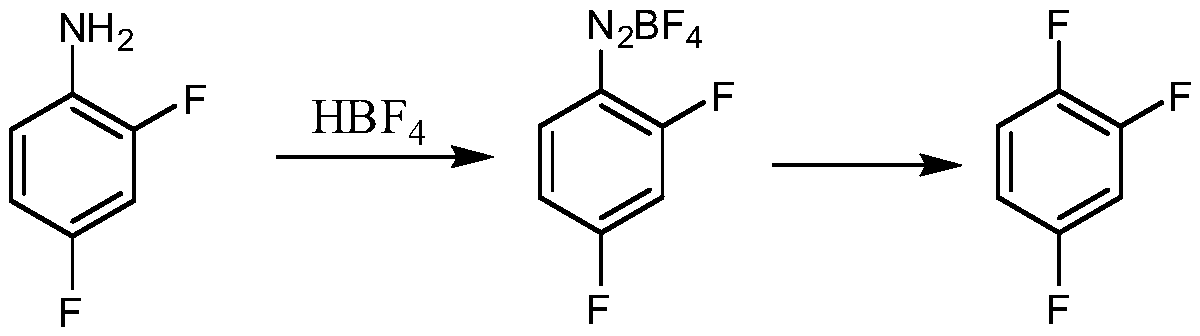
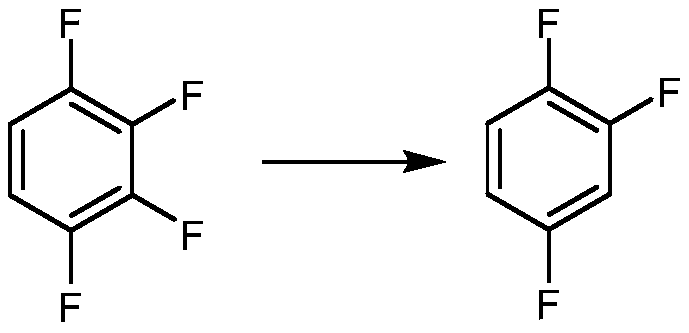
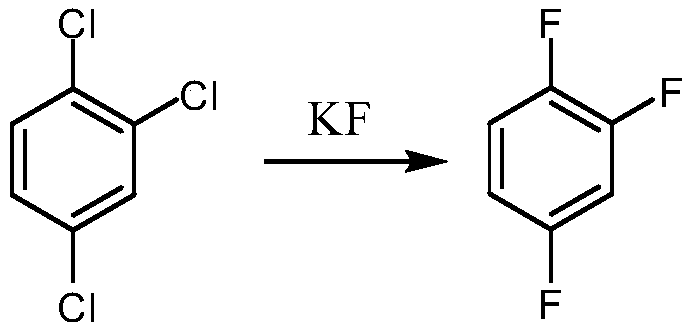
Synthetic method for 1,2,4-trifluorobenzene
A synthesis method and technology of trifluorobenzene, applied in chemical instruments and methods, preparation of nitro compounds, preparation of amino compounds, etc., can solve the problems of difficult fluorination and low total yield, and achieve mild operating conditions and low cost of raw materials , The effect of low safety risk factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Add 165g of 2,4-dichlorofluorobenzene to a 500mL four-neck flask, use 70.7g of fuming nitric acid (98% in mass fraction) and 70.7g of concentrated sulfuric acid (98% in mass fraction) to form a mixed acid, Add mixed acid dropwise at 60°C, keep warm at the same temperature for 3 hours after dropping, let stand for half an hour, separate the layers, wash the organic layer with water and alkali respectively, and obtain 205.8g of 2,4-dichloro-5-fluoronitrobenzene by layering, GC The purity is 99.5%, and the molar yield is 98.0%.
[0038] (2) Add 200g of 2,4-dichloro-5-fluoronitrobenzene and 200g of sulfolane to a 500mL four-neck flask, heat up to 100°C, remove water under reduced pressure, and add 121.5g of spray-dried potassium fluoride, 2g after dehydration for 1 hour Tetrabutylammonium bromide, heated up to 170-180°C, followed by GC, when the raw material was less than 3%, the reaction was terminated, rectified, and 146.5g of 2,4,5-trifluoronitrobenzene was obtained,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com