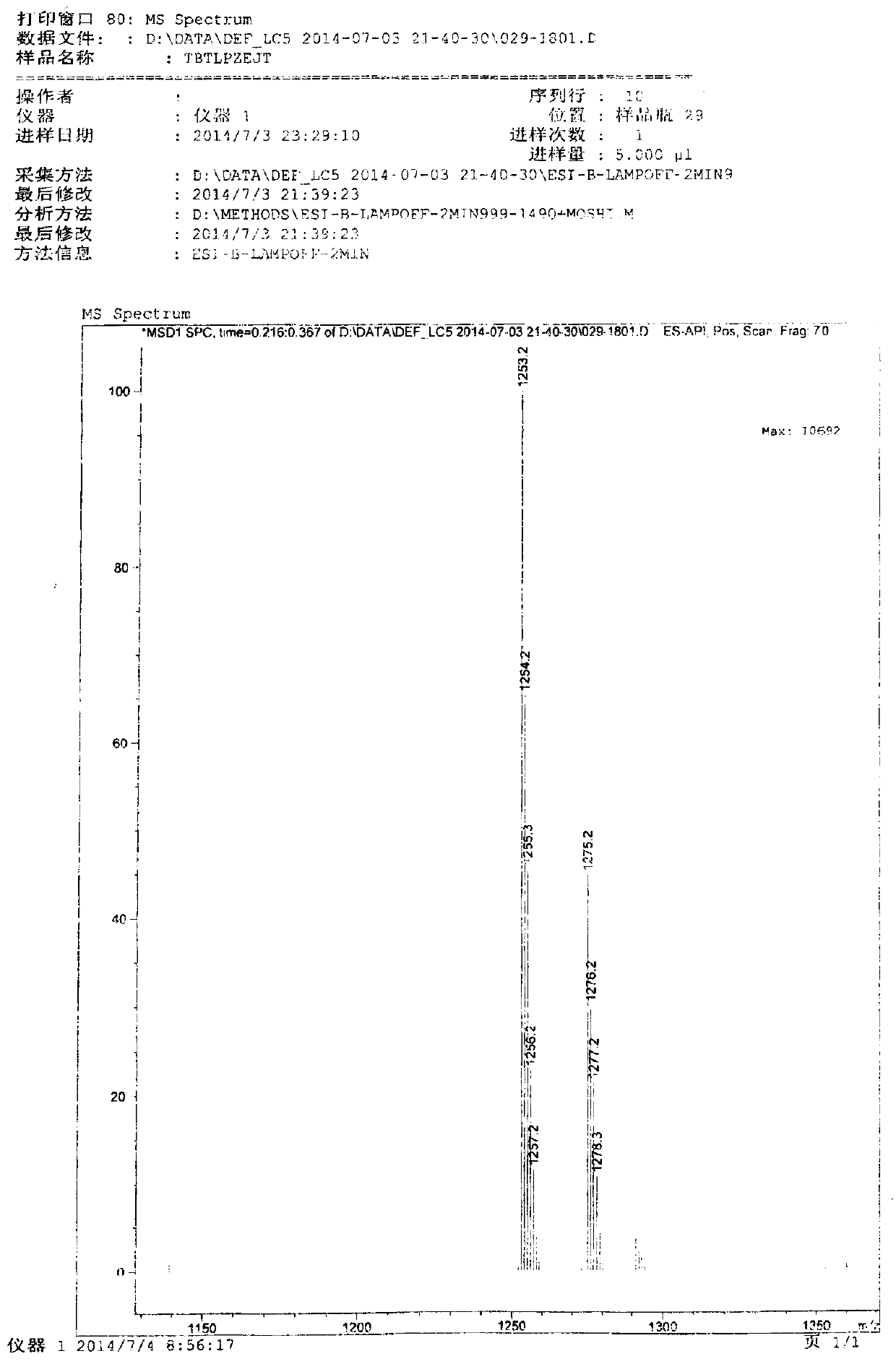
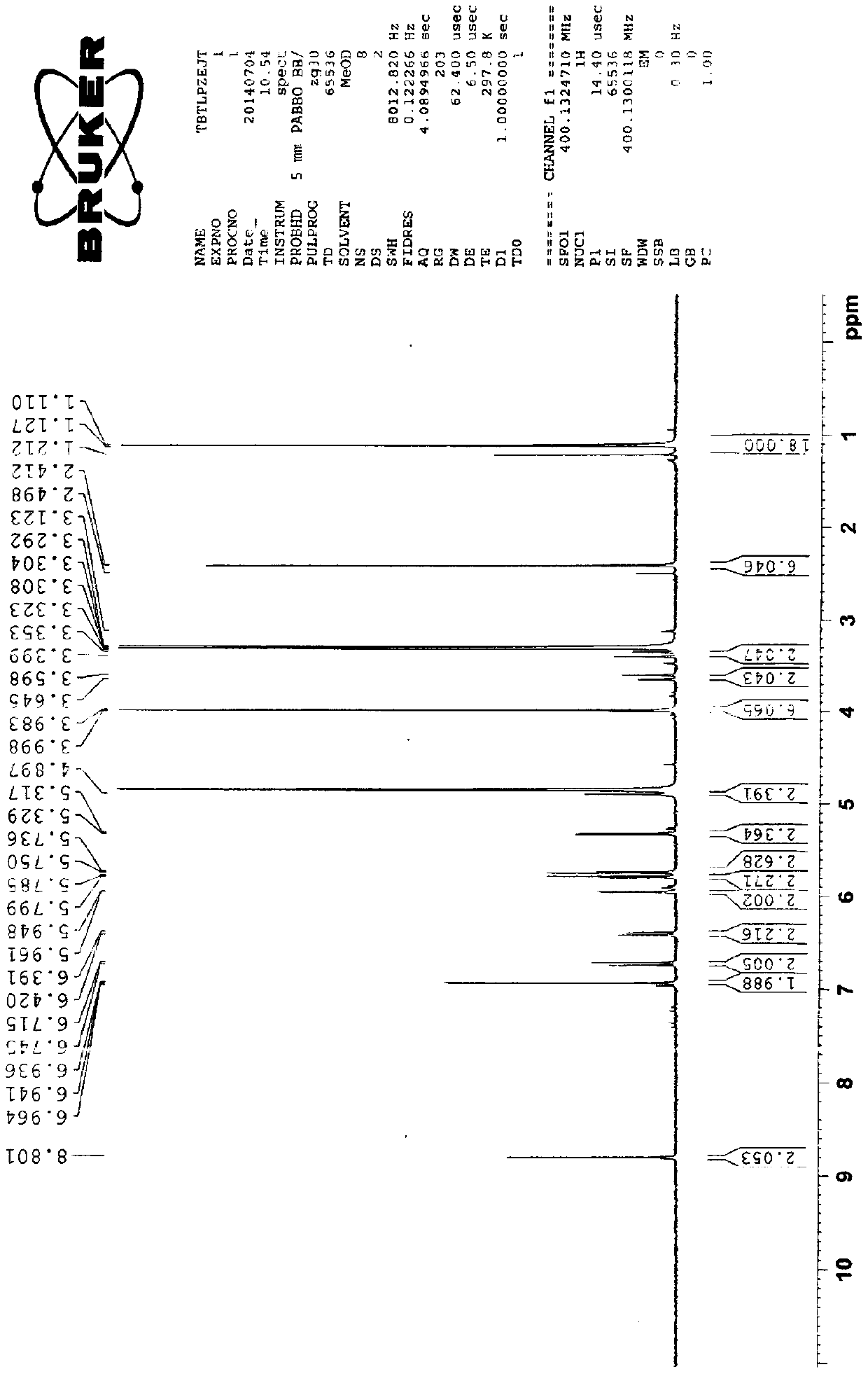
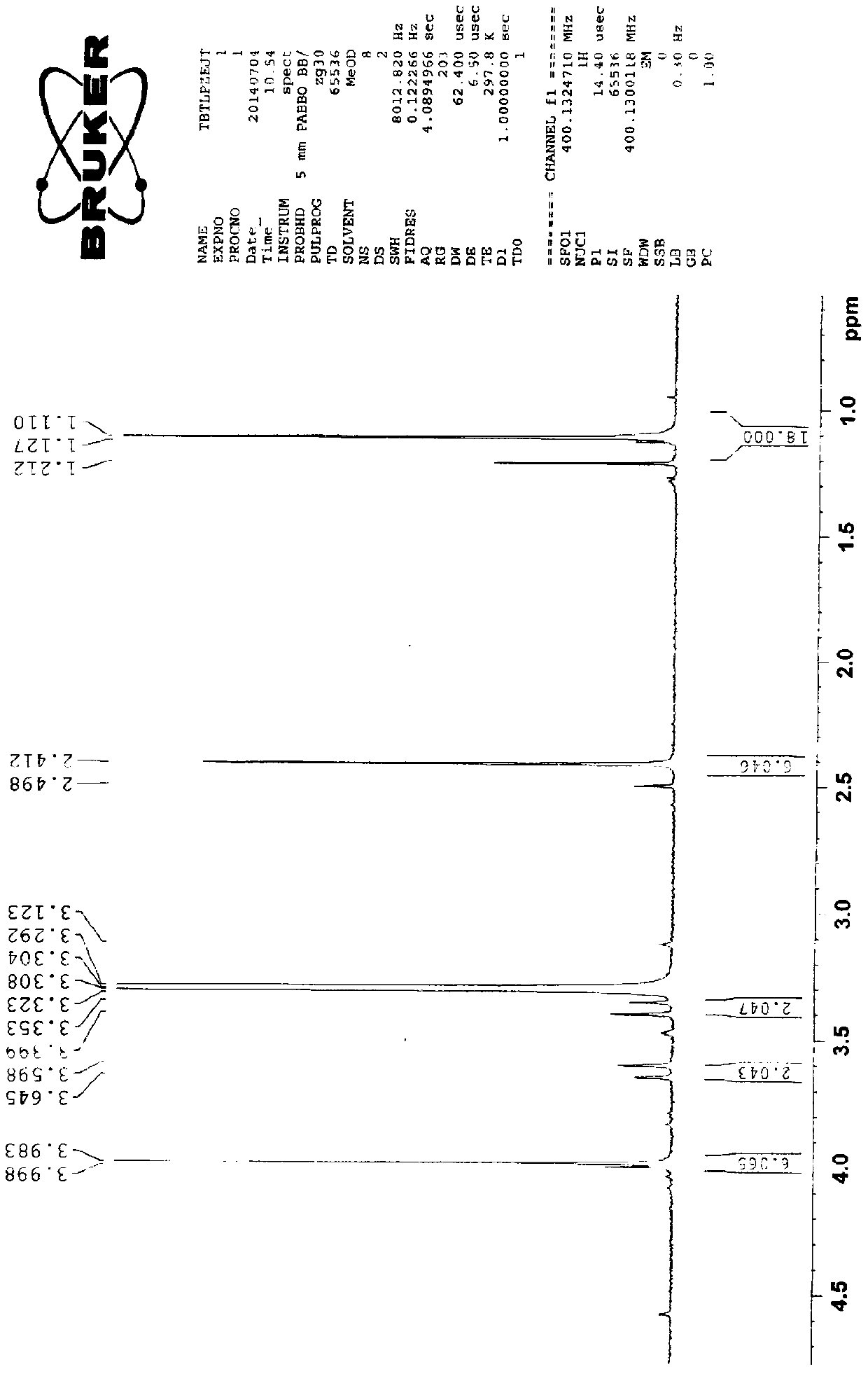
Preparation method of cefditoren pivoxil dimer
A technology of cefditoren pivoxil and ester dimer, which is applied in the direction of organic chemistry, can solve the problems of low yield and cumbersome steps, and achieve the effect of simple steps, simple raw materials, and safe clinical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of cefditoren pivoxil dimer
[0030] Add 125ml of N,N-dimethylformamide (DMF) and 22.2g (0.04mol) of cefditoren sodium into the reaction vessel, stir to dissolve, and lower the temperature to below -80°C.
[0031] Add 0.06 mol of iodomethyl pivalate, and control the temperature below -50°C to react for 20 minutes.
[0032] Then add 0.06 mol of iodomethyl pivalate, and control the temperature below -50°C to react for 20 minutes.
[0033] Finally, 0.04 mol of iodomethyl pivalate was added, and the temperature was controlled below -50°C until the reaction was complete.
[0034] During the reaction, triethylamine needs to be added to control the pH between 7.5-8.5. After the reaction, add 350ml of ethyl acetate and 270ml of pure water to the reaction solution, stir, leave to stand for stratification, and discard the lower aqueous layer. The organic layer was stirred and washed with 0.1% sodium bicarbonate solution and water successively, separated into layers,...
Embodiment 2
[0036] Preparation of cefditoren pivoxil dimer
[0037] Add 150 ml of acetonitrile and 22.2 g (0.04 mol) of cefditoren sodium into the reaction vessel, stir to dissolve, and lower the temperature to below -80°C.
[0038] Add 0.06 mol of iodomethyl pivalate, and control the temperature below -40°C to react for 30 minutes.
[0039] Then add 0.06 mol of iodomethyl pivalate, and control the temperature below -40°C to react for 30 minutes.
[0040] Finally, 0.06 mol of iodomethyl pivalate was added, and the temperature was controlled below -40°C until the reaction was complete.
[0041] During the reaction process, pyridine needs to be added to control the pH between 7.5-8.5. After the reaction, add 350ml ethyl acetate and 270ml pure water to the reaction solution, stir, let stand to separate layers, discard the lower aqueous layer, and discard the organic layer. Stir and wash with 0.5% sodium hydroxide solution and water successively, let stand to separate layers, discard the wa...
Embodiment 3
[0043] Preparation of cefditoren pivoxil dimer
[0044] Add 120 ml of tetrahydrofuran and 22.2 g (0.04 mol) of cefditoren sodium into the reaction vessel, stir to dissolve, and lower the temperature to below -80°C.
[0045] Add 0.10 mol of iodomethyl pivalate, and control the temperature below -35°C to react for 28 minutes.
[0046] Then add 0.10 mol of iodomethyl pivalate, and control the temperature below -35°C to react for 28 minutes.
[0047] Finally, 0.04 mol of iodomethyl pivalate was added, and the temperature was controlled below -35°C until the reaction was complete.
[0048]During the reaction, tributylamine needs to be added to control the pH between 7.5-8.5. After the reaction, add 350ml of ethyl acetate and 270ml of pure water to the reaction solution, stir, leave to stand for stratification, and discard the lower aqueous layer. The organic layer was stirred and washed with 1% sodium bicarbonate solution and water successively, and the layers were allowed to sta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com