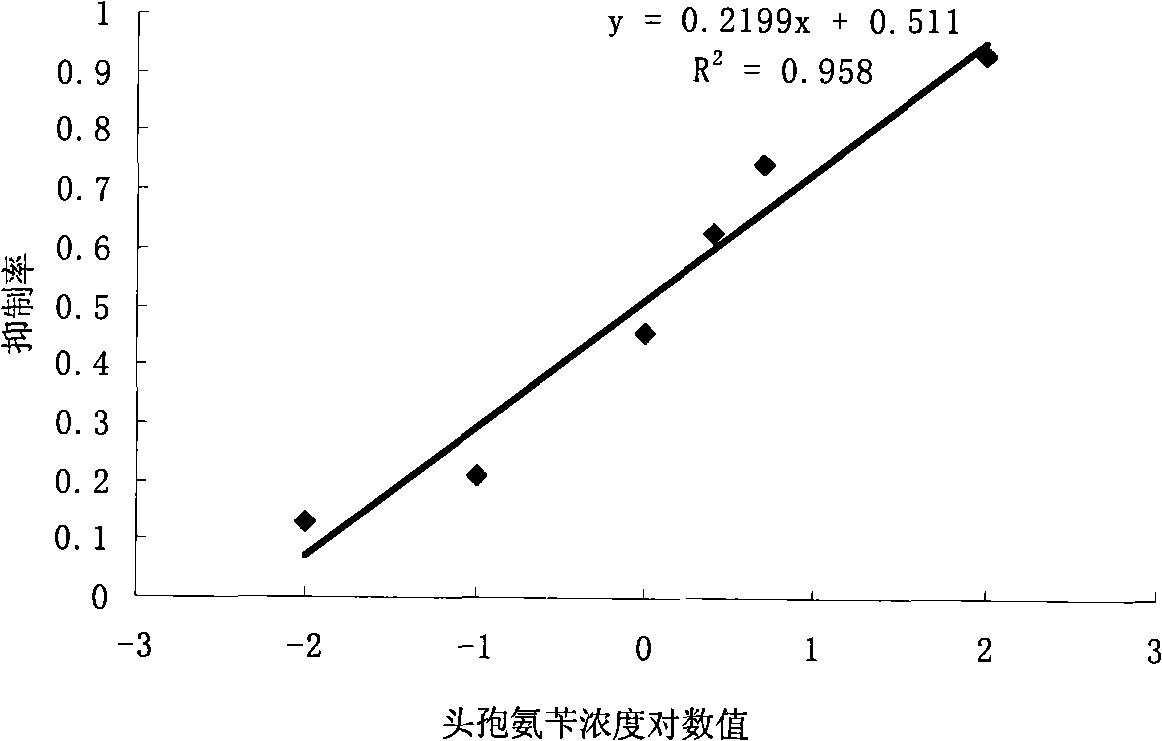
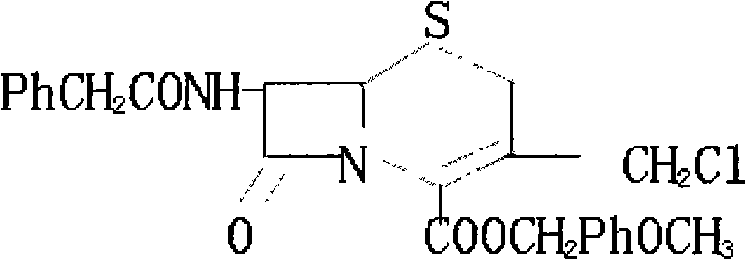
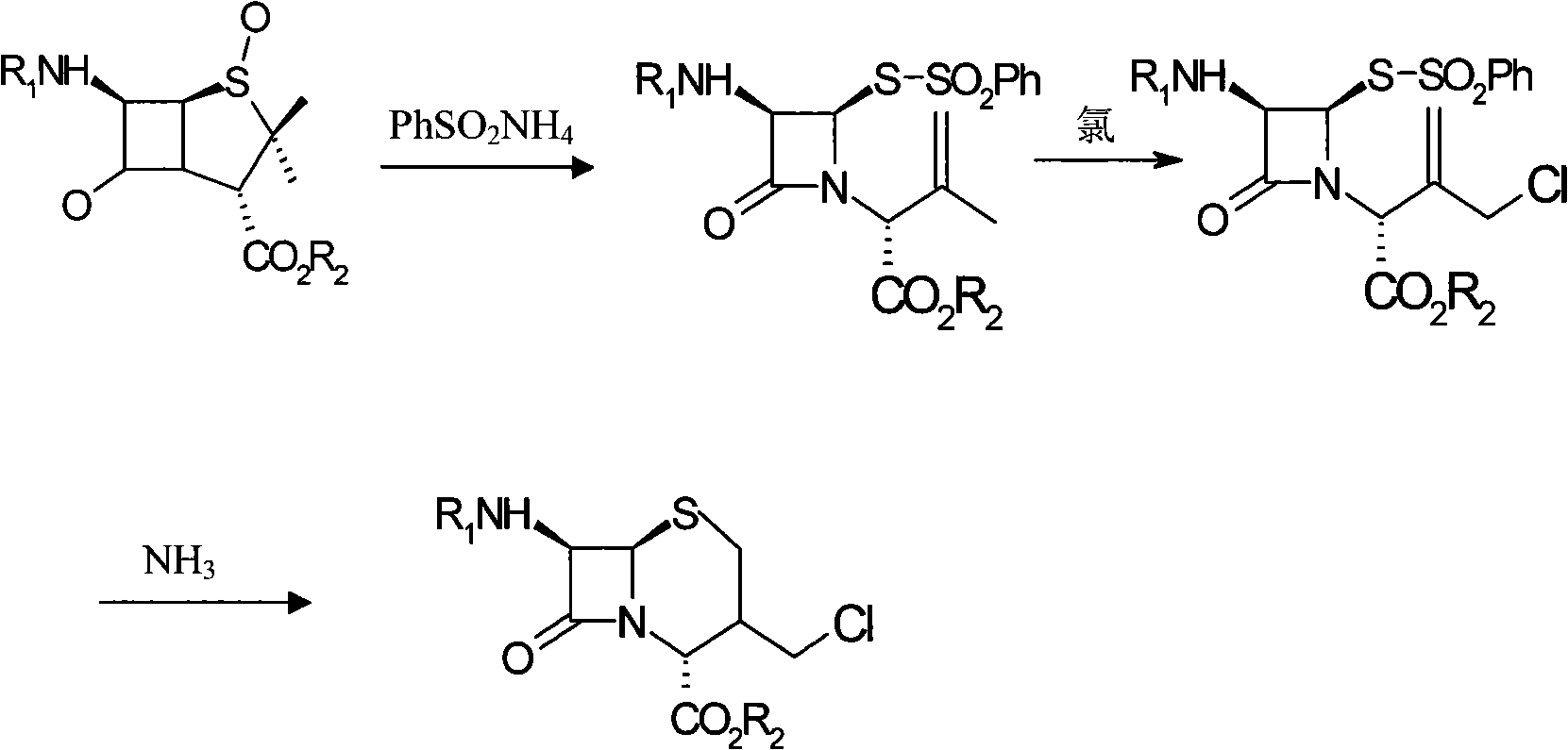
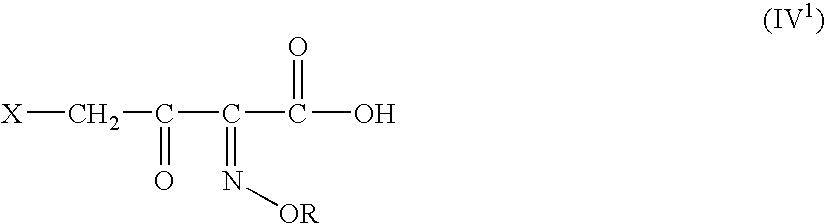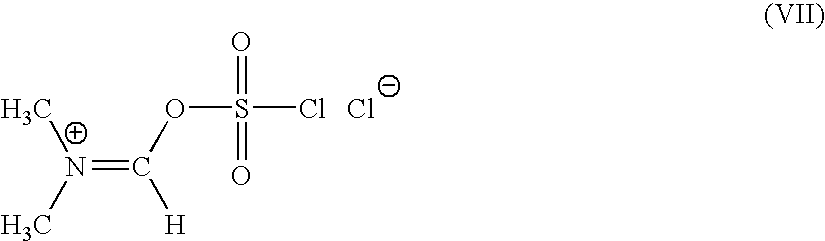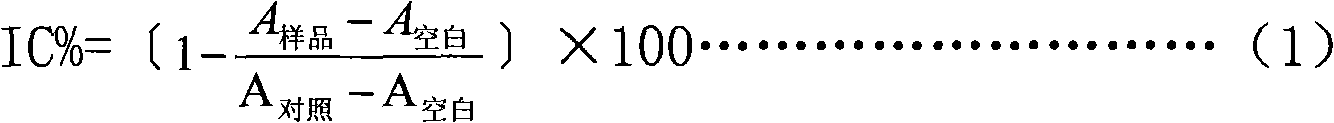Patents
Literature
136 results about "Cephalosporin Antibiotic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
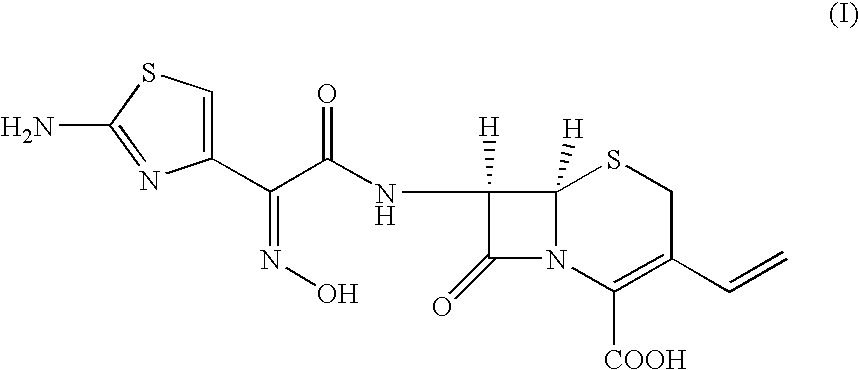
A synthetic or semi-synthetic beta-lactam antibiotic derived from Acremonium (Cephalosporium) fungi with bactericidal activity. Cephalosporin antibiotics bind to and inactivate penicillin-binding proteins (PBPs) located on the inner membrane of the bacterial cell wall. Inactivation of PBPs interferes with the cross-linkage of peptidoglycan chains necessary for bacterial cell wall strength and rigidity. This interrupts bacterial cell wall synthesis and results in the weakening of the bacterial cell wall, eventually causing cell lysis.
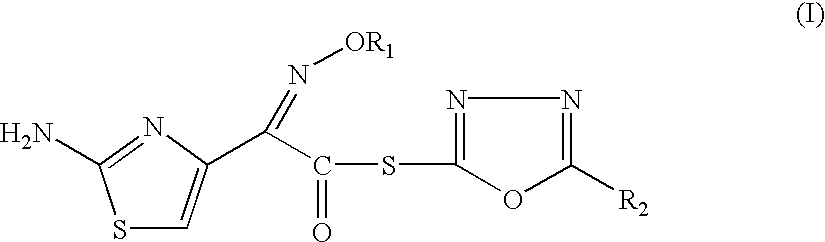
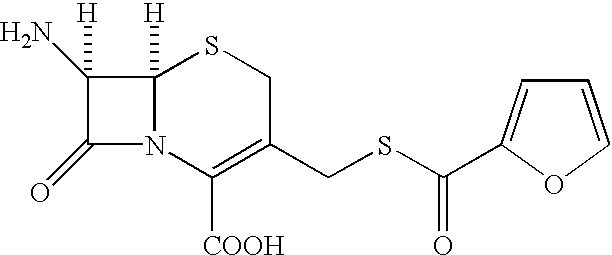
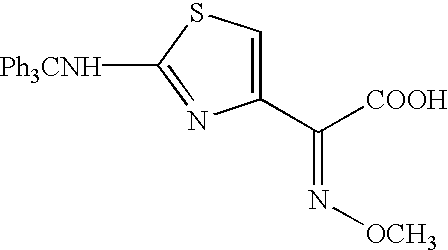
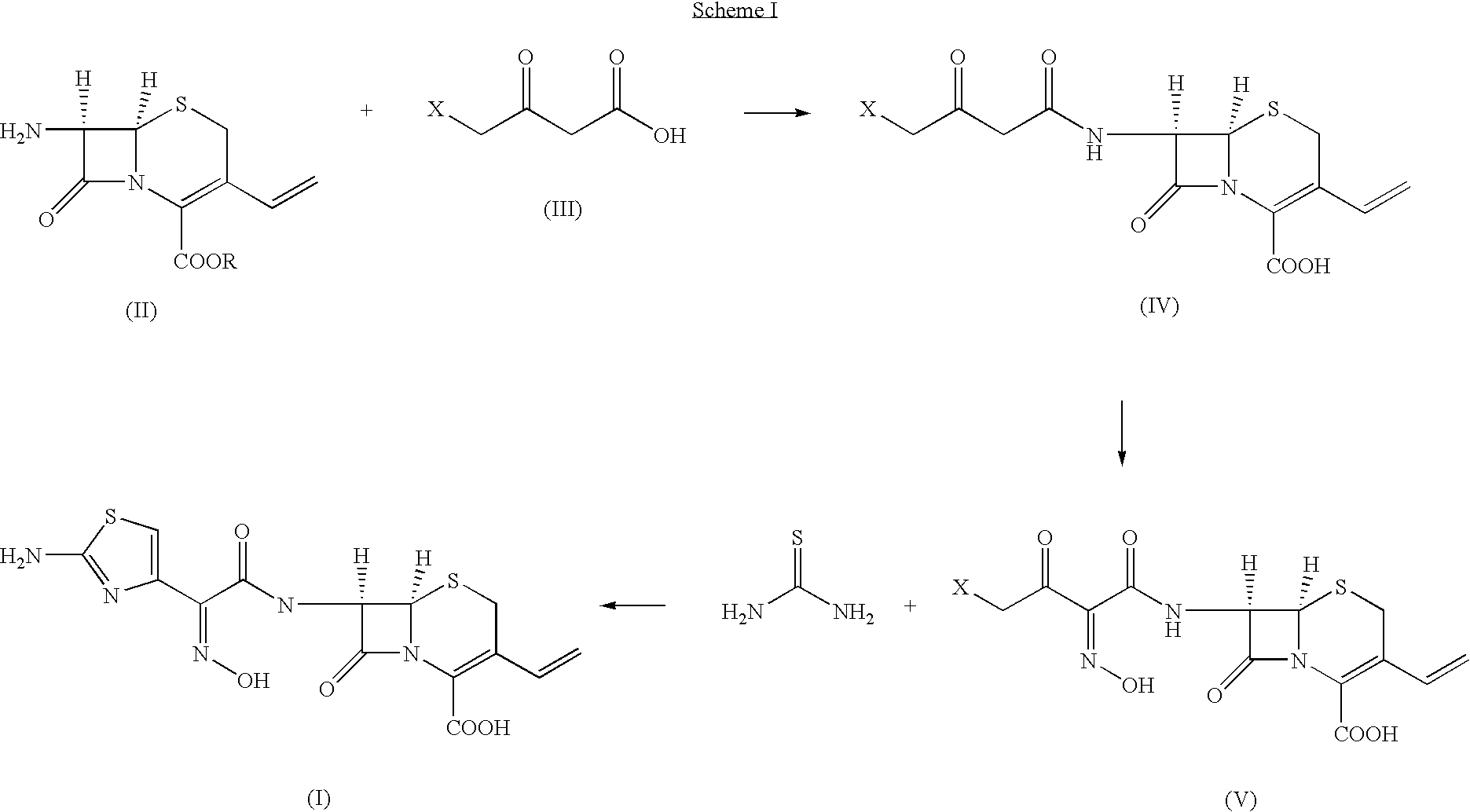
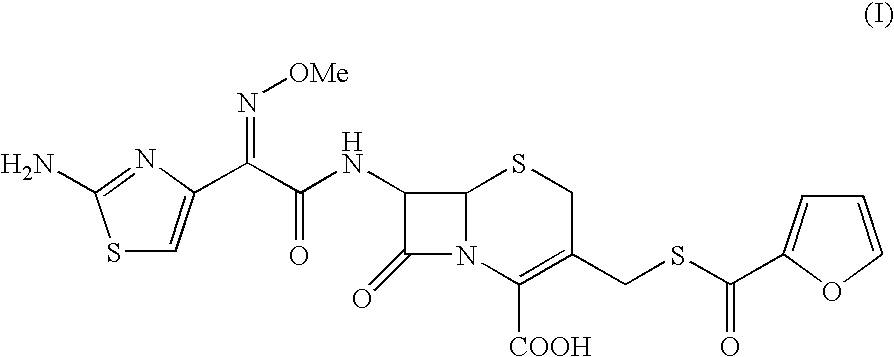
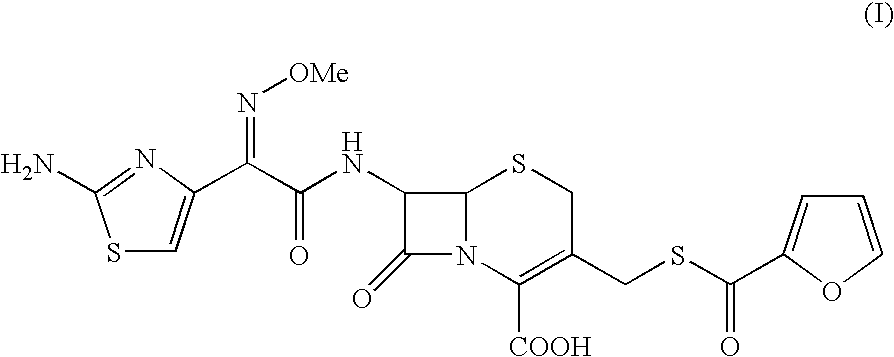
Thioester derivatives of thiazolyl acetic acid and their use in the preparation of cephalosporin compounds
InactiveUS6388070B1High purityHigh yieldOrganic chemistryBulk chemical productionAcetic acidHydrogen
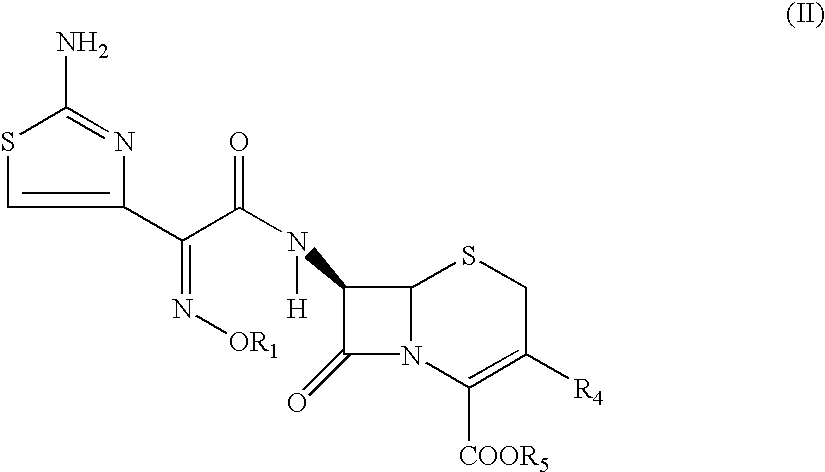
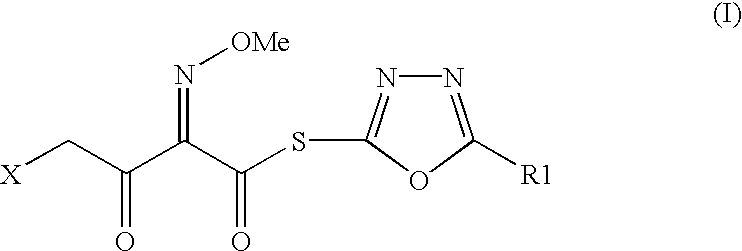
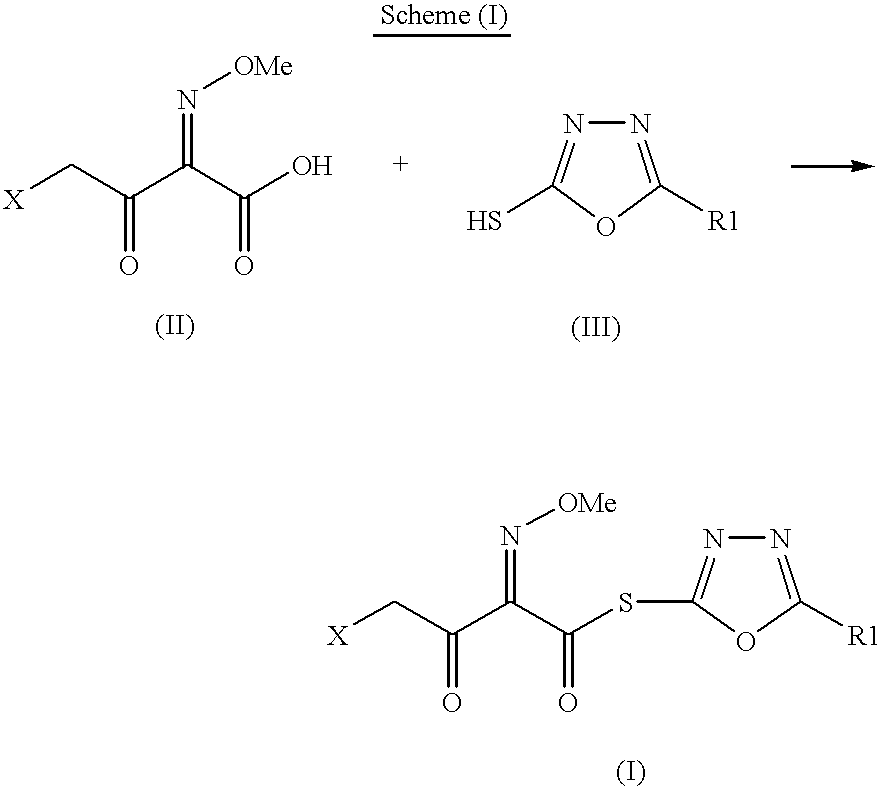
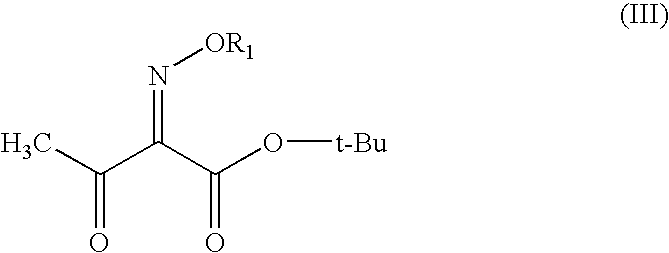
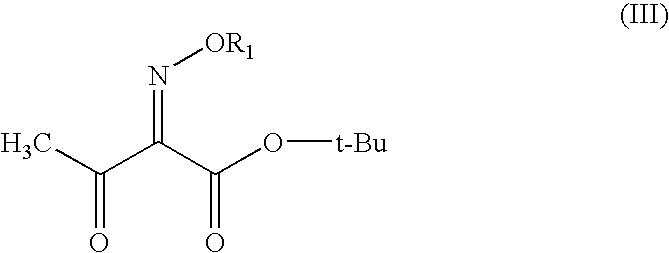
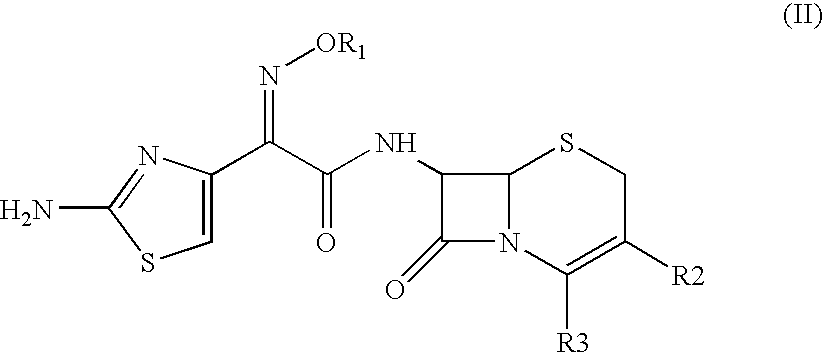
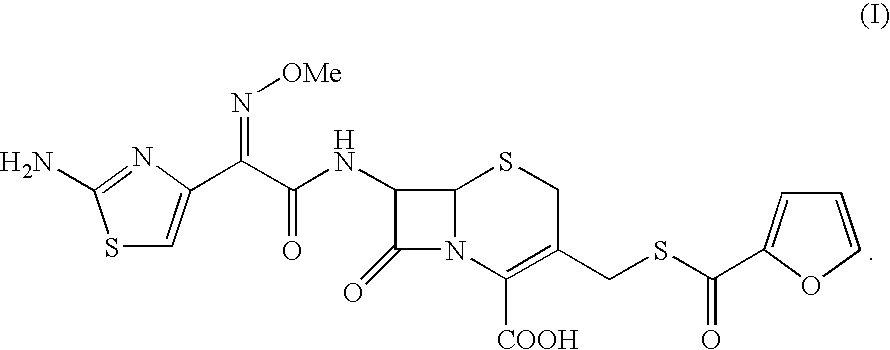
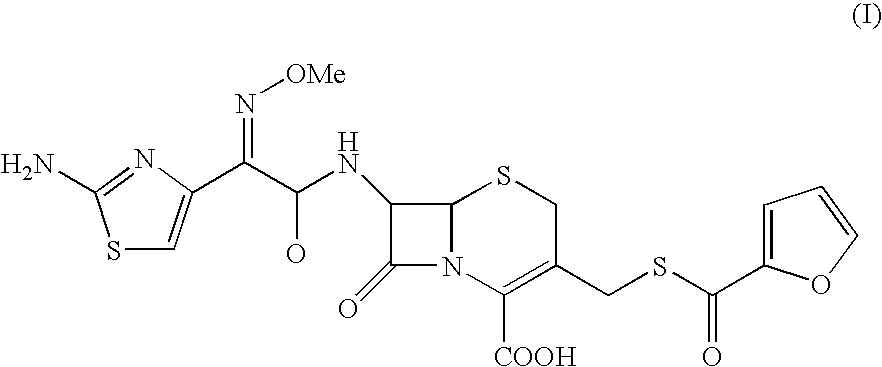
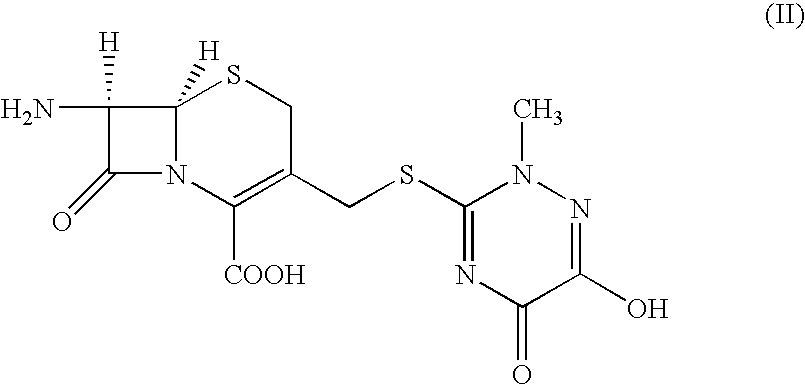
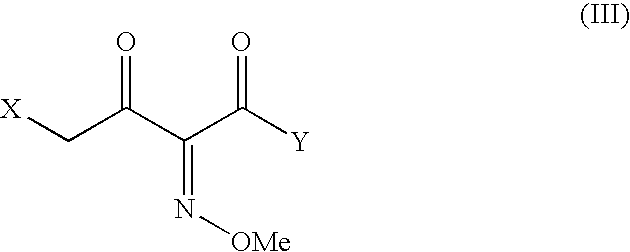
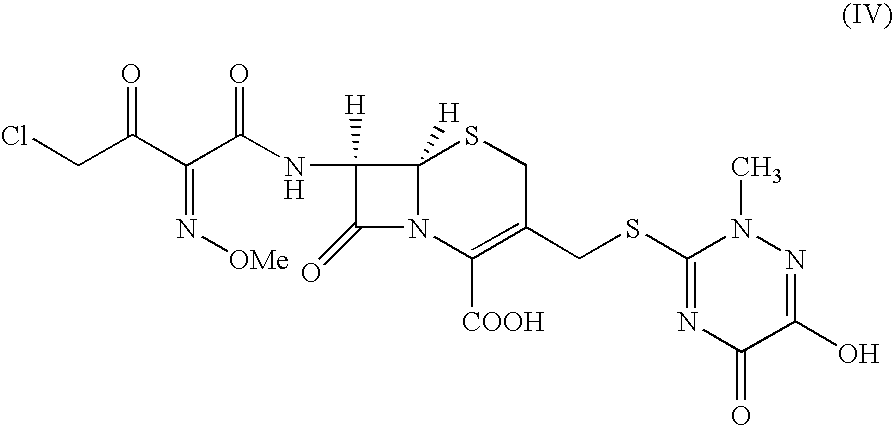
The present invention provides novel thioester derivatives of thiazolyl acetic acid of the general formula (I),wherein, R1 represents H, trityl, CH3, or CRaRbCOOR3, in which Ra and Rb, independently of one another, represents hydrogen or methyl and R3 represents H or C1-C7 alkyl; and R2 represents C1-C4 alkyl or phenyl. The invention also provides a method for preparation of the thioester derivatives and reaction of the thioester derivatives with cephem carboxylic acids to produce cephalosporin antibiotic compounds having general formula (II),wherein, R1 represents H, trityl, CH3, or CRaRbCOOR3, in which Ra and Rb, independently of one another, represents hydrogen or methyl and R3 represents H or C1-C7 alkyl; R4 is CH3, -CH=CH2, CH2OCH3, CH2OCOCH3,and R5 is H or a salt or a carboxylic protecting group, comprising,acylating a compound of formula (III),wherein, R4 and R5 are defined as above, and R6 is H or trimethylsilyl;with a compound of formula (I).
Owner:ORCHID CHEM & PHARM LTD
Process for preparation of cefdinir
InactiveUS6093814ALow priceManufacturing timeAntibacterial agentsOrganic active ingredientsP-Toluenesulfonic acidCefdinir
PCT No. PCT / KR96 / 00250 Sec. 371 Date May 18, 1998 Sec. 102(e) Date May 18, 1998 PCT Filed Dec. 26, 1996 PCT Pub. No. WO97 / 24358 PCT Pub. Date Jul. 10, 1997The present invention relates to a novel crystalline cefdinir intermediate having formula (II) which can be used very usefully for preparing a cephalosporin antibiotics, cefdinir, in which Ph represents phenyl, p-TsOH represents p-toluenesulfonic acid, and DMAC represents N,N-dimethylacetamide, to a process for preparation thereof and to a process for preparing cefdinir using the compound of formula (II). According to the present invention, cefdinir can be prepared in an excellent color and purity and with a good yield.
Owner:HANMI SCI CO LTD
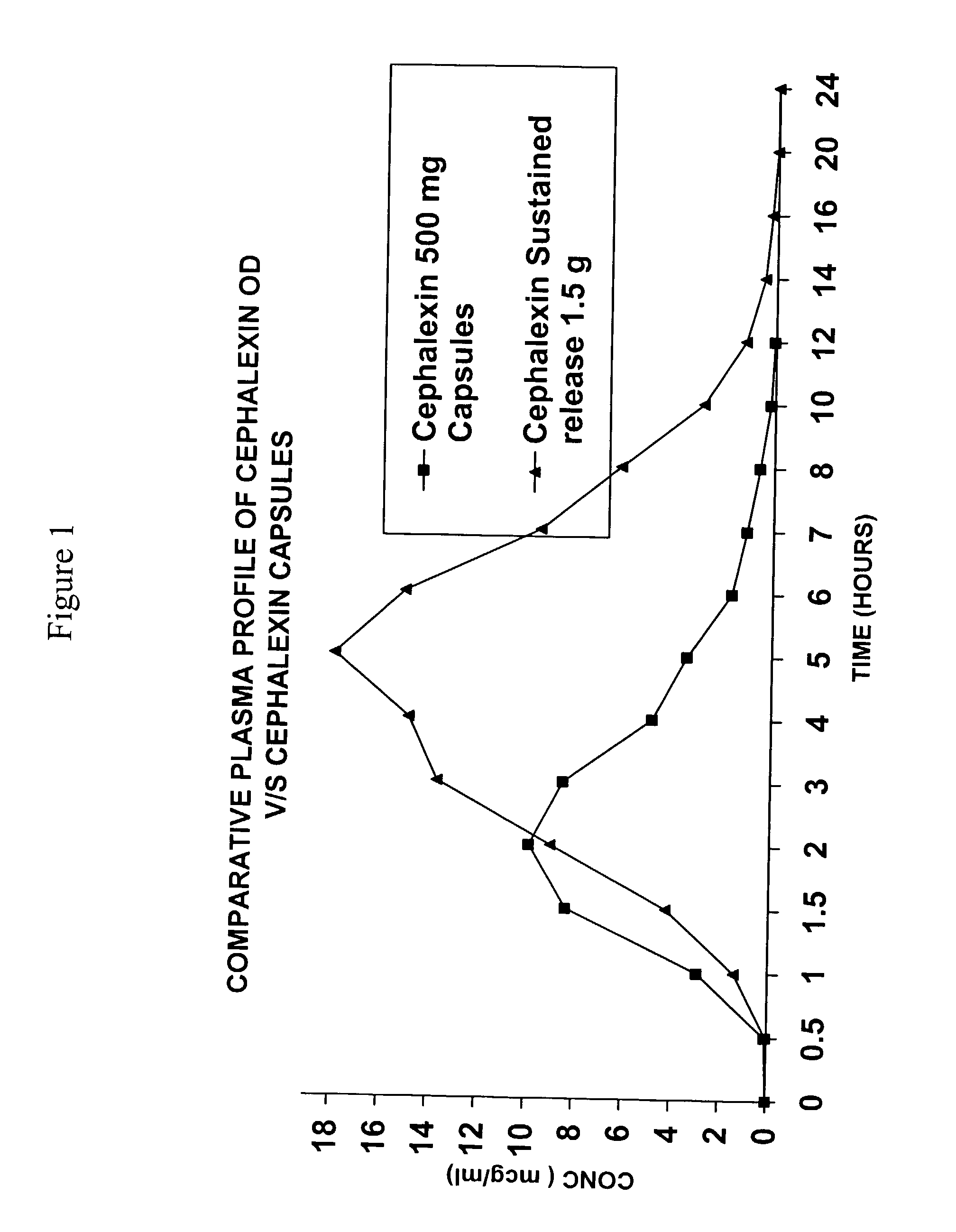
Sustained release pharmaceutical composition of a cephalosporin antibiotic
InactiveUS20040033262A1Sustained releaseMaintain good propertiesBiocideOrganic active ingredientsCombinatorial chemistryBULK ACTIVE INGREDIENT
This invention relates to a sustained release pharmaceutical composition comprising at least a cephalosporin antibiotic, a mixture of polymers and other pharmaceutically acceptable excipients; in the composition, polymers are selected from mixture of galactomannans and neutral swellable polymers, which releases the active ingredient in a predetermined manner.
Owner:ORCHID HEALTH CARE A DIV OF ORCHID CHEM & PHARMA
Preparation of new intermediates and their use in manufacturing of cephalosporin compounds
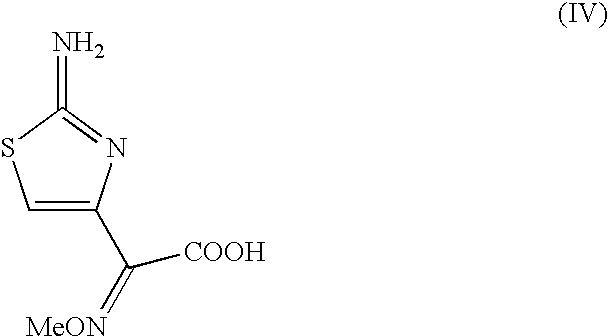
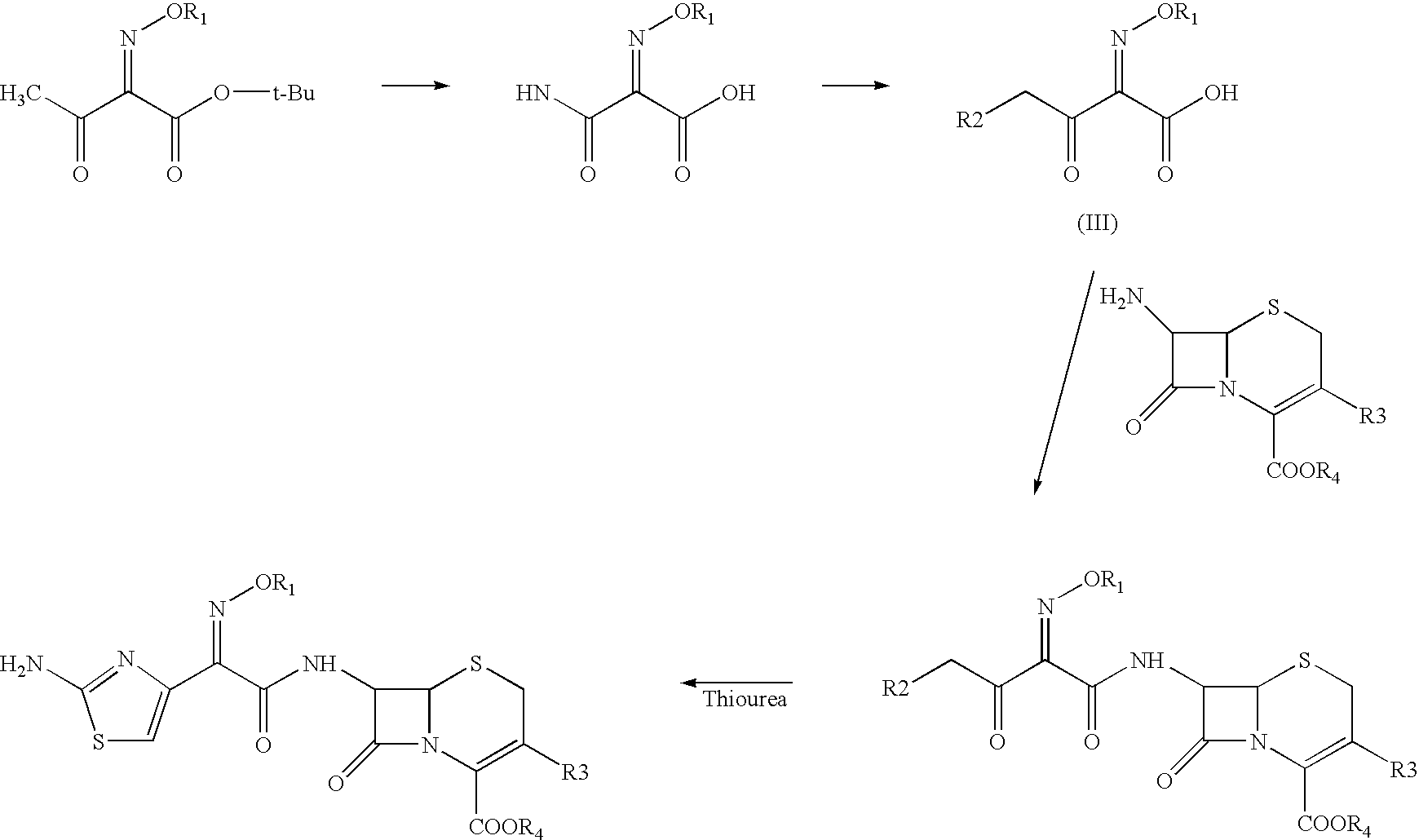
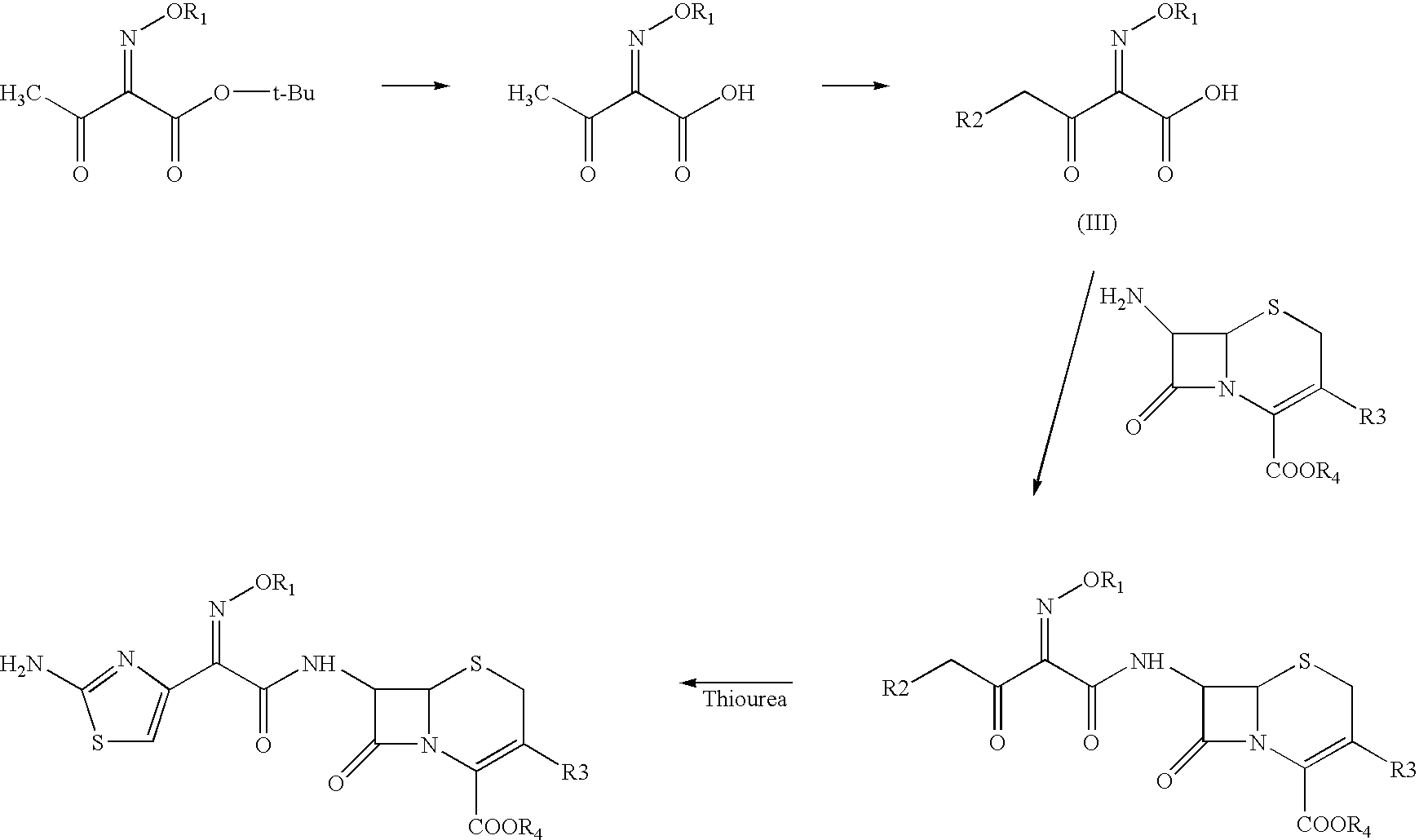
The present invention provides new thioester derivatives of 4-halogeno-2-methoxyimino-3-oxo-butyric acid of the general formula (I), also, the invention provides a method by which the said thioester derivatives can be prepared by reacting 4-halogeno-2-methoxyimino-3-oxo-butyric acid of the general formula (II) with 2-mercapto-5-substituted-1,3,4-oxadiazoles of the general formula (III) in a solvent, in the presence of DMF / POCl3 and in presence of an organic base and if desired the so obtained thioester derivatives so obtained are reacted with 7-amino-cephem carboxylic acids of the general formula (V) to produce condensed products which are insitu reacted with thiourea to get cephalosporin antibiotic compounds having the general formula (VI).
Owner:ORCHID CHEM &PHARMA LIMITED INDIA
Novel intermediates for synthesis of cephalosporins and process for preparation of such intermediates
InactiveUS20060135761A1Easily hydrolysableSulfuric acid esters preparationBulk chemical productionCefmenoximeAntibiotic Y
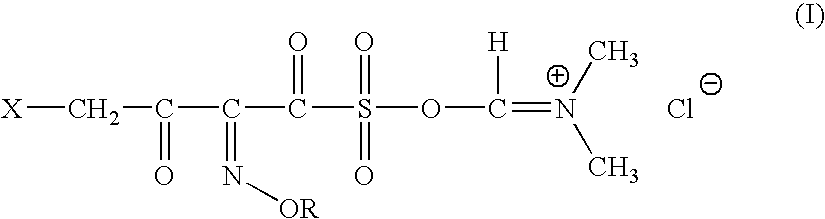
A novel 4-halo-2-oxyimino-3-oxo butyric acid-N,N-dimethyl formiminium chloride chlorosulfate of formula (I) useful in the preparation of cephalosporin antibiotics wherein X is chlorine or bromine; R is hydrogen, C1-4 alkyl group, an easily removable hydroxyl protective group, —CH2COOR5, or —C(CH3)2COOR5, wherein R5 is hydrogen or an easily hydrolysable ester group. The compound of formula (I) is prepared by reacting 4-halo-2-oxyimino-3-oxobutyric acid of formula (IV1), wherein X, R and R5 are as defined above, with N,N-dimethylformiminium chloride chlorosulphate of formula (VII) in an organic solvent at a temperature ranging from −30° C. to −15° C. The cephalosporins that may be prepared from the intermediate include cefdinir, cefditoren pivoxil, cefepime, cefetamet pivoxil, cefixime, cefmenoxime, cefodizime, cefoselis, cefotaxime, cefpirome, cefpodoxime proxetil, cefquinome, ceftazidime, cefteram pivoxil, ceftiofur, ceftizoxime, ceftriaxone and cefuzonam.
Owner:LUPIN LTD
Novel amorphous hydrate of a cephalosporin antibiotic
InactiveUS20060094703A1Improve bioavailabilityUseful for developmentOrganic active ingredientsOrganic chemistryOrganic solventCarboxylic acid
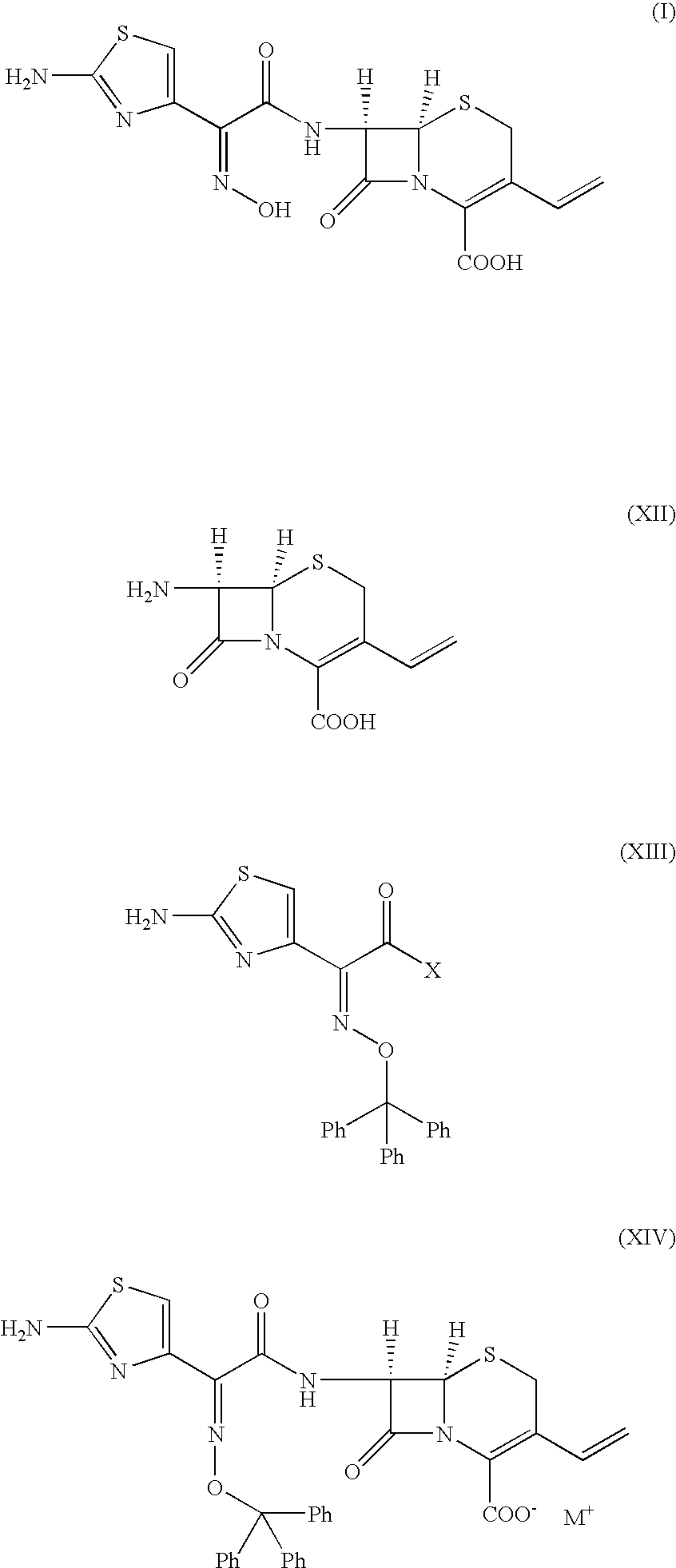
A process for the preparation of cefdinir of the formula (I) the said process comprising the steps of: i) condensing 7-amino-3-cephem-4-carboxylic acid of the formula (XII) wherein R1 is as defined above with compound of the formula (XIII) in the presence of a tertiary amine and an organic solvent, followed by treatment with a base to produce a salt of compound formula (XIV), wherein M+ is a counter ion and ii) hydrolyzing the compound of the formula (XIV) using an acid in the presence of a solvent to produce cefdinir of formula (I).
Owner:ORCHID CHEM & PHARM LTD
Process for the preparation of cephalosporin intermediate and its use for the manufacture of cephalosporin compounds
InactiveUS6919449B2High yieldHigh purityAntibacterial agentsUrea derivatives preparationThioureaBiological activation
The present invention relates to a method for the preparation of cephalosporin antibiotic of the formula (II), which comprises hydrolyzing and halogenating the ester of formula (III) by photochemical irradiation in one pot using a halogenating agent in the absence or presence of a solvent, to produce compound of formula (I), activating the 4-halogeno-2-substitutedimino-3-oxo-butyric acid of formula (I) using conventional activation agents gives compound of formula (IV), condensing the activated compound of the formula (IV) with 7-amino cephem derivative of the formula (V) to produce a compound of formula (VI), and cyclizing the compound of formula (VI) with thiourea to give cephalosporin compounds of the formula (II).
Owner:ORCHID CHEM & PHARM LTD
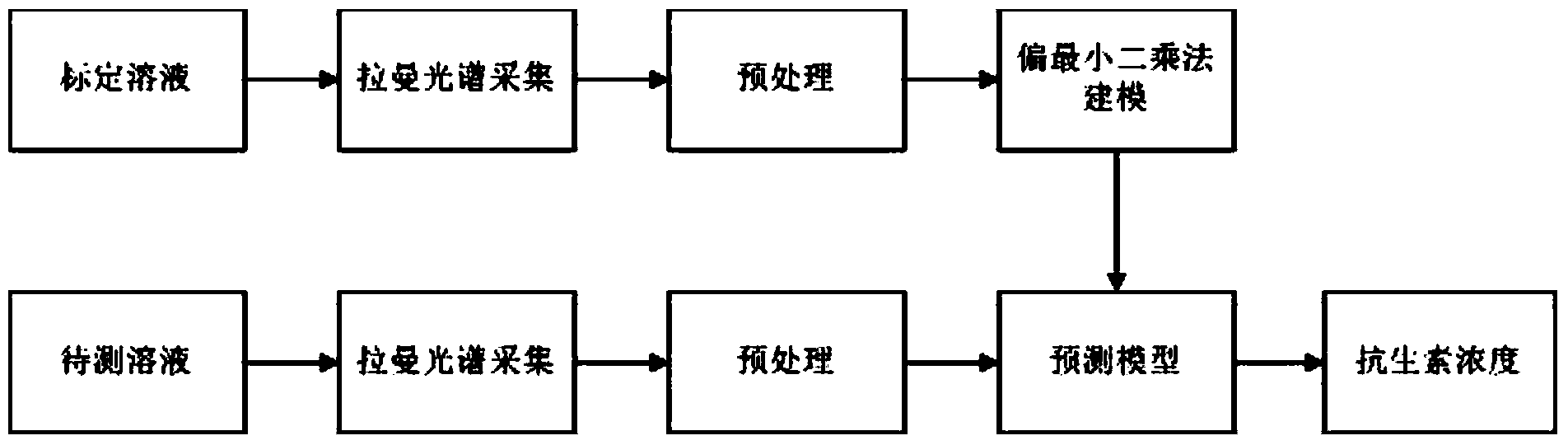
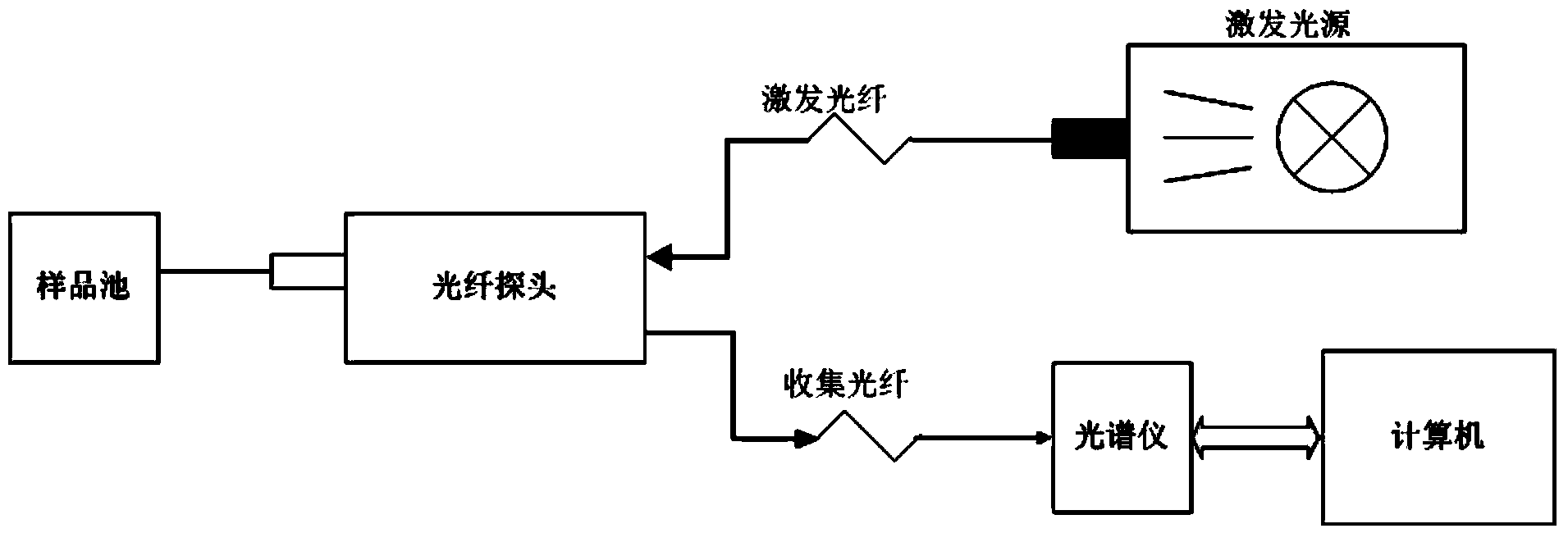
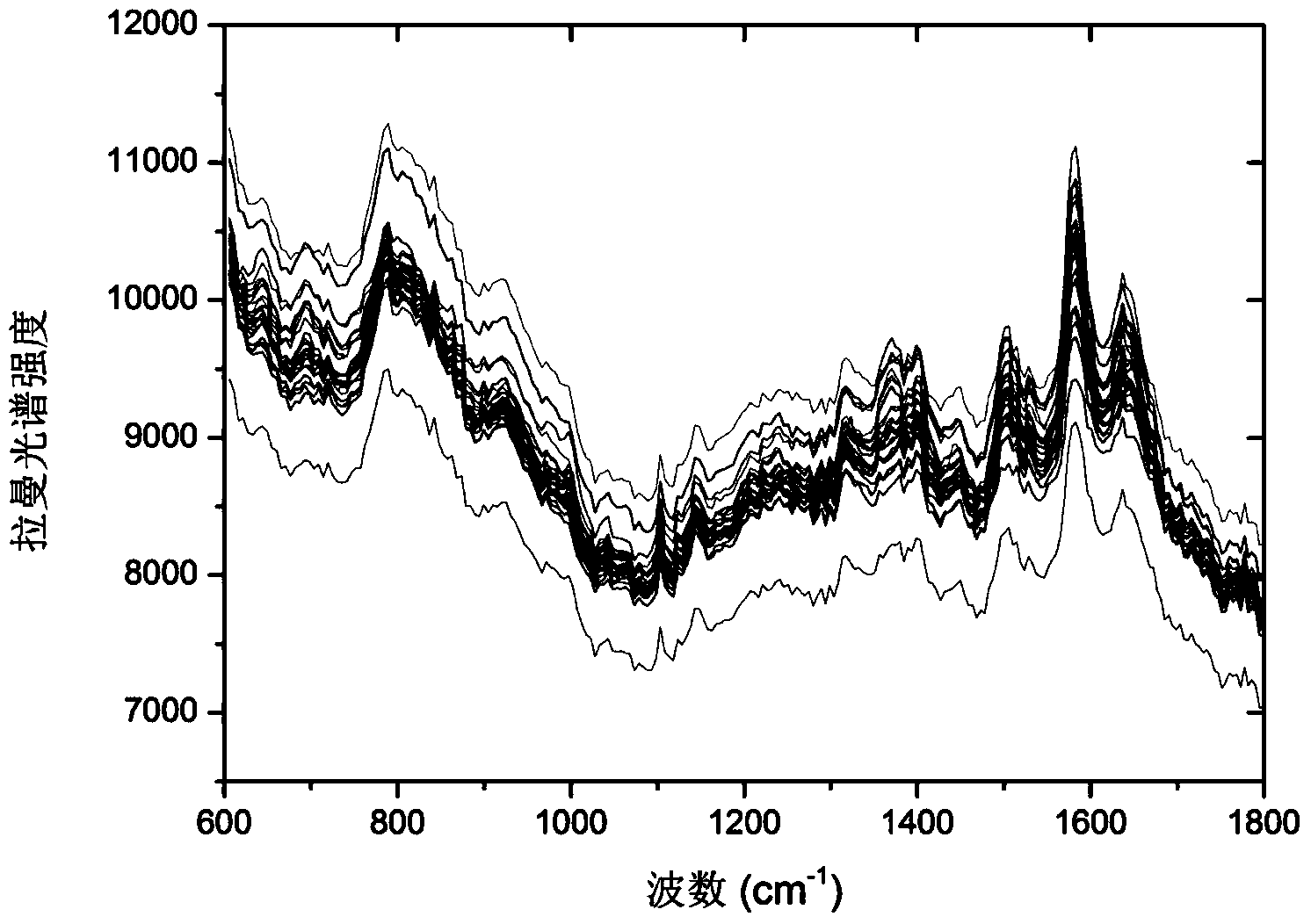
Concentration detection method for mixed solution of cephalosporin antibiotics based on Raman spectrum
InactiveCN103411955AHigh sensitivityWide detection rangeRaman scatteringAntibiotic YCephalosporin Antibiotic
The invention discloses a concentration detection method for a mixed solution of cephalosporin antibiotics based on Raman spectra. The concentration detection method comprises the following steps: (1) measuring a Raman spectrum of a mixed solution of cephalosporin antibiotics with known concentration; (2) preprocessing the Raman spectrum of the mixed solution of the cephalosporin antibiotics with the known concentration in the step (1); (3) processing the preprocessed Raman spectrum of the mixed solution of the cephalosporin antibiotics in the step (2) by a partial least square method so as to obtain a corrected prediction model; (4) acquiring a Raman spectrum of a mixed solution of to-be-measured cephalosporin antibiotics, and carrying out preprocessing; (5) inputting the Raman spectrum preprocessed in the step (4) into the prediction model so as to obtain the concentration of the mixed solution of the cephalosporin antibiotics. According to the concentration detection method, the concentration of the mixed solution of the multivariable cephalosporin antibiotics is detected by virtue of the Raman spectra. The method is quick and accurate, overcomes the limitations of the prior art effectively, and has important significances on fast food safety inspection and real-time monitoring.
Owner:TIANJIN UNIV
Preparation method and application of polyvinyl alcohol immobilized acylase
The invention discloses a preparation method of polyvinyl alcohol immobilized acylase. The preparation method comprises the steps: preparing a mixed aqueous solution from polyvinyl alcohol and a cross-linking agent, adding acylase into the mixed aqueous solution of the polyvinyl alcohol and the cross-linking agent, uniformly mixing to obtain an acylase polyvinyl alcohol gel aqueous solution, and carrying out formation and solidification process treatment on the acylase polyvinyl alcohol gel aqueous solution to obtain the polyvinyl alcohol immobilized acylase. The polyvinyl alcohol immobilized acylase is used for synthesizing a cephalosporin antibiotics midbody 7-ACA (Aminocephalosporanic Acid) or 7-ADCA (Aminodesacetoxycephalosporanic Acid). According to the preparation method disclosed by the invention, as PVA (Polyvinyl Alcohol) is used for immobilizing biological enzyme, the enzyme activity recovery rate can reach above 80 percent, immobilized acylase is also more stable and can be reused for above 50 times, so that the application cost of the acylase in the production of the 7-ACA and the 7-ADCA is greatly lowered, and the production efficiency and the product quality are improved.
Owner:JIANGSU HUITENG BIOMEDICAL TECH
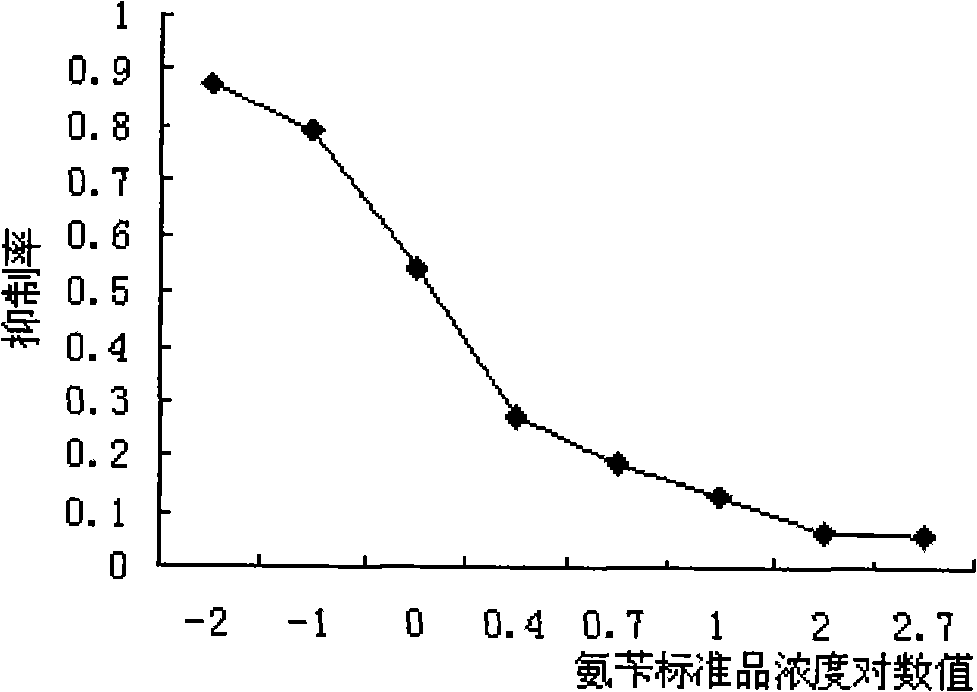
ELISA detection method for quantitative determination of cephalosporin antibiotic content in animal-derived food
InactiveCN101315372AThe pre-processing process is simpleHigh sensitivityColor/spectral properties measurementsBiotechnologyAntiendomysial antibodies
The invention relates to an enzyme-linked immune detection method for quantitatively detecting the content of spore antibiotic in animal food and belongs to the immune analysis field. The method comprises the steps of adopting the polyclonal antibody of the spore antibiotic with a group-specific antibody as a detection reagent; preparing an enzyme-linked immune detection kit; by using cephalexin as a standard, measurating the concentration of different spore class antibiotics and the relative value of the cephalexin standard through an experiment; and providing the measurated correction factors. The multi-residue detection of the enzyme-linked immune detection kit is expected to be realized. The method has the advantages of simple pretreatment, high sensitivity, high accuracy, etc.
Owner:JIANGNAN UNIV
Method for synthesizing 7-phenylacetylamino-3-chloromethyl cephalosporin alkyl acid p-methoxybenzyl ester
InactiveCN101525340AFew reaction stepsSimple and fast operationOrganic chemistryPenicillinThiosulfinate
The invention belongs to the field of cephalosporin antibiotic medicaments, and in particular relates to a method for synthesizing 7-phenylacetylamino-3-chloromethyl cephalosporin alkyl acid p-methoxybenzyl ester (GCLE). The technical proposal is that the method comprises the following steps: reacting penicillin sulfoxide ester with ammonium benzene sulfinate and 2-mercaptobenzothiazole in dichloromethane, and steaming out a solvent at normal pressure to generate aza-cyclobutanone thiosulfinate intermediate; adding dichloromethane to the intermediate after cooling, stirring and introducing saturated brine ice for cooling, adding trichloro isocyanic acid for reaction so as to generate an allylic chlorination product of the aza-cyclobutanone thiosulfinate; and reducing the pressure and drying the allylic chlorination product by distillation, adding dimethyl formamide to the product, stirring and introducing saturated brine ice for cooling, adding ammonia for reaction, adding water and dichloromethane to the mixture, mixing and stirring the mixture, standing for layering, transferring a dichloromethane layer at the bottom layer to another reactor, and steaming out the solvent at the normal pressure to obtain a dry product which is the GCLE. The method has the advantages of mild reaction condition, few reaction steps, simple operation, short production cycle, and improved production efficiency.
Owner:SHANDONG FANGXING SCI & TECH DEV
Process for preparing cefepime
A novel process is disclosed for the preparation of Cefepime, a cephalosporin antibiotic, using novel new intermediates of the general Formula, where X represents Bromine or Chlorine atom This process comprises the step of cyclizing the bromo or chloro intermediate with thiourea to produce Cefepime of high purity. A process to prepare bromo or chloro intermediate comprising the acylation of 7-Amino-3-[(1-methyl-1-pyrrolidinium) methyl]-3-cephem-4-carboxylate with 4-halo-2-methoxyimino-3-oxobutyric acid halide is also described.
Owner:HANDA VIJAY KUMAR +2
Cephalosporin compound and a process for its preparation
A process is disclosed for the preparation of 7-amino-3-(2-furanylcarbonylthiomethyl)-3-cephem-4-carboxylic acid of the formula This compound is useful as an intermediate for the preparation of ceftiofur cephalosporin antibiotic used for bovine respiratory infections.
Owner:AUROBINDO PHARMA LTD
Amorphous hydrate of a cephalosporin antibiotic
InactiveUS7244842B2Improve bioavailabilityUseful for developmentOrganic active ingredientsOrganic chemistryOrganic solventCarboxylic acid
A process for the preparation of cefdinir of the formula (I) the said process comprising the steps of: i) condensing 7-amino-3-cephem-4-carboxylic acid of the formula (XII) wherein R1 is as defined above with compound of the formula (XIII) in the presence of a tertiary amine and an organic solvent, followed by treatment with a base to produce a salt of compound formula (XIV), wherein M+ is a counter ion and ii) hydrolyzing the compound of the formula (XIV) using an acid in the presence of a solvent to produce cefdinir of formula (I).
Owner:ORCHID CHEM & PHARM LTD
Process for the preparation of cephalosporin antibiotic
InactiveUS20060058281A1Easy to condenseImpurity formation is highOrganic active ingredientsArsenic organic compoundsCephalosporin AntibioticOrganic chemistry
An improved one-pot process for the preparation of Ceftiofur of the formula (I) or its salt, without isolating intermediate compound.
Owner:ORCHID CHEM & PHARM LTD
Method for removing bacterial endotoxins in cephalosporin antibiotics by utilizing macroporous adsorption resins
The invention discloses a method for removing bacterial endotoxins in cephalosporin antibiotics by utilizing macroporous adsorption resins, comprising the following steps: firstly filtering the cephalosporin antibiotics solution containing the bacterial endotoxins and removing solid insoluble matters; then utilizing the pretreated macroporous adsorption resins to carry out static adsorption or dynamic adsorption on the cephalosporin antibiotics solution; and finally crystallizing, drying and grinding the solution after adsorption treatment, thus obtaining the product with bacterial endotoxins in accordance with the quality standard. The method has the advantages of high bacterial endotoxins removal rate, good selectivity, easy desorption, repeated use, low fluid resistance and easy magnification.
Owner:东瑞(南通)医药科技有限公司
Process for preparing cefquinome sulfate
InactiveCN101307064AGood treatment effectLow toxicityAntibacterial agentsOrganic chemistryCefotaxime7-ACA
The invention discloses a method for preparing cefquinome sulfate, belonging to the cephalosporin antibiotics special for animals. The method comprises the following: A. a step of preparing 7-amino-cefquinome, during which, iodotrimethylsilane reacts with 5, 6, 7, 8-tetrahydroquinoline, and a reaction product reacts with 7-amino-cephalosporanic acid, thereby obtaining the 7-amino-cefquinome; B. a step of preparing the cefquinome sulfate, during which, the 7-amino-cefquinome reacts with AE active ester after the 7-amino-cefquinome is dissolved and is decolored by sulphuric acid, and the cefquinome sulfate is obtained after the reaction product is subject to extraction, suction filtering, leaching and drying. The method uses 7-ACA which is widely used in industry, is low in cost and is easily obtained as a raw material, without using cefotaxime acid with unstable service quality; meanwhile, the method has a mild reaction condition and simple operation, reduces the dosage of the 5, 6, 7, 8-tetrahydroquinoline which is an important intermediate, and greatly improves the product quality with the yield of 64.1 percent and the purity of 99.7 percent, thereby the method is suitable for industrial production.
Owner:崔增学
Method for preparing cephalosporium acremonium proteome
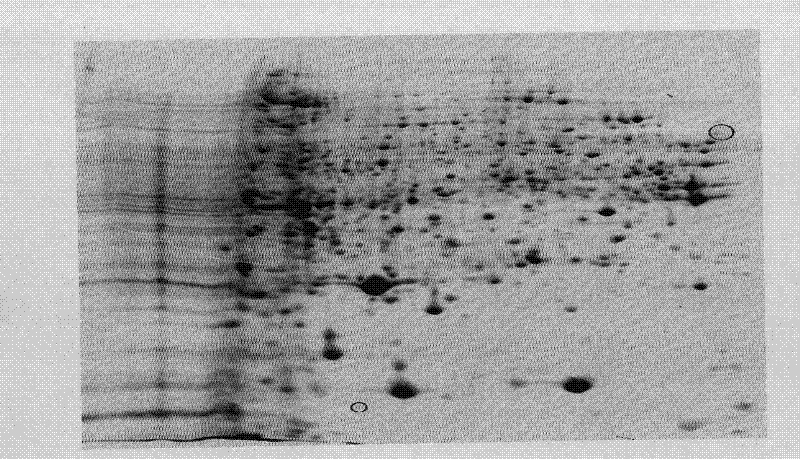
InactiveCN102443047AOptimized Molecular BreedingOptimal Metabolic EngineeringPeptide preparation methodsTotal proteinAntibiotic Y
The invention discloses a method for preparing cephalosporium acremonium proteome, which comprises the following steps that: cephalosporium acremonium thalli are ground into powder with liquid nitrogen, and then, total protein samples are extracted by a trichloroacetic acid (TCA)-acetone precipitation method; the obtained total protein samples are mixed with hydrated solution, hydration sampling is carried out, immobilized pH gradient (IPG) solid phase rubber strips are adopted, next, isoelectric focusing is carried out, the rubber strips are balanced after the isoelectric focusing completion and are then transferred onto sodium dodecyl sulfonate-polyacrylamide gel electrophoresis (SDS-PAGE) gel, electrophoresis is carried out, the electrophoresis is stopped when bromophenol blue indicators reach the bottom edge, and the gel is obtained; and the transmission scanning is carried out with a gel scanning instrument after the obtained gel is dyed, and the obtained images are analyzed by using software. The cephalosporin C has the important place in the cephalosporin antibiotics production, so the study on the cephalosporium acremonium proteome can be carried out, the foundation is laid for optimizing the molecular breeding and the metabolic engineering of cephalosporin producing strains, and the important significance is realized.
Owner:SHANGHAI INST OF PHARMA IND
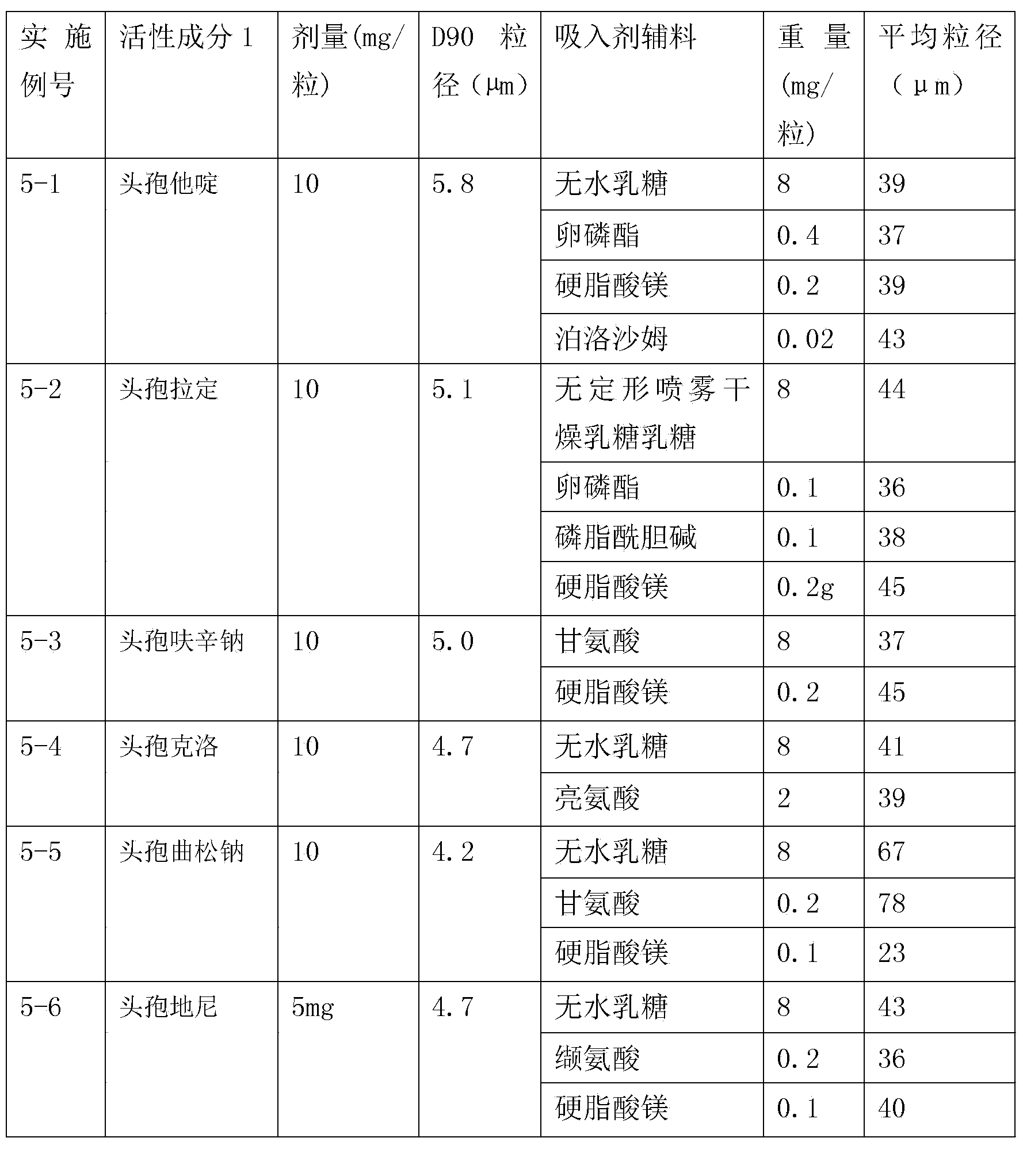
Inhalation preparation containing cephalosporin antibiotic
The invention relates to an inhalation preparation containing cephalosporin antibiotic and applications thereof. The cephalosporin antibiotic is made into an inhalation dosage form, and thus the preparation can treat pulmonary bacterial infection with less dosage and has a certain curative effect on asthma and chronic obstructive pneumonia.
Owner:TIANJIN JINYAO GRP
Compound inhalation preparation containing cephalosporin antibiotic and glucocorticoid
The invention relates to a compound inhalation preparation containing cephalosporin antibiotic and glucocorticoid and applications thereof. The cephalosporin antibiotic is made into an inhalation dosage form, so that the preparation can treat pulmonary bacterial infection with less dosage form and has a certain curative effect on asthma and chronic obstructive pneumonia at the same time.
Owner:TIANJIN JINYAO GRP
Process for the preparation of cephalosporin antibiotic
InactiveUS7345169B2Easy to condenseOrganic active ingredientsArsenic organic compoundsCephalosporin AntibioticPhotochemistry
An improved one-pot process for the preparation of Ceftiofur of the formula (I) or its salt, without isolating intermediate compound
Owner:ORCHID CHEM & PHARM LTD
Detection method for antibiotic resistance genes
ActiveCN111100935AAchieving Simultaneous DetectionLow costMicrobiological testing/measurementDNA/RNA fragmentationCephalosporin AntibioticDrug resistance
The invention provides a detection method for antibiotic resistance genes. The method can simultaneously detect the presence or level of multiple antibiotic resistance genes (such as KPC and other genes capable of causing bacterial drug resistance to carbapenem antibiotics, CTX-M and other genes capable of causing drug resistance to beta-lactam antibiotics, AAC and other genes capable of causing drug resistance to cephalosporin antibiotics, and drug resistance genes capable of causing bacterial drug resistance to other types of antibiotics) in nucleic acid molecules in samples. A probe set anda kit including the probe set of one or more kinds are also provided. The probe set and kit can be used for implementing the method. In addition, the kit is also provided; the kit can simultaneouslydetect the presence or level of the multiple antibiotic resistance genes (such as KPC and other genes capable of causing bacterial drug resistance to carbapenem antibiotics, CTX-M and other genes capable of causing drug resistance to beta-lactam antibiotics, AAC and other genes capable of causing drug resistance to cephalosporin antibiotics and the drug resistance genes capable of causing bacterial drug resistance to other types of antibiotics) in the nucleic acid molecules in the samples in a round of reaction.
Owner:XIAMEN UNIV
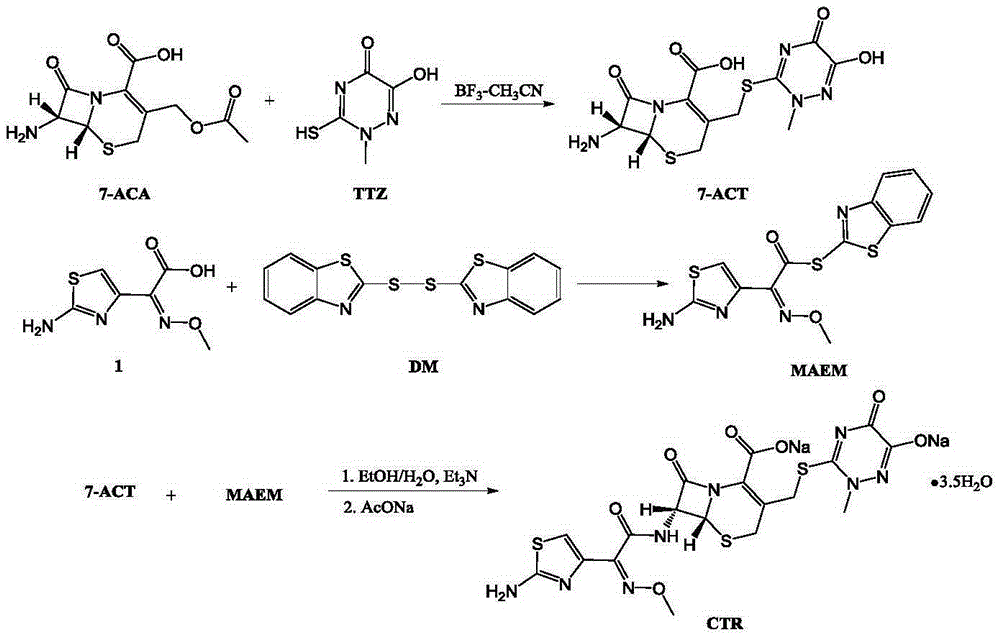
Original-quality ceftriaxone sodium and pharmaceutical preparation thereof
ActiveCN105418641AHarm reductionToxicAntibacterial agentsOrganic active ingredientsChloroformateAntibiotic Y
The invention discloses original-quality ceftriaxone sodium and a pharmaceutical preparation thereof. The key technology and industrialization of the third generation of cephalosporin antibiotics active ester intermediate wins the second prize of National Scientific and Technological Progress Award, and the third generation of cephalosporin antibiotics intermediate AE active ester is a key factor for affecting the internal quality of the ceftriaxone sodium. A preparation method comprises the steps that 1, boron trifluoride-acetonitrile serves as a catalyst, and on the condition that acetonitrile serves as solvent, a triazine ring is reacted with 7-ACA to generate 7-ACT; 2, triethylamine and aminothiazoly loximate are added into the solvent, a chloroformate activator is dropwise added slowly during cooling mixing, the 7-ACT is added for a one-pot reaction after stirring is conducted, and ceftriaxone is obtained; 3, a salt-forming agent is added, and the ceftriaxone sodium is obtained. According to the preparation method, use of a condensing agent with higher price is avoided, the process route is shortened, operation is easy, the reaction condition is mild, the product yield is high, the purity is good, and industrial production is easy.
Owner:广东金城金素制药有限公司 +1
Process for the preparation of a cephalosporin antibiotic
An improved process for the preparation of ceftriaxone sodium comprising the steps of: i) reacting the 3-cephem derivative of formula (II) with halo acid derivative of formula (III) wherein X represents halogen and Y represent halogen in the presence of silylating agent and methylene chloride at −25 to 10° C., to produce (IV), ii) quenching the reaction by pouring the reaction mixture into water or in a aqueous solution of sodium carbonate, iii) preparing sodium salt solution of (IV) by adding sodium carbonate and separating the organic layer, iv) cyclizing the sodium salt of (IV) in the aqueous solution with thiourea at a temperature in the range of 0 to 30° C., v) adjusting the pH to 1.5 to 2.5 to precipitate the ceftriaxone free acid, vi) converting the ceftriaxone free acid to sodium salt using sodium-2-ethyl hexanoate in water and vii) precipitating and isolating the ceftriaxone sodium.
Owner:ORCHID CHEM & PHARM LTD
Monoclonal antibody, enzyme-linked immunosorbent assay method and kit for detecting cephalosporin antibiotics
ActiveCN104558187AHigh recognition sensitivityExcellent recognition sensitivityMicroorganism based processesTissue cultureAntibiotic YCephalosporin Antibiotic
The invention discloses a specific monoclonal antibody capable of resisting various cephalosporin antibiotics such as ceftiofur, ceftriaxone and the like. The invention further discloses an enzyme-linked immunosorbent assay method and kit for detecting the various cephalosporin antibiotics such as the ceftiofur, the ceftriaxone and the like. According to the invention, the monoclonal antibody is secreted by a hybridoma cell 4D5 of which the preservation number is CCTCC No. C201341. Compared with the prior art, the monoclonal antibody, prepared by the invention, can be used for distinguishing the various cephalosporin antibiotics such as the ceftiofur, the ceftriaxone and the like at the same time. The enzyme-linked immunosorbent assay method and kit disclosed by the invention have the advantages of high detection efficiency, high sensitivity, high precision, high accuracy and the like.
Owner:HUAZHONG AGRI UNIV
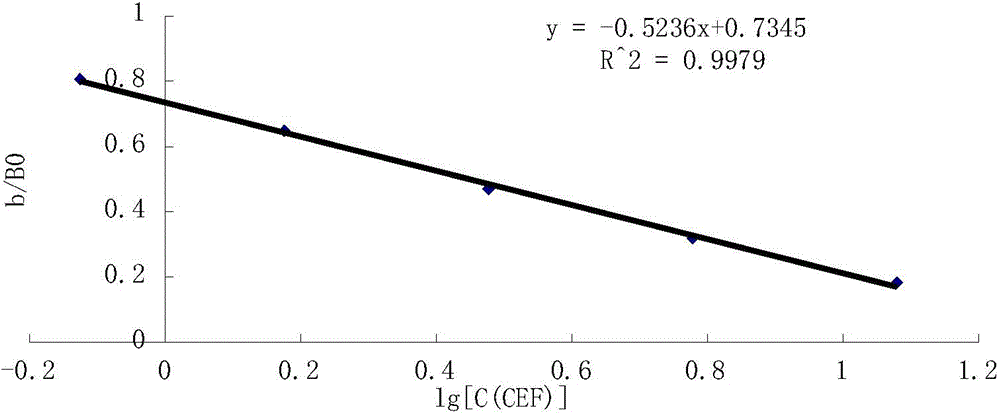
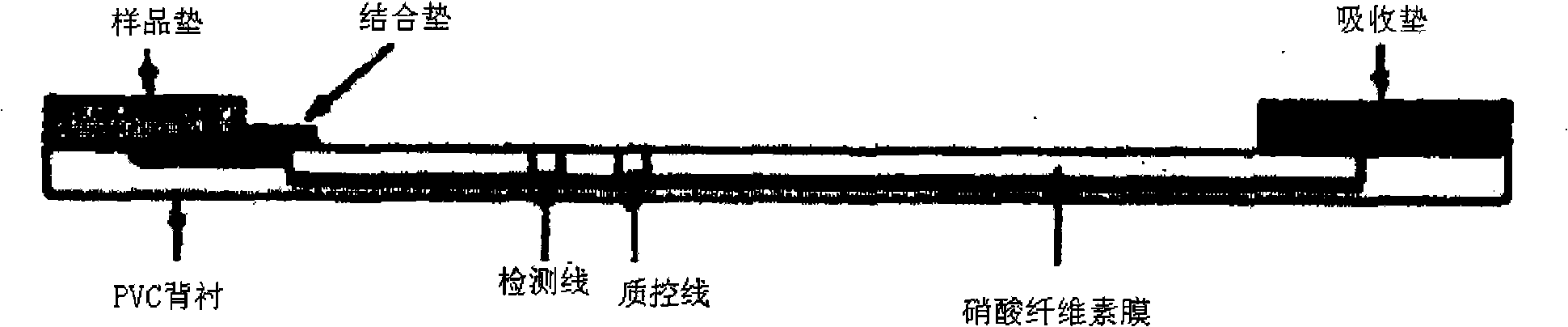
Preparation method of immune chromatography test paper for detecting cephalosporin antibiotic
InactiveCN101315364ASuitable for on-site testingSimple and fast operationBiological testingCelluloseReagent strip
The invention provides a method for preparing an immunity chromatographic test strip for testing cephalosporins antibiotics, and relates to the immunity testing field. The test strip is composed of a sample pad, a combined pad, a cellulose nitrate membrane, a water absorbing pad and a PVC backing; the sample pad, the combined pad, the cellulose nitrate membrane and the water absorbing pad are sequentially stuck to the PVC backing; the combined pad is coated with a group-specific anti-cephalosporins antibiotic antibody colloidal gold label of anti-cephalosporins antibiotics; and the cellulose nitrate membrane is sequentially coated with a cefalexin-OVA test line and a goat anti-rabbit IgG quality control line. The method prepares the test trip by assembling the test preparation of the combination of the group-specific antibody of the anti-cephalosporins antibiotics and the chromogenic colloidal gold, and carries out a multi-residue screening test of the cephalosporins antibiotics with low cost and rapidness; and the portable test strip which can be used for the cephalosporins antibiotic residue tests of different samples is the application of the immunity chromatographic analysis method.
Owner:JIANGNAN UNIV
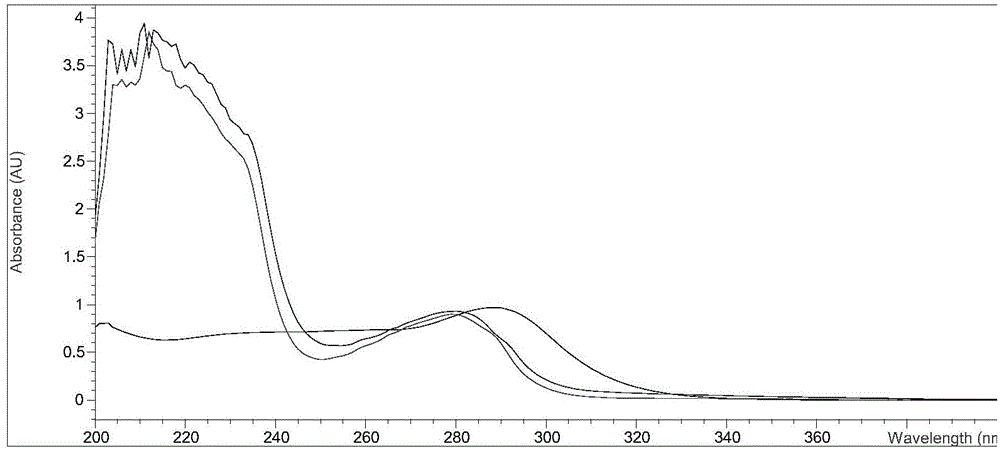
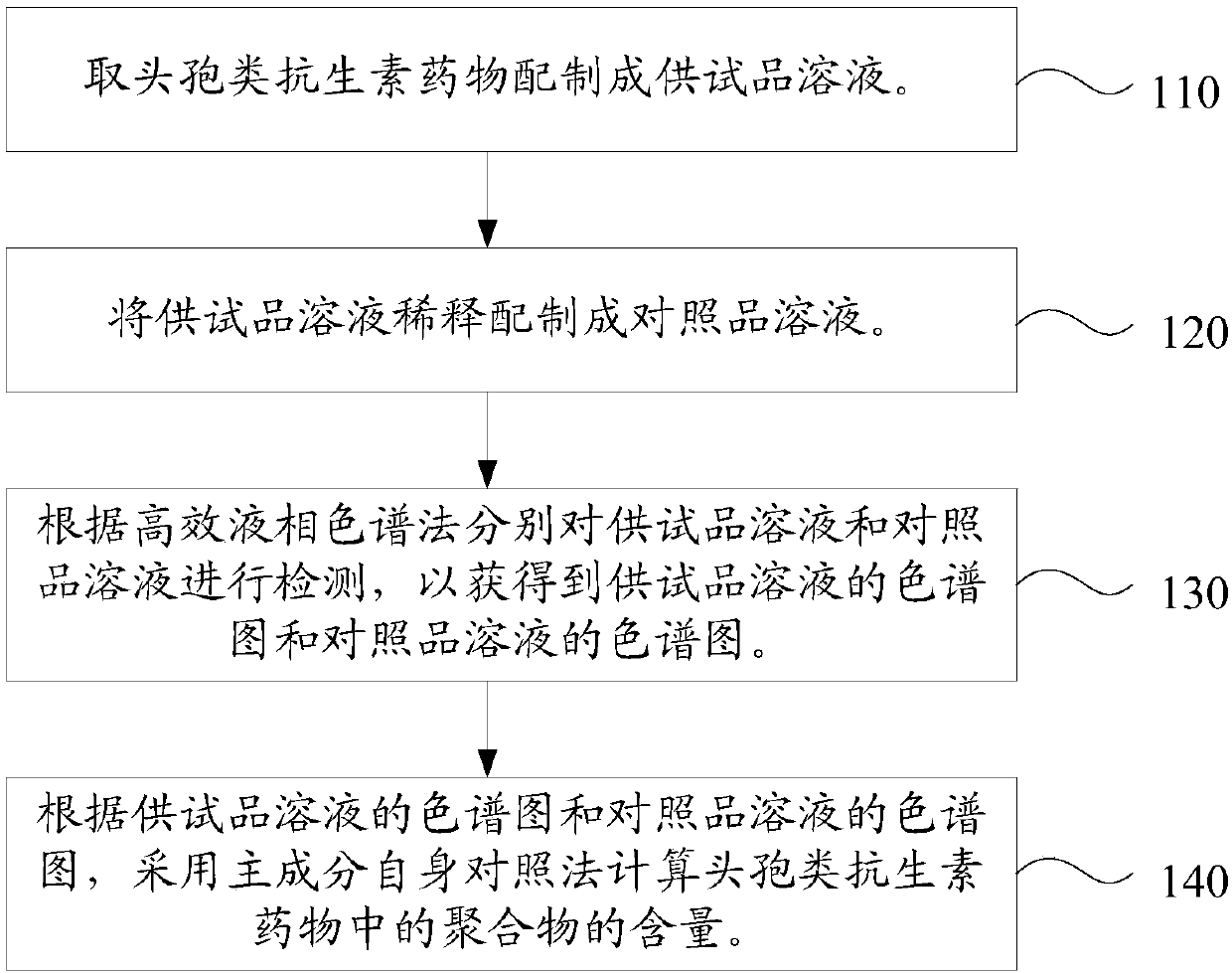
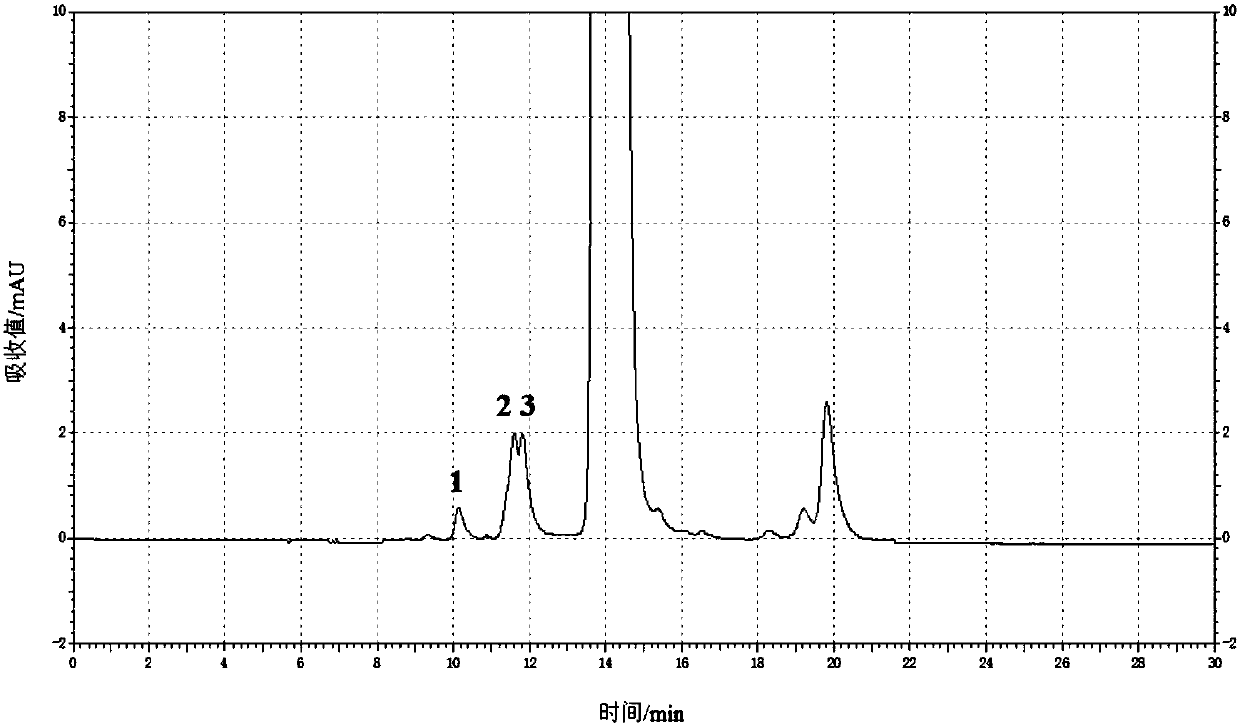
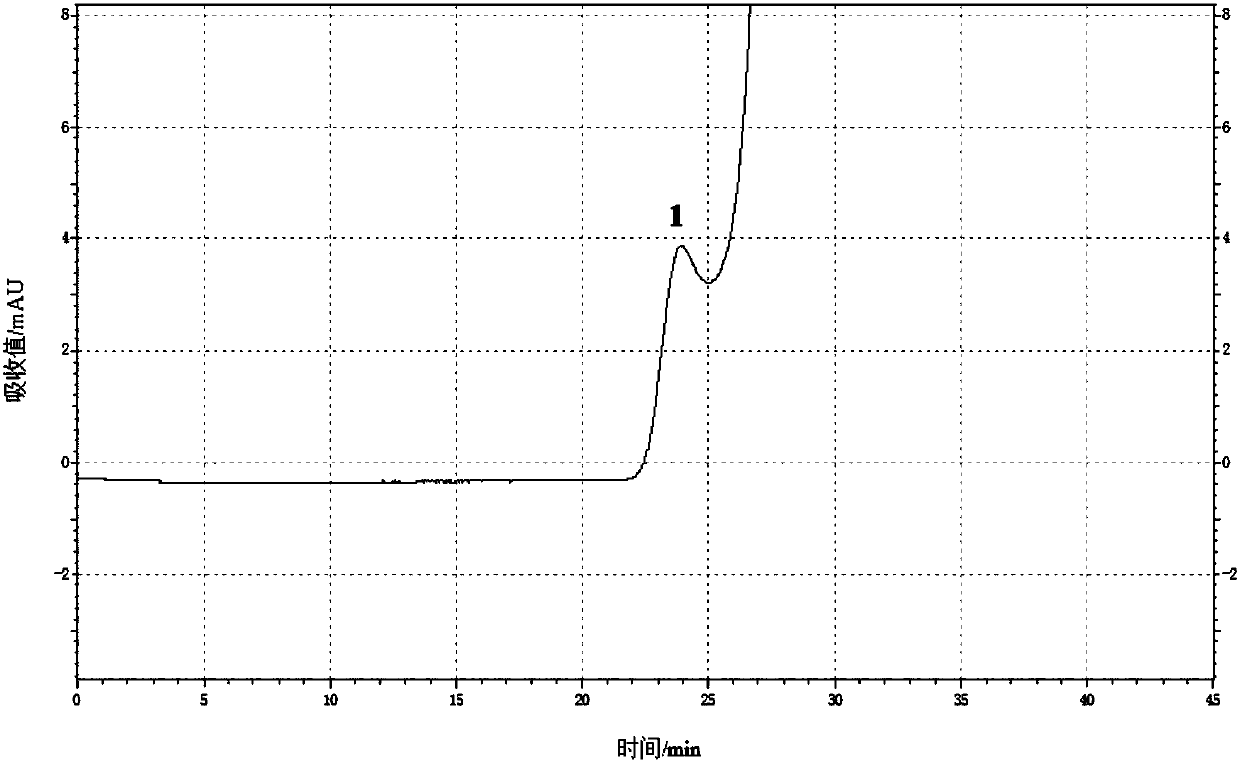
Content determination method of polymer in cephalosporin antibiotic drug
The invention relates to a content determination method of a polymer in a cephalosporin antibiotic drug. The content determination method of the polymer in the cephalosporin antibiotic drug comprisesthe following steps: detecting a test sample solution and a reference solution respectively according to a high-performance liquid chromatography, so as to obtain a chromatogram of the test sample solution and a chromatogram of the reference solution, wherein the test sample solution is a solution containing the cephalosporin antibiotic drug, the reference solution is a diluted solution of the test sample solution and chromatographic conditions are as follows: a mobile phase is a mixed solution of a water solution of ammonium acetate and acetonitrile, and a chromatographic column is a gel chromatographic column; calculating the content of the polymer in the cephalosporin antibiotic drug by adopting a main component self-control method according to the chromatogram of the test sample solution and the chromatogram of the reference solution. An experiment proves that the content determination method has relatively high accuracy in determination of the content of the polymer in the cephalosporin antibiotic drug.
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
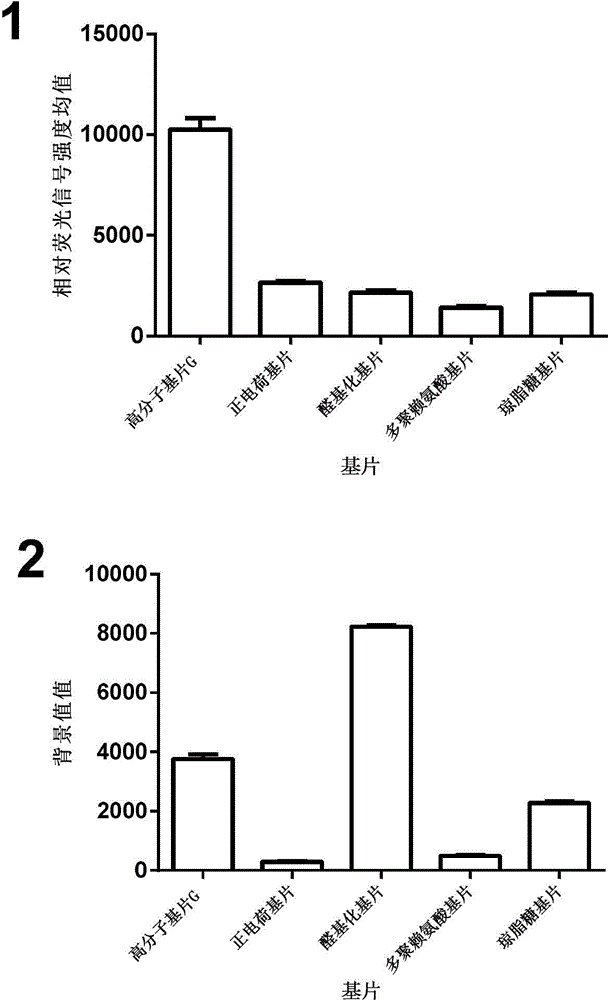
Antibody chip kit and method for detecting residual cephalosporin antibiotics in food
The invention discloses an antibody chip kit for detecting residual cephalosporin antibiotics in food, and belongs to the technical field of the drug residue detection. The kit comprises a chip, an antibody, a second antibody marked by Cy3, and an extraction reagent, the antibody is composed of a cefalexin monoclonal antibody and a ceftiofur monoclonal antibody, and the cefalexin monoclonal antibody is secreted by hybridoma 3A6 with a preservation number of CCTCC NO: C201340; the ceftiofur monoclonal antibody is secreted by hybridoma 4D5 with a preservation number of CCTCC NO: C201341, and the chip fixes cefalexin coating antigen and cefalexin coating antigen. The invention further discloses an antibody chip method for detecting residual cephalosporin antibiotics in food, the method can be used for simultaneously detecting 8 kinds of cephalosporins, and has the advantages of high accuracy, high precision, high efficiency, etc.
Owner:HUAZHONG AGRI UNIV
Original development quality ceftazidime and medicine preparation thereof
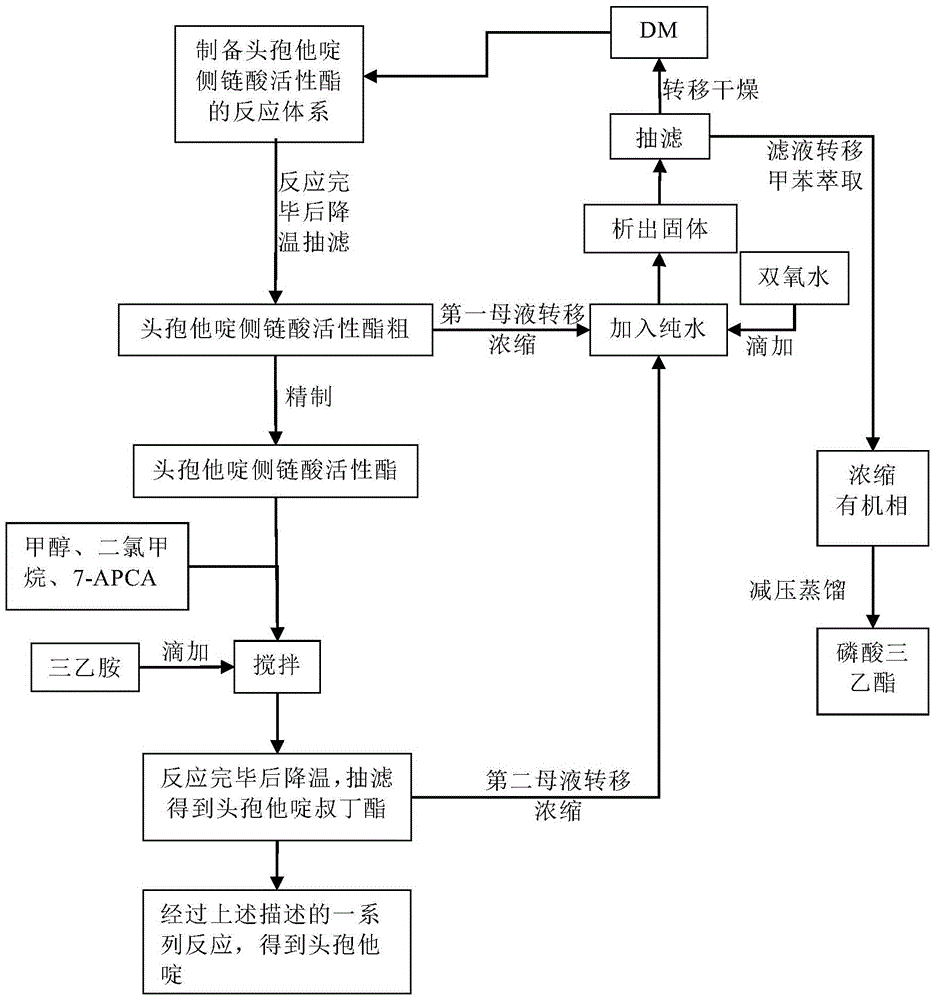
The invention discloses original development quality ceftazidime and a medicine preparation thereof. The third-generation cephalosporin antibiotics active ester midbody key technology and industrialization obtains the second prize of National Scientific and Technological Progress Award. The cephalosporin antibiotics active ester belongs to a key factor for influencing the internal quality of the cephalosporin. A preparation method comprises the following steps that (a) mixed solvents are added into ceftazidime side chain acid, dibenzothiazyl disulfide, aniline and 2-picoline; triethyl phosphate is dripped for reaction; (b) a coarse product is refined to obtain ceftazidime side chain acid active ester, and the first mother liquid is recovered; (c) the material is added into a mixed solvent for neutralizing 7-APCA; triethylamine is dripped; the temperature reduction is performed for crystal separation and filtering to obtain ceftazidime tert-butyl ester; the second mother liquid is recovered; (d) the ceftazidime tert-butyl ester is subjected to hydrolysis and purification, and then, the ceftazidime is obtained. The original development quality ceftazidime has the advantages that high-toxicity triphenylphosphine is not used; waste liquid and waste slag can be sufficiently recovered and reutilized; the method is safe; the cost is low; the yield is high; the industrial production is facilitated.
Owner:广东金城金素制药有限公司 +1
Medicament for treating lower respiratory tract infection and preparation method thereof
InactiveCN103127430AGood curative effectNo side effects were foundAntibacterial agentsAntiviralsPinelliaAntibiotic Y
The invention provides a medicament for treating lower respiratory tract infection and a preparation method thereof, aiming at solving problems of poor effect on some pathogenic bacteria, difficult control of non-bacterial infection, high price and the like for preventing lower respiratory tract infection by using cephalosporin antibiotics in the prior art; the medicament comprises the following components by weight: 10-30 parts of fritillaria thun-bergli, 20-40 parts of almond, 10-15 parts of loquat leaves, 15-30 parts of white mulberry root bark, 6-10 parts of radix stemonae, 10-30 parts of rhizoma phragmitis, 10-15 parts of radix bupleuri, 6-10 parts of pinellia ternate, 10-15 parts of scutellaria, 30-60 parts of radix astragali, 10-15 parts of platycodon grandiflorum, 30-60 parts of gypsum, 10-15 parts of radix ophiopogonis, 20-40 parts of licorice, and 10-15 parts of citrus peel. The medicament of the invention adopts Chinese herbal medicine as main components, and has the advantages of no drug tolerance, easy control of non-bacterial infection, and low cost.
Owner:CHENGDU JIANJIANG PHARMA FACTORY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com