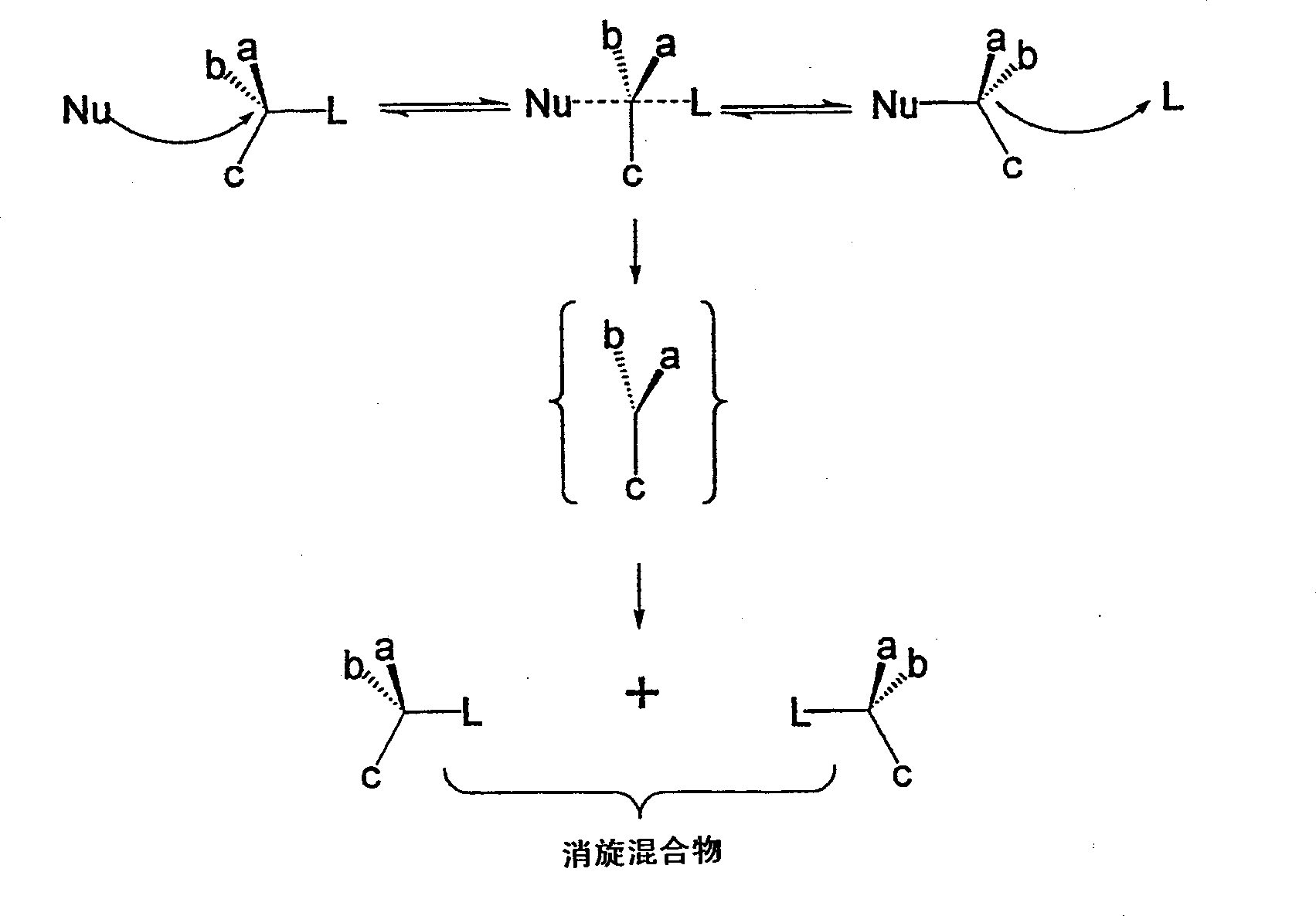
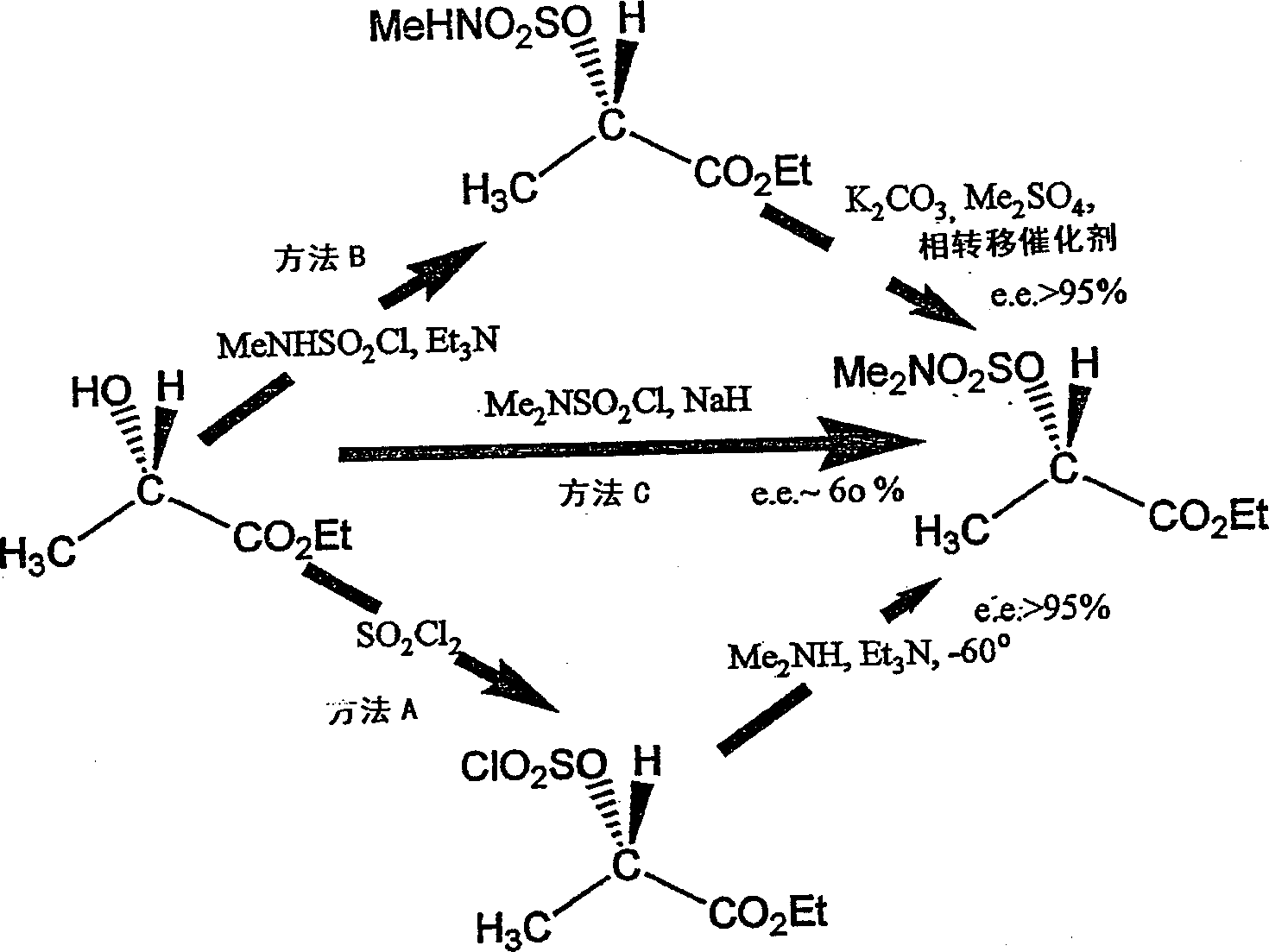
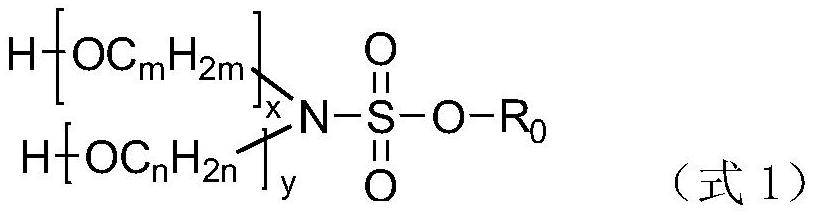
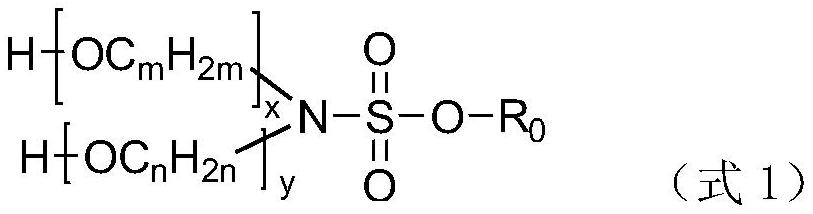
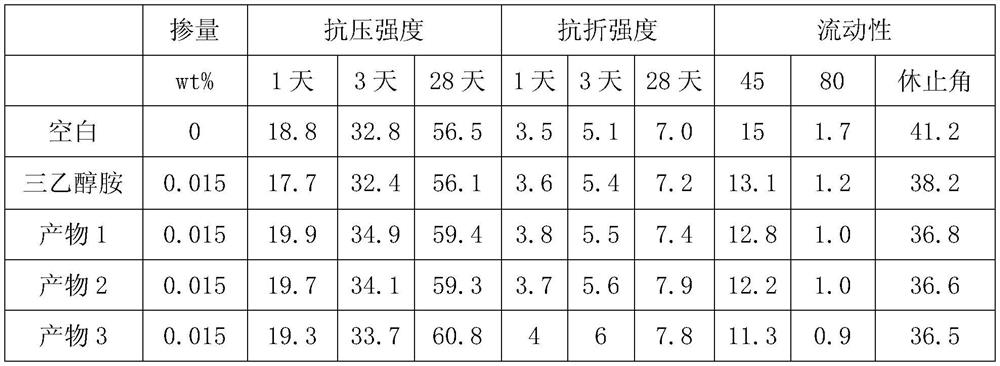
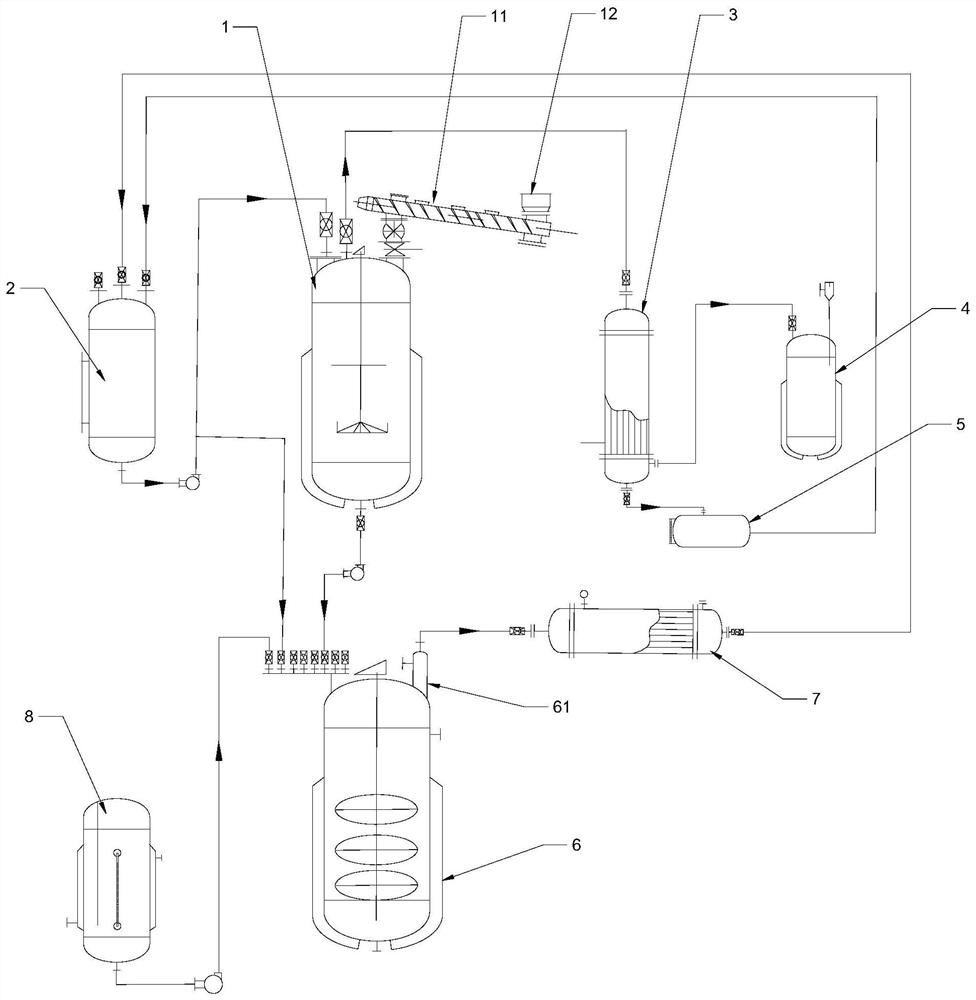
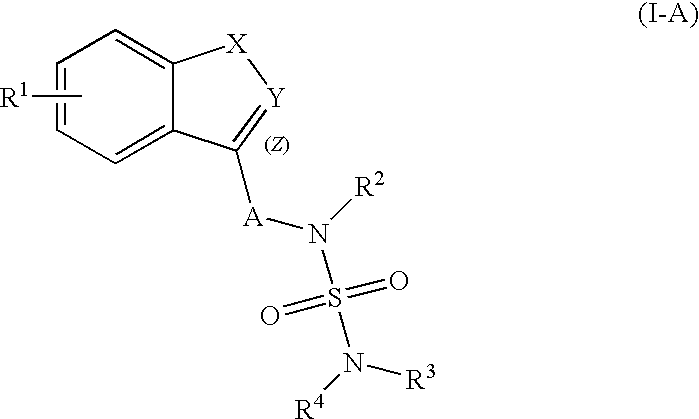
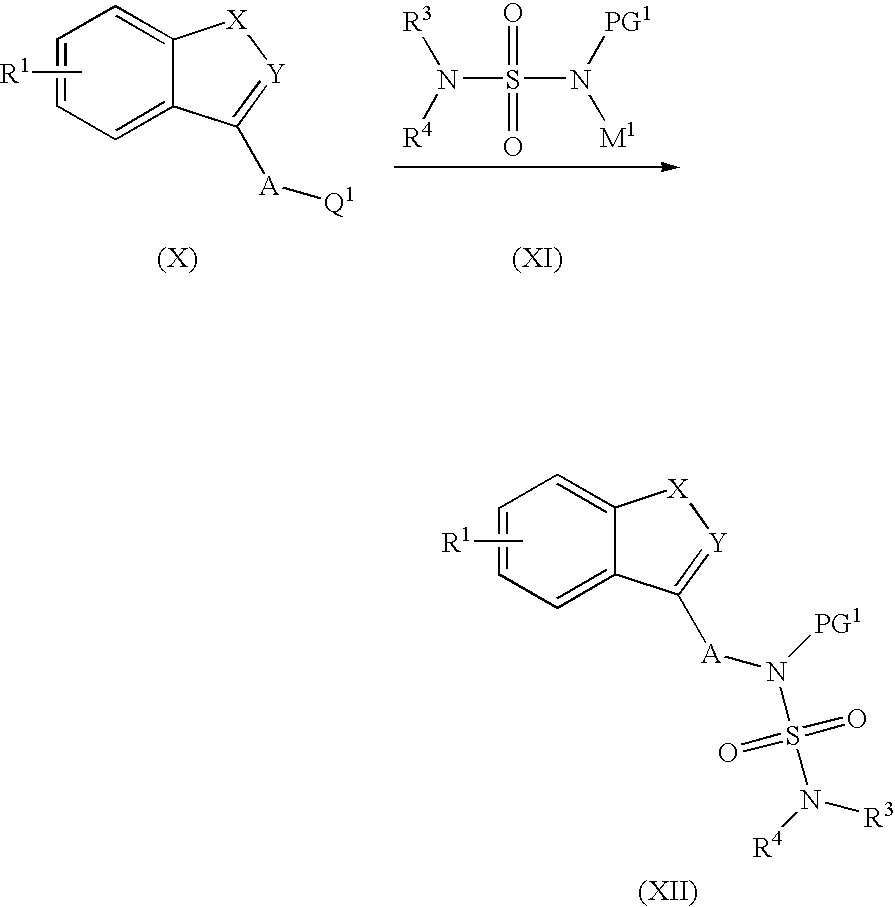
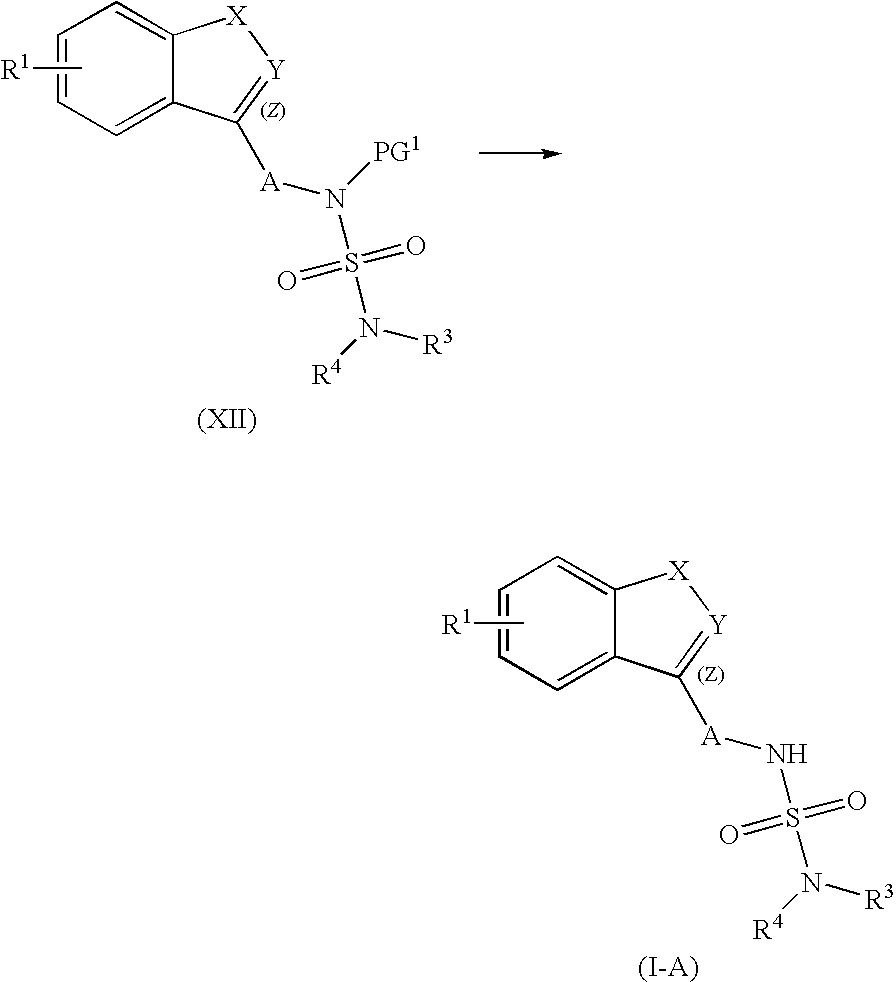
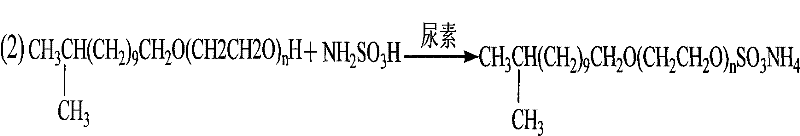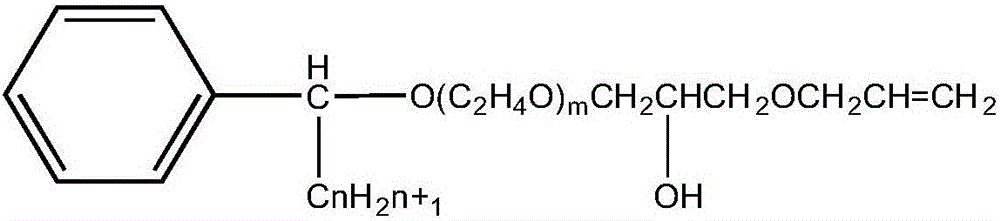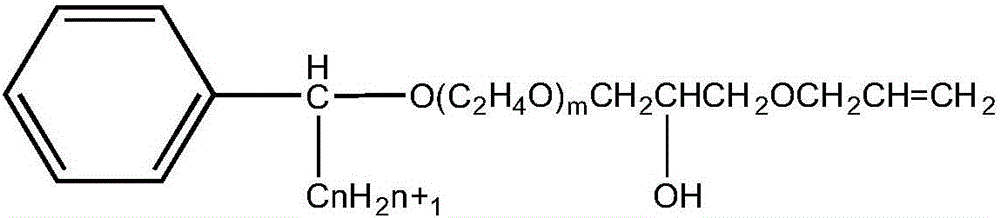Patents
Literature
84results about "Sulfuric acid amide preparation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
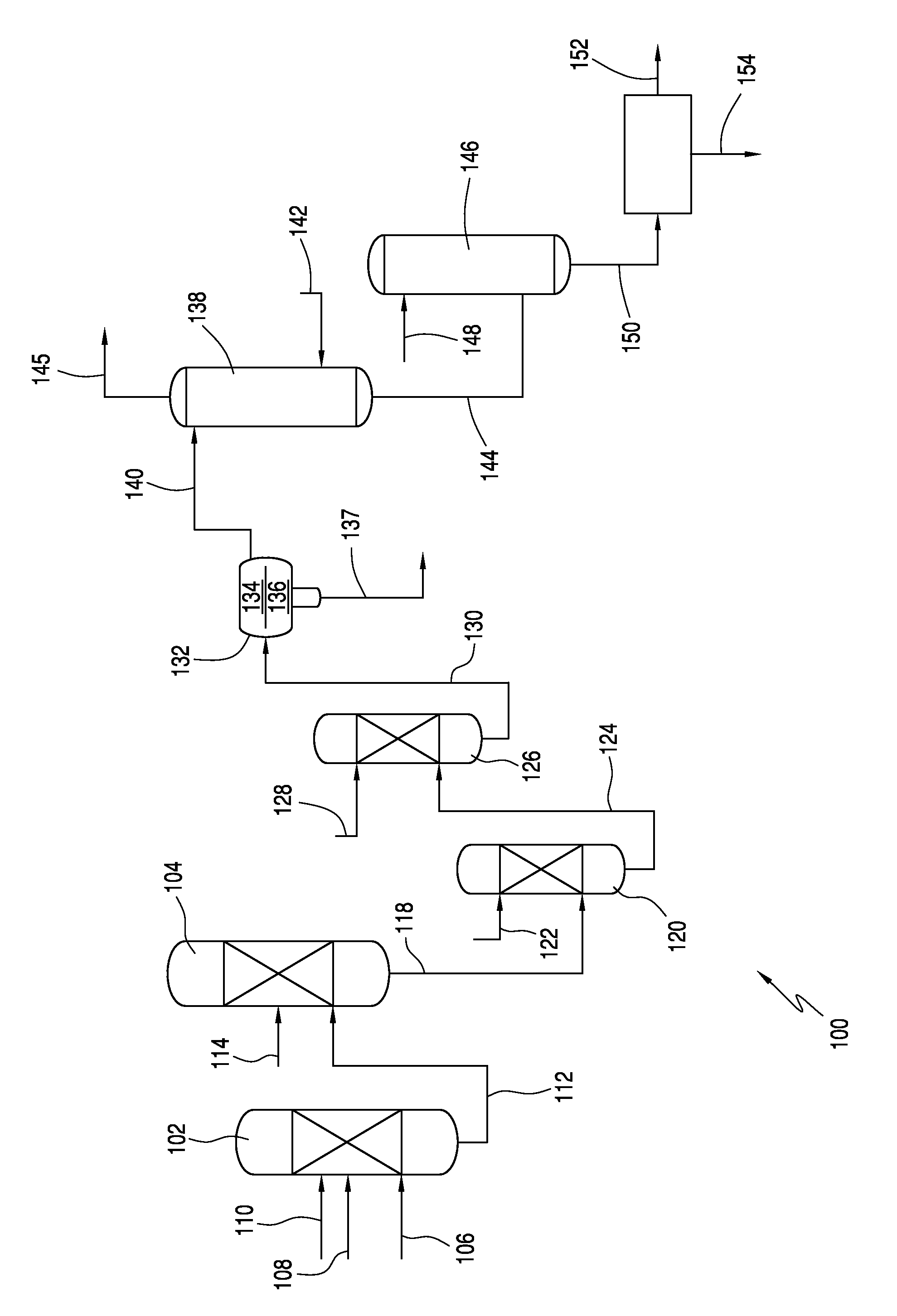
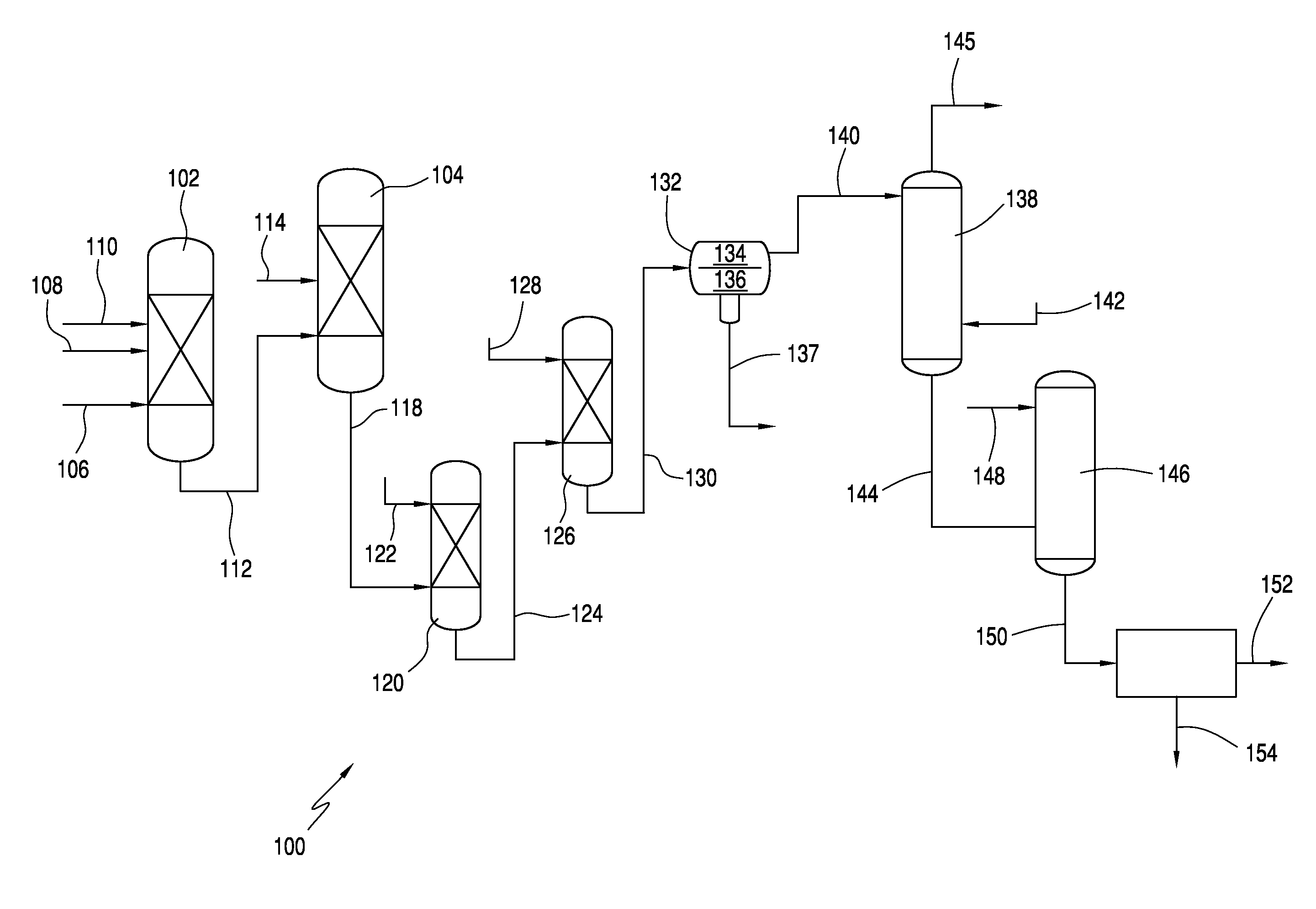
Process for producing acesulfame potassium
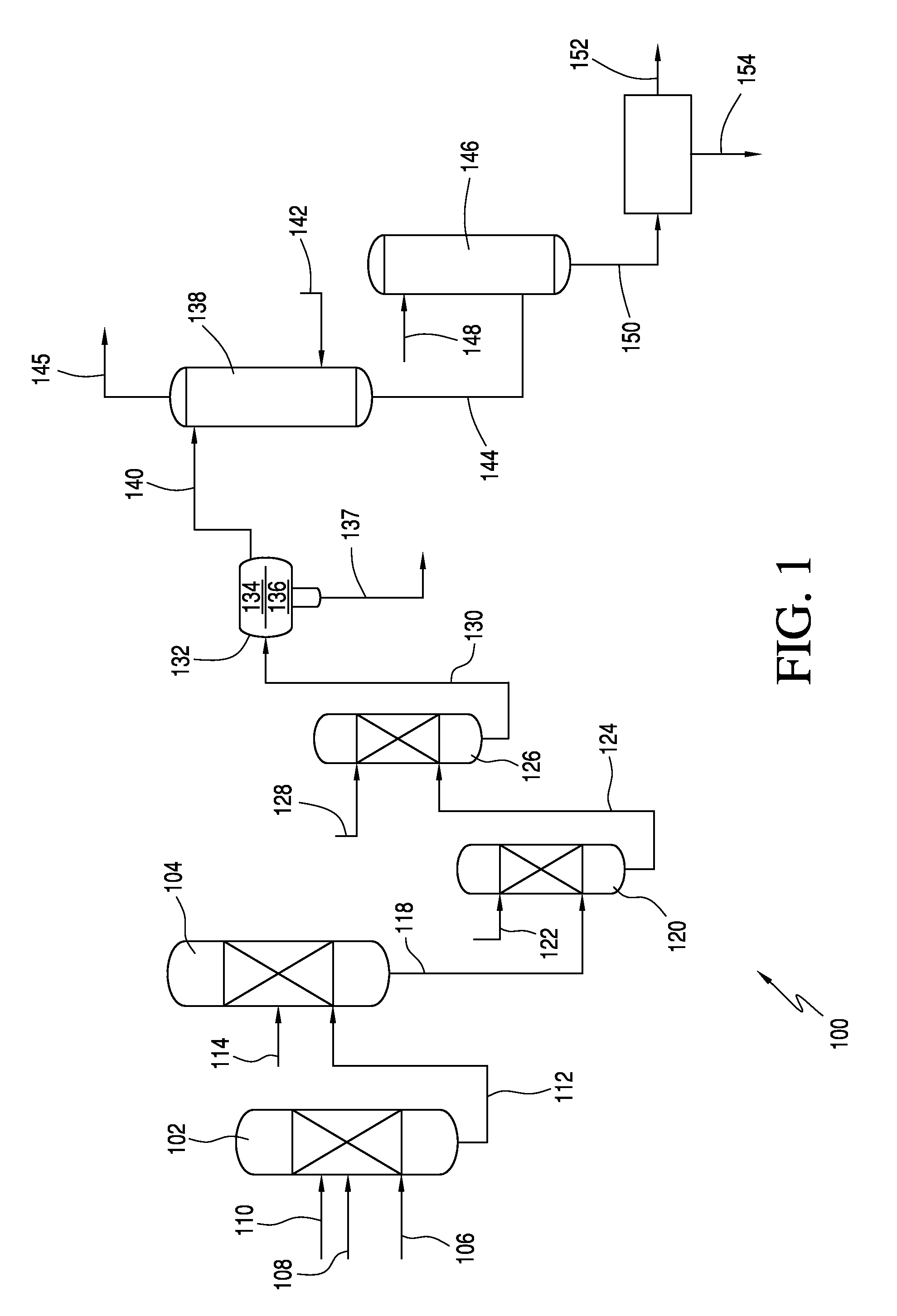
ActiveUS9024016B2Organic compound preparationSulfuric acid amide preparationAcetic acidSulfamic acid
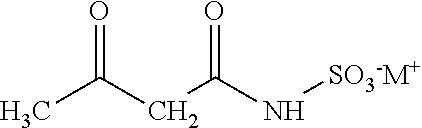
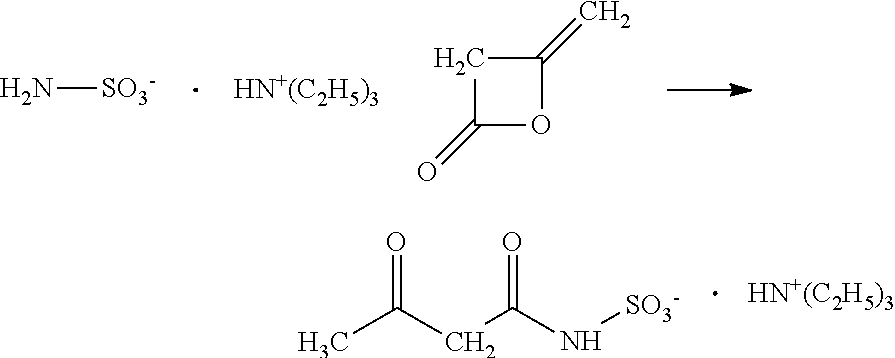
In one embodiment, the invention relates to processes for producing acesulfame potassium. In one embodiment, the process comprises the step of reacting a first reaction mixture to form an amidosulfamic acid salt such as a trialkyl ammonium amidosulfamic acid salt. The first reaction mixture comprises sulfamic acid, an amine, and smaller amounts, if any, acetic acid, e.g., less than 1 wt % (10000 wppm). In terms of ranges, the first reaction mixture may comprise from 1 wppm to 1 wt % acetic acid. The process further comprises the step of reacting the amidosulfamic acid salt with diketene to form an acetoacetamide salt. In preferred embodiments, the amidosulfamic acid salt formation reaction is conducted at pH levels from 5.5 to 7.0. The process further comprises the step of deriving the acesulfame-K from the acetoacetamide salt.
Owner:CELANESE SALES GERMANY
Process for Producing Acesulfame Potassium
ActiveUS20130331565A1Organic compound preparationSulfuric acid amide preparationAcetic acidSulfamic acid
In one embodiment, the invention relates to processes for producing acesulfame potassium. In one embodiment, the process comprises the step of reacting a first reaction mixture to form an amidosulfamic acid salt such as a trialkyl ammonium amidosulfamic acid salt. The first reaction mixture comprises sulfamic acid, an amine, and smaller amounts, if any, acetic acid, e.g., less than 1 wt % (10000 wppm). In terms of ranges, the first reaction mixture may comprise from 1 wppm to 1 wt % acetic acid. The process further comprises the step of reacting the amidosulfamic acid salt with diketene to form an acetoacetamide salt. In preferred embodiments, the amidosulfamic acid salt formation reaction is conducted at pH levels from 5.5 to 7.0. The process further comprises the step of deriving the acesulfame-K from the acetoacetamide salt.
Owner:CELANESE SALES GERMANY
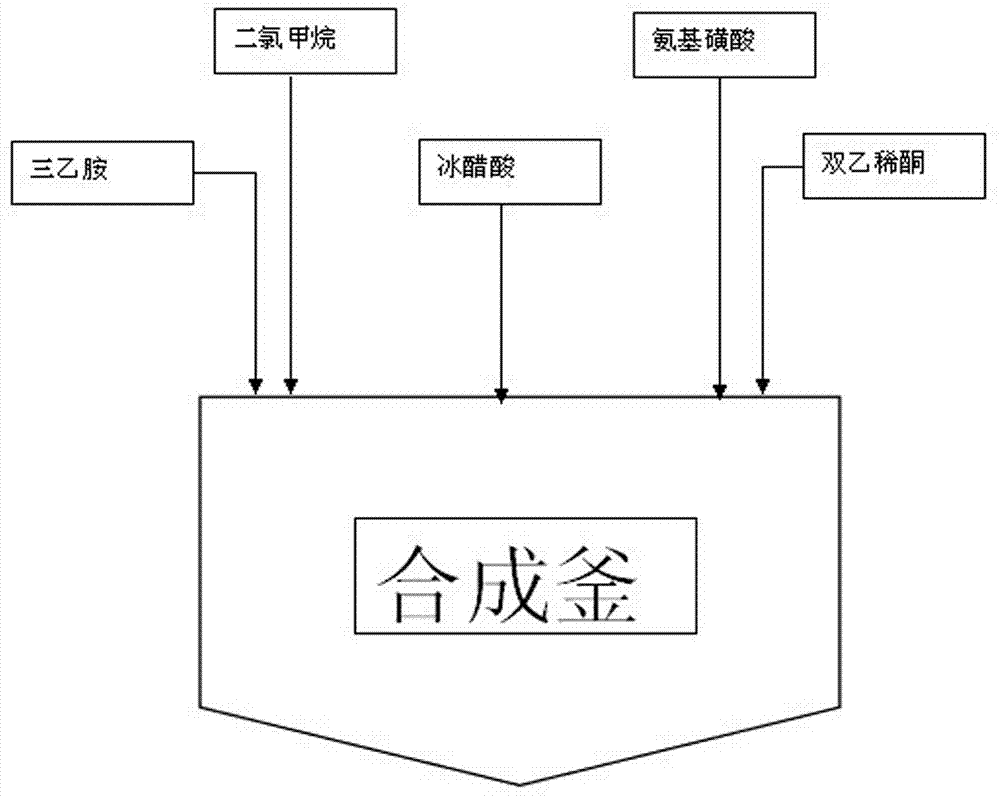
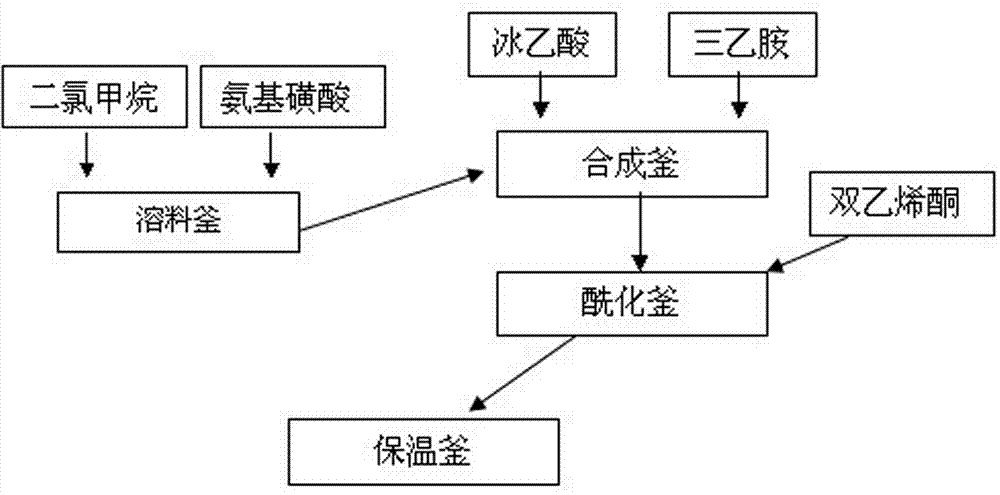
Acesulfame synthesis and acylation production process
InactiveCN103570592AReduce labor intensityImprove continuitySulfuric acid amide preparationAcetic acidEconomic benefits
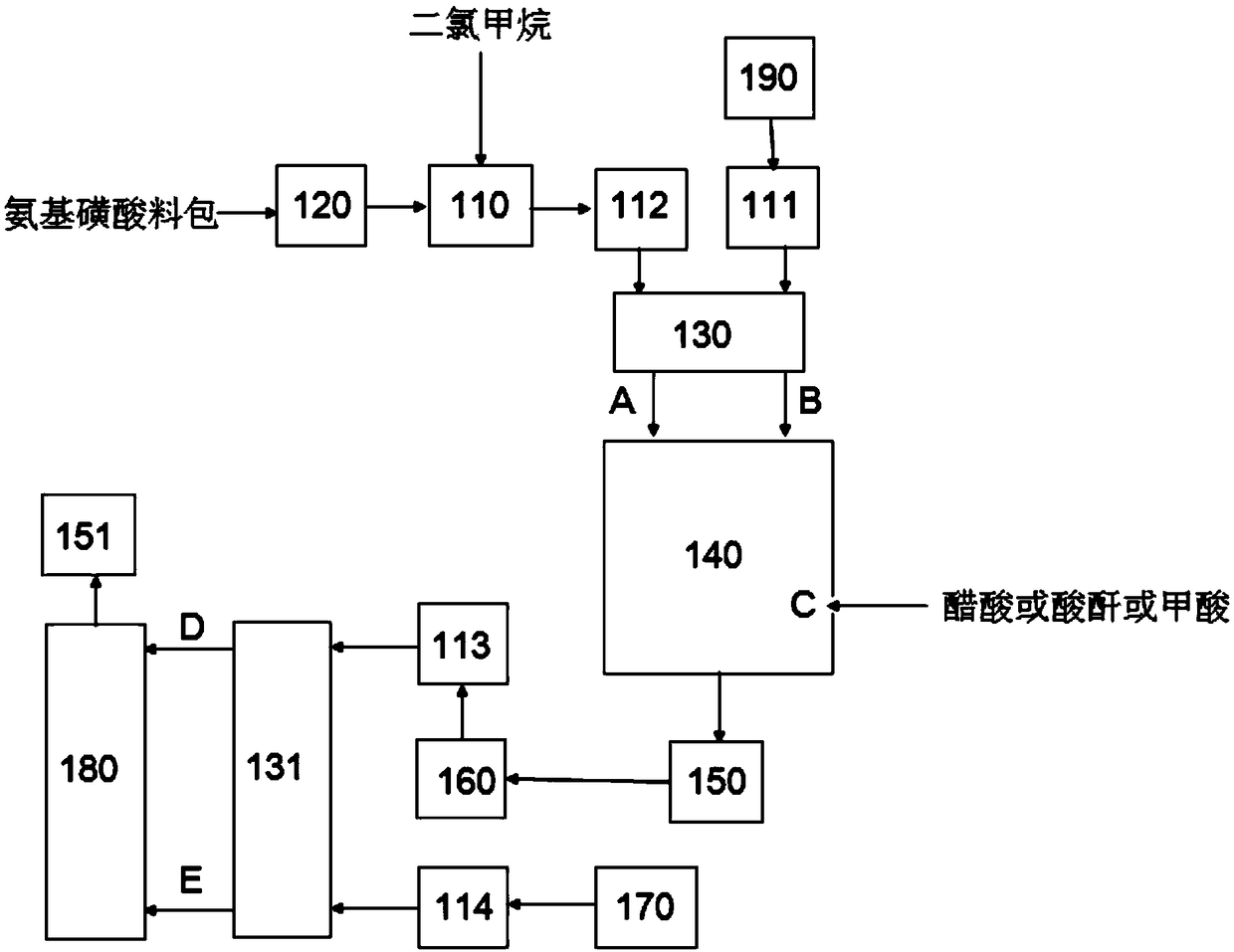
The invention relates to an acesulfame synthesis and acylation production process, which comprises the following steps of a, putting a sulfamic acid and dichloromethane into a dissolving kettle, and pumping a mixture into a synthesis kettle; b, adding dropwise triethylamine into the synthesis kettle, adding dropwise a glacial acetic acid, and performing natural reaction for 1 hour after the glacial acetic acid is added dropwise; c, placing reactants in the synthesis kettle into an acylation kettle, and adding dropwise diketene for an acylation reaction; and d, after the diketene is added dropwise, circulating a product subjected to the acylation reaction for 1 hour in the acylation kettle, placing into a heat-preservation kettle, performing natural reaction for 1 hour to generate an acetoacetyl-N-sulfonate intermediate, and feeding the acetoacetyl-N-sulfonate intermediate to a sulfonation section for use. The acesulfame synthesis and acylation production process has the advantages that 1, a stepwise reaction mode is adopted, so that compared with a conventional single-kettle production process, the method has the characteristics of production operation continuity and high reaction stability; 2, a multi-kettle stepwise production mode is adopted, so that the production efficiency is greatly improved, the reaction efficiency of the whole production flow is optimized, and the economic benefits of an enterprise are significantly improved.
Owner:ANHUI JINGHE IND
Method for Producing Imide Compound
ActiveUS20120020867A1Difficult to obtainHigh selectivityNitrosyl chlorideAmino preparation from aminesImideAlkaline earth metal
Disclosed is a method for producing “a salt or a complex comprising imide and an organic base”, characterized by reacting halogenated sulfuryl or halogenated phosphoryl with ammonia in the presence of an organic base. According to this method, a target imide compound can be produced in a high yield while significantly suppressing the production of by-products. Further, by reacting the obtained imide compound with an alkali metal hydroxide or an alkaline earth metal hydroxide, an imide metal salt can be easily derived.
Owner:CENT GLASS CO LTD
Acesulfame potassium synthesis section acylation reaction process
ActiveCN105198778AIncrease temperatureShort reaction timeSulfuric acid amide preparationDichloromethaneAcesulfame potassium
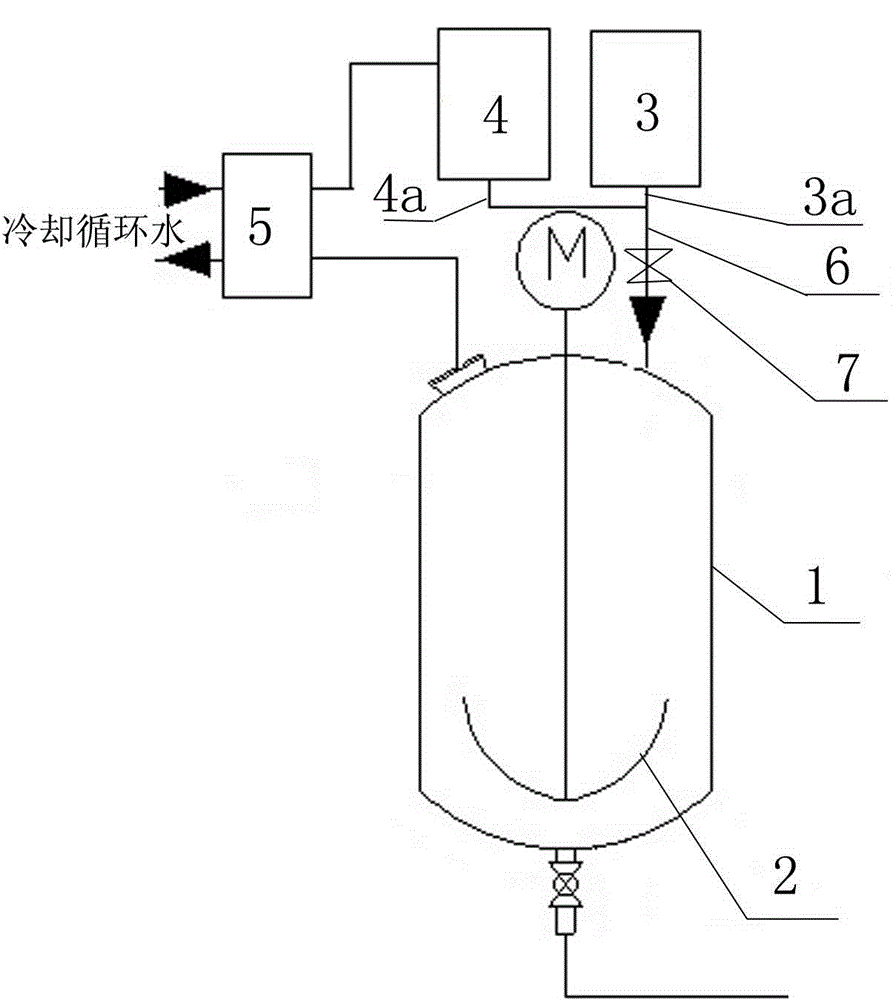
The invention discloses an acesulfame potassium synthesis section acylation reaction process. The process includes the following steps that a, a stirring machine of an acylation kiln and circulating cooling water flowing through a plate heat exchanger are started; b, objects obtained through the synthesis reaction of sulfamic acid and triethylamine are placed in an acylation kiln. C, dichloromethane and diketene with the volume ratio of 1 to 1 are evenly mixed, and a mixture of dichloromethane and diketene is dropwise added into the acylation kiln according to the ingredient amount, wherein the dropwise adding flow is 500 L / H; d, vaporized dichloromethane returns to a container for containing dichloromethane after passing through the plate heat exchanger, the mixture of dichloromethane and diketene is dropwise added into the acylation kiln, the acylation reaction temperature is raised, the reaction time is shortened, and the production efficiency is improved; meanwhile, dichloromethane is evaporated through the acylation reaction, evaporated dichloromethane is liquefied after being cooled through circulating cooling water, and liquefied dichloromethane returns to the container for containing dichloromethane to be recycled. The process is simplified, and the energy consumption is lowered.
Owner:ANHUI JINGHE IND
Binary or ternary fluorine-containing sulfimide alkali metal salt and ionic liquid and applications thereof
InactiveCN102786443AEasy to separate and purifyHigh yieldElectrolytic capacitorsSecondary cellsElectrochemical windowHydrolysis
The invention discloses a method for preparing binary or ternary fluorine-containing sulfimide alkali metal salts, a method for preparing ionic liquid by the binary or ternary fluorine-containing sulfimide alkali metal salts, and applications of the alkali metal salts and ionic liquid as electrolytes in carbon-based super capacitors, secondary lithium (ion) batteries, and the like. The method for preparing the binary or ternary fluorine-containing sulfimide alkali metal salts provided by the invention is short in operation steps, easy for product separation and purification, and high in product yield and purity; the binary or ternary fluorine-containing sulfimide lithium provided by the invention has good thermal stability and hydrolysis resistance; a nonaqueous electrolytic solution of the binary or ternary fluorine-containing sulfimide lithium has high conductivity and lithium ion transference number, and also exhibits good oxidation resistance and good compatibility with widely-used electrode materials; meanwhile, the ionic liquid containing the binary or ternary fluorine-containing sulfimide anions exhibits the properties of low viscosity and high conductivity, and has a wide electrochemical window.
Owner:HUAZHONG UNIV OF SCI & TECH +1
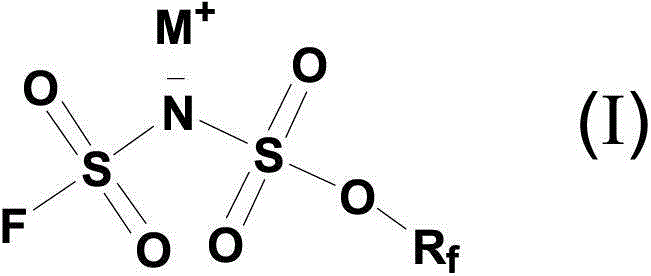
Fluorosulfuryl-containing compound, and intermediate, preparation method and application thereof
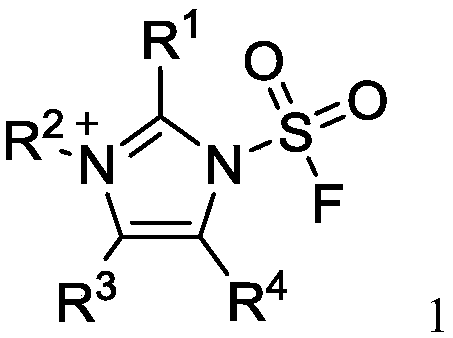
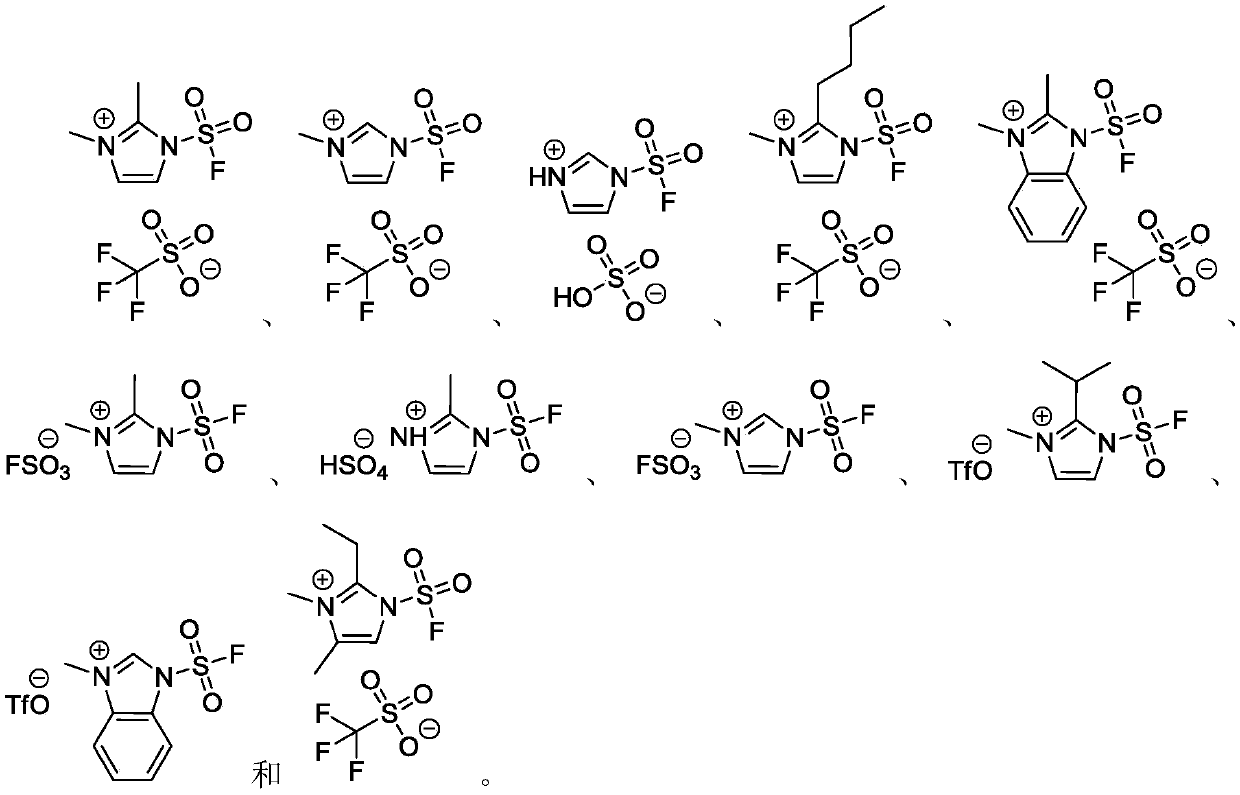
InactiveCN110590609AWide substrate adaptabilityLow toxicityOrganic compound preparationSulfuric acid amide preparationChemical transformationReagent
The invention discloses a fluorosulfuryl-containing compound, and an intermediate, preparation method and application thereof. The fluorosulfuryl-containing compound disclosed by the prevention includes cations and anions, and the cations are shown as a formula 1 (please see the specifications for the formula). The fluorosulfuryl-containing compound can react with a substrate to efficiently synthesize a fluorosulfuryl product, is low in toxicity, easy to prepare and convenient to use, and is in a stable solid state at the normal temperature; and in addition, the substrate of the compound has extremely high adaptability and can include a phenolic compound and an amine compound, and the fluorosulfuryl-containing compound is a unique solid form reagent capable of realizing chemical transformation at present and thus has important academic and application value.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
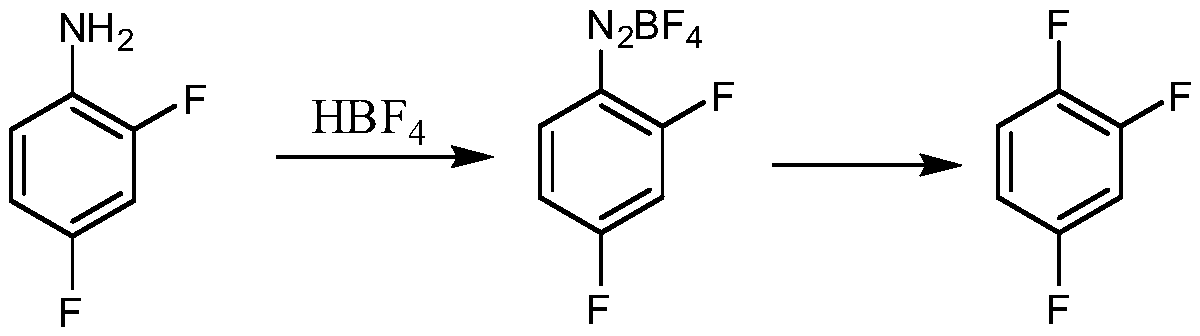
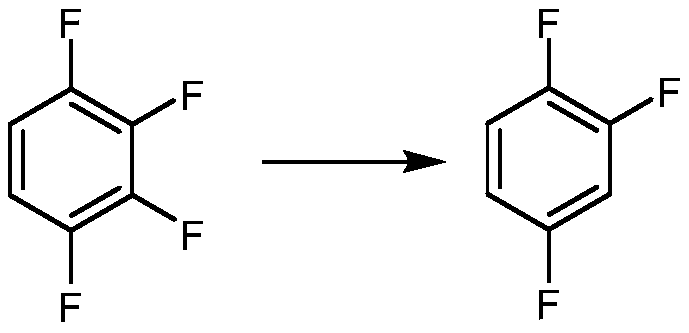
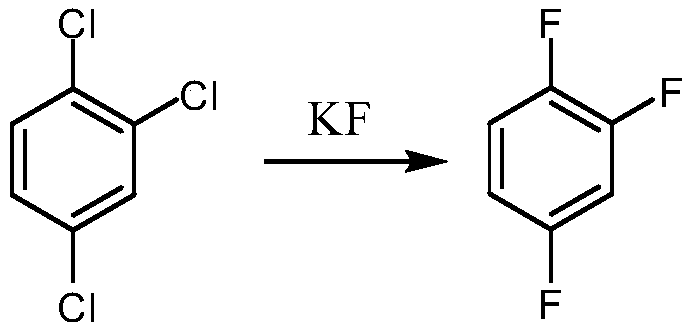
Synthetic method for 1,2,4-trifluorobenzene
ActiveCN110498730ALow costEasy to realize industrializationOrganic compound preparationSulfuric acid amide preparationNitrosylsulfuric acidPotassium fluoride
The invention provides a synthetic method for 1,2,4-trifluorobenzene, belongs to the field of pesticide, medicine, and liquid crystal material intermediate preparation, and solves the problem of harshreaction conditions of a current method for synthesizing 1,2,4-trifluorobenzene. The synthetic method for the 1,2,4-trifluorobenzene is characterized by comprising the following steps: performing nitration by using 2,4-dichlorofluorobenzene as a raw material and nitric acid as a nitrating agent to form 2,4-dichloro-5-fluoronitrobenzene in the presence of sulfuric acid; dissolving the 2,4-dichloro-5-fluoronitrobenzene into an organic solvent, adding potassium fluoride and a first catalyst, and performing fluorination under the catalysis of the first catalyst to obtain 2,4,5-trifluoronitrobenzene; dissolving the 2,4,5-trifluoronitrobenzene into a solvent, and performing hydrogenation reduction with hydrogen under the catalysis of a second catalyst to obtain 2,4,5-trifluoroaniline; and performing a reaction on the 2,4,5-trifluoroaniline and sulfuric acid, after a salt is formed, performing a diazotization reaction on the salt and nitroso-sulfuric acid, performing a deamination reductionreaction with sodium hypophosphite under the catalysis of a copper salt, and finally performing steam distillation to obtain the 1,2,4-trifluorobenzene. The method provided by the invention has the advantages of mild reaction conditions and the like
Owner:ZHEJIANG LINJIANG CHEM
Alkali metal salt of (sulfonyl fluoride)( multi-fluorine alkoxy sulfonyl) imine and ionic liquids
ActiveCN104151206ANo pollution in the processReduce usageSecondary cellsSulfuric acid amide preparationSulfoniumSulfonyl fluoride
The invention provides a method for preparing an alkali metal salt of (sulfonyl fluoride)( multi-fluorine alkoxy sulfonyl) imine, and preparing corresponding ionic liquids through a replacement reaction of the alkali metal salt and an ammonium salt, a phosphor salt, a sulfonium salt and the like. The invention provides an electrolyte material of a secondary lithium battery or carbon-based super capacitor based on an ionic liquid of an asymmetric (sulfonyl fluoride)( multi-fluorine alkoxy sulfonyl) imine negative ion. The electrolyte material has good compatibility with electrode materials of LiCoO2, LiFePO4, Li, graphite, Li4Ti5O12 and active carbon and the like.
Owner:武汉市瑞华新能源科技有限公司
Ionic liquid, preparation method and application
ActiveCN107602424AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsIonic liquidIon
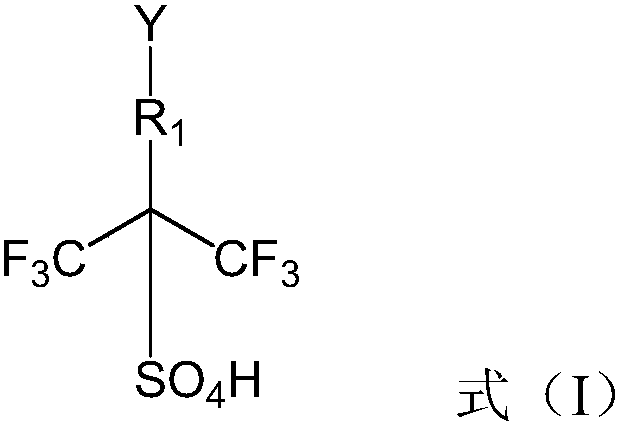
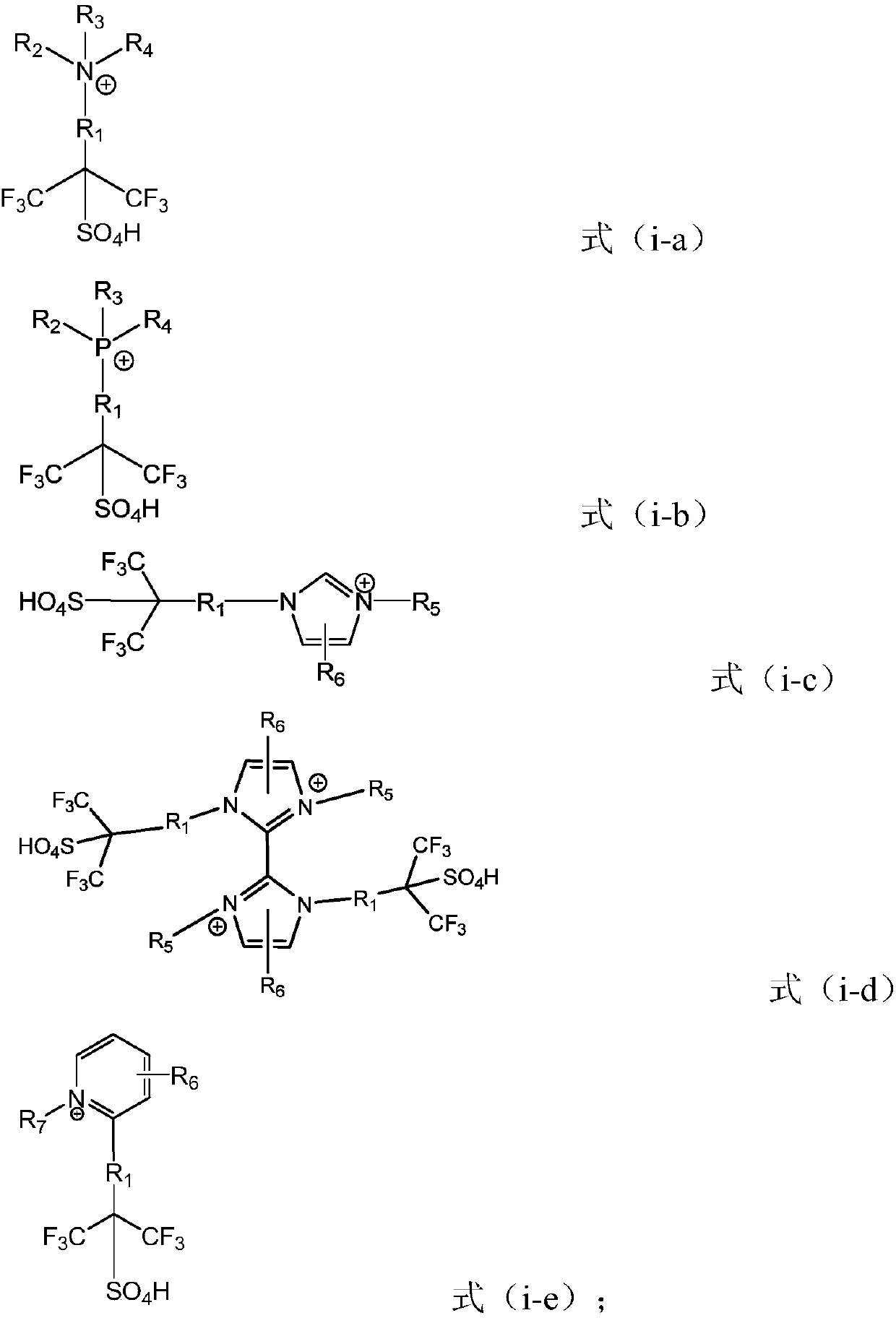
The invention relates to an ionic liquid. Cations of the ionic liquid contain hexafluoroisopropyl sulfonic acid groups. According to the ionic liquid provided by the invention, the hexafluoroisopropylsulfonic acid groups are introduced into the cation part of the ionic liquid to give a super acid center to the ionic liquid, through changing anion types, the ionic liquid of which the acidity is greater than that of 98% concentrated sulfuric acid is obtained, and the highest level of the hamlet acidity (H0) in the obtained ionic liquid can reach -14.13.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Binary fluorine-containing sulfimide and preparation method of alkali metal salt of binary fluorine-containing sulfimide
InactiveCN104193655AEasy to separate and purifyHigh yieldSulfonic acid amide preparationSulfuric acid amide preparationElectrolysisElectrochemical window
The invention discloses a method for preparing binary or ternary fluorine-containing sulfimide, a method for preparing an ionic liquid from an alkali metal salt of the binary or ternary fluorine-containing sulfimide, and an application of the alkali metal salt and the ionic liquid as electrolyte in a carbon-based super capacitor, a secondary lithium (ion) battery, and the like. The method for preparing the alkali metal salt of the binary or ternary fluorine-containing sulfimide is simple and short in operation step, a product is easy to separate and purify, and the yield and the purity of the product are both high; the binary or ternary fluorine-containing sulfimide lithium disclosed by the invention is good in thermal stability and hydrolysis resistance, a non-water electrolysis liquid of the binary or ternary fluorine-containing sulfimide lithium is relatively high in conductivity and lithium ion transference number, relatively good in oxidation resistance and good in compatibility with electrode materials which are used widely; and meanwhile the ionic liquid containing binary or ternary fluorine-containing sulfimide anions is low in viscosity, high in conductivity and wide in electrochemical window.
Owner:HUAZHONG UNIV OF SCI & TECH +1
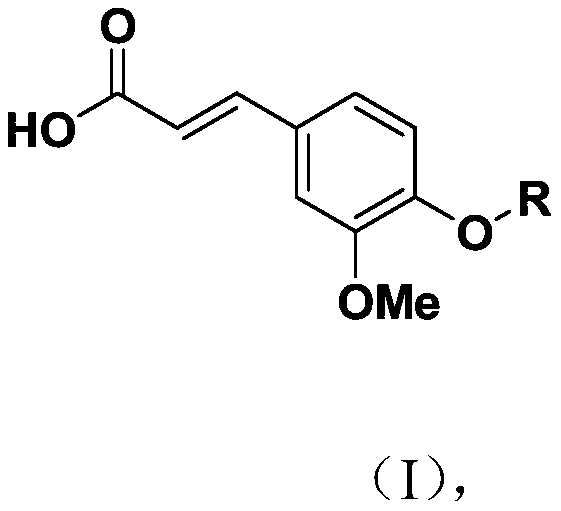
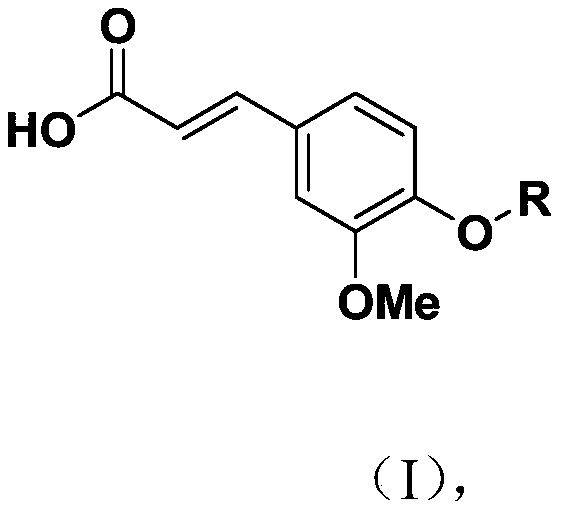
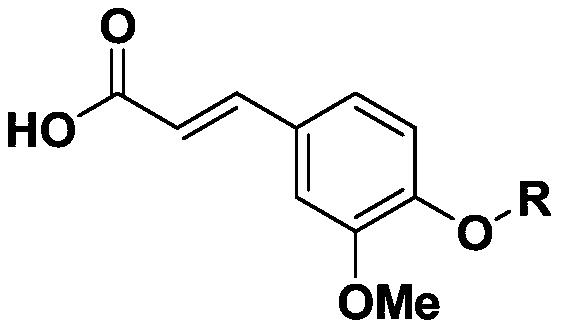
Novel ferulic acid sulfonate derivative and preparation method and application thereof
PendingCN110590615APharmaceutically activeRealize resource utilizationSulfonic acid esters preparationSulfuric acid amide preparationSulfonateResource utilization
The invention discloses a novel ferulic acid sulfonate derivative and a preparation method and application thereof. A structural formula of the novel ferulic acid sulfonate derivative is as shown in aformula (I) in the specification, wherein R is selected from groups as shown in the specification. The compound has potential pharmaceutical activity, ferulic acid as the preparation raw material canbe obtained by a natural extraction method which has a positive effect on realization of resource utilization of spartina alterniflora. The obtained ferulic acid sulfonate derivative has potential pharmaceutical activity.
Owner:南京施倍泰生物科技有限公司
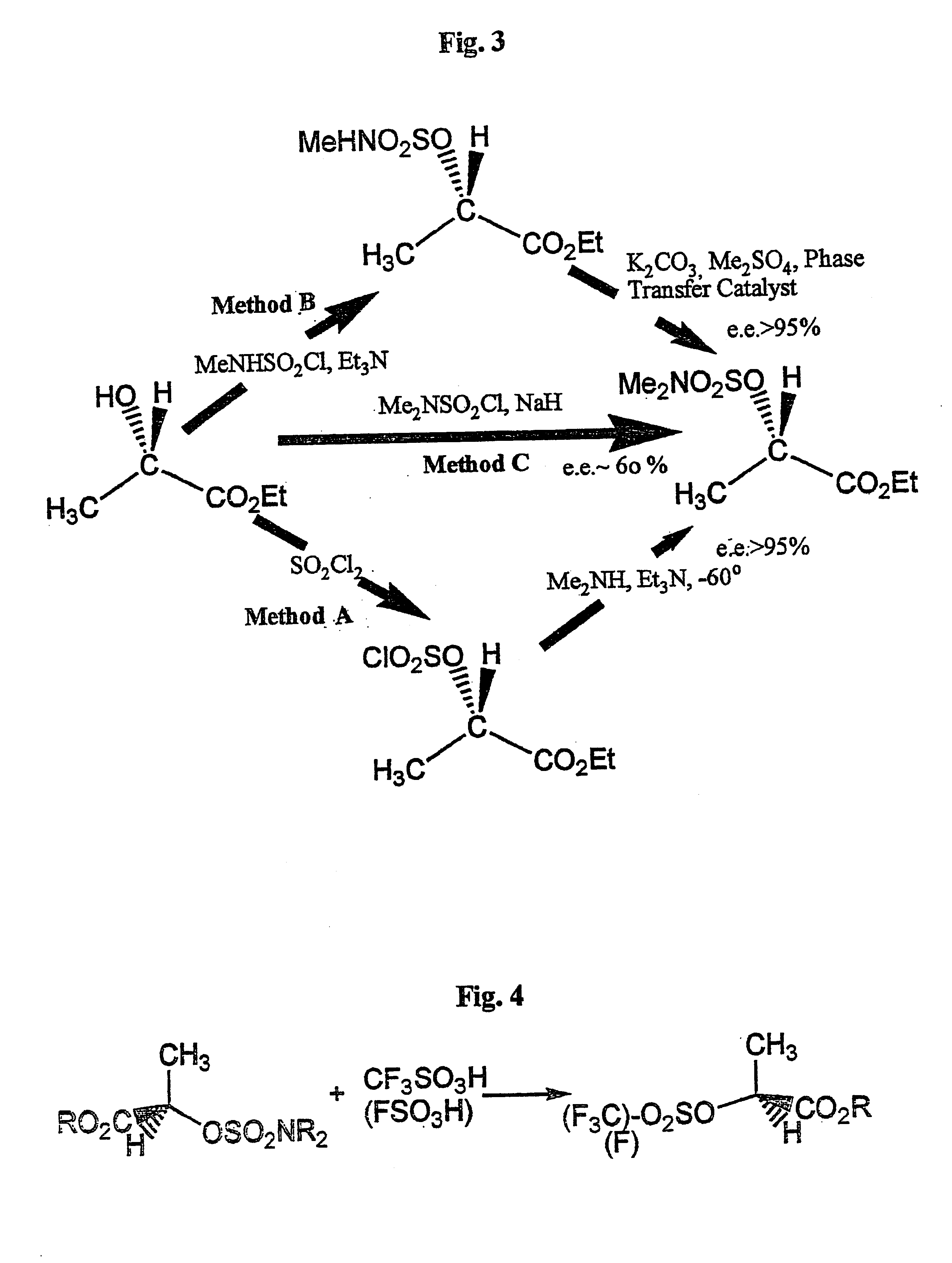
Conversion of a hydroxy group in certain alcohols into a fluorosulfonate ester or a trifluoromethylsulfonate ester
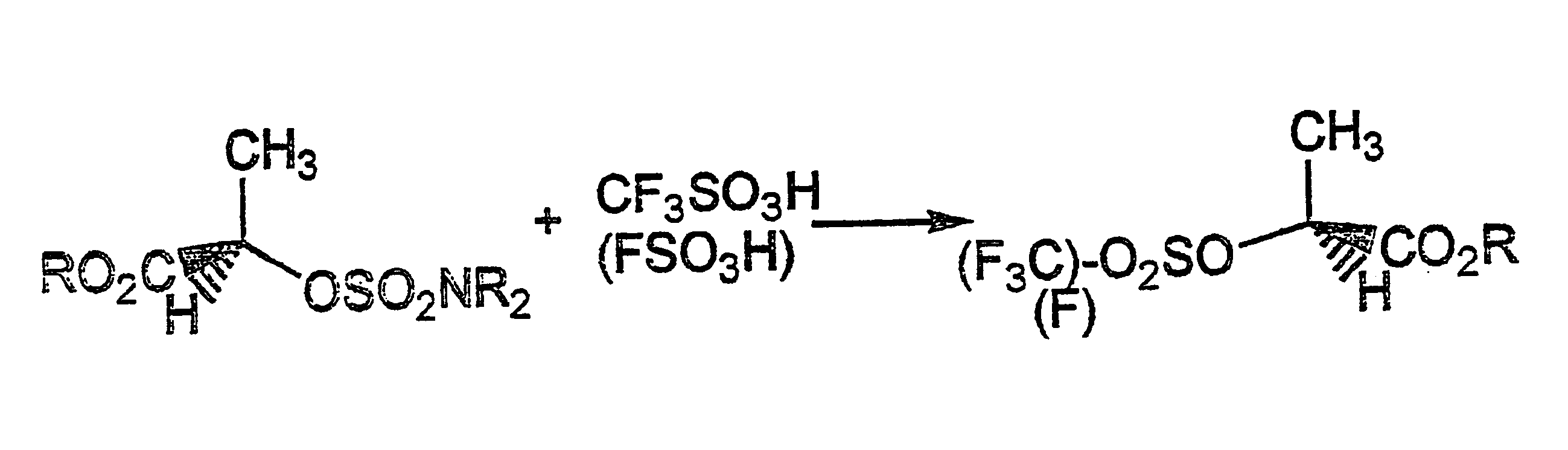
InactiveUS6395918B1Easy to disassembleOrganic compound preparationOrganic chemistry methodsSulfonateAlcohol
The present invention provides a method of converting a hydroxy group in alcohols containing an electron withdrawing group into perfluoroalkane sulfonate and fluorosulfonate esters, which are good leaving groups, with inversion of configuration where the hydroxyl-bearing carbon is chiral. The method consists of converting an alcohol to an O--N,N-dialkylsulfamate ester and reacting it with a perfluoroalkansulfonic or fluorosulfonic acid. The method has applications in the synthesis of pharmaceutical and agrochemical compounds.
Owner:LOEWENTHAL HANS JACOB EDGAR +1
Preparation method of isotridecanol polyoxyethylene ether sulfate
InactiveCN102126990AReduce energy consumptionEasy to operateTransportation and packagingFibre treatmentEpoxySulfate
The invention discloses a preparation method of isotridecanol polyoxyethylene ether sulfate. The method comprises the following steps of: adding epoxy ethane into isotridecanol serving as an initiator in the presence of a basic catalyst by adopting a one-step method and undergoing a polymerization reaction to obtain isotridecanol polyoxyethylene ether; and undergoing a sulfating reaction on the isotridecanol polyoxyethylene ether and sulfamic acid in a programmed temperature raising manner under the catalytic action of urea, and adding a NaOH aqueous solution until the pH value of a reactant is 5-8 to obtain isotridecanol polyoxyethylene sulfate, wherein the molar ratio of the isotridecanol to the epoxy ethane is 1:(3-7), the molar ratio of the isotridecanol polyoxyethylene ether to the sulfamic acid is 1:(0.9-1.5) and the molar ratio of the isotridecanol polyoxyethylene ether to the urea is 1:(0.2-0.8). The preparation method has the characteristics of reduced energy consumption, light product color, good product quality, no three wastes, easiness of operation, small equipment investment and the like.
Owner:ZHEJIANG HECHENG CHEM
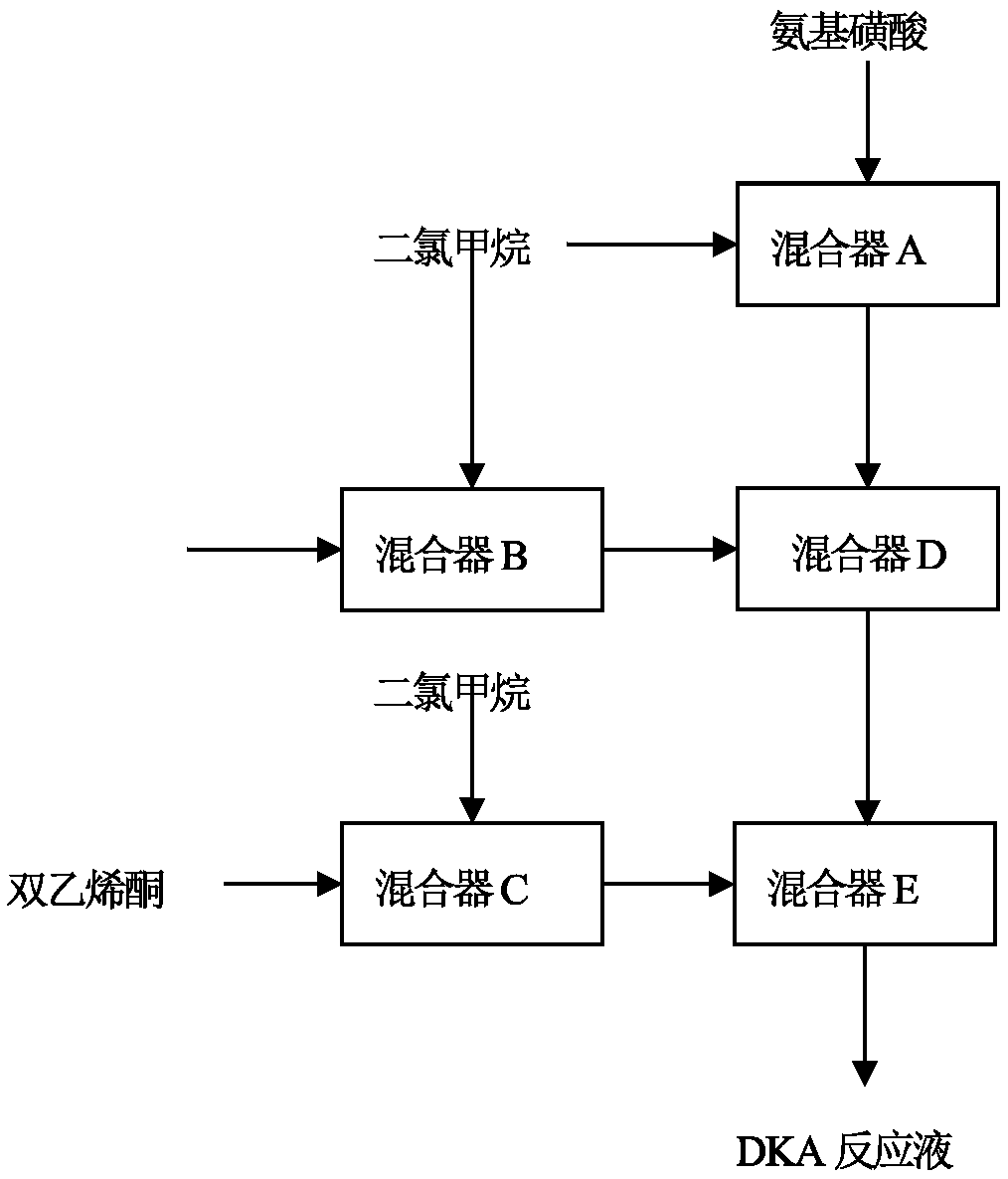
Continuous preparation method of acetoacetamido triethylamine sulfonate
PendingCN111377834ASimple processLow costOrganic compound preparationSulfuric acid amide preparationReaction temperatureDiketene
The invention discloses a continuous preparation method of acetoacetamido triethylamine sulfonate, comprising the following steps of: continuously mixing and dissolving sulfamic acid and dichloromethane, continuously neutralizing with a triethylamine solution to obtain a neutralization reaction solution, introducing the neutralization reaction solution and diketene into a continuous reactor, and carrying out addition acylation reaction to obtain a DKA reaction solution; wherein the molar ratio of n (sulfamic acid) to n (dichloromethane) in the continuous mixing and dissolving process is 1: (1-20), and the temperature is 0-40 DEG C; in the continuous neutralization, the pH of the neutralization is equal to 7-9, and the neutralization temperature is 10-40 DEG C; in the addition acylation reaction, the molar ratio of n (sulfamic acid) to n (diketene) is 1: (0.5-1.5), the acylation reaction temperature is 0-30 DEG C, and the acylation retention time is 0.01 s to 30 min. The invention provides a process for continuously preparing a DKA solution. The process is simple, low in cost, continuous in the whole process and high in yield.
Owner:NANTONG ACETIC ACID CHEM +1
Use of compounds for decreasing activity of hormone-sensitive lipase
Use of compounds to inhibit hormone-sensitive lipase, pharmaceutical compositions comprising the compounds, methods of treatment employing these compounds and compositions, and novel compounds. The present compounds are inhibitors of hormone-sensitive lipase and may be useful in the treatment and / or prevention of medical disorders where a decreased activity of hormone-sensitive lipase is desirable.
Owner:NOVO NORDISK AS
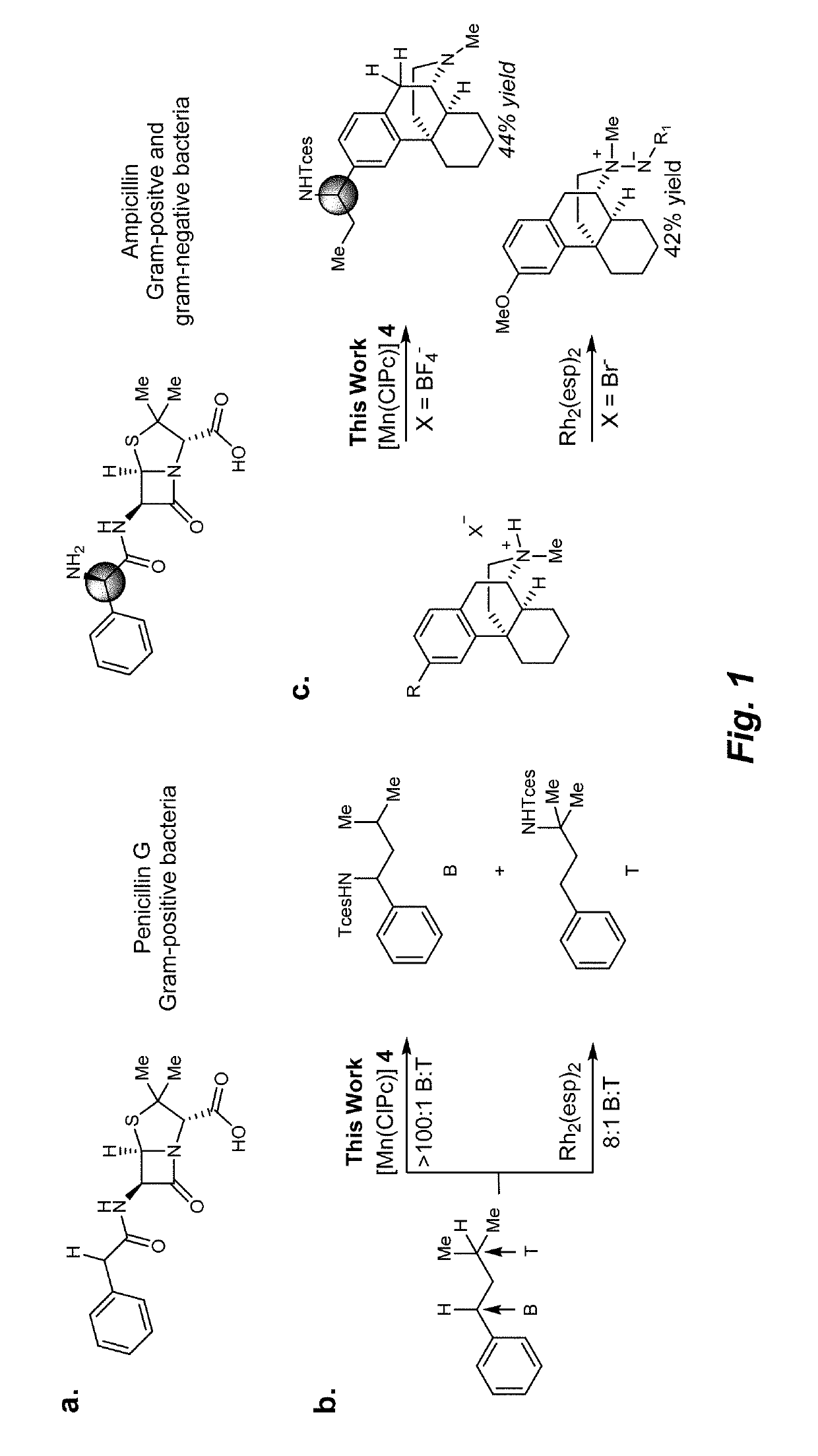
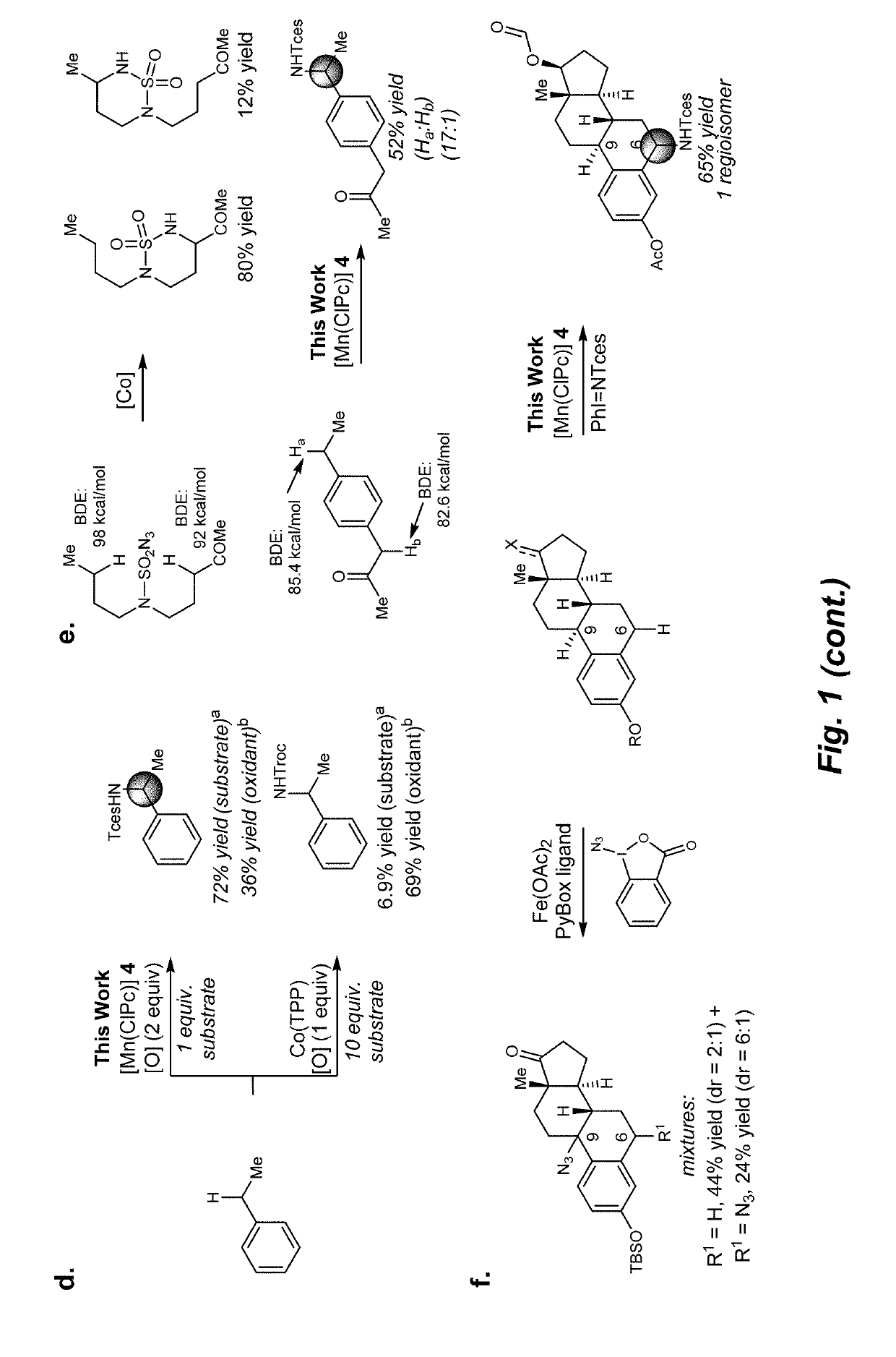
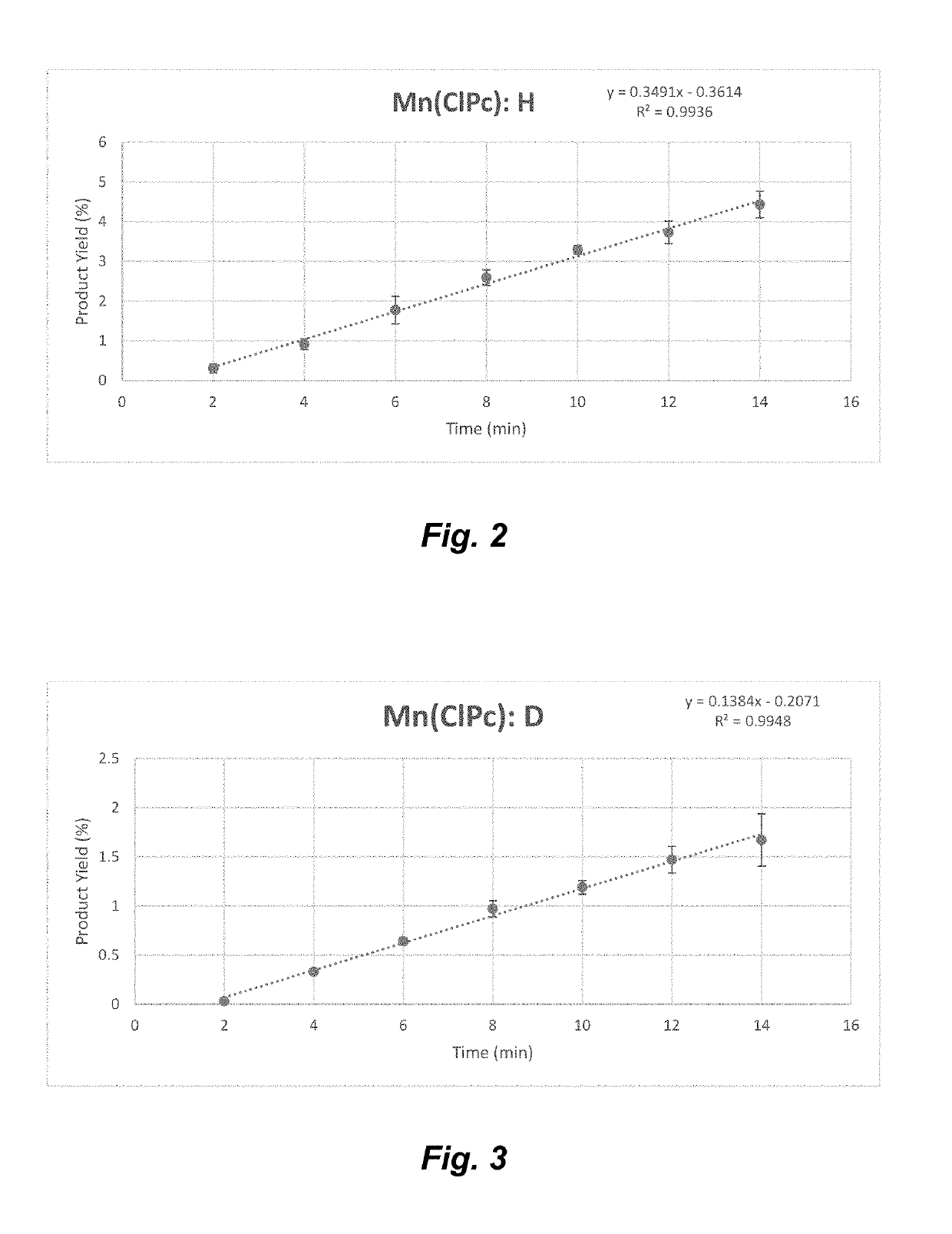
Manganese (III) catalyzed c--h aminations
ActiveUS20190106448A1Organic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsRate-determining stepSite selectivity
Reactions that directly install nitrogen into C—H bonds of complex molecules are significant because of their potential to change the chemical and biological properties of a given compound. Selective intramolecular C—H amination reactions that achieve high levels of reactivity, while maintaining excellent site-selectivity and functional-group tolerance is a challenging problem. Herein is reported a manganese perchlorophthalocyanine catalyst [MnIII(ClPc)] for intermolecular benzylic C—H amination of bioactive molecules and natural products that proceeds with unprecedented levels of reactivity and site-selectivity. In the presence of Brønsted or Lewis acid, the [MnIII(ClPc)]-catalyzed C—H amination demonstrates unique tolerance for tertiary amine, pyridine and benzimidazole functionalities. Mechanistic studies indicate that C—H amination proceeds through an electrophilic metallonitrene intermediate via a stepwise pathway where C—H cleavage is the rate-determining step of the reaction. Collectively these mechanistic features contrast previous base-metal catalyzed C—H aminations.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Compounds and uses thereof for decreasing activity of hormone-sensitive lipase
Use of compounds to inhibit hormone-sensitive lipase, pharmaceutical compositions comprising the compounds, methods of treatment employing these compounds and compositions, and novel compounds. The present compounds are inhibitors of hormone-sensitive lipase and may be useful in the treatment and / or prevention of medical disorders where a decreased activity of hormone-sensitive lipase is desirable.
Owner:NOVO NORDISK AS
Tubular reaction synthetic method and apparatus for continuously producing acesulfame intermediate
ActiveCN108822004AHigh yieldEasy to operateAmino preparation from aminesSulfuric acid amide preparationRemote controlPropeller
The invention relates to tubular reaction synthetic method and apparatus for continuously producing acesulfame intermediate. The apparatus includes a sulfamic acid blending tank and a first metering pump, a spiral propeller, a synthetic tubular reactor, an acylation tubular reactor, a triethylamine metering tank and a second metering pump, a first tubular mixer, a second tubular mixer, an triethylamine sulfamate metering tank and a third metering pump, a diketene metering tank and a firth metering pump, a first heat insulation intermediate tank, and a second heat insulation intermediate tank.The tubular reaction synthetic apparatus is short in reaction period, allows continuous production and is low in production consumption, increases yield of the intermediate, reduces production cost and has competitiveness. The apparatus is high in automation degree and allows remote control, is safe and reliable and can increase productivity and efficiency.
Owner:东营市科维生物技术有限公司
Alpha-phenyl alkyl alcohol polyoxyethylene ether hydroxypropyl allyl ether as well as derivative and preparation method thereof
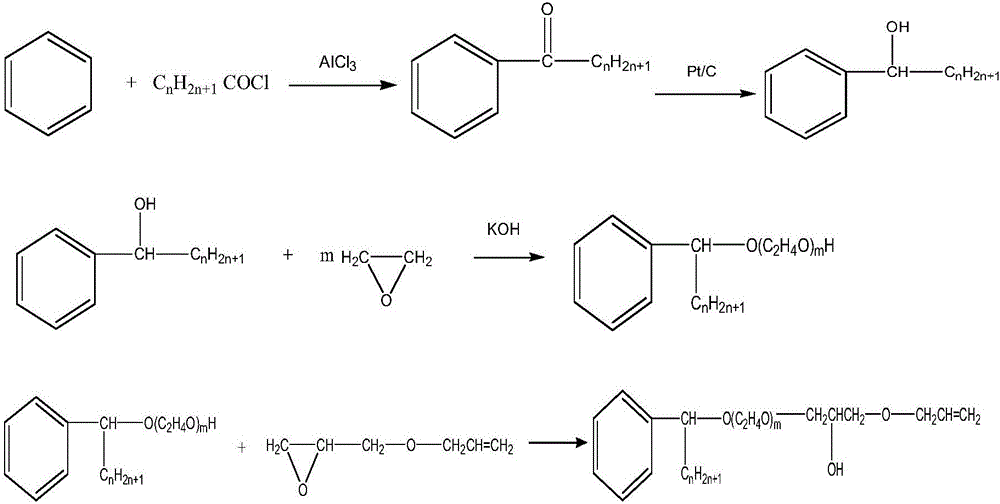
ActiveCN105968338AStable in natureGood water solubilitySulfuric acid amide preparationEther preparation from oxiranesSolubilityAlcohol
The invention discloses alpha-phenyl alkyl alcohol polyoxyethylene ether hydroxypropyl allyl ether as well as a derivative and a preparation method thereof. Alpha-phenyl alkyl alcohol polyoxyethylene ether hydroxypropyl allyl ether is a reaction type emulsifying agent; and the molecular structure comprises a hydrophobic group and an active reaction group, wherein the hydrophobic group is an alpha-phenyl alkyl chain segment, and the active reaction group is a polyoxyethylene ether allyl chain segment. The APEO emulsifying agent, namely alpha-phenyl alkyl alcohol polyoxyethylene ether hydroxypropyl allyl ether and the derivative thereof have the characteristics of stable performance, good water solubility, excellent wetting, emulsifying and dispersing performance, few foams and the like and further have the original performance of nonionics, furthermore, the application is excellent, and the efficiency is relatively high; and furthermore, the molecular structure contains terminal-group double bond, and the APEO emulsifying agent has active chemical properties and can be used as a synthetic intermediate of a special surfactant.
Owner:NANTONG HANTAI CHEM
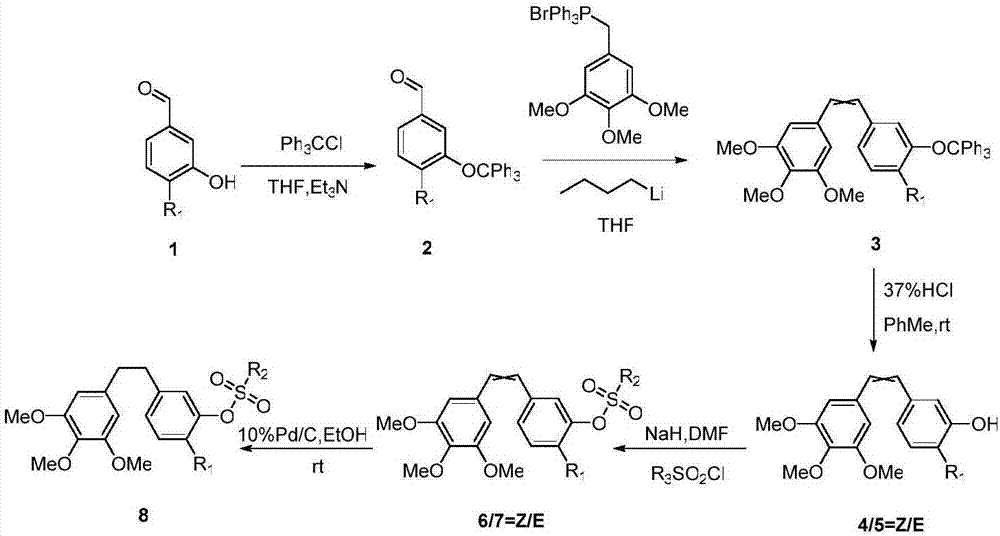
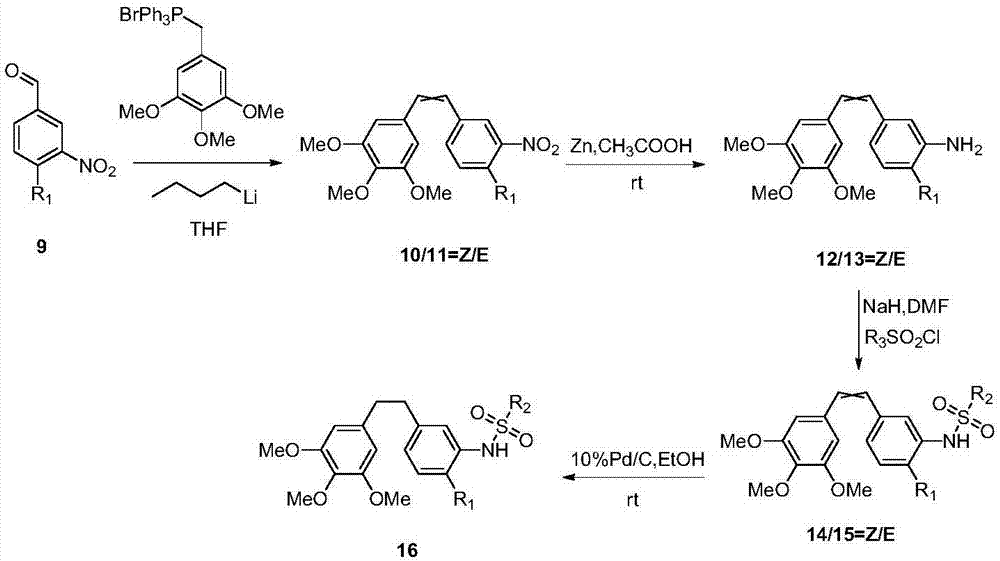
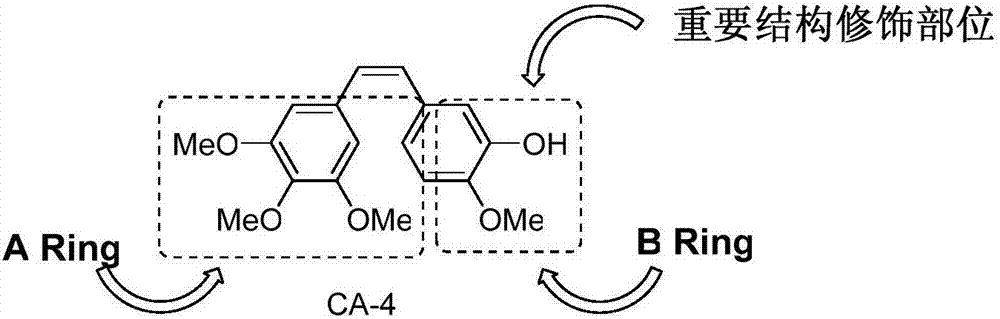
CA-4 antitumor drug, synthesizing method and application thereof
ActiveCN107382796AImprove targeting activityAntibacterial agentsSenses disorderNatural productAlkoxy group
The invention discloses a CA-4 antitumor drug, a synthesizing method and an application thereof. The CA-4 antitumor drug is formed in the manner of introducing the alkoxy or fluorine-containing alkoxy into 4' site of natural product combretastatin and functionally chemical modifying 3' site thereof. The CA-4 antitumor drug disclosed by the invention has higher capacity of restraining tubulin gathering and can be used for anti-tumor therapy.
Owner:浙江华理生物制药有限公司
Conversion of hydroxy group in certain alcohols into fluorosulfonate ester or trifluoromethylsulfonate ester
The present invention provides a method of converting a hydroxy group in alcohols containing an electron withdrawing group into perfluoroalkanesulphonate (or salt) and fluorosulphonate esters (or salt), which are good leaving groups, with inversion of configuration where the hydroxyl-bearing carbon is chiral. The method consists of converting said alcohol to an O-N,N-dialkylsulphamate ester (or salt) and reacting said O-N,N-dialkylsulphamate ester (or salt) with a perfluoroalkanesulphonic acid or fluorosulphonic acid. Such a method is useful for preparing chiral compounds for pharmaceutical and agrochemical use.
Owner:汉斯·雅各布·埃德加·勒文塔尔 +1
Preparation method and application of N, N-disubstituted sulfamic acid compound
PendingCN114196005AImprove adsorption capacityReduced reunion tendencySulfuric acid amide preparationAlkaneDimer
The invention discloses a preparation method of an N, N-disubstituted sulfamic acid compound, which specifically comprises the following steps: by taking a sulfamic acid compound as a substrate, carrying out addition reaction on the sulfamic acid compound and alkylene oxide to prepare the N, N-disubstituted sulfamic acid compound, and reacting the N, N-disubstituted sulfamic acid compound with the alkylene oxide to prepare the N, N-disubstituted sulfamic acid compound. The N, N-disubstituted sulfamic acid compound is a dihydroxyalkyl sulfamic acid compound or a dimer alkoxy sulfamic acid compound. The invention also discloses an application of the N, N-disubstituted sulfamic acid compound prepared by the method. In the invention, sulfamic acid or salt or ester is used as a substrate and is subjected to addition condensation with alkylene oxide. The method has the advantages of easily available raw materials, low cost, short synthesis route, mild process conditions and simple post-treatment.
Owner:NANJING BAOCHUN CHEMICAL INDUSTRY CO LTD
Method for Producing Chlorosulfonyl Isocyanate
ActiveUS20070286789A1Easy to controlSulfonic acid amide preparationCyanic/isocyanic acidSulfur trioxideChlorosulfonyl isocyanate
A method for producing chlorosulfonyl isocyanate by reaction of sulfur trioxide with cyanogen chloride, wherein chlorosulfonyl isocyanate or a solution including chlorosulfonyl isocyanate is used as a reaction solvent, sulfur trioxide and cyanogen chloride which are respectively diluted with the chlorosulfonyl isocyanate or the solution including chlorosulfonyl isocyanate are added at the same time to a reaction system in an almost equimolar amount under reflux. By the production method of present invention, chlorosulfonyl isocyanate can be produced from sulfur trioxide and cyanogen chloride in which the yield of the chlorosulfonyl isocyanate is high, the method has excellent operability, number of equipments is reduced, and time for controlling the temperature is saved.
Owner:NIPPON SODA CO LTD
Sodium cyclamate production process and device
PendingCN113387849ALow costEasy to operateSulfuric acid amide preparationFood ingredientsProcess engineeringSodium cyclamate
The invention discloses a sodium cyclamate production process and device, the device comprises a neutralization kettle, a reaction kettle and a dilute amine kettle, a discharge port of the dilute amine kettle is connected with a feed port of the neutralization kettle, the device also comprises a first condenser, the first condenser is connected with a gas discharge port arranged in the neutralization kettle, the first condenser is connected with a dilute amine tank, and the diluted amine tank is connected with a diluted amine kettle. The reaction kettle is provided with an amine outlet, the amine outlet is connected with a second condenser, the second condenser is connected with the dilute amine kettle, and a discharge port of the dilute amine kettle is connected with a feed port of the reaction kettle. According to the sodium cyclamate production process and device provided by the invention, the process operation is simple, and the yield is as high as 95-99%; the device adopts a circulating system, so that cyclohexylamine can be recycled, and the material cost is reduced.
Owner:金城化学(江苏)有限公司
Novel stilbene derivative and preparation method thereof
The invention provides a novel stilbene derivative. The novel stilbene derivative is a compound with a general formula I or II as described in the specification or a salt formed by the compound with the general formula I or II as described in the specification and inorganic acid or organic acid. In the general formula I or II, X represents a hydrogen atom or halogen atom; R represents a substituent selected from a group consisting of C1-5 alkyl groups, C2-4 alkenyl groups, C2-4 alkynyl groups, C3-6 cycloalkyl groups, five-to-twelve-component aryl groups, two-to-five-component heteralkyl groups, three-to-six-component hetercycloalkyl groups, five-to-twelve-component heteraryl groups, substituted three-to-six-component cycloalkanes, substituted five-to-twelve-component aryl groups and substituted five-to-twelve-component heteralkyl groups; or the substituent represented by R is a first substituent formed by a spacer group and any one selected from a group consisting of C3-6 cycloalkanes, three-to-six-component hetercycloalkanes, five-to-twelve-component aryl groups and five-to-twelve-component heteraryl groups, and the spacer group comprises -CH2-, -(CH2)2-, -CH=CH-, -CH2O-, -CH(OCH3)- and -CH2S-.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Calcium cyclohexylsulfamate preparation method
InactiveCN106083661AMeet food requirementsSimple processSulfuric acid amide preparationCalcium hydroxideDecomposition
The invention discloses a calcium cyclohexylsulfamate preparation method, which comprises: (1) neutralizing: carrying out a reaction on cyclohexylamine and aminosulfonic acid at a temperature of less than 80 DEG C under the pH value of 6.8-7.0; (2) concentrating: concentrating the reaction product obtained in the step (1) to obtain a concentrated product, wherein stirring is started after the concentrating is performed for 2-3 h; (3) carrying out a reaction: carrying out a reaction on the concentrated product and cyclohexylamine at a temperature of 60-150 DEG C to obtain a half finished product; (4) recovering: recovering the partial cyclohexylamine, and shortening the decomposition time; (5) adding an alkali: adding calcium hydroxide and water to the half finished product obtained in the step (3), and carrying out a replacement reaction to obtain a product mixture; (6) adjusting: adjusting the product mixture; and (7) crystallizing the adjusted product mixture, and separating to obtain the calcium cyclohexylsulfamate. According to the present invention, the preparation method has characteristics of simple process, easily-available raw materials, low energy consumption, high yield, high product purity, easy use, and high conversion rate.
Owner:JIANGSU LIANWEI BIOCHEM IND CO LTD
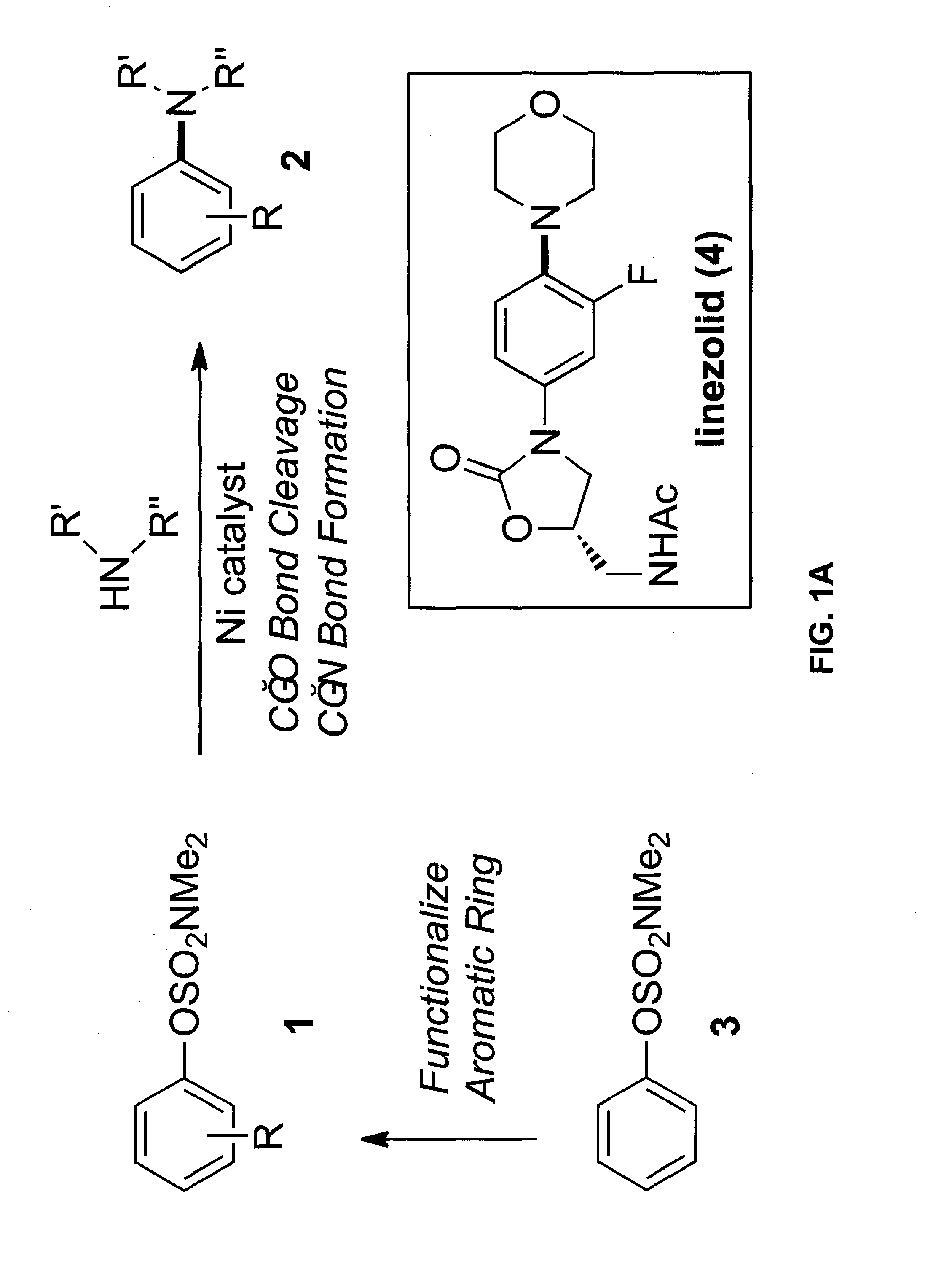
Amination of Aryl Alcohol Derivatives
ActiveUS20130289270A1Good substrateEfficient accessSilicon organic compoundsCarbamic acid derivatives preparationArylCarbamate
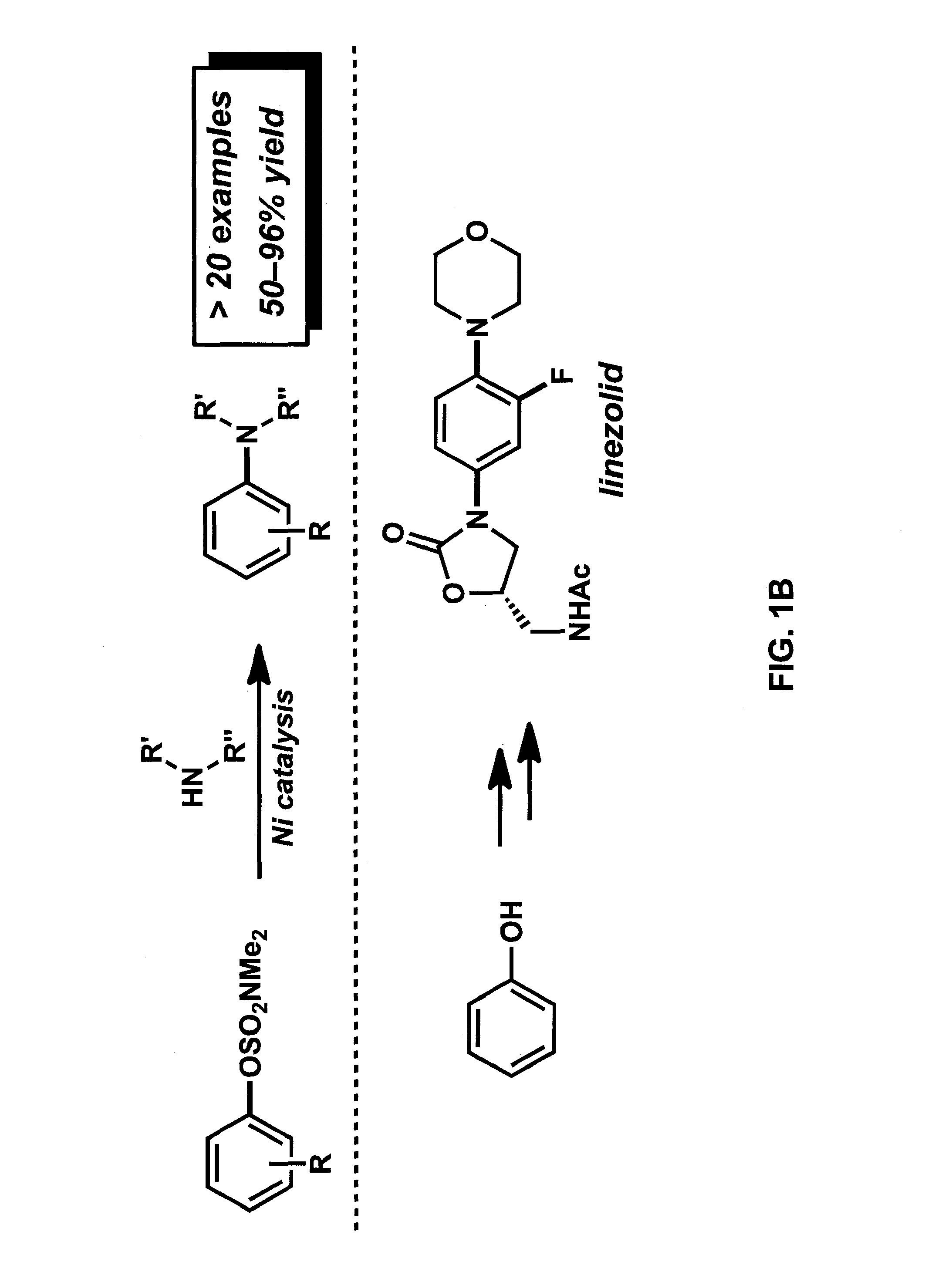
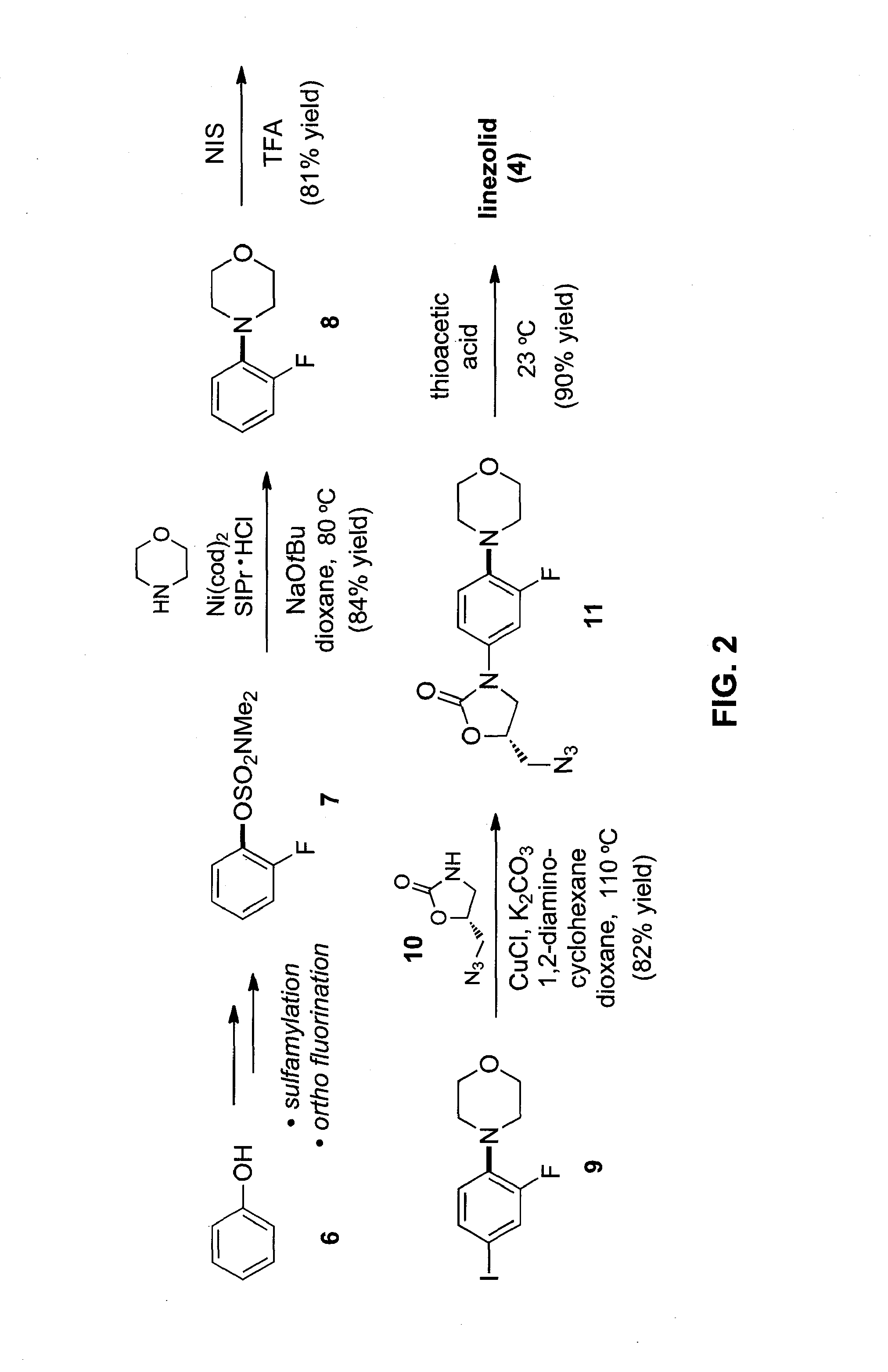
Embodiments of the invention provide methods and materials for chemical cross-coupling reactions that utilize aryl alcohol derivatives as cross-coupling partners. Embodiments of the invention include methods for the amination of aryl sulfamates and carbamates, which are attractive cross-coupling partners, particularly for use in multistep synthesis. Illustrative embodiments include versatile means to use simple derivatives of phenol as precursors to polysubstituted aryl amines, as exemplified by a concise synthesis of the antibacterial drug linezolid.
Owner:RGT UNIV OF CALIFORNIA
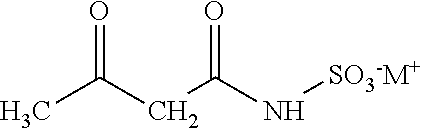
Preparation method of acetoacetamide-N-sulfonic acid triethylamine salt
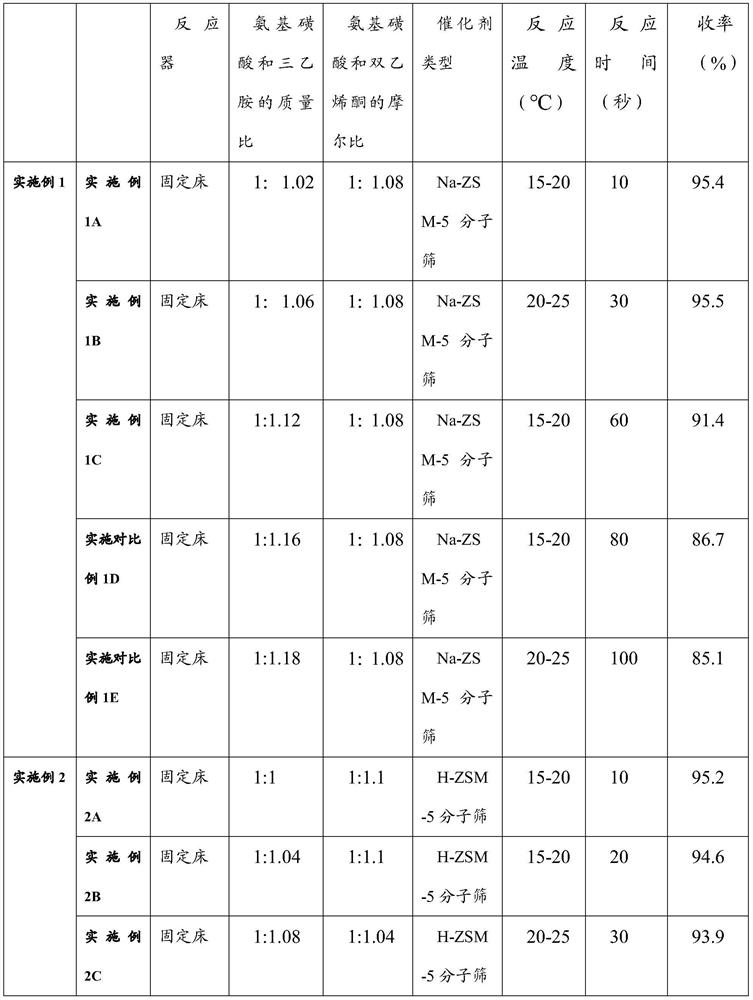
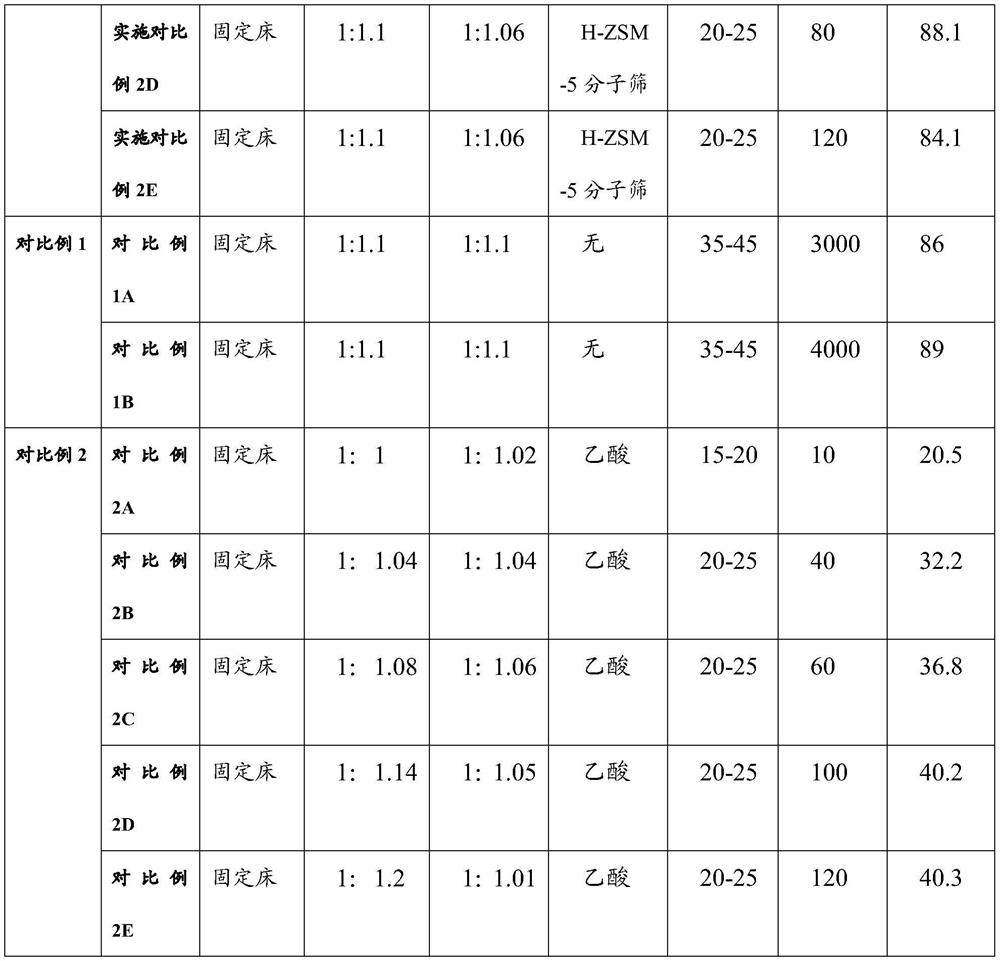
ActiveCN113454056ASimple post-processingGood conditionMolecular sieve catalystsOrganic compound preparationPtru catalystFixed bed
The invention provides a preparation method of acetoacetamide-N-sulfonic acid triethylamine salt, which comprises the following steps: dissolving sulfamic acid in first dichloromethane to prepare a first reaction solution; dissolving triethylamine in second dichloromethane to prepare a second reaction solution, and adding the second reaction solution into the first reaction solution for amination reaction to form an ammonium sulfamate solution; filling a zeolite catalyst into a fixed bed reactor, sequentially introducing the ammonium sulfamate solution and diketene into the fixed bed reactor, and reacting under preset conditions to form the acetoacetamide-N-triethylamine sulfonate solution. On one hand, the post-treatment process of the product is simplified, so that the final product acesulfame is better in appearance, and the use feeling is remarkably improved; on the other hand, large-scale continuous production of the acetoacetamide-N-sulfonic acid triethylamine salt is realized, the reaction time is greatly shortened, the reaction yield is improved, and the production cost of acesulfame potassium is further reduced.
Owner:ANHUI JINGHE IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com