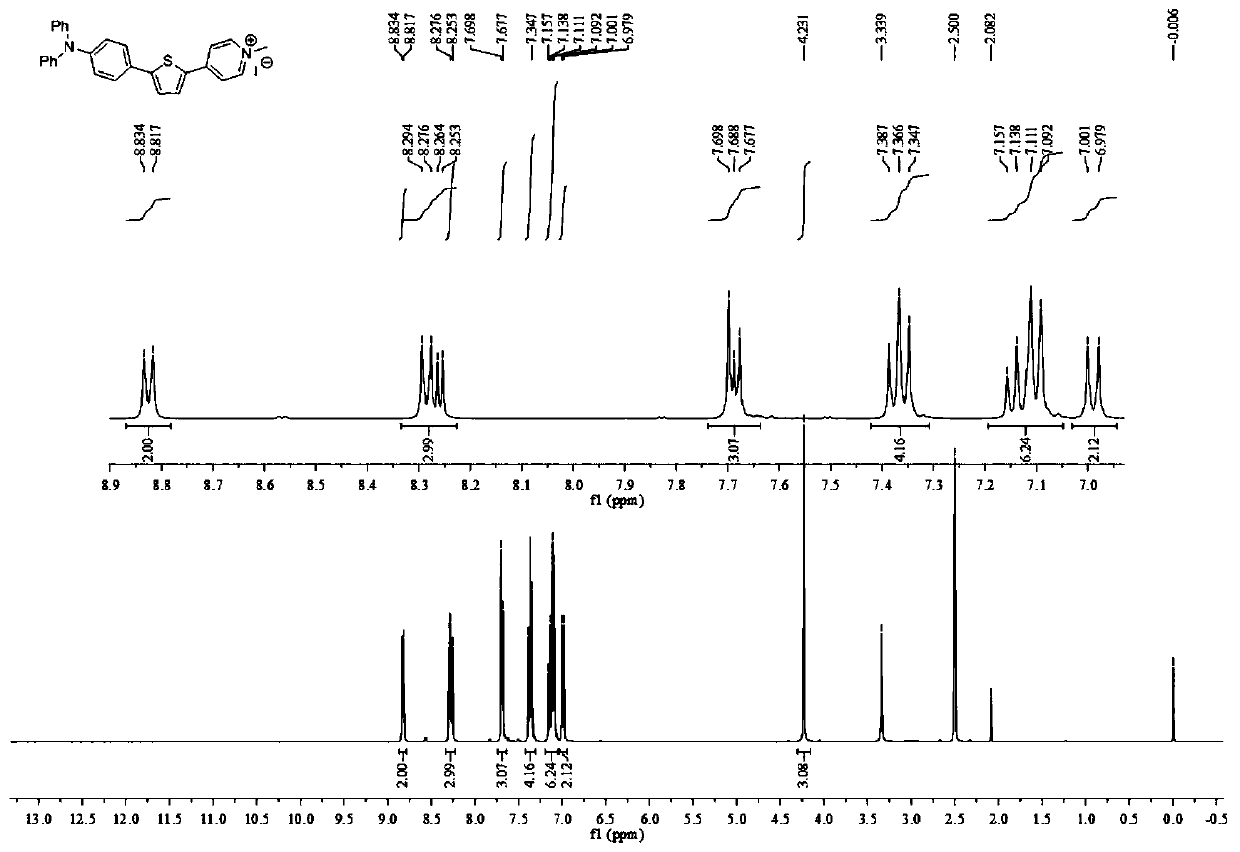
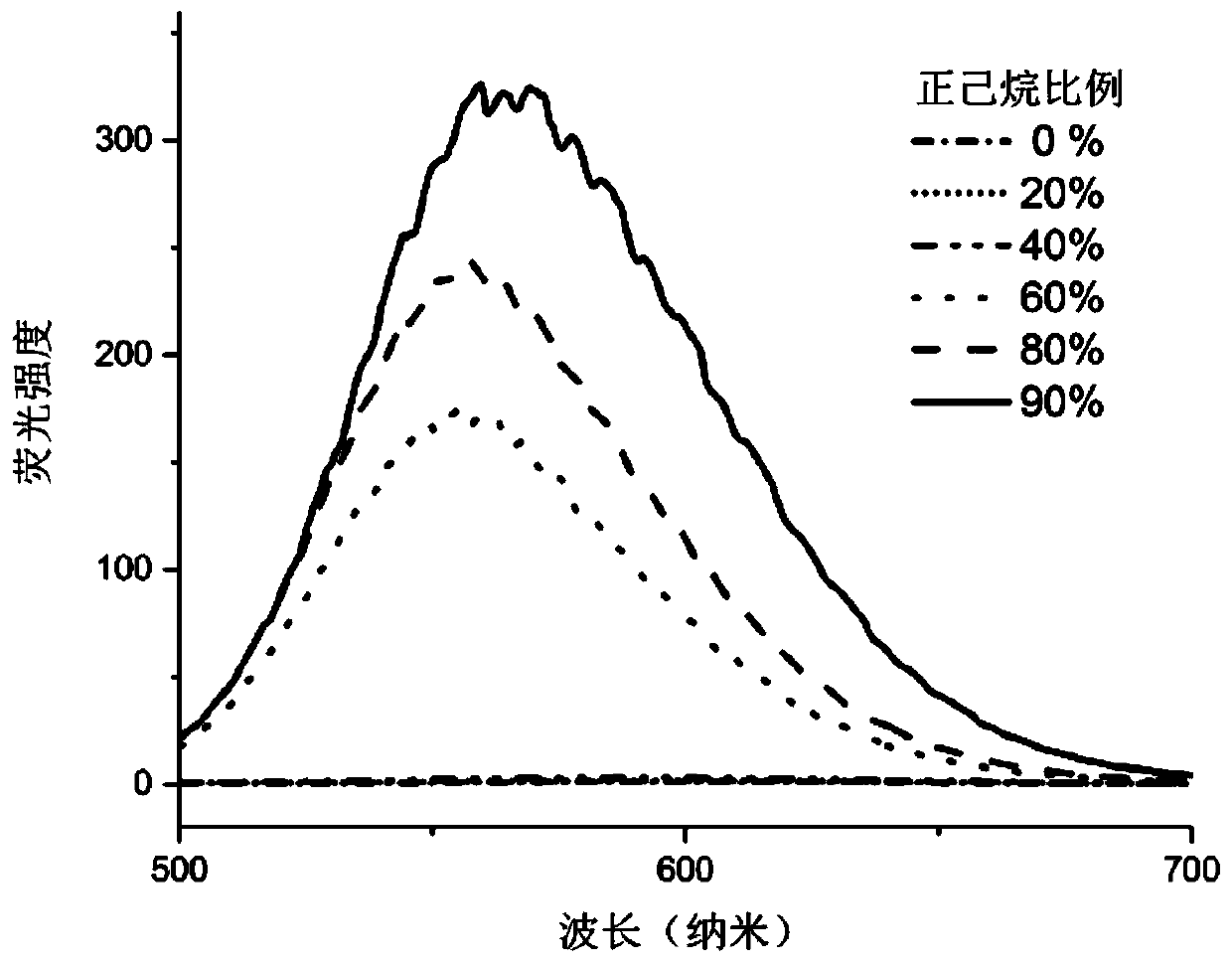
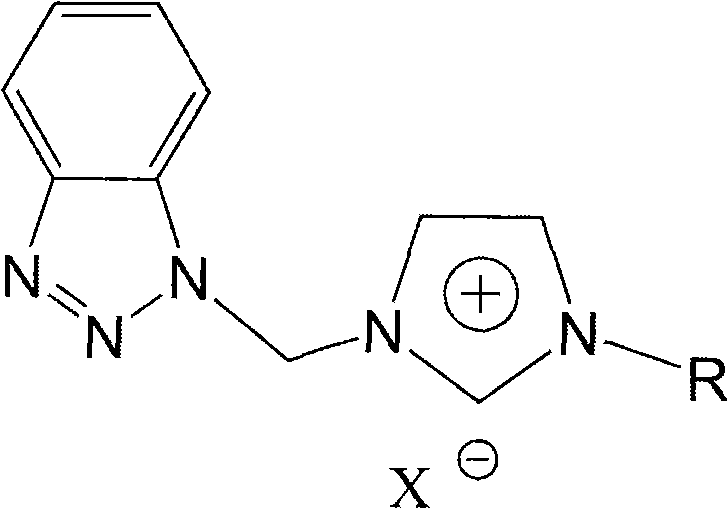
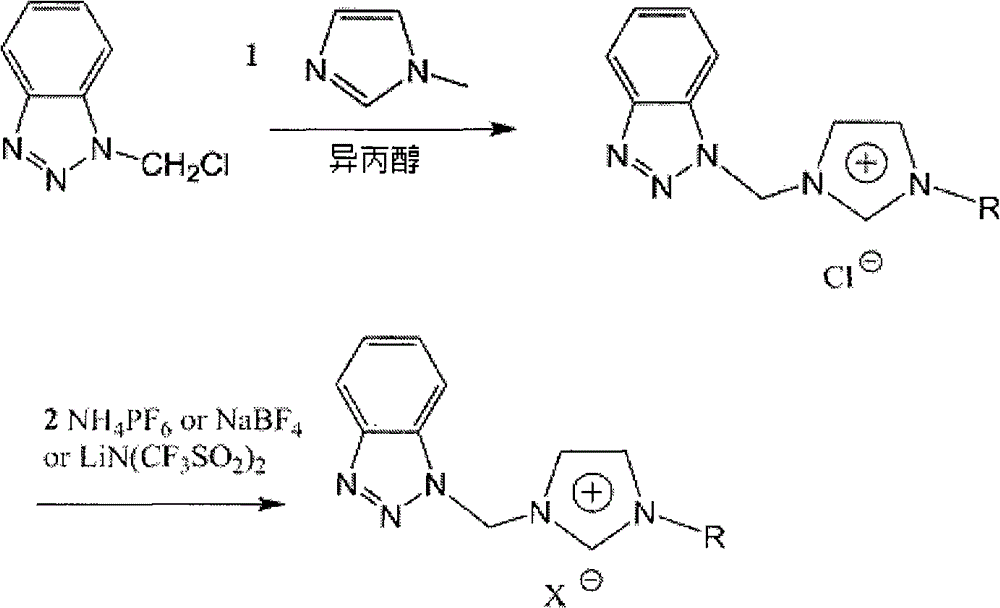
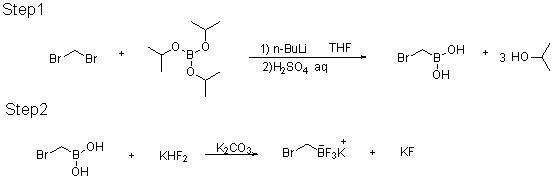
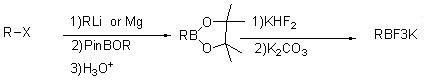
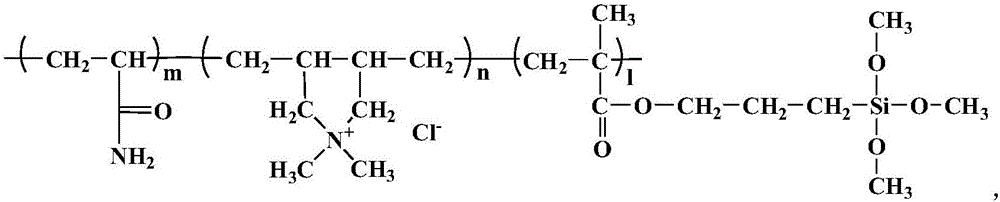
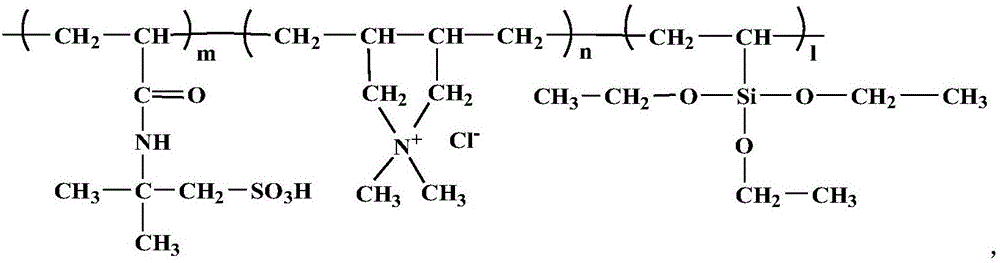
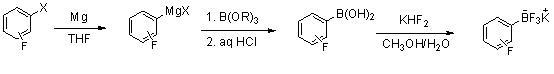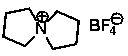Patents
Literature
507 results about "Fluoroboric acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fluoroboric acid or tetrafluoroboric acid (archaically, fluoboric acid) is an inorganic compound with the chemical formula [H⁺][BF₄⁻], where H⁺ represents the solvated proton. The solvent can be any suitably Lewis basic entity. For instance, in water, it can be represented by H₃OBF₄ (oxonium tetrafluoroborate), although more realistically, several water molecules solvate the proton: [H(H₂O)ₙ⁺][BF₄⁻]. The ethyl ether solvate is also commercially available: [H(Et₂O)ₙ⁺][BF₄⁻], where n is most likely 2. Unlike strong acids like H₂SO₄ or HClO₄, the pure unsolvated substance does not exist (see below).
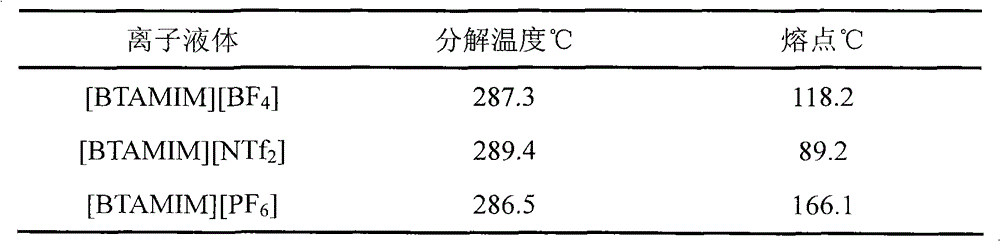
Room temperature ionic liquid containing unsaturated double bond and its prepn and application
InactiveCN1417407AGroup 5/15 element organic compoundsPulping with organic solventsSulfate radicalsTriflic acid
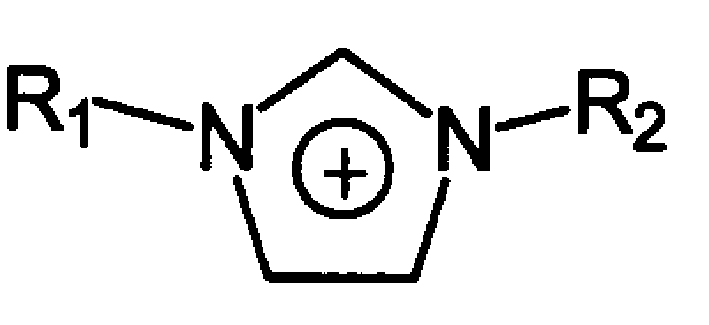
The room temperature ionic liquid containing unsaturated double bond has the general expressino of A+B-, where A+ contains R1 being hydroxyl with 1-4 carbon atoms and R2 containing 2-20 carbon atoms and at least one double bond; and B- is one of anions, including chlorate radical, bromate radical, iodate radical, acetate radical, sulfate radical, nitrate radical, tetrafluorobromate radical, etc. Its preparation is to mixture and react olefin halide R1X and N-alkyl imidazole to obtain ionic liquid dialkyl imidazolium halide. The present invention also relates to the application of the ionic liquid in dissolving cellulose and preparing cellulose derivative.
Owner:山东中科恒联生物基材料有限公司
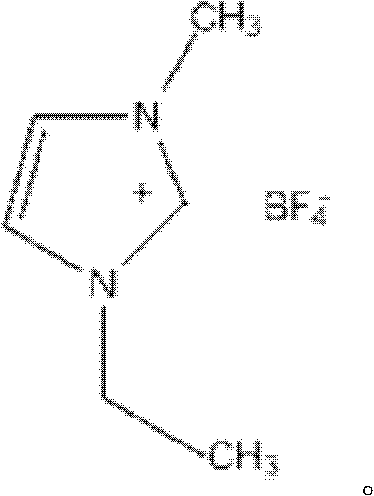
Method of esterifying in ion liquid [Hmim]+ BF4-
InactiveCN1405140ASynthetic method is simpleLow costOrganic compound preparationCarboxylic acid esters preparationSolventIon
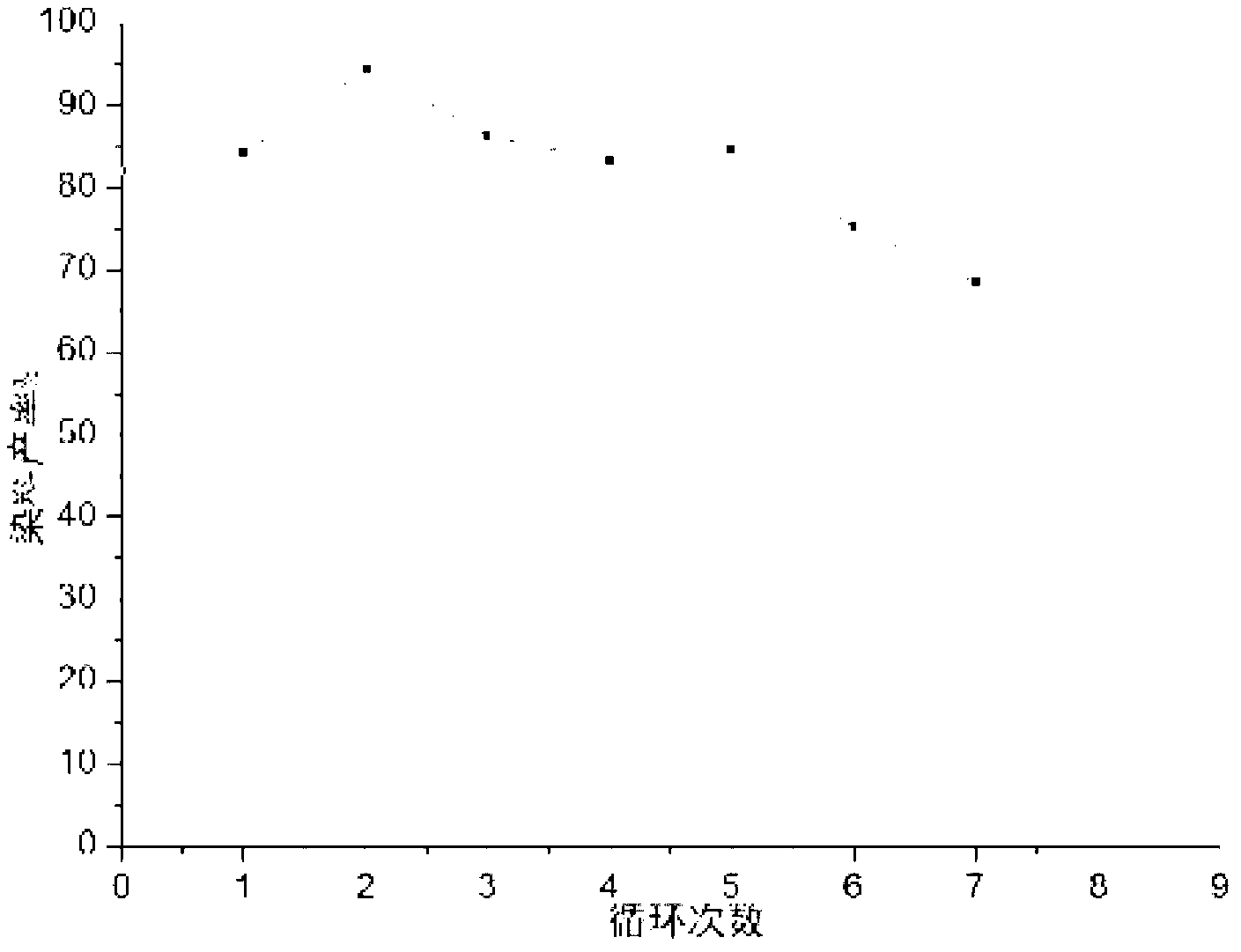
The invention relates to a method for making esterification in ion liquid [Hmim](+)BF4(-), i,e, methyl imidazole salt tetrafluoroborate which can be used as solvent and catalyst. in the esterification reaction the fatty acid or aromatic acid and straight chain alcohol or branched chain alcohol are undergone the processes of stirring, heating, standing still and gravity settling so as to obtain the esterified product. Its conversion rate is 80-100%, its selectivity is 100%, and its conversion rate is high, and its catalyst can be repeatedly and circularly used, etc.
Owner:EAST CHINA NORMAL UNIVERSITY
Diatomaceous Ionic Gel Separation Layer for Energy Storage Devices and Printable Composition Therefor
InactiveUS20140017558A1Low vapor pressureHybrid capacitor separatorsHybrid capacitor electrolytesElectrical conductorTetrafluoroborate
Representative embodiments provide a liquid or gel separator utilized to separate and space apart first and second conductors or electrodes of an energy storage device, such as a battery or a capacitor. A representative liquid or gel separator comprises a plurality of particles selected from the group consisting of: diatoms, diatomaceous frustules, diatomaceous fragments, diatomaceous remains, and mixtures thereof; a first, ionic liquid electrolyte; and a polymer or, in the printable composition, a polymer or a polymeric precursor. Another representative embodiment further comprises a second electrolyte different from the first electrolyte; the first and second electrolytes comprise zinc tetrafluoroborate salt in 1-ethyl-3-methylimidalzolium tetrafluoroborate ionic liquid; and the polymer comprises polyvinyl alcohol (“PVA”) or polyvinylidene fluoride (“PVFD”). Additional components, such as additional electrolytes and solvents, may also be included.
Owner:NTHDEGREE TECH WORLDWIDE
Fire retardant compositions with reduced aluminum corrosivity
InactiveUS6905639B2Reduced-tendency to corrode various metalBroaden applicationFireproof paintsAntifouling/underwater paintsBiopolymerFerrous Gluconate
Corrosion-inhibited fire retardant compositions and methods of making and using the same are provided. The corrosion-inhibited fire retardant compositions are comprised of at least one fire retardant component, at least one biopolymer having a particle size diameter of less than about 100 microns, and a corrosion inhibiting system. The corrosion inhibiting system is comprised of at least one corrosion inhibiting compound selected from a group of compounds including azoles, insoluble ferric pyrophosphate, soluble ferric pyrophosphate, ferrous oxalate, ferric citrate, ferrous sulfate, ferric ammonium citrate, soluble ferric orthophosphate, insoluble ferric orthophosphate, ferric ammonium oxalate, ferric ammonium sulfate, ferric bromide, ferric sodium oxalate, ferric stearate, ferric sulfate, ferrous acetate, ferrous ammonium sulfate, ferrous bromide, ferrous gluconate, ferrous iodide, ferric acetate, ferric fluoroborate, ferric hydroxide, ferric oleate, ferrous fumarate, ferrous oxide, ferric lactate, ferric resinate and any combination thereof. In a specific embodiment, the corrosion-inhibited fire retardant composition includes a xanthan biopolymer.
Owner:PERIMETER SOLUTIONS LP
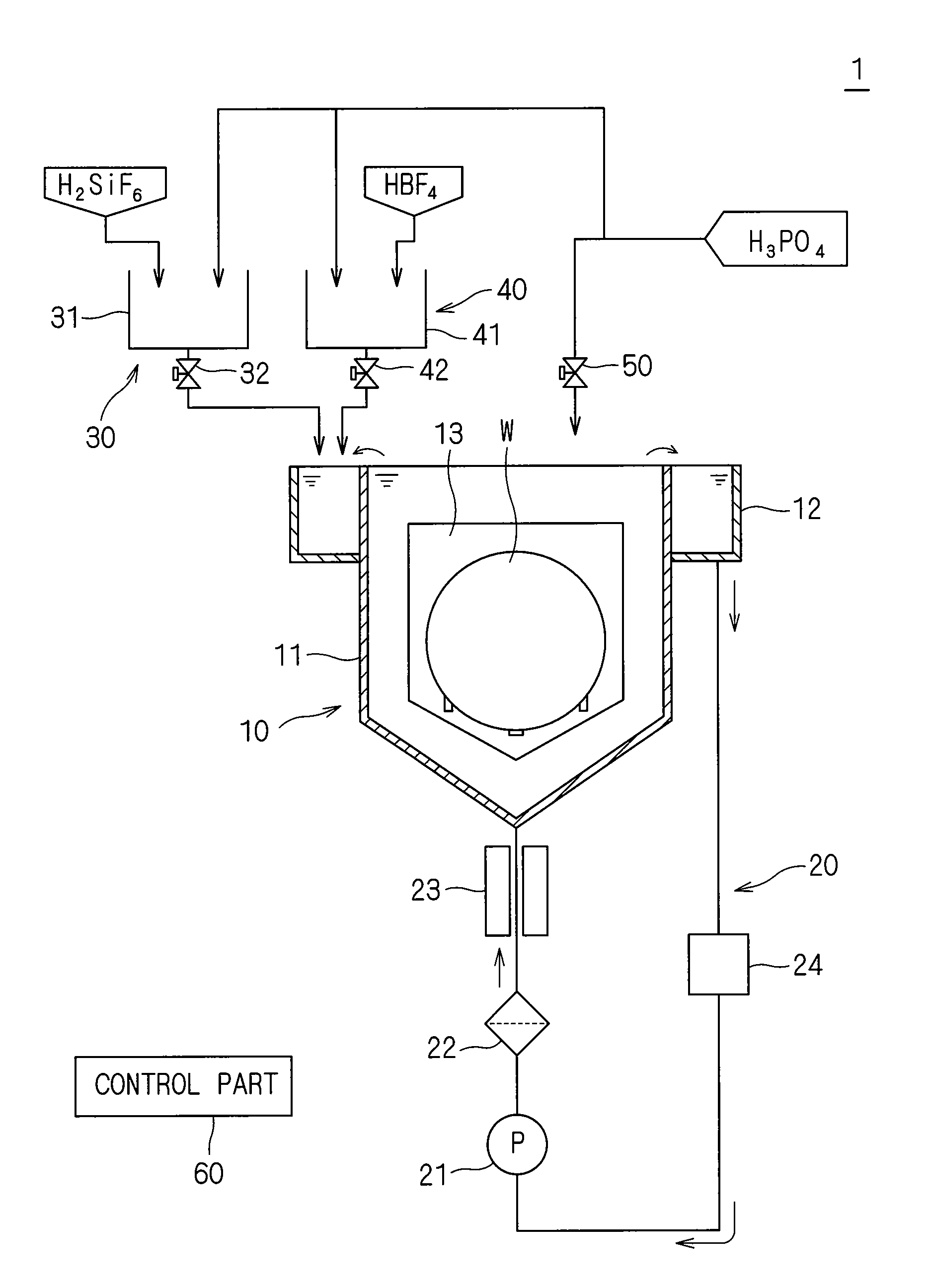
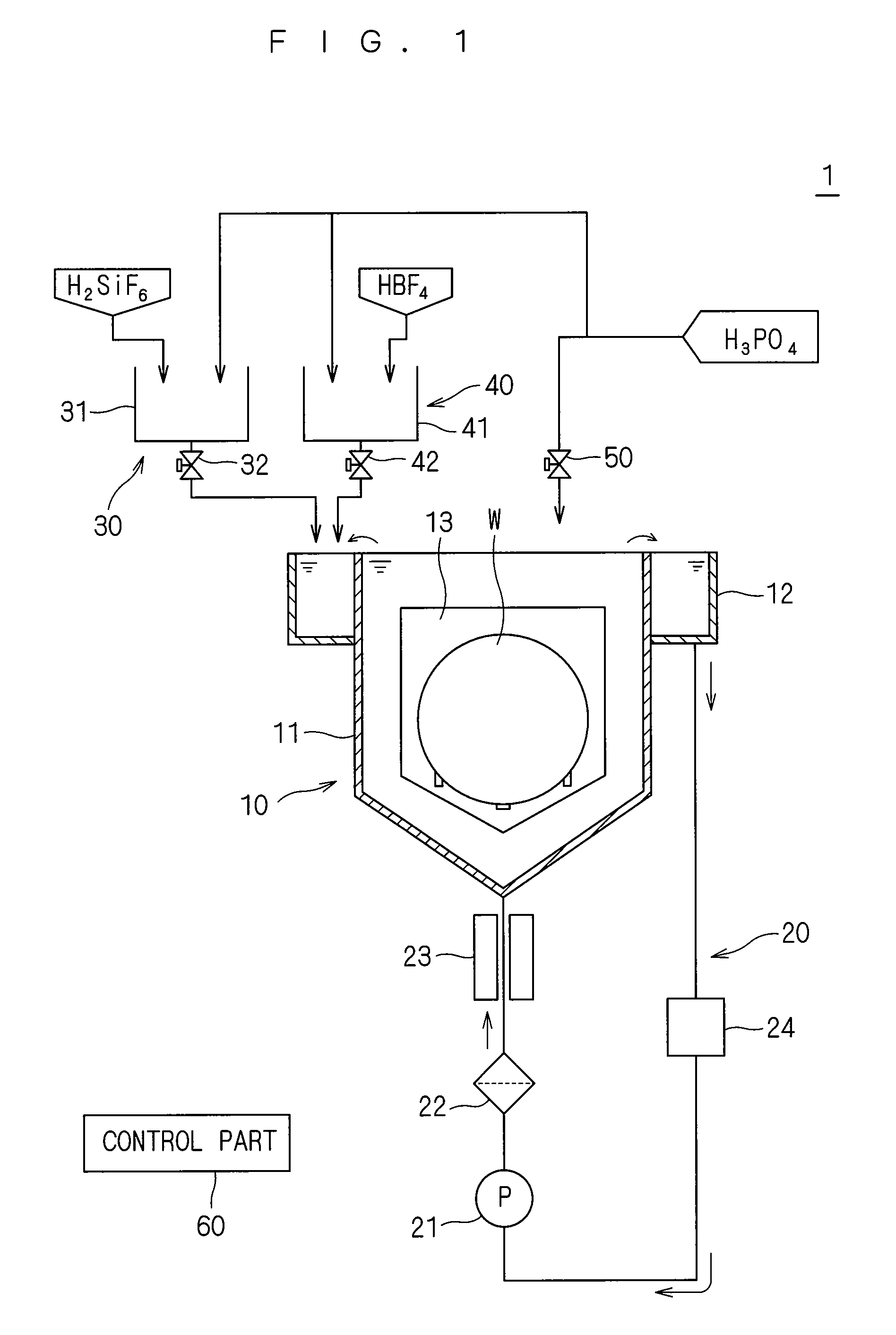
Substrate processing apparatus and substrate processing method for performing etching process with phosphoric acid solution
ActiveUS20090081881A1Constant characteristicConstant solutionDecorative surface effectsSemiconductor/solid-state device manufacturingDecompositionPhosphoric acid
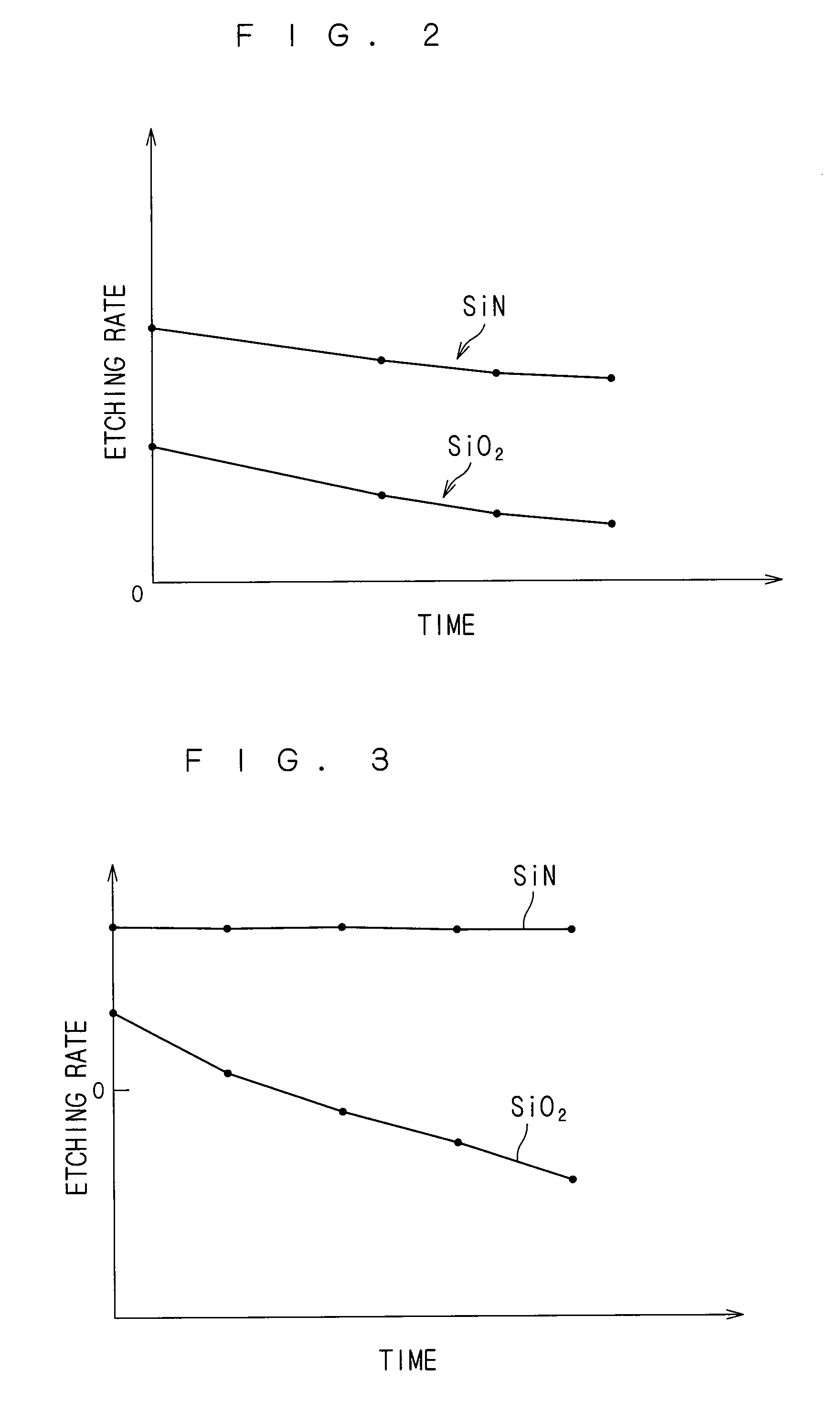
An additive containing a hexafluorosilicic acid solution (H2SiF6+H2O) is sequentially inputted into a phosphoric acid solution pooled in an immersion bath from an additive input mechanism. Further, a trap agent containing a fluoroboric acid solution (HBF4+H2O) is inputted into the phosphoric acid solution from a trap agent input mechanism. F− which accelerates etching of a silicon nitride film is added as appropriate by sequentially inputting the additive and siloxane which increases by the sequential input is etched with hydrofluoric acid generated by decomposition of the fluoroboric acid, to thereby suppress a significant increase in the concentration of siloxane. This makes it possible to maintain respective initial etching rates of the silicon nitride film and a silicon oxide film.
Owner:DAINIPPON SCREEN MTG CO LTD
Coating composition
InactiveUS7063735B2Improve corrosion resistanceAnti-corrosive paintsMetallic material coating processesHafniumFluoroboric acid
A coating composition comprising an aqueous mixture containing inorganic particles, a catechol compound, and one or more fluoroacids. The preferred fluoroacids are selected from fluorotitanic acid, fluorozirconic acid, fluorosilicic acid, fluoroboric acid, fluorostannic acid, fluorogermanic acid, fluorohafnic acid, fluoroaluminic acid or salts of each thereof. The invention is also directed to a coating on a metal substrate. The coating comprises silica particles attached to the metal substrate through a metal-oxide matrix. The metal-oxide matrix comprises a metal selected from titanium, zirconium, silicon, hafnium, boron, aluminum, germanium, or tin, and a catechol compound.
Owner:HENKEL KGAA
Two-photon fluorescent probe capable of targeting mitochondria and preparation method and application of probe
ActiveCN109970630AIntense two-photon excited fluorescenceGood aggregation-induced luminescence effectOrganic chemistryPhotodynamic therapyO-Phosphoric AcidFluorescence
The invention discloses a two-photon fluorescent probe capable of targeting mitochondria and a preparation method and application of the probe. The structure of the two-photon fluorescent probe is shown as a formula I, wherein R represents a conjugated structure, R' represents alkyl or aryl, R'' represents hydrogen, methyl or methoxy, X represents halogen anions or bi-trifluoromethane sulfonimideanions or hexafluoro-phosphoric acid anions or tetra-fluoroboric acid anions or trifluoromethanesulfonic acid anions. The compound has stronger two-photon excitation fluorescence, a good aggregation-induced luminescence effect, a great mitochondrial targeting function and excellent photoinduced singlet oxygen generation capability.
Owner:SICHUAN UNIV
Benzotriazole group-containing ionic liquid and its preparation method and use
InactiveCN102746279AImprove thermal stabilityCorrosion resistanceOrganic chemistryAdditivesImideBenzene
The invention discloses a benzotriazole group-containing ionic liquid and its preparation method and use. The benzotriazole group-containing ionic liquid is characterized in that cations are imidazolium cations; anions are hexafluorophosphate radical, tetrafluoroborate radical or bis(trifluoromethylsulfonyl)imide anions; and an imidazole ring-substituted end group contains a benzotriazole group. The benzotriazole group-containing ionic liquid can be used as a lubricant additive or a lubricating grease additive.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
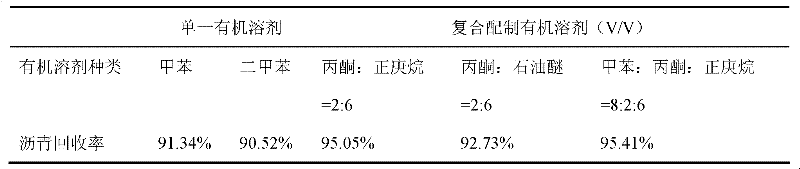
Ionic liquid for assisting oil-sand separation and separation method
ActiveCN102391185ALow viscosityReduce transportation energy consumptionOrganic chemistryLiquid hydrocarbon mixture productionTetrafluoroborateOrganic solvent
The invention relates to ionic liquid for assisting oil-sand separation and a separation method. According to the separation method, the ionic liquid is 1-ethyl-3-methylimidazole tetrafluoroborate; the range of the mass of added ionic liquid is 1-5 times of the mass of oil sand, and the ratio of the volume of an organic solvent to the mass of the oil sand is 1-12 ml / g so as to extract asphalt; the organic solvent and the ionic liquid simultaneously enter an extraction device so as to carry out extraction separation on the oil sand, and the asphalt separation is carried out under a temperature range of 15-60 DEG C; the organic solvent is collected through distilling at a temperature of 70-200 DEG C, and the rest organic product after distilling is an asphalt product; the residual sand and the ionic liquid are subjected to water cleaning by a small amount of water after oil-sand separation, the residual sand product is cleanly discharged; and the ionic liquid and the water can be recycled through fractionation by distillation. The residual sand and the ionic liquid are cleaned by the small amount of water after the oil-sand separation, thus, water is saved, and further, the loss of ionic liquid reagents is little; and in addition, the assisting separation method has high efficiency for the recovery of the asphalt which can reach 95%, and the residual organic matters in the oil sand is little.
Owner:TIANJIN UNIV
Method for preparing potassium trifluoroborate series compounds
InactiveCN102060867ASolve corrosiveAvoid affecting yieldGroup 3/13 element organic compoundsOrganic baseBoronic acid
The invention relates to synthesis of organic compounds, and provides a method for preparing potassium trifluoroborate series compounds. The method comprises the following steps of: adding organic boric acid or organic borate and solvent (THF (tetrahydrofuran), or MTBE (Methyl Tertiary Butyl Ether), or ethyl acetate, or methanol) into a reaction kettle lined with tetrafluoroethylene plastic at room temperature; adding potassium bifluoride and water at normal temperature, stirring for 1 to 12 hours, and reacting to prepare a solid-liquid mixture; adding solid potassium ion containing inorganic or organic alkali into the solid-liquid mixture after the reaction is completed, neutralizing until the pH is between 7 and 9, and continuously stirring for 1 to 5 hours; directly filtering to obtain a solid coarse product after stirring is completed; dissolving the coarse product with solvent, filtering and concentrating, adding nonpolar solvent, and pulping to obtain high-quality RBF3K series compounds. The method is easy to operate, has mild reaction conditions, and can realize scale-up production; and the product prepared by the method has high yield and excellent purity, and the cost is greatly reduced.
Owner:大连联化医药技术有限公司
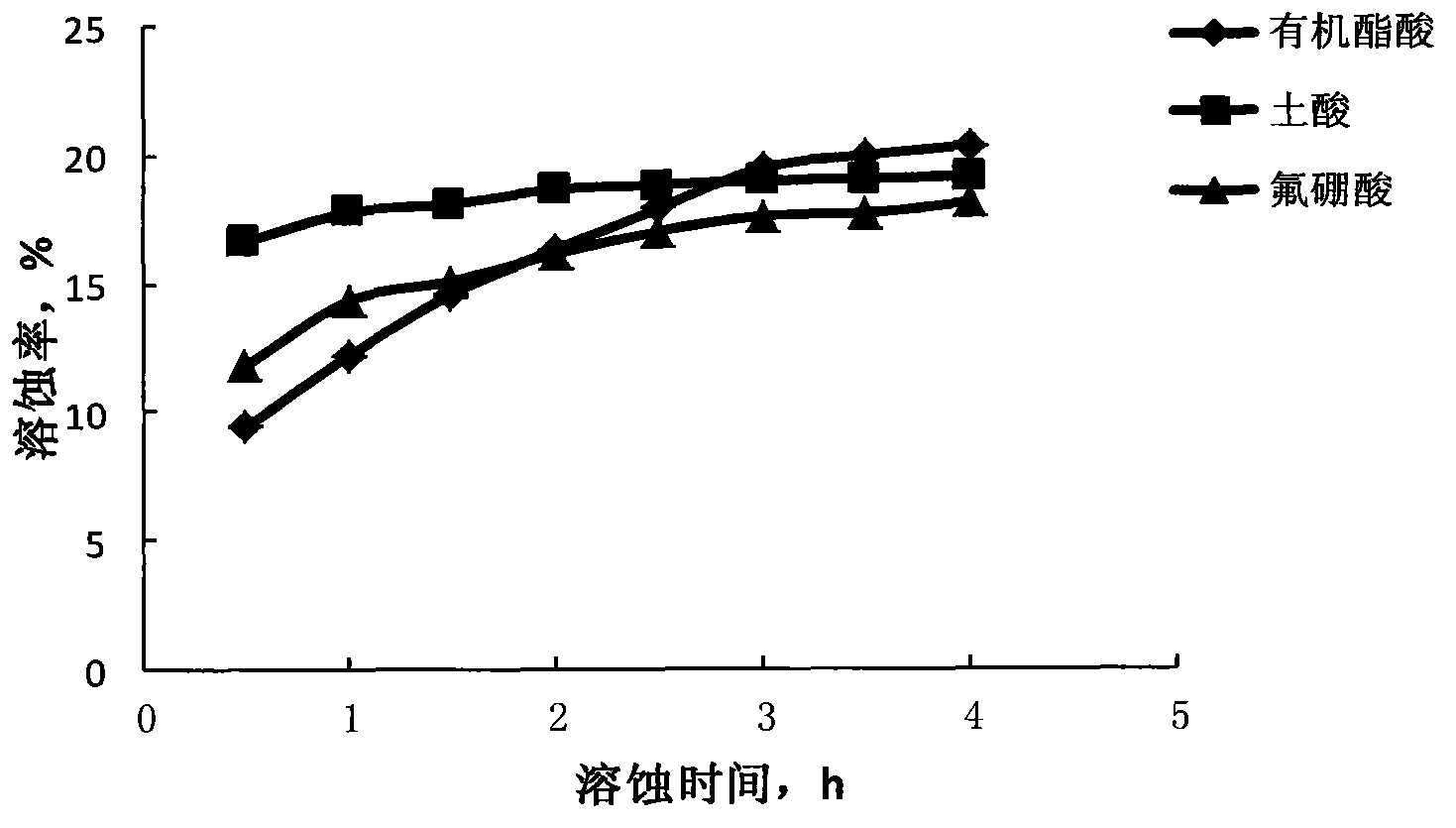
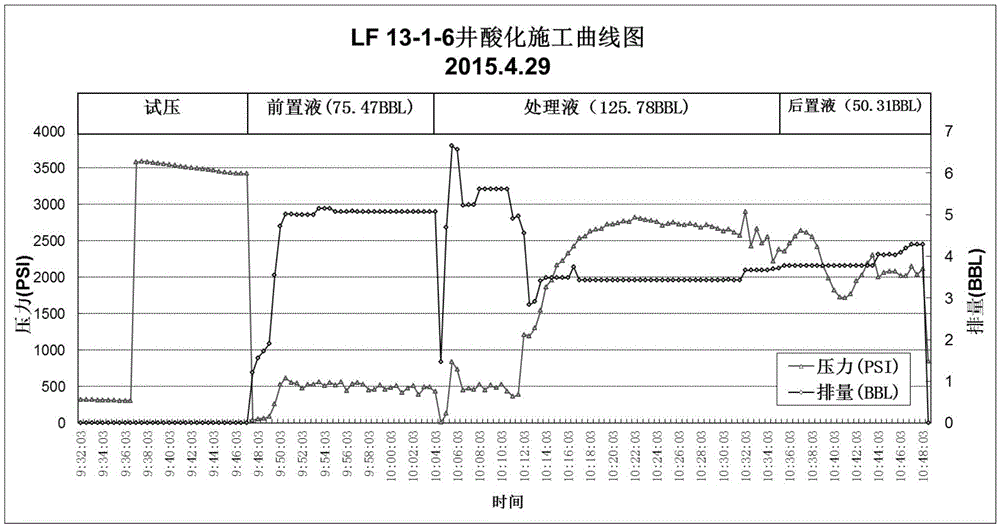
Fluoroboric acid blocking remover and acidification de-blocking method
The invention discloses a fluoroboric acid blocking remover and an acidification de-blocking method. The blocking remover is composed of prepad acid, subject acid and afterpad acid. The prepad acid and the afterpad acid are prepared from the same constituents which include, by mass, 10% of hydrochloric acid, 0.8-1.5% of clay stabilizer, 0.2-1.0% of ferrous stabilizer, 0.25-1.0% of corrosion inhibitor, 0.4-1.0% of water block removal agent, 0.2-1.0% of mutual solvent and the balance water. The subject acid is prepared from, by mass, 6% of acetic acid, 2% of fluoroboric acid, 0.8-1.5% of clay stabilizer, 0.2-1.0% of ferrous stabilizer, 0.25-1.0% of corrosion inhibitor, 0.4-1.0% of water block removal agent, 0.2-1.0% of mutual solvent and the balance water. By the adoption of the fluoroboric acid blocking remover, de-blocking of a low-yield unconventional tight sandstone oil and gas reservoir caused by water blocking reaction, inorganic scale and inflow fluid emulsification can be achieved effectively.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for preparing azo dye with alkalescent arylamine serving as diazotization ingredient
ActiveCN103265820AReduce manufacturing costReduce pollutionMonoazo dyesDisperse dyePolyethylene glycol
The invention provides a method for preparing an azo dye with alkalescent arylamine serving as a diazotization ingredient. The method is characterized in that in a diazotization reaction, ethyl acetate, propyl acetate, butyl acetate, acetone, glycol dimethyl ether or polyethylene glycol 200 is used as a diazotization reaction solvent instead of concentrated sulfuric acid, fluoroboric acid or naphthalene disulfonic acid is used as a diazo salt stabilizer instead of concentrated sulfuric acid, a diazo salt is filtered and separated, filtrate is directly applied to a next diazotization reaction of the alkalescent arylamine, and the diazo salt is applied to a coupling reaction so as to synthesize disperse dyes. Compared with the traditional preparation methods, the method has the advantages that the dosage of the concentrated sulfuric acid can be reduced greatly during the diazotization reaction of the alkalescent arylamine, so that the production cost of the azo dye with the alkalescent arylamine serving as the diazotization ingredient is reduced greatly, the environmental pollution can be reduced from the source, the pollution retreatment of a production end is avoided, and thus, the method has broad application prospects.
Owner:DALIAN UNIV OF TECH
Beryllium fluoroborate non linear optical crystal and its growing method and use
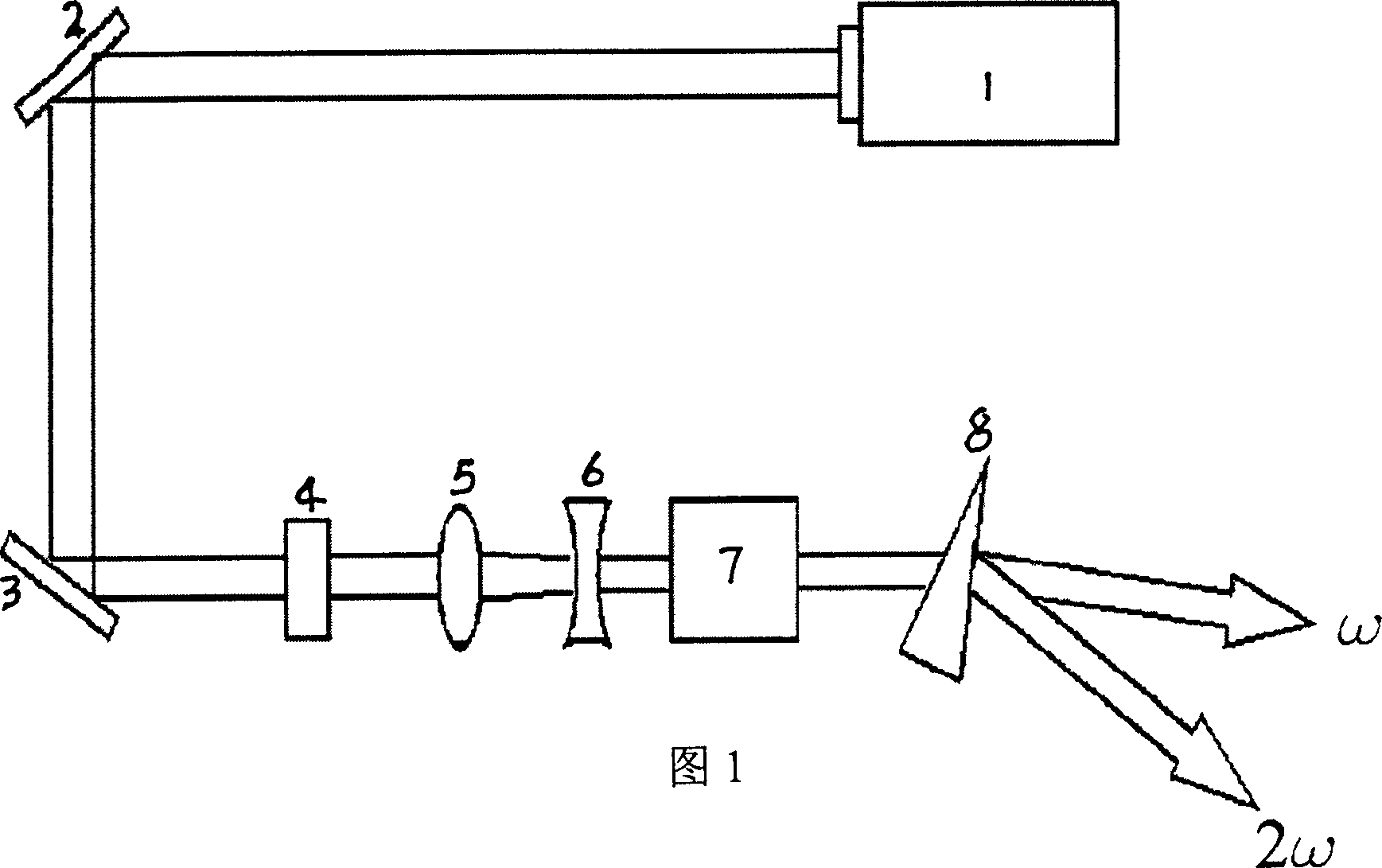
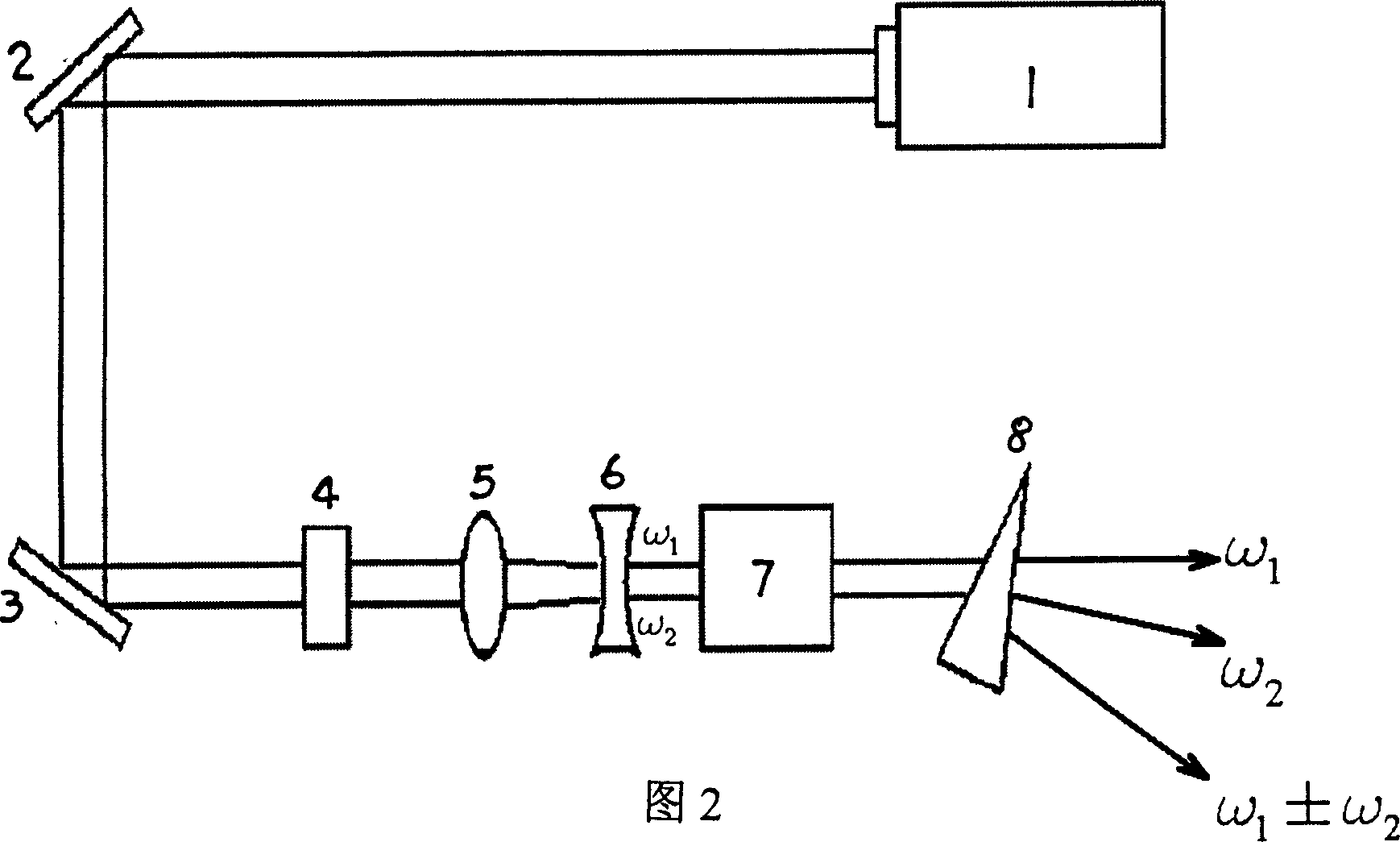
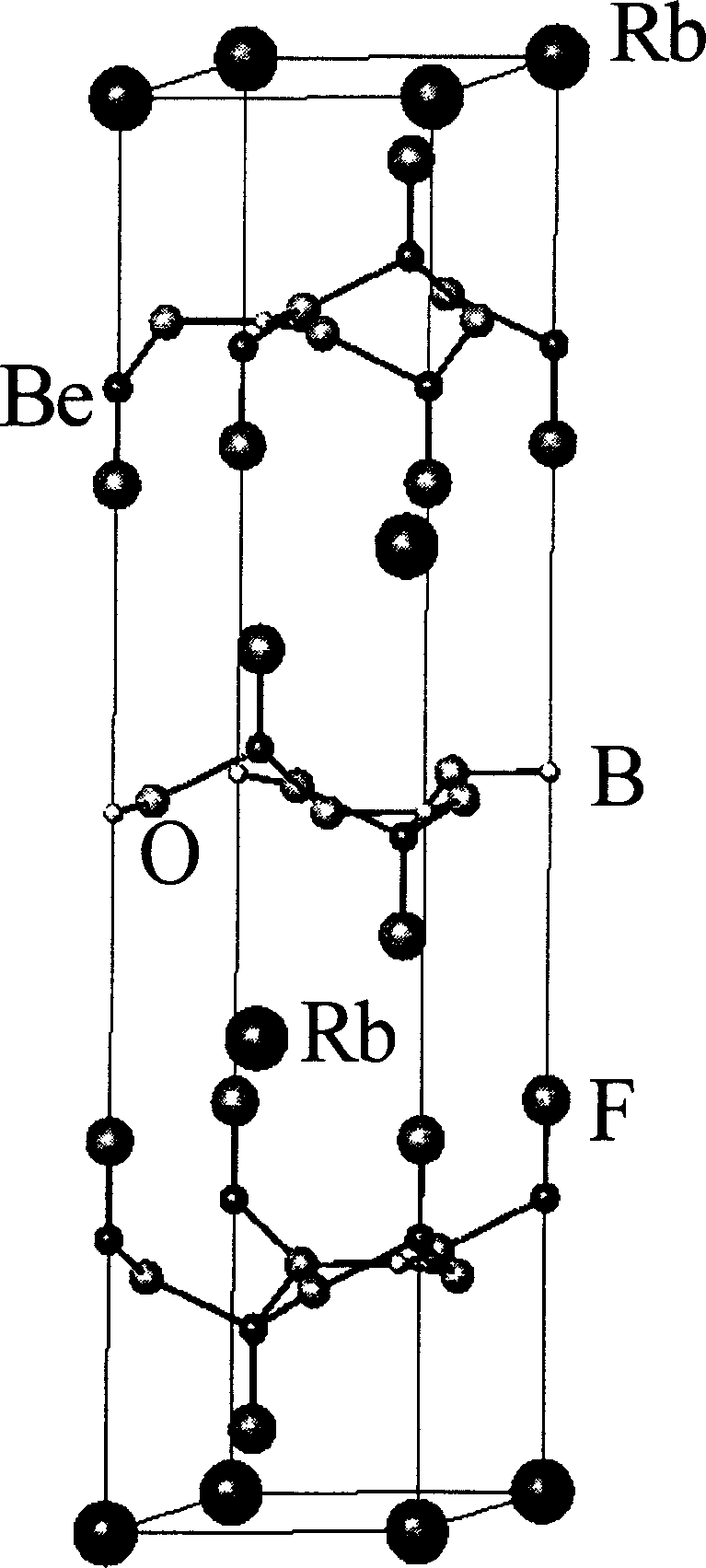
InactiveCN1904148AEasy and fast growing methodPioneering Nonlinear Optics ApplicationsPolycrystalline material growthFrom frozen solutionsWave bandNonlinear optics
This invention relates to a kind of glucinium borofluoride non-linear optic crystal. The molecular formula is MBe2BO3F2,M=Rb or Cs. Applying fused salt growth preparation process: first mix glucinium borofluoride with flux in proportion, to rise temperature to 750-800DEG C, then cool down to 2-10DEG C over saturation temperature; Add seed crystalline in above mentioned high temperature solution, rotate the seed crystalline style, cool down to saturation temperature and then cool it slowly; lift out the crystal from solution and cool down to room temperature to gain the output. The feature of this invention: nonlinear optical effect; wide lucency wave band, ultraviolet cut-off edge reach 150nm, no deliquescence, not dissoluble to dilute hydrochloric acid and nitric acid, good chemical stability, fit for ultraviolet wave band laser frequency conversion need, can be used for making nonlinear optics, can implement N days: double, triple, quadruple, or quintuple frequency multiplication YAG laser output or even lower than 200nm.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Two-dimensional transition metal carbonitride as well as preparation method and application thereof
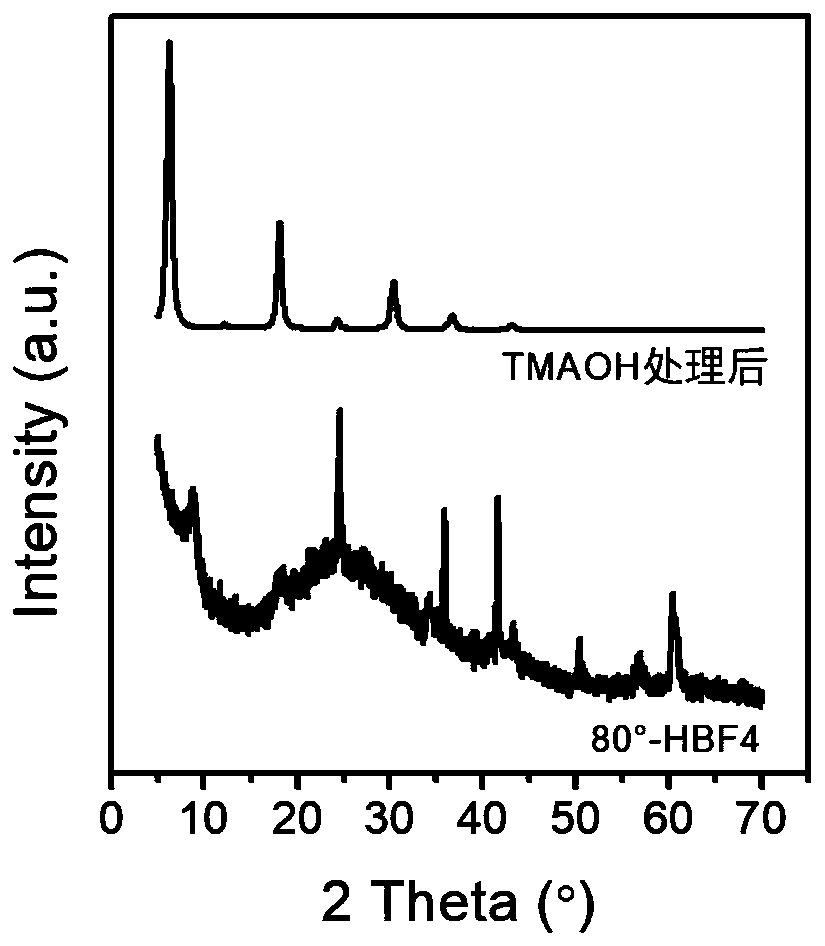
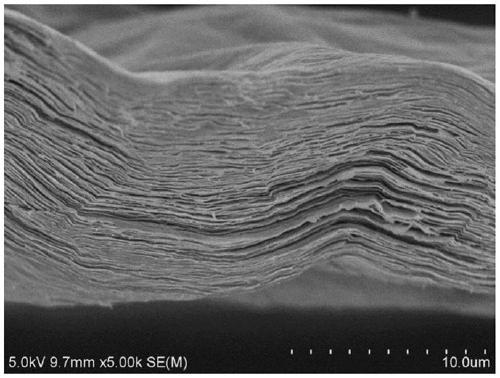
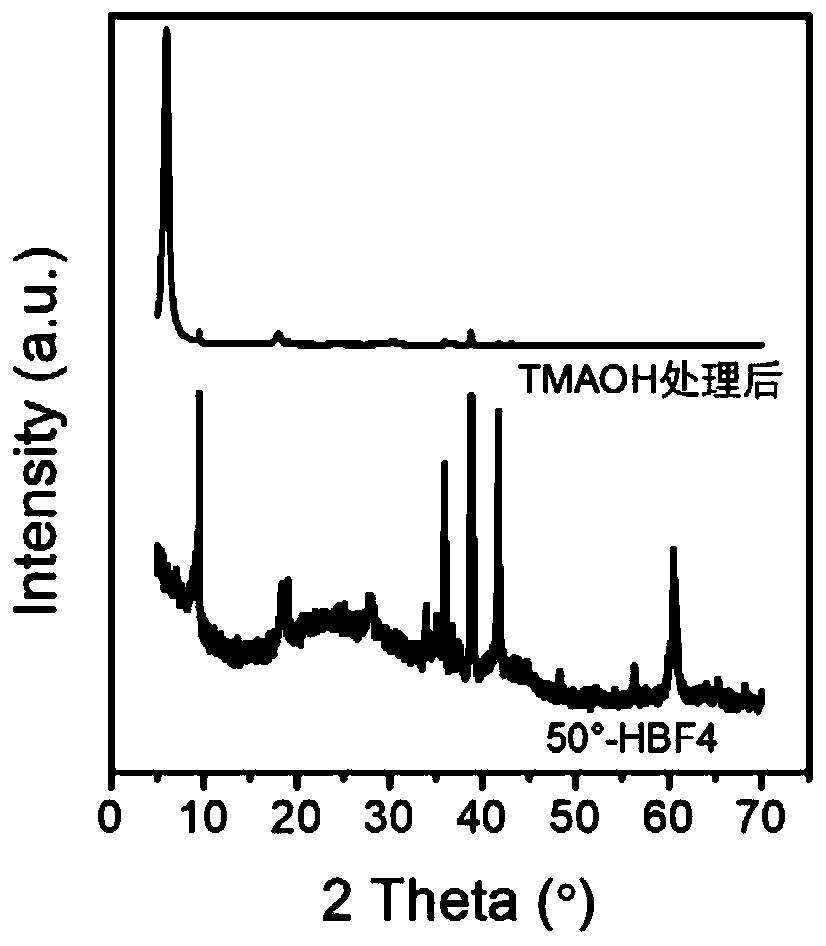
ActiveCN111498850AEasy to filmHigh specific capacitanceNitrogen compoundsTitanium carbideEtchingFluoroboric acid
The invention discloses a two-dimensional transition metal carbonitride and a preparation method and application thereof, and belongs to the technical field of two-dimensional materials. The method comprises the following steps: adding an MAX phase material into a fluoroboric acid aqueous solution for normal-pressure etching; then washing and adding the MXene into an aqueous solution of tetraalkylammonium hydroxide for post-treatment, then washing a treatment product to obtain a two-dimensional transition metal carbonitride (MXene) material; and dispersing the material into water through handcranking, oscillation, stirring, high-speed dispersion or ultrasonic dispersion to form a stable dispersion liquid. According to the method, without use of hydrofluoric acid, the method is safer and more environmentally friendly compared with hydrofluoric acid etching; and the obtained MXene material has good application in the fields of supercapacitors, lithium ion batteries, electromagnetic shielding and electro-catalysis.
Owner:JIANGNAN UNIV
Sand prevention multi-branched polymer for oil-water well and preparation method of sand prevention multi-branched polymer
The invention discloses a sand prevention multi-branched polymer for an oil-water well and a preparation method of the sand prevention multi-branched polymer. The multi-branched polymer is prepared by taking an oxidation-reduction system as an initiator to enable free radical copolymerization of monomer acrylamide or 2-acrylamido-2-methyl propanesulfonate, double-bond organic silane coupling agent and cationic monomer dimethyl diallyl ammonium chloride according to a free radical polymerization principle. The polymer is high in compatibility with systems of hydrochloric acid, ammonium chloride, multi-hydrogen acid, fluoroboric acid, mud acid and the like, thereby allowing addition into sandstone acidizing fluid to reduce after-acidization sand outflow risks so as to realize integration of sand prevention and yield increase. Moreover, due to adoption of a low-temperature aqueous solution free radical polymerization method, high simplicity and convenience in operation are realized.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA)
Retardance and low damage acid solution system for high temperature condensate oil gas reservoir acidification
The present invention discloses a retardance and low damage acid solution system for high temperature condensate oil gas reservoir acidification. The acid solution system is suitable for acidification modification of high temperature deep well, particularly condensate gas reservoir, and comprises the following components, by weight, 0.5-3 parts of hydrofluoric acid or 6-12 parts of fluoroboric acid, 8-10 parts of an organic ester, 0.01-0.05 part of a catalyst, 0.1-1 part of a corrosion inhibitor, 0.1-1 part of an iron ion stabilizer, 0.1-1 part of a clay stabilizer, 1-2 parts of a cleanup additive, and 80-100 parts of water. According to the present invention, effects of retardance and corrosion inhibition of the acid solution system can be well achieved at a high temperature, and water blocking can be released and acid solution backflow can be easily achieved with the finally produced methanol and the carbon dioxide.
Owner:SOUTHWEST PETROLEUM UNIV
Prepn process of 1-fluoronaphthalene
ActiveCN1887833AShort synthetic routeMild conditionsHalogenated hydrocarbon preparationNitriteNitrous acid ester
The preparation process of 1-fluoronaphthalene includes the following steps: (1) reacting 1-naphthylamine with nitrous acid, nitrous acid ester or nitrite in acid medium to obtain diazo salt; (2) reacting the diazo salt with fluoroboric acid or its salt or fluorophosphoric acid or its salt to obtain diazo fluoroborate or diazo fluorophosphate; and (3) heating the diazo fluoroborate or diazo fluorophosphate to decompose to obtain 1-fluoronaphthalene. The process has short synthesis path, less side products, mild reaction condition, easy control, relatively low cost, great production capacity, high product purity and other advantages, and the product is used as medicine intermediate.
Owner:SHANGHAI CHEMSPEC CORP
Fluxing medium growing method for fluoroboric acid calcium non-linear optical crystal
InactiveCN101113531AFast light transmission bandImprove mechanical propertiesPolycrystalline material growthBy pulling from meltNonlinear optical crystalRoom temperature
The invention relates to a preparation method of a calcium fluoborate nonlinear optic crystal; calcium fluoborate and a flux are mixed, and heated to 900 - 1170 DEG C at a heating rate of 20- 100 DEG C / hour, and kept in constant temperature for 5- 50 hours, and then cooled to a temperature 2- 10 DEG C higher than saturate temperature, and a mixing melt of calcium fluoborate and the flux is obtained; the mass ratio between calcium fluoborate and the flux is 1: 0.1-1, and the flux is a LiF-CaO-B2O3 system, wherein the molar ratio of LiF: CaO: B2O3 is 1-0.5 : 0-0.37 : 0-0.2; a crystallon arranged on a crystallon rod is dropped to the mixing melt prepared through the steps and cooled to saturate temperature at the same time, the crystallon rod is rotated at a rotating rate of 5-50 revolutions / minute, then the temperature is slowly reduced at a rate of 0.2-3 DEG C / day, the obtained crystal is lifted from the liquid surface and cooled to room temperature at a cooling rate of 5- 100 DEG C / hour, and the obtained calcium fluoborate is a nonlinear optic crystal. The method has the advantages of simple operation, fast growing speed, easy growing and low cost.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Electrolytic solution for electric double layer capacitor, electric double layer capacitor using the same, and manufacturing method therefor
InactiveUS20120044614A1Reduce functionImprove leakageHybrid capacitor electrolytesHybrid capacitor electrodesNonaneSulfolane
Provided are an electrolytic solution for an electric double layer capacitor capable of providing an electric double layer capacitor having stable quality, an electric double layer capacitor using the electrolytic solution, and a manufacturing method for the electric double layer capacitor. The electrolytic solution includes a supporting electrolyte, sulfolane, and a linear sulfone. It is preferred that the electrolytic solution further include an organic fluorine compound. Further, it is preferred that the supporting electrolyte contain 5-azoniaspiro[4.4]nonane tetrafluoroborate, and the content of 5-azoniaspiro[4.4]nonane tetrafluoroborate be 1.5 to 3.6 mol / dm3.
Owner:SEIKO INSTR INC
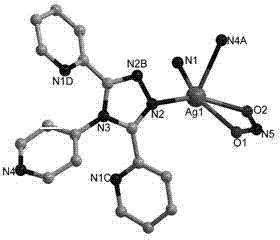
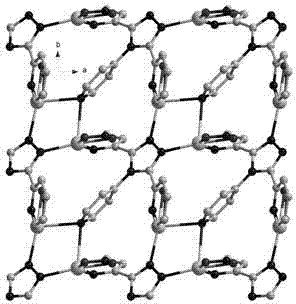
Metallic silver coordination polymer with two-dimensional lamellar structure, and preparation and application thereof
InactiveCN102516270AWith selective switchingEasy to manufactureAnion exchanger materialsSilver organic compoundsNitrite ionBenzoic acid
The invention relates to a 3,5-di(2-pyridyl)-4-(4-pyridyl)-1,2,4-triazolyl-silver (I) coordination polymer, and a preparation and application thereof. The compound is synthesized by the following steps: under normal temperature and pressure, mixing AgNO2 acetonitrile solution and ligand L chloroform solution, and keeping stirring for half an hour, and standing in a dark place to volatilize for about one week, thereby obtaining the colorless lumpy monocrystal product. The invention has the advantages of simple preparation technique, short reaction time, easy after-treatment and high yield. The experiment proves that nitrite ions in the material can selectively react with tetrafluoroborate ions, hexafluorosilicate ions, nitrate ions and perchlorate in an anion exchange mode, and benzoate ions and acetate ions can not perform similar anion exchange reaction. The material overcomes the limitation to the existing anion exchange material, the exchange process is simple and easy to implement, and thus, the invention is hopeful to practical application in the field of ion exchange materials.
Owner:TIANJIN NORMAL UNIVERSITY
Low-temperature electrolyte for supercapacitor and preparation method thereof
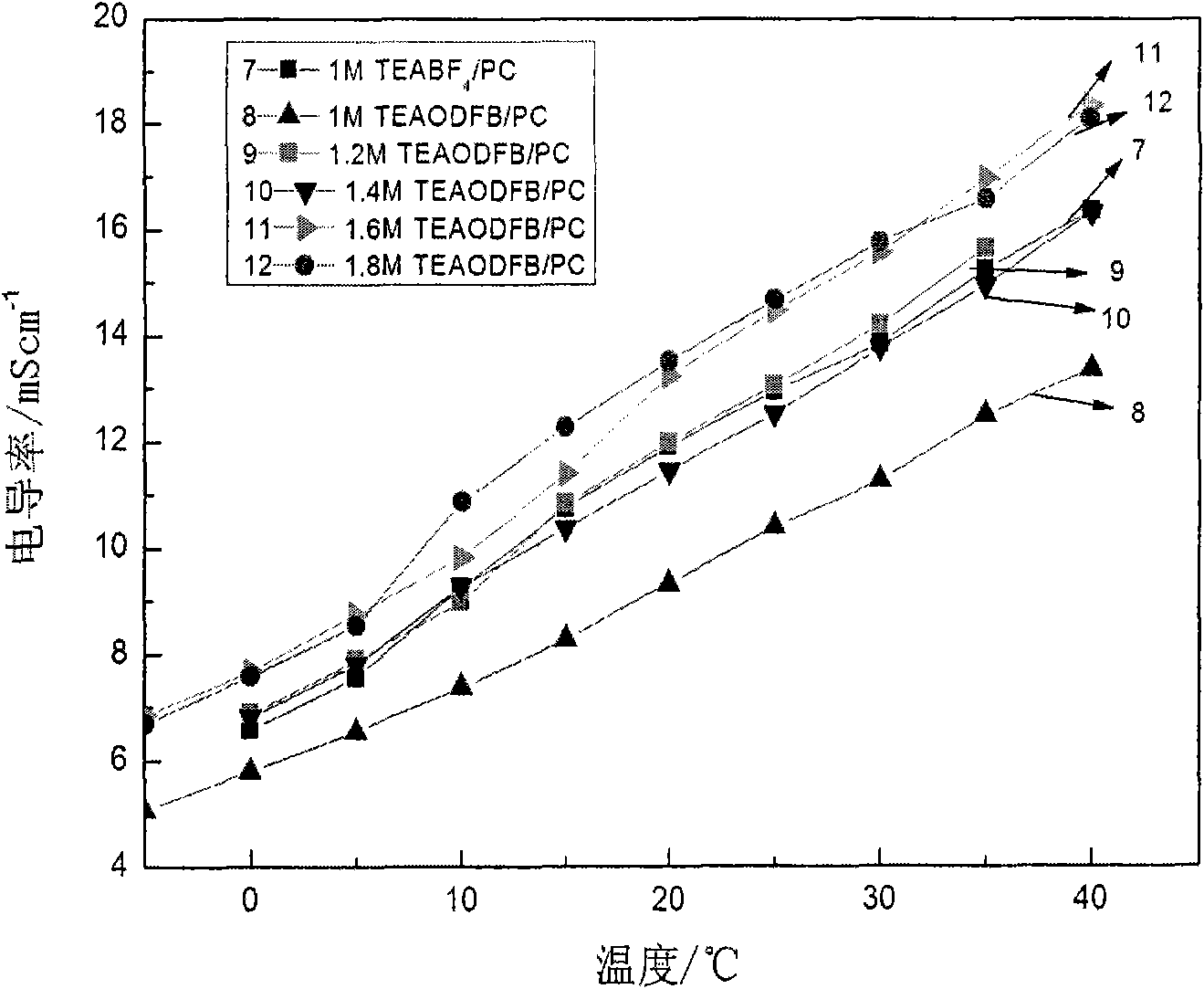
InactiveCN101593625ALow melting pointImprove solubilityElectrolytic capacitorsSulfolanePropionitrile
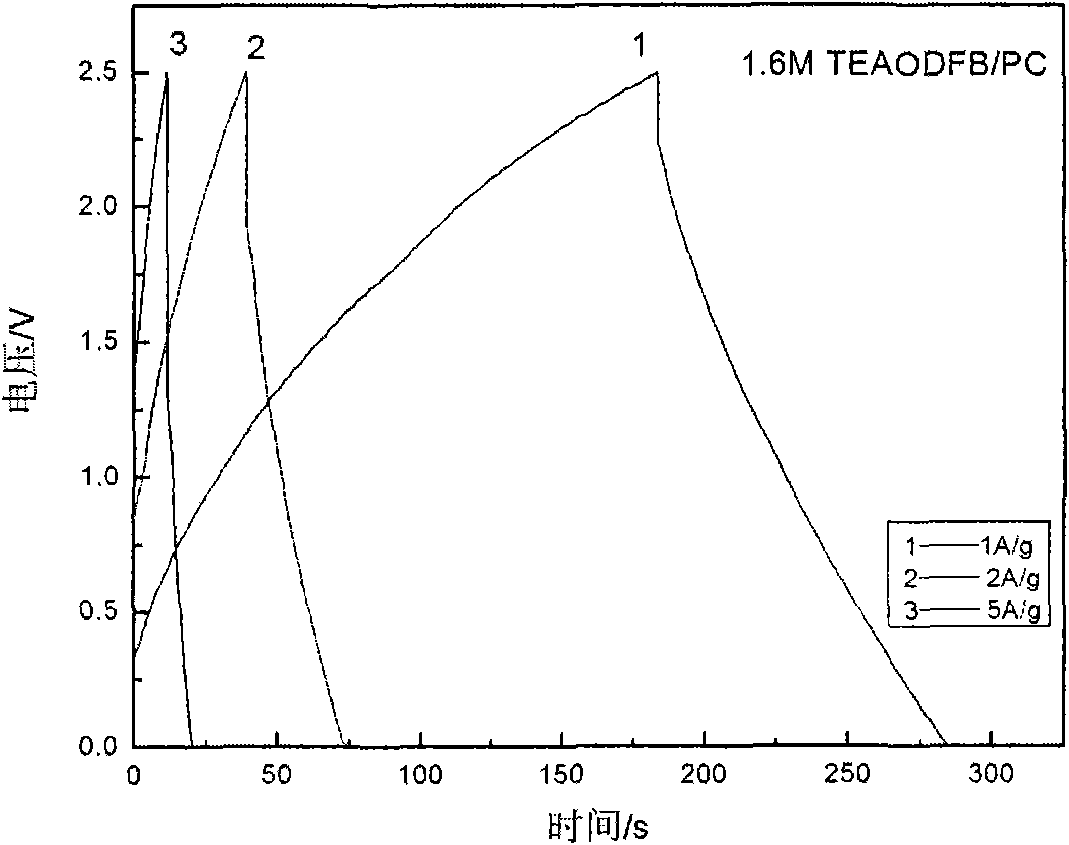
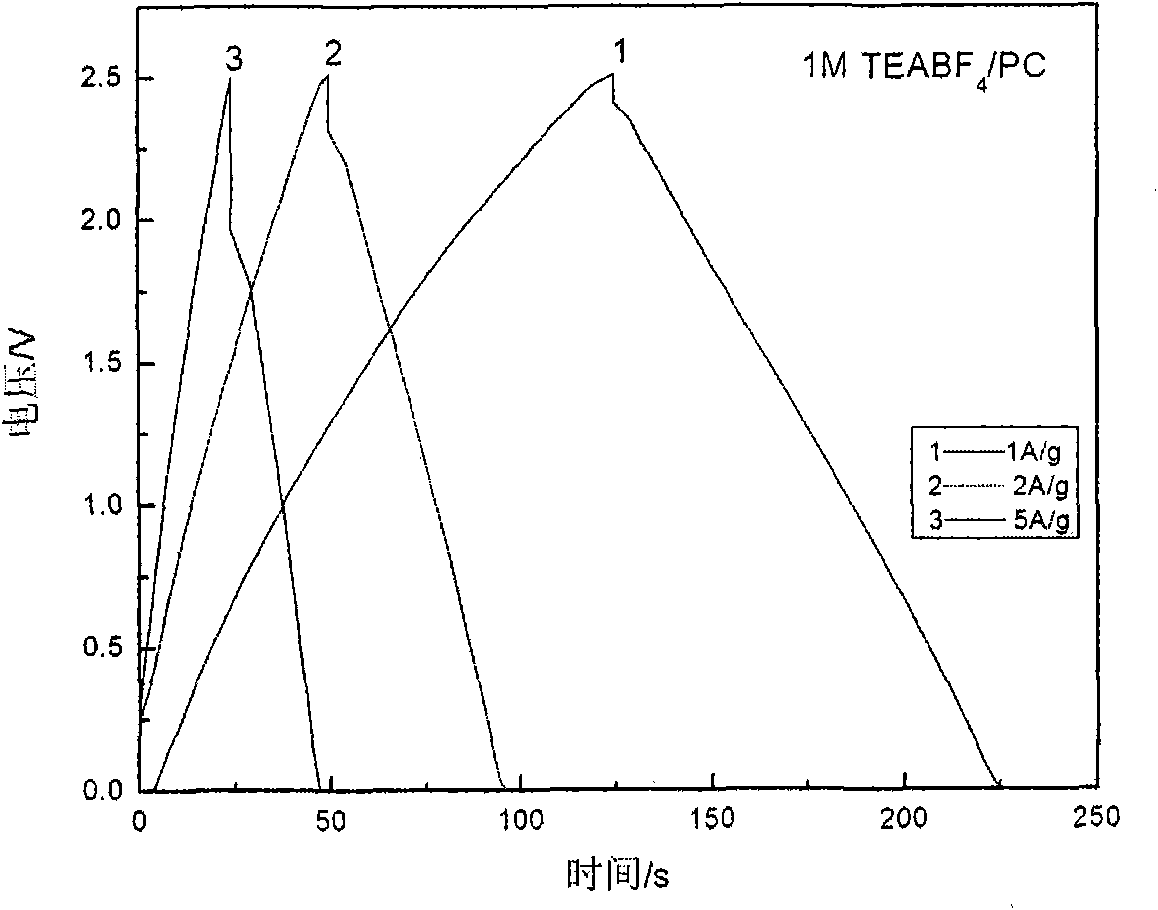
The invention discloses low-temperature electrolyte for a supercapacitor and a preparation method thereof. The electrolyte comprises solute and non-aqueous organic solvent. The solute is ionic liquid tetraethylammonium-oxalate-difluoro-borate (TEAODFB) which is battery-grade TEAODFB obtained by purifying a product of reaction of a chlorine-containing compound, an oxalate-containing compound and a fluoroboric acid containing compound in acetonitrile or carbonic ester medium by adopting a method of reduced pressure evaporation or low-temperature recrystallization. The non-aqueous organic solvent is one or the combination of acetonitrile, propionitrile, methoxy propionitrile, ethylene carbonate, propylene carbonate, dimethyl carbonate, diethyl carbonate, methyl ethyl carbonate, gamma-butyrolactone, N,N-dimetbylformamide, tetrahydrofuran and sulfolane. The concentration of the adopted electrolyte is 0.8 to 2mol / L. The obtained low-temperature electrolyte has high specific capacity and charge / discharge cyclic life at the temperature of 30 DEG C below zero.
Owner:CENT SOUTH UNIV +1
Oil and water well polyacid blocking remover
InactiveCN105482801AFix damagePrevent contamination from cloggingDrilling compositionCalcium in biologySilicic acid
The invention relates to an oil and water well polyacid blocking remover. The blocking remover comprises 10-15% of hydrochloric acid, 6-8% of fluoroboric acid, 12-15% of modified silicic acid, 1-2% of anti-swelling agent, 1-2% of an iron ion stabilizer, 1-2% of a demulsifying cleanup additive, 1-2% of a water damage treatment agent, 2-4% of a corrosion inhibitor, 6-8% of a precipitation inhibitor, and the balance of water. Inorganic acids in the blocking remover are hydrochloric acid and fluoroboric acid, and hydrochloric acid can corrode the calcareous components of reservoir; and fluoroboric acid can corrode solid phase components, and has a loose sandstone bonding effect. An organic acid in the blocking remover is modified silicic acid, and can solve organic obstruction in a drilling fluid. The blocking remover can solve the problems of loose sandstone reservoir mud and drilling fluid pollution and obstruction and low corrosion rate of low-permeability reservoir acid solution blocking removers of present oil wells, and also has a low skeleton corrosion effect.
Owner:CHINA NAT OFFSHORE OIL CORP +1
Tetraethyl tetrafluo ammonium fluoroborate preparation method
The present invention relates to the preparation process of tetraethyl ammonium tetrafluoroborate as electronic level reagent in producing electronic elements. The preparation process includes recrystallization to obtain refined boric acid, preparing fluoroboric acid with hydrofluoric acid and boric acid in the equal molar ratio, exchange reaction of tetraethyl ammonium halide and fluoroboric acid at 10-50 deg.c and alcohol in the presence of organic solvent, eliminating hydrogen halide at 30-90 deg.c and reduced pressure condition, recrystallization and low temperature decompression drying to obtain ultimate product tetraethyl ammonium tetrafluoroborate. The ultimate product reaches the quality requirement of: purity not lower than 99.5 %, water content not more than 10 ppm, Fe not more than 1.0 ppm, Si not more than 3.0 ppm, Na not more than 3.0 ppm, and K not more than 3.0 ppm.
Owner:陈耀华 +2
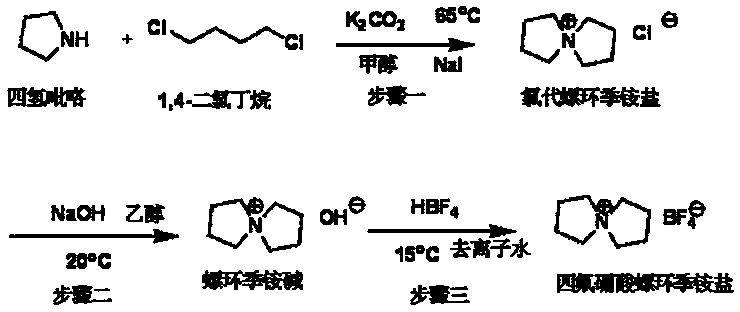
Synthetic method for spiro quaternary ammonium tetrafluoroborate
InactiveCN104277045AReduce pollutionSuitable for industrial scale productionOrganic chemistryTetrafluoroborateSodium iodide
The invention discloses a rapid simple efficient method for synthesizing a high-purity spiro quaternary ammonium tetrafluoroborate. The method comprises the following steps: step 1, adding a carbonate, 1,4-dichlorobutane, tetrahydro pyrrole, sodium iodide and a methanol solvent into a reaction kettle to perform reaction, so as to obtain a chloro spiro quaternary ammonium salt; step 2, dissolving the chloro spiro quaternary ammonium salt into ethanol in a reaction kettle, then adding sodium hydroxide to perform ion exchange, and evaporating the solvent to finally obtain a spiro quaternary ammonium base; and step 3, dissolving the spiro quaternary ammonium base into deionized water in a reaction kettle, then adding fluorboric acid for neutralization, then adding active carbon for refluxing decoloring, and then performing pumping filtration, evaporation condensation and drying, so as to finally obtain the high-purity spiro quaternary ammonium tetrafluoroborate with extremely low water content. The step 1 also can be performed as following: adding the raw materials into a ball milling tank for ball milling reaction, so as to obtain the chloro spiro quaternary ammonium salt. The provided synthetic method for the spiro quaternary ammonium tetrafluoroborate is relatively suitable for industrial large-scale production.
Owner:HUNAN ZHENGYUAN ENERGY STORAGE MATERIALS & DEVICE INST
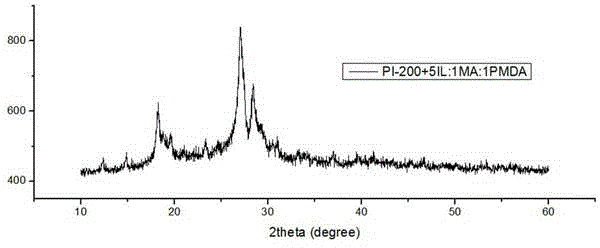
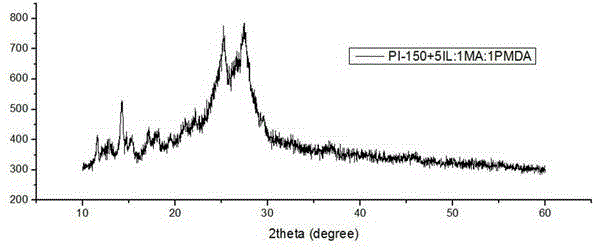
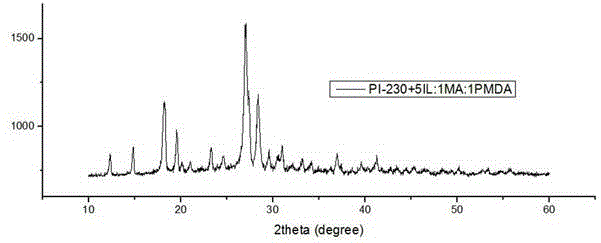
Method for preparing polyimide by utilizing ionic liquid and application of polyimide prepared by method
ActiveCN103819672AReduce manufacturing costHas visible light absorption propertiesWater/sewage treatment by irradiationOrganic-compounds/hydrides/coordination-complexes catalystsTetrafluoroborateSolar light
The invention discloses a method for preparing polyimide (PI) by utilizing ionic liquid. According to the method, the PI is obtained by performing polymerization on two organic monomers in an ionic liquid solution at the polymerization temperature of 100 DEG C to 300 DEG C, wherein in the two monomers, the first monomer is aromatic amine or heterocyclic amine containing three amino function groups and the second monomer is aromatic anhydride or heterocyclic anhydride containing an anhydride functional group; the molar ratio of the first monomer to the second monomer is (0.2-5):1; the ionic liquid is imidazole tetrafluoroborate or tetrabutyl phosphorus imidazolium salt; the proportion relationship between the ionic liquid and the sum of the monomers is that the molar ratio of the ionic liquid to the monomers is (1.6-5):1. By the method, an organic photocatalytic material with a visible light absorption property is obtained through thermal polymerization of simple specific monomers, water can be decomposed by solar light to produce hydrogen, and a wide application prospect is achieved.
Owner:NANJING UNIV +1
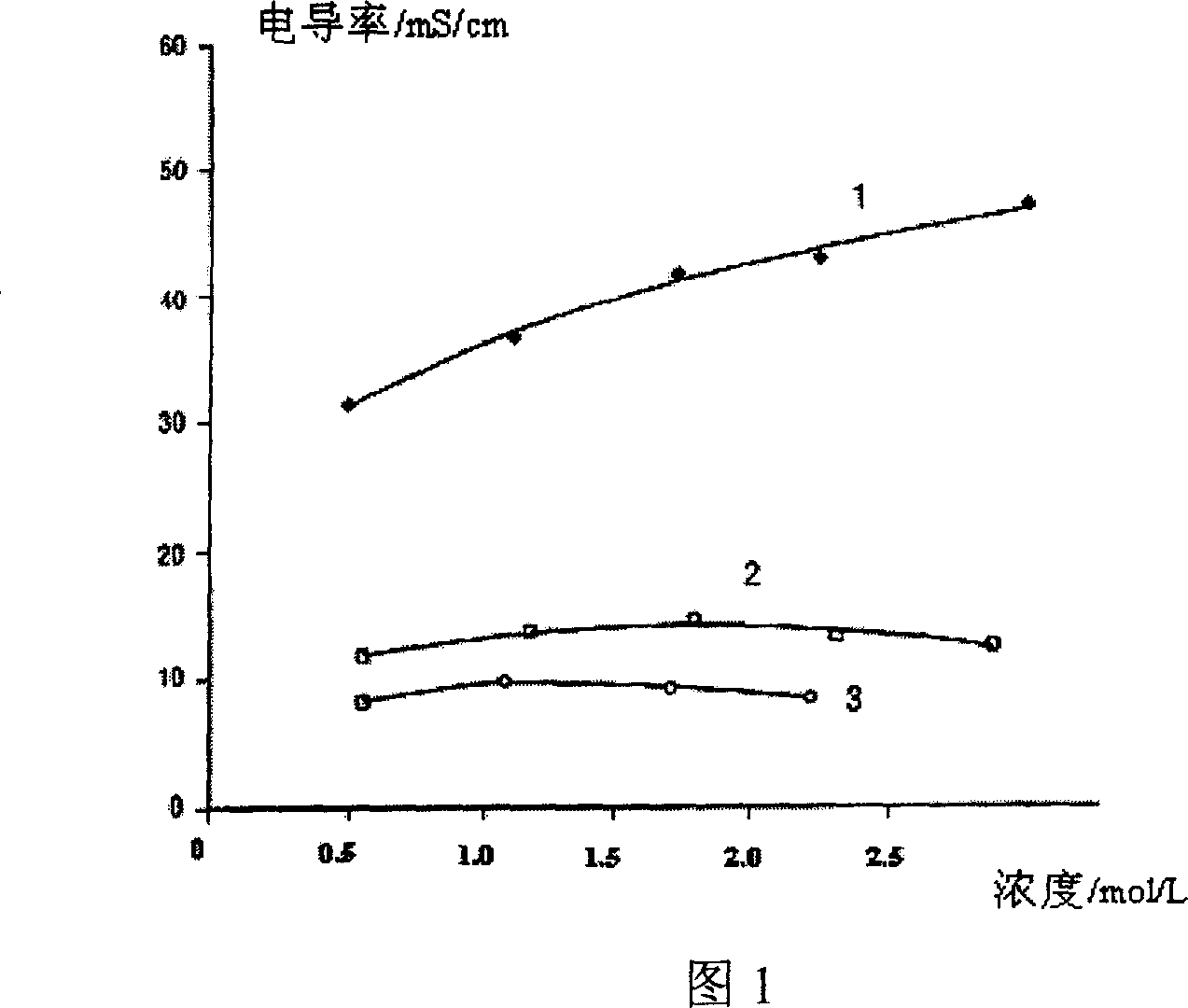
High temperature electrolyte for super capacitor
The invention relates to a super-capacitor high-temperature electrolyte. Wherein, its solute is N-trialkyl-N-alkoxy acyl fluoroboric acid or phosphorofluoric acid or Fraude's reagent, or trifluoromethyl sulfonic acid; and the solvent is aprotic solvents; its density in room temperature is 0.8-2.0mol / l; the aprotic solvents are acetonitrile, ethyl cyanide, methacrylonitrile, gamma- butyrolactone, gamma- valerolactone, vinylene carbonate, propylene carbonate, N, N- dimethyl formamide, 1- dimethyl formamide-2- pyrrolidone, methylenedioxy ethane, 2-methoxy ether, tetrahydrofuran, dioxolane, dimethyl carbonate, diethyl carbonate, diethyl carbonate, or dimethyl sulfoxide. And the inventive capacitor has high capacity and high service life at 85Deg. C.
Owner:锦州凯美能源有限公司
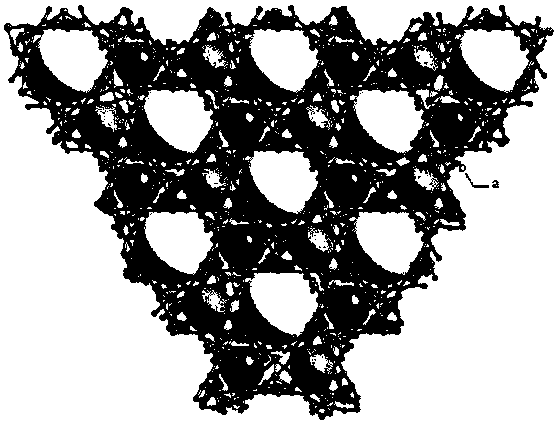
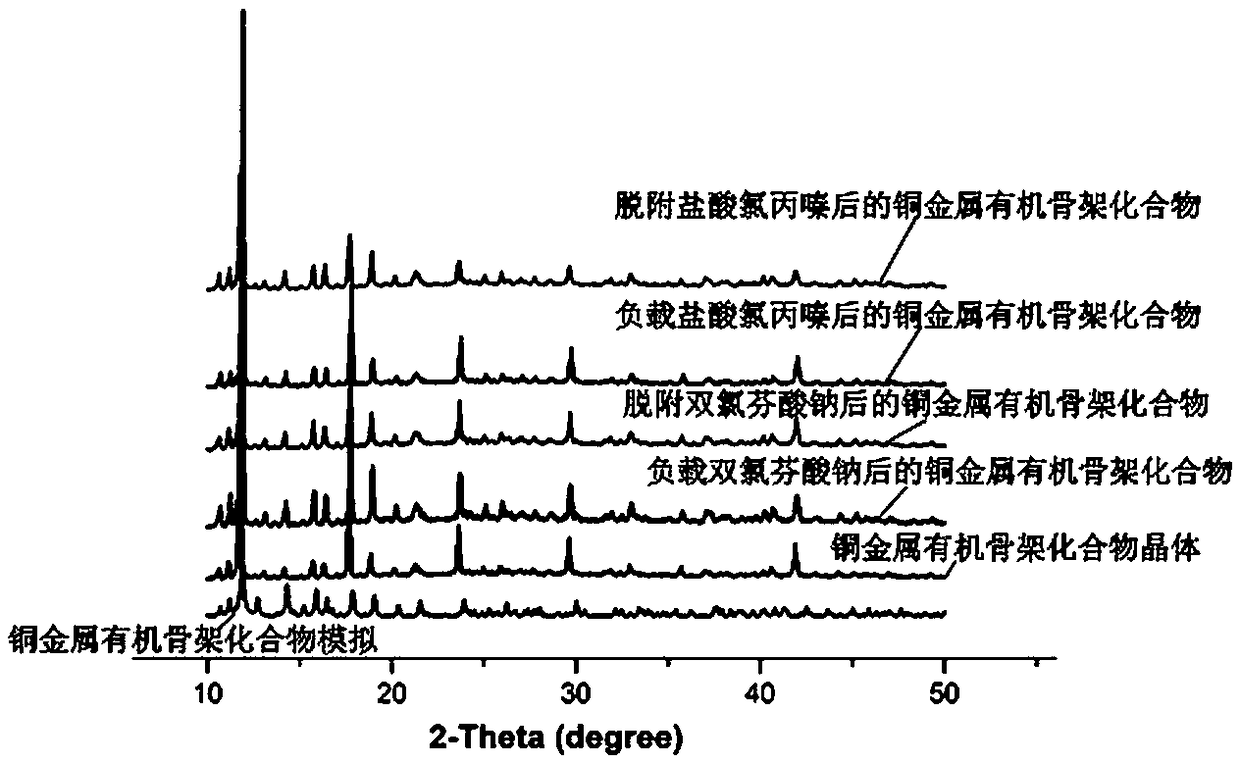
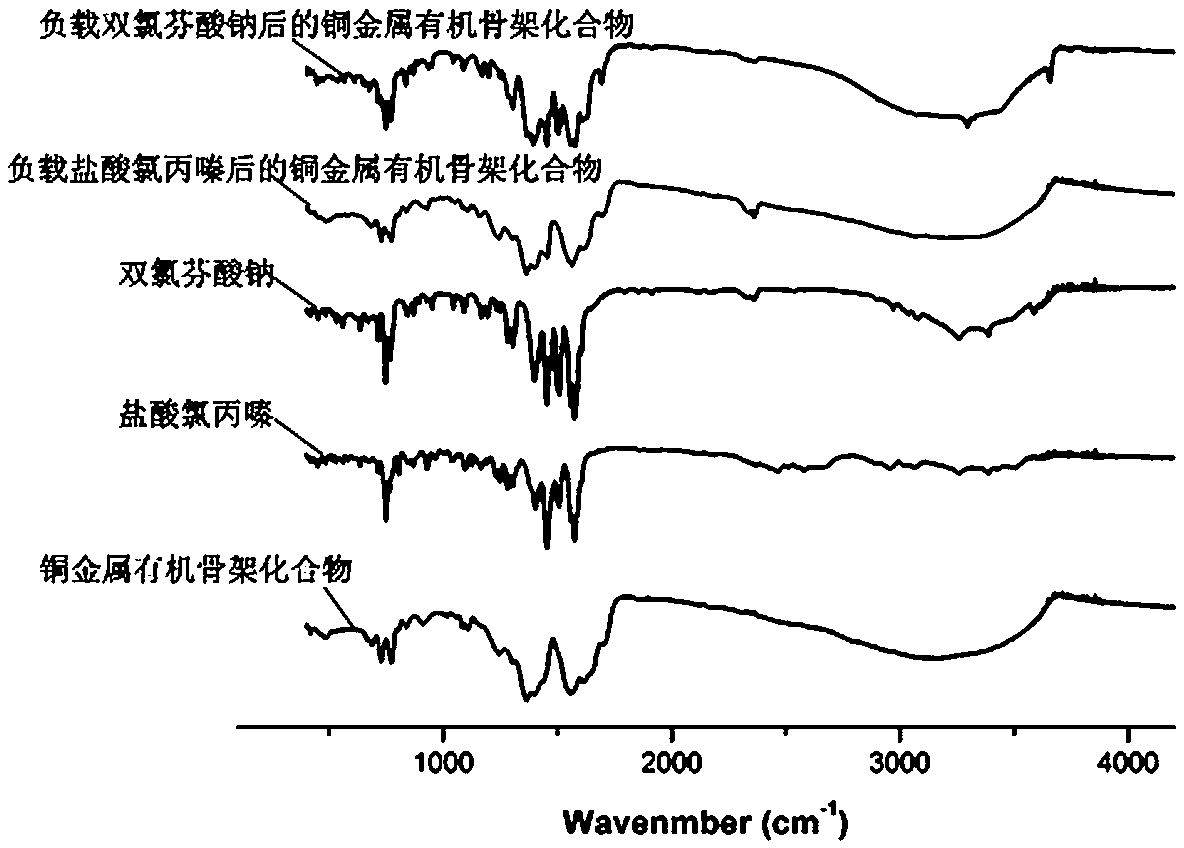
Preparation method and application of copper metal organic framework compound
ActiveCN108421531ASimple manufacturing methodHigh yieldOther chemical processesWater contaminantsBenzoic acidDesorption
The invention discloses a preparation method and application of a copper metal organic framework compound. The preparation method comprises the steps of: 1) mixing copper nitrate with 2, 5-bis(3', 5'-dicarboxyphenyl)-benzoic acid, then adding a solvent and performing stirring, then adding fluoroboric acid and performing stirring to obtain a mixed solution A; 2) placing a glass bottle with the sealed mixed solution A obtained in step 1) at 85DEG C-95DEG C for reaction for 8-12h to obtain a crystal B; 3) cleaning the crystal B obtained in step 2) with ethanol three times, then soaking the crystal B in ethanol, and finally drying the crystal in an oven, thus obtaining the copper metal organic framework compound. The preparation method provided by the invention is simple and high in yield. Thecompound prepared by the method provided by the invention has good stability to water and good adsorption ability to drug pollutants, and also has certain desorption ability and recycling ability, and therefore has potential application in adsorption of drug residue in environmental water.
Owner:GUANGDONG MEDICAL UNIV
Preparation method of rhodium compounds
InactiveCN105585596AEasy to operateHigh purityRhodium organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsTetrafluoroborateDimmer
The invention discloses a preparation method of rhodium compounds. According to the method, bis(1.5-cyclooctadiene) rhodium(I) tetrafluoroborate compounds are synthesized in two steps, firstly, 1.5-cyclooctadiene rhodium chloride dimmers are synthesized with rhodium chloride trihydrate as raw materials; secondly, the bis(1.5-cyclooctadiene) rhodium(I) tetrafluoroborate compounds are synthesized with the 1.5-cyclooctadiene rhodium chloride dimmers as raw materials. The one-way total yield is 93% or above, operation is easy, industrial production can be achieved, and certain economic benefits are achieved.
Owner:JIANGXI HANS PRECIOUS METALS CO LTD
Ionic liquid with high electrochemical stability and preparing method thereof
InactiveCN101210000ALower melting temperatureImprove electrochemical stabilityOrganic chemistrySulfate radicalsOrganic synthesis
The invention discloses a functional ionic liquid that has high electrochemical stability and a preparation method thereof. Positive ion of the ionic liquid is selected from nitrile-functionalized quaternary ammonium positive ion and negative ion thereof from one of chlorine, bromine, iodine, fluoborate, fluorophosphates, sulfate radical, nitrate radical, trifluoroacetic radical, trifluorochloromethane sulfonic acid group, bi (perfluoroalkyl group sulfonyl) imino negative ion, dinitrile amic radical and saccharin acid radical. Nitrile functional ionic liquid, the negative ion of which is halogen negative ion, is firstly prepared through reaction of pentagons or hexatomic tertiary amine with the alkyl halide that has nitrile functional groups, then ionic liquid that contains different negative ions is prepared by exchange of the negative ions. The ionic liquid has very low melting temperature, can bear the high temperature of 150 DEG C and be stable to water, hardly has vapor pressure but has good electrochemical stability, thereby the invention can be taken as solvent, catalyst ligand, electrochemical electrolyte, etc. that are applied in the fields of organic synthesis, catalytic reaction and lithium-ion battery, etc.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Ammonium fluoroborate modified Y-type molecular sieve and preparation method thereof
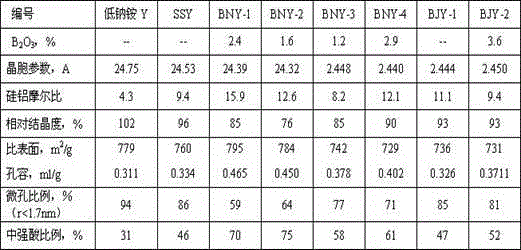
ActiveCN104556124AMolecular sieve catalystsFaujasite aluminosilicate zeoliteMolecular sievePhysical chemistry
The invention discloses a preparation method for a modified Y-type molecular sieve. The preparation method comprises the following steps: preparing an ammonium fluoroborate solution; (2) putting an industrially produced Y molecular sieve in the ammonium fluoroborate solution in the step (1), carrying out an aluminization removal and boron supplement reaction in the slurry system: NH4BF4+Al<3+>+Na<+>=NaAlF4 (a large-particle precipitate)+B<3+>+NH4<+>, and carrying out solid-liquid separation and drying after the reaction; (3) adding the molecular sieve dried in the step (2) into an alkaline solution to be pulped, and separating, washing and drying after the pulping to prepare the modified Y-type molecular sieve. The invention provides a modification method for carrying out aluminization removal and boron supplement on the Y-type molecular sieve by a combination of a boron-containing compound and the alkaline solution, so that a boron-containing modified Y-type molecular sieve which is medium / high in strong acid content is prepared.
Owner:CHINA PETROLEUM & CHEM CORP +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com