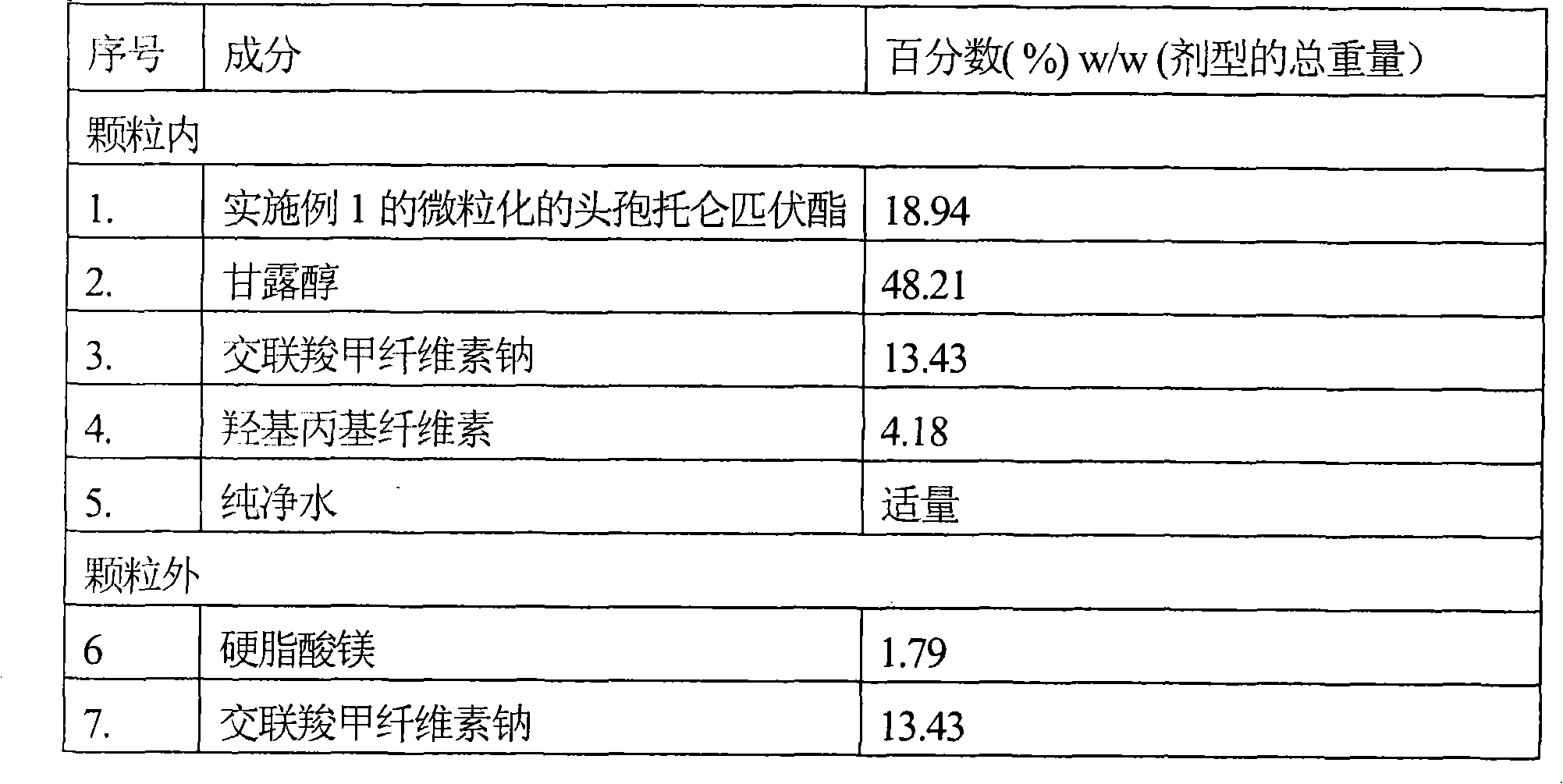
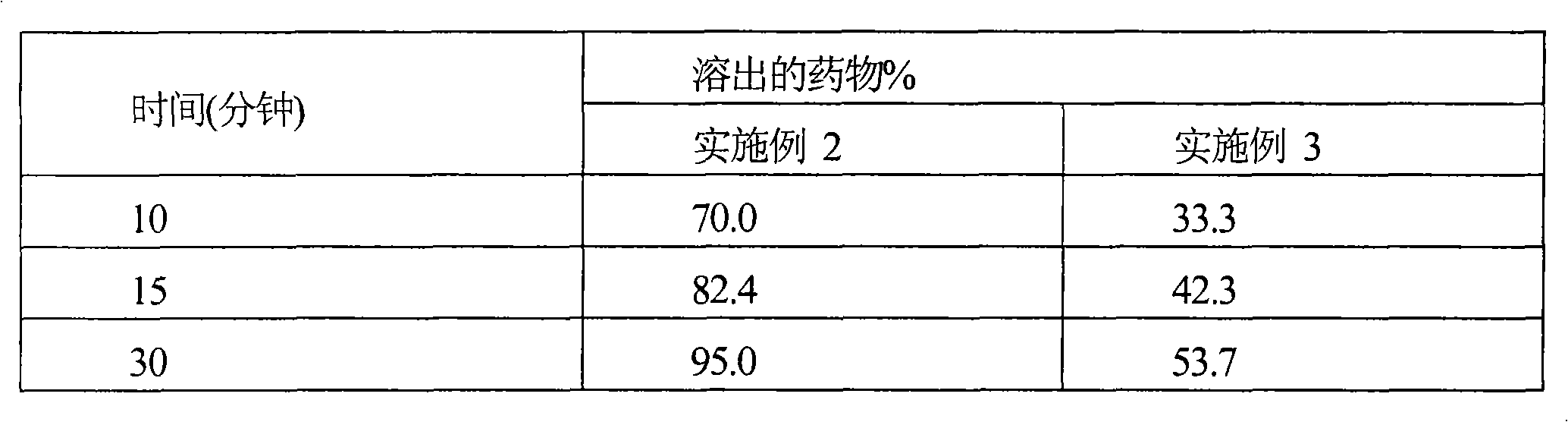
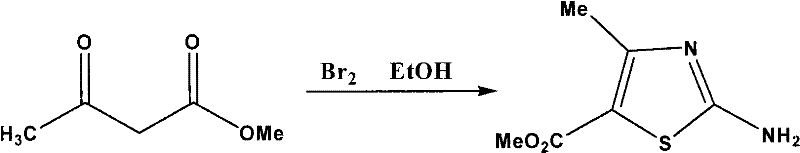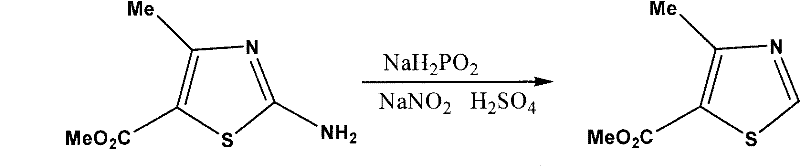Patents
Literature
41 results about "Cefditoren" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
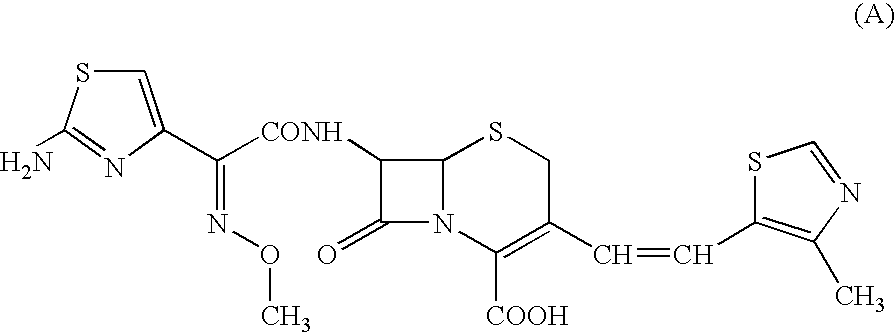
Cefditoren is used to treat a wide variety of bacterial infections.
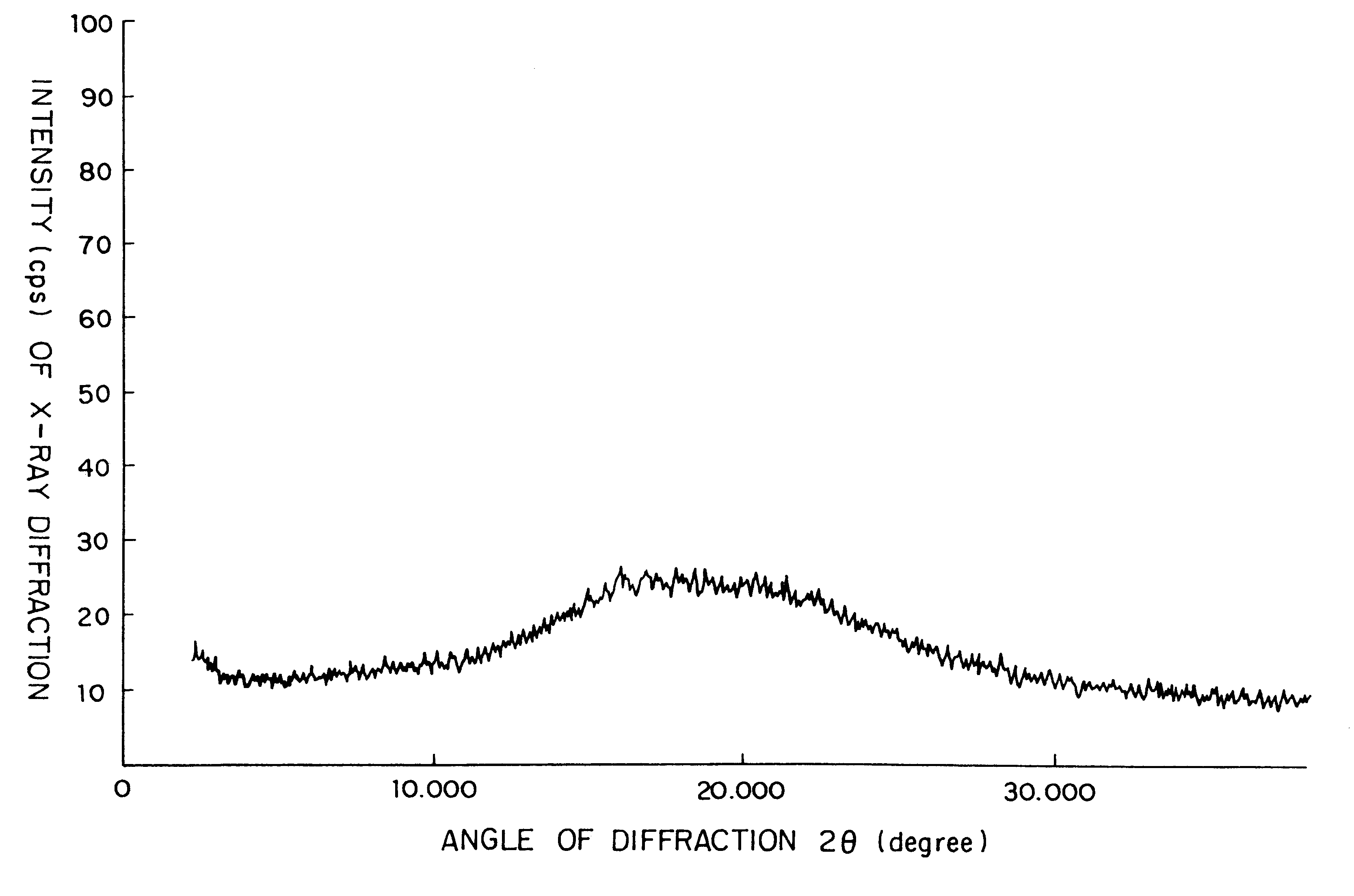
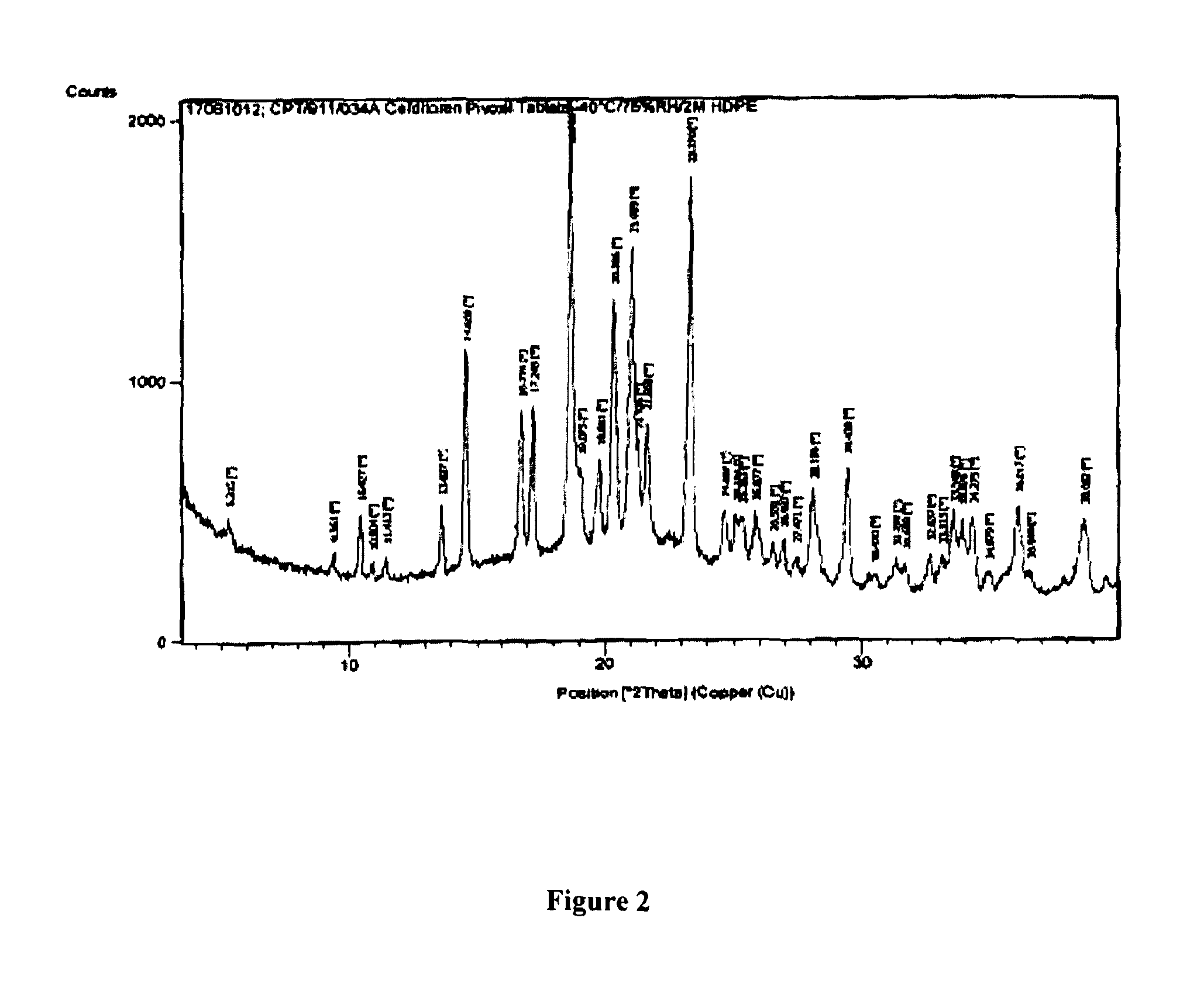
Crystalline substance of cefditoren pivoxyl and the production of the same
InactiveUS6441162B2High purityGood storage stabilityAntibacterial agentsOrganic active ingredientsOrganic solventCefditoren
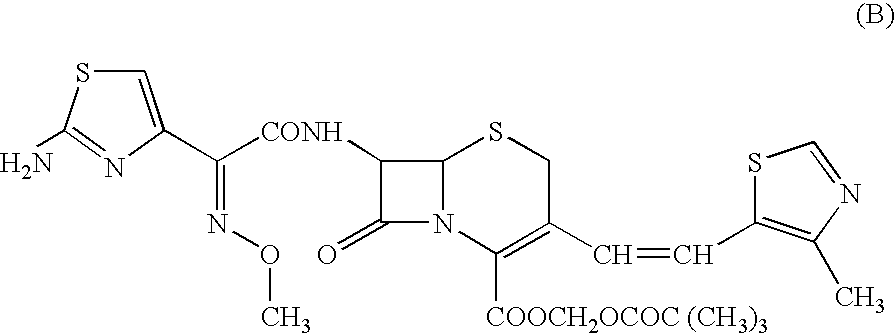
A crystalline substance of Cefditoren pivoxyl is provided which has a high purity and enhanced thermal stability on storage. This crystalline Cefditoren pivoxyl may be prepared by a process comprising a step of dissolving an amorphous form of Cefditoren pivoxyl in an anhydrous, first organic solvent capable of dissolving said amorphous form well therein, and steps of replacing the first organic solvent component of the resulting solution by an anhydrous alkanol of 1 to 5 carbon atoms as a second organic solvent, in such a manner that the firstly prepared solution of Cefditoren pivoxyl in the first organic solvent is mixed with a volume of the alkanol and then is concentrated below 15° C. under reduced pressure, and so on. Thereby, the process proceeds so as to produce a solution containing 50 mg / ml to 250 mg / ml of Cefditoren pivoxyl dissolved in the alkanol alone. From the latter solution, crystals of Cefditoren pivoxyl are induced to deposit by addition of water at a temperature of 0-10° C. The resulting admixture of the concentrated solution of Cefditoren pivoxyl in alkanol with added water and the deposited Cefditoren pivoxyl is then agitated 10° C. or below, to effect a complete crystallization of Cefditoren pivoxyl.
Owner:MEIJI SEIKA KAISHA LTD
Process for the preparation of cefditoren
InactiveUS20060173175A1Process stabilityPromote commercializationOrganic chemistryEnzymatic hydrolysisSodium iodide
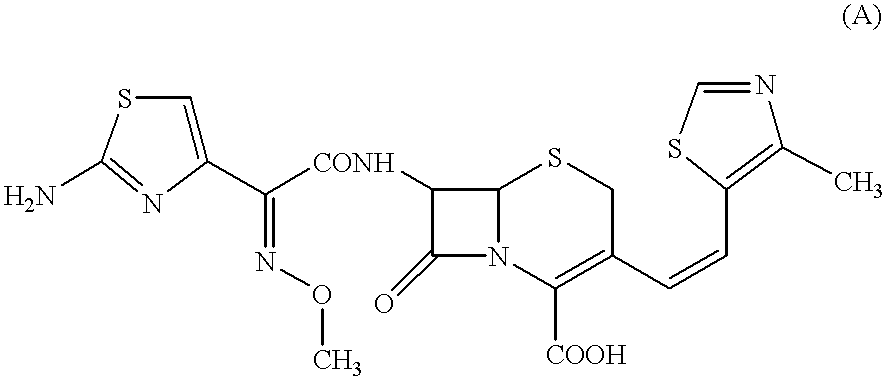
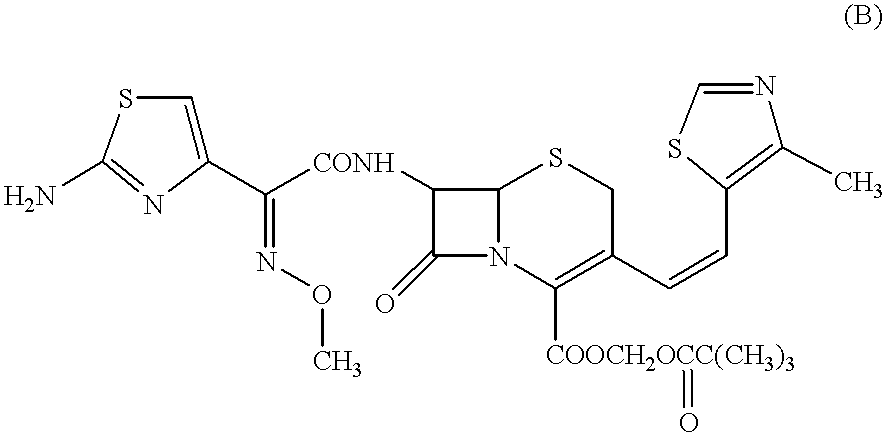
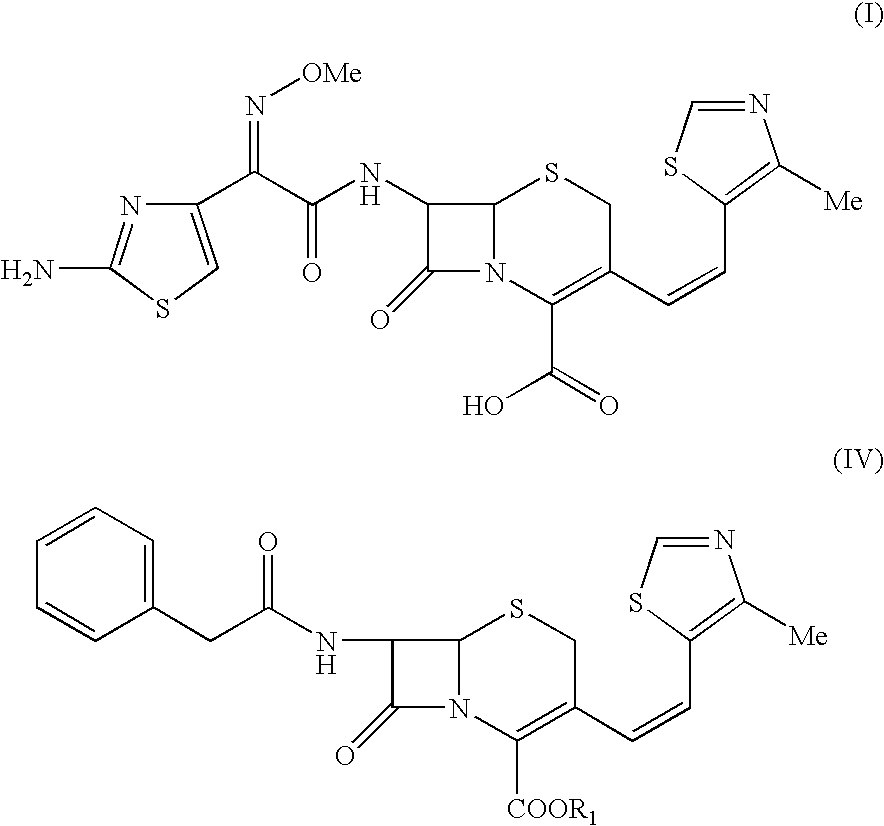
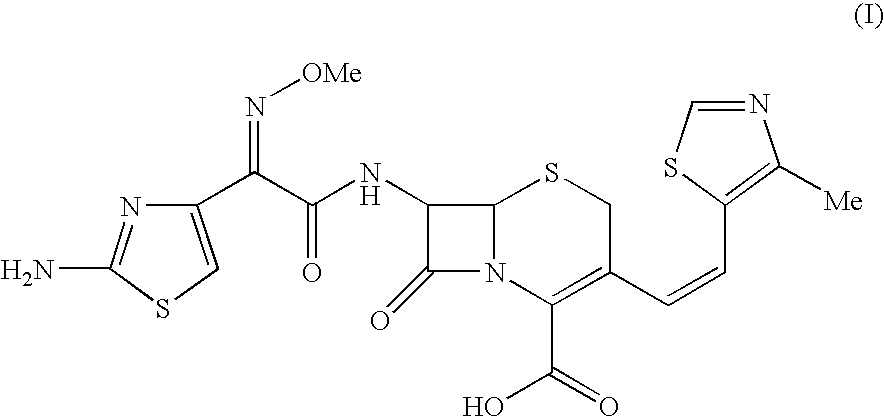
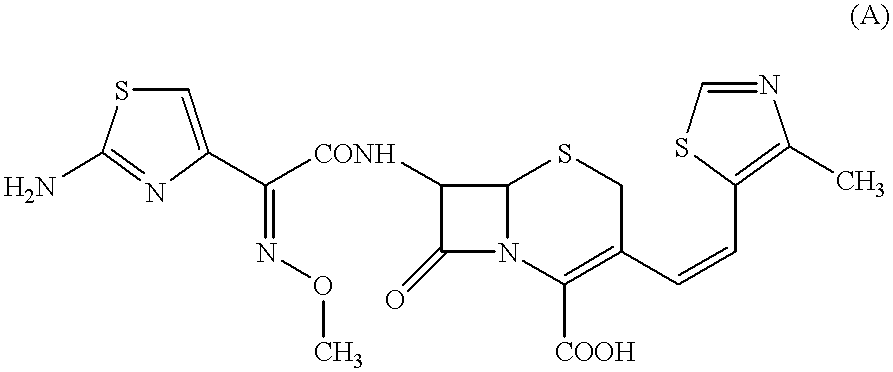
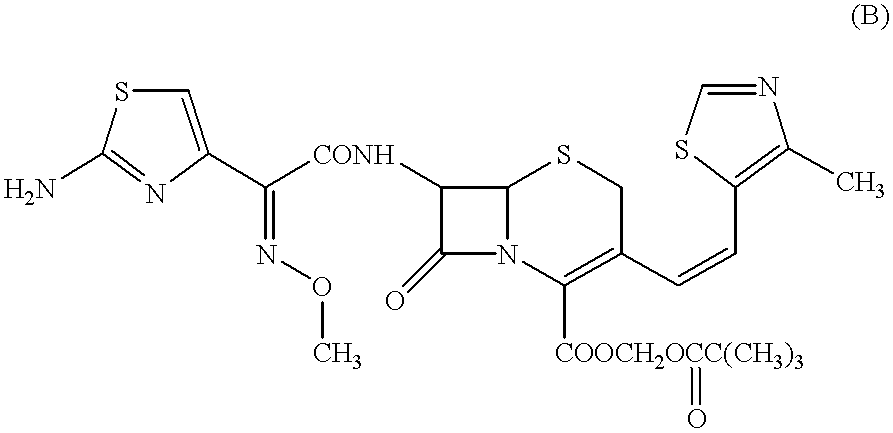
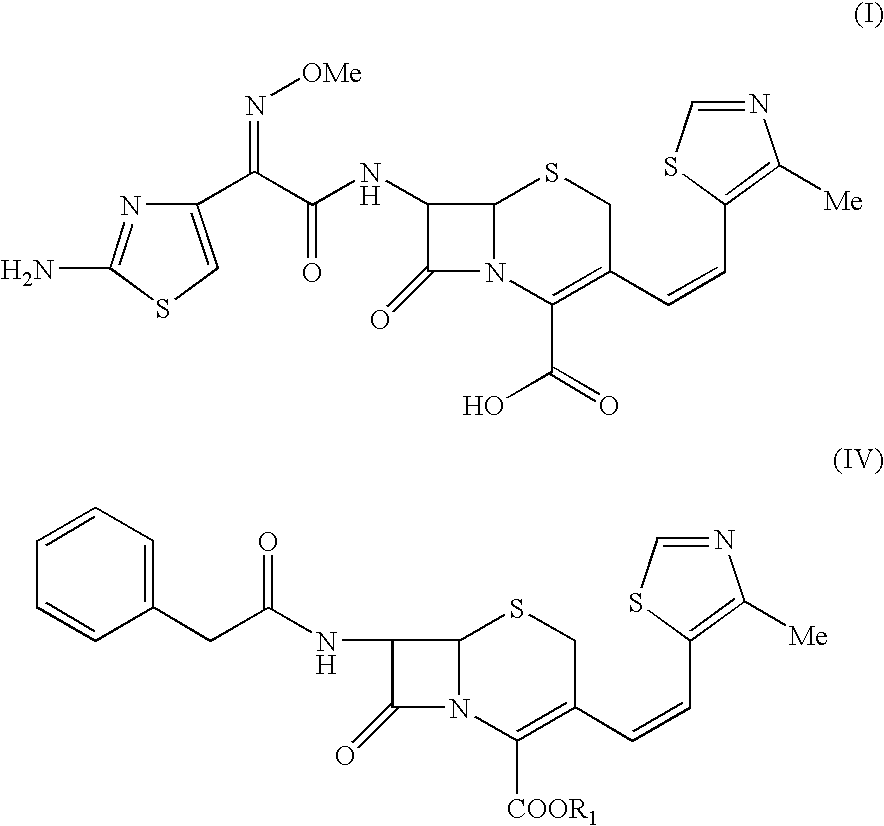
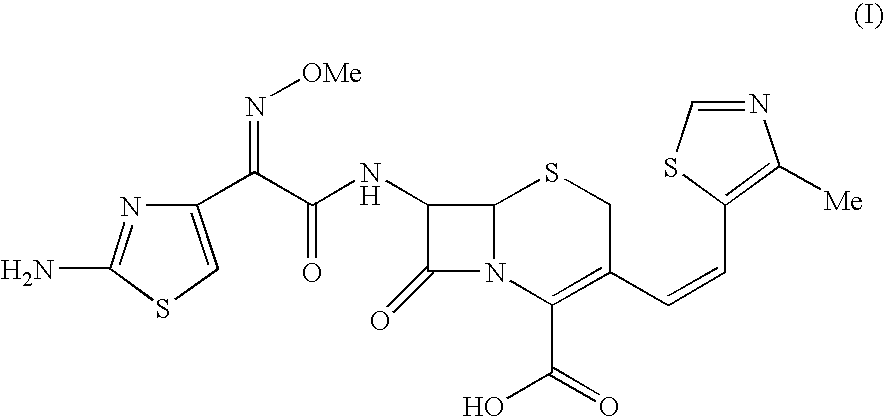
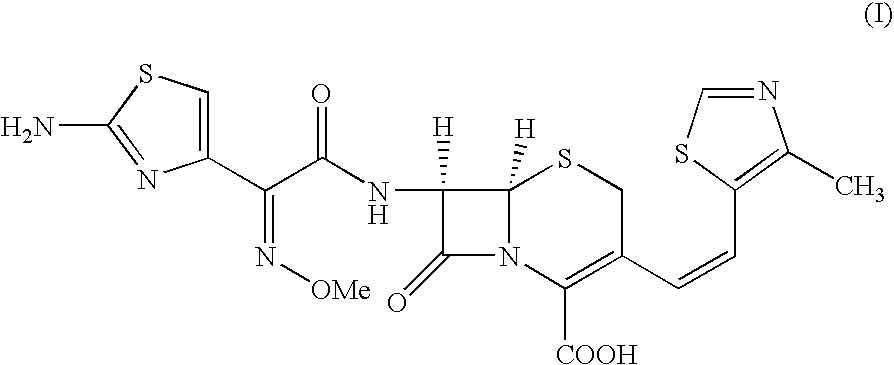
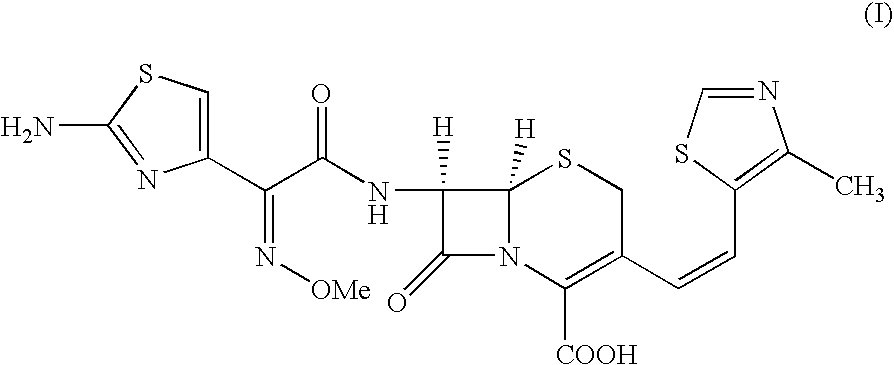
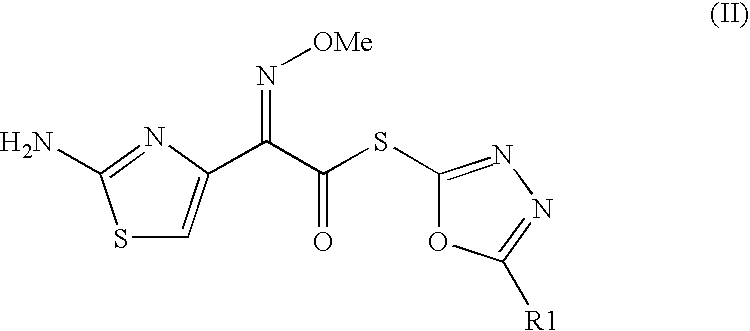
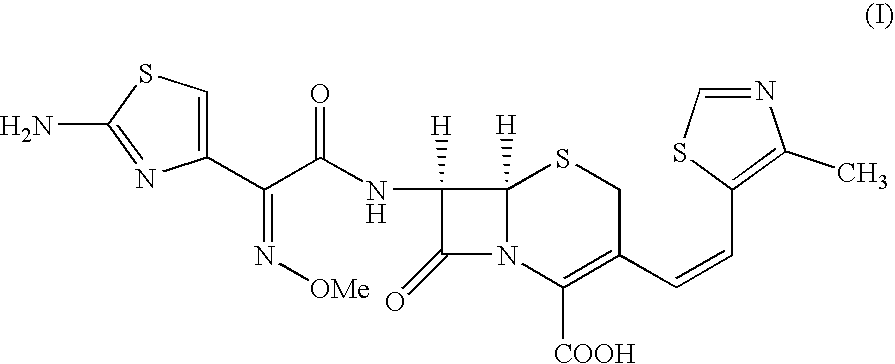
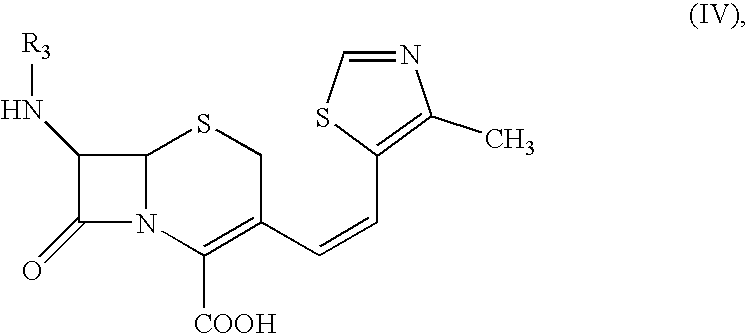
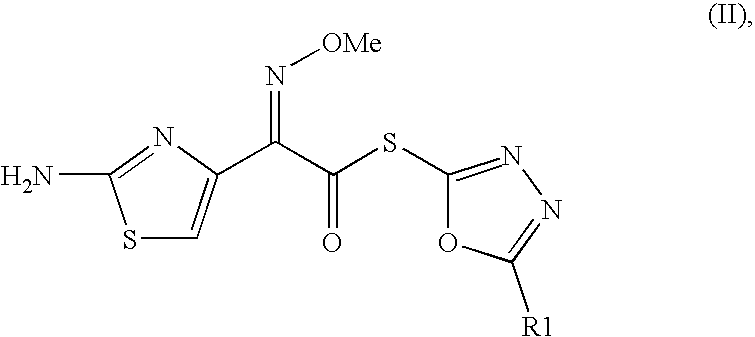
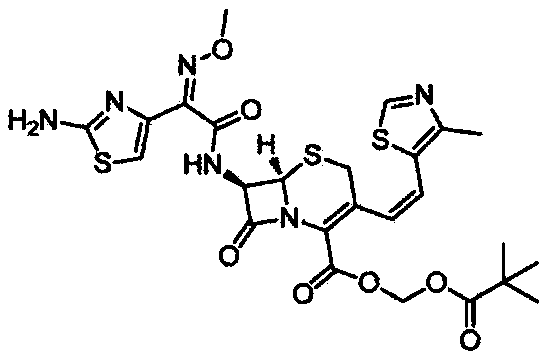
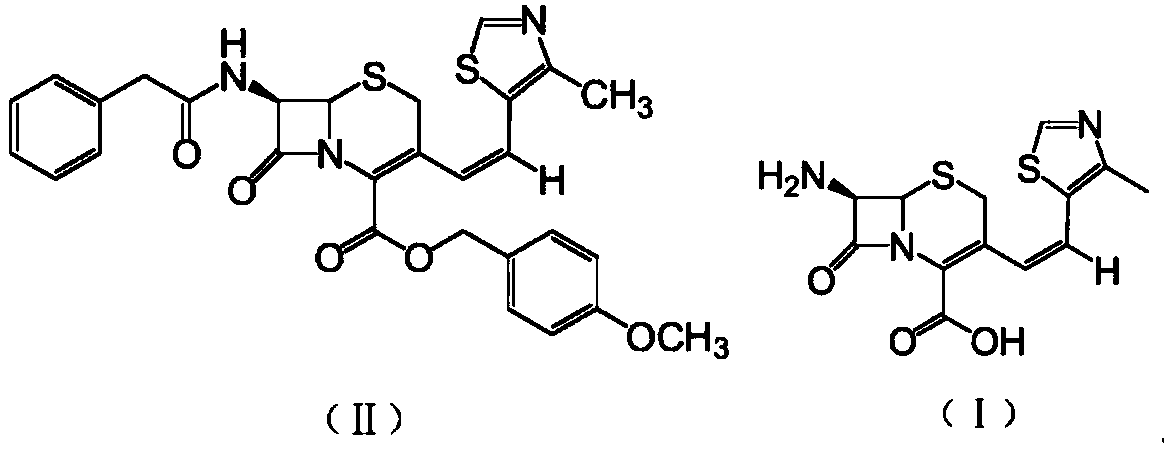
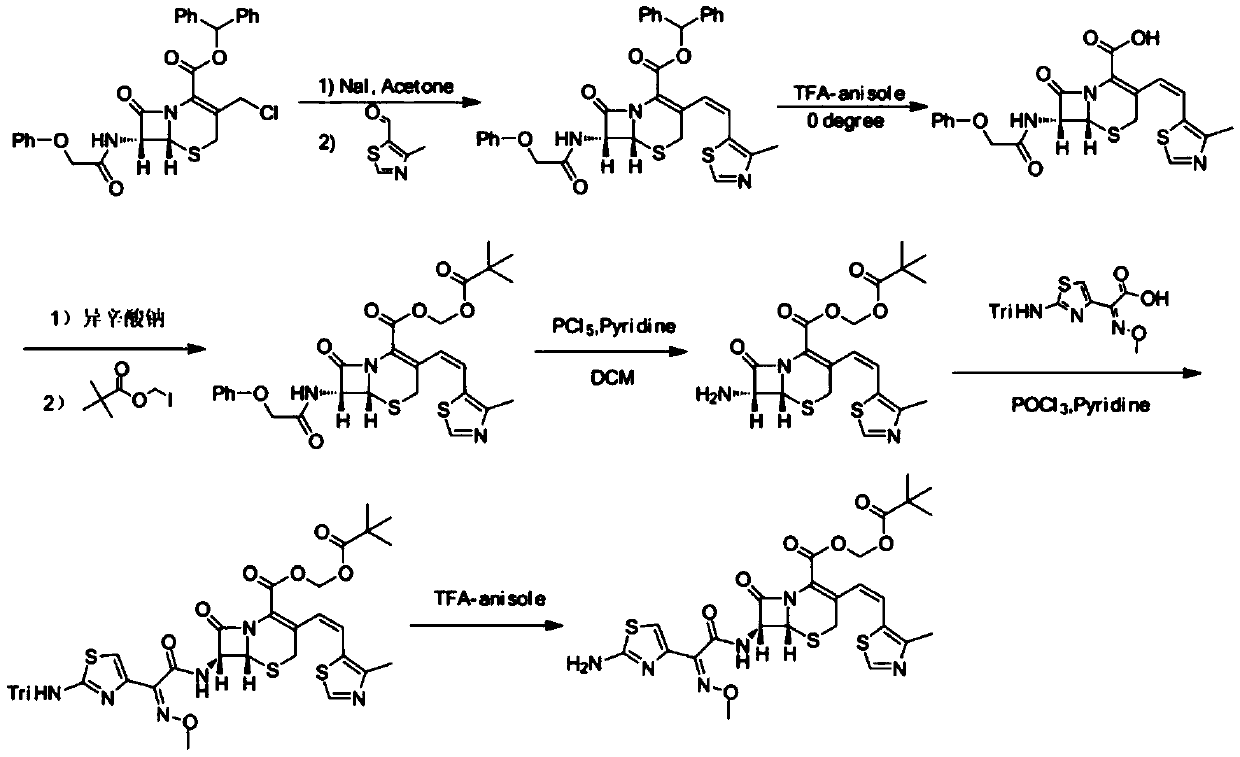
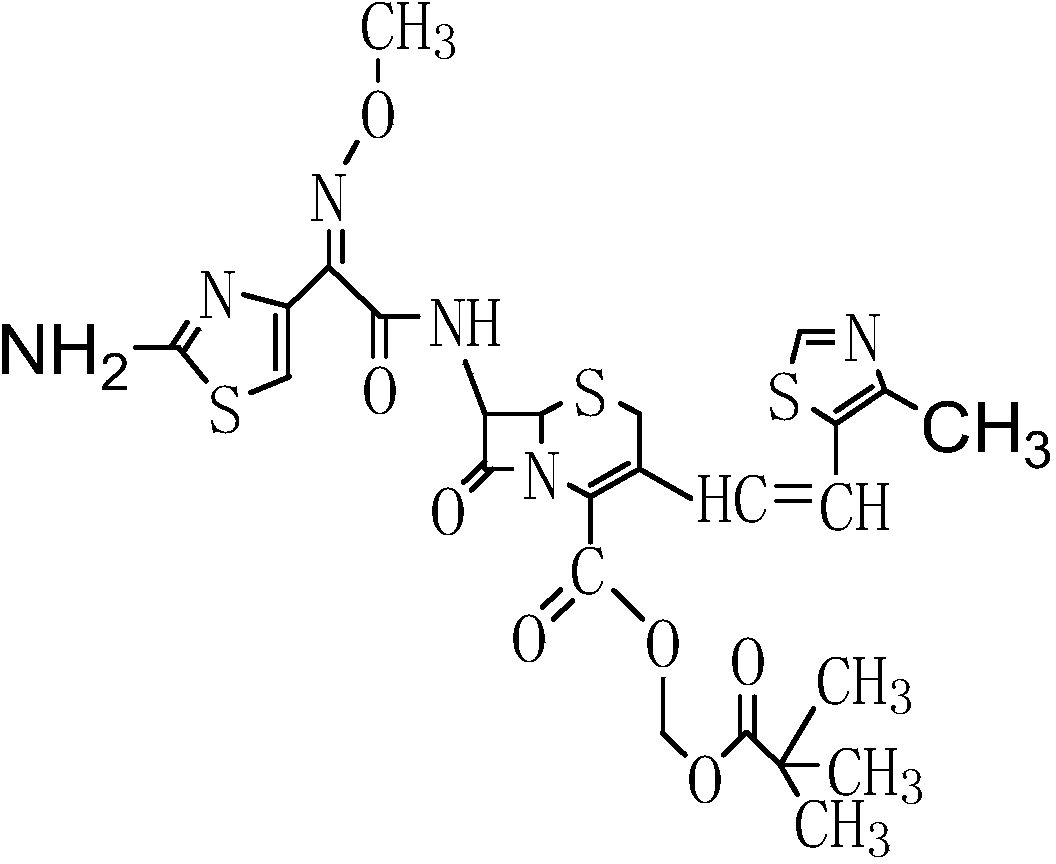
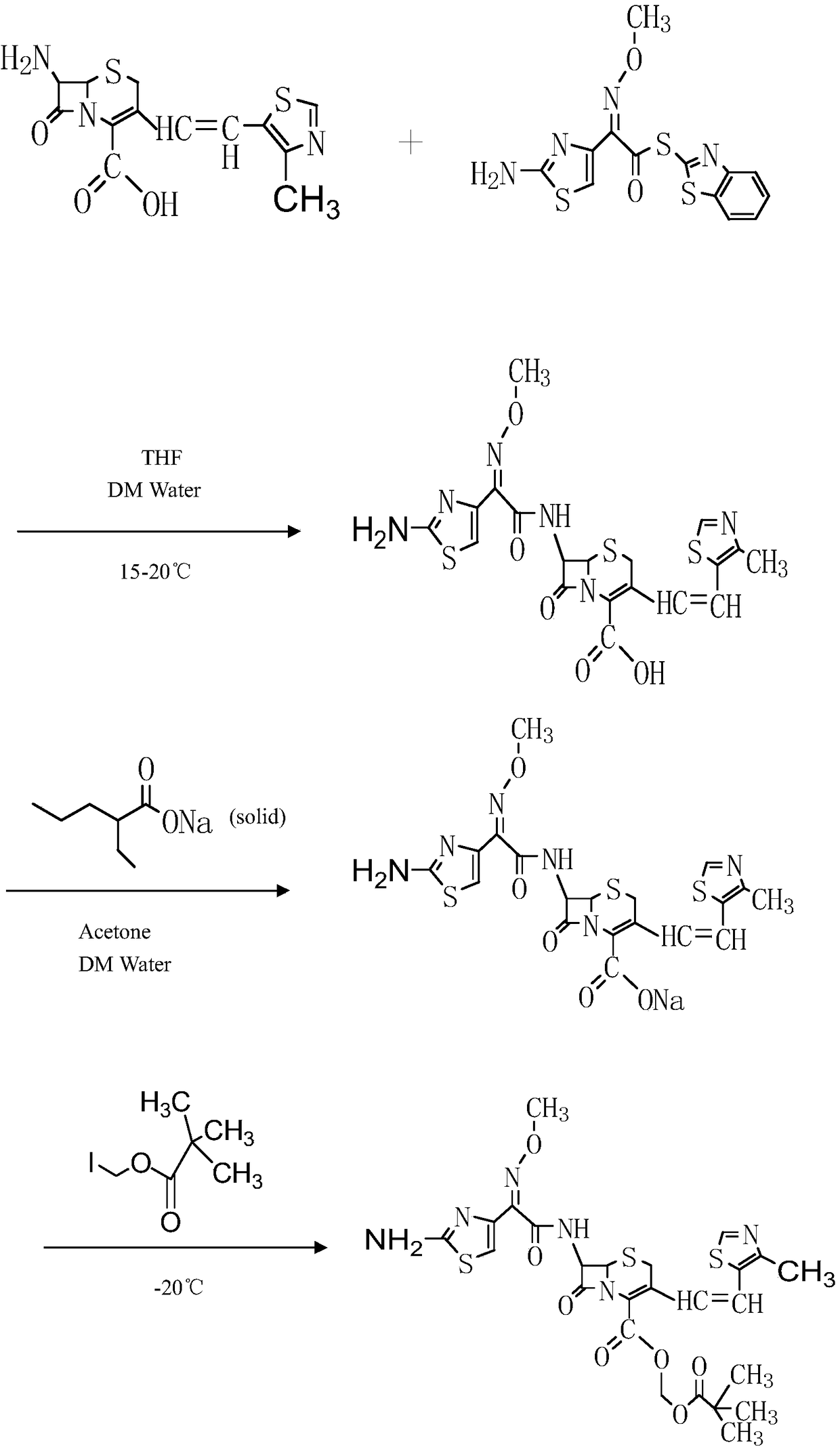
The present invention relates to an improved process for the preparation of Cefditoren of formula (I), the said process comprising the steps of: i) converting the compound of formula (II) to a compound of the formula (III) using TPP and sodium iodide in the presence of THF, water, and base; ii) reacting the compound of formula (III) with 4-methyl-5-formyl-thiazole to produce a compound of formula (IV); iii) deesterifying the compound of the formula (IV) to yield compound of formula (V); iv) converting the compound of formula (V) to compound of formula (VI) in the presence of a base and solvent; v) converting the compound of formula (VI) into compound of formula (VII) by enzymatic hydrolysis; and vi) reacting compound of formula (VII) with compound of formula (VIII) in the presence of solvent and base to produce compound of formula (I).
Owner:ORCHID CHEM & PHARM LTD
Crystalline substance of cefditoren pivoxyl and the production of the same
InactiveUS6294669B1Reduce pressureHigh purityAntibacterial agentsOrganic active ingredientsOrganic solventCefditoren
As a novel substance is provided such a new, crystalline substance of Cefditoren povoxyl which has a high purity and an enhanced thermal stability on storage. This crystalline Cefditoren pivoxyl may be prepared by a process comprising a step of dissolving amorphous substance of Cefditoren pivoxyl in an anhydrous, first organic solvent capable of dissolving said amorphous substance well therein, and steps of replacing the first organic solvent component of the resulting solution by an anhydrous alkanol of 1 to 5 carbon atoms as a second organic solvent, in such a manner that the firstly prepared solution of Cefditoren pivoxyl in the first organic solvent is mixed with a volume of the alkanol and then is concentrated below 15° C. under reduced pressure, and so on. Thereby, the process proceeds so as to produce a solution containing 50 mg / ml to 250 mg / ml of Cefditoren pivoxyl dissolved in the alkanol alone. From the latter solution, crystals of Cefditoren pivoxyl are induced to deposit by addition of water at a temperature of 0-10° C. The resulting admixture of the concentrated solution of Cefditoren pivoxyl in alkanol with added water and the deposited Cefditoren pivoxyl is then agitated 10° C. or below, to effect a complete crystallization of Cefditoren pivoxyl.
Owner:MEIJI SEIKA PHARMA CO LTD
Preparation method of cefditoren pivoxil
ActiveCN104513256ASources are cheap and readily availableEasy to operateOrganic chemistrySodium bicarbonateCefditoren
The invention belongs to the technical field of medicines and particularly relates to a preparation method of cefditoren pivoxil. The preparation method particularly includes following steps: (1) carrying out a reaction between cefditoren mother nucleus 7ATCA and AE-activated ester with dichloromethane as a solvent under an alkaline condition at 0-5 DEG C; (2) performing extraction with pure water and adding a sodium iso-octoate / acetone solution to obtain cefditoren sodium; (3) carrying out a reaction between the cefditoren sodium and iodomethyl pivalate under the alkaline condition at -40 DEG C to obtain a cefditoren pivoxil solution; (4) adding pure water to separate out a crystal to obtain a crude product of the cefditoren pivoxil. The technical scheme also comprises steps of dissolving the crude product of the cefditoren pivoxil in a mixed solution including dichloromethane and anhydrous ethanol, washing the material solution with a 1% sodium bicarbonate solution and pure water, collecting an organic phase, and performing a pressure-reducing evaporate-drying process to obtain the cefditoren pivoxil being higher than 99% in purity and less in impurities. The preparation method is simple in operation, is easy to control, is high in yield, allows the raw material to be obtained easily and is suitable for industrialized large-scale production.
Owner:LUNAN BETTER PHARMA
Crystallographically stable amorphous cephalosporin compositions and process for producing the same
InactiveUS6342493B1Good water solubilityImprove solubilityAntibacterial agentsPowder deliveryOral medicationCefditoren
Processes are provided for the preparation of orally administrable, yellow and powdery compositions essentially consisting of particles composed of a homogeneous mixture of an amorphous Cefditoren pivoxil substance with a water soluble high-molecular additive. These compositions can be produced by dissolving crystalline Cefditoren pivoxil substance and the water-soluble high-molecular additive in an aqueous solution of an acid, then neutralizing the resultant solution, to co-precipitate the product, and drying the thus precipitated product, followed by recovering the product in the form of the above-mentioned particles.
Owner:MEIJI SEIKA KAISHA LTD
Preparation process of cefamandole nafate
The invention discloses a preparation process of cefamandole nafate. The preparation process comprises steps of: heating and stirring 7-amino cephalosporanic acid, 5-mercapto-1-methyltetrazole and a catalyst boron trifluoride acetonitrile complex for a reaction; and carrying out a cooling post-treatment to obtain cefditoren nuclear parent; conducting a heating reflux reaction on the cefditoren nuclear parent and a silanizing agent until the solution turns to a clarified state; adding N, N-dimethyl aniline under the protection of inert gas at a low temperature, dropwise adding D-(-)-O-formyl mandeloyl chloride for reaction, and carrying out post-treatment to obtain formyl cefamandole acid; and reacting the formyl cefamandole acid with an organic acid salt, and recrystallizing to obtain the cefamandole nafate. By the above way, the preparation process of cefamandole nafate provided by the invention employs a simple and easily implemented process to obtain high-yield cefditoren nuclear parent with low impurity content; dichloromethane is used as a solvent to obtain the cefamandole acid with greatly enhanced color grade and yield; and dosage of activated carbon in the post-treatment is obviously reduced, so as to reduce the production cost.
Owner:苏州盛达药业有限公司
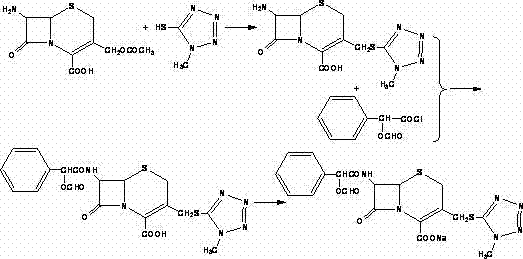
Method for synthesizing cefditoren pivoxil
The invention relates to a method for synthesizing cefditoren pivoxil. The method comprises that D-7ACA reacts with an oxidizing reagent to produce a compound 1, the compound 1 is protected through silanization to produce a compound 2, 4-methylthiazole-5-methanol and NaI undergo an iodination reaction in the presence of a small amount of sulfuric acid for catalysis, triphenylphosphine is added into the reaction system and undergoes a reaction to produce a compound 3, the compound 3 is added into the compound 2 liquid and undergoes a reaction, the reaction product is concentrated, methanol anda small amount of concentrated hydrochloric acid are added into the concentrated product, the concentrated product is deprotected and crystallized to form cefditoren mother nucleuses, 7-ATCA and an AEactive ester undergo a reaction under alkaline conditions, the reaction product is crystallized to form a cefditoren sodium wet product, the cefditoren sodium wet product is added into iodomethyl pivalate and undergoes a reaction in the presence of a phase transfer catalyst and the product is crystallized to form a cefditoren pivoxil crude product. In preparation of the compound 1, cefditoren sodium and cefditoren pivoxil, single solvents are used and are easy to recover. The method has the advantages of simple operation, high product conversion rate, few impurities and low production cost and is suitable for industrial production of cefditoren pivoxil.
Owner:QILU ANTIBIOTICS PHARMA
Preparation method of Cefditoren Pivoxil
The invention relates to a preparation method of Cefditoren Pivoxil. The preparation method comprises steps as follows: 7-ACA (3-acetyloxymethyl-5-thio-7-amino-8-oxy-1-nitrogen heterobicyclic octyl-2-ene-2 carboxylic acid) is taken as a starting raw material and is subjected to iodination and Wittig reaction after silanization protection, and a Cefditoren parent nucleus 7-ATCA (7-amino-3-[(Z)-2-(4-methyl-5-thiazole) vinyl]-3-cephem-4-carboxylic acid) is generated; after amino protection of aminothiazole ethyl gallate, a compound 2 is produced from 7-ATCA under catalysis of AlMe3; the compound2 is subjected to an esterification reaction with iodomethyl pivalate under actions of a phase transfer catalyst and an acid adsorbent, the amino protection is removed, and a target product CefditorenPivoxil is obtained. According to the preparation method, reaction conditions are mild, product purity and yield are high, the process is stable, amplification is easy, and the method is applicable to industrial production.
Owner:SHANDONG YUXIN PHARMA CO LTD
Method for preparing cefditoren pivoxil
The invention discloses a method for preparing cefditoren pivoxil in the technical field of medicine, which comprises: dissolving a cefditoren pivoxi crude product in a water-soluble solvent; preparing a cefditoren pivoxil organic phase; preparing a cefditoren pivoxil aqueous phase; and adjusting the pH value of the solution, performing acid extraction treatment and menstruum crystallization treatment, and obtaining the cefditoren pivoxil with the purity of 98.1 to 98.8 percent. The method ensures that the cefditoren pivoxil can obtain high-purity products according with pharmacopoeia through purification under stable condition by simple operation.
Owner:SHANGHAI JIAO TONG UNIV
Amorphous cefditoren pivoxil composition and process for producing the same
A composition of an amorphous cefditoren pivoxil excellent in stability and releasability; and a process for producing the amorphous composition. The composition comprises cefditoren pivoxil and a pharmaceutically acceptable organic polymer, and is characterized by being obtained by pulverizing crystalline cefditoren pivoxil in the presence of a pharmaceutically acceptable organic polymer and making the cefditoren pivoxil amorphous.
Owner:MEIJI SEIKA KAISHA LTD
Micronized cefditoren pivoxil composition
The invention relates to a medicament composition and method of preparing the same. The medicament composition includes micronized particles of cefditoren pivoxil, wherein the particles have the d0.5 from 1[mu]m to 40 [mu]m. The invention also relates to method for preparing micronized cefditoren pivoxil particles.
Owner:RANBAXY LAB LTD
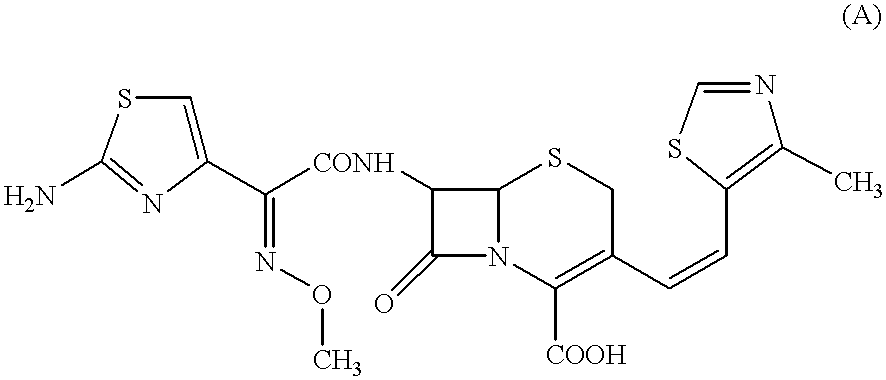
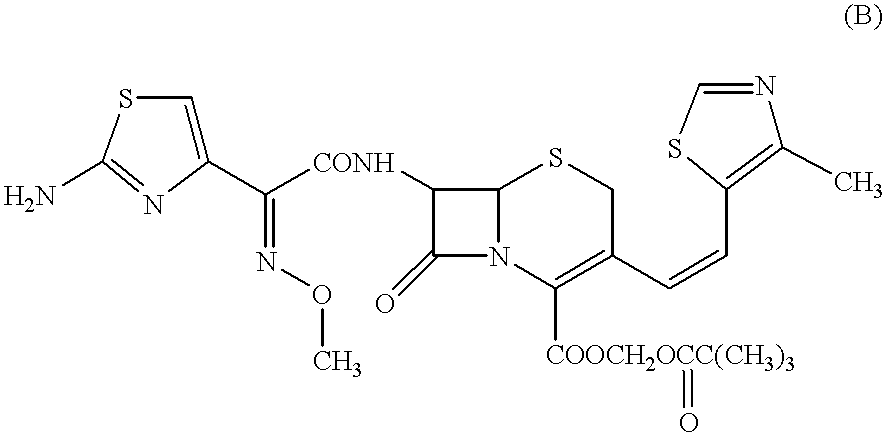
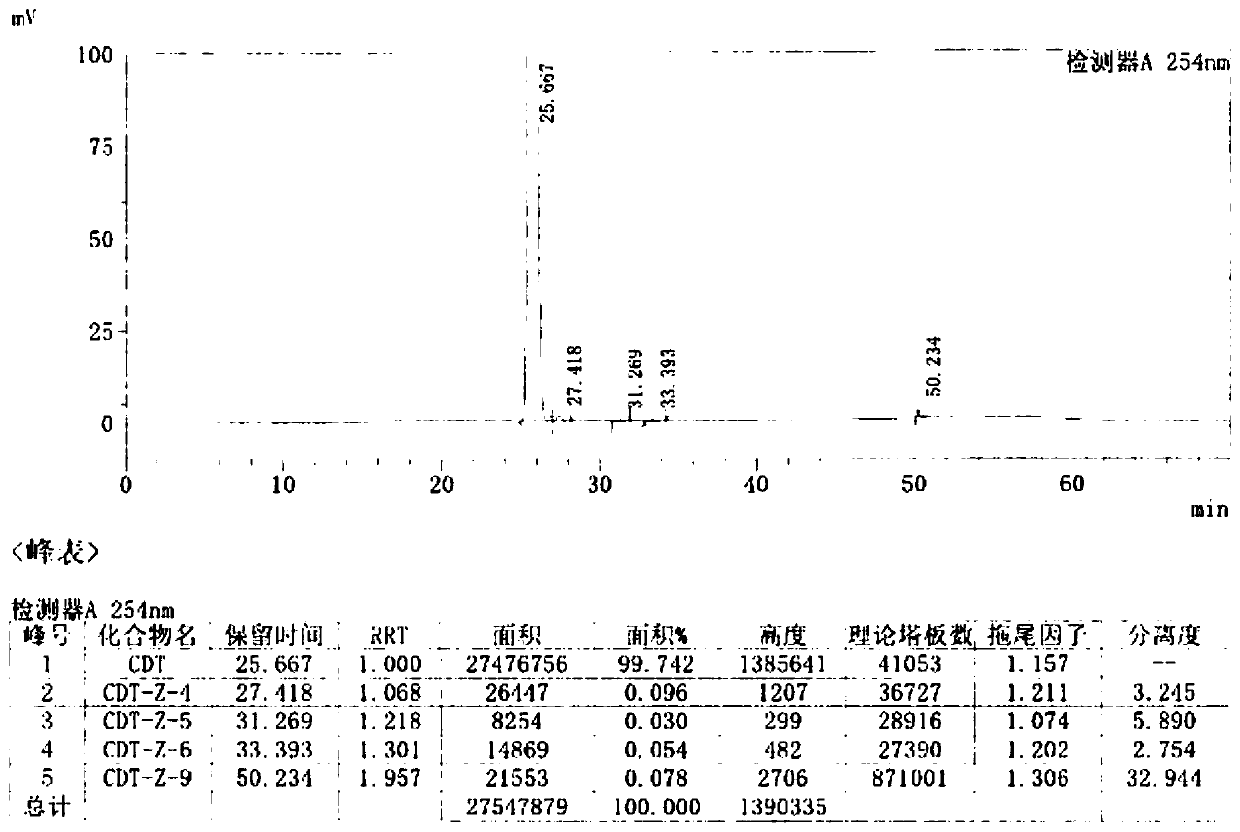
Preparation methods of Cefditoren acid delta 3 isomer and cefditoren pivoxil delta 3 isomer
The invention discloses preparation methods of Cefditoren acid delta 3 isomer and cefditoren pivoxil delta 3 isomer. The cefditoren acid delta 3 isomer is prepared from the following steps that pure water and tetrahydrofuran are added in a reaction vessel, the temperature is lowered to 0-5 DEG C, a 7-ATCA delta 3 isomer and AE active ester (MAEM) are added, after adding is completed, then organicalkali is dropwise added to control pH of 8.0-8.5, the organic alkali is needed to be additionally added to keep the pH of 8.0-8.5, dichloromethane and water are added after reaction is completed, stirring and extraction are conducted, still standing and layering are conducted, a lower organic layer is discarded, the pH of an upper water layer is 3.0-3.5, stirring and filtering are conducted, andwashing and drying are conducted to obtain a product. The content of the cefditoren acid delta 3 isomer and the cefditoren pivoxil delta 3 isomer which are prepared through the preparation method canreach above 93%.
Owner:CHONGQING MEDICAL & PHARMA COLLEGE
Crystalline substance of cefditoren pivoxyl and the production of the same
InactiveUS20020002279A1High purityGood storage stabilityAntibacterial agentsOrganic active ingredientsOrganic solventCefditoren
A crystalline substance of Cefditoren pivoxyl is provided which has a high purity and enhanced thermal stability on storage. This crystalline Cefditoren pivoxyl may be prepared by a process comprising a step of dissolving an amorphous form of Cefditoren pivoxyl in an anhydrous, first organic solvent capable of dissolving said amorphous form well therein, and steps of replacing the first organic solvent component of the resulting solution by an anhydrous alkanol of 1 to 5 carbon atoms as a second organic solvent, in such a manner that the firstly prepared solution of Cefditoren pivoxyl in the first organic solvent is mixed with a volume of the alkanol and then is concentrated below 15° C. under reduced pressure, and so on. Thereby, the process proceeds so as to produce a solution containing 50 mg / ml to 250 mg / ml of Cefditoren pivoxyl dissolved in the alkanol alone. From the latter solution, crystals of Cefditoren pivoxyl are induced to deposit by addition of water at a temperature of 0-10° C. The resulting admixture of the concentrated solution of Cefditoren pivoxyl in alkanol with added water and the deposited Cefditoren pivoxyl is then agitated 10° C. or below, to effect a complete crystallization of Cefditoren pivoxyl.
Owner:MEIJI SEIKA KAISHA LTD
Preparation method of cefditoren pivoxil dimer
ActiveCN110183468AClinical Safe Use GuaranteeThe preparation method is simple and easyOrganic chemistryOrganic solventCefditoren
The invention discloses a preparation method of a cefditoren pivoxil dimer. The preparation method is characterized in that an organic solvent and cefditoren sodium are added to a reaction container,and are stirred and dissolved, the temperature is reduced to subzero 20-subzero 50 DEG C, iodomethyl pivalate is added in batches, and iodomethyl pivalate is added again after reacting for a certain time after each addition of iodomethyl pivalate; inorganic or organic base is required to be added to control pH at 7.5-8.5 during the reaction, and subsequent separation and purification are performedafter the reaction to obtain the product. The preparation method has simple steps, simple raw materials and low cost, and is suitable for large-scale preparation. The content of the cefditoren pivoxil dimer prepared with the preparation method provided can reach 92.0% or higher, a theoretical basis is provided for safe use of drugs, effective data support is provided for the quality standard of cefditoren pivoxil, and effective guarantee is provided for safe clinical use of drugs.
Owner:CHONGQING MEDICAL & PHARMA COLLEGE
Noncrystalline antibacterial composition containing cefditoren pivoxil
According to the present invention, there is provided a solid dispersion composition which can maintain amorphous cefditoren pivoxil in a suspension for a long period of time. The present invention is a solid dispersion composition comprising at least 0.1 mg of a sugar ester fatty acid on the basis of an amount equivalent to 100 mg efficacy of cefditoren pivoxil.
Owner:MEIJI SEIKA KAISHA LTD
Process for the preparation of Cefditoren
InactiveUS7459550B2Process stabilityPromote commercializationOrganic chemistryEnzymatic hydrolysisSodium iodide
Owner:ORCHID CHEM & PHARM LTD
Process for the preparation of cefditoren using the thioester of thiazolylacetic acid
The present invention provides a process for the preparation of Cefditoren of formula (I) which comprises acylating 7-amino-cephem carboxylic acids of the general formula (IV), where R3 is hydrogen or trimethylsilyl with thioester derivatives of the formula (II), where R1 represents C1-C4 alkyl or phenyl in an organic solvent in the presence of an organic base at a temperature in the range of -10 ° C. to 30 ° C.
Owner:ORCHID CHEM & PHARM LTD
Odor-proof garment fabric
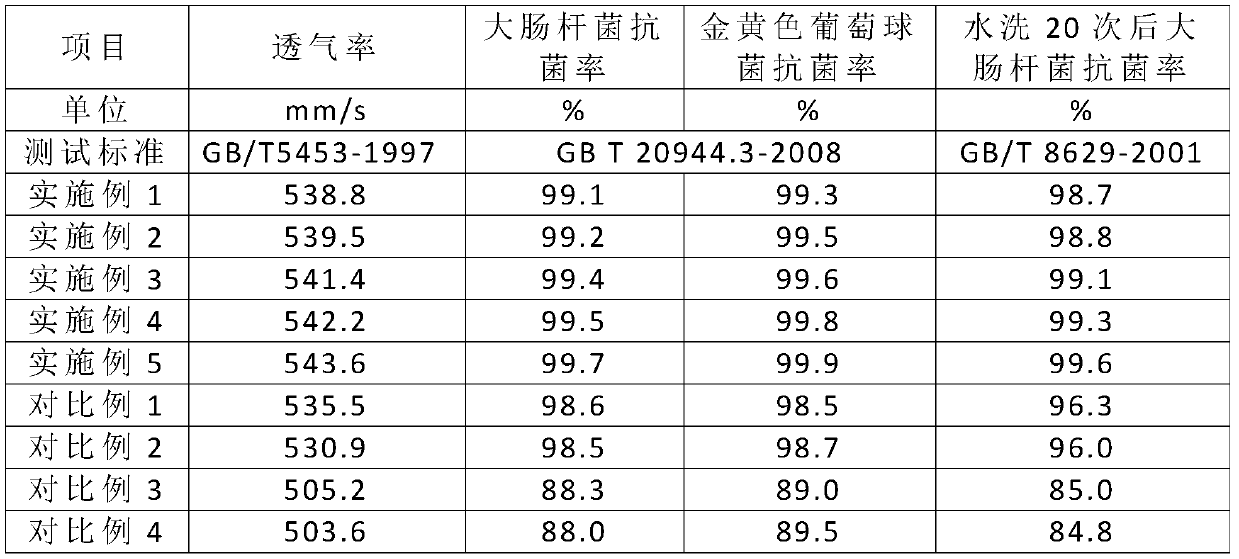
ActiveCN111020841AImprove wearing comfortImprove pass rateWeft knittingHeating/cooling textile fabricsEpoxyIminodiacetic acid
Owner:迈科凯普(杭州)生物科技有限公司
Pharmaceutical compositions of cefditoren pivoxil
InactiveUS20130143857A1Enhanced drug releaseAntibacterial agentsOrganic active ingredientsCefditorenPharmaceutical medicine
The present invention relates to a pharmaceutical composition comprising Cefditoren pivoxil, water soluble high molecular weight substance and one or more pharmaceutically acceptable excipient wherein weight ratio of high molecular weight substance to Cefditoren pivoxil is greater than 1:4 and a process for preparation thereof.
Owner:LUPIN LTD
Amorphous antibiotic composition comprising cefditoren pivoxil
InactiveUS20070053973A1Use of materialHighly orally absorbable amorphousAntibacterial agentsOrganic active ingredientsMedicineCefditoren
According to the present invention, there is provided a solid dispersion composition which can maintain amorphous cefditoren pivoxil in a suspension for a long period of time. The present invention is a solid dispersion composition comprising at least 0.1 mg of a sugar ester fatty acid on the basis of an amount equivalent to 100 mg efficacy of cefditoren pivoxil.
Owner:MEIJI SEIKA KAISHA LTD
Preparation method of high-purity cefditoren pivoxil
PendingCN110655527ATake advantage ofMild and complete reactionOrganic chemistrySodium bicarbonateCefditoren
The invention belongs to the technical field of medicines, and particularly relates to a preparation method of high-purity cefditoren pivoxil. The preparation method comprises following specific steps: controlling the temperature of cefditoren sodium (1) and iodomethyl pivalate (2) to be -30 DEG C to-35 DEG C for reaction to obtain a cefditoren pivoxil (3) solution; extracting with an organic phase / water mixed solvent, washing with a 0.1% sodium bicarbonate aqueous solution, crystallizing with an organic phase inert solvent, and carrying out ethyl acetate crystal transformation purification onan obtained crude product to obtain the high-purity cefditoren pivoxil of which the purity is greater than 99.5% and the individual impurity content is less than 0.1%, thereby reaching the USP bulk drug standard of the variety. The technical scheme discloses in the invention further provides a refining method of cefditoren pivoxil, a crystalline cefditoren pivoxil product is obtained by directlydissolving and crystallizing with ethyl acetate, and the refining method is simple to operate, easy to control, high in yield, good in quality and suitable for industrial production.
Owner:BEIJING JIMEITANG MEDICINE RES CO LTD
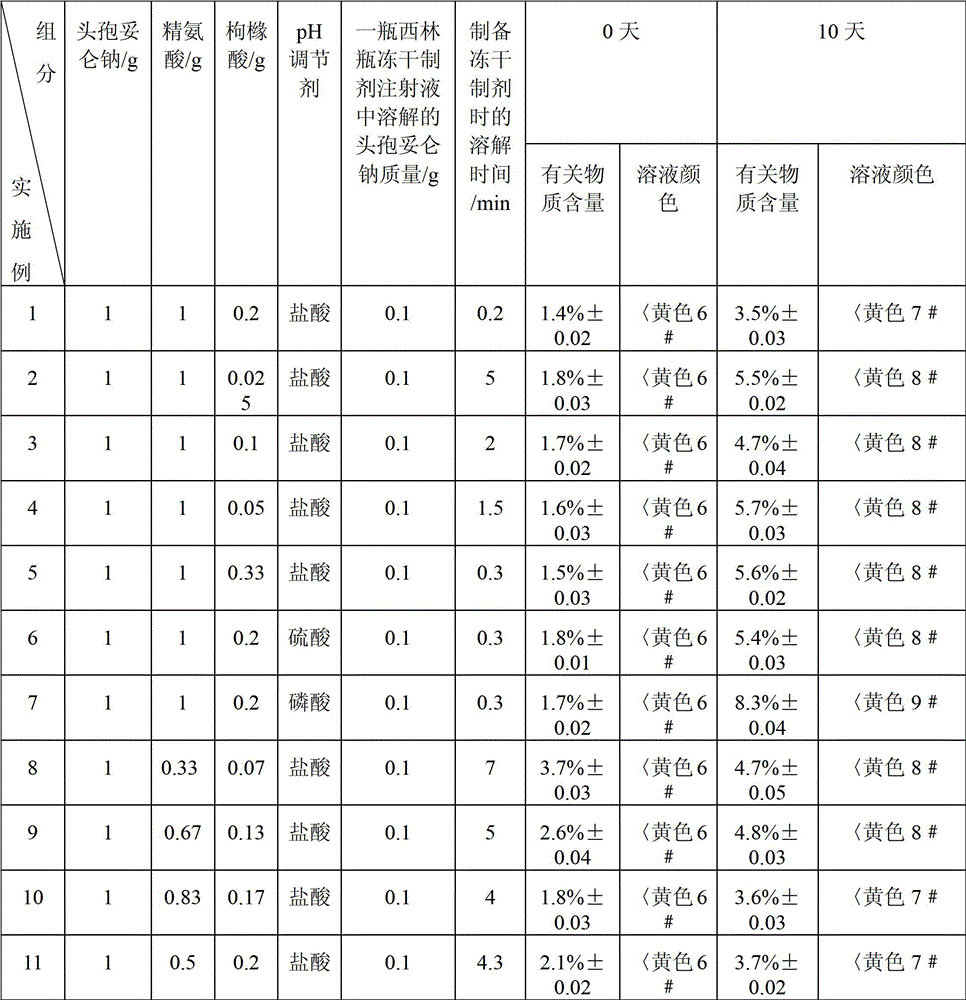
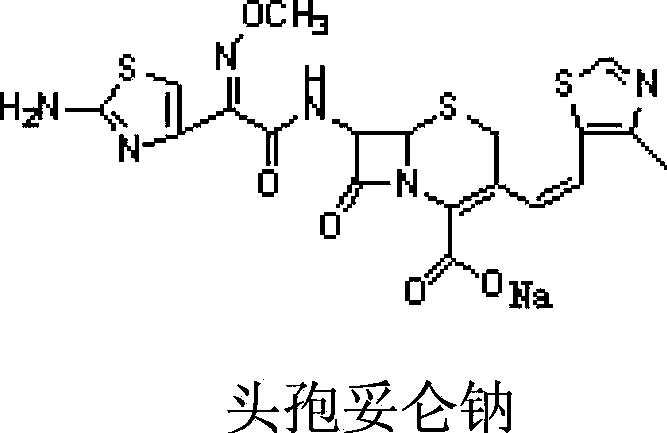
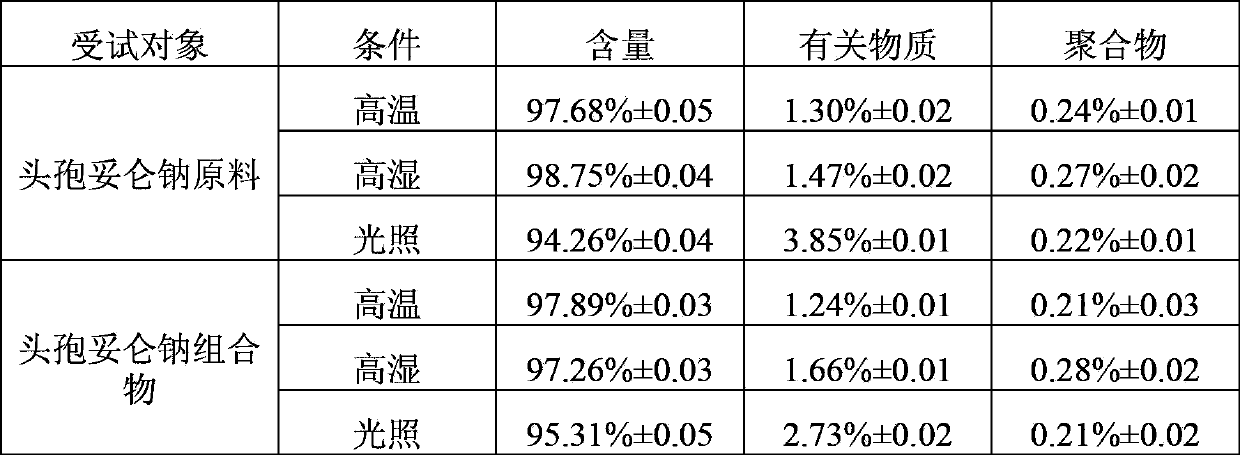
Freeze-dried preparation of cefditoren sodium for injection and preparation method thereof
ActiveCN103860484BGood solubilization effectImprove stabilityAntibacterial agentsPowder deliveryArginineCefditoren
The invention relates to the field of freeze-dried preparation for injection. The invention particularly discloses a cefditoren sodium freeze-dried preparation for injection and a preparing method thereof. The cefditoren sodium freeze-dried preparation for injection comprises cefditoren sodium, a dissolution-assisting agent and a proper amount of a pH regulator, wherein the dissolution-assisting agent is a mixture of arginine and citric acid. The cefditoren sodium freeze-dried preparation for injection adopts citric acid and arginine to be combined as the dissolution-assisting agent, and the dissolution-assisting agent has quite good dissolution-assisting property on cefditoren sodium, obviously improves the dissolution rate in water for injection, shortens the dissolution time, and improves the stability of the cefditoren sodium freeze-dried preparation; and the cefditoren sodium freeze-dried preparation can achieve the stability equal to the cefditoren sodium raw material.
Owner:SICHUAN KELUN PHARMA CO LTD
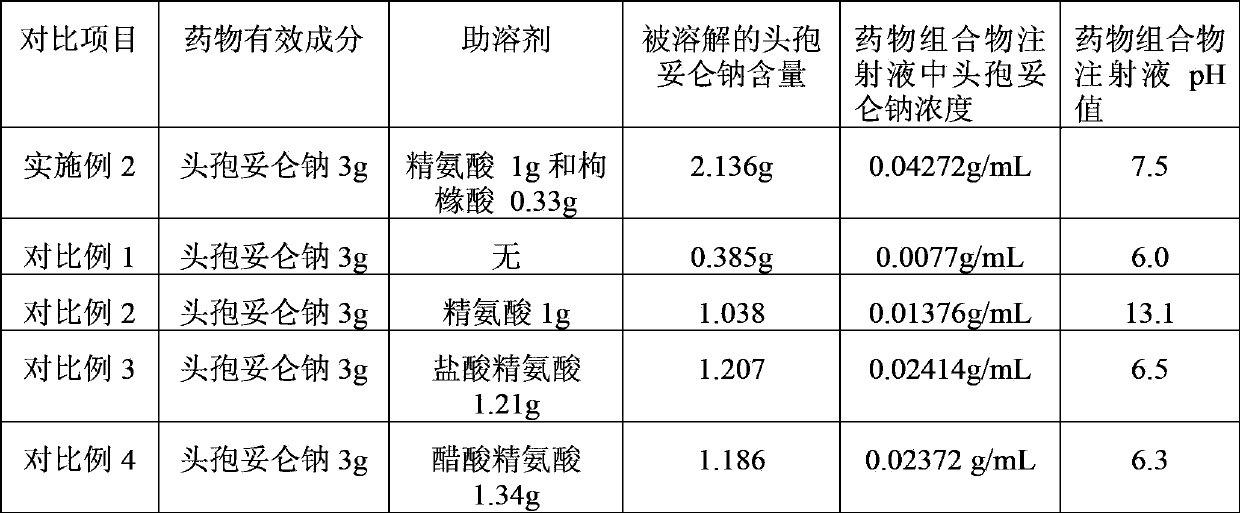
Cefditoren sodium containing-pharmaceutical composition
ActiveCN103860563AImprove solubilityIncrease concentrationAntibacterial agentsOrganic active ingredientsOrganic acidSolubility
The invention discloses a cefditoren sodium-containing pharmaceutical composition which is composed of cefditoren sodium and a cosolvent, the cosolvent is a mixture of arginine and a pharmacologically acceptable hydroxyl-containing solid organic acid, and the hydroxyl-containing solid organic acid has 4-12 carbon atoms. The cefditoren sodium-containing pharmaceutical composition compounds the hydroxyl-containing solid organic acid and the arginine to use as the cosolvent, and has a very good cosolubility, the concentration of the cefditoren sodium in an injection is improved, compared with cefditoren sodium raw material, the solubility of the cefditoren sodium-containing pharmaceutical compositioncan be as high as 554.8%; and at the same time, the stability of the cefditoren sodium-containing pharmaceutical composition is good and is equal to that of the cefditoren sodium raw material.
Owner:SICHUAN KELUN PHARMA CO LTD
Process for the preparation of cefditoren using the thioester of thiazolylacetic acid
The present invention provides a process for the preparation of Cefditoren of the formula (I)which comprises acylating 7-amino-cephem carboxylic acids of the general formula (IV)where R3 is hydrogen or trimethylsilyl with thioester derivatives of the formula (II)wherein R1 represents C1-C4 alkyl or phenyl in an organic solvent in the presence of an organic base at a temperature in the range of -10° C. to 30° C.
Owner:ORCHID CHEM & PHARM LTD
A kind of preparation method of cefditoren pivoxil
The invention relates to a preparation method of Cefditoren Pivoxil. The preparation method comprises steps as follows: 7-ACA (3-acetyloxymethyl-5-thio-7-amino-8-oxy-1-nitrogen heterobicyclic octyl-2-ene-2 carboxylic acid) is taken as a starting raw material and is subjected to iodination and Wittig reaction after silanization protection, and a Cefditoren parent nucleus 7-ATCA (7-amino-3-[(Z)-2-(4-methyl-5-thiazole) vinyl]-3-cephem-4-carboxylic acid) is generated; after amino protection of aminothiazole ethyl gallate, a compound 2 is produced from 7-ATCA under catalysis of AlMe3; the compound2 is subjected to an esterification reaction with iodomethyl pivalate under actions of a phase transfer catalyst and an acid adsorbent, the amino protection is removed, and a target product CefditorenPivoxil is obtained. According to the preparation method, reaction conditions are mild, product purity and yield are high, the process is stable, amplification is easy, and the method is applicable to industrial production.
Owner:SHANDONG YUXIN PHARMA CO LTD
A kind of preparation method of cefditoren pivoxil
Owner:LUNAN BETTER PHARMA
Method for preparing cefditoren pivoxil
The invention discloses a method for preparing cefditoren pivoxil in the technical field of medicine, which comprises: dissolving a cefditoren pivoxi crude product in a water-soluble solvent; preparing a cefditoren pivoxil organic phase; preparing a cefditoren pivoxil aqueous phase; and adjusting the pH value of the solution, performing acid extraction treatment and menstruum crystallization treatment, and obtaining the cefditoren pivoxil with the purity of 98.1 to 98.8 percent. The method ensures that the cefditoren pivoxil can obtain high-purity products according with pharmacopoeia throughpurification under stable condition by simple operation.
Owner:SHANGHAI JIAO TONG UNIV
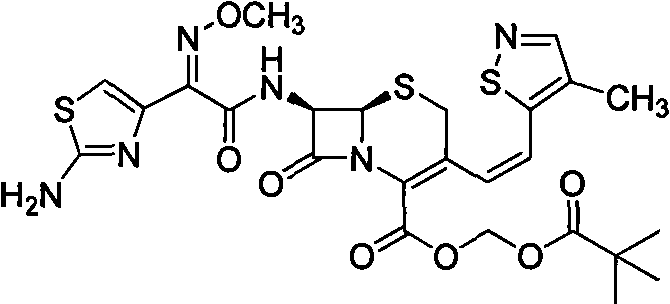
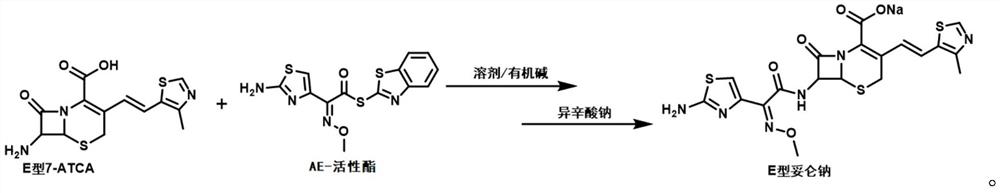
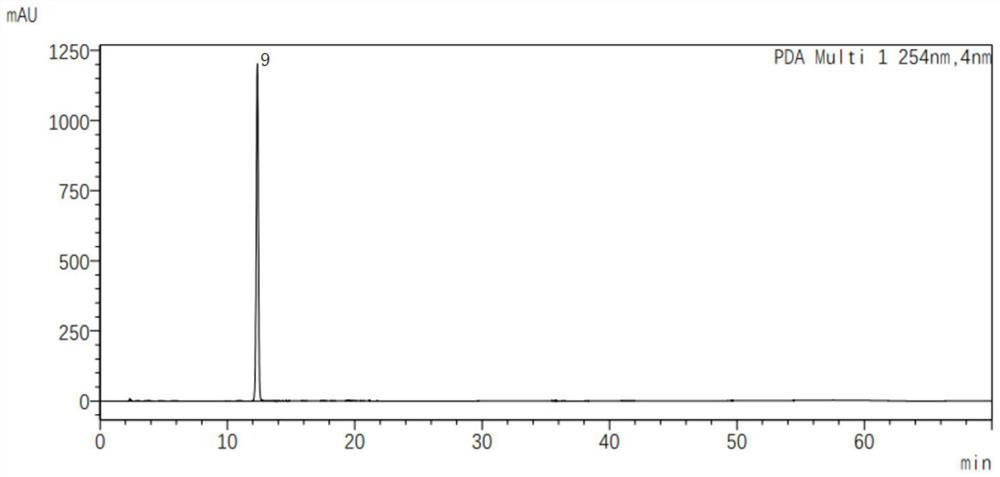
Preparation method of E-type cefditoren sodium
The invention relates to the technical field of synthesis of drug related substances, and particularly discloses a preparation method of E-type cefditoren sodium. The preparation method of the E-type cefditoren sodium comprises the following steps: cooling a mixed solution composed of water and acetone to 0-5 DEG C, adding E-type 7-ATCA and AE active ester, adjusting the pH value to 8-8.5 by using organic alkali, heating to 15-25 DEG C, reacting, and maintaining the pH value of the reaction solution at 8-8.5 in the reaction process; and after the reaction is finished, adding an acetone solution of sodium isooctoate, growing crystals, carrying out suction filtration, and drying to obtain the E-type cefditoren sodium. According to the preparation method of the E-type cefditoren sodium provided by the invention, the E-type cefditoren sodium with high purity and high yield can be obtained, the whole preparation method is simple to operate, the reaction condition is mild, and the finally obtained E-type cefditoren sodium completely meets the requirement of serving as a cefditoren pivoxil reference substance and has good application prospects. Important data support is provided for ensuring the quality detection of the cefditoren pivoxil.
Owner:湖北凌晟药业股份有限公司
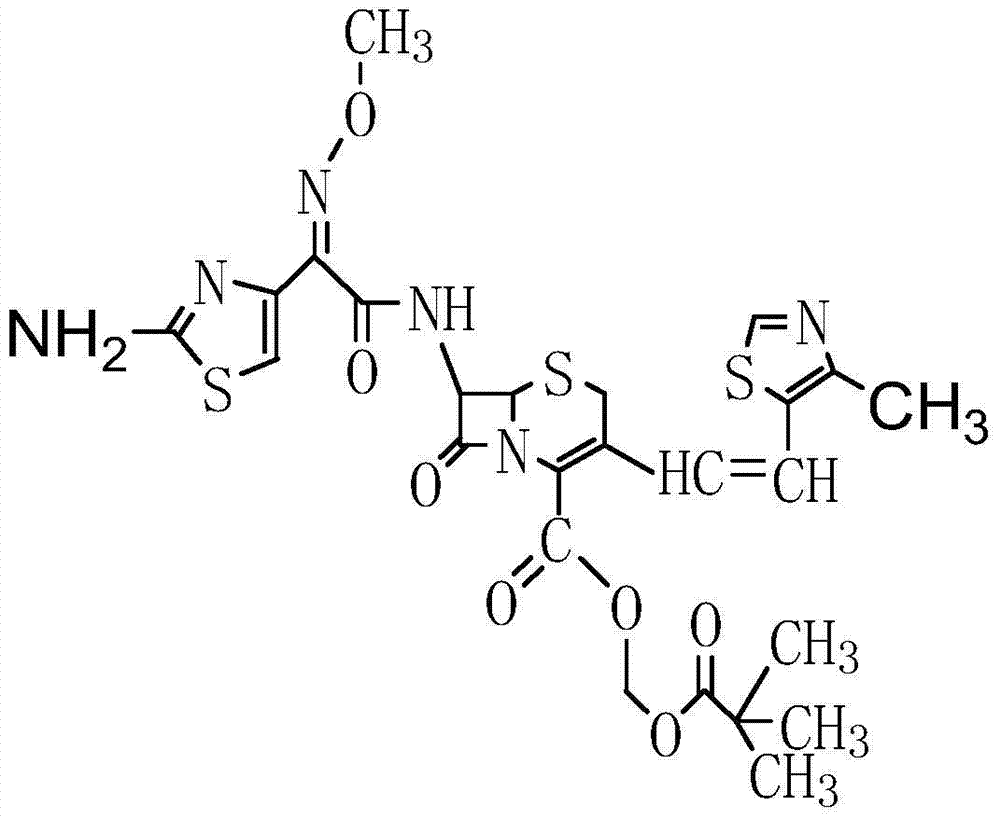
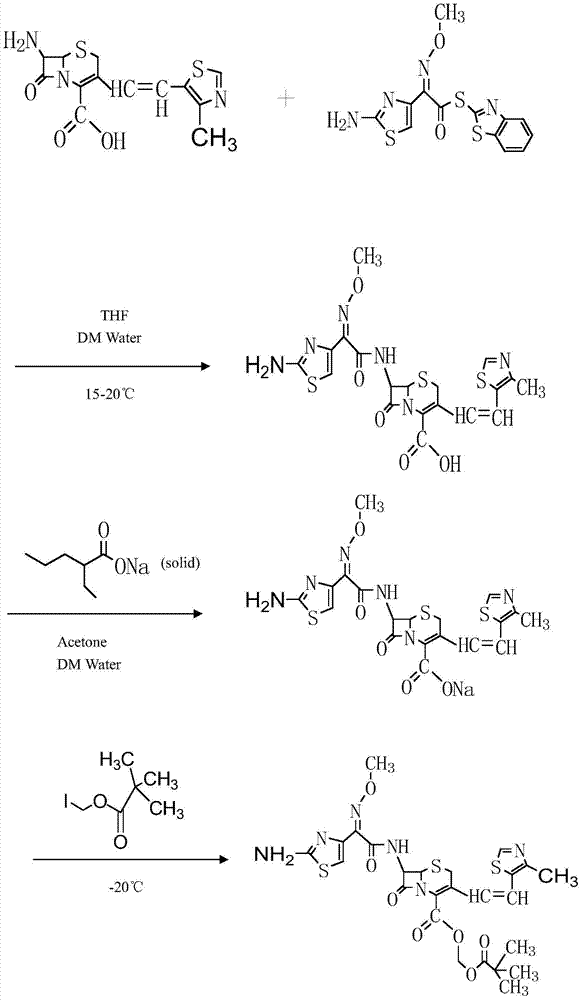
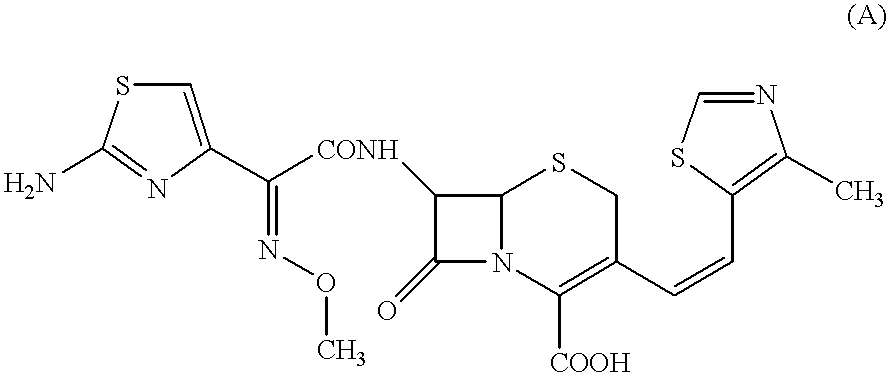
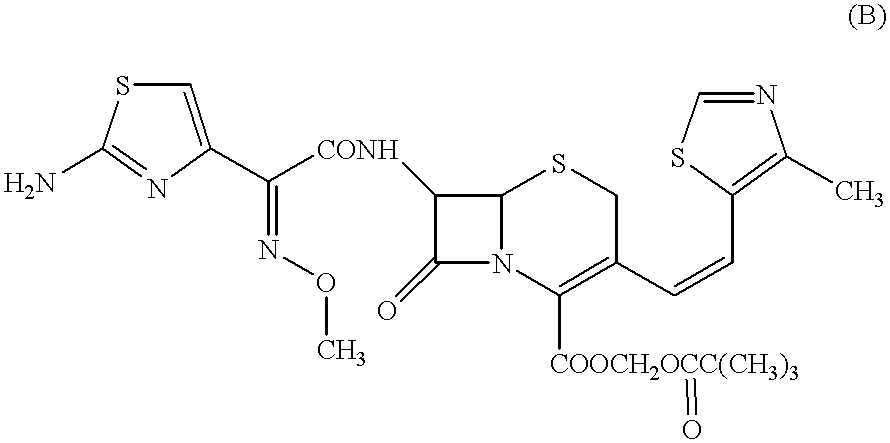
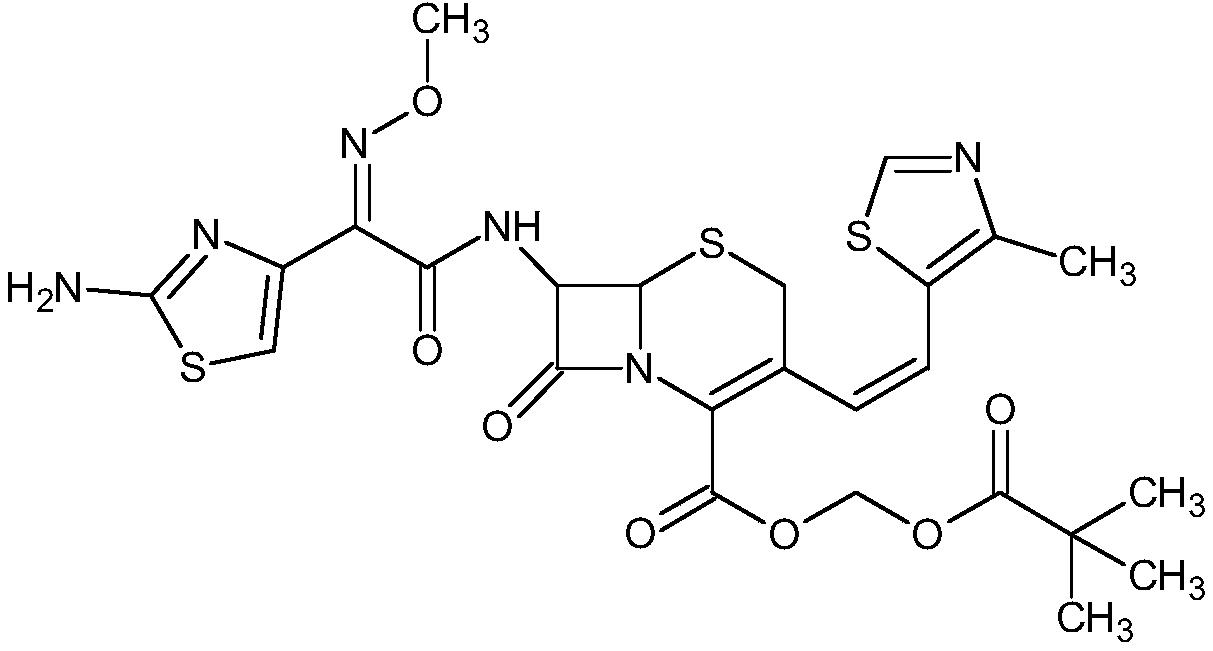
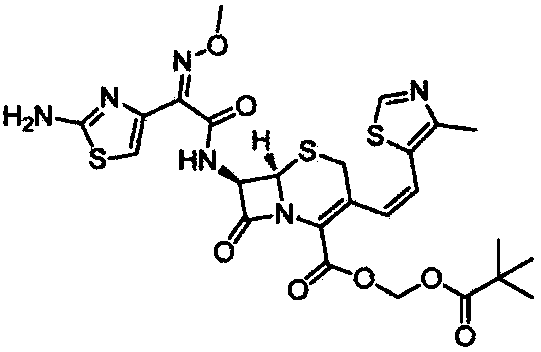
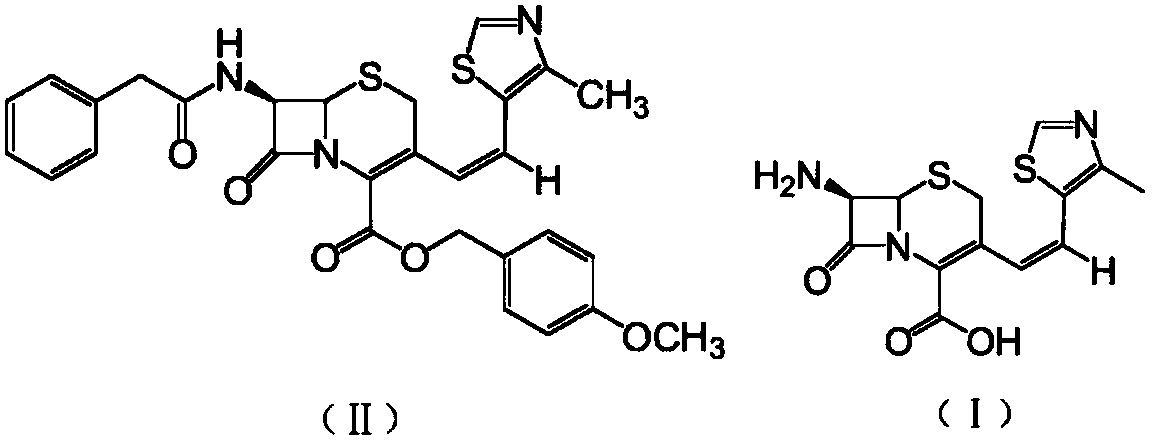
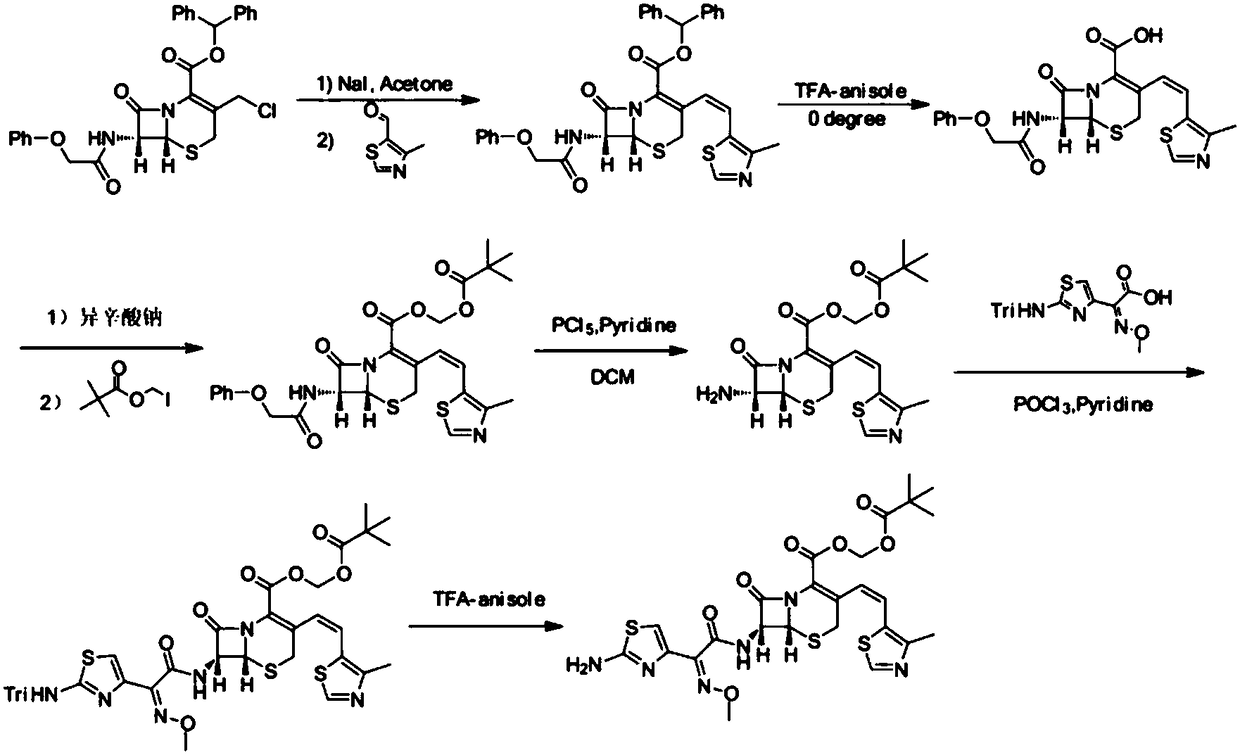
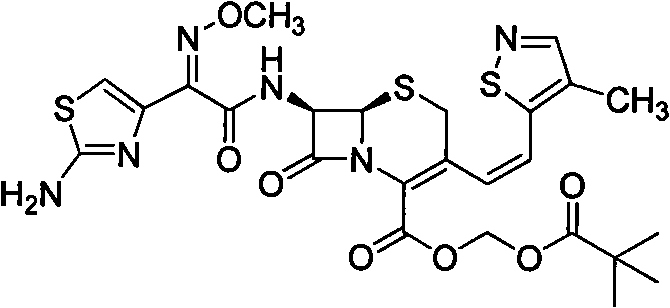
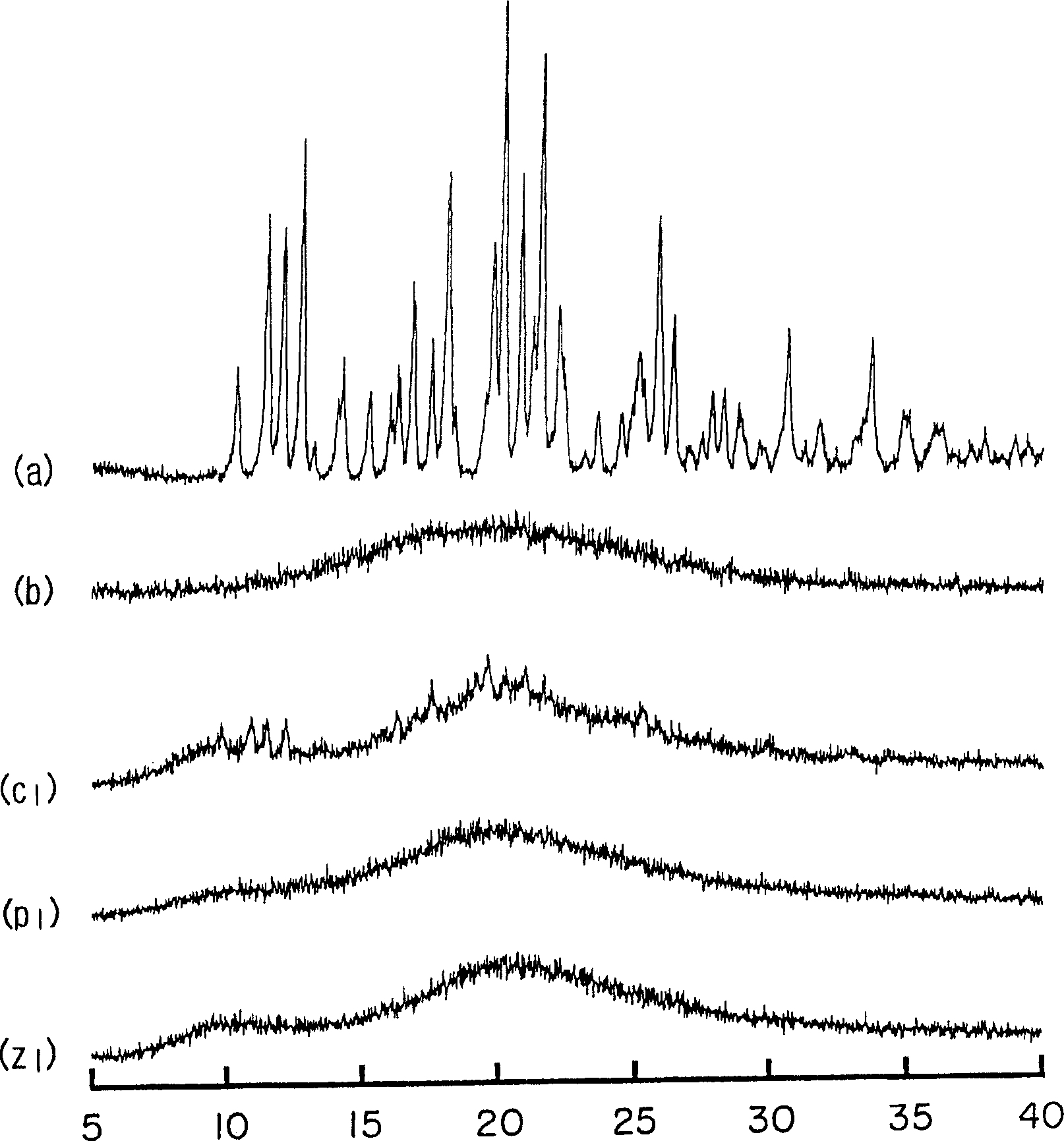
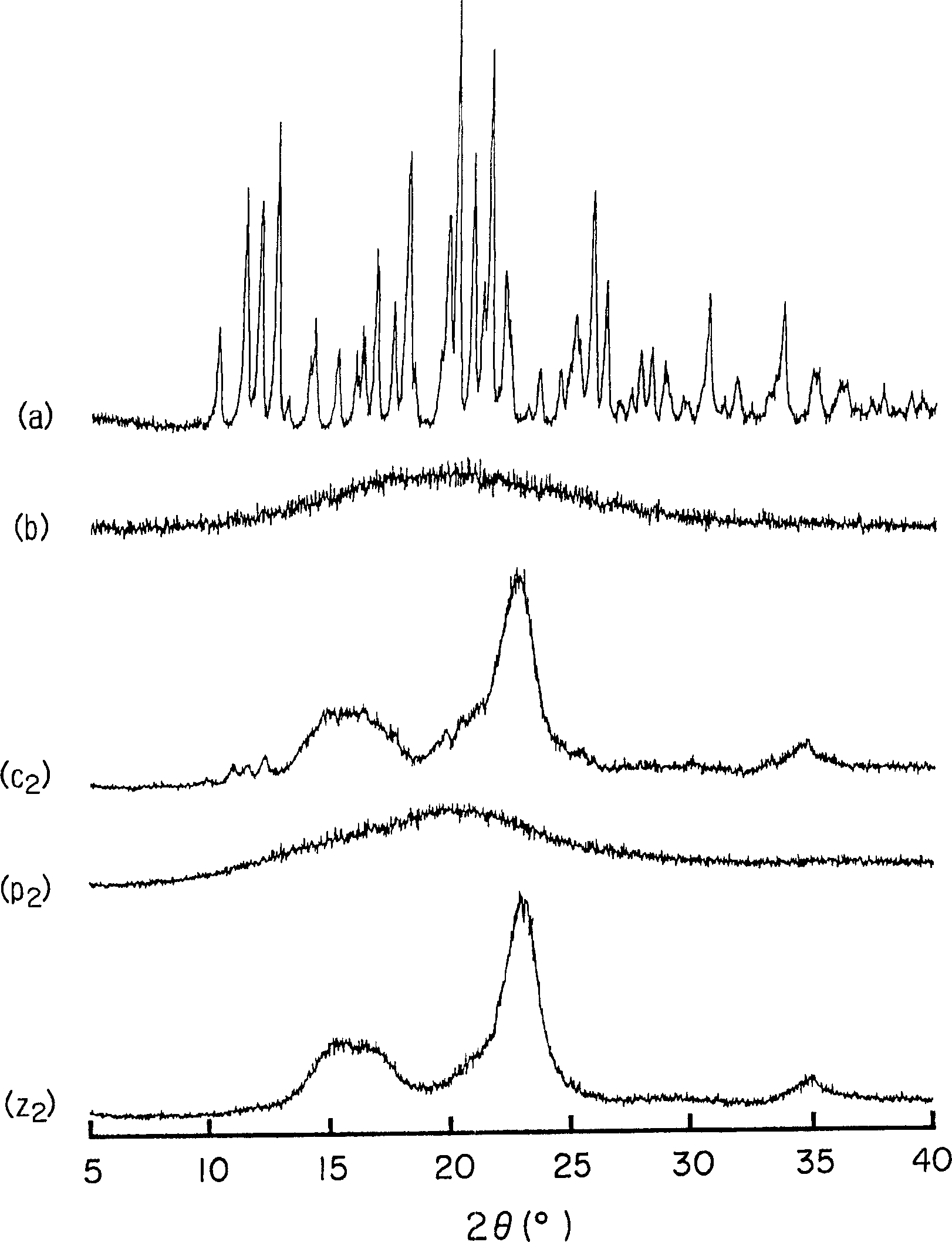
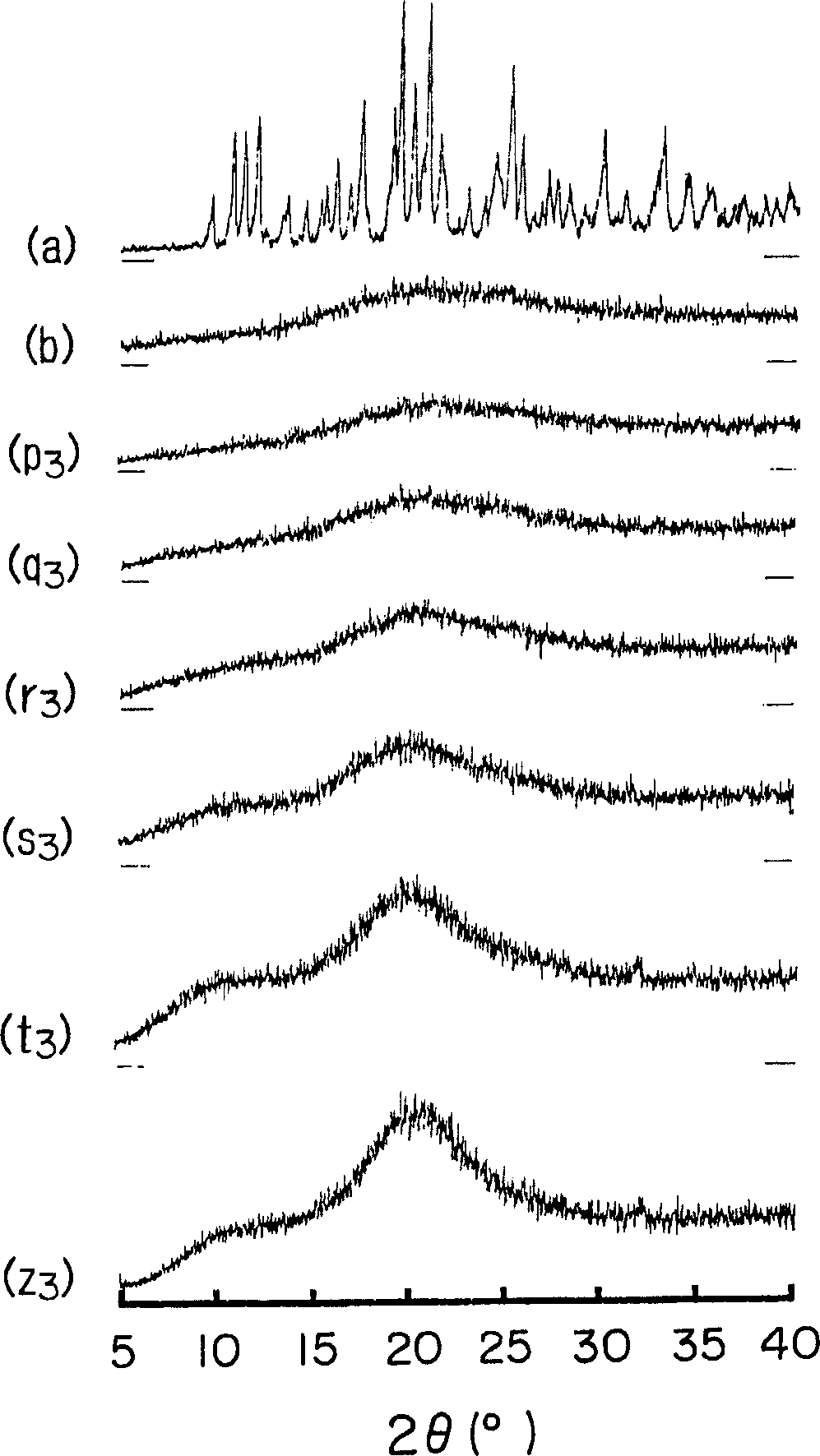
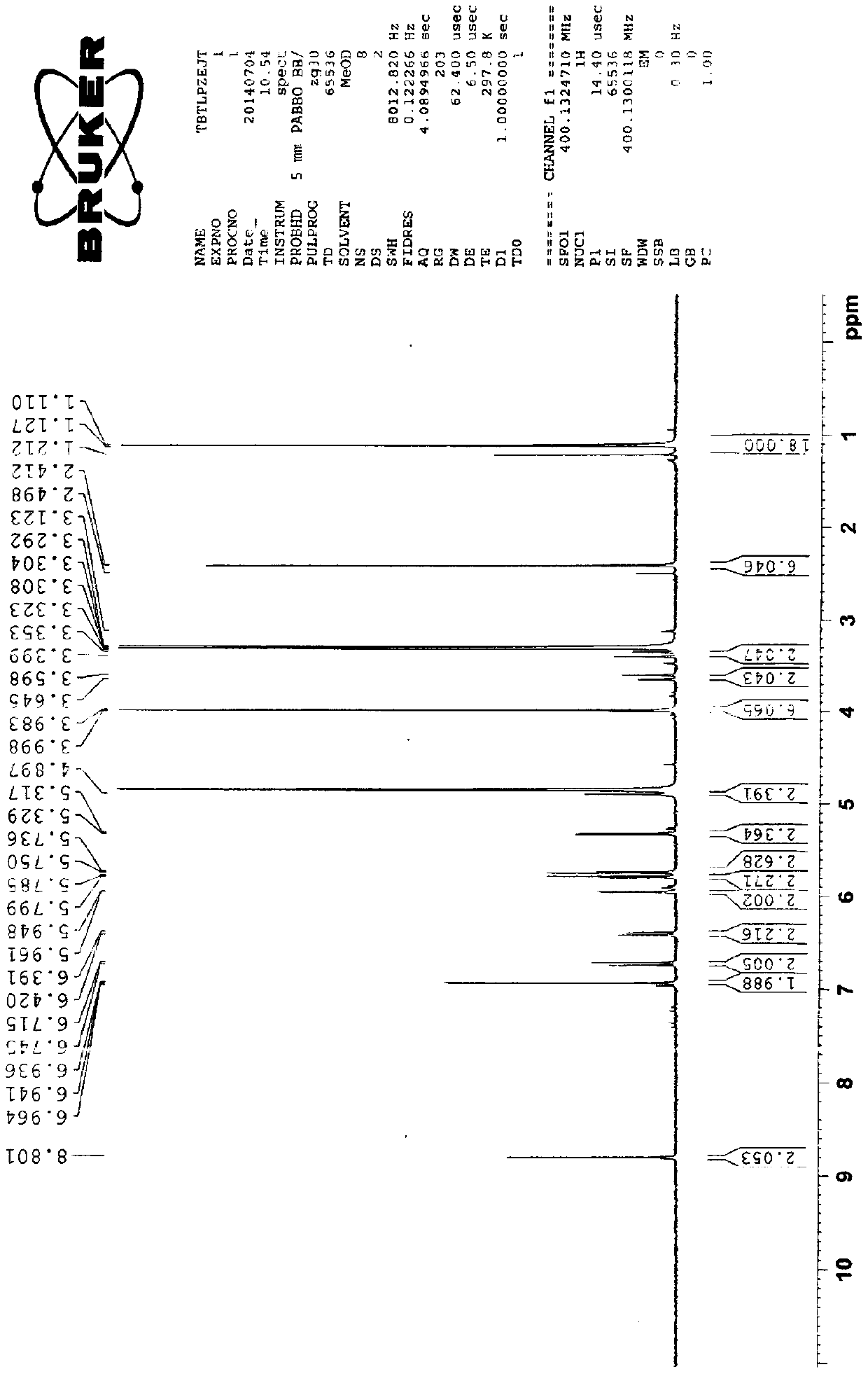
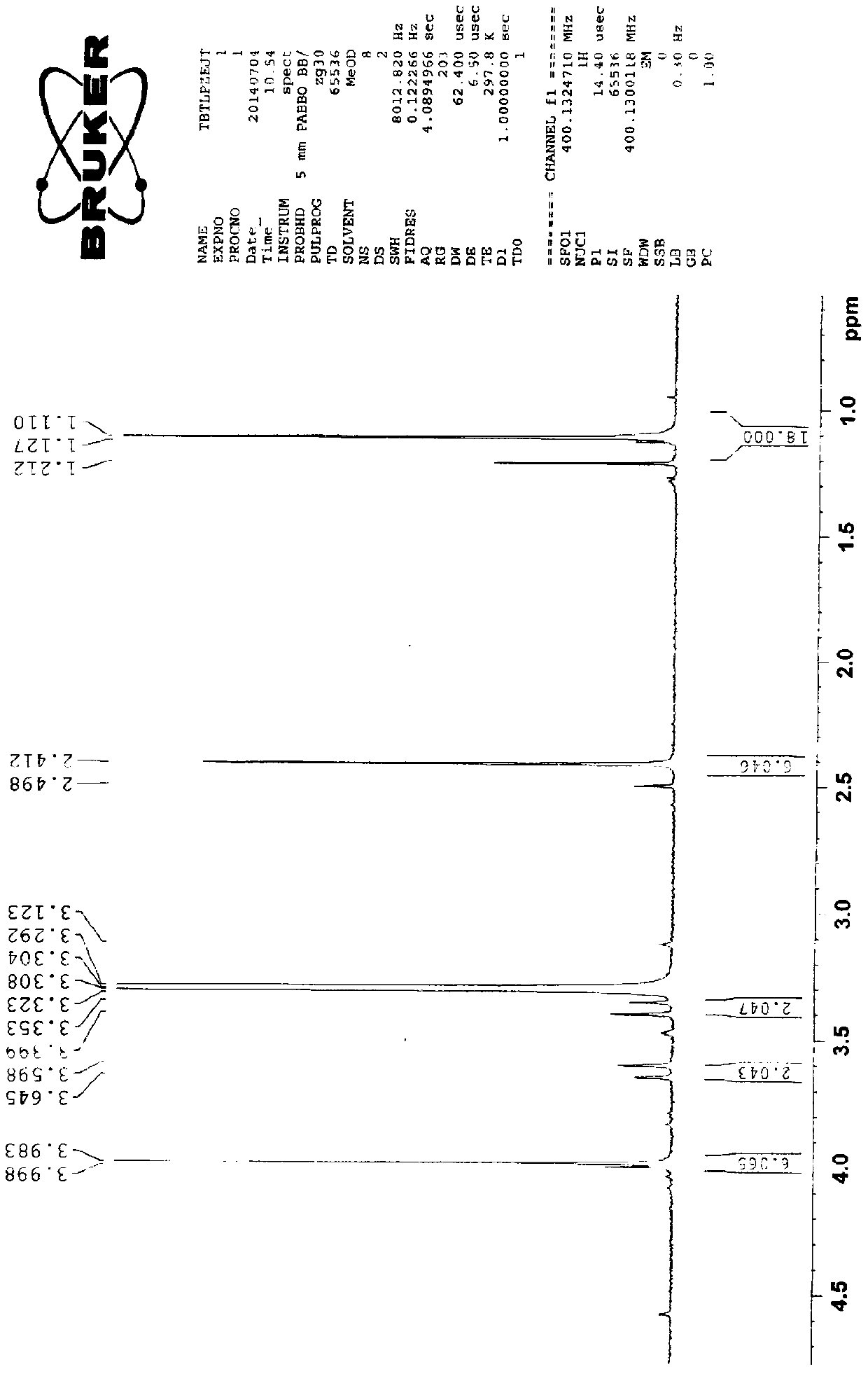
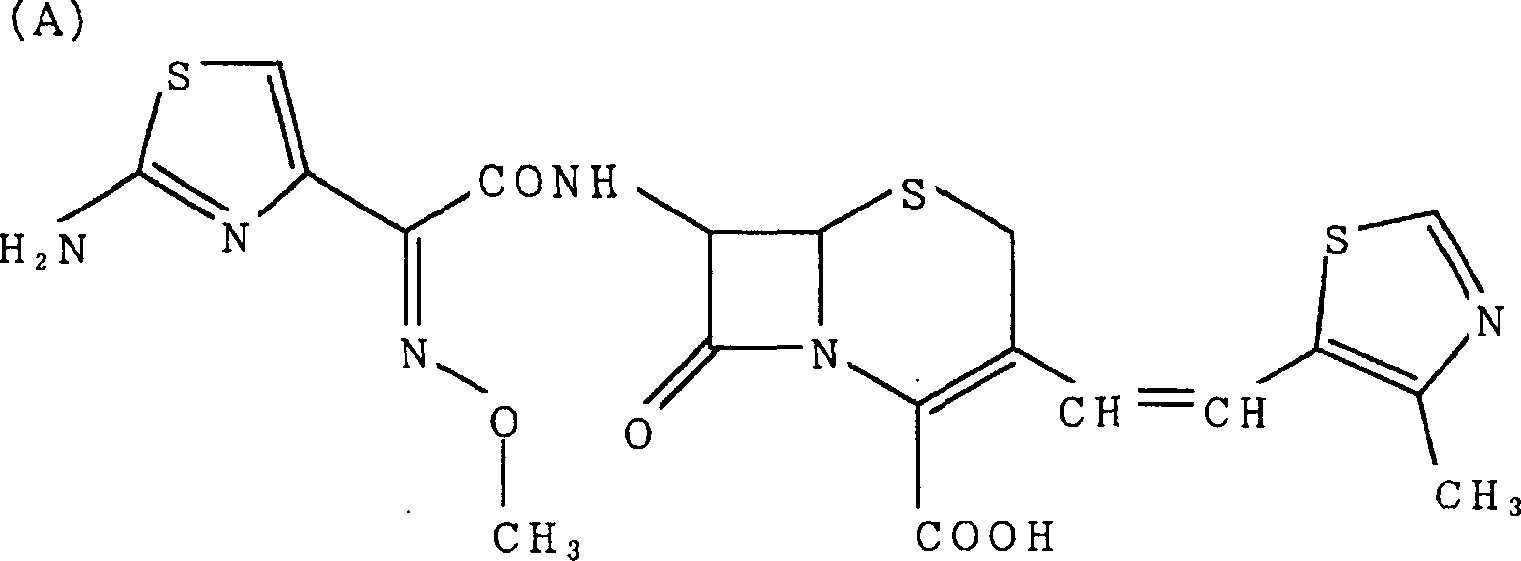
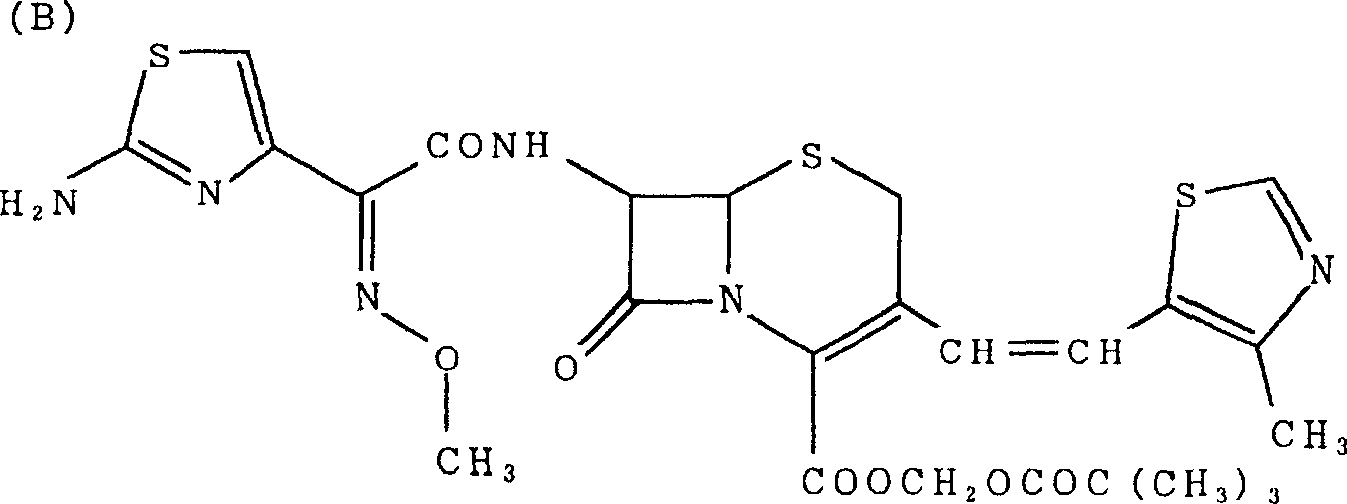
Preparation method of cefditoren pivoxil [delta]3 isomer
The invention relates to a preparation method of a cefditoren pivoxil [delta]3 isomer, and belongs to the technical field of drug intermediate synthesis. The invention aims to solve the problems of low isomerization and difficulty in separation of existing synthetic products, and provides the method. The method comprises the following steps: adding 7-ATCA, BSA and a first organic alkali into a first non-water-soluble organic solvent to carry out an isomerization reaction; after the reaction is finished, adding water, standing and separating liquid; collecting a water phase, and adding acid into the collected water phase to adjust the pH value to 2.0 to 3.0 to obtain a 7-ATCA[delta]3 isomer shown in the specification; in the presence of a second organic alkali, carrying out a condensation reaction on the 7-ATCA[delta]3 isomer and AE active ester to obtain a corresponding cefditoren [delta]3 isomer shown in the specification, and reacting the cefditoren [delta]3 isomer with iodomethyl pivalate to obtain the cefditoren pivoxil [delta]3 isomer. The method has the advantages that the isomerization rate is high, the yield and purity of the product can be improved, and the separation operation is easy.
Owner:浙江东邦药业有限公司
Synthetic technology of cefditoren pivoxil intermediate
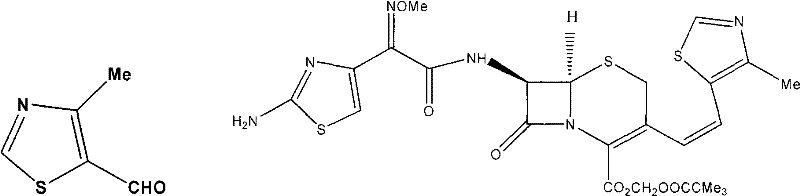
The invention which belongs to the medicine processing field concretely relates to a synthetic technology of a cefditoren pivoxil intermediate. The synthetic technology of the cefditoren pivoxil intermediate provided in the invention has the advantages of simple operation, no pollution and high yield. The technology of the invention comprises the following steps: preparation of 2-amino-4-methyl-5-methylthiazole formate, preparation of 4-methyl-5-methylthiazole formate and preparation of 4-methyl-5-formylthiazole.
Owner:王颢
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
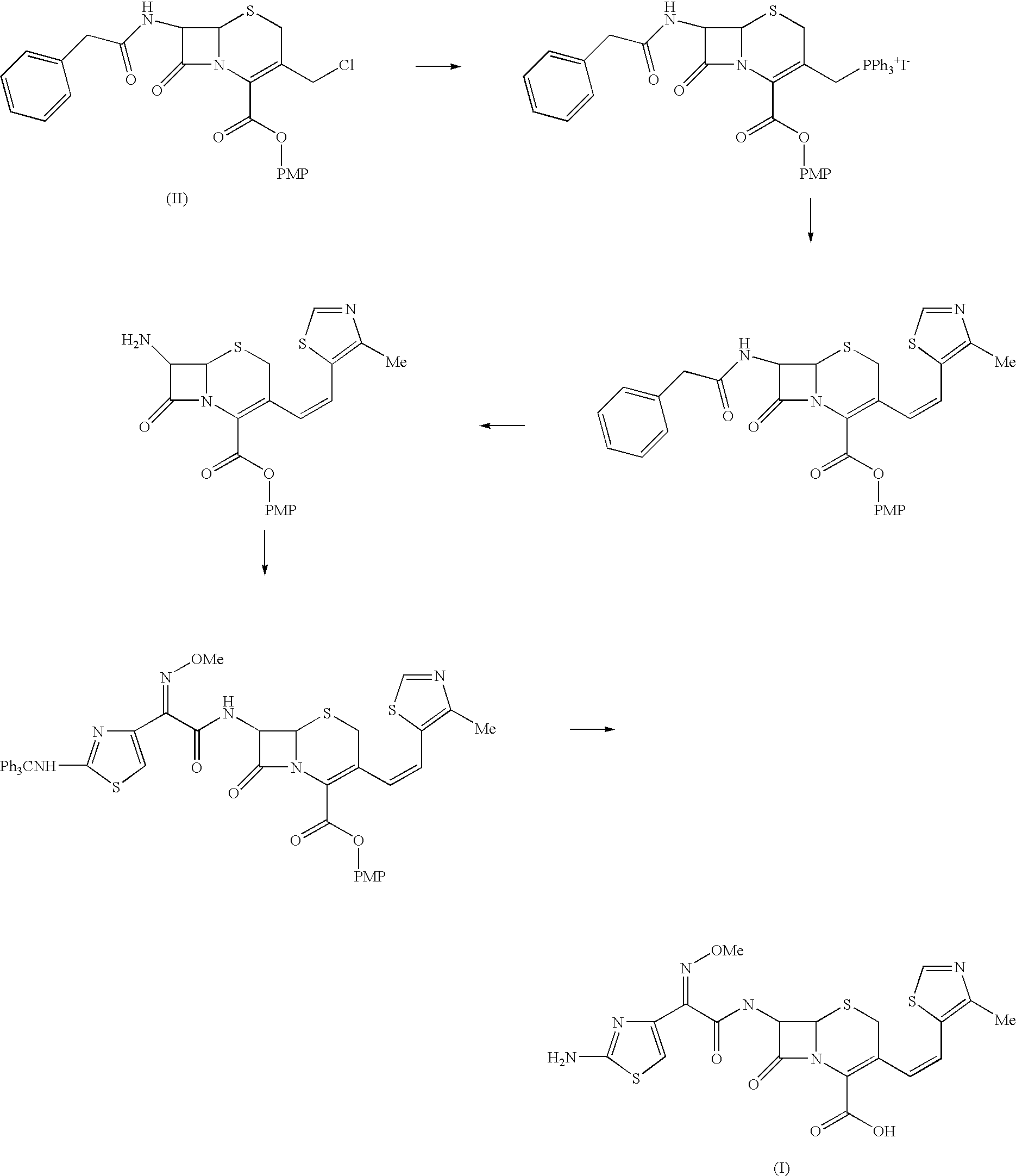

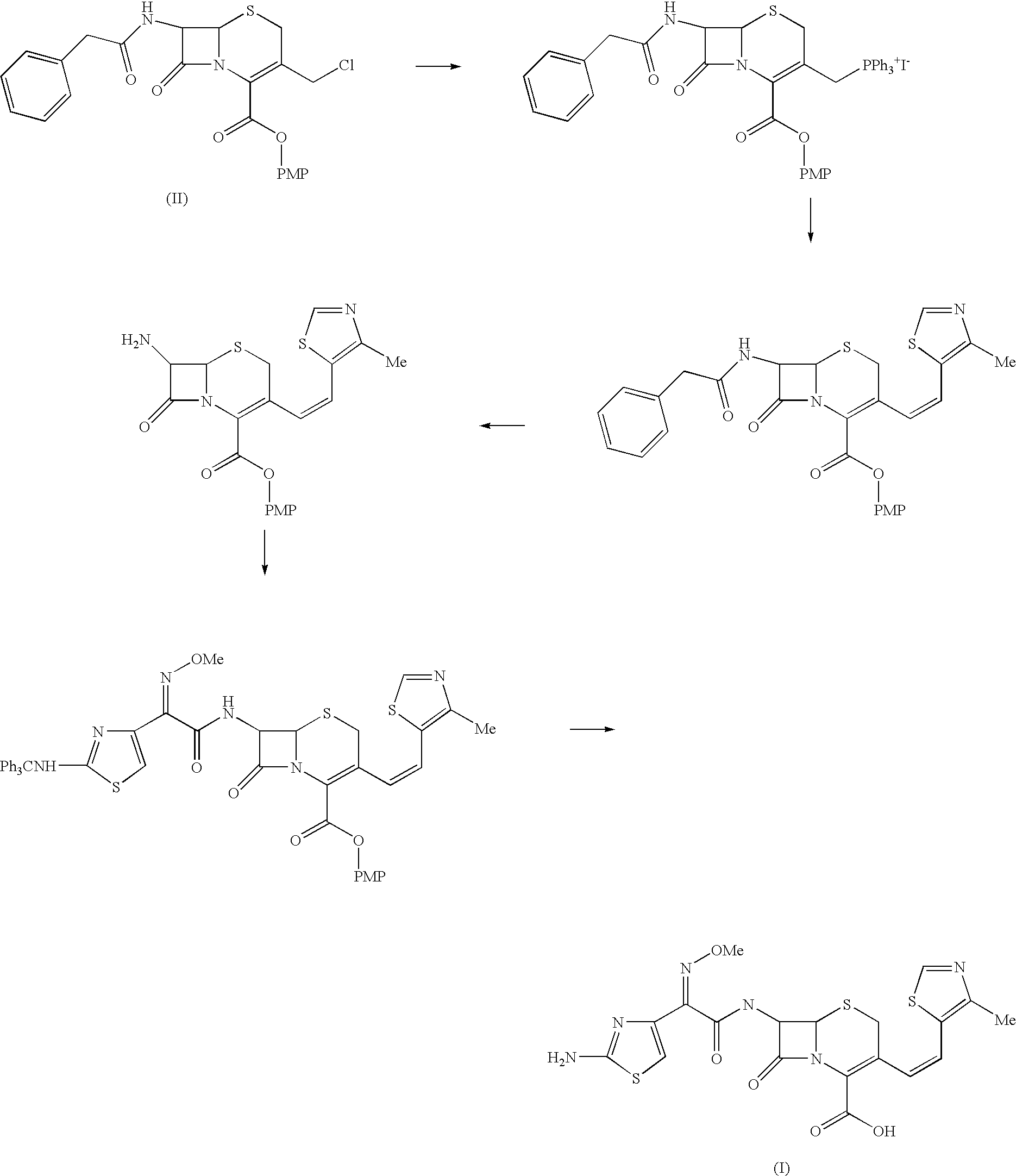
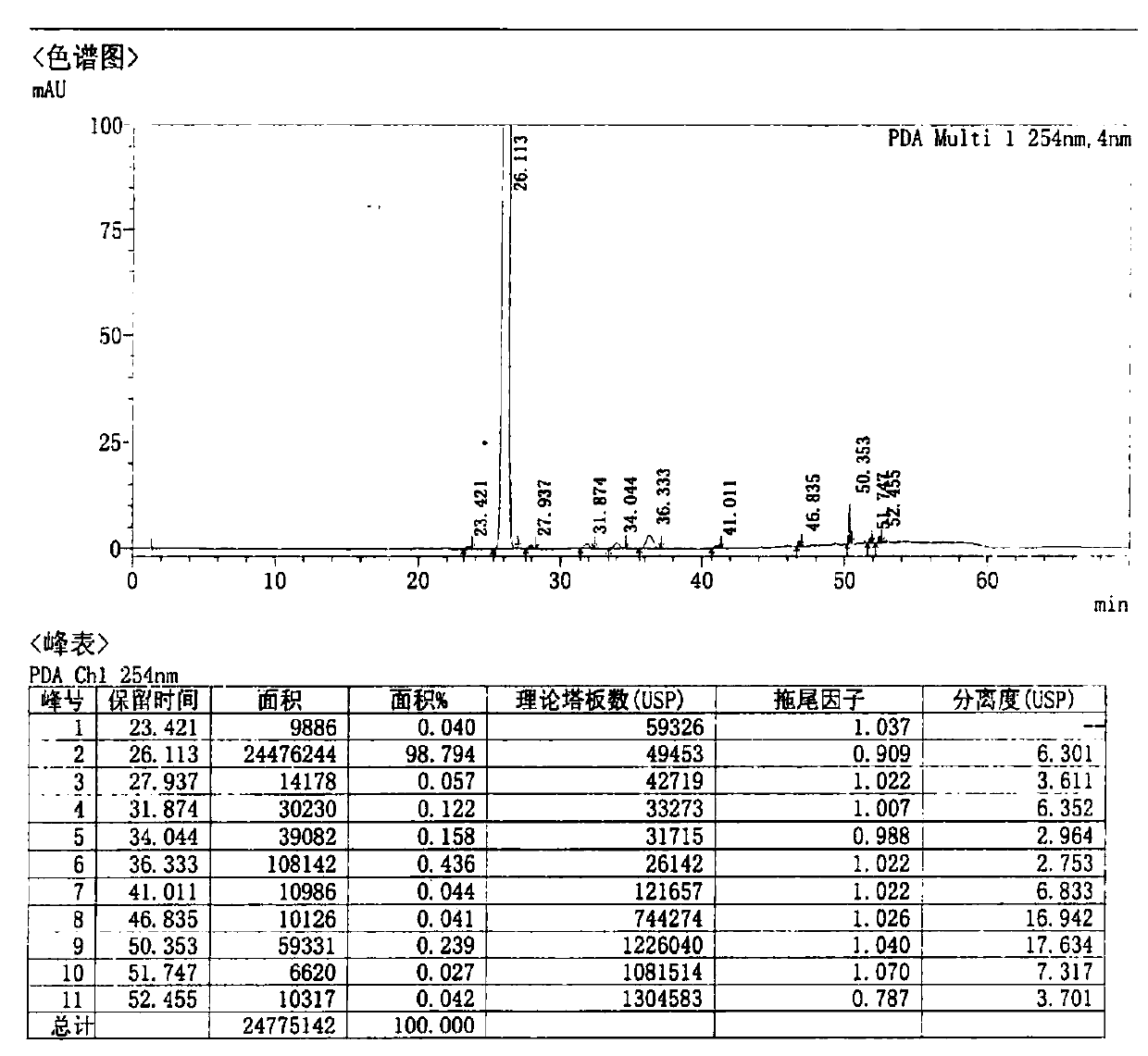
![Preparation method of cefditoren pivoxil [delta]3 isomer Preparation method of cefditoren pivoxil [delta]3 isomer](https://images-eureka.patsnap.com/patent_img/6ee1f42d-3563-41b4-900f-ff71f1b82eeb/FDA0002371118920000011.png)
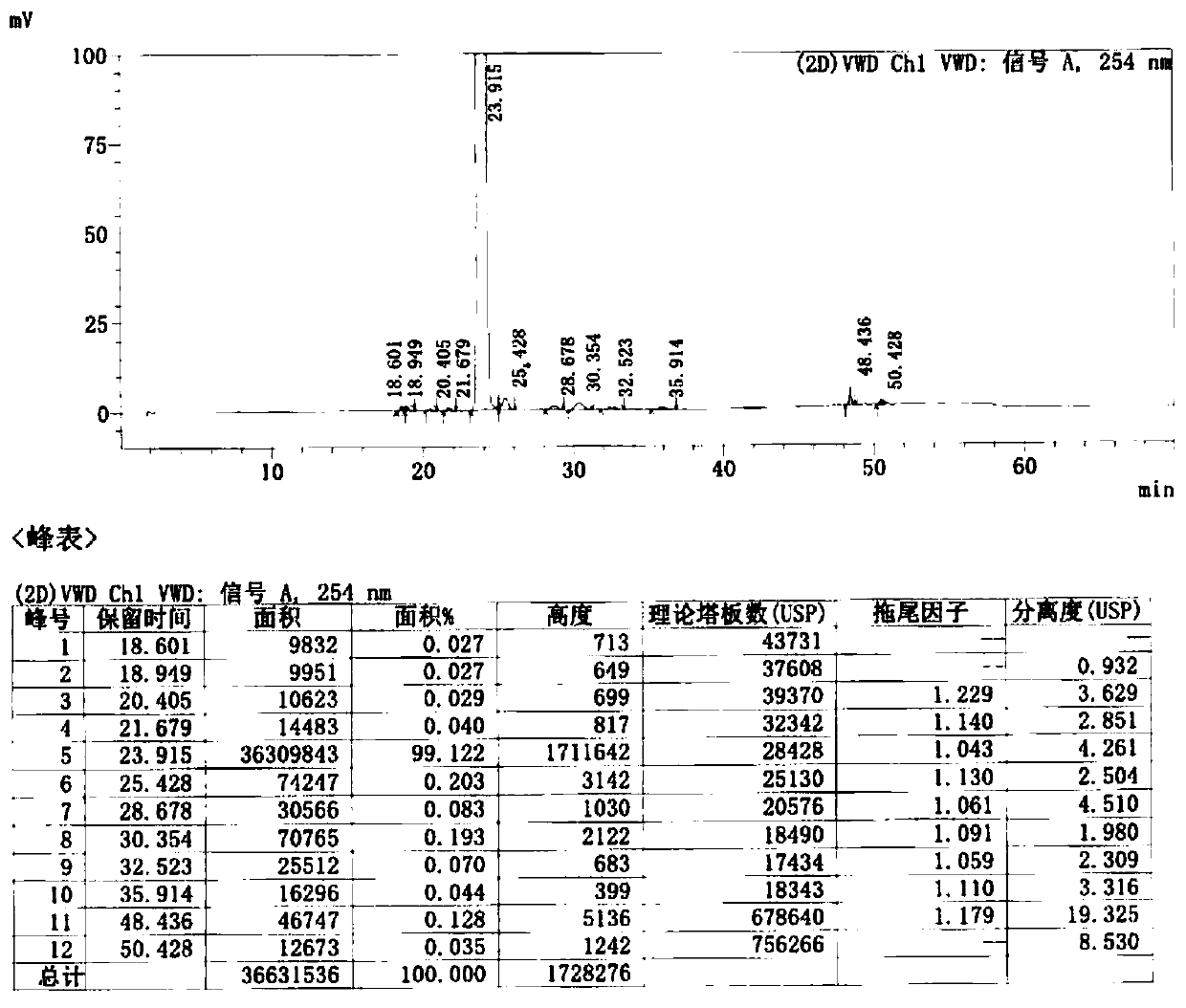
![Preparation method of cefditoren pivoxil [delta]3 isomer Preparation method of cefditoren pivoxil [delta]3 isomer](https://images-eureka.patsnap.com/patent_img/6ee1f42d-3563-41b4-900f-ff71f1b82eeb/FDA0002371118920000012.png)
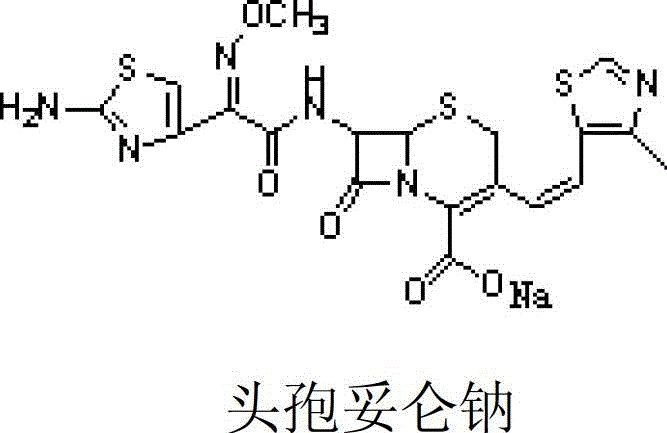
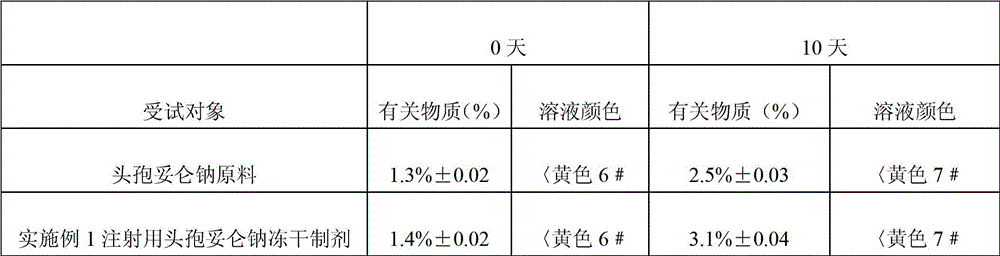
![Preparation method of cefditoren pivoxil [delta]3 isomer Preparation method of cefditoren pivoxil [delta]3 isomer](https://images-eureka.patsnap.com/patent_img/6ee1f42d-3563-41b4-900f-ff71f1b82eeb/FDA0002371118920000013.png)