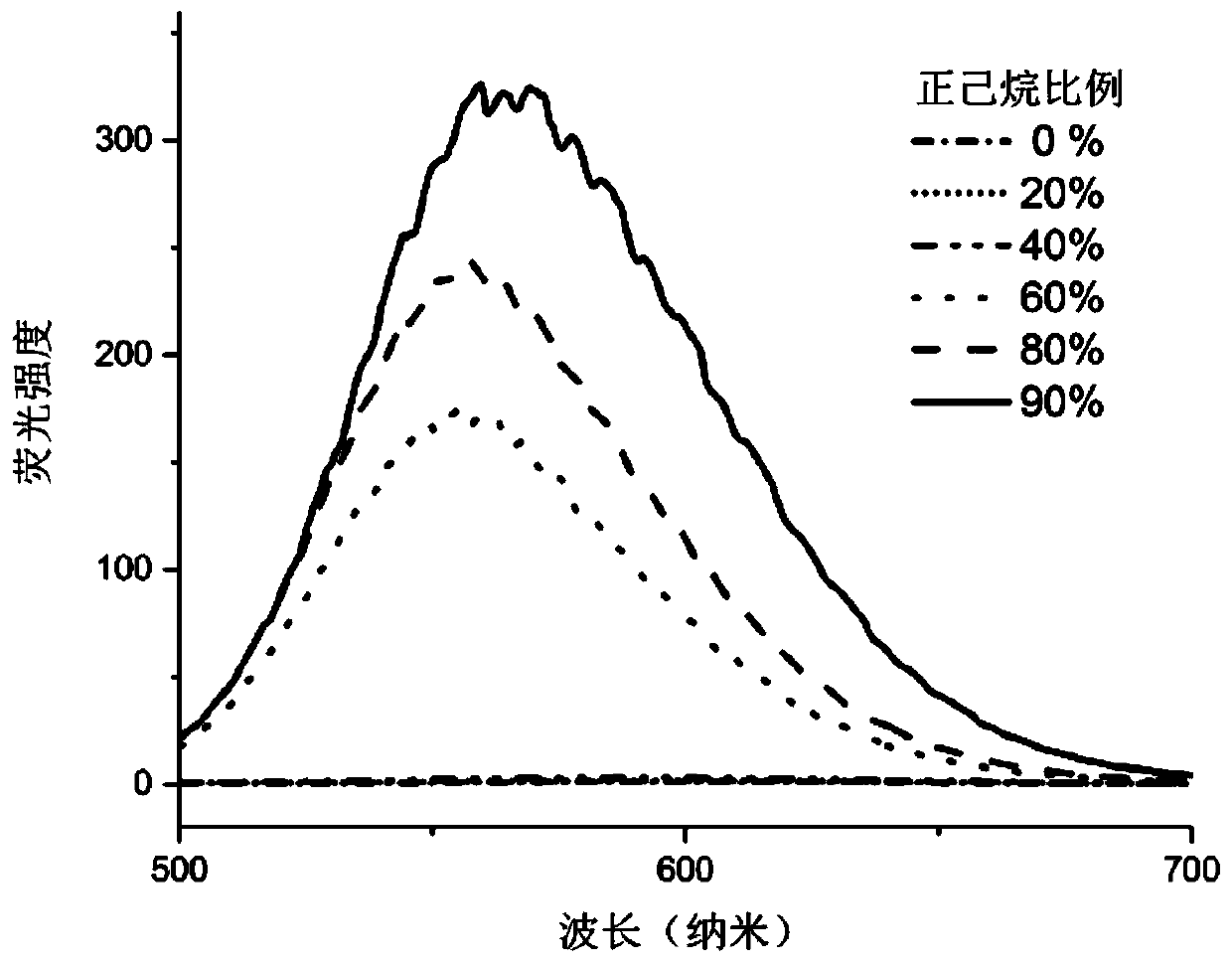
Two-photon fluorescent probe capable of targeting mitochondria and preparation method and application of probe
A reaction and anion technology, applied in the field of biomedicine, can solve the problems of excessive background fluorescence interference, aggregation-induced quenching, shallow penetration depth, etc., and achieve the effects of strong two-photon excitation fluorescence, easy availability of raw materials, and simple method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The synthesis of formula III compound, wherein Its reaction formula is as follows:
[0042]
[0043] Specifically: under the protection of argon, p-bromoiodobenzene (3.4g, 12mmol), 4-pyridineboronic acid (1.3g, 10mmol), tetrakis (triphenylphosphine) palladium (0.58g, 0.5mol) and sodium carbonate (3.2g, 30mmol) into a 250mL three-neck flask. Toluene (100 mL), ethanol (30 mL) and water (10 mL) were added to deoxygenate and stirred at room temperature for 10 minutes, then heated to reflux to 110° C. and stirred for 24 h. Then, it was filtered, concentrated, extracted with saturated brine, dried, and purified by column chromatography to obtain the product 4-pyridylbromobenzene (58% yield).
Embodiment 2
[0045] The synthesis of formula IV compound, wherein R" is hydrogen; its reaction formula is as follows:
[0046]
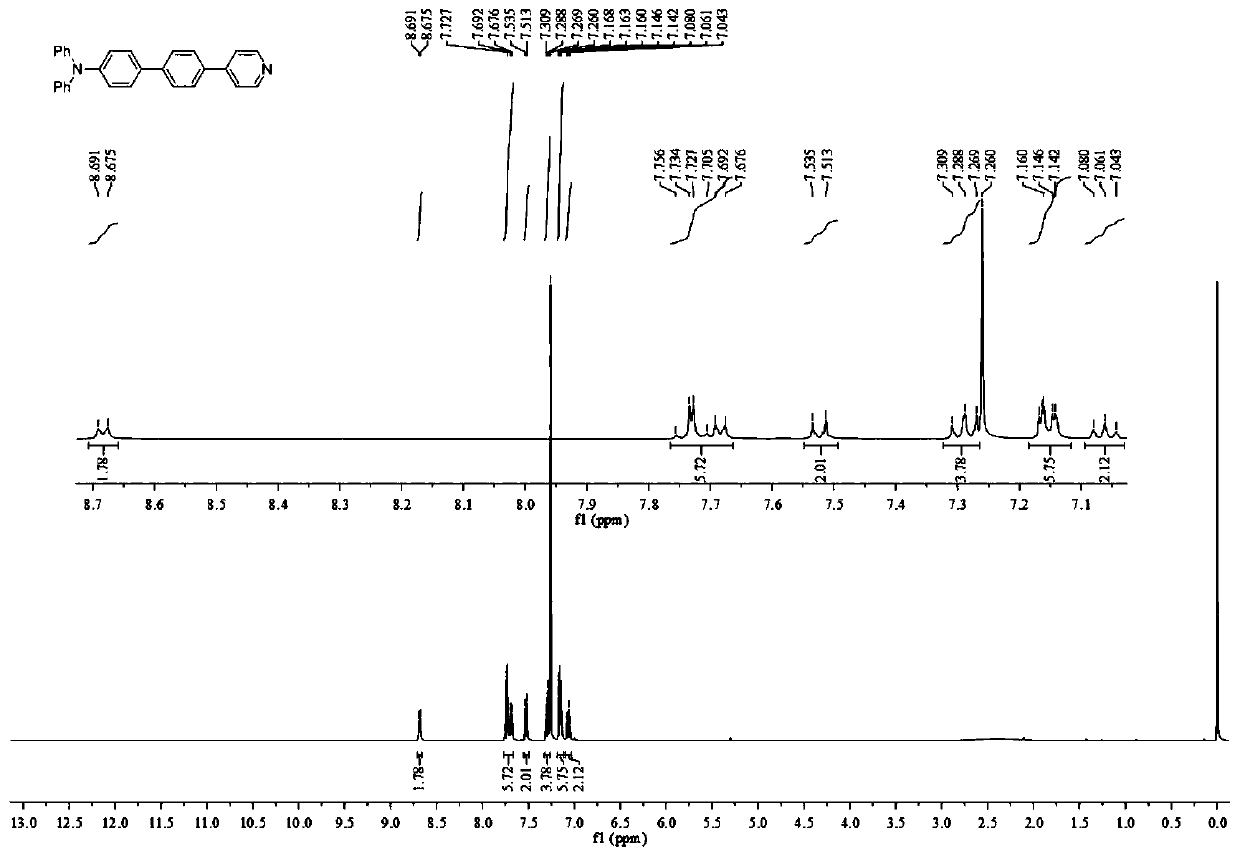
[0047] Specifically: under the protection of argon, triphenylamine borate (2.24g, 7mmol), 4-pyridylbromobenzene (1.8g, 7.7mmol), tetrakis (triphenylphosphine) palladium (0.4g, 0.35mol) and carbonic acid Sodium (2.2g, 21mmol) was added into a 250mL three-necked flask. Toluene (70 mL), ethanol (20 mL) and water (7 mL) were added to deoxygenate and stirred at room temperature for 10 minutes, then heated to reflux to 110° C. and stirred for 24 h. Then, it was filtered, concentrated, extracted with saturated brine, dried, and purified by column chromatography to obtain the product 4-pyridinetriphenylaminobenzene (69% yield).
Embodiment 3
[0049] The synthesis of formula V compound, wherein R " is hydrogen, R ' is methyl; Its reaction formula is as follows:
[0050]
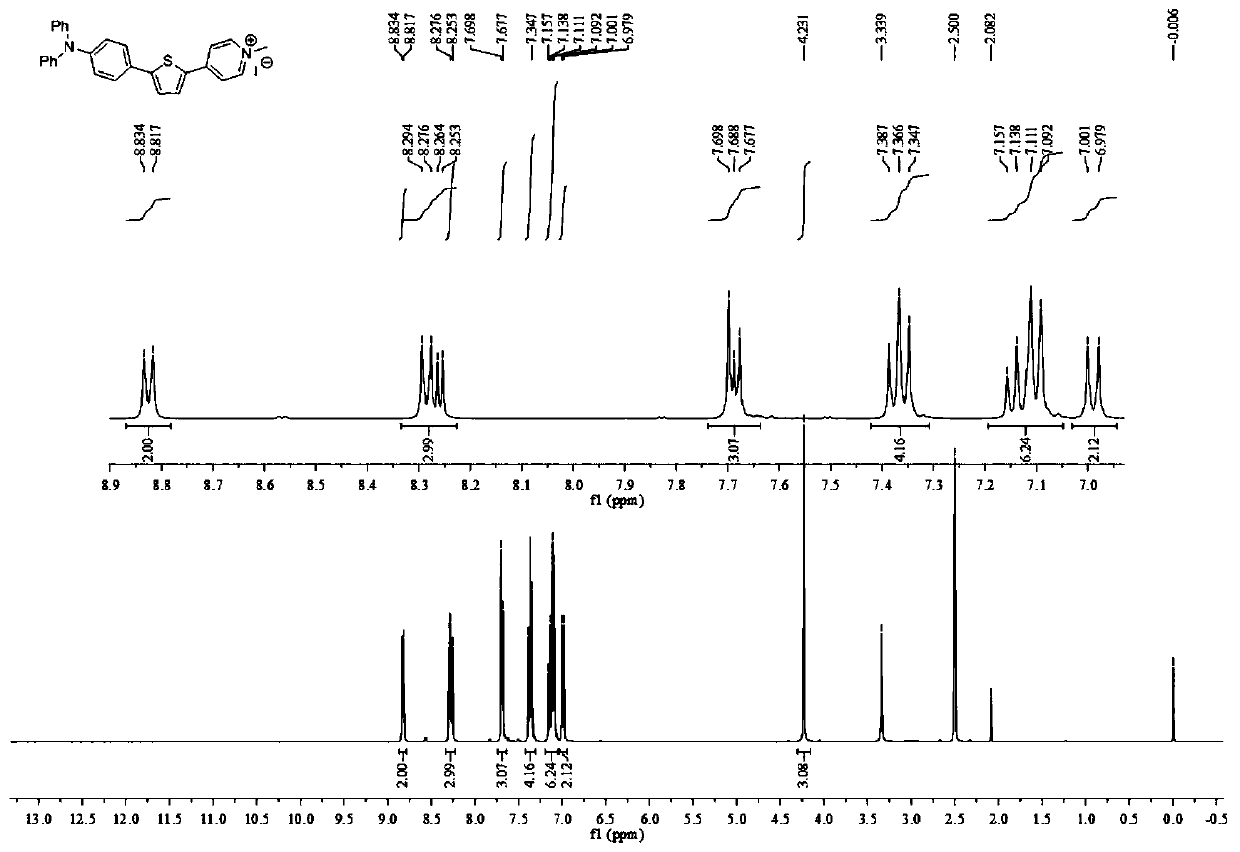
[0051] Specifically: under argon protection, 4-pyridinetriphenylaminobenzene (1.4g, 3.6mmol) and methyl iodide (2.3mL, 36mmol) were dissolved in acetone, stirred at room temperature for 24h, and filtered to obtain the product 4-( 1-picoline)trianilinobenzene (74% yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com