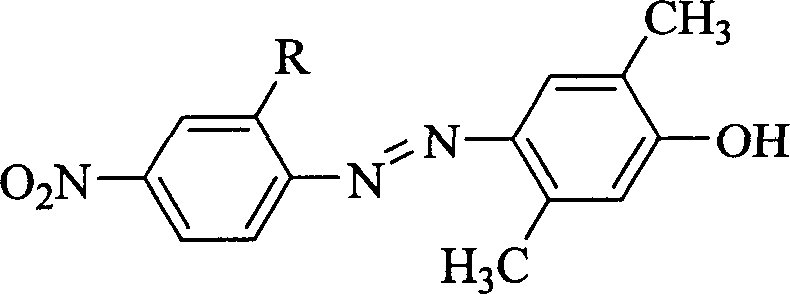
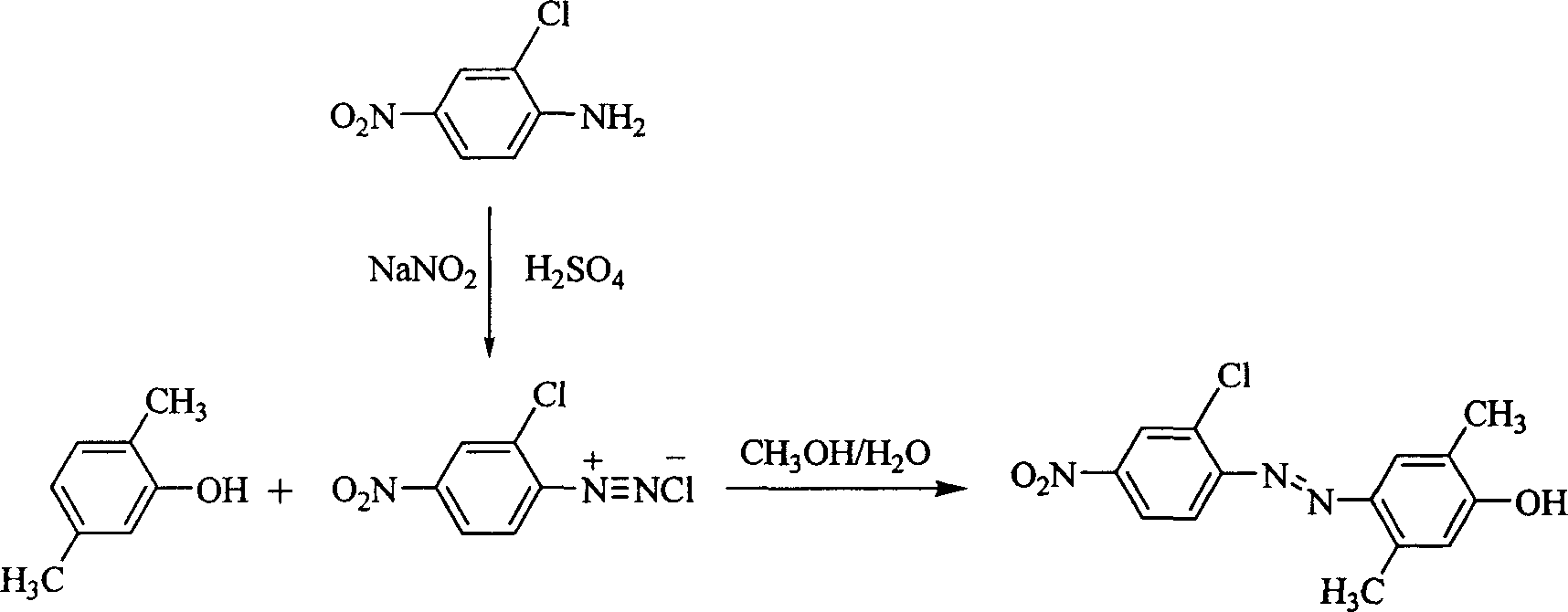
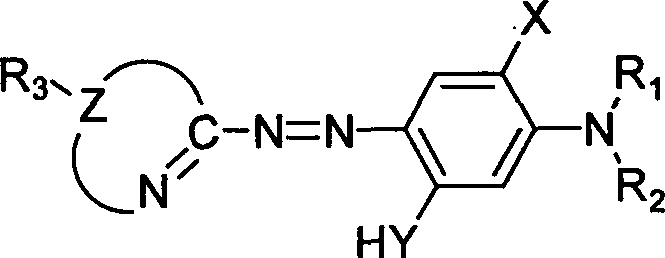
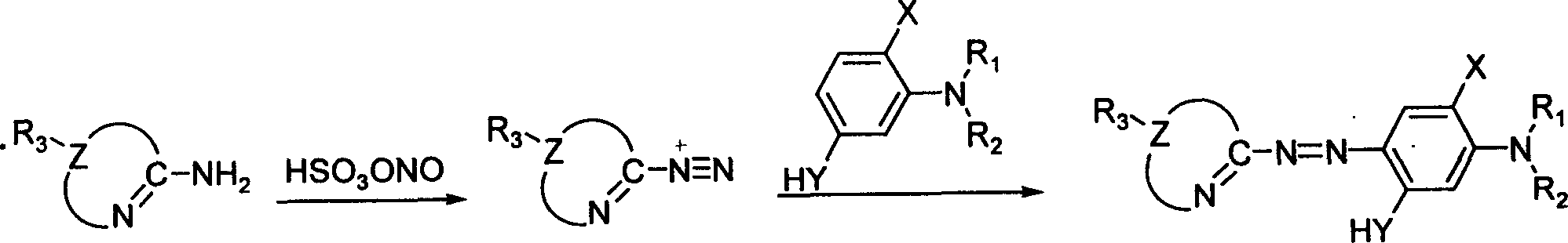
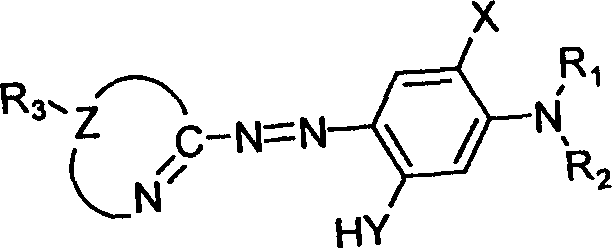
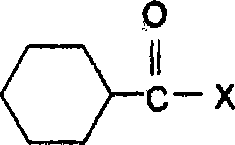
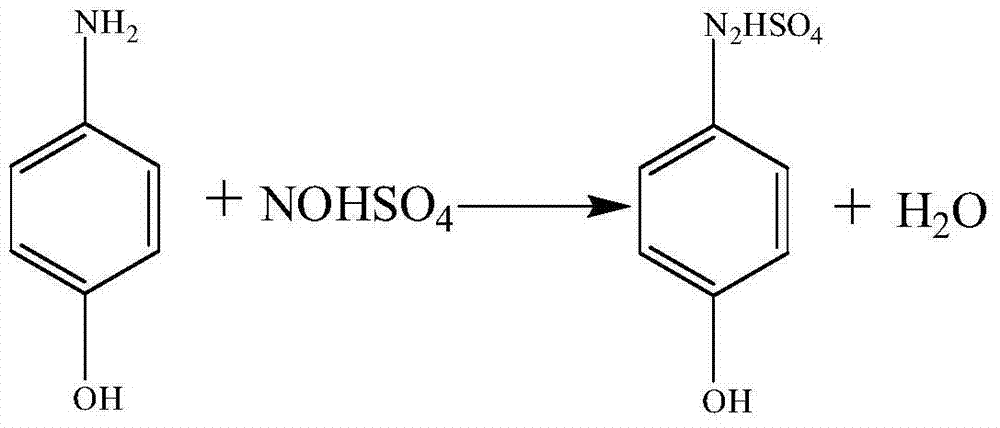
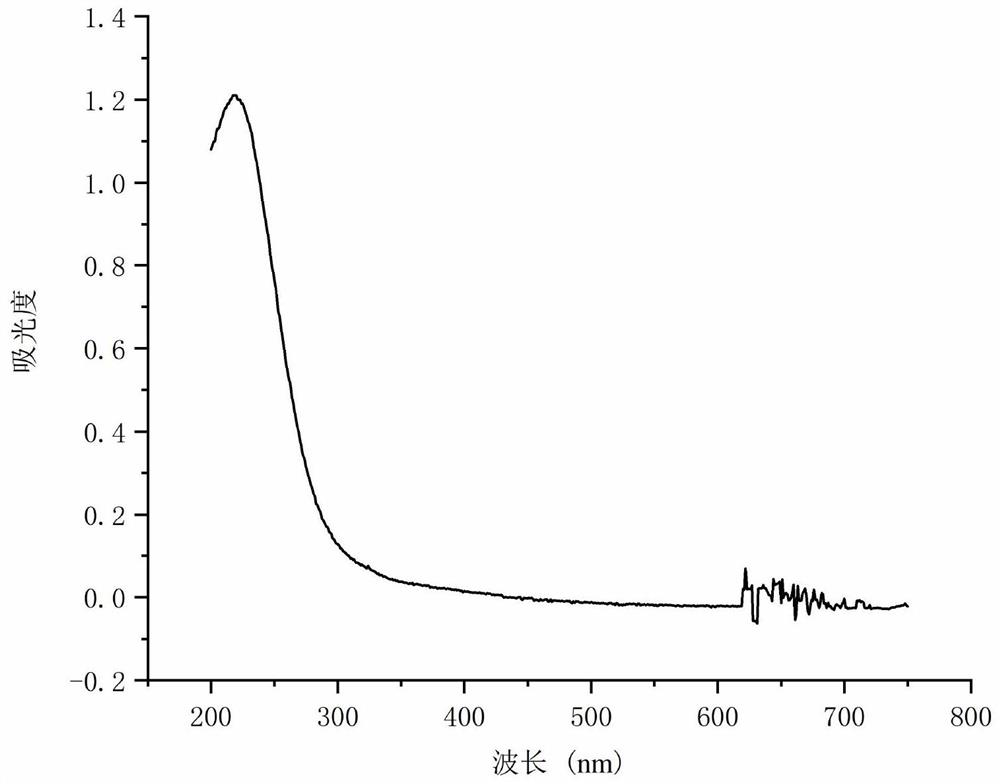
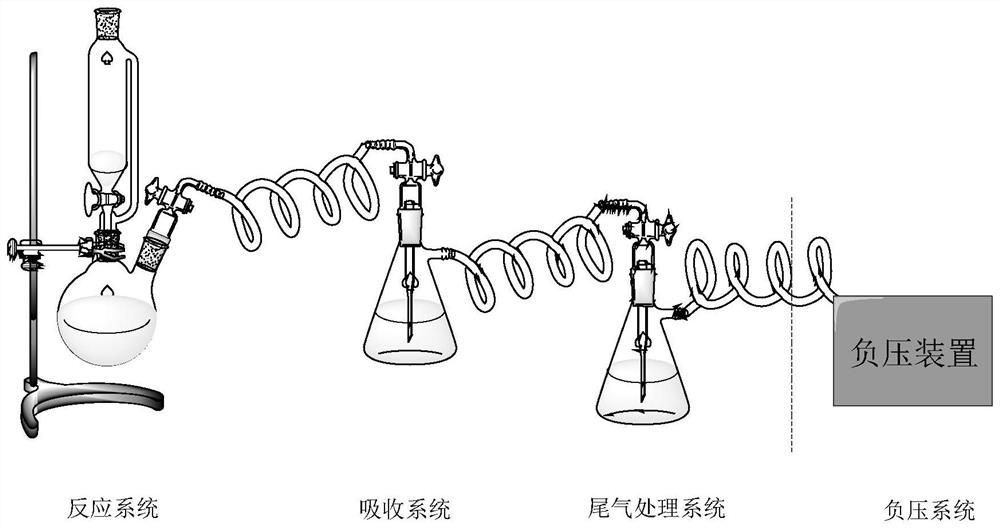
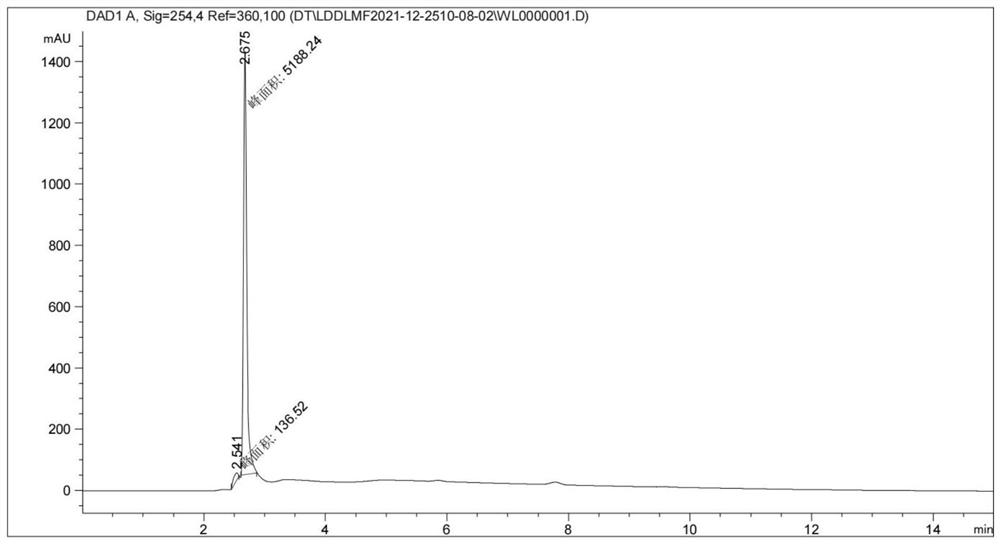
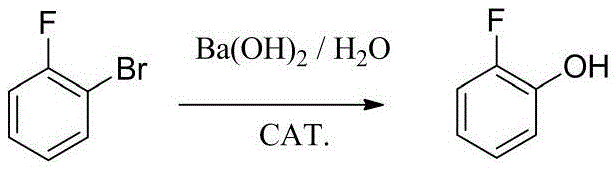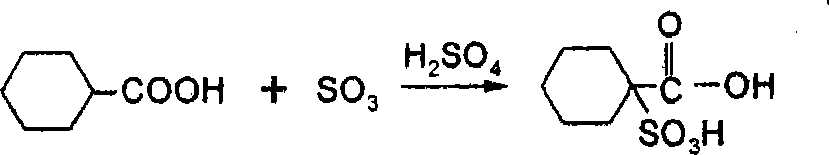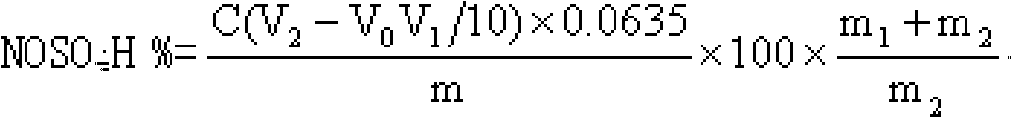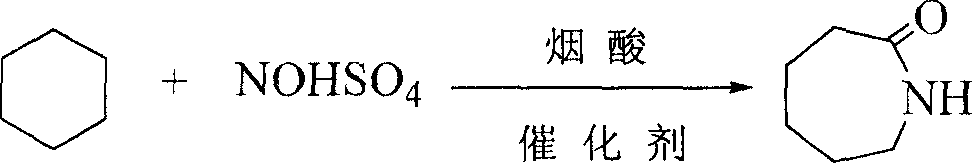Patents
Literature
43 results about "Nitrosylsulfuric acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nitrosylsulfuric acid is the chemical compound with the formula NOHSO₄. It is a colourless solid that is used industrially in the production of caprolactam, and was formerly part of the lead chamber process for producing sulfuric acid. The compound is the mixed anhydride of sulfuric acid and nitrous acid.
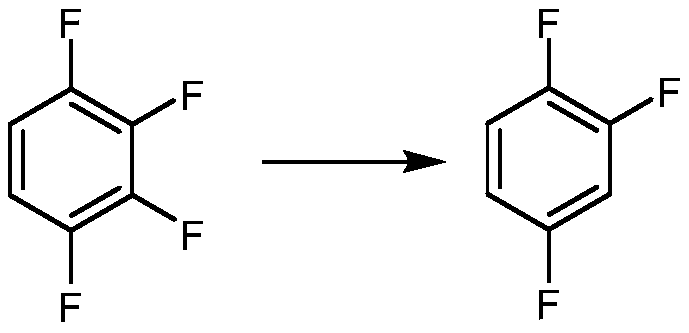
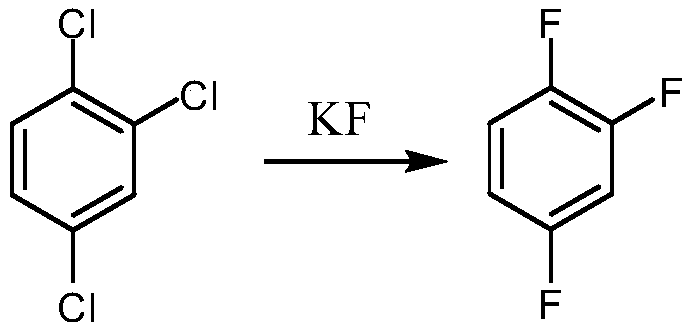
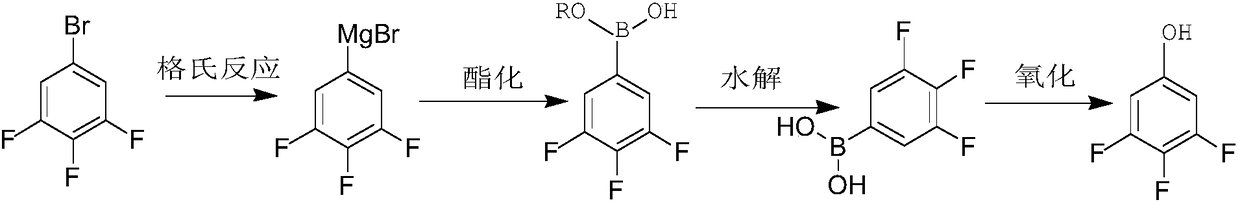
Synthetic method for 1,2,4-trifluorobenzene
ActiveCN110498730ALow costEasy to realize industrializationOrganic compound preparationSulfuric acid amide preparationNitrosylsulfuric acidPotassium fluoride
The invention provides a synthetic method for 1,2,4-trifluorobenzene, belongs to the field of pesticide, medicine, and liquid crystal material intermediate preparation, and solves the problem of harshreaction conditions of a current method for synthesizing 1,2,4-trifluorobenzene. The synthetic method for the 1,2,4-trifluorobenzene is characterized by comprising the following steps: performing nitration by using 2,4-dichlorofluorobenzene as a raw material and nitric acid as a nitrating agent to form 2,4-dichloro-5-fluoronitrobenzene in the presence of sulfuric acid; dissolving the 2,4-dichloro-5-fluoronitrobenzene into an organic solvent, adding potassium fluoride and a first catalyst, and performing fluorination under the catalysis of the first catalyst to obtain 2,4,5-trifluoronitrobenzene; dissolving the 2,4,5-trifluoronitrobenzene into a solvent, and performing hydrogenation reduction with hydrogen under the catalysis of a second catalyst to obtain 2,4,5-trifluoroaniline; and performing a reaction on the 2,4,5-trifluoroaniline and sulfuric acid, after a salt is formed, performing a diazotization reaction on the salt and nitroso-sulfuric acid, performing a deamination reductionreaction with sodium hypophosphite under the catalysis of a copper salt, and finally performing steam distillation to obtain the 1,2,4-trifluorobenzene. The method provided by the invention has the advantages of mild reaction conditions and the like
Owner:ZHEJIANG LINJIANG CHEM
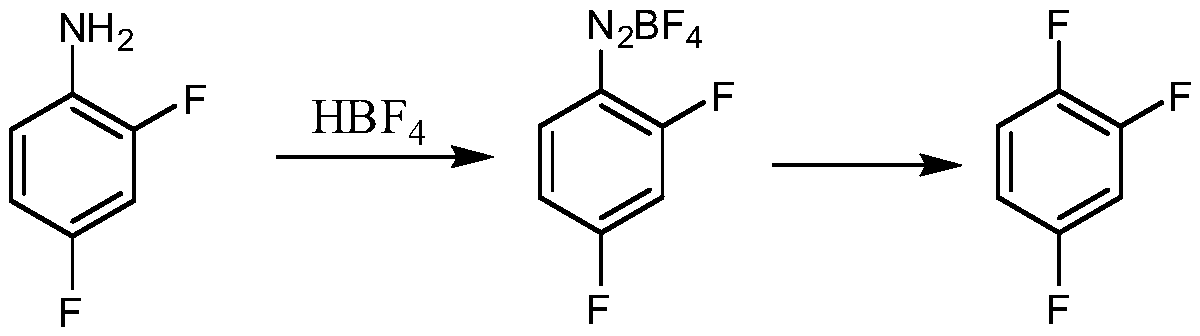
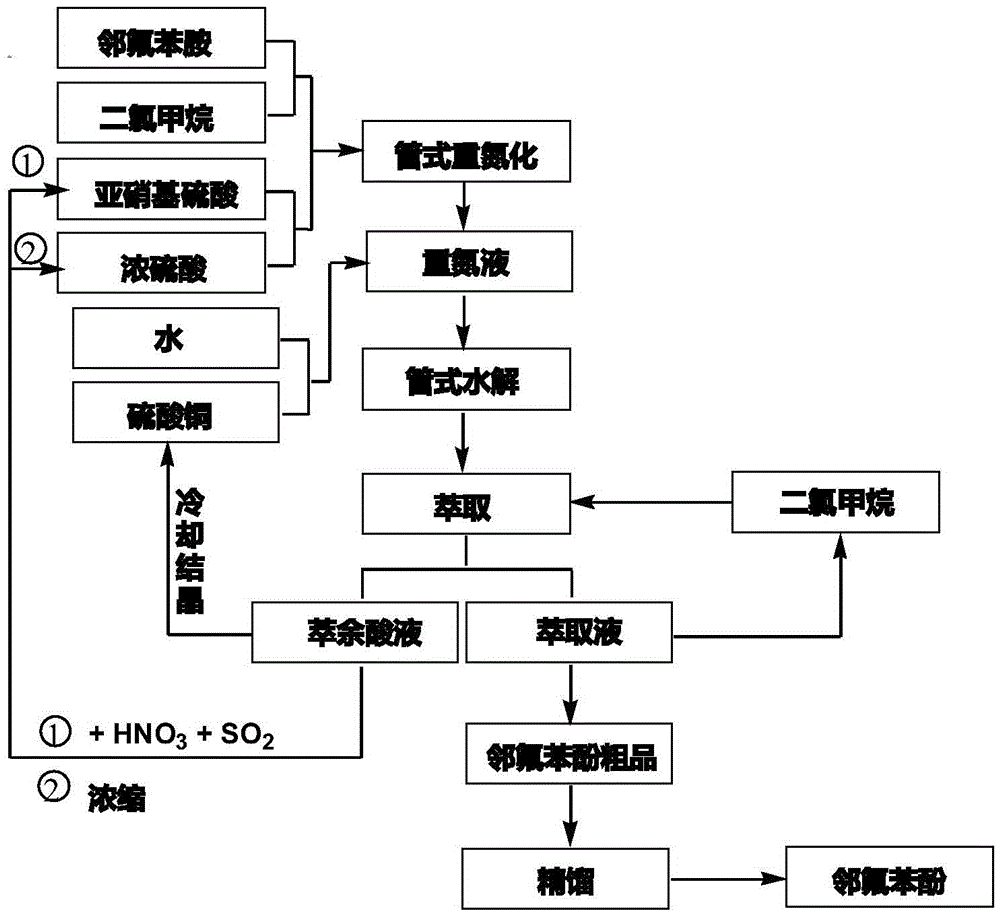
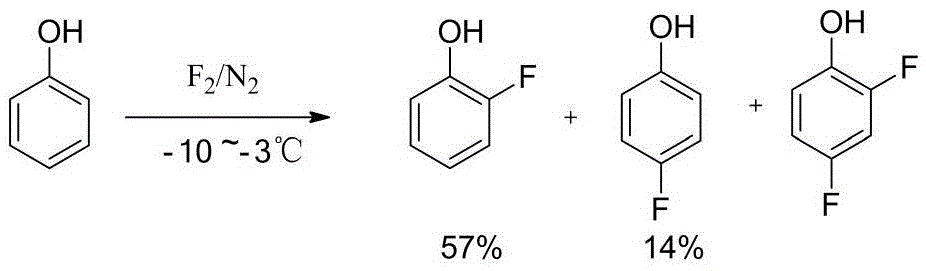
Tubular continuous o-fluorophenol production method
InactiveCN105481654ASolve the phenomenon of uneven temperature distributionAchieve recyclingOrganic chemistryOrganic compound preparationNitrosylsulfuric acidAfter treatment
The present invention discloses a tubular continuous o-fluorophenol production method comprising tubular diazo reaction of a nitrosyl sulfuric acid solution and an o-fluoroaniline solution in a tubular reactor to obtain a diazo solution; tubular hydrolysis reaction of the diazo solution and a copper sulphate water solution in the tubular reactor to obtain a hydrolysis solution; and after-treatment of the hydrolysis solution to obtain o-fluorophenol. The method uses the tubular reactor for the tubular diazo reaction and the tubular hydrolysis reaction to solve the phenomenon of material backmixing and uneven temperature distribution of kettle-type reaction, reduces the incidence of side effects, and improves the yield of the product.
Owner:ZHEJIANG LINJIANG CHEM
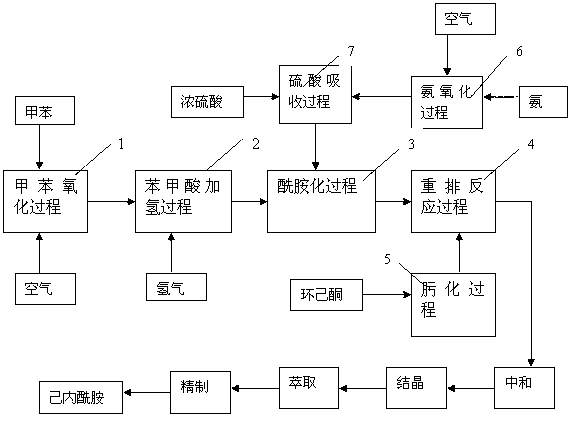
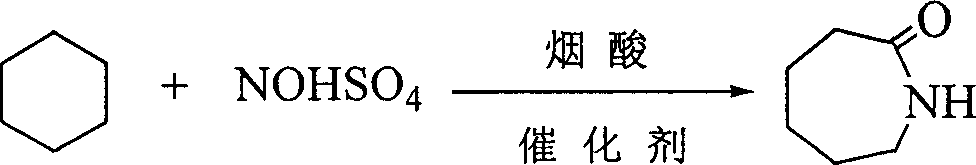
Caprolactam production method
ActiveCN103012262AIncrease productionLow costLactams preparationBulk chemical productionPalladium on carbonBenzoic acid
The present invention provides a caprolactam production method, which comprises the following processes that: toluene is oxidized under an effect of a cobalt salt catalyst to generate benzoic acid, benzoic acid is subjected to hydrogenation under an effect of a palladium carbon catalyst to generate cyclohexanecarboxylic acid, the cyclohexanecarboxylic acid and nitrosylsulfuric acid are subjected to an amidation reaction in an amidation reactor to generate an amide liquid, cyclohexanone is subjected to an oximation reaction to generate cyclohexanone oxime, the amide liquid and the cyclohexanone oxime are subjected to a rearrangement reaction in a rearrangement reactor, and the reaction product is subjected to processes such as neutralization, crystallization, extraction and refinement to prepare the ??caprolactam, wherein a rearrangement reaction temperature is 70-130 DEG C, pressure is 0-1.0 MPa, and a molar ratio of the acid in the amide liquid to the cyclohexanone oxime is 1-2:1. With the present invention, combination of the two processes such as a toluene method and a cyclohexanone-hydroxylamine method is achieved, a caprolactam yield is increased without increase of ammonium sulfate by-production, nicotinic acid consumption and ammonium sulfate by-production are reduced, the production process is simplified, and construction investment and production cost are saved.
Owner:CHINA PETROLEUM & CHEM CORP
Single diazo compound, its preparation method and use
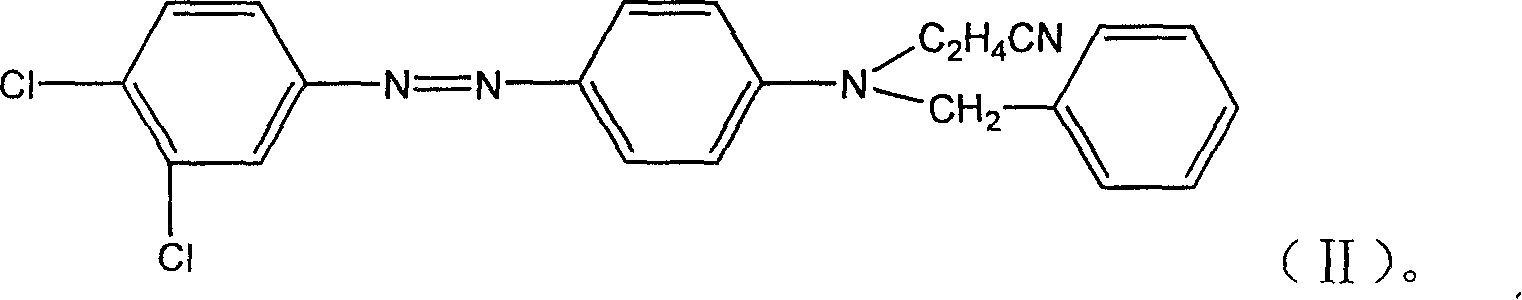
InactiveCN1869004AHigh color fastnessEffective dispersionMonoazo dyesOrganic chemistryNitrosylsulfuric acidColour fastness
A monoazo compound used for the disperse yellow dye with high dye fastness is prepared through diazotizing reaction between dichlorophenylamine and nitrosylsulfuric acid in sulfuric acid to obtain diazonium salt, coupling reaction on N-cyanoethyl-N- benzylphenylamine and post-treating.
Owner:闰土控股集团有限公司
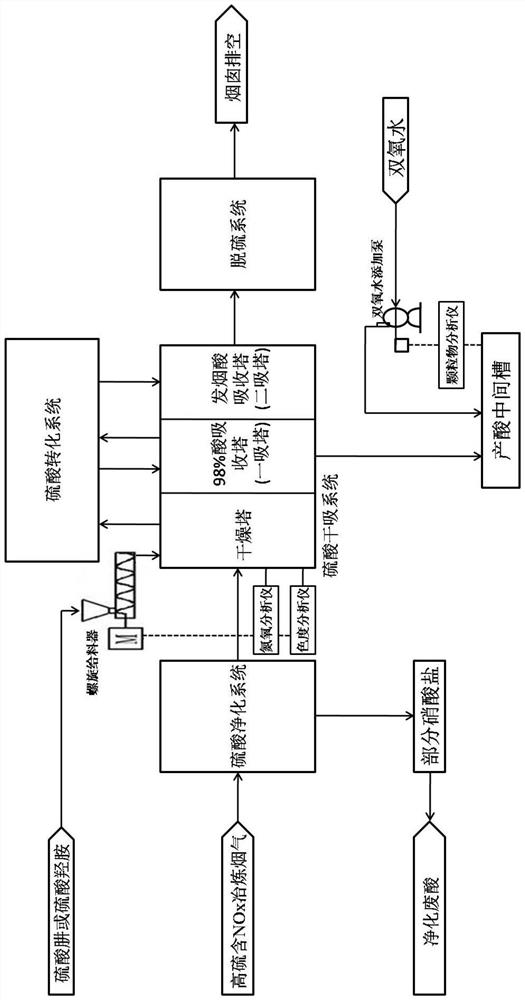
Preparation method of nitrosyl sulfuric acid and method for separating sulfuric acid and phosphoric acid in industrial waste acid
ActiveCN105417509AAchieve reuseEmission reductionNitrogen compoundsAlkali metal sulfite preparationNitrosylsulfuric acidO-Phosphoric Acid
The invention discloses a preparation method of nitrosyl sulfuric acid and a method for separating sulfuric acid and phosphoric acid in industrial waste acid. The preparation method comprises the following steps: (1), mixing sulfur with a sulfuric acid solution A, performing warming reaction to generate sulfur dioxide gas, and performing centrifugal treatment on waste acid after reaction to obtain waste acid containing a lot of phosphoric acid; and (2), drying the sulfur dioxide gas, and enabling the dried sulfur dioxide gas to react with a mixed nitric acid solution B in a multistage-series falling-film absorption tower, thereby obtaining nitrosyl sulfuric acid. According to the preparation method of nitrosyl sulfuric acid, disclosed by the invention, industrial waste acid containing nitric acid and sulfuric acid is used as a reaction raw material to realize waste recycling, and meanwhile the purpose of concentrating the phosphoric acid in the waste acid is achieved, so that an economical and effective treatment method is provided for the industrial waste acid.
Owner:ZHEJIANG LINJIANG CHEM
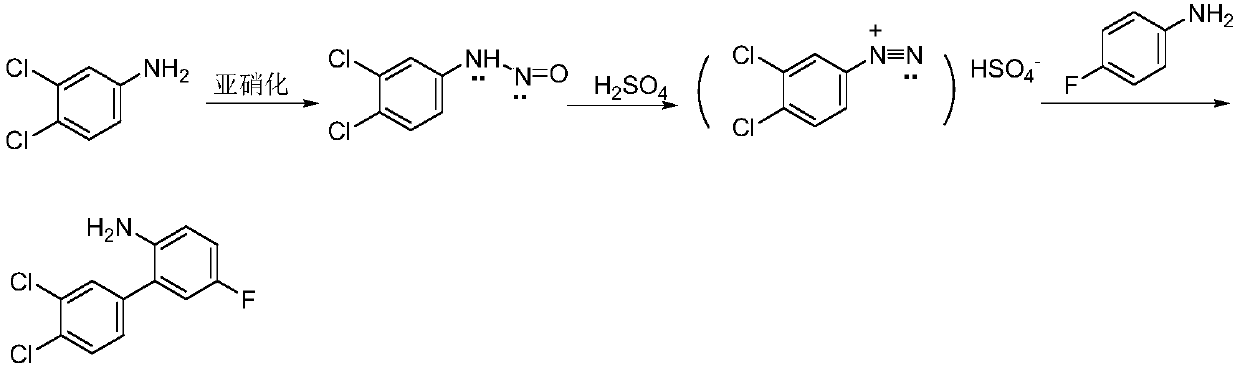
Method for synthesizing 3, 4-dichloro-2-amino-5-fluorobiphenyl
InactiveCN111072492AHigh selectivityAchieving processing powerAmino preparation from aminesAlkali metal sulfite/sulfate purificationNitrosylsulfuric acidNitroso
The invention discloses a method for synthesizing 3, 4-dichloro-2-amino-5-fluorobiphenyl. The method comprises the following steps: carrying out diazotization reaction on substrate 3, 4-dichloroaniline and nitroso sulfuric acid to prepare diazonium salt; carrying out a coupling reaction on the diazonium salt and 4-fluoroaniline to obtain the product 3, 4-dichloro-2-amino-5-fluorobiphenyl; sodium chloride needing landfill treatment is not generated in the reaction process, and the method has the characteristics of mild conditions, simple operation, high raw material conversion rate and productselectivity, low energy consumption and the like, and is suitable for industrial production; byproduct sodium sulfate generated by the reaction is a brownish yellow solid; a colorless sodium sulfate product is obtained after resin adsorption decoloration and thermal evaporation concentration crystallization combined process treatment, harmless treatment and resource utilization of sodium sulfate-containing waste salt slag are realized in the whole process, the produced sodium sulfate product reaches the industrial-grade anhydrous sodium sulfate product standard, the economic benefit is improved, and the environmental pollution is avoided.
Owner:ZHEJIANG UNIV OF TECH
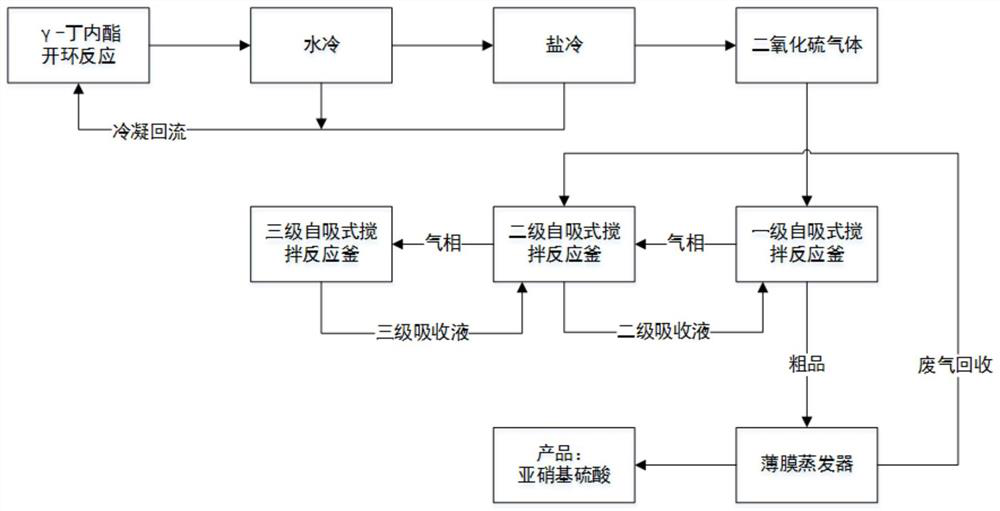
Production method and application of nitroso-sulfuric acid
ActiveCN106379875ASolve pollutionSolve the costNitrogen compoundsAlkali metal nitrate preparationNitrosoNitrosylsulfuric acid
The invention provides a production method of nitroso-sulfuric acid, comprising the following steps: a, heating and dehydrating waste acid produced by diazotization deamination so as to obtain concentrated waste acid, and supplementing concentrated sulfuric acid to prepare mixed acid; b, introducing a gas mixture of exhaust gas produced by a nitrobenzene compound chloro-nitro reaction and the air into a mixed acid spray tower for absorption, detecting nitroso-sulfuric acid prepared in the spray tower and directly pumping qualified nitroso-sulfuric acid into a storage tank of nitroso-sulfuric acid; c, preparing a sodium hydroxide aqueous solution, absorbing tail gas which is not completely absorbed through the spray tower for the preparation of sodium nitrate and sodium chloride; d, adding nitric acid into the tail gas absorption liquid after tail gas absorption until pH of the solution is acidic, regulating pH to neutral level by the use of sodium hydroxide, heating and concentrating the absorption liquid, cooling for crystallization, precipitating out sodium nitrate solid, and continuously carrying out evaporative crystallization to obtain sodium chloride solid. The nitroso-sulfuric acid can be used as a diazotization reagent for the diazotization reaction of weak-base amine containing electron-withdrawing group and heterocyclic amine. The technology has advantages of reasonable design, low energy consumption and simple operation control.
Owner:ZHEJIANG LINJIANG CHEM
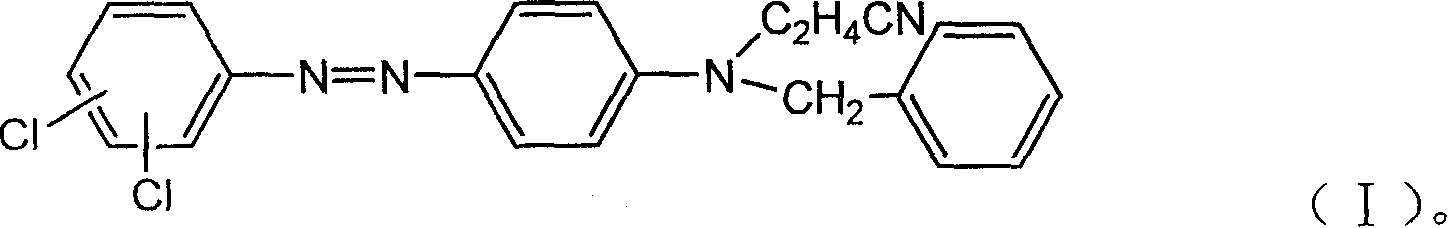
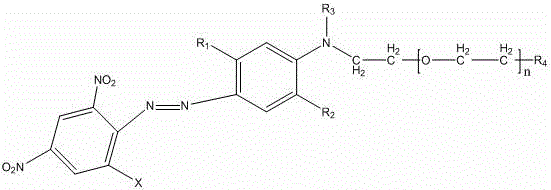
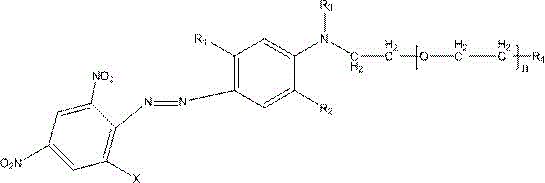
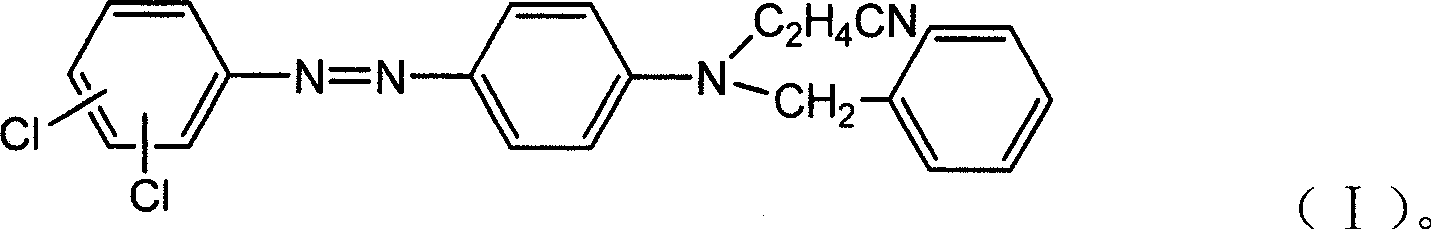
Strong-alkali-resistant moderate-temperature disperse violet dye as well as compound mixture and preparation method thereof
ActiveCN106118119AHas the function of strong alkali resistanceBright lusterMonoazo dyesNitrosylsulfuric acidDisperse dye
The invention discloses a strong-alkali-resistant moderate-temperature disperse violet dye as well as a compound mixture and a preparation method thereof. The strong-alkali-resistant moderate-temperature disperse violet dye is a compound of which the structure is as shown in a structural formula (I), and the compound is prepared in the way that 2-bromo-4,6-dinitroaniline or 2-chloro-4,6-dinitroaniline is diazotized by use of nitrosyl sulfuric acid, and the diazotized product is subjected to coupling reaction with m-substituted and o-substituted-N-aniline emulsion. The strong-alkali-resistant moderate-temperature disperse violet dye is environment-friendly in production process, can be applied to moderate-temperature dyeing of such hydrophobic fibers as polyester, nylon and spandex and blended fabrics of the hydrophobic fibers, is bright-colored, can replace disperse violet 28, disperse violet 31 and disperse violet 93, and can be compounded with azo-type disperse dyes and anthraquinone disperse dyes to achieve the functions of adjusting color tone and increasing effect so as to meet the demands of different application conditions. The strong-alkali-resistant moderate-temperature disperse violet dye is high in promotion performance, has favorable fastness in water washing, insolation, sublimation and the like, is excellent in comprehensive performance and has a wide application prospect.
Owner:烟台澳土复合材料有限公司
Synthesis of caprolactam and its oligomer
The present invention is synthesis process of caprolactam and its oligomer. Cyclohexane as initial material is nitrosated with nitroso sulfuric acid inside fuming sulphuric acid medium and in the presence of catalyst to synthesize caprolactam and its oligomer directly. The present invention has greatly simplified technological process, lowered cost and greatly raised resource utilization.
Owner:XIANGTAN UNIV
D-pi-A structure nonlinear optical chromophore, and its synthesizing method
InactiveCN1850788ANonlinear Optical Performance ImprovementHigh transparencyOrganic chemistryNitrosylsulfuric acidPhenol
This invention discloses D- pi-A structure nonlinear optics chromophore and ts synthesizing method. Its structure general formula is shown as (1), and R is fluorin, chlorine or bromine and other halogen atoms. Nitroaniline compound contains halogen atoms is react with nitrolys-sulfuric acid to generate diazo salt, then it is coupled reacted with 2, 5-dimethyl phenol to generate the species chromophore compound. Its synthesis technique is simple, yield is high, the material is easy to get, and reaction condition is wild. This kind of compound has higher second order nonlinear optics capability and excellent light permeability at visible light wave band, it can be used to blue green wave band laser double frequency field.
Owner:ZHEJIANG UNIV
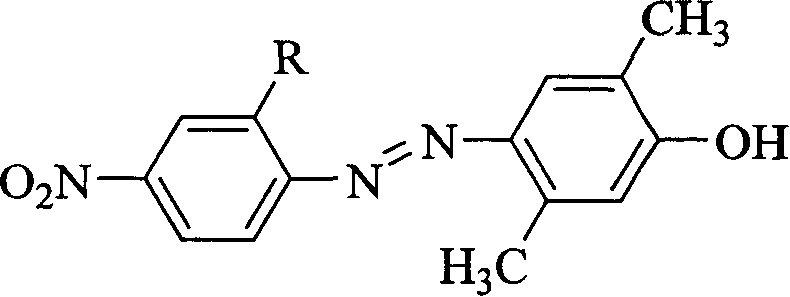
Heterocyclyl-azo-dialkyl aminophenol pigments and process for synthesizing the same
InactiveCN1557878AWell formedThe instrument is simpleMonoazo dyesSodium acetateNitrosylsulfuric acid
The present invention is heterocyclic azo-dialkyl phenol dye and its synthesis, and relates to chemical product and its preparation technology. The synthesis process includes dissolving diazo component in organic solvent through stirring, adding concentrated inorganic acid at certain temperature, adding nitrosulfonic acid slowly to obtain diazo solution; and through dissolving the coupling component, urea and sodium acetate in organic solvent via stirring at certain temperature, dropping the diazo solution slowly into the reaction system through stirring, letting stand for a night, filtering, washing and drying, azo dye is prepared. The dye has high dissolvability, good optical property, high light and heat stability, and the process has high yield, simple reaction system and easily controlled reaction condition.
Owner:HEILONGJIANG UNIV
Method for preparing caprolactam
ActiveCN101168525AImprove conversion rateHigh selectivityLactams preparationNitrosylsulfuric acidSide reaction
The invention discloses a caprolactam preparation method. In fuming sulfuric acid and hexahydrobenzoic acid or the mixed anhydride substance produced in the derivant reaction, a certain quantity of substance containing caprolactam is added, nitroso sulfuric acid is added in the mixed anhydride containing caprolactam to perform the nitrosation reaction, the product is further hysrolyzed to obtain caprolactam-sulphuric acid solution, and the hexahydrobenzoic acid or the derivant whose reaction is not accomplished is circulated to use. The method can suppress the side reaction of the sulfonationduring the nitrosation process, and realize the selectivity of the main sulfonation reaction under the precondition that the total acid consumption and the by-product are not increased.
Owner:CHINA PETROLEUM & CHEM CORP +1
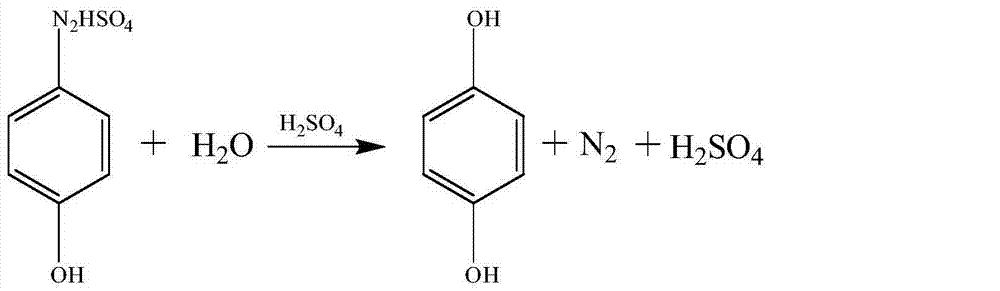
Hydroquinone synthesis method
InactiveCN104744217AEasy to cleanOrganic chemistryOrganic compound preparationNitrosylsulfuric acidSynthesis methods
The invention discloses a preparation method of hydroquinone (called as HQ for short). The preparation method comprises 1, diazotization reaction, 2, hydrolysis reaction, 3, extraction reaction and 4, purification reaction. Nitroso-sulfuric acid is used for the hydroquinone diazotization reaction so that the whole reaction system does not contain waste acid and through simple treatment, the hydrolyzed waste acid can be recycled and thus the preparation method is clean and environmentally friendly, does not produce a sodium salt in the reaction process and guarantees acid recycle.
Owner:JIANGSU YANGNONG CHEM GROUP +2
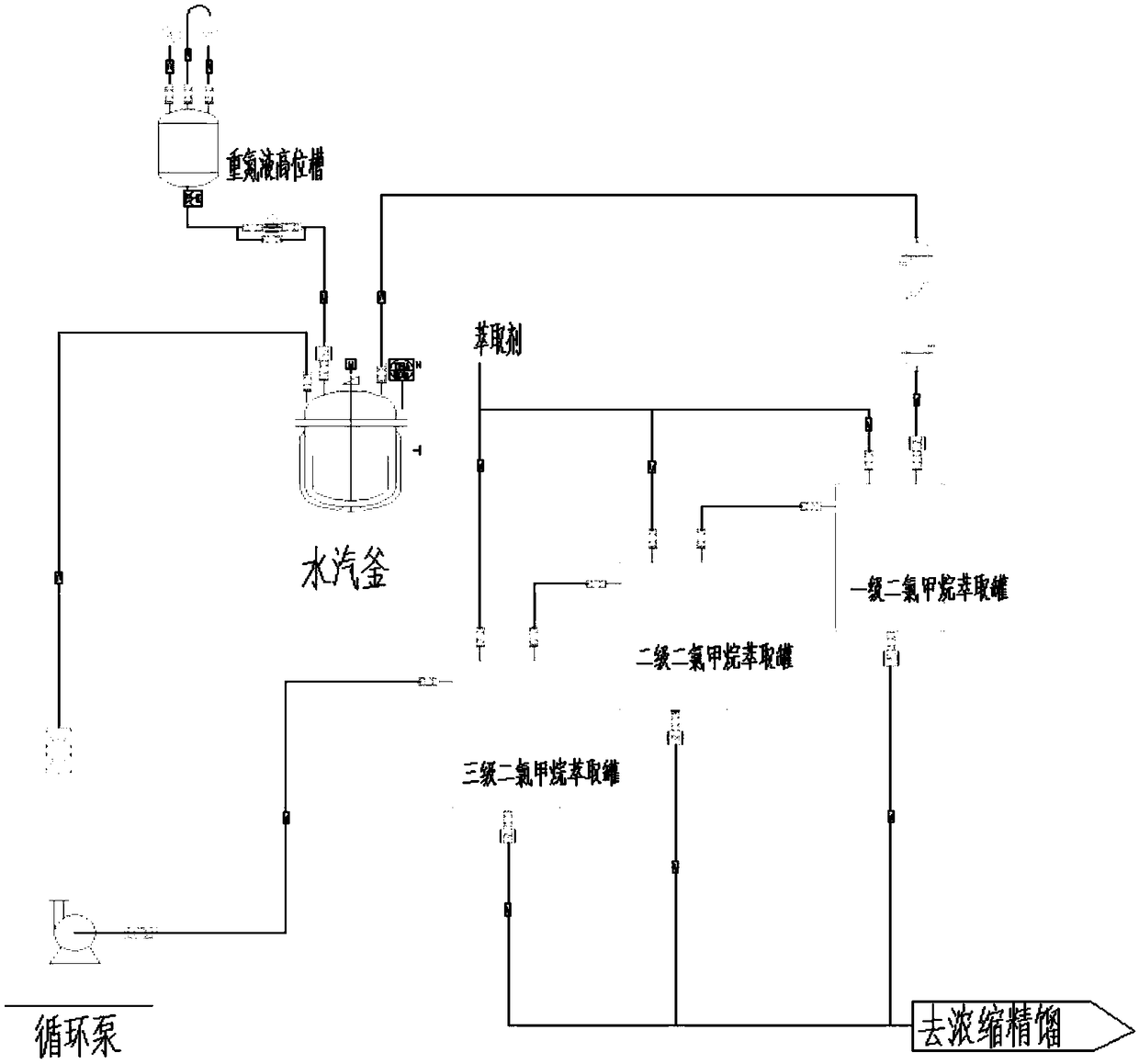
Synthesis method of 3, 4, 5-trifluorophenol
InactiveCN109456150ALow costSimple processOrganic compound preparationAmino compound preparationNitrosylsulfuric acidThree stage
The invention discloses a synthesis method of 3, 4, 5-trifluorophenol. The synthesis method includes following steps: taking 3, 4, 5-trifluorobromobenzene as a raw material, adding a certain volume ofammonia water and cuprous complexing catalyst, allowing heat-insulating reaction in a high-pressure kettle, and performing aftertreatment to obtain 3, 4, 5-trifluoroaniline; allowing 3, 4, 5-trifluoroaniline to be salified with sulfuric acid, being in diazotization reaction with nitroso-sulfuric acid, and hydrolyzing under copper salt catalysis to obtain 3, 4, 5-trifluorophenol. A hydrolysis device is introduced into a three-stage dichloromethane extraction device. Sulfuric acid concentration of a system is ensured to be constant, so that reaction yield is increased substantially. The synthesis method is easy-to-obtain in raw material and simple in process; the catalyst and the sulfuric acid are used for several times, so that raw material cost is low; the synthesis method is easy for industrialization and has good application prospect.
Owner:ZHEJIANG LINJIANG CHEM
Method for testing nitroso-sulfuric acid
InactiveCN101769873AInhibit side effectsNo side effectsMaterial analysis by observing effect on chemical indicatorNitrosylsulfuric acidNitroso
The invention relates to a method for testing nitroso-sulfuric acid, and belongs to the field of chemical engineering experiments. The invention adopts a method of diluting first and then testing so as to greatly avoid the side reaction of the nitroso-sulfuric acid and water in solution of oxidant when high-content nitroso-sulfuric acid is measured and greatly enhance the accuracy of testing because of no side reaction. Non-toxic and non-irritating nitrogen oxide gas does not pollute the environment and do harm to the health of an operator.
Owner:HANGZHOU LONGSHAN CHEM CO LTD
A kind of production method and application of nitrosyl sulfuric acid
ActiveCN106379875BCreate economic valueEasy to operateNitrogen compoundsAlkali metal nitrate preparationNitrosylsulfuric acidHeterocyclic amine
The invention provides a production method of nitroso-sulfuric acid, comprising the following steps: a, heating and dehydrating waste acid produced by diazotization deamination so as to obtain concentrated waste acid, and supplementing concentrated sulfuric acid to prepare mixed acid; b, introducing a gas mixture of exhaust gas produced by a nitrobenzene compound chloro-nitro reaction and the air into a mixed acid spray tower for absorption, detecting nitroso-sulfuric acid prepared in the spray tower and directly pumping qualified nitroso-sulfuric acid into a storage tank of nitroso-sulfuric acid; c, preparing a sodium hydroxide aqueous solution, absorbing tail gas which is not completely absorbed through the spray tower for the preparation of sodium nitrate and sodium chloride; d, adding nitric acid into the tail gas absorption liquid after tail gas absorption until pH of the solution is acidic, regulating pH to neutral level by the use of sodium hydroxide, heating and concentrating the absorption liquid, cooling for crystallization, precipitating out sodium nitrate solid, and continuously carrying out evaporative crystallization to obtain sodium chloride solid. The nitroso-sulfuric acid can be used as a diazotization reagent for the diazotization reaction of weak-base amine containing electron-withdrawing group and heterocyclic amine. The technology has advantages of reasonable design, low energy consumption and simple operation control.
Owner:ZHEJIANG LINJIANG CHEM
A kind of alkali-resistant disperse blue dye, its compound mixture and preparation method thereof
Owner:烟台澳土复合材料有限公司
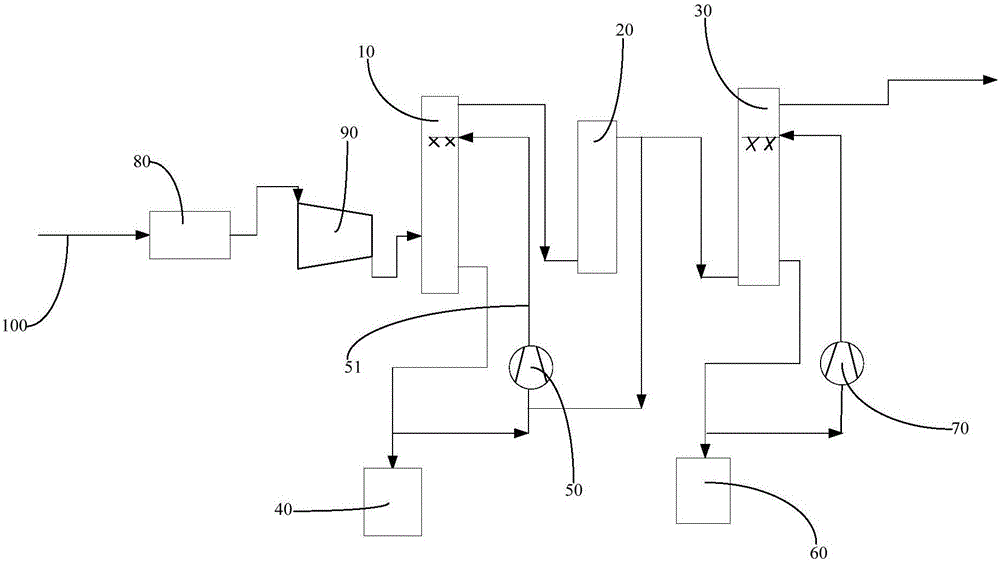
Flue gas purification system and method
InactiveCN106178913ALow running costLess investmentGas treatmentDispersed particle separationNitrosylsulfuric acidNitric oxide
The invention relates to the field of coal-fired flue gas pollutant purification, and discloses a flue gas purification system and method. The gas purification system comprises a desulphurization tower (10), an oxidation tower (20) and a denitration tower (30) which are communicated in sequence. Oxygen is contained in the oxidation tower. The desulphurization tower is provided with a flue gas inlet used for being communicated with a flue gas delivery pipeline (100), and a first supply device used for supplying a nitrosylsulfuric acid solution into a tower body of the desulphurization tower. The denitration tower is provided with an inlet used for being communicated with an outlet of the oxidation tower, and a second supply device used for supplying a nitric acid solution into a tower body of the denitration tower. According to the gas purification system, as the nitrosylsulfuric acid solution and the nitric acid solution are adopted to remove sulfur dioxide, nitric oxide and mercury, compared with the mode that an FGD process is adopted to carry out desulfurization and then an SCR process is adopted to carry out denitration in the prior art, operating cost is obviously lowered, and equipment investment is small; besides, the occupied area of equipment is small, and the application range is wide.
Owner:CHINA SHENHUA ENERGY CO LTD +3
Fipronil production process
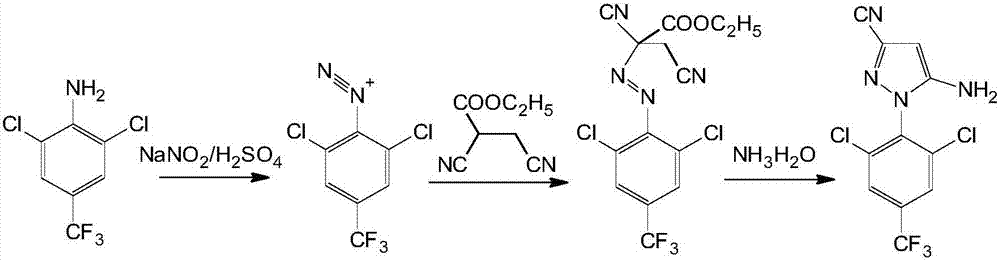
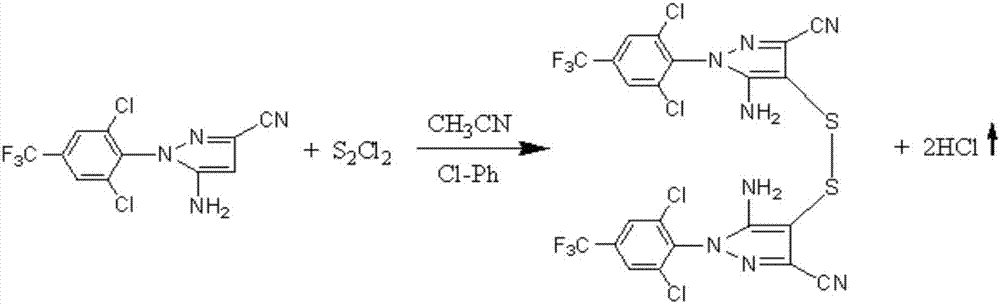
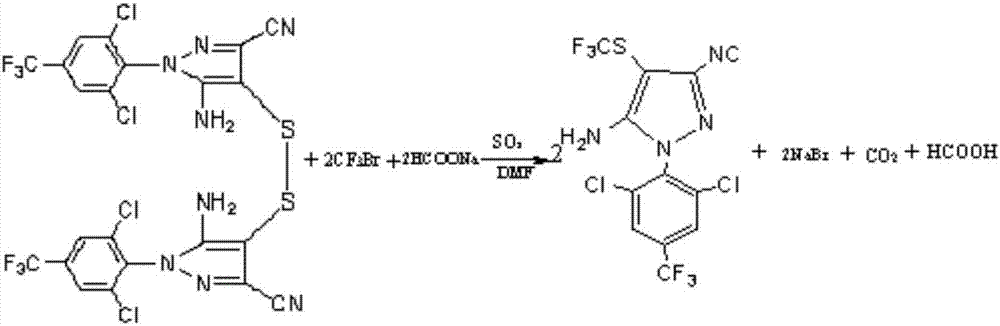
A fipronil production process comprises the following steps that 1, concentrated sulfuric acid and sodium nitrite are used as starting raw materials, reaction is performed in a glacial acetic acid solvent to generate nitrosylsulfuric acid, then a diazo-reaction with arylamine is performed to generate arylamine diazonium salt, then a condensation reaction with dicyan ester is performed, and finally a rearrangement reaction with concentrated ammonia water is performed in an ammonia water medium to generate pyrazol; 2, the pyrazol is dissolved in an acetonitrile solvent, 13 sulfur is rapidly added at the temperature of 20 DEG C for reaction, stirring continues for 30 minutes after the temperature no longer rises, and cooling, filtration and drying are performed to obtain a sulfide product; 3, the sulfide is dissolved in N,N-dimethyl formamide, sodium formate is added and bromotrifluoromethane is introduced under the effect of a catalyst sulfur dioxide for reaction, and a fipronil sulfoxide product is obtained; 4, the fipronil sulfoxide is dissolved in a dichloromethane solvent, an oxidizing agent prepared from sulfuric acid and calcium peroxide is added to perform redox reaction, and alkaline washing, washing, crystallization, centrifugation and drying are performed to obtain a fipronil product. Compared with the prior art, the fipronil production process has the advantages that the process is reasonable and free of risk, the fipronil production process purity is improved, the production efficiency is improved, the production cost is reduced, and the environmental pollution is reduced.
Owner:连云港埃森化学有限公司
Strong-alkaline-resistant disperse blue dye, compounded mixture thereof, and preparation method thereof
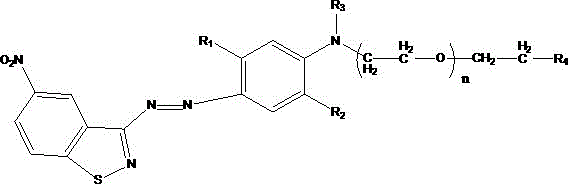
ActiveCN105907126ASolve pollutionSolve the problem of energy consumptionMonoazo dyesDisperse dyeNitrosylsulfuric acid
The invention discloses a strong-alkaline-resistant disperse blue dye, a compounded mixture thereof, and a preparation method thereof. The strong-alkaline-resistant disperse blue dye is a compound represented as the structural formula (I), which is prepared by diazotizing 3-amino-5-nitrobenzoisothiazole with nitrosyl sulfuric acid and then performing a coupling reaction with a m / o-substituted-N-substituted aniline emulsion. The strong-alkaline-resistant disperse blue dye has green and environment-friendly production process. A test result proves that the dye can be used for dyeing hydrophobic fibers, such as Dacron, nylon, spandex and the like and blended fabrics thereof, can form bright gloss, and can replace disperse blue 165 and disperse blue 56. The disperse blue dye can be compounded with an azo-type disperse dye and an anthraquinone-type disperse dye in different ratios to regulate the tone of the dyes and improve effects, thereby satisfying demands under different application conditions. The dye has high improvement-ability, is excellent in various fastnesses with water washing, sun-exposure and sublimation, has excellent comprehensive performances and has a wide application prospect.
Owner:烟台澳土复合材料有限公司
Preparation method of p-chlorophenylhydrazine sulfate
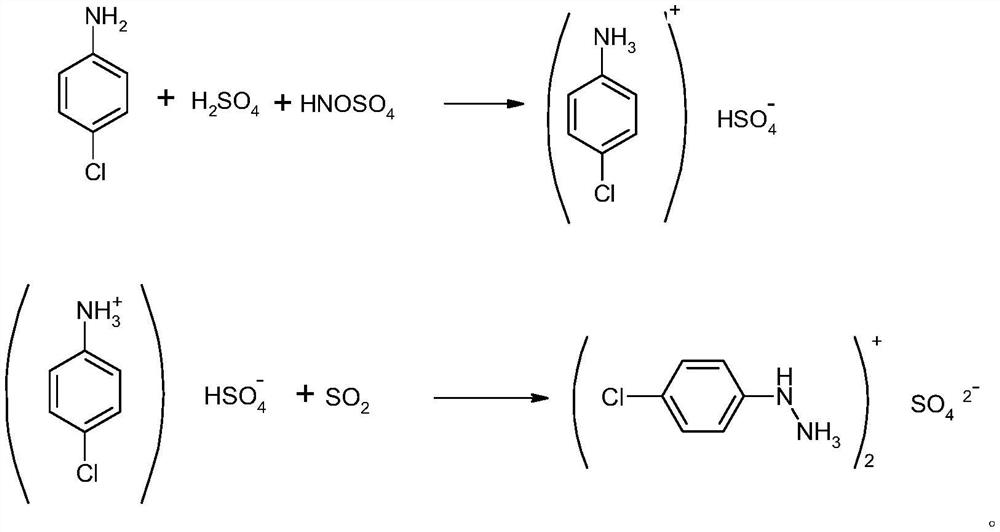
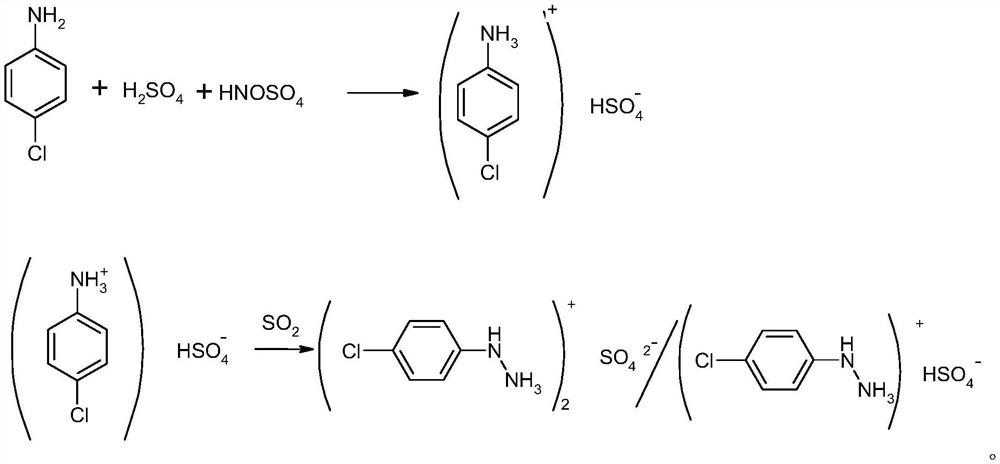
InactiveCN112939805AReduce wasteRaw materials can be recycledHydrazine preparationP-chloroanilineNitrosylsulfuric acid
The invention discloses a preparation method of p-chlorophenylhydrazine sulfate, which comprises the following steps of by taking p-chloroaniline as a raw material, carrying out diazotization, reduction, acidolysis and crystallization centrifugation to obtain the p-chlorophenylhydrazine sulfate. According to diazotization, sulfate reacts with aniline, and then reacts with nitrososulfuric acid at low temperature to generate diazonium liquid; the obtained diazonium liquid reacts with sulfur dioxide under a certain pressure to generate an intermediate, then pressure is released, heating and refluxing are conducted for 1 hour, cooling, crystallizing and centrifuging are conducted to obtain p-chlorophenylhydrazine sulfate, the centrifuged mother liquor isadsorbed by carbon fibers, and the mother liquor is reused in diazotization synthesis; the purity of the p-chlorobenzene phenylhydrazine sulfate is more than 97%, the yield is more than 85%, compared with the prior art, the process has the advantages that no other metal cations are introduced, the centrifuged mother liquor can be applied to diazotization synthesis after being treated, no wastewater is generated, clean production is realized, and the requirements of safe production of current industrial production are met.
Owner:JIANGSU YOUJIA CHEM
Solvent-free coupling synthesis process of disperse blue 360
The invention discloses a solvent-free coupling synthesis process of disperse blue 360. The process comprises the following steps: adding 2-amino-5-nitrothiazole into an organic acid medium, controlling the temperature, dropwise adding nitroso-sulfuric acid and carrying out a diazotization reaction to obtain a diazonium solution; adding N, N-diethyl m-methylaniline into a coupling reactor to carryout solventless reaction, cooling to -10 to 10 DEG C, adding the diazonium solution to carry out coupling reaction until the coupling is finished, filtering and washing to obtain a crude product; andobtaining a high-purity fine blue crystal, namely the disperse blue 360 through a crystal transformation process. According to the solvent-free coupling synthesis process of disperse blue 360, pure disperse blue 360 crystals can be obtained, the yield is high, the produced organic acid and crystal transformation solvent can be completely recycled, energy is saved, waste is reduced, the productioncost is greatly reduced, multiple purposes are achieved, and the process has good practical significance for achieving large-scale clean production.
Owner:JIANGSU HANSYN PHARMA
Recycling method of nitrogen oxide gas in diazotization reaction process
PendingCN114272738AAvoid secondary pollutionSimple methodOrganic chemistryDispersed particle separationNitrosylsulfuric acidNitroso
The invention belongs to the field of industrial waste gas treatment, and relates to a method for recycling nitrogen oxide gas in a diazotization reaction process. The method comprises the following steps: introducing NOX gas generated by reaction of a diazotization reagent into an absorption device filled with concentrated sulfuric acid until the content of nitroso sulfuric acid in absorption liquid is 60-96 wt%; placing primary arylamine in a sulfuric acid solution, stirring to form a turbid liquid, dropwise adding the obtained absorption liquid into the turbid liquid, and reacting to prepare a diazonium salt solution; the nitrogen oxide gas generated in the diazotization reaction process can be absorbed, the absorption liquid can still serve as a diazotization reagent to be applied to the diazotization reaction, waste gas recycling is achieved, the process is simple, and high economic benefits are achieved.
Owner:NANJING UNIV OF SCI & TECH
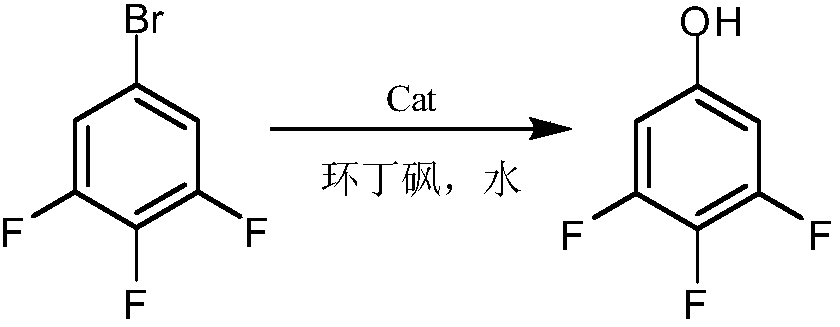
Green synthesis method of 3,4,5-trifluorobromobenzene
InactiveCN112624895AReduce processing costsReduce usageHalogenated hydrocarbon preparationMetal/metal-oxides/metal-hydroxide catalystsNitrosylsulfuric acidNitroso
The invention discloses a green synthesis method of 3,4,5-trifluorobromobenzene. The green synthesis method comprises the following steps: adding 2,3,4-trifluoroaniline and water into a reactor, adding hydrobromic acid and hydrogen peroxide, and carrying out a hybrid reaction to obtain 2,3,4-trifluoro-6-bromoaniline after the reaction is ended; slowly adding a sulfuric acid solution of nitroso sulfuric acid into the prepared 2,3,4-trifluoro-6-bromoaniline, and carrying out a diazotization reaction to obtain a diazonium salt solution; and adding sodium hypophosphite and porous material-loaded copper oxide into the prepared diazonium salt solution as catalysts, conducting reacting, and sequentially carrying out distillation, alkali washing, water washing and reduced-pressure rectification on reaction liquid after the reaction is finished so as to obtain a target product. According to the method, nitrososulfuric acid replaces sodium nitrite to serve as a diazotization reagent, so no high-salt wastewater is discharged, production cost is reduced, and the method is more environmentally friendly; and hydrobromic acid serves as a bromine source, and hydrogen peroxide is added, so the utilization rate of bromine atoms is high, the adding amount of hydrobromic acid is reduced, and the amount of three wastes generated in implementation of the method is remarkably reduced.
Owner:SHANGYU XIES CHEM IND
Method for testing nitroso-sulfuric acid
InactiveCN101769873BInhibit side effectsNo side effectsMaterial analysis by observing effect on chemical indicatorNitrosylsulfuric acidNitroso
The invention relates to a method for testing nitroso-sulfuric acid, and belongs to the field of chemical engineering experiments. The invention adopts a method of diluting first and then testing so as to greatly avoid the side reaction of the nitroso-sulfuric acid and water in solution of oxidant when high-content nitroso-sulfuric acid is measured and greatly enhance the accuracy of testing because of no side reaction. Non-toxic and non-irritating nitrogen oxide gas does not pollute the environment and do harm to the health of an operator.
Owner:HANGZHOU LONGSHAN CHEM CO LTD
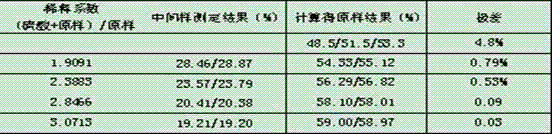
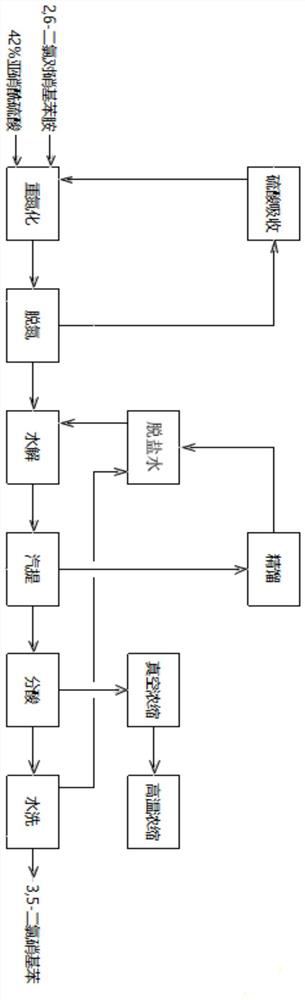
Production process of 3,5-dichloronitrobenzene
PendingCN112500295ANo pollution in the processHigh yieldOrganic compound preparationAmino compound preparationNitrosylsulfuric acidNitrobenzene
The invention provides a production process of 3,5- dichloronitrobenzene, and belongs to the field of preparation of pesticide intermediates. The method comprises the following steps: adding nitrosylsulfuric acid with the mass fraction of 42% into a reaction kettle, conducting cooling to 5 DEG C, sequentially adding 2,6-dichloro-4-nitroaniline or 4,6-dichloro-o-nitroaniline, isopropanol and desalted water to respectively complete diazotization, denitrification and hydrolysis processes, wherein the temperature of the whole process is not higher than 45 DEG C; conducting steam extraction with water vapor at 100 DEG C to recover isopropanol and acetone, separating diluted sulfuric acid and 3,5-dichloronitrobenzene from a reaction solution after steam extraction, washing 3,5-dichloronitrobenzene with water, carrying out hydrogenation reduction on the washed 3,5-dichloronitrobenzene to produce 3,5-dichloroaniline, carrying out vacuum concentration and high-temperature concentration on thediluted sulfuric acid to obtain 97% concentrated sulfuric acid to prepare nitrosyl sulfuric acid, and separating a sulfuric acid concentrated distillate through an R / O membrane to prepare desalted water, and recycling the desalted water in the process. The process provided by the invention has the advantages of high yield, no pollution and low cost, and is a green production process.
Owner:德兴市德邦化工有限公司
Synthesis of caprolactam and its oligomer
The present invention is synthesis process of caprolactam and its oligomer. Cyclohexane as initial material is nitrosated with nitroso sulfuric acid inside fuming sulphuric acid medium and in the presence of catalyst to synthesize caprolactam and its oligomer directly. The present invention has greatly simplified technological process, lowered cost and greatly raised resource utilization.
Owner:XIANGTAN UNIV
Single diazo compound, its preparation method and use
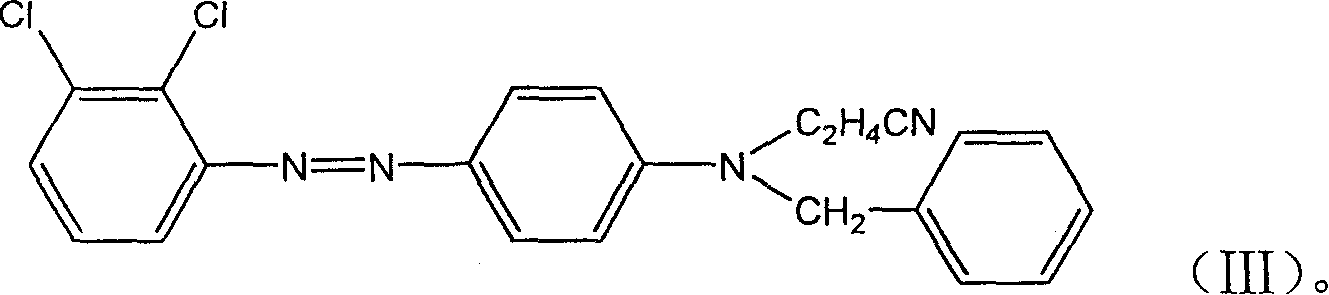
InactiveCN100503556CHigh color fastnessEffective dispersionMonoazo dyesOrganic chemistryNitrosylsulfuric acidDisperse yellow
A monoazo compound used for the disperse yellow dye with high dye fastness is prepared through diazotizing reaction between dichlorophenylamine and nitrosylsulfuric acid in sulfuric acid to obtain diazonium salt, coupling reaction on N-cyanoethyl-N- benzylphenylamine and post-treating.
Owner:闰土控股集团有限公司
Wet denitration method for high-sulfur flue gas
PendingCN113893671ANo reduction in production qualityPromote reductionGas treatmentNitrogen preparationNitrosylsulfuric acidNitroso
The invention discloses a wet denitration method for high-sulfur flue gas. The method comprises the following steps of 1) purifying, 2) conducting conversion and absorption, (3) discharging gas, and 4) conducting acid production treatment. High-sulfur flue gas self-denitration is carried out by adopting the characteristics that a purified dilute acid aqueous solution absorbs part of nitrogen oxides and concentrated sulfuric acid, and sulfur trioxide reacts with the nitrogen oxides to generate nitroso sulfuric acid. Advanced treatment of nitrososulfuric acid and iron nitrososulfate in the concentrated sulfuric acid is focused, and nitrososulfuric acid and iron nitrososulfate are reduced into nitrogen, water and sulfuric acid or ferrous sulfate through a medicament, so that the final purposes of sulfuric acid wet denitration and no adverse byproducts are achieved, and a complete closed-loop process is formed. According to the denitration system disclosed by the invention, advanced treatment processes such as reduction and purification of nitrososulfuric acid or iron nitrososulfate are also added. Not only are NOHSO4 and Fe(NO)SO4 reduced completely, but also the adverse effect of excess of a small amount of reducing agent is eliminated thoroughly, and the production quality of sulfuric acid is not reduced practically.
Owner:CINF ENG CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com