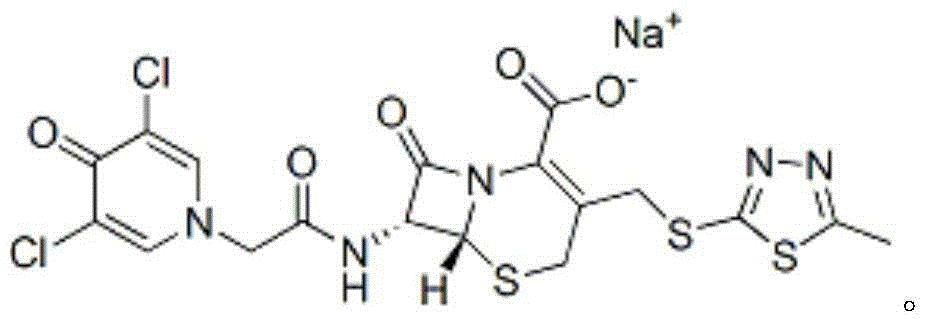
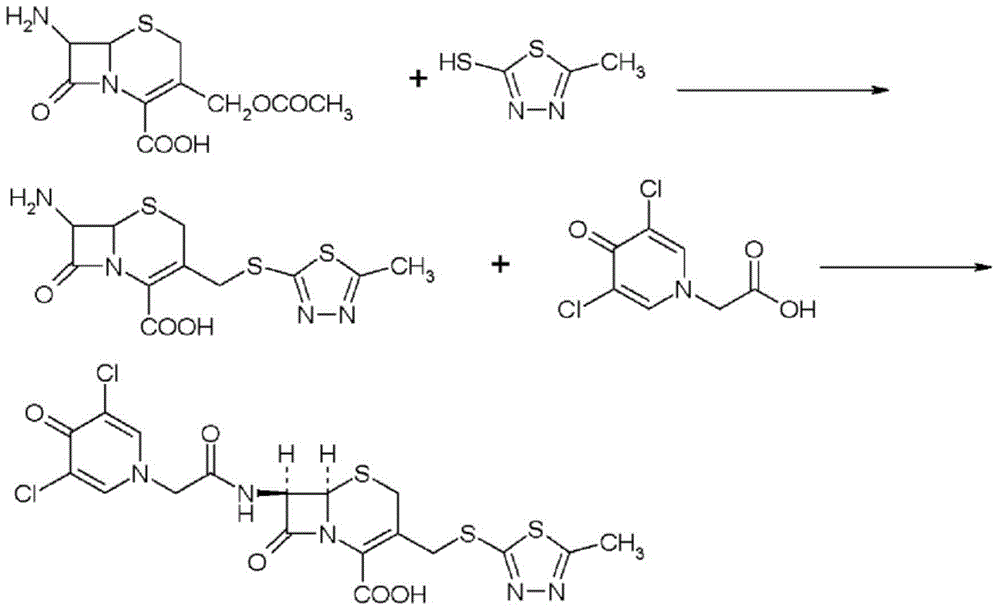
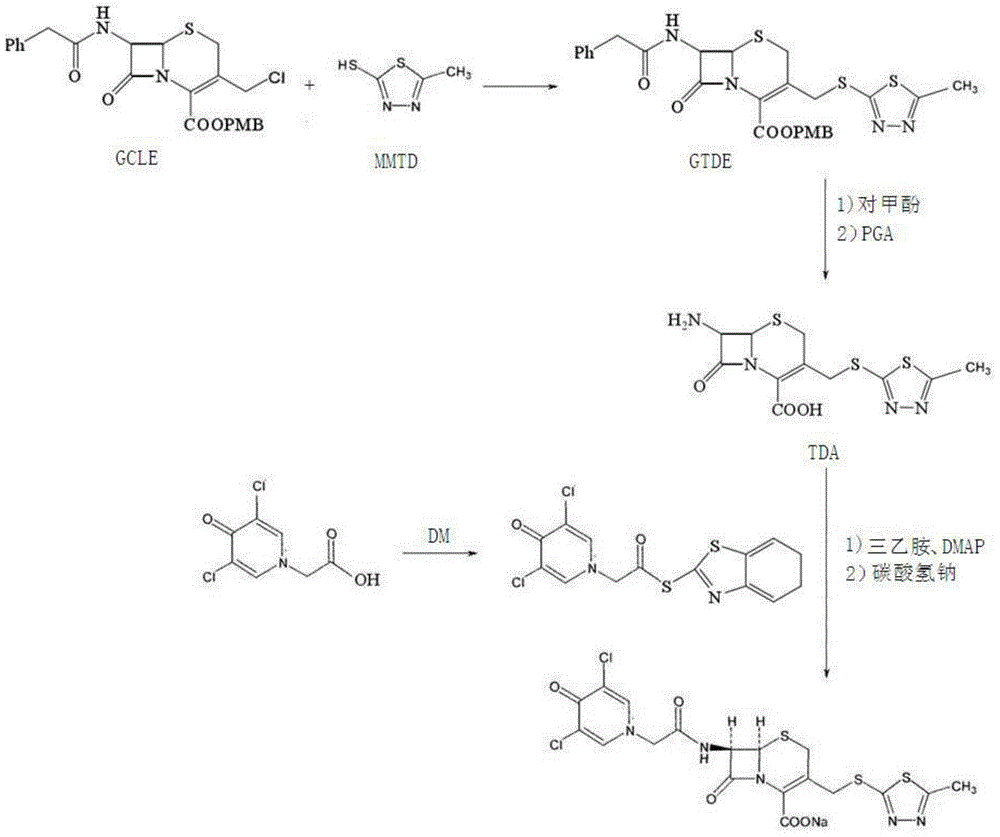
Preparation method for cephalosporin anti-infective drug
An anti-infection and cephalosporin technology, applied in the direction of organic chemistry, etc., can solve the problems of low yield of cefoxitone, unsuitable for large-scale industrial production, and high synthesis cost, and achieve easy operation of reaction conditions, good appearance and color, and production low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1) p-methoxybenzyl 7-phenylacetamido-3-(2-methyl-1,3,4-thiadiazol-5-yl)thiomethyl-3-cephem-4-carboxylate ( GTDE) preparation
[0030] Add 600mL acetone into the dry reaction vessel, heat to 50°C, add 102.5gGCLE (content 95%, technical grade, 0.2mol) into the acetone, stir to dissolve, add 29.1g 2-mercapto-5-methyl-1, 3,4-Thiadiazole (MMTD, 0.22mol), the reaction temperature is controlled at 50-55°C, and the reaction time is 2 hours. After the reaction is completed, add water to crystallize, filter with suction, wash with a small amount of water, and dry under vacuum below 40°C. 112.8 g GTDE (0.192 mol), yield 96%, HPLC purity 99.2%.
[0031] 2) Preparation of 7-amino-3-(2-methyl-1,3,4-thiadiazol-5-yl)thiomethyl-3-cephem-4-carboxylic acid (TDA)
[0032]Add 112.8g GTDE (0.192mol) and 225.6g p-cresol prepared in step 1) into the reaction kettle, control the temperature at 45-50°C, stir and react for 6 hours; cool to 40°C after the reaction is complete, and add 564g aceti...
Embodiment 2
[0038] 1) p-methoxybenzyl 7-phenylacetamido-3-(2-methyl-1,3,4-thiadiazol-5-yl)thiomethyl-3-cephem-4-carboxylate ( GTDE) preparation
[0039] Add 600mL acetone into the dry reaction vessel, heat to 50°C, add 198.2gGCLE (content 98.3%, technical grade, 0.4mol) into the acetone, stir to dissolve, add 63.5g 2-mercapto-5-methyl-1, 3,4-Thiadiazole (MMTD, 0.48mol), the reaction temperature is controlled at 50-55°C, and the reaction time is 3 hours. After the reaction is completed, add water to crystallize, filter with suction, wash with a small amount of water, and dry under vacuum below 40°C. 223.9 g GTDE (0.38 mol), yield 95%, HPLC purity 98.9%.
[0040] 2) Preparation of 7-amino-3-(2-methyl-1,3,4-thiadiazol-5-yl)thiomethyl-3-cephem-4-carboxylic acid (TDA)
[0041] Add 223.9g GTDE (0.38mol) and 2239g p-cresol prepared in step 1) into the reaction kettle, control the temperature at 45-50°C, stir and react for 1 hour; cool to 40°C after the reaction is complete, add 3360g ethyl ace...
Embodiment 3
[0047] 1) p-methoxybenzyl 7-phenylacetamido-3-(2-methyl-1,3,4-thiadiazol-5-yl)thiomethyl-3-cephem-4-carboxylate ( GTDE) preparation
[0048] Add 1000mL acetone into the dry reaction vessel, heat to 50°C, add 263gGCLE (content 92.5%, industrial grade, 0.5mol) into the acetone, stir to dissolve, add 76.02g 2-mercapto-5-methyl-1,3 , 4-Thiadiazole (MMTD, 0.575mol), the reaction temperature is controlled at 50-55°C, and the reaction time is 3 hours. After the reaction is completed, add water to crystallize, filter with suction, wash with a small amount of water, and dry under vacuum below 40°C to obtain 284.6 g GTDE (0.485 mol), yield 97%, HPLC purity 99.3%.
[0049] 2) Preparation of 7-amino-3-(2-methyl-1,3,4-thiadiazol-5-yl)thiomethyl-3-cephem-4-carboxylic acid (TDA)
[0050] Add 284.6g GTDE (0.485mol) and 1707.6g p-cresol prepared in step 1) into the reactor, control the temperature at 45-50°C, stir and react for 3 hours; cool to 40°C after the reaction is complete, and add 28...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com