Method and kit for cross-over lymphatic toxicity experiment
A lymphatic toxicity and experimental technology, applied in the field of cross-lymphatic toxicity experiments, can solve the problems of limited development, counting and typing errors, carcinogenicity, etc., and achieve the effects of improving sensitivity and accuracy, reducing experimental costs, and expanding the reaction system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 The method and kit of cross lymphocytotoxicity experiment of the present invention
[0036] 1. Kit composition
[0037] 1. Reagents
[0038] Lymphocyte separation solution (50ml) was purchased from Tianjin Haoyang Biological Products Technology Co., Ltd.
[0039] Cell counting blank plates (20 pieces) were purchased from SHIH-YUNG Medical Equipment Company.
[0040] Rabbit complement (1 tube), One Lambda Company.
[0041] Positive serum (100 μl) was purchased from Tianjin Xiupeng Biotechnology Company.
[0042] Trypan blue staining solution or neutral red staining solution (500ul) was purchased from Beyond Biotechnology Co., Ltd.
[0043] PBS buffer (100ml)
[0044] Normal saline (50ml)
[0045] 2. Required equipment
[0046] 37°C water bath (incubator), centrifuge, ordinary optical microscope, 8-tube tube, EP tube, 1-10μl micro-sampler, etc.
[0047] 2. Detection method
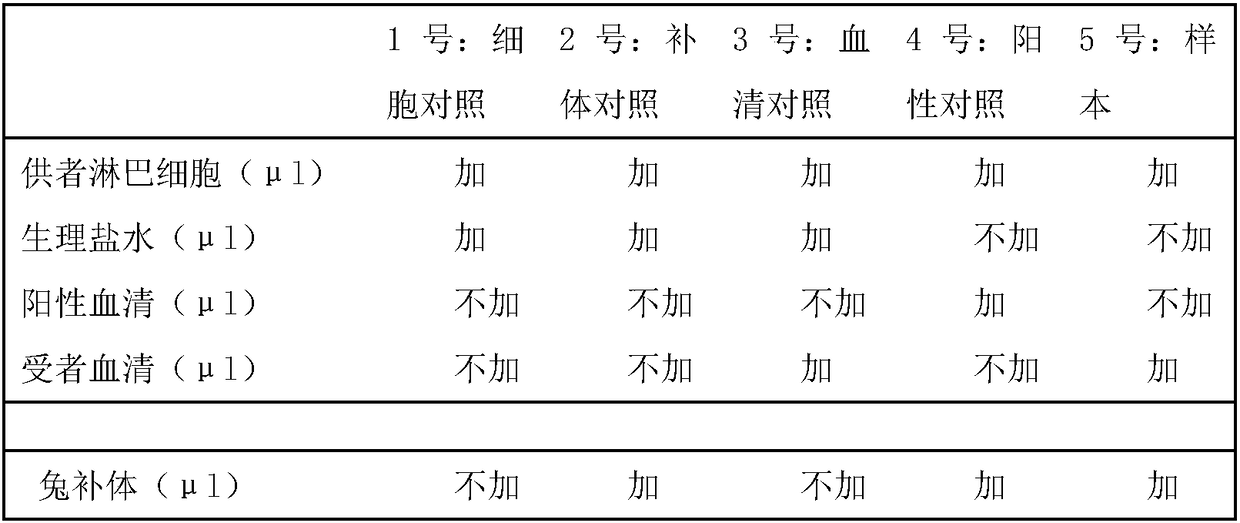
[0048] 1. Specimen
[0049] Take 1 ml of venous anticoagulated whole blood ...
Embodiment 2
[0076] 1. Detection method
[0077] Except that the amount of trypan blue was increased from 2 ul to 5 ul after the reaction was completed, the rest of the steps were the same as in Example 1.
[0078] 2. Accuracy verification
[0079] 1. Take 20 cases of venous anticoagulated whole blood from transplant donors, and use the traditional fluorescent staining detection method and the method of the present invention to carry out cross-lymphocytotoxicity test detection.
[0080] 2. Test results
[0081] The test results are as follows:
[0082]
[0083] It can be seen from the above table that the detection consistency between the method of the present invention and the traditional fluorescence detection method is 100% (20 / 20), indicating that the method of the present invention can accurately detect whether there is anti-donor lymphocyte antibody in the sample to be seen.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com