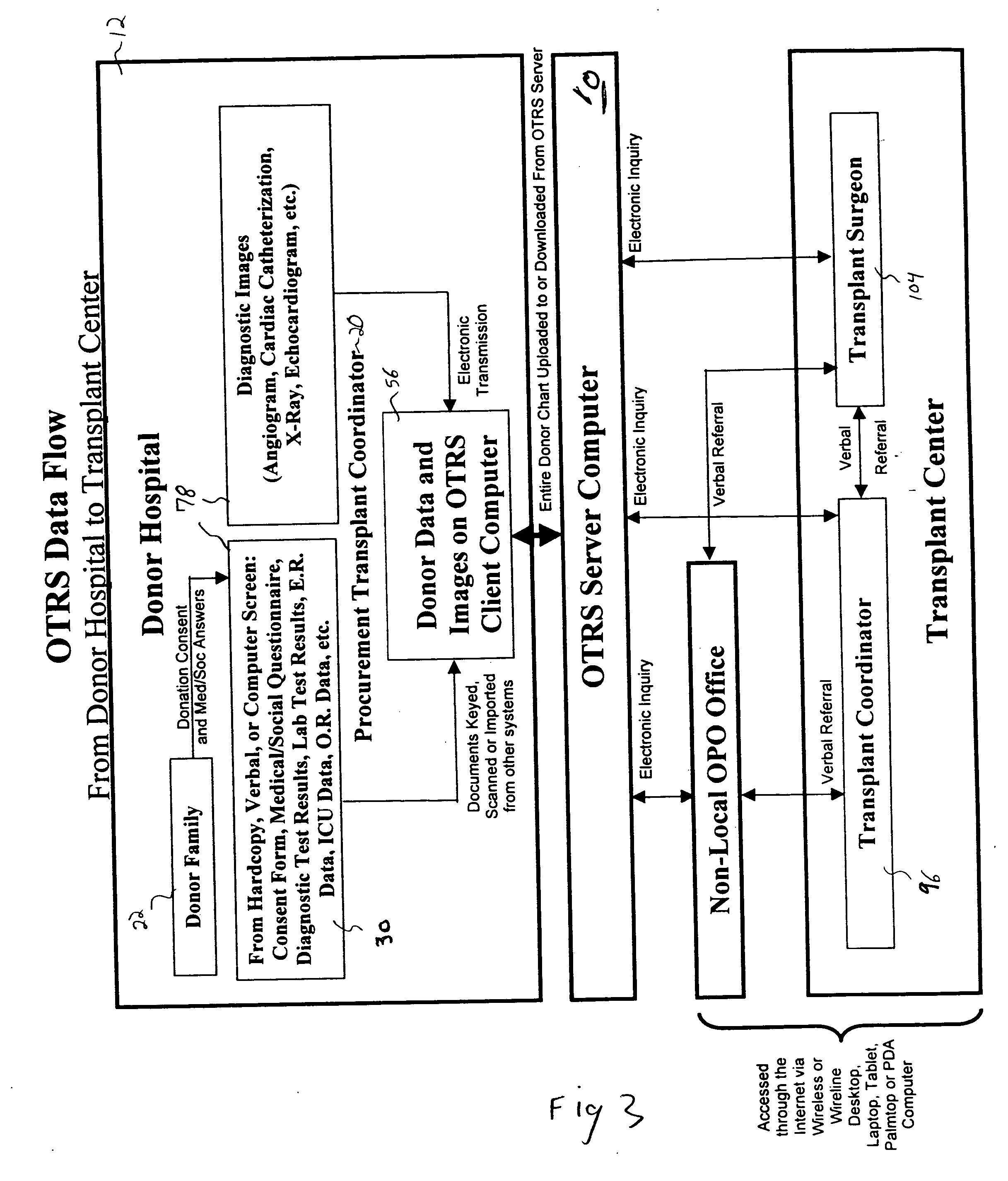
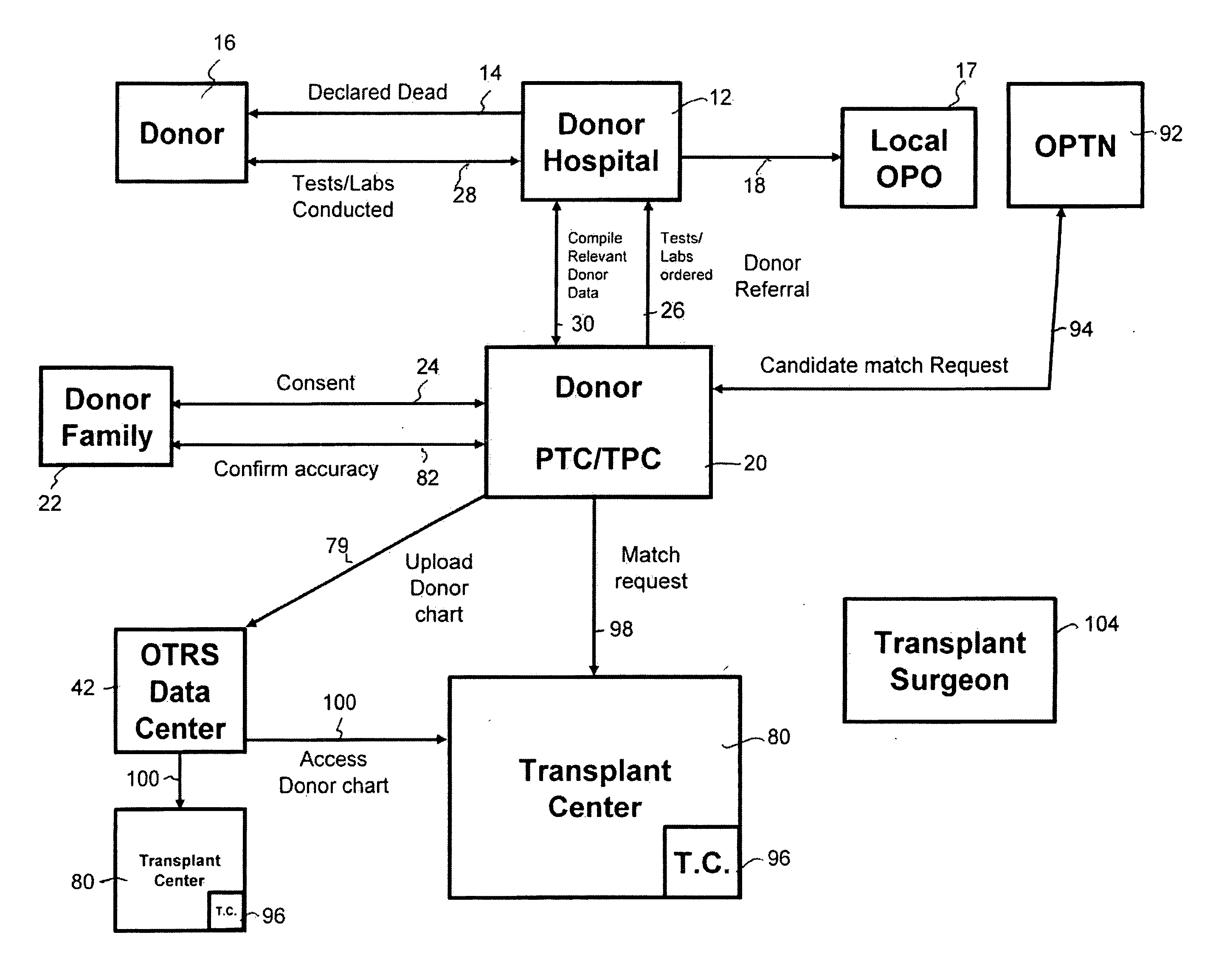
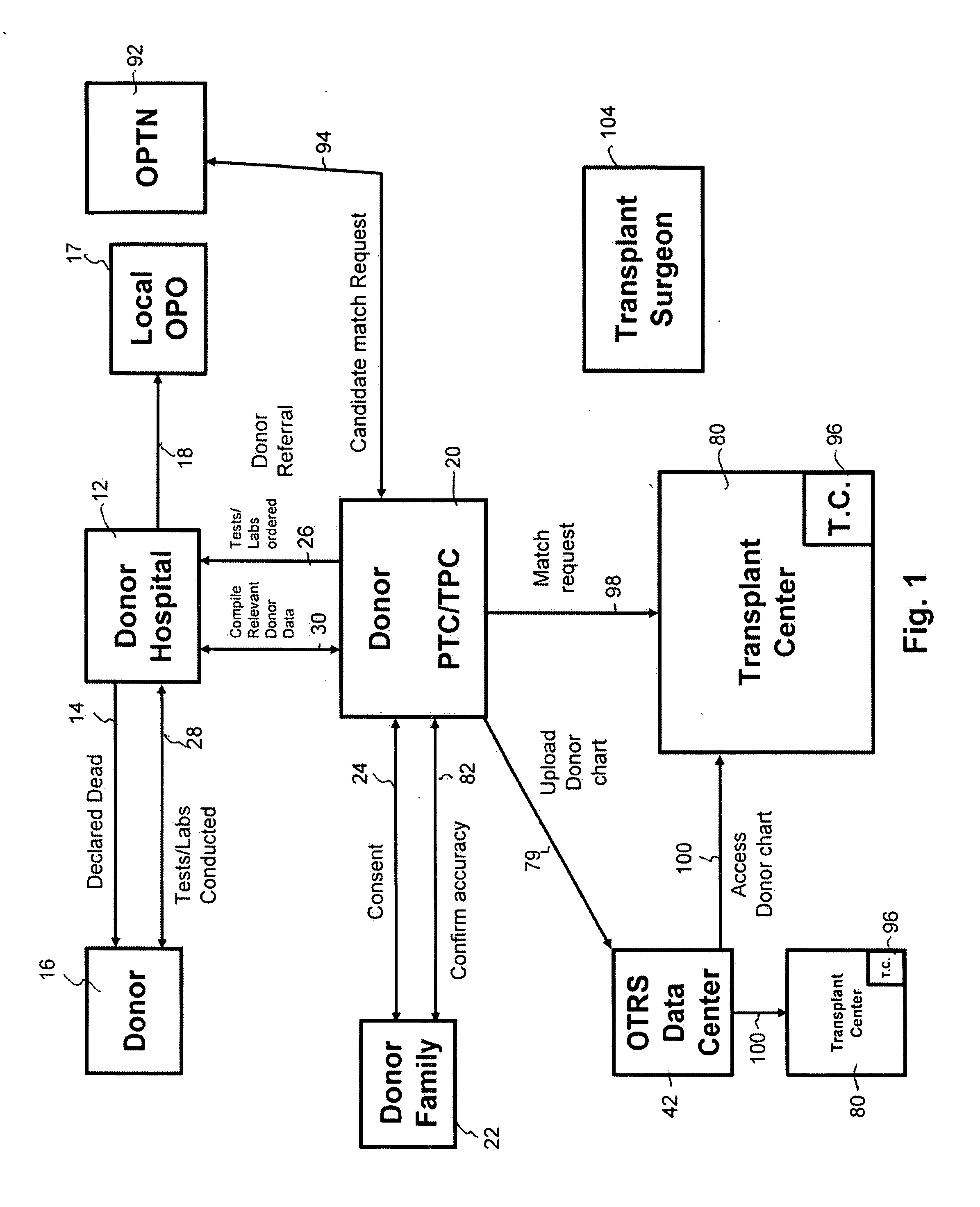
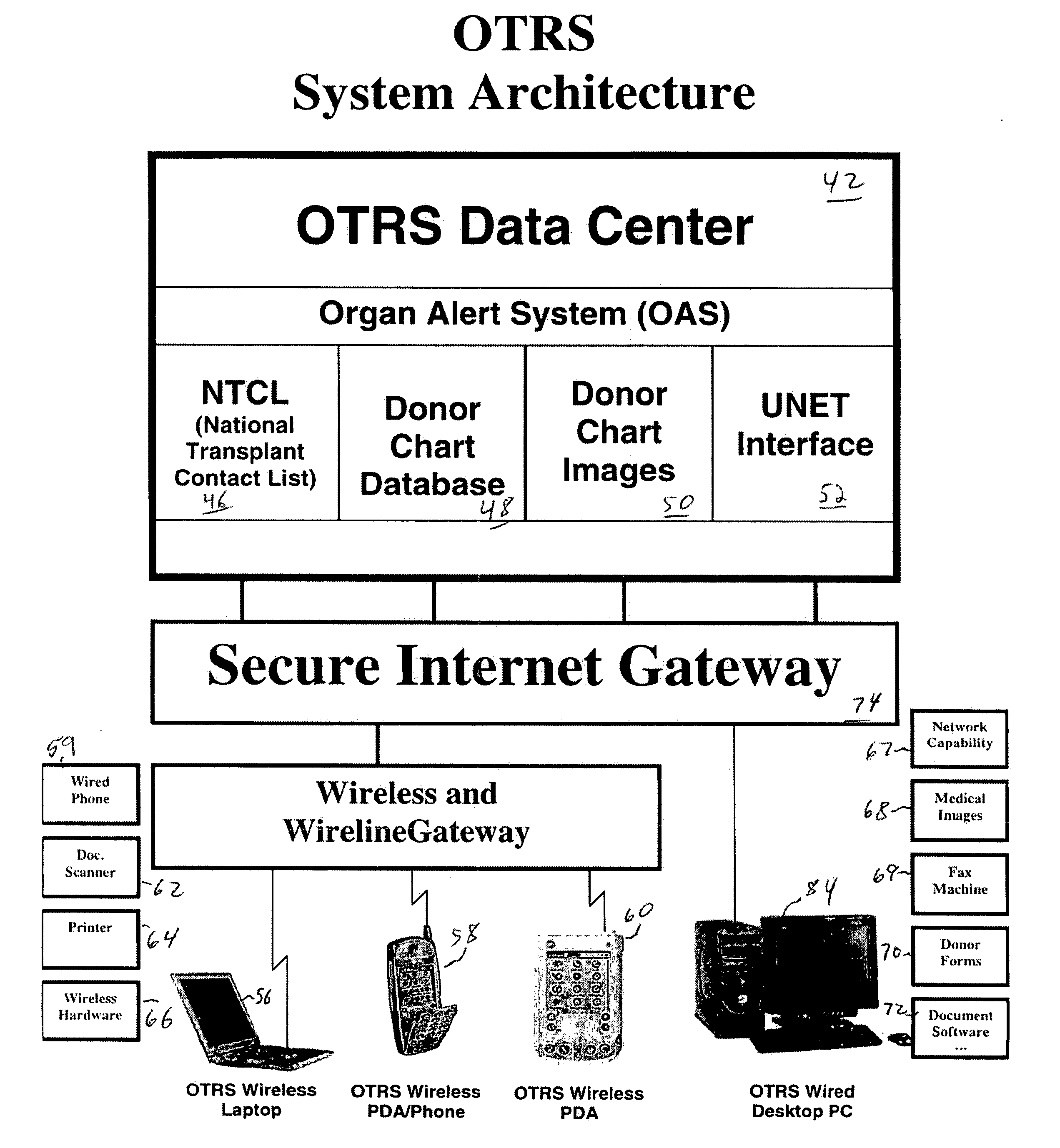
Secure network gateway for accessible patient data and transplant donor data
InactiveUS20060031098A1Shorten the timeQuality improvementSpecial service provision for substationTelemedicineThird partyPatient data
A method of organizing and making available transplant donor data, the method comprising the steps of: (a) providing a secure database to store transplant donor data; (b) providing access to a selective third party to at least one of upload and view transplant donor data and download and view transplant donor data; and (c) generating an authorization code required to access the transplant donor data by the selective third party.
Owner:KALTHOFF ROBERT MICHAEL +1
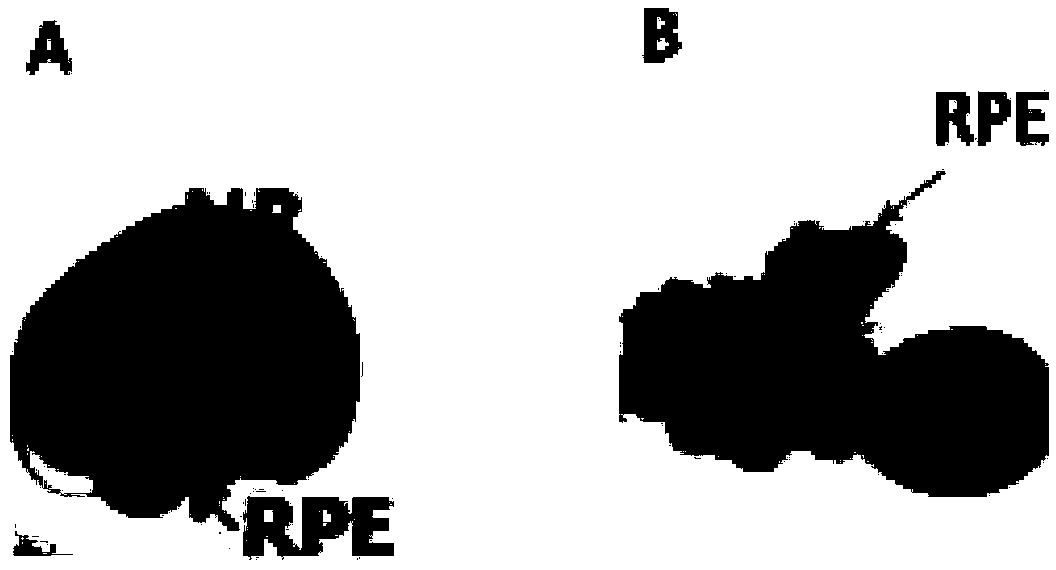
Preparation and amplification culture method of human multipotential stem cell sourced human retinal pigment epithelium cells
PendingCN108728413ATypical Morphological FeaturesAvoid damageCell dissociation methodsCulture processDiseaseEmbryo
The invention discloses a preparation and amplification culture method of human multipotential stem cell sourced human retinal pigment epithelium cells. The method comprises the following steps of collecting a 3D-RPE sphere from the human multipotential stem cell sources; performing mechanical separation, removing non-RPE cells or agglomerates containing no pigments; remaining RPE cell sheets containing the pigments; performing enzymolysis digestion on the pigment-containing RPE cell sheets to obtain RPE unicellular suspension; thus obtaining the human multipotential stem cell sourced human retinal pigment epithelium cells. The features of the human RPE cells prepared by the method are similar to the features of human embryo sourced RPE cells; the typical RPE cell form features are realized; the normal physiological functions are shown. The human RPE cells prepared by the method can realize the subculture; the mass amplification is realized; the seed cells are provided for the study and treatment of retinal diseases; benefits are brought to blind patients; the problem of important defects of limited retinal pigment epithelium cells and RPE transplanting donor lack in the prior artcan be solved; great significance is realized.
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV
Use of endovascular hypothermia in organ and/or tissue transplantations
InactiveUS20070213793A1Lower potentialDecreasing oxygen demandDiagnosticsSurgerySurgeryTissue transplantation
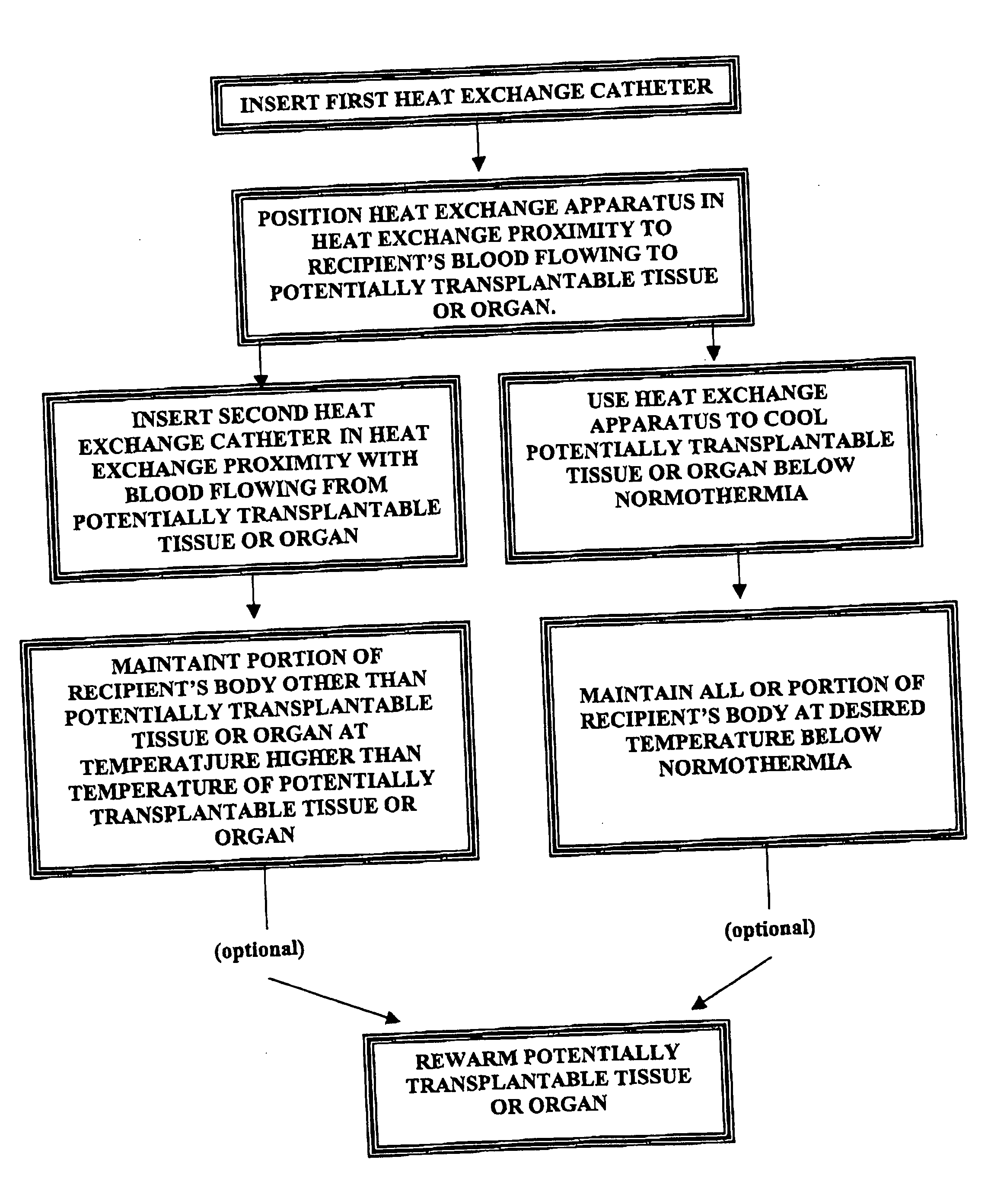
Methods for (a) preventing hypoxic damage to a potentially transplantable organ or tissue prior to explanation of that organ or tissue from the body of a mammalian transplant donor and (b) preventing rejection of a transplanted organ or tissue in a human or veterinary transplant recipient. The methods comprise placing a heat exchange apparatus in the vasculature of the donor or recipient and using that heat exchange apparatus to cool at least a portion of the body of the donor or recipient to a temperature below normothermia (e.g. below normothermia and sometimes between about 30° C. and about 36° C.).
Owner:ZOLL CIRCULATION
Secure network gateway for accessible patient data and transplant donor data
InactiveUS20050102161A1Shorten the timeQuality improvementSpecial service provision for substationTelemedicineCause of deathPatient data
A method of accessing transplant donor data from a remote location, the method comprising the steps of: (a) accessing a database over a network containing transplant donor data that includes information specific to a potential transplant donor; (b) reviewing at least one of information specific to the potential transplant donor, cause of death of the potential transplant donor, and time of death of the potential transplant donor; and (c) acting on the reviewed transplant donor data to establish qualification to at least one of an organ and a tissue available for transplant.
Owner:KALTHOFF ROBERT MICHAEL +1
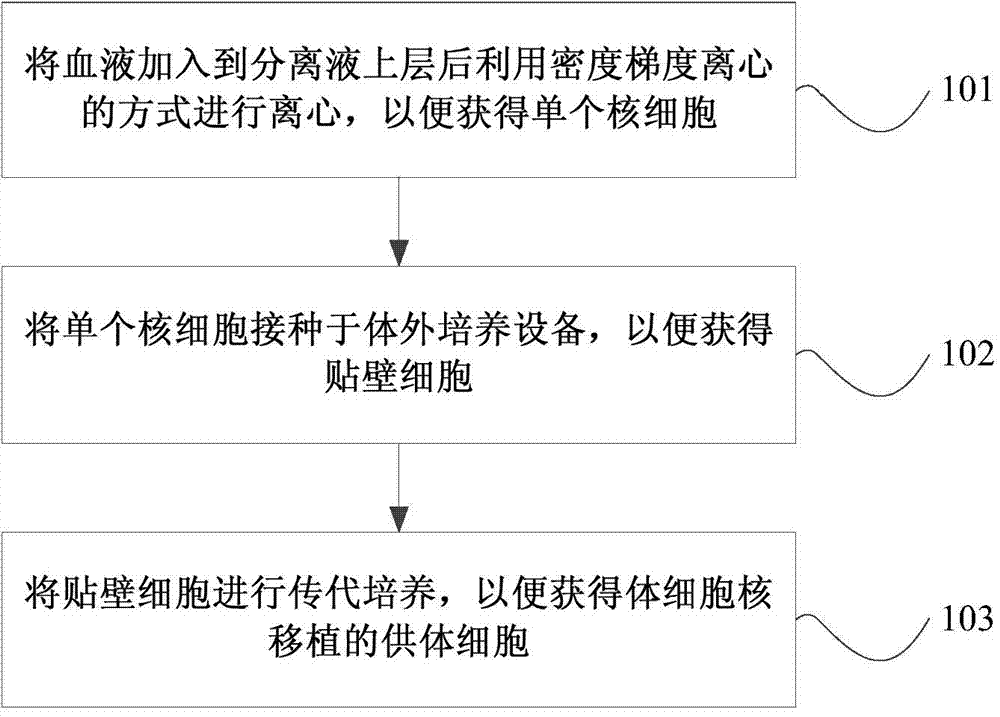
Method for separating cells from blood and cultivating the cells and method for cloning non-human animal
ActiveCN104513807AEasy to collectLess stressBlood/immune system cellsGenetic engineeringSomatic cellBiology
The invention provides a method for separating cells from blood and cultivating the cells and a method for cloning non-human animals. The method for separating cells from blood and cultivating the cells comprises following steps: (1) adding the blood to an upper layer of a separation liquid and performing centrifugation in a manner of density gradient centrifugation for obtaining mononuclear cells; (2) inoculating the mononuclear cells onto an in-vitro cultivation device containing an adherent cell culture medium to obtain adherent cells; and (3) performing serial subcultivation to the adherent cells to obtain somatic cell nuclear transplanted donor cells. The somatic cell nuclear transplanted donor cells obtained through the method can be directly used for manually cloning the non-human animals. The method is simple in operation processes, is easy to carry out, is low in cost, is less in stress stimulation and harm in animals, and can ensure a complete appearance of the animals and improve animal welfare.
Owner:深圳华大基因农业控股有限公司
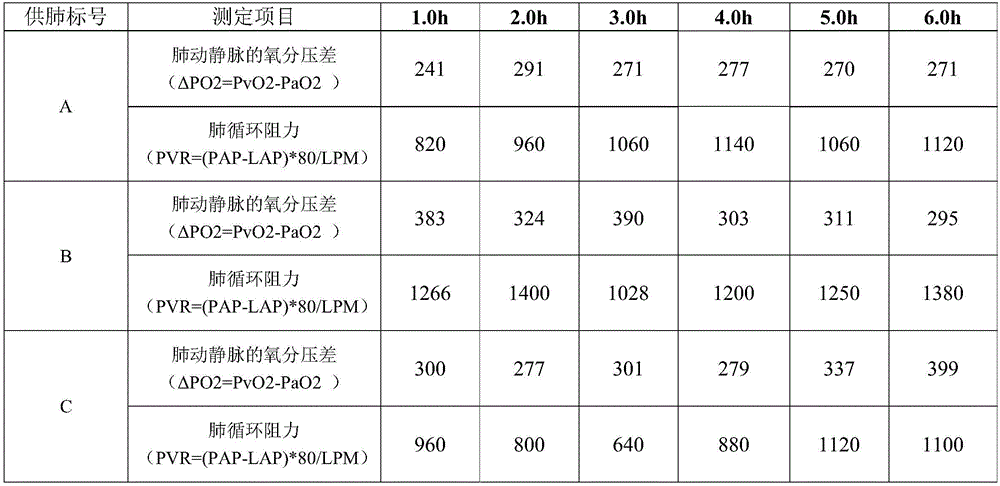
Lung perfusion fluid for perfusing transplant donor lung and preparation method for lung perfusion fluid
InactiveCN105918308AMeet perfusionMeet preservation needsDead animal preservationPulmonary complianceLung perfusion
The invention provides lung perfusion fluid for perfusing a transplant donor lung and a preparation method for the lung perfusion fluid. The lung perfusion fluid can be used for providing good lung protecting and resuscitating actions and can meet the requirements of the transplant donor lung on perfusion and preservation; meanwhile, the time of perfusion is relatively long, and the donor lung still can retain good lung compliance and oxygenation capability 6 hours after the donor lung is subjected to perfusion by the lung perfusion fluid provided by the invention; and finally, the preparation method for the lung perfusion fluid is simple, and raw materials are readily available and are economical and practical, so that the lung perfusion fluid is suitable for being popularized and applied to general laboratories or hospitals.
Owner:SHANGHAI CHEST HOSPITAL
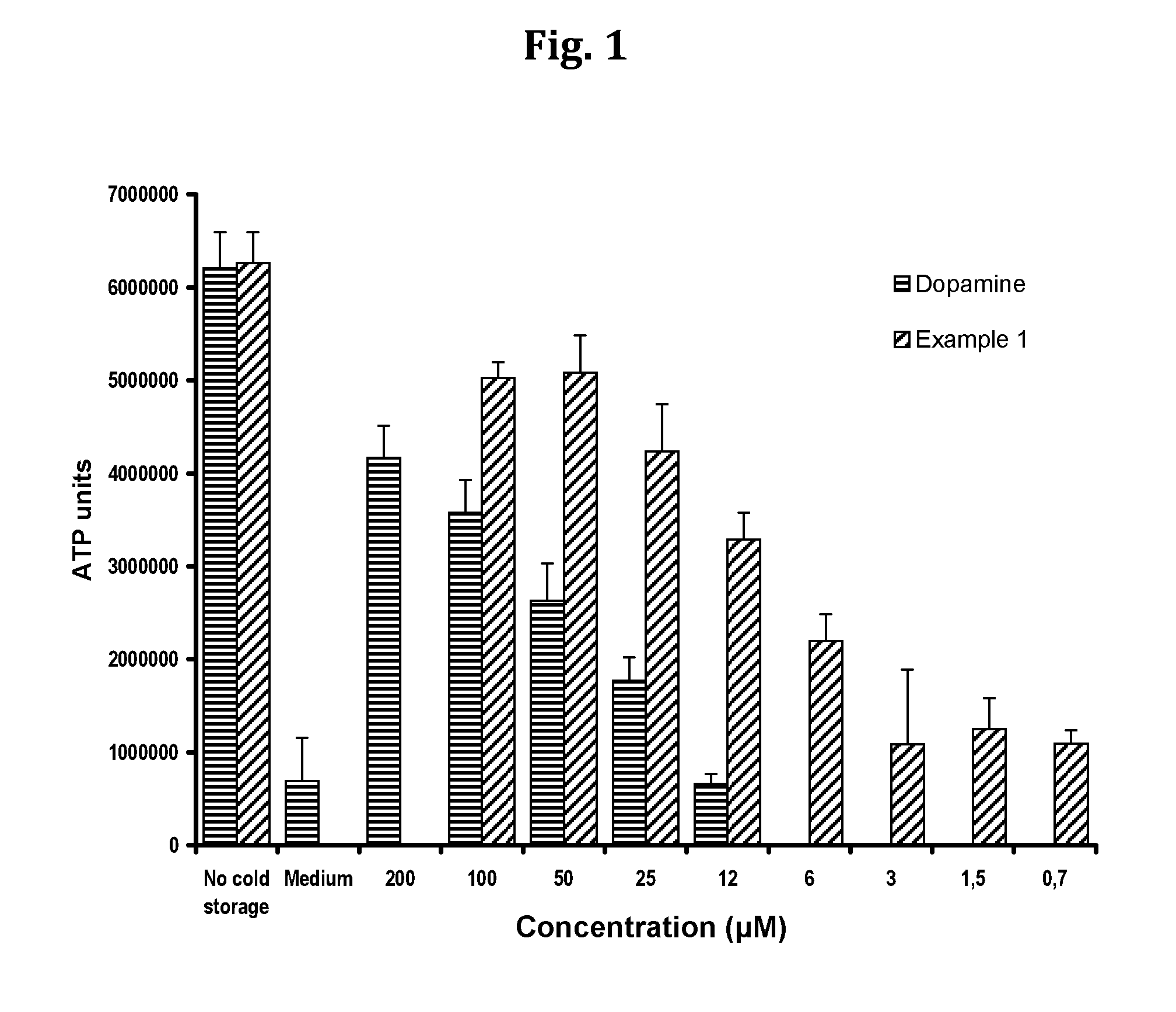
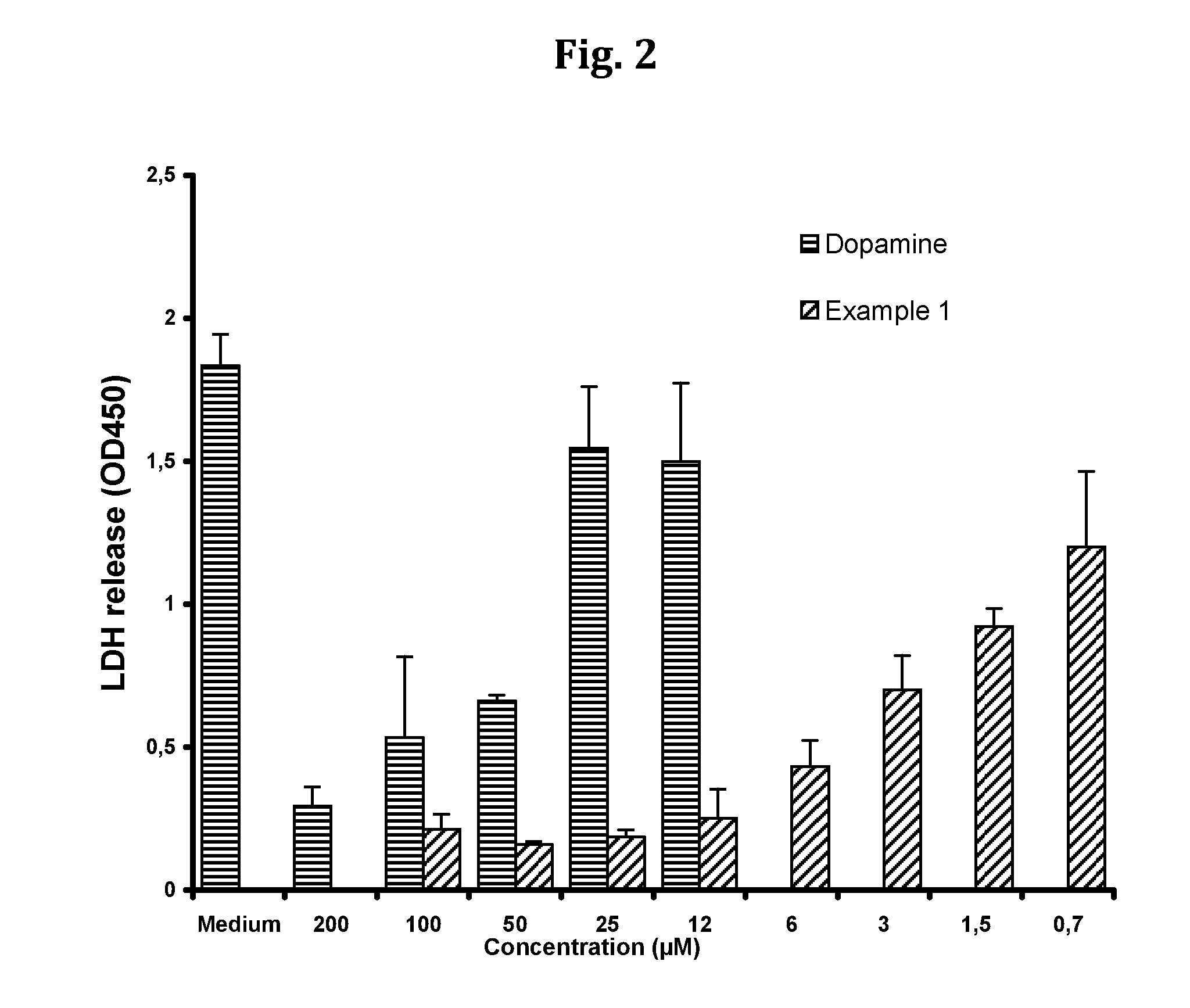
Compositions and methods for improved organ transplant preservation and acceptance
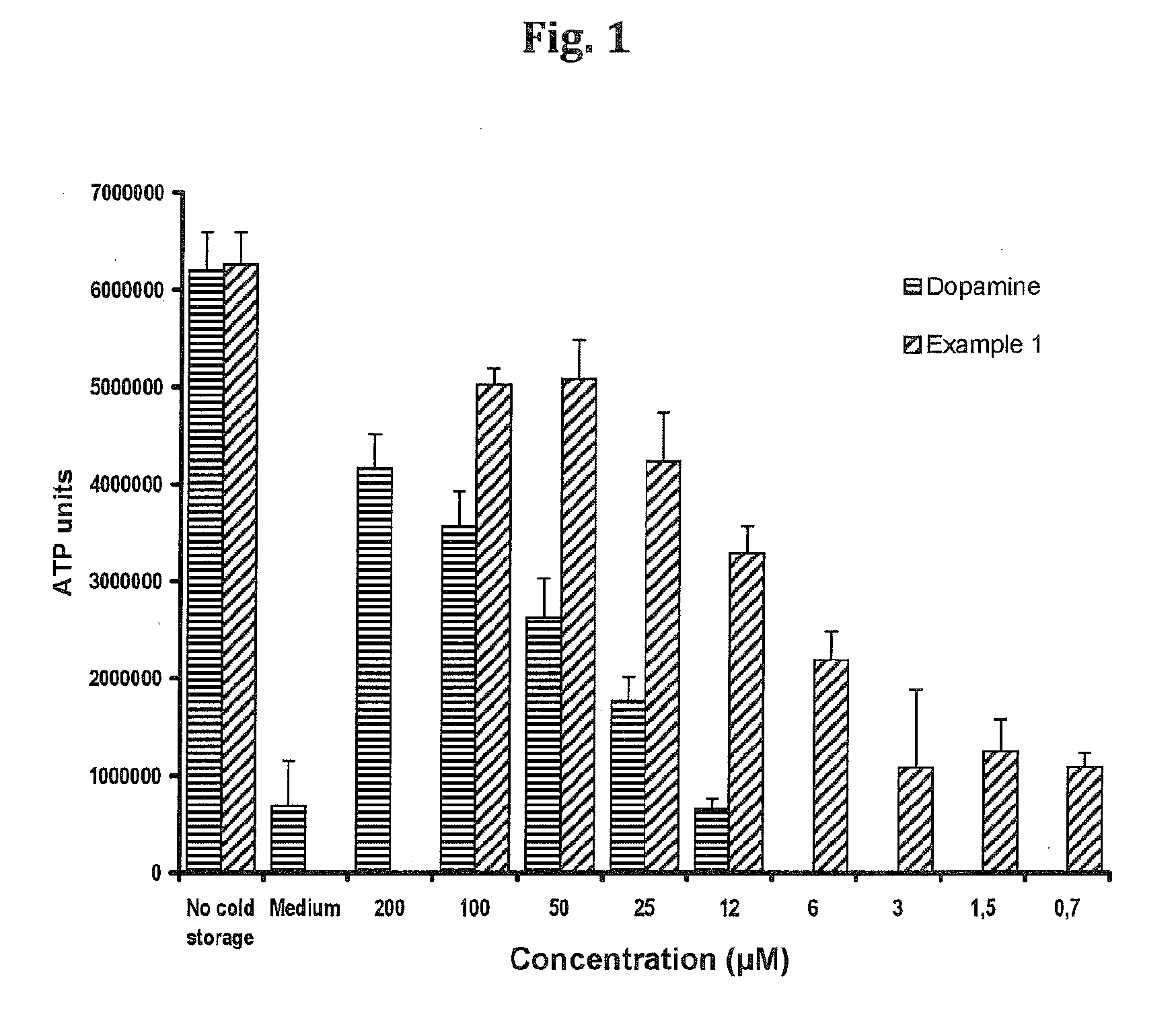
InactiveUS20130142866A1Minimise cold preservation injuryMinimize injuryBiocideDead animal preservationOrgan transplantingDopamine
The invention provides a novel aqueous composition for the storage and preservation of transplants, such as organ or tissue allografts. The composition comprises the compound N-octanoyl dopamine in solubilised form. The composition may also be administered as a pre-treatment of transplant donors. Moreover, it may be used in transplant recipients, optionally in combination with immunosuppressants.
Owner:NOVALIQ GMBH
Methods for determining the risk of acute graft versus host disease
ActiveUS20150301022A1Increased riskBiocidePhosphorous compound active ingredientsCandidate donorSub populations
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +4
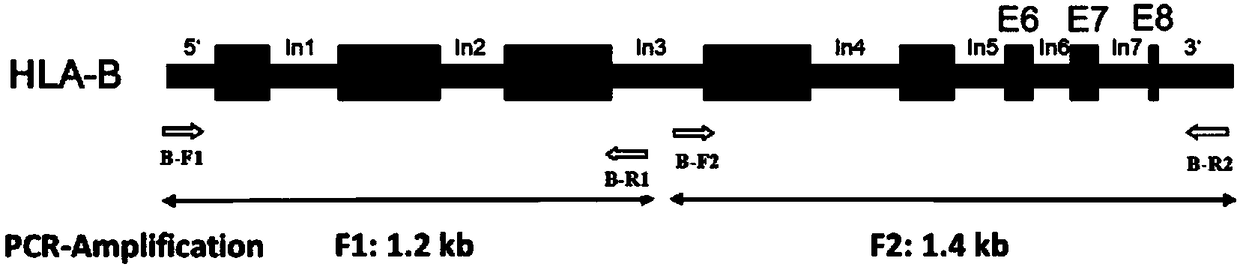
Primer group, kit and method for amplification of human leukocyte antigen-B (HLA)-B gene, and primer group, kit and method for genotyping of HLA-B gene
ActiveCN108531568AIncrease heightShorten the lengthMicrobiological testing/measurementDNA/RNA fragmentationPatient survivalOrgan transplantation
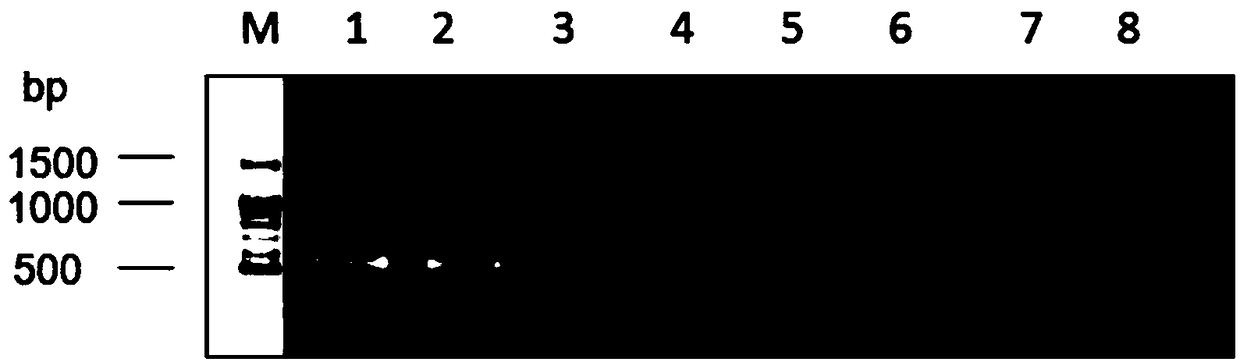
The invention discloses a primer group, a kit and a method for amplification of a human leukocyte antigen-B (HLA)-B gene, and the primer group, the kit and the method for genotyping of the HLA-B gene,belonging to the field of gene detection. According to the primer group, provided by the invention, for the amplification of the HLA-B gene, at least two sets of primers are designed according to theeight exon regions of the HLA-B gene; the primers are used for performing polymerase chain reaction (PCR) amplification on the HLA-B gene, so that a product having a gene fragment length of less than1.5 kb is obtained; after the HLA-B gene is subjected to the PCR amplification by the primers, the gene fragment length of the amplified product is less than 1.5 kb, so that the requirement for template integrity is reduced, the amplification efficiency is high, amplification reaction time is short, and the cost is low. The primer groups, the kits and the methods can meet the needs of amplification or genotyping of the HLA-B gene in mass samples, provide a more accurate basis for clinical HLA matching, provide suitable transplant donors for patients, reduce the rejection in a transplantationprocess and improve the success rate of organ transplantation and the patient survival rate.
Owner:BEIJING NUOSHI KANGYING MEDICAL TECH
Method for inducing selectively suppressed immune response to transplanted tissue or cells
InactiveUS7625557B2Raise the possibilitySuppress immune rejectionUltrasonic/sonic/infrasonic diagnosticsCompounds screening/testingAbnormal tissue growthDendritic cell
Transimmunization methods incorporating skin immunologic challenges are described for either selectively suppressing the immune response of recipients of transplanted tissue or cells or monitoring induced anti-cancer immunity. In one embodiment, skin from the transplant donor is allografted to the transplant recipient to induce an immunological response to the transplanted skin. A quantity of blood is taken from the recipient and treated to render the T cells in the blood apoptotic and to induce differentiation of blood monocytes into dendritic cells. The treated blood is incubated and administered to the recipient to induce formation of suppressor T cell clones which reduce the number of T cells attacking the transplanted tissue or organ. This tolerogenic approach can be complemented by also feeding the immature dendritic cells apoptotic or necrotic cells from the organ donor. In a second embodiment, dendritic cells loaded with tumor antigens are injected intradermally to monitor the anti-cancer immunity induced by Transimmunization.
Owner:YALE UNIV
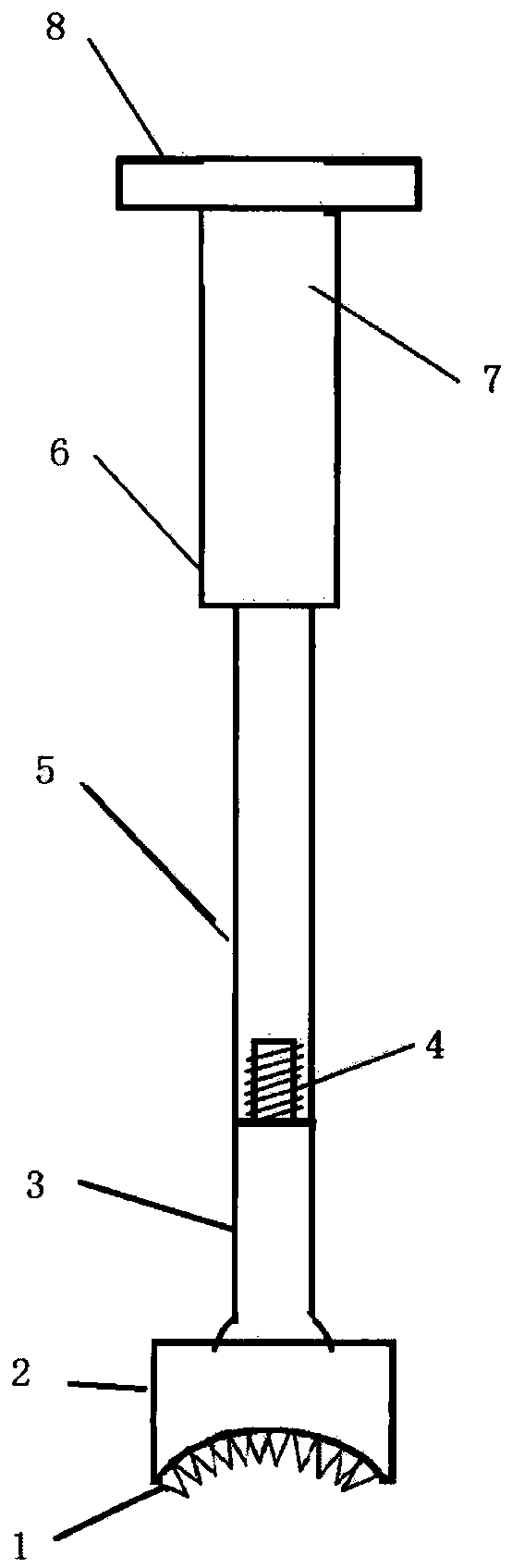
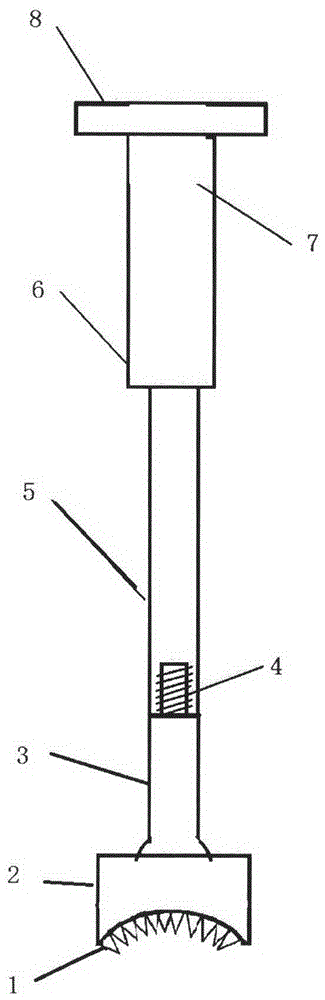
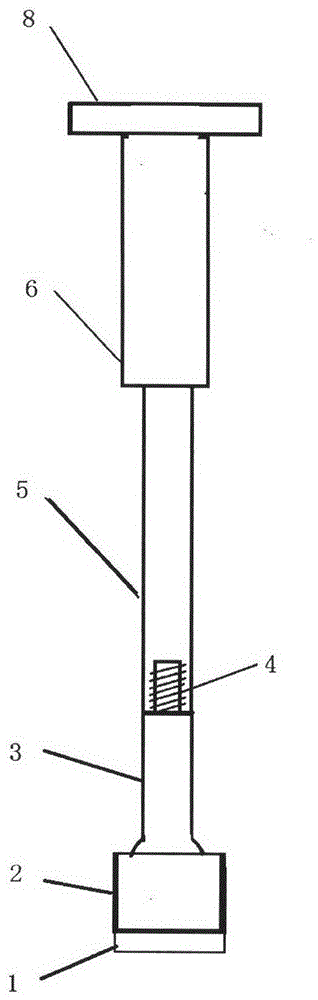
Medical forming device for gristle transplanting receptor spongy bone bed
The invention relates to a medical forming device for a gristle transplanting receptor spongy bone bed. Multiple knife edges are connected to a knife head base connected with a knife head connecting rod through a universal ball joint. The knife head connecting rod is connected with a handle connecting rod and a handle, the handle is connected with a handle stopping block, and the handle stopping block is connected with a manual sliding impacting device or an electric impacting device. The defects that in the prior art, because a bone knife, a bone chisel and other simple tools are used for manually trimming, the bone cutting face is not flat, randomness is high, and the bone cutting face of a receptor and the bone cutting face of a donor are prone to being mismatched, which is adverse to bone healing are overcome. The receptor spongy bone bed can be formed through one-off impacting, so that forming efficiency is improved, operation time is shortened, and the form of the receptor spongy bone bed is kept uniform. The manual sliding impacting device and the electric impacting device can be selected for use, so that the form of the spongy bone, after cutting, below the transplanted donor gristle is completely matched with that of the receptor sponge bone bed, a transplanted donor and a receptor are completely matched, and bone healing is facilitated.
Owner:THE AFFILIATED DRUM TOWER HOSPITAL MEDICAL SCHOOL OF NANJING UNIV
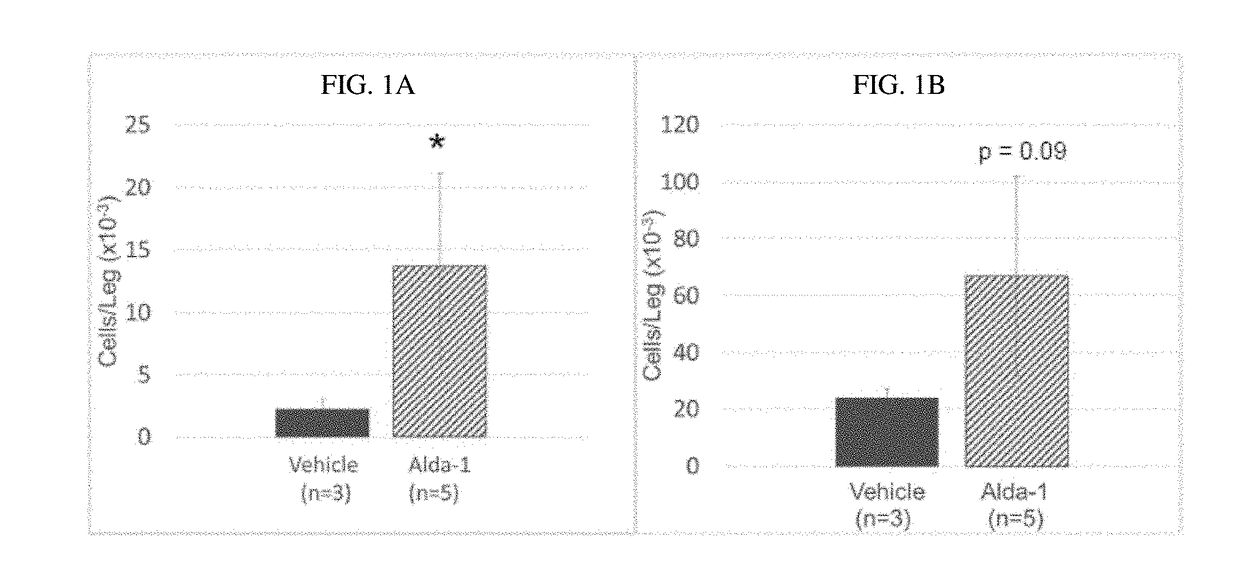
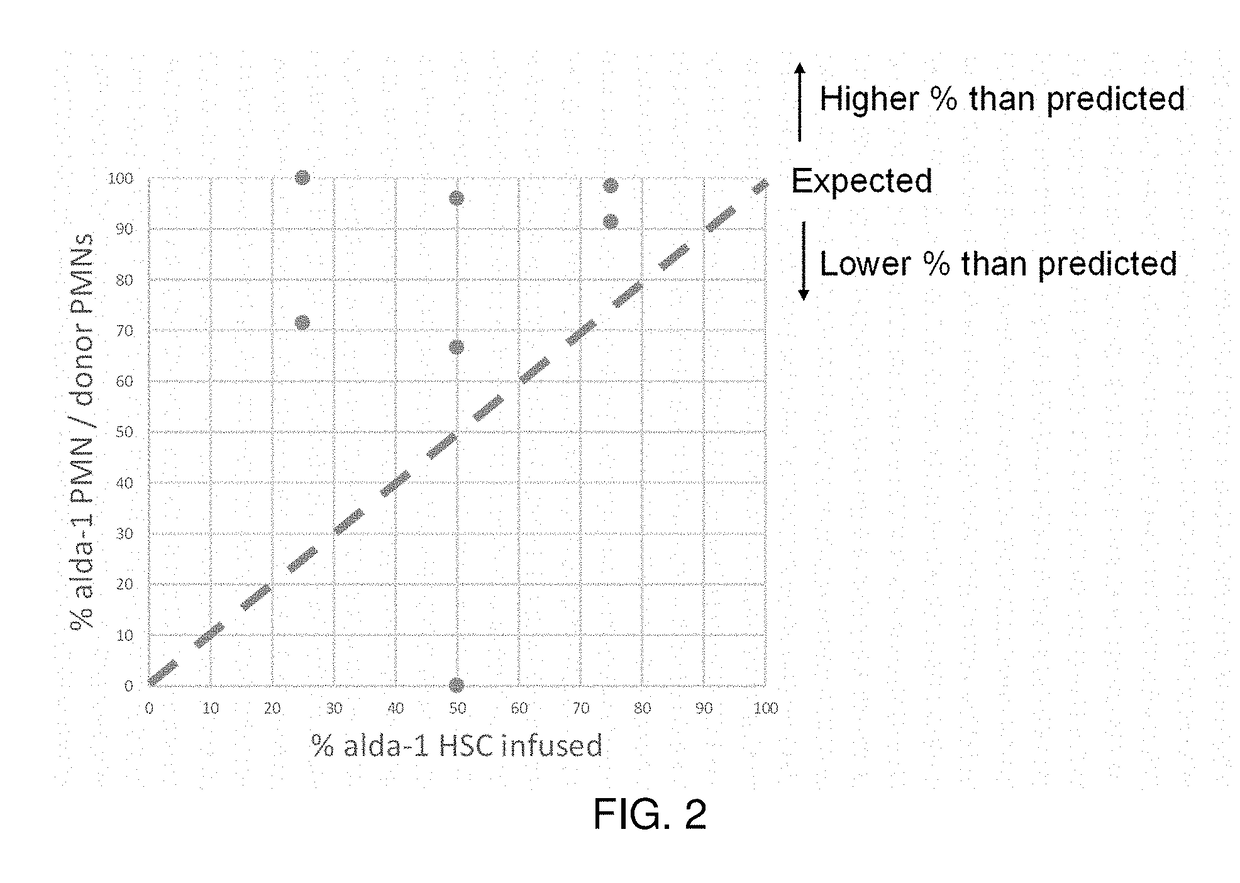
Mitochondrial aldehyde dehydrogenase-2 modulators for protecting, expanding and increasing the potency of hematopoietic stem cells
InactiveUS20190083457A1Improve effectivenessIncreasing the potency of hematopoietic cellsBlood/immune system cellsCell culture active agentsDiseaseHematopoietic cell
The present disclosure provides methods of protecting and expanding hematopoietic cells. The present disclosure also provides methods of increasing the potency of hematopoietic cells. Aspects of the methods include contacting a starting population of hematopoietic cells with a therapeutically effective amount of at least one ALDH2 agonist. Aspects include in vivo, in vitro and ex vivo methods of protecting, expanding and increasing the potency of hematopoietic cells. Aspects of the methods include treating an individual who is undergoing chemotherapy or radiation treatment for cancer, has been exposed to damaging toxins, has a genetic disease that leads to HSC damage, bone marrow failure, an autoimmune disease, or development of hematologic malignancies. Aspects of the methods also include treating a healthy individual who is a stem cell transplant donor.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Human umbilical cord tissue cells for inhibiting adverse immune response in histocompatibility-mismatched transplantation
Cell-based compositions and methods of their use to inhibit an adverse immune response such as graft versus host disease or rejection of transplanted tissue in a transplant recipient that is histocompatibility mismatched to the transplant donor are disclosed. The compositions and methods utilize postpartum-derived cells, such as cells derived from the placenta or umbilicus.
Owner:DEPUY SYNTHES PROD INC
Method for inducing selectively suppressed immune response to transplanted tissue or cells
InactiveUS7988951B2Raise the possibilitySuppress immune rejectionCompounds screening/testingUltrasonic/sonic/infrasonic diagnosticsAbnormal tissue growthT-cell apoptosis
Transimmunization methods incorporating skin immunologic challenges are described for either selectively suppressing the immune response of recipients of transplanted tissue or cells or monitoring induced anti-cancer immunity. In one embodiment, skin from the transplant donor is allografted to the transplant recipient to induce an immunological response to the transplanted skin. A quantity of blood is taken from the recipient and treated to render the T cells in the blood apoptotic and to induce differentiation of blood monocytes into dendritic cells. The treated blood is incubated and administered to the recipient to induce formation of suppressor T cell clones which reduce the number of T cells attacking the transplanted tissue or organ. This tolerogenic approach can be complemented by also feeding the immature dendritic cells apoptotic or necrotic cells from the organ donor. In a second embodiment, dendritic cells loaded with tumor antigens are injected intradermally to monitor the anti-cancer immunity induced by Transimmunization.
Owner:YALE UNIV
Kidney transplant donor-specific urine source cell and its DNA preparation method and application thereof
PendingCN110305835AEasy to sequence and analyzeImprove separation efficiencyMicrobiological testing/measurementArtificial cell constructsGenomicsPenicillin
The invention relates to a kidney transplant donor-specific urine source cell and its DNA preparation method, and an application in kidney transplant donor genomics background analysis. The preparation method of the urine source cell of the invention comprises the following steps: (1) taking a middle section of urine of a kidney transplant acceptor, centrifuging the material, and discarding a supernatant; (2) adding a phosphate buffer solution containing a penicillin streptomycin mixture or a primary cell antibiotic to a lower layer obtained by centrifugation, performing resuspending, and thencentrifuging the material again, discarding the supernatant; (3) adding a urine source cell culture medium to a lower layer precipitate obtained by centrifugation, and performing resuspending to obtain a cell suspension; and (4) inoculating the obtained cell suspension into a culture vessel, and performing the cell amplification in vitro to obtain the kidney-transplant donor-specific urine sourcecell. The method obtains the donor-specific urine source cell based on kidney transplant postoperative acceptor urine, obtains donor DNA, performs donor genomics background analysis, is safe, non-invasive, low-cost, and highly efficient for separation, and is easy to obtain a sufficient amount of DNA.
Owner:北京博富瑞基因诊断技术有限公司
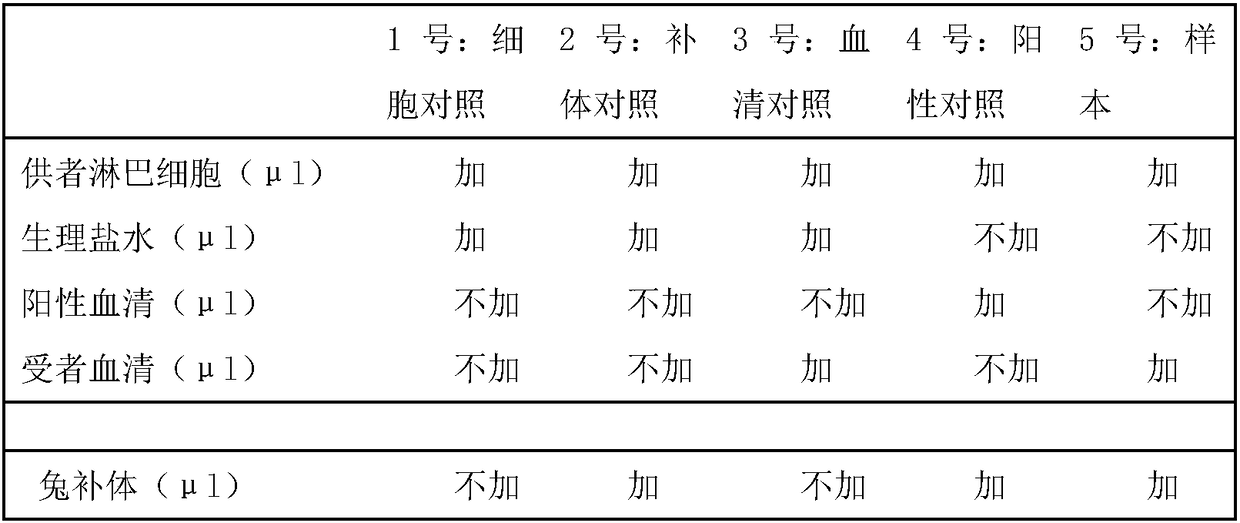
Method and kit for cross-over lymphatic toxicity experiment
InactiveCN108663512ASpeed up the reaction processHigh sensitivityMaterial analysisSerum igeMedicine
The invention discloses a method for a cross-over lymphatic toxicity experiment. The method comprises the following steps: (1) taking the venous anticoagulant whole blood of a transplant donor and theserum of a transplant recipient, and separating donor lymphocytes from the venous anticoagulant whole blood of the transplant donor; (2) taking a donor lymphocyte fluid, carrying out staining and marking, and adjusting the cell concentration; (3) taking and dissolving lyophilized complement; (4) taking five reaction tubes, sequentially adding two or three of the donor lymphocytes, normal saline,positive serum and the recipient serum, carrying out uniform mixing, standing the obtained mixture, adding a fifth reagent rabbit complement, uniformly mixing the mixture and the rabbit complement, and standing the new mixture; and (5) staining the new mixture, and loading the obtained sample. The invention also discloses a detection kit for the cross-over lymphatic toxicity experiment. The methodand the kit have the advantages of low toxicity, low cost, high detection accuracy and excellent clinical application prospect.
Owner:SICHUAN PROVINCIAL PEOPLES HOSPITAL
Method for preparing fecal microbiota transplantation capsule containing benzoylaconine by utilizing human fecal microbiota fermented aconite roots
InactiveCN110742929AMaximum releaseGood heartAntipyreticAnalgesicsIntestinal microorganismsCurative effect
The invention relates to a method for preparing a fecal microbiota transplantation capsule containing benzoylaconine by utilizing human fecal microbiota fermented aconite roots. Fecal microbiota transplantation (FMT) means that a functional flora in healthy human feces is transplanted into gastrointestinal tracts of a patient to re-establish a new intestinal flora so as to realize treatment on intestinal and parenteral diseases. Clinical application discovers that a required curative effect cannot be achieved after certain patients take the prepared aconite roots, one of main reasons causing individual differences is incomplete intestinal flora of the patient, and toxic substances in the prepared aconite roots cannot be normally metabolized into non-toxic active ingredients, the active ingredients have low utilization, and the required curative effect of the prepared aconite roots cannot be achieved. Healthy high-quality feces of a fecal microbiota transplant donor are utilized, are used for simulating intestinal microbes in vitro to achieve an effect of transforming the prepared aconite roots, aconitine and other toxic ingredients in the prepared aconite roots can be transformed into nontoxic metabolitic products through proper fermentation conditions, and the content of benzoylaconine and other active ingredients is increased. The fecal microbiota capsules prepared from fecalmicrobiota fermentation liquid can be used in fecal microbiota transplantation treatment in clinical application.
Owner:广东南芯医疗科技有限公司
Methods for determining the risk of acute graft versus host disease
ActiveUS10151745B2Enhance and regulate activityRapid responseDisease diagnosisBiological testingCandidate donorSub populations
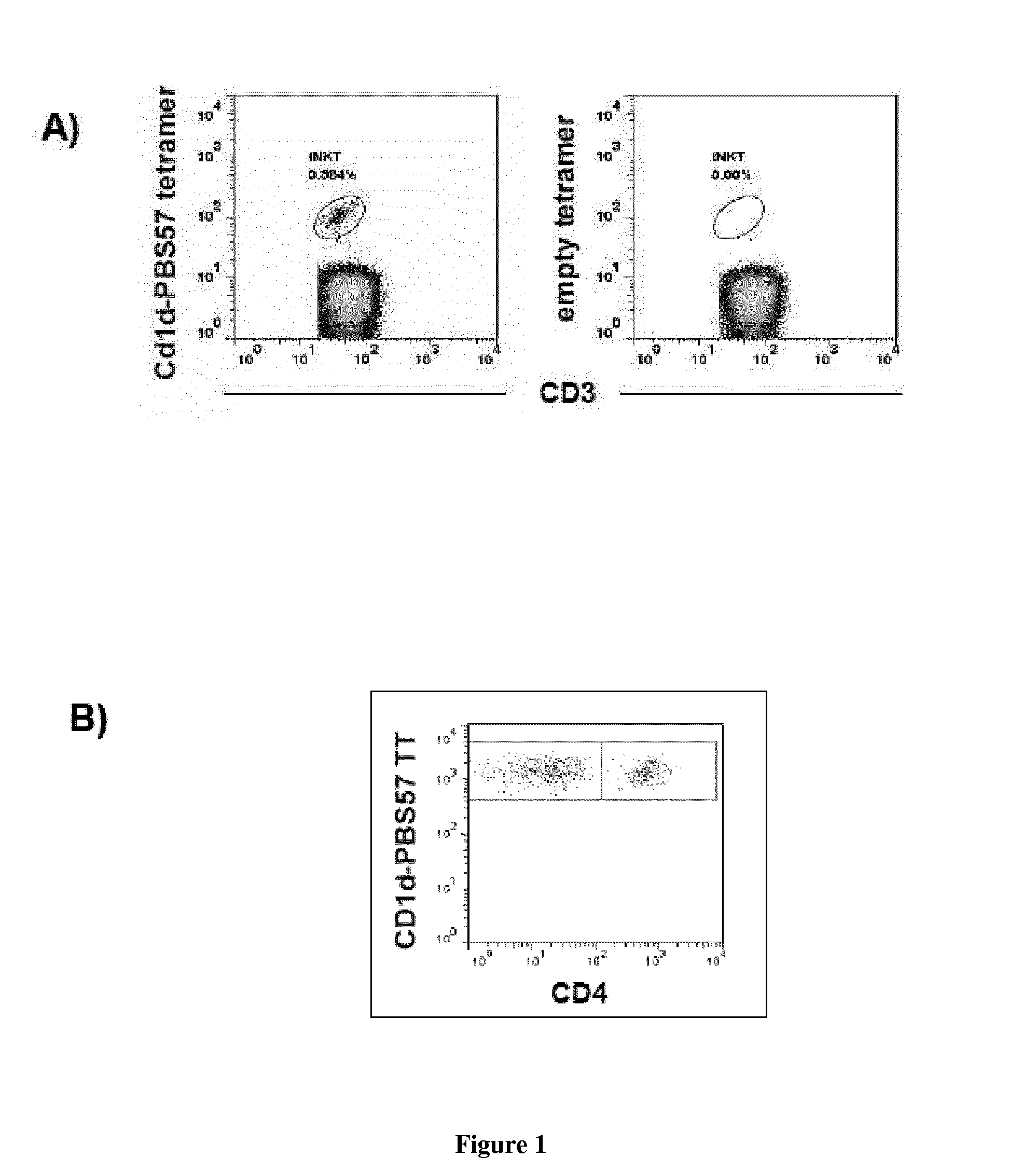
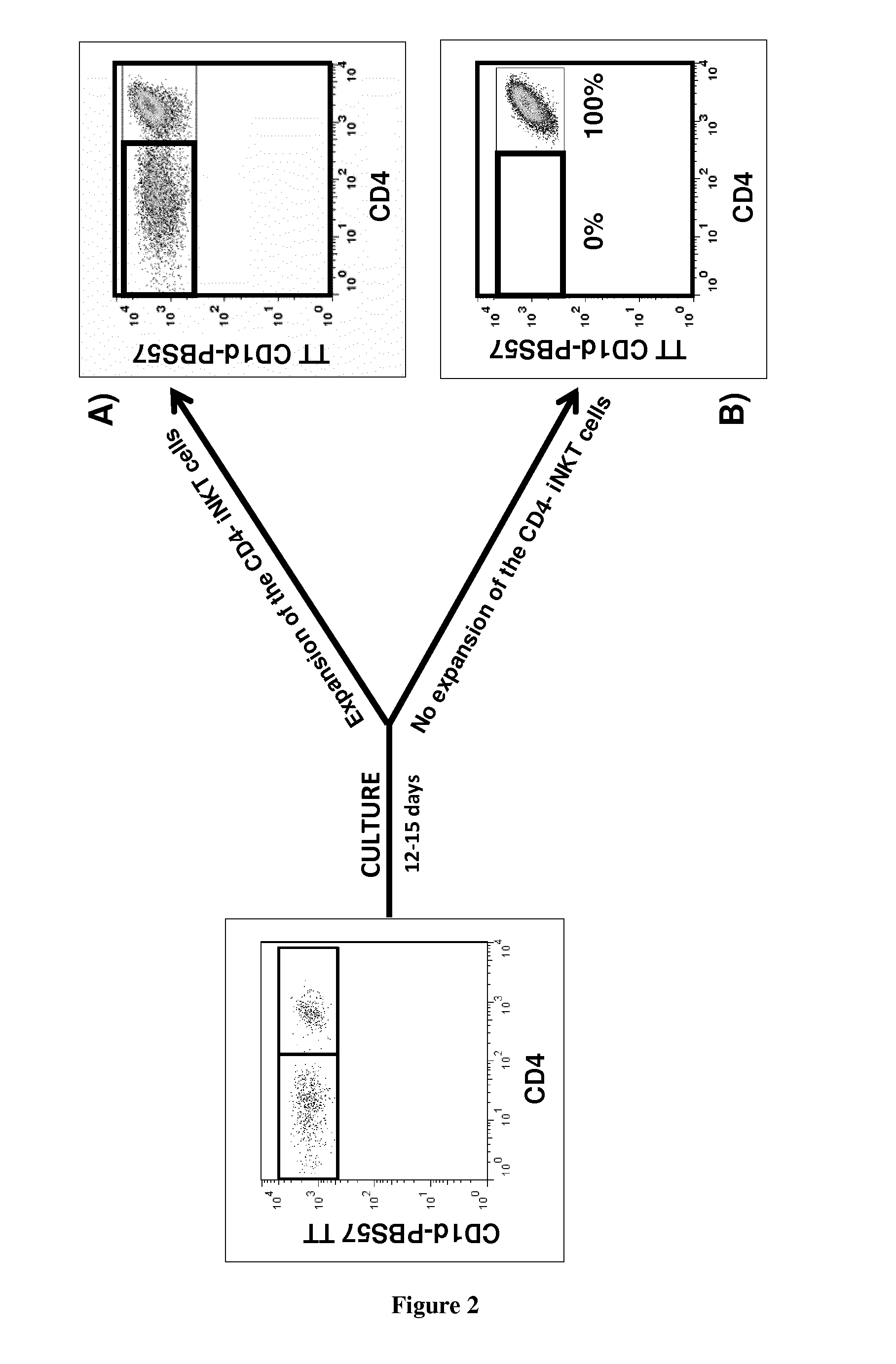
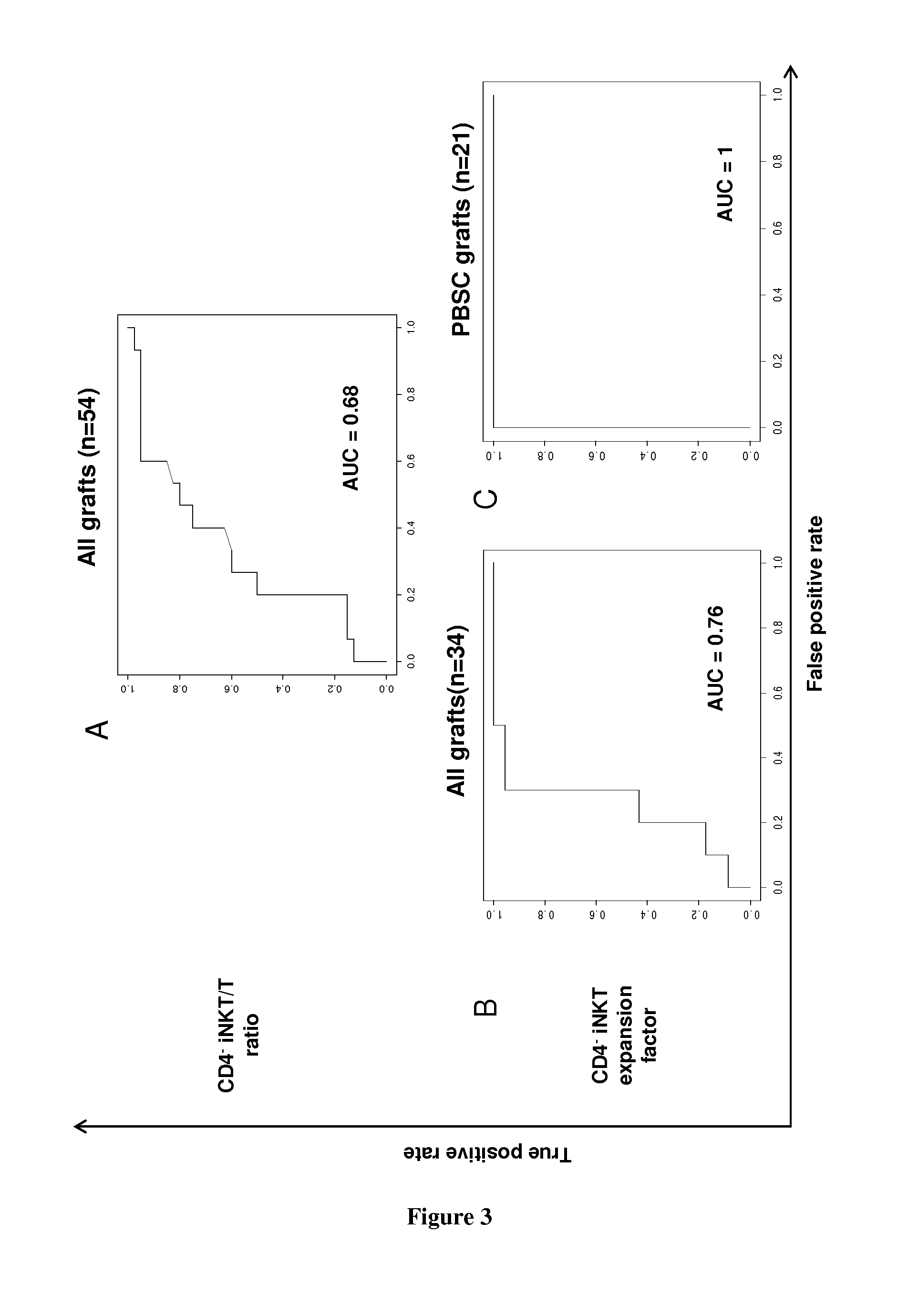
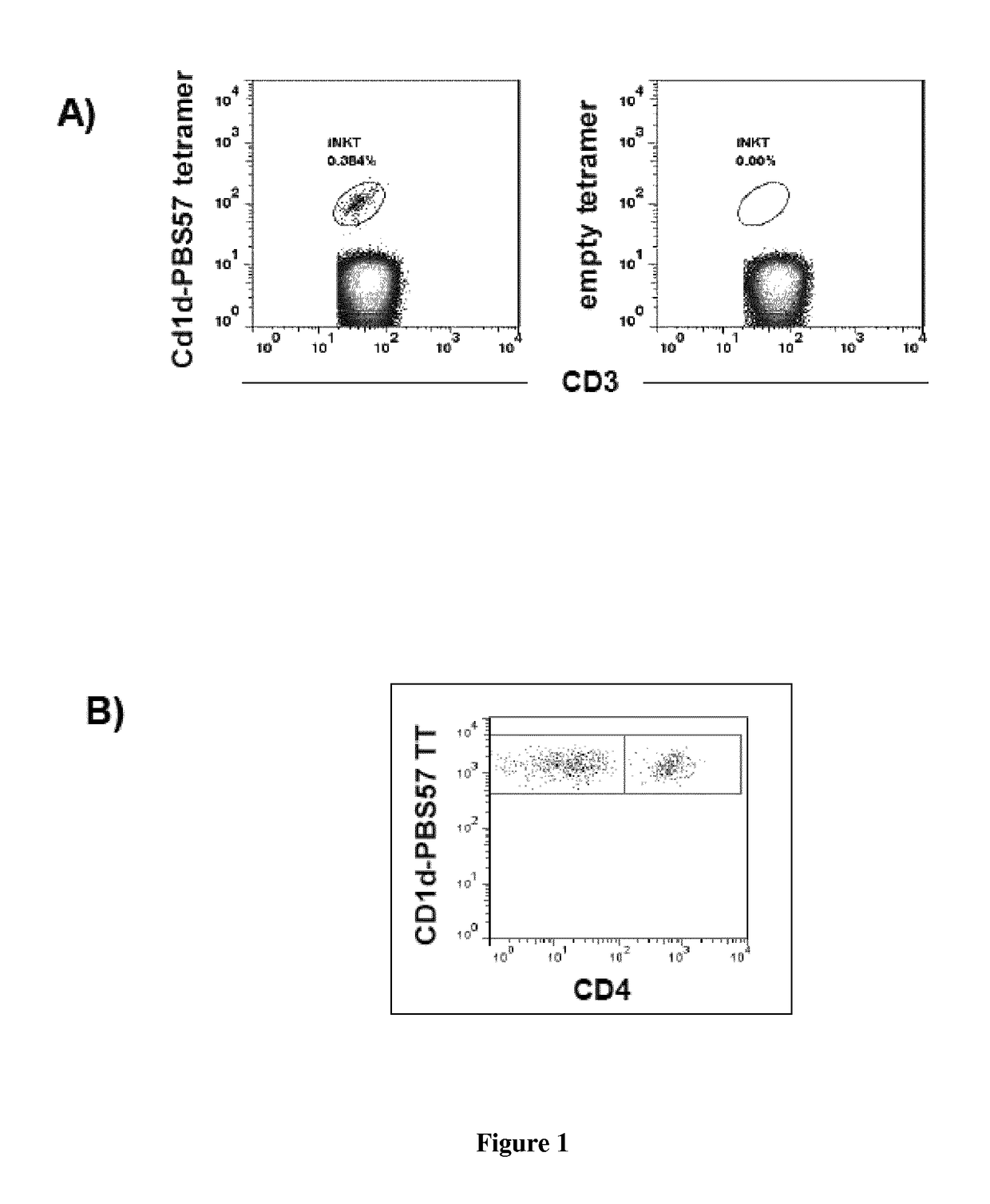
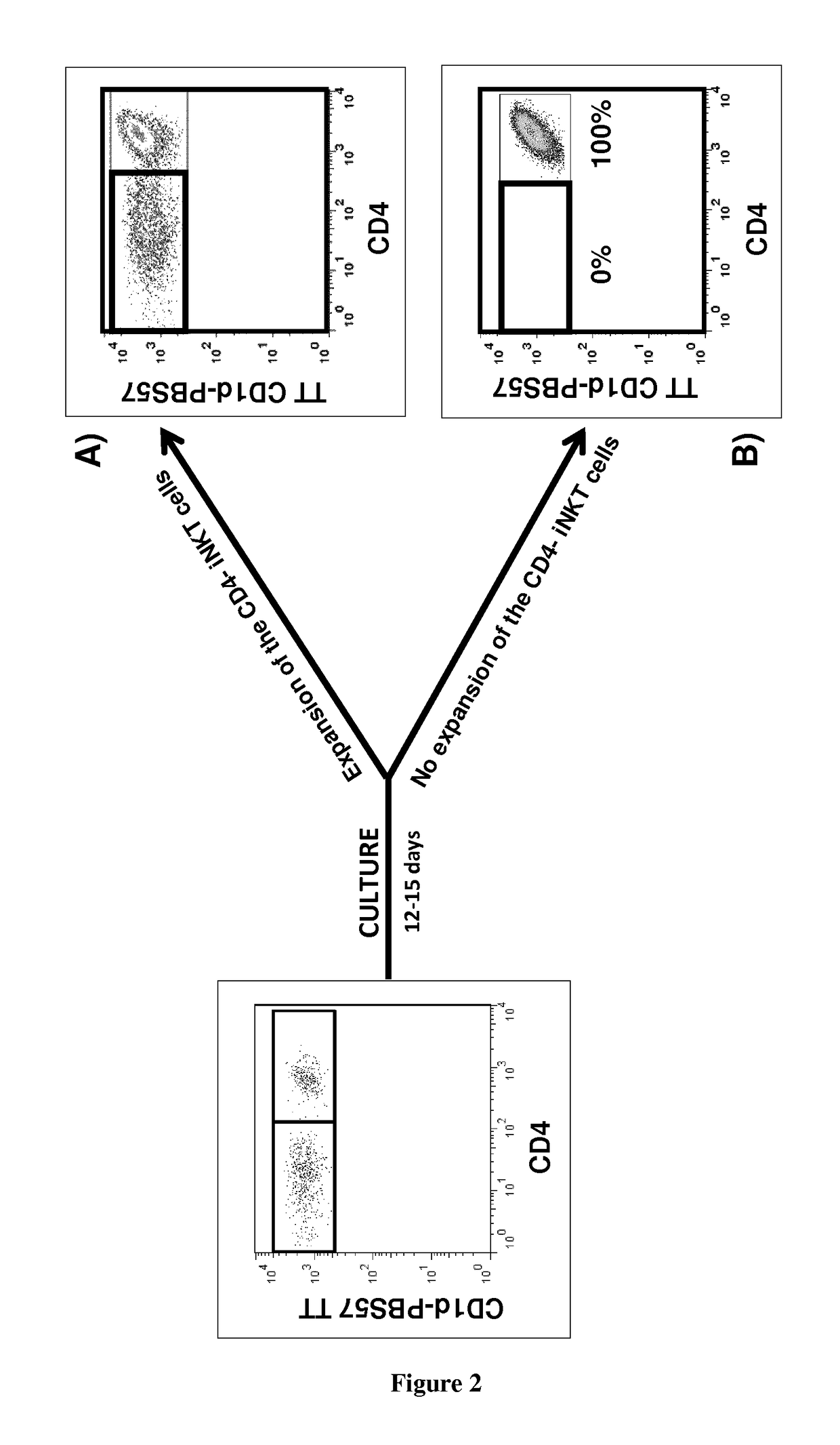
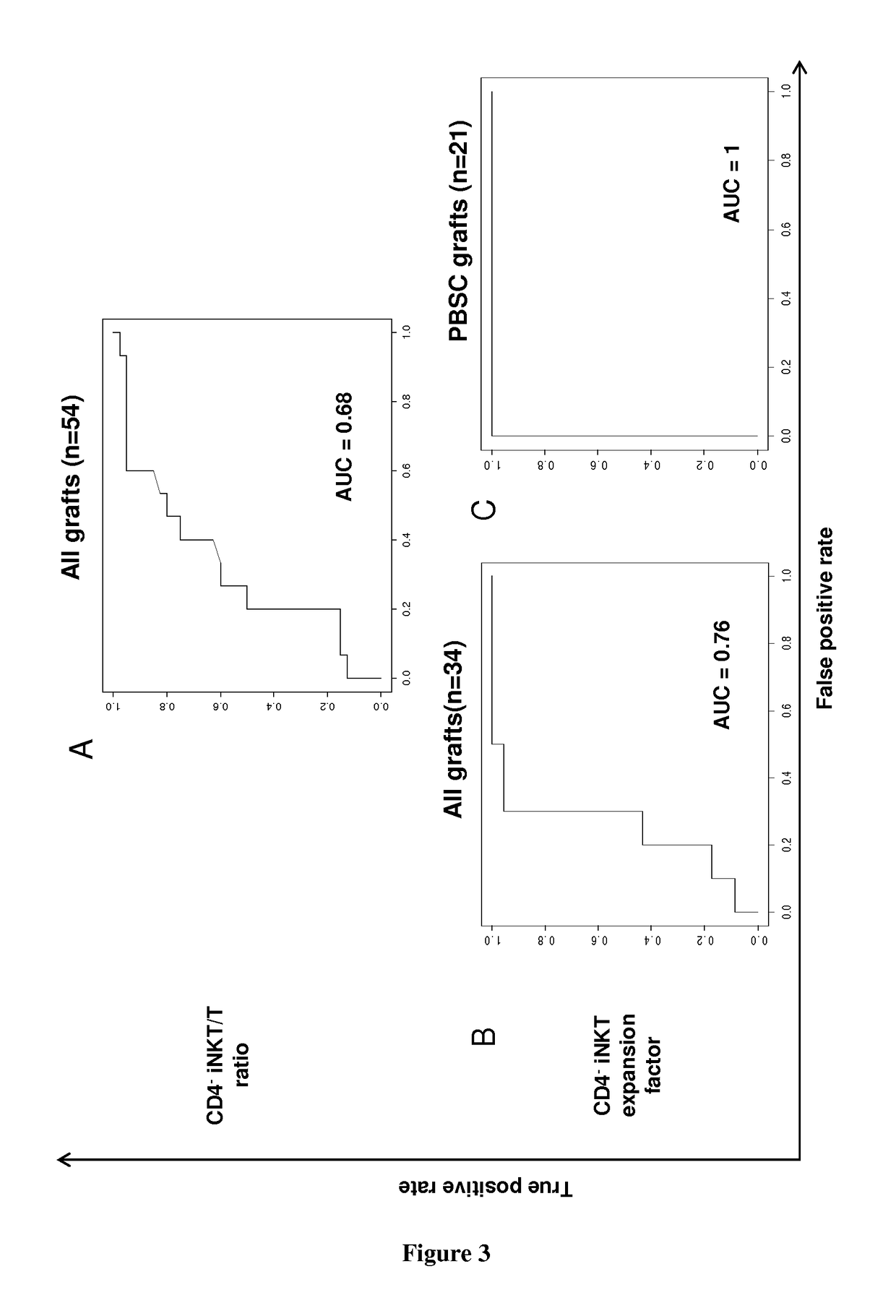
The present invention relates to a method for determining whether a candidate human transplant donor is at risk of inducing acute graft versus host disease (aGVHD) in a human transplant recipient, which may in turn allow the selection of a donor exhibiting no risk for the recipient. The present invention also relates to a method for adjusting the immunosuppressive treatment administered to a human transplanted recipient following its graft transplantation after having performing the method for determining risk of the invention. The methods comprise expanding the candidate donor's iNKT cells (invariant NKT cells) and determining the presence or absence of expansion of the CD4(−) iNKT cell sub-population. In particular, CD3+CD4− TCRV[alpha]24V[beta]11 cells are determined. Kits are disclosed.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +4
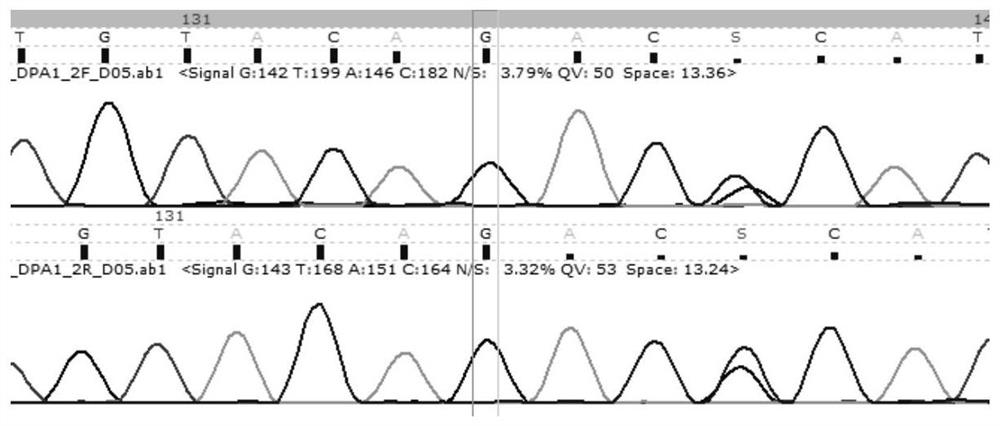
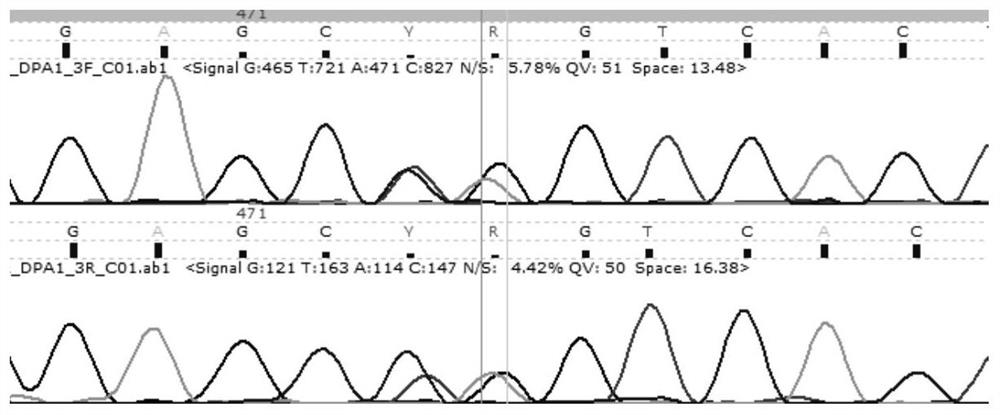
HLA-DPA1 gene typing kit
PendingCN111662967AAccurate allelic typingComplete and reliable allelic typing methodMicrobiological testing/measurementDNA/RNA fragmentationTyping methodsHematopoietic stem cell transplantation
The invention discloses an HLA-DPA1 gene typing kit. The HLA-DPA1 gene typing kit comprises two amplification primers and four sequencing primers shown in SEQ ID NO. 3, 4, 8, 9, 10 and 11. The kit canserve as an independent and widely-applied identification method, can successfully achieve accurate typing identification of HLA-DPA1 sites, can complete full-length amplification of HLA-DPA1 genes at a time, and then conducts double-end sequencing on 2 and 3 exons. Compared with the prior art, the HLA-DPA1 gene typing kit has the advantages that the steps are simple, the typing speed is high, typing is accurate, a report can be issued within three days, and the kit can be compatible with first-generation and second-generation sequencing platforms. The improvement of a typing method is beneficial for improving the accuracy of HLA matching of hematopoietic stem cell transplantation donors and recipients, therefore, more suitable transplantation donors are selected, the rejection reaction in the transplantation process is reduced, and evidence can be provided for clinicians to diagnose disease occurrence.
Owner:银丰基因科技有限公司 +1
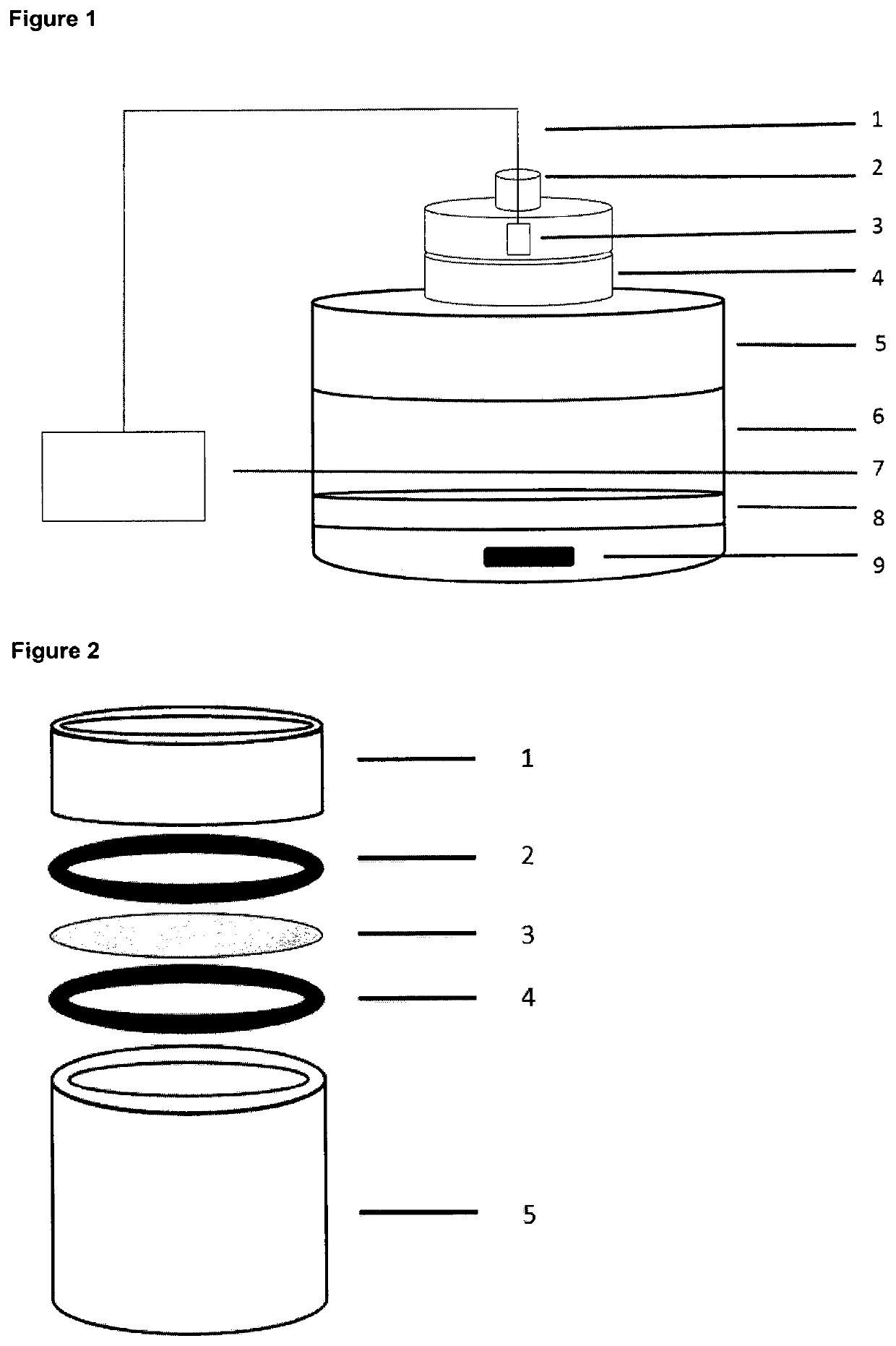
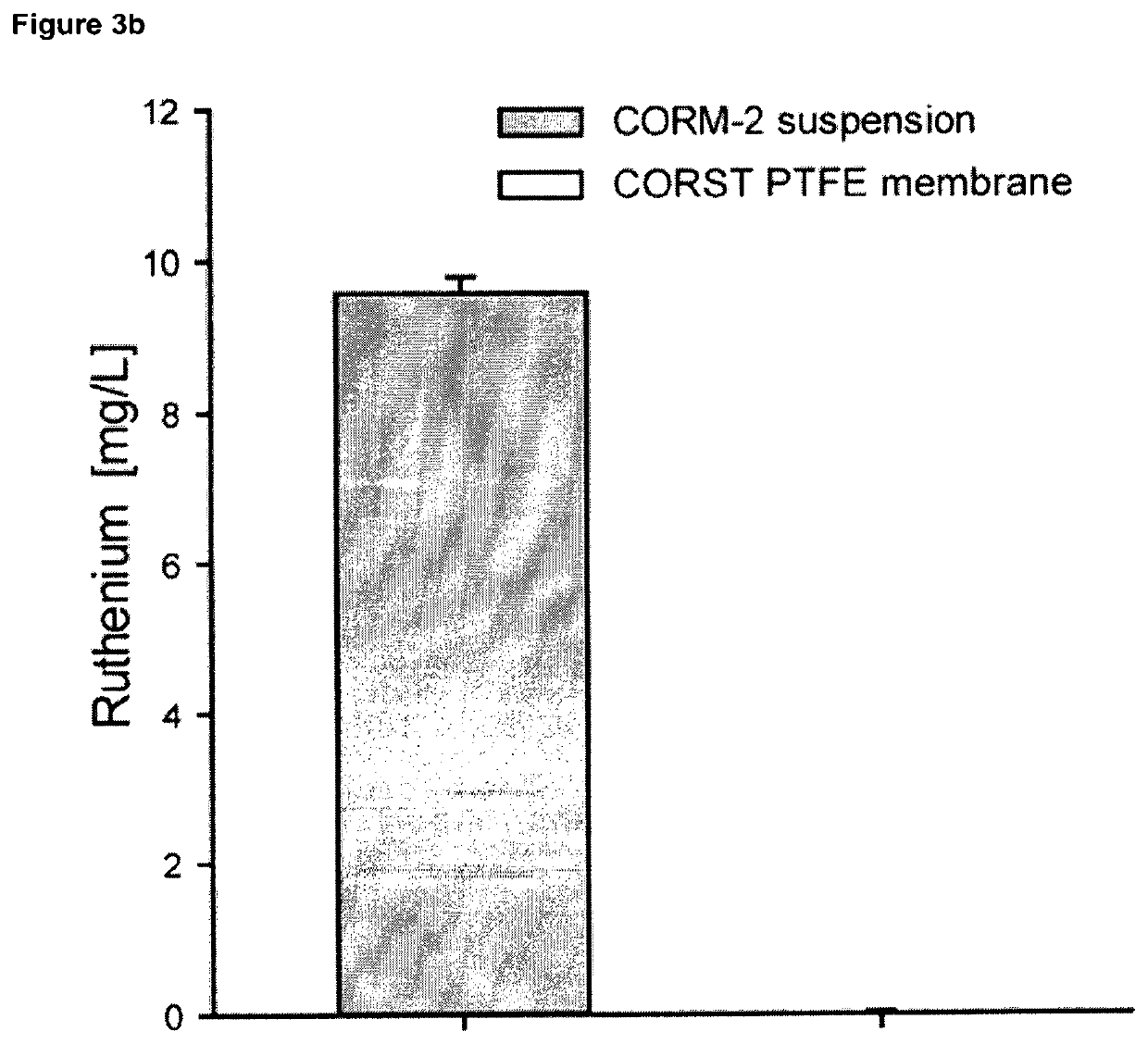
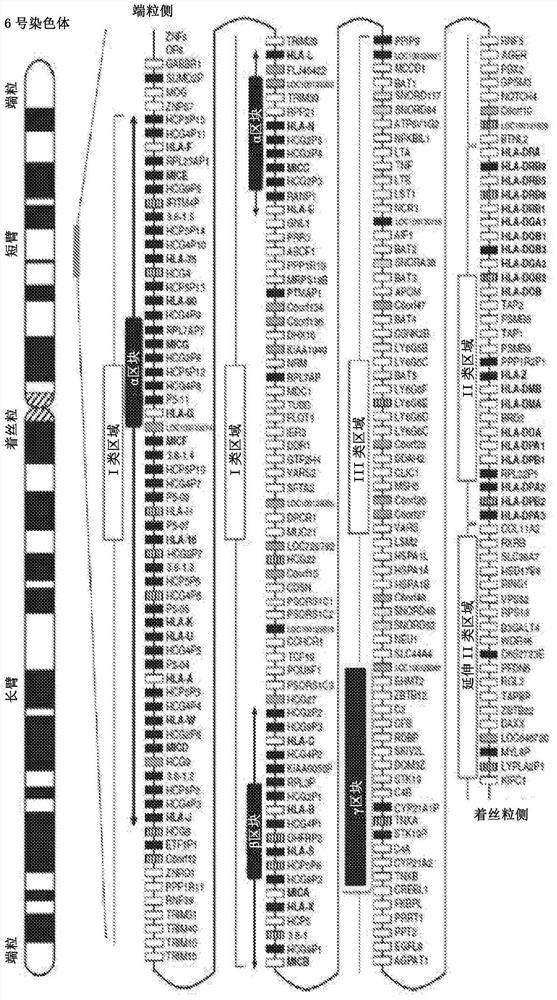
Gas delivery device comprising a gas releasing molecule and a gas permeable membrane
The present invention relates to a gas delivery device comprising a gas releasing molecule and a gas permeable membrane. The invention also relates to the use of the gas delivery device for delivery of gas to an extracorporeal transplant, extracorporeal cells, a brain-dead transplant donor or foodstuff and in therapy. The invention further relates to the use of a gas permeable and liquid and solid impermeable membrane to separate a gas releasing molecule and its non-gaseous degradation products from an extracorporeal transplant, extracorporeal cells, a brain-dead transplant donor or foodstuff.
Owner:JULIUS MAXIMILIANS UNIV WURZBURG
Method for screening MSAP sites relevant to regeneration in vitro of lycium ruthenicum
The invention discloses a method for screening MSAP sites relevant to regeneration in vitro of lycium ruthenicum. The method comprises the steps that (1) sterile seedlings are cultured; (2) leaf explants are inoculated, and thus calluses and regenerated plants are obtained; (3) donor plants and leaf-regenerated plants are acclimated and transplanted; (4) DNA of the calluses, DNA of transplanted and non-transplanted donor plant leaves and DNA of transplanted and non-transplanted regenerated plant leaves are extracted; (5) all the types of extracted DNA are subjected to double enzyme digestion through combination of EcoR I and Msp I or combination of EcoR I and Hpa II, and double-chain connectors are connected; (6) a PCR pre-amplification reaction is conducted; (7) a selective PCR amplification reaction is conducted; (8) capillary electrophoresis is conducted; (9) statistic analysis is conducted, and the MSAP sites relevant to regeneration in vitro of lycium ruthenicum leaves are screened; and (10) specific bands are recovered, sequenced and analyzed. According to the method for screening the MSAP sites relevant to regeneration in vitro of the lycium ruthenicum, the MSAP sites relevant to leaf regeneration can be screened rapidly and are unrelated to transplanting and acclimatizing; and excavation of the type of the sites has important significance to finally disclose dedifferentiation and redifferentiation mechanisms of woody plant leaves, and the content of plant epigenetics is enriched.
Owner:SHENYANG AGRI UNIV
Primer group, kit and method for HLA-DRB1 gene high-resolution typing
ActiveCN108588205AShorten the lengthReduced integrity requirementsMicrobiological testing/measurementDNA/RNA fragmentationTypingOrgan transplantation
The invention discloses a primer group, kit and method for HLA-DRB1 gene typing, and belongs to the field of gene detection. According to the primer group for HLA-DRB1 gene high-resolution typing, primers are designed correspondingly according to exons 2 and exons 3 of HLA-DRB1 genes, and the primers are utilized to conduct PCR amplification on the HLA-DRB1 genes. The primers conduct PCR amplification on the HLA-DRB1 genes, thus the gene fragment length of an amplified product is smaller than 1 kb, the requirement for the integrity of a template is reduced, the amplification efficiency is high, the amplification reaction time is short, the cost is reduced, the gene typing requirement of the large-scale sample HLA-DRB1 genes can be met, the more accurate basis is provided for clinical HLA matching, a proper transplant donor is provided for a patient, the rejection reaction in the transplant process is reduced, and the success rate of organ transplantation and the survival rate of the patient are increased.
Owner:BEIJING NUOSHI KANGYING MEDICAL TECH
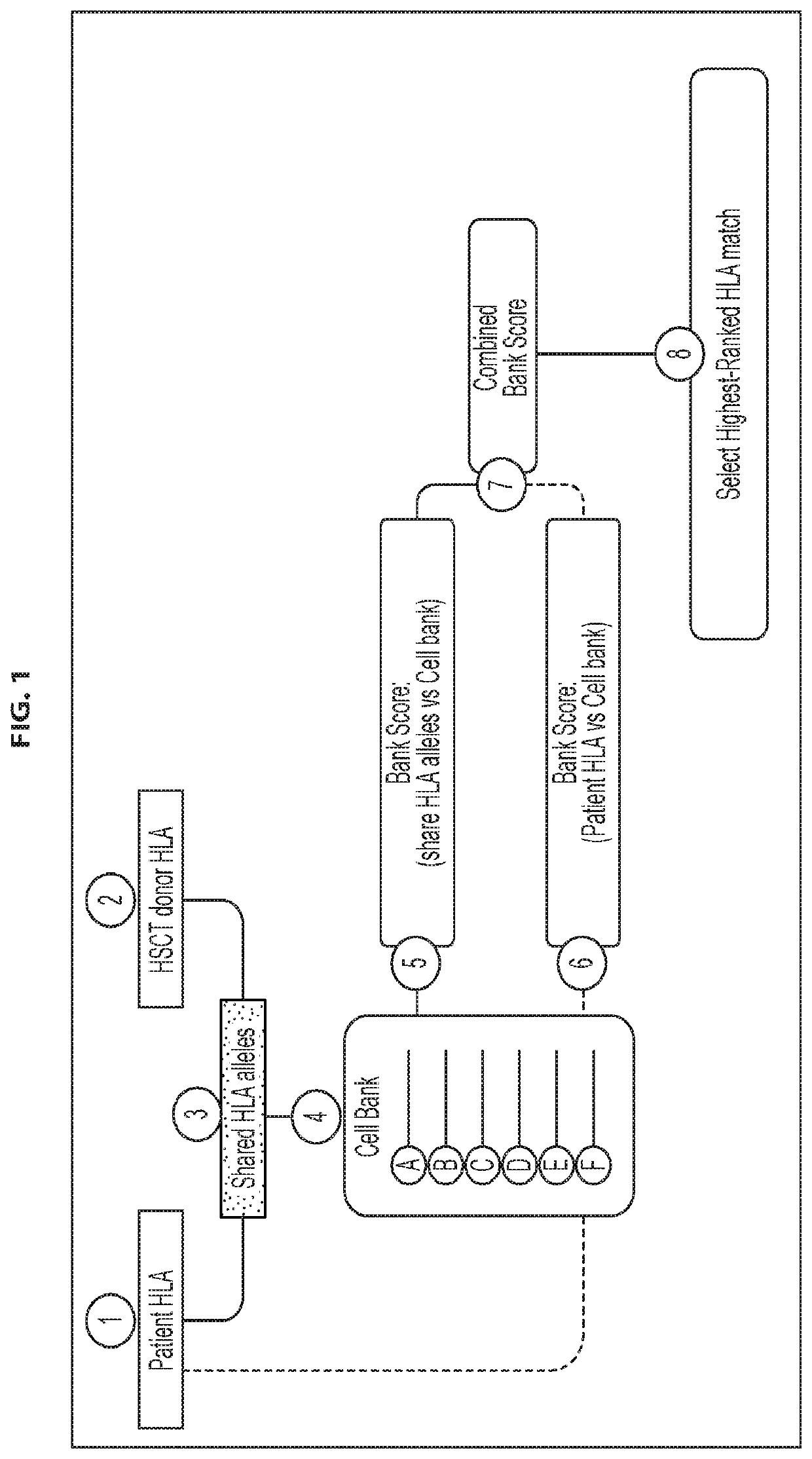
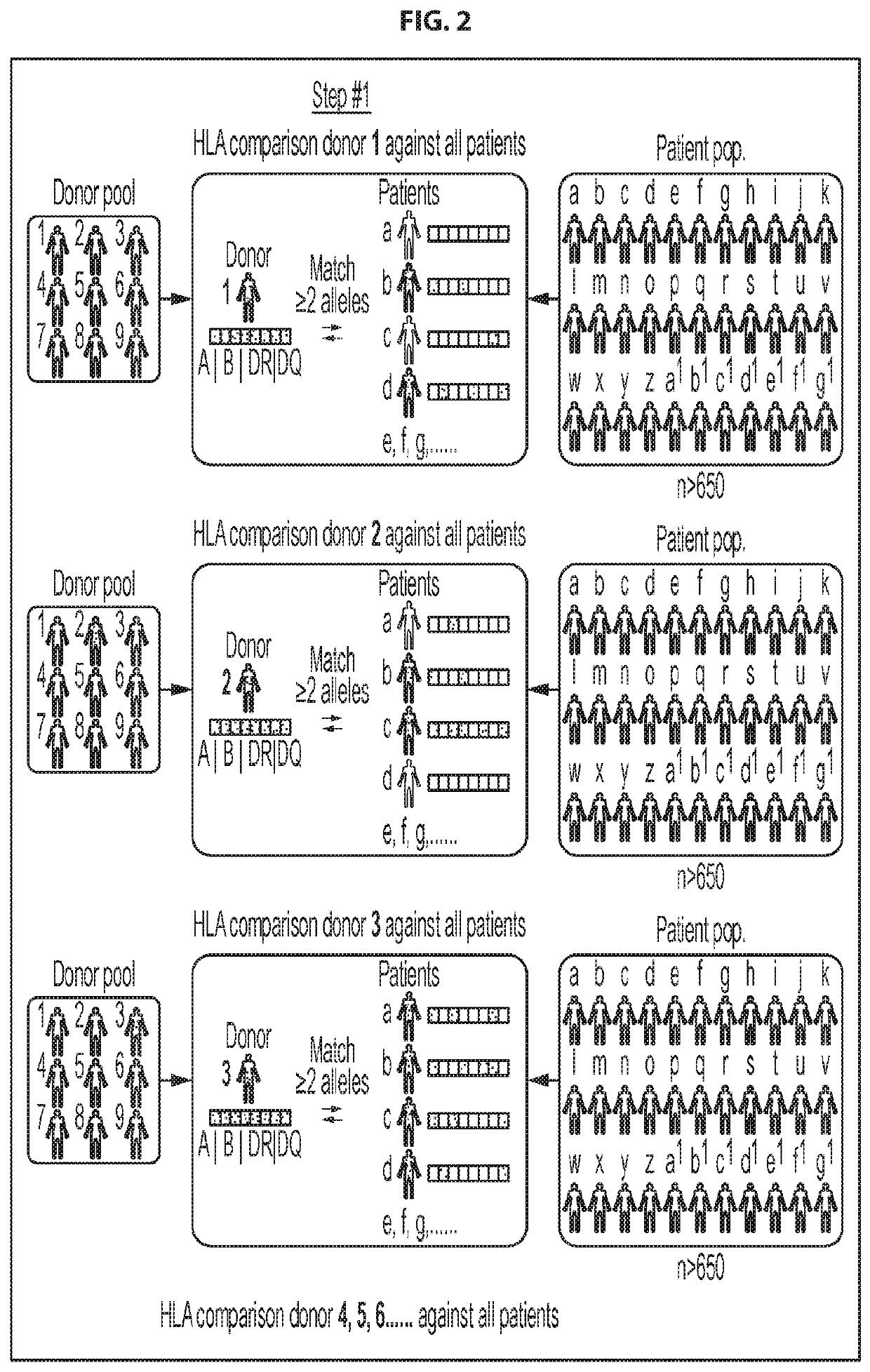
Antigen-specific t cell banks and methods of making and using the same therapeutically
PendingUS20220257654A1Increase the number ofBioreactor/fermenter combinationsBiological substance pretreatmentsDiseaseTransplant Procedure
Embodiments of the disclosure include methods of identifying and selecting suitable donors for use in constructing donor minibanks of antigen-specific T cell lines; donor minibanks of antigen-specific T cell lines; and donor banks made up of a plurality of such minibanks. The present disclosure includes methods of treating a disease or condition comprising administering at least one antigen-specific T cell line from such a donor minibank or donor bank to patient (e.g., who has received transplanted material from a transplant donor in a transplant procedure), and methods for selecting the best HLA-matched antigen-specific T cell line in the donor minibank for a particular patient.
Owner:BAYLOR COLLEGE OF MEDICINE
Method for inducing selectively suppressed immune response to transplanted tissue or cells
InactiveUS20100183735A1Raise the possibilitySuppress immune rejectionCompounds screening/testingEnergy modified materialsAbnormal tissue growthDendritic cell
Transimmunization methods incorporating skin immunologic challenges are described for either selectively suppressing the immune response of recipients of transplanted tissue or cells or monitoring induced anti-cancer immunity. In one embodiment, skin from the transplant donor is allografted to the transplant recipient to induce an immunological response to the transplanted skin. A quantity of blood is taken from the recipient and treated to render the T cells in the blood apoptotic and to induce differentiation of blood monocytes into dendritic cells. The treated blood is incubated and administered to the recipient to induce formation of suppressor T cell clones which reduce the number of T cells attacking the transplanted tissue or organ. This tolerogenic approach can be complemented by also feeding the immature dendritic cells apoptotic or necrotic cells from the organ donor. In a second embodiment, dendritic cells loaded with tumor antigens are injected intradermally to monitor the anti-cancer immunity induced by Transimmunization.
Owner:YALE UNIV
Cancellous bone bed shaper for medical cartilage graft recipients
The invention relates to a medical forming device for a gristle transplanting receptor spongy bone bed. Multiple knife edges are connected to a knife head base connected with a knife head connecting rod through a universal ball joint. The knife head connecting rod is connected with a handle connecting rod and a handle, the handle is connected with a handle stopping block, and the handle stopping block is connected with a manual sliding impacting device or an electric impacting device. The defects that in the prior art, because a bone knife, a bone chisel and other simple tools are used for manually trimming, the bone cutting face is not flat, randomness is high, and the bone cutting face of a receptor and the bone cutting face of a donor are prone to being mismatched, which is adverse to bone healing are overcome. The receptor spongy bone bed can be formed through one-off impacting, so that forming efficiency is improved, operation time is shortened, and the form of the receptor spongy bone bed is kept uniform. The manual sliding impacting device and the electric impacting device can be selected for use, so that the form of the spongy bone, after cutting, below the transplanted donor gristle is completely matched with that of the receptor sponge bone bed, a transplanted donor and a receptor are completely matched, and bone healing is facilitated.
Owner:THE AFFILIATED DRUM TOWER HOSPITAL MEDICAL SCHOOL OF NANJING UNIV
Gas delivery device comprising a gas releasing molecule and a gas permeable membrane
Owner:JULIUS MAXIMILIANS UNIV WURZBURG
major histocompatibility complex single nucleotide polymorphism
ActiveCN105899675BMicrobiological testing/measurementMaterial analysis by electric/magnetic meansMedicineNucleotide
Owner:ILLUMINA INC
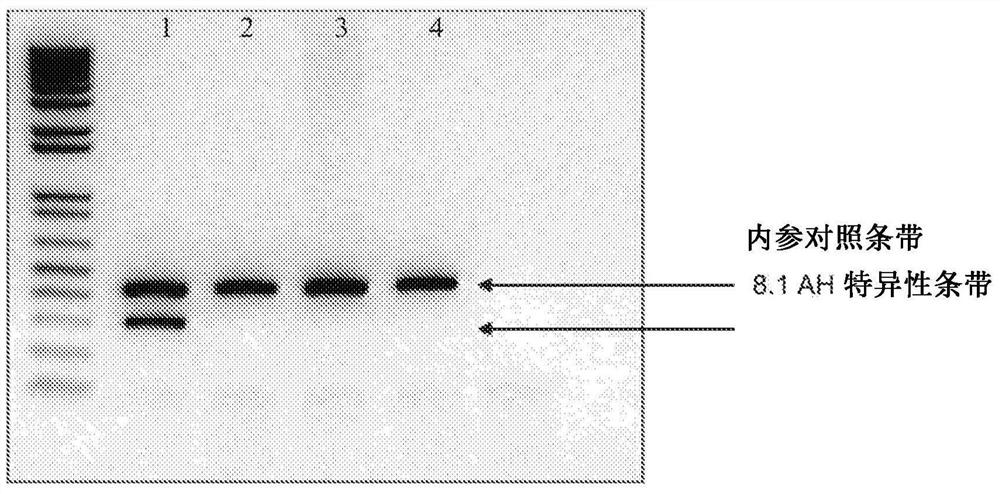
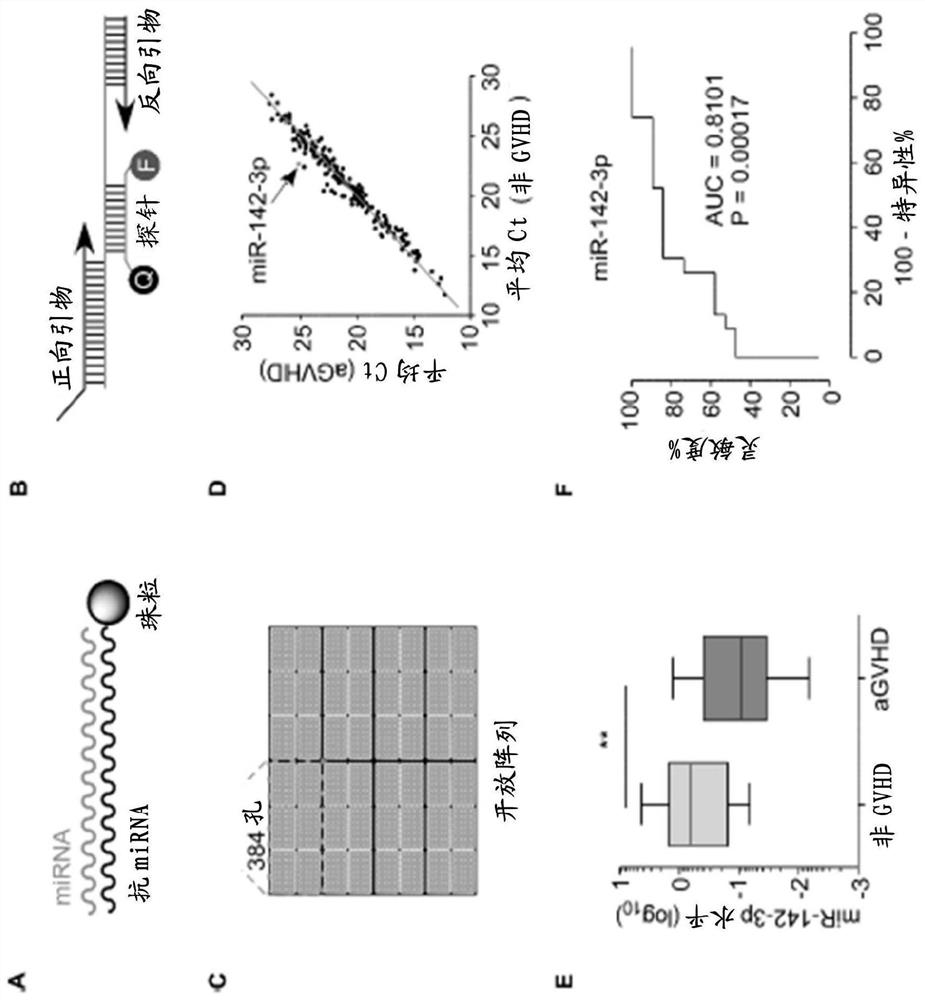
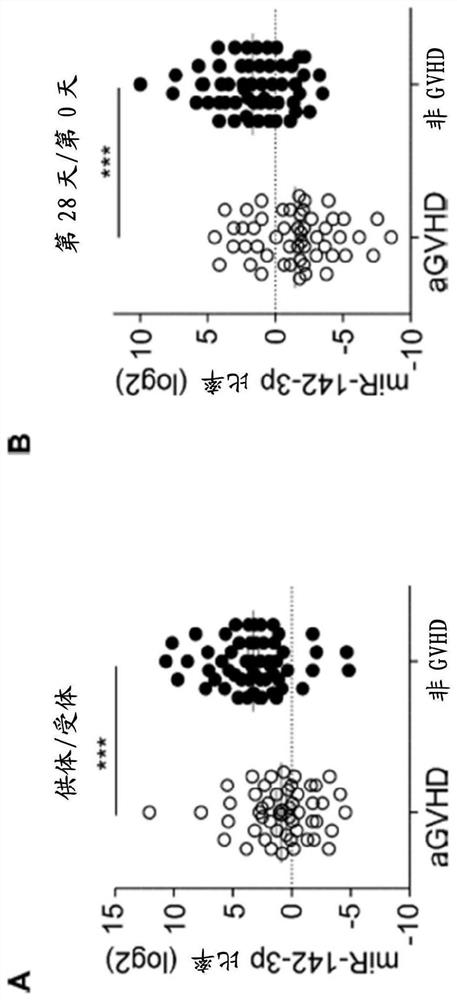
Compositions and methods for donor selection and prognosis of acute graft-versus-host disease
PendingCN112004944AMicrobiological testing/measurementUnknown materialsHost diseaseAcute graft versus host disease
Provided are compositions and methods for donor selection and prognosis of acute graft-versus-host disease. Provided is a method, which comprises obtaining a first biological sample from a first subject at risk of GVHD; detecting a level of miR-142-3p in the sample; and determining a risk of, prognosis of, or diagnosis of GVHD in the first subject. Provided also is a method for selecting a stem cell transplant donor, which comprises obtaining a first biological sample from a first subject and a second biological sample from a second subject, detecting a level of miR-142-3p in the first biological sample and the second biological sample; determining a ratio of the level of miR-142-3p in the second biological sample to the level of miR-142-3p in the first biological sample; determining the likelihood of the first subject will develop GVHD based on the ratio; and selecting a transplant donor based on the ratio.
Owner:DUKE UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com