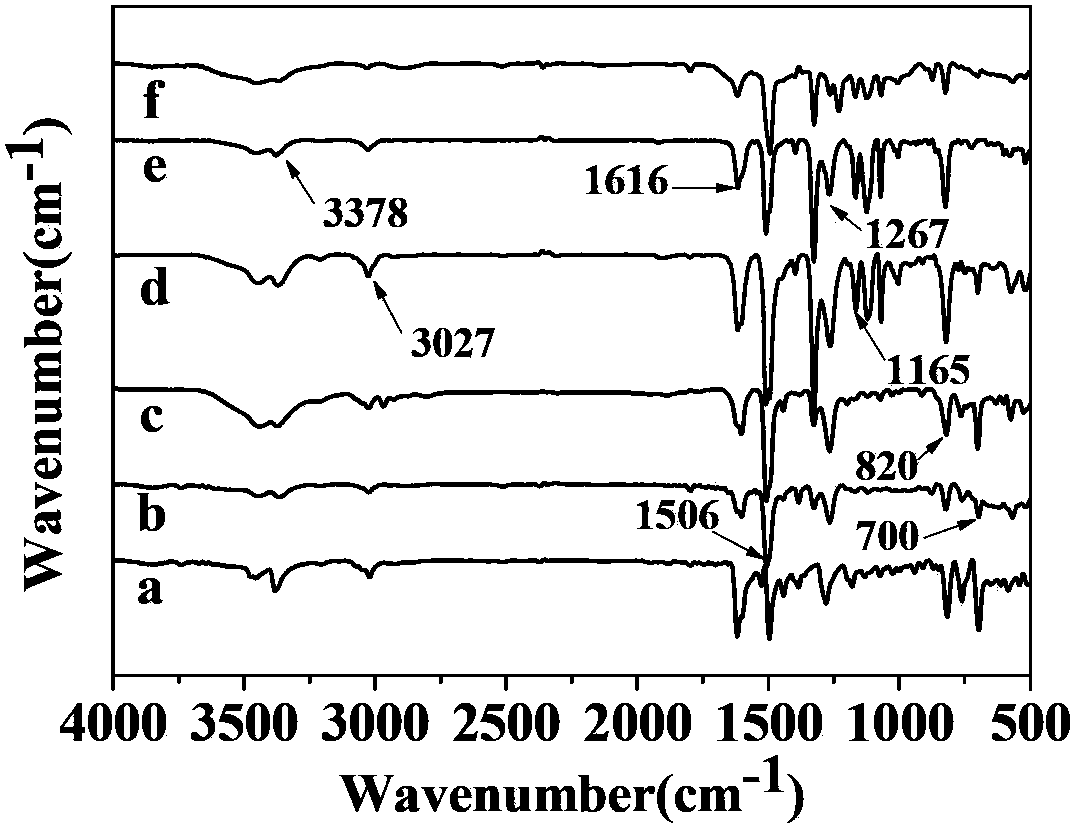
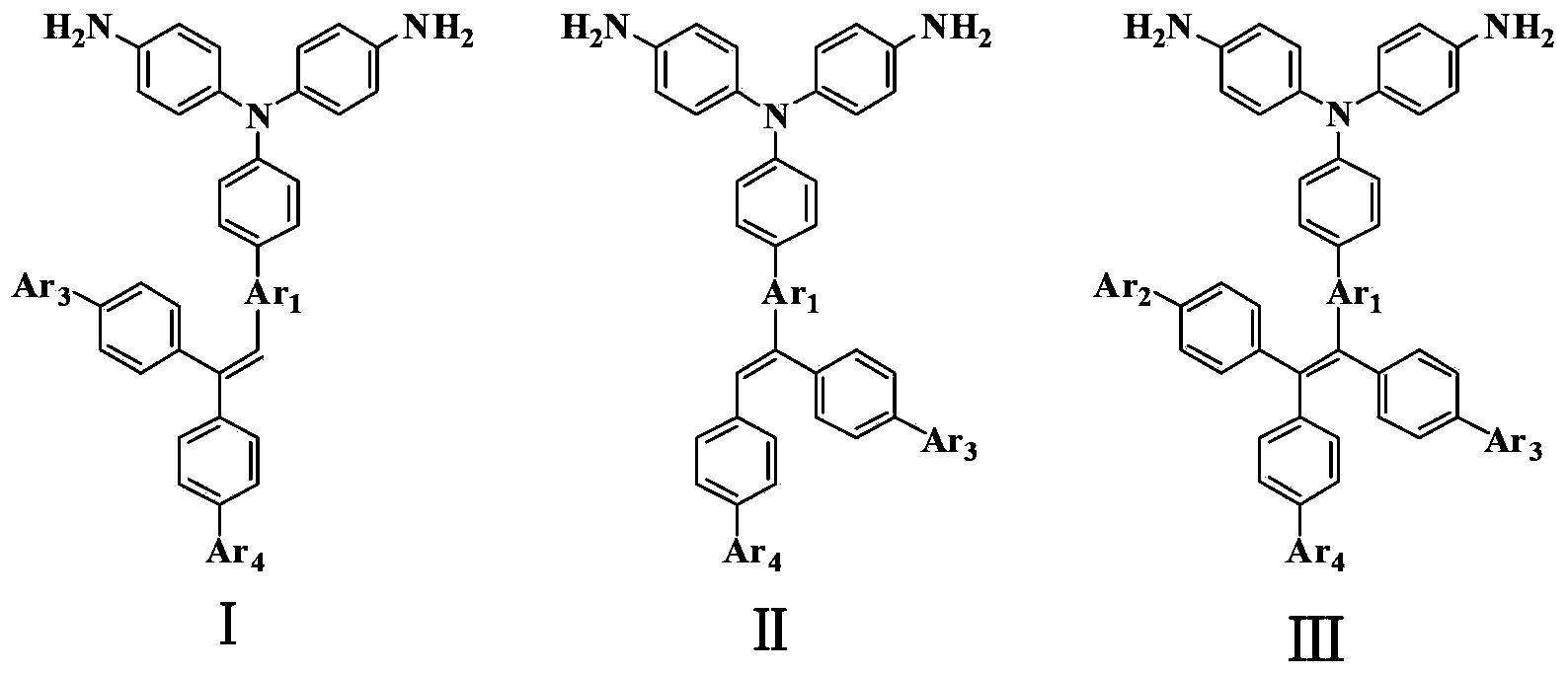
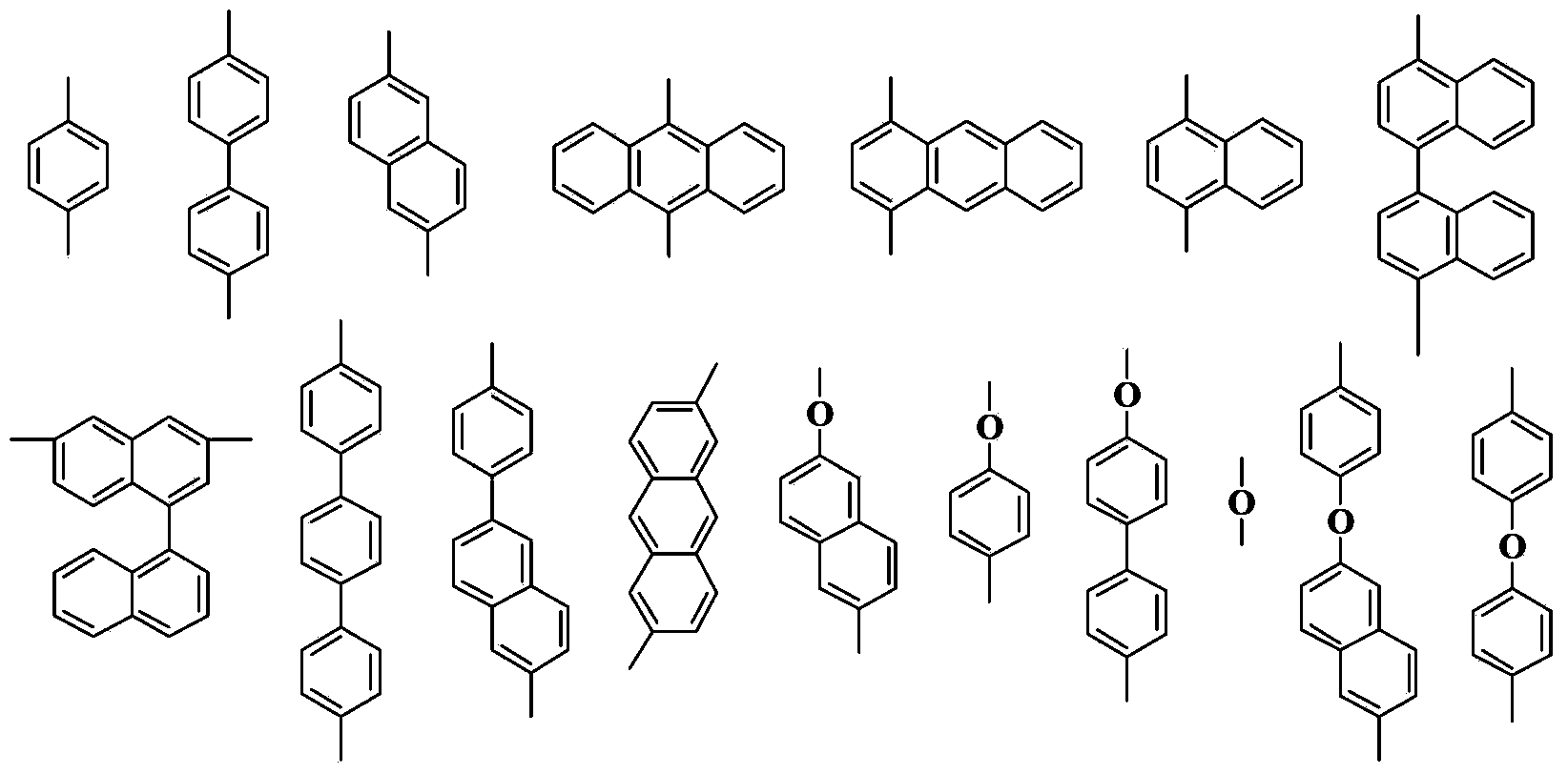
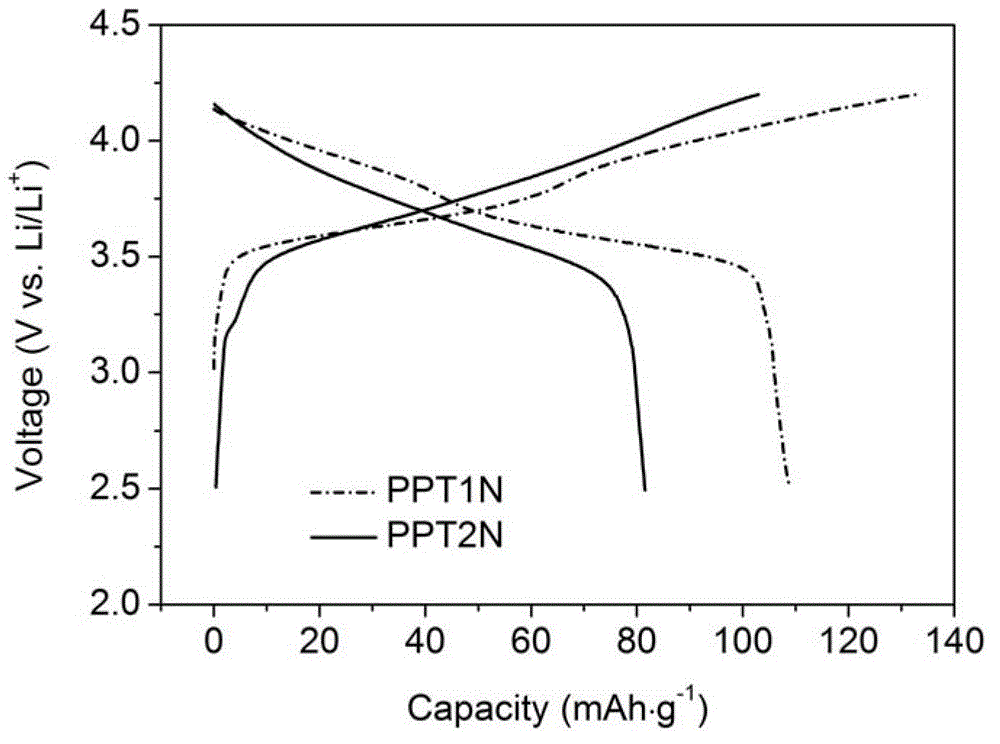
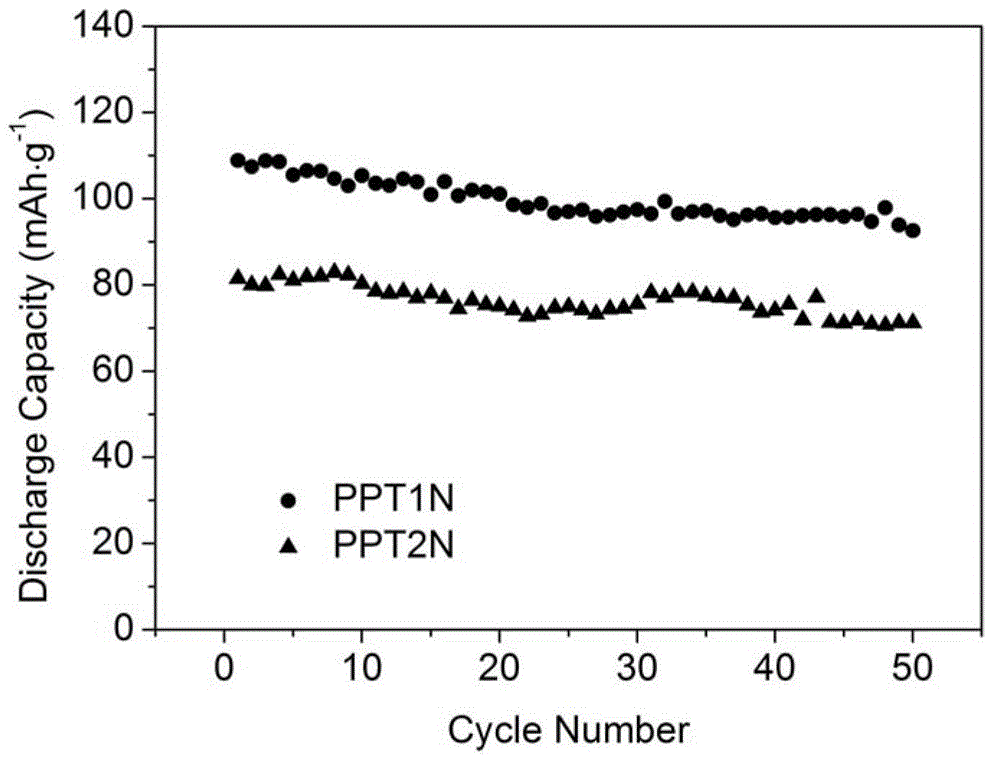
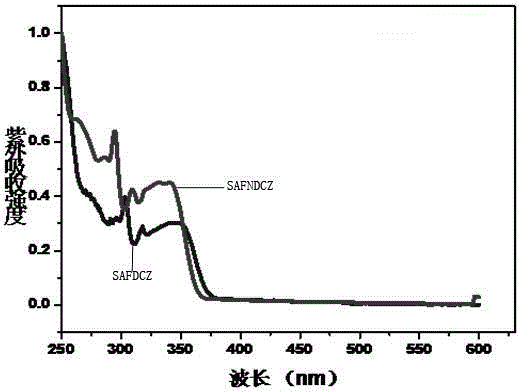
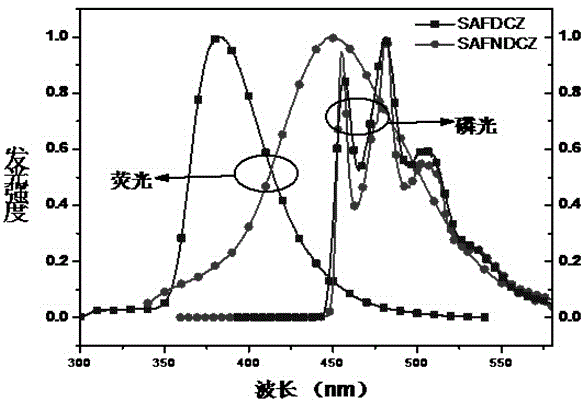
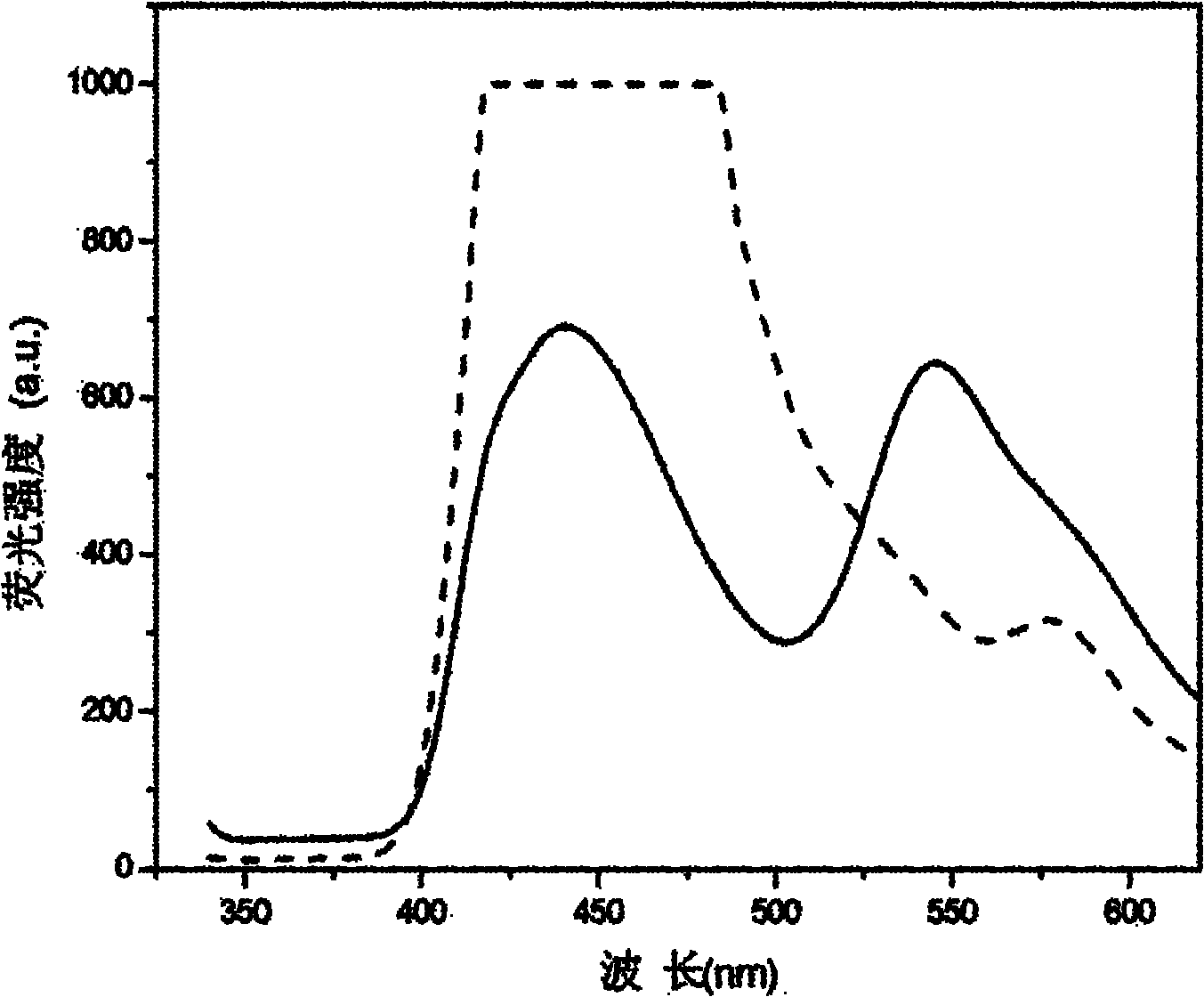
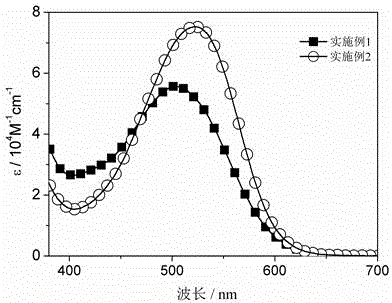
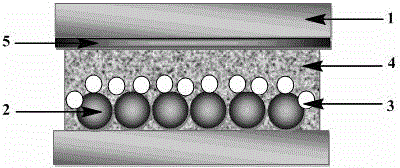
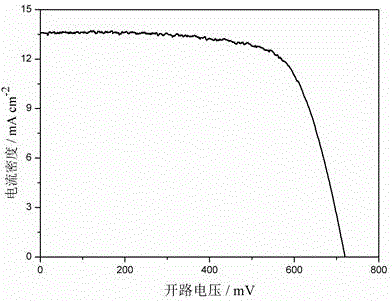
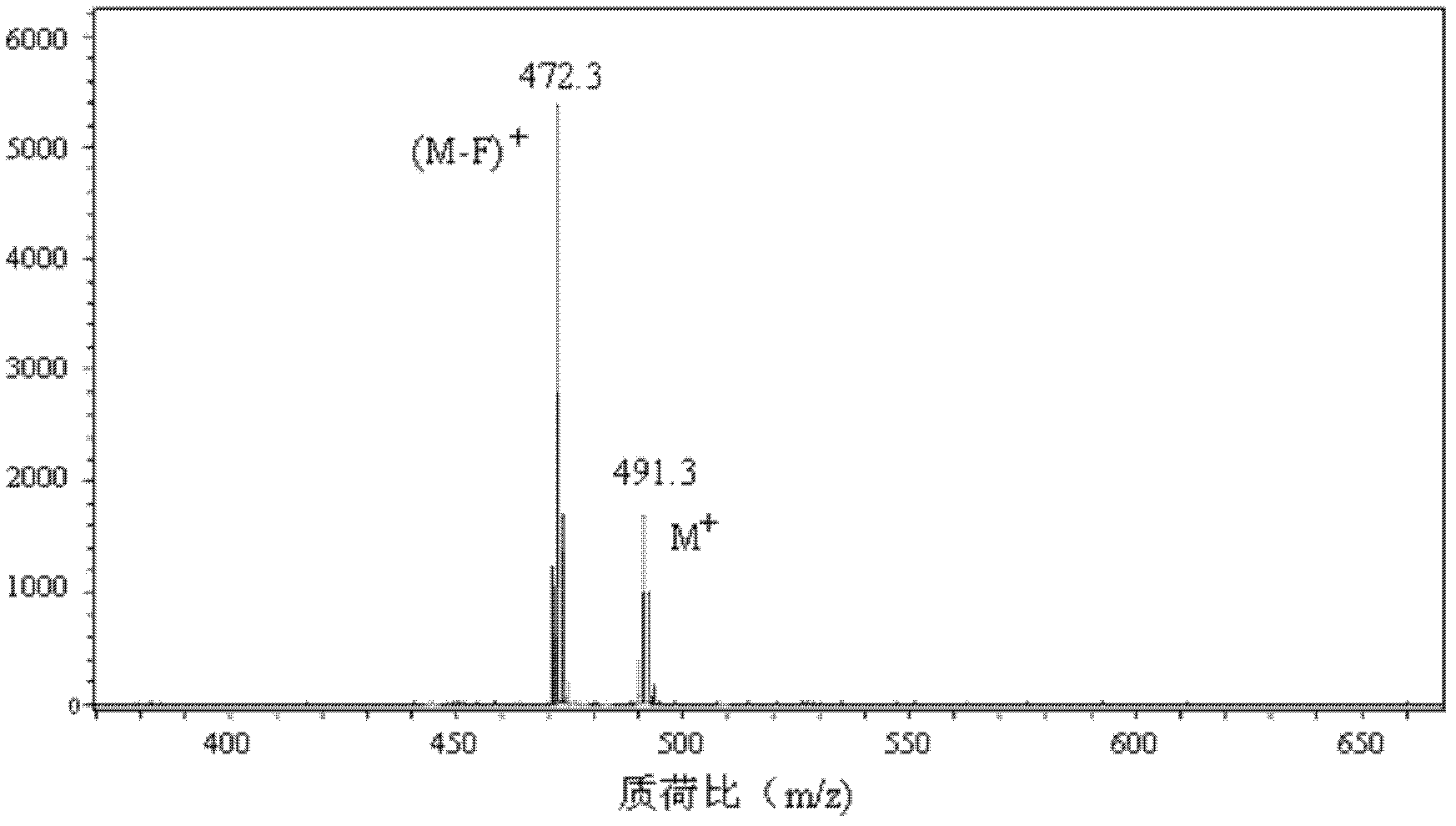Patents
Literature
869 results about "Triphenylamine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Triphenylamine is an organic compound with formula (C₆H₅)₃N. In contrast to most amines, triphenylamine is nonbasic. Its derivatives have useful properties in electrical conductivity and electroluminescence, and they are used in OLEDs as hole-transporters.
Oligoaniline compound
ActiveCN101679206AImprove conductivityImprove featuresOrganic chemistrySolid-state devicesPhosphoric Acid EstersCarboxyl radical
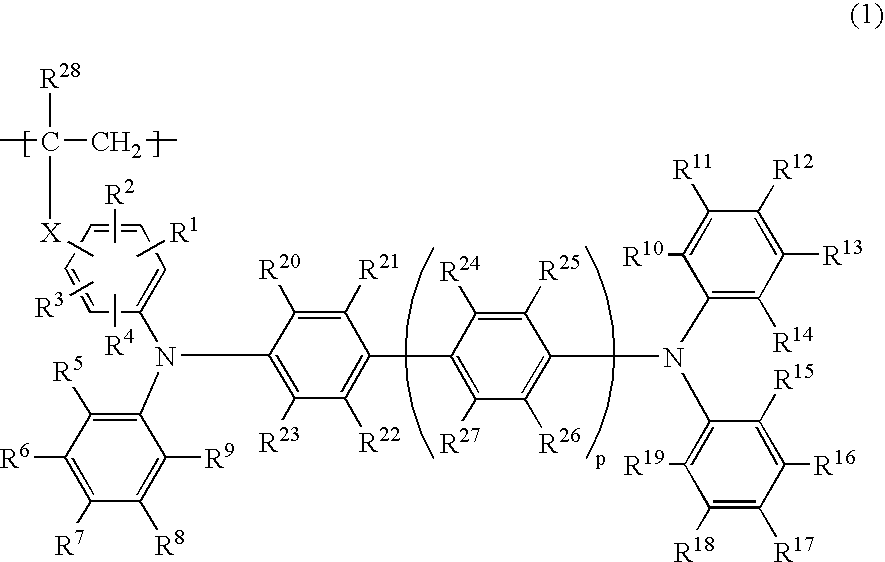
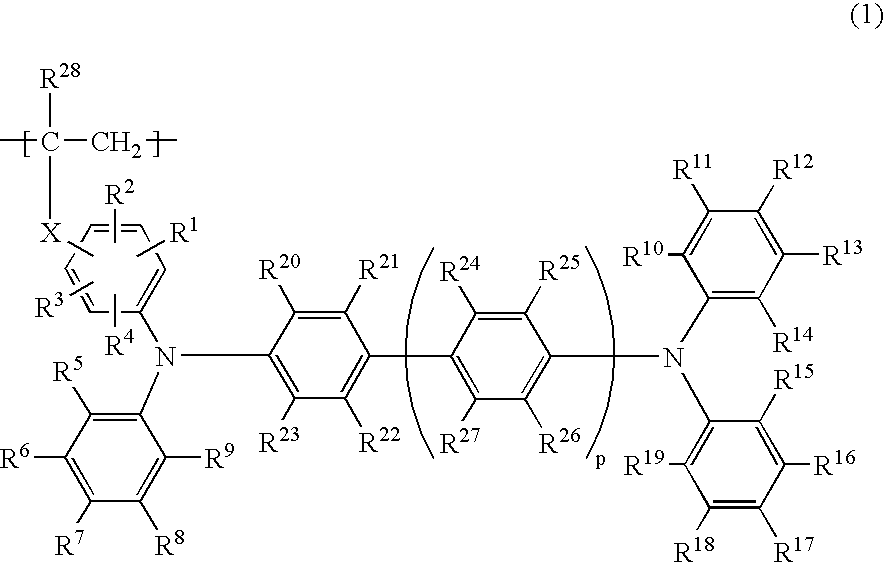
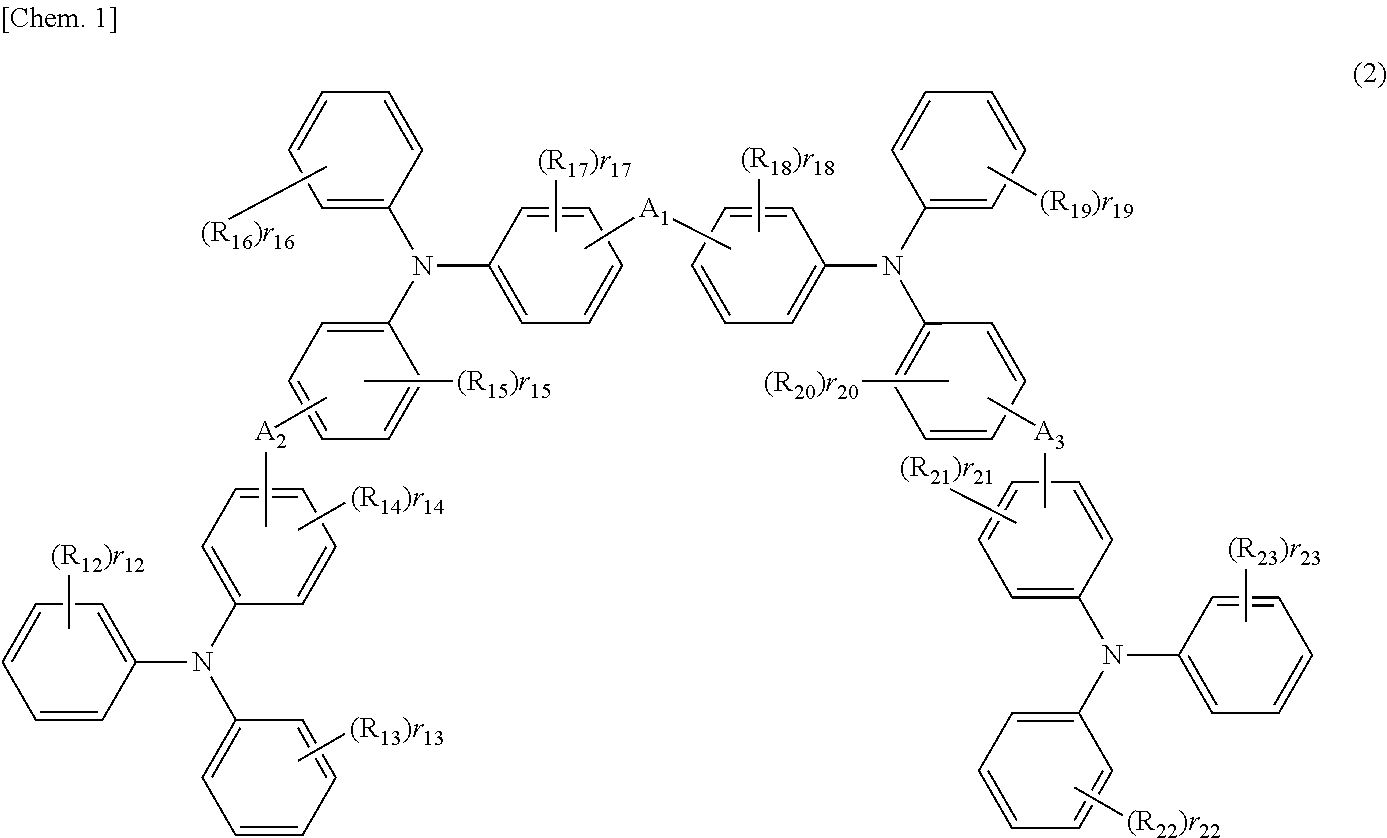
Any of the oligoaniline compounds with a triphenylamine structure represented by the formula (1) exhibits satisfactory light emitting efficiency and brightness performance when used in either an OLEDdevice or a PLED device, and is further satisfactory in the solubility in organic solvents so as to be applicable to various coating methods. Each of R<1> and R<2> independently is a hydrogen atom, anoptionally substituted monovalent hydrocarbon group, t-butoxycarbonyl, etc.; each of R<3> to R<34> independently is a hydrogen atom, hydroxyl, silanol, thiol, carboxyl, a phosphoric group, a phosphoric ester group, ester, thioester, amido, nitro, an optionally substituted monovalent hydrocarbon group, etc.; and each of m and n is an integer of 1 or greater provided that they satisfy the relationship m+n=20.
Owner:NISSAN CHEM CORP
Aggregation-induced emission molecule as well as preparation method and use thereof
InactiveCN103194215AControl Conjugate LengthEfficient luminous efficiencyAmino preparation from aminesSolid-state devicesStructural formulaLight-emitting diode
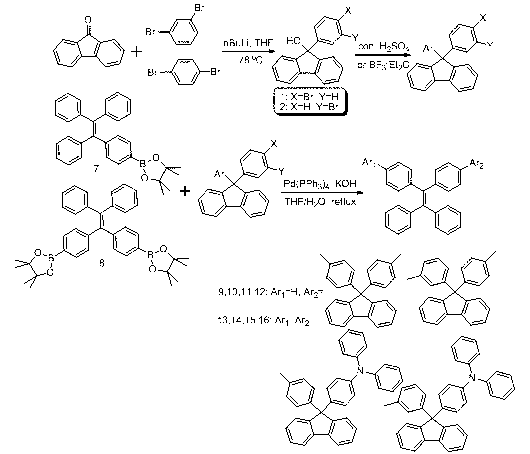
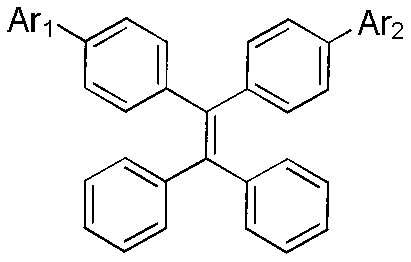
The invention discloses an aggregation-induced emission molecule as well as a preparation method and use thereof. The aggregation-induced emission molecule has a structural formula (shown in the description), wherein when Ar1 and Ar2 are same groups, Ar2 is a group shown in the description; and when Ar2 is hydrogen, Ar1 is a group shown in the description. The preparation method comprises the steps of: starting from a group shown in the description, wherein X=br, and Y=H or X=H, and Y=Br and tetraphenyl ethylene boric acid ester, obtaining derivatives of fluorene containing toluene and triphenylamine units by utilizing an acid-induced intramolecular dehydration reaction, linking tetraphenyl ethylene to a ninth site of the fluorine in a conjugated manner by utilizing a Suzuki reaction, and finally obtaining a target compound. According to the compound disclosed by the invention, the heat stability and the aggregation-induced emission property are good, the solid fluorescence quanta of the compound are high in yield and are emitted by blue lights, and the compound can be applied to a luminescent layer material of a blue-light inorganic light emitting diode; and the reaction conditions of the preparation method are mild, and the yield is high.
Owner:WUHAN UNIV
Terpyridyl derivative with electroluminescent and electrochromic characteristics and complex thereof
InactiveCN102532003AObvious organic electroluminescent propertiesObvious electrochromic propertiesCobalt organic compoundsIron organic compoundsDonor groupAniline
The invention discloses a derivative with electroluminescent and electrochromic characteristics and a complex thereof. Specifically, a series of mono-branched and poly-branched structures are obtained by taking 4-phenyl terpyridyl as a terminal group and connecting electron-attracting groups (such as nitro, aldehyde group and carboxyl group) or push donor groups (such as hydroxyl, a diphenylamine derivative and a triphenylamine derivative). A general formula is shown in the specifications. The triple pyridine derivative disclosed by the invention has superior light stability, superior thermal stability and remarkable electroluminescent characteristic; a metal complex of the derivative has remarkable triplet luminous characteristic, and can be applied to an OLED (Organic Light Emitting Diode) luminous material; and more importantly, the metal complex has superior electrochromic characteristic, and is suitable to be taken as a smart off-color material.
Owner:SUZHOU UNIV OF SCI & TECH
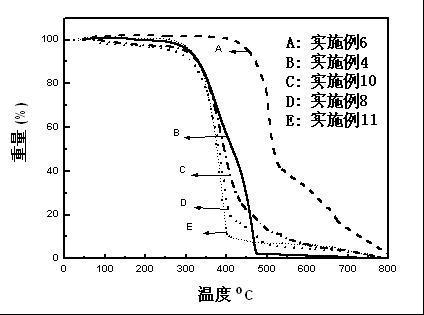
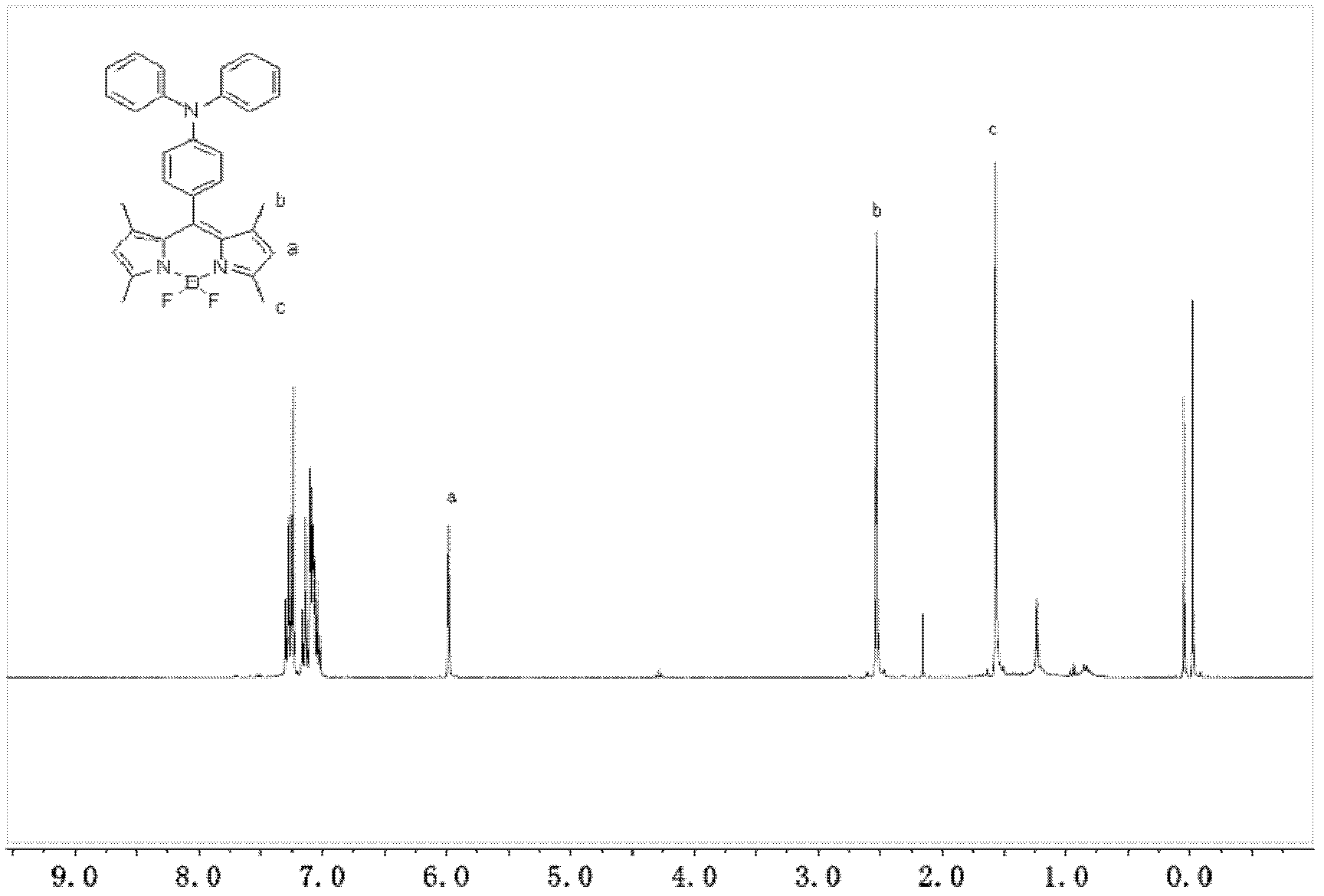
1,3,5,7-tetramethyl-8-triphenylamine base pyrrole methane-boron difluoride complex compound and preparation method thereof
InactiveCN102321109AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsMethylene DichlorideOrganic synthesis
The invention relates to a 1,3,5,7-tetramethyl-8-triphenylamine base pyrrole methane-boron difluoride complex compound and a preparation method thereof, which belong to the technical field of organic synthesis. The existing photocatalysts have few varieties and low conversion rate, and the existing bodipy dye has low infrared fluorescent efficiency and small Stokes displacement. The invention provides the 1,3,5,7-tetramethyl-8-triphenylamine base pyrrole methane-boron difluoride complex compound. The preparation method comprises the steps that: 4-formoxyl tetramethyl and 2,4-dimethyl pyrrole are dissolved in organic solvents, trifluoroacetic acid or monoprop is used as catalysts, and a reaction system is formed; 2,3-dichloro-5,6-dicyan-1,4-para-benzoquinone with the same mol ratio as the 4-formoxyl tetramethyl is taken, is dissolved in the methylene dichloride and is added into the reaction system; and one of triethylamine, triisopropyl amine and N,N-diisopropylethylamine is added into the reaction system, boron trifluoride etherate is added under the ice bath, and final products are generated.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Novel diamine compound, and preparation method and application thereof
ActiveCN104341311AThe synthesis process is simpleEasy to purifyAmino preparation from aminesOrganic compound preparationSynthesis methodsNitrobenzene
The invention discloses a novel diamine compound, and a preparation method and an application thereof. The preparation method of the novel functional diamine compound comprises the following steps: a large conjugate structure comprising benzophenone carbonyl group is obtained; the ketone carbonyl group is subjected to a Wittig or Wittig-Horner reaction, such that a large conjugate system with a triphenylethylene / tetraphenylethylene structure and comprising a halogen atom is obtained; the halogen atom is further subjected to a Suzuki reaction or a plurality steps of reactions, such that a monoamine compound comprising a triphenylethylene / tetraphenylethylene large conjugate system is obtained; the monoamine compound is subjected to a reaction with halogenated nitrobenzene, such that a dinitro monomer comprising triphenylamine and the triphenylethylene / tetraphenylethylene large conjugate system is obtained; and the dinitro monomer is reduced into the novel diamine compound, such that the novel functional diamine compound comprising triphenylamine and a triphenylethylene / tetraphenylethylene structure is obtained. The synthesis method provided by the invention is simple. Purification is easy. The method is suitable for industrial productions. The synthesized diamine compound has a significant aggregation-induced emission property, and can be used for synthesizing high-performance and functional polymers such as polyamide, polyimide, polyamideimide, polyesterimide, and the like.
Owner:SUN YAT SEN UNIV
Compound, display panel and display device
ActiveCN110028459AGroup 4/14 element organic compoundsGroup 5/15 element organic compoundsChemical structureDisplay device
The invention provides a compound with a D-(pi)-sigma-(pi)-A chemical structure. The compound has a chemical structure shown as formula (I), wherein L1 and L2 are selected from at least one of a single bond, C1-C20 alkylene, C3-C20 cycloalkylene, C3-C20 heterocyclylene, C6-C40 arylidene, C4-C40 heteroarylene, C10-C60 fused arylidene or C10-C60 fused heteroarylene; an electrondonor D is selected from C1-C20 alkyl, C3-C20 cycloalkyl, C1-C20 alkoxyl, C3-C20 heterocyclyl, C6-C40 aryl, C4-C40 heteroaryl, C10-C60 fused arylidene, C10-C60 fused heteroarylene, C12-C40 carbazyl and a derivative group of C12-C40 carbazyl, C12-C40 diphenylamine and a derivative group of C12-C40 diphenylamine, C18-C60 triphenylamine and a derivative group of C18-C60 triphenylamine, and C12-C40 acridinyl and a derivative group of C12-C40 acridinyl; an electron acceptor A is selected from a nitrogen-containing heterocyclic substituent, a cyan-containing substituent, a triaryl boron substituent, a benzophenone substituent, a heteroaromatic ketone substituent, a sulfone substituent and a phosphorus-containing oxyl substituent. The invention provides a series of bipolar materials with the D-(pi)-sigma-(pi)-A structure, and luminance and luminous efficiency EQE can be improved.
Owner:WUHAN TIANMA MICRO ELECTRONICS CO LTD
Thermal activation delayed fluorescence high-molecular compound and preparation method and application thereof
ActiveCN106589324AHigh triplet energy levelGood hole transport performanceLuminescent compositionsQuantum yieldSide chain
The invention discloses a thermal activation delayed fluorescence high-molecular compound which is prepared by using asymmetric thermal activation delayed fluorescence micromolecules as luminescent monomer and applying a Suzuki polymerization method. A strategy of connecting TADF micromolecules through a side chain is adopted, and polyspirofluorene, fluorene, carbazole, benzene or triphenylamine is used as a main chain, so that high triplet-state energy level of the main chain is guaranteed, the main chain can be ensured to have good hole transmission performance, prepared TADF high molecules can well inherit TADF characteristics of the micromolecules, and photoluminescence quantum yield and inverse intersystem crossing constant of certain high molecules are even higher than those of the micromolecules. The synthetic high-molecular compound has quite high glass transition temperature and thermal decomposition temperature and has quite good film-forming performance. When the high-molecular compound is applied in electroluminescent devices, highest current efficiency (38.6 cd / A), power efficiency (14.3 lm / W) and external quantum efficiency (16.1%) of current delayed fluorescence high molecules can be acquired by doping a main body and assistant TADF micromolecules.
Owner:WUHAN UNIV
Dendrimer with triphenylamine core, organic memory device having the same, and manufacturing method thereof
InactiveUS20080248330A1Simple processLow costOrganic chemistryOrganic compound preparationDendrimerHeteroatom
A dendrimer according to example embodiments may include a triphenylamine core, wherein a conjugated dendron having no heteroatoms is coupled to the triphenylamine core. An organic memory device according to example embodiments may include an organic active layer between a first electrode and a second electrode, wherein the organic active layer includes the dendrimer according to example embodiments. A barrier layer may be provided between the first and second electrodes. A method of manufacturing the organic memory device according to example embodiments may include forming an organic active layer between a first electrode and a second electrode, the organic active layer including the dendrimer according to example embodiments.
Owner:SAMSUNG ELECTRONICS CO LTD
Photoconductor Overcoat Having Radical Polymerizable Charge Transport Molecules Containing Two Ethyl Acrylate Functional Groups and Urethane Acrylate Resins Containing Six Radical Polymerizable Functional Groups
ActiveUS20150168908A1Improve wear resistanceExtended service lifeConductive materialSynthetic resin layered productsElectrical conductorUrethane acrylate
An improved overcoat layer for an organic photoconductor drum of an electrophotographic image forming device is provided. The overcoat layer is prepared from a curable composition including a triphenylamine charge transport containing two ethyl acrylate functional groups and a urethane resin containing six radical polymerizable functional groups. The amount of the triphenylamine charge transport containing two ethyl acrylate functional groups in the curable composition is about 20 to about 80 percent by weight. The amount of the urethane resin containing six radical polymerizable functional groups in the curable composition is about 20 to about 80 percent by weight. This overcoat layer improves wear resistance of the organic photoconductor drum without negatively altering the electrophotographic properties, thus protecting the organic photoconductor drum from damage and ultimately providing a photoconductor with a longer useful life when compared to other organic photoconductors commercially available.
Owner:LEXMARK INT INC
Application of triphenylamine derivative polymer as lithium ion batteries cathode material
ActiveCN102751501AImprove cycle stabilitySignificant charge and discharge voltage platformCell electrodesBiopolymerCharge discharge
The invention provides an application of a triphenylamine derivative polymer as a lithium ion batteries cathode material, belonging to the technical field of lithium ion battery. The triphenylamine derivative is a triphenylamine derivative polymer containing triphenylamine and thiophene structure. By introducing an electroactive conjugated monomer in the polytriphenylamine backbone, the microscopic morphology of the polymer is significantly improved, the conductivity and conjugate capability of the polymer are enhanced, so that the cathode material has high specific capacity, excellent cycling stability, significant charge-discharge voltage platform, and good rapid charge and discharge performance, etc.
Owner:ZHEJIANG UNIV OF TECH
Triphenylamine spirofluorene derivatives and uses thereof
ActiveCN104892578AImprove electroluminescence performanceHigh glass transition temperatureOrganic chemistrySolid-state devicesChemical structureAcceptor
The invention discloses triphenylamine spirofluorene derivatives and uses thereof. The general chemical structure of the triphenylamine spirofluorene derivatives is shown in the specification, wherein triphenylamine spirofluorene is the main body, and A is carbazole, alpha-carboline, beta-carboline, gamma-carboline, pyridine, diphenylamine, dimethoxydiphenylamine, dimethyldiphenylamine or 3,6-di-tert-butylcarbazole. The derivatives are good in thermal stability and hole conductivity. By simple methods, the degree of conjugation of the main body material is effectively controlled, the molecular weights of compounds are increased, the triplet state energy level and the glass-transition temperature of a material are largely increased, and the threshold voltage is obviously reduced. Polarity can be adjusted by adjusting donor / acceptor electronic groups. The bipolar performance is improved by adjusting the intensity of the donor / acceptor electronic groups. Compared with common phosphorescence main body materials, performance of devices prepared from the derivatives is improved and the derivatives can be widely applied in the field of organic electroluminescence.
Owner:SUZHOU JOYSUN ADVANCED MATERIALS CO LTD
Organic solar cell material and preparation thereof
InactiveCN101525334AImprove solubilityImproved solar spectral responseOrganic chemistryFinal product manufactureOrganic solar cellDecomposition
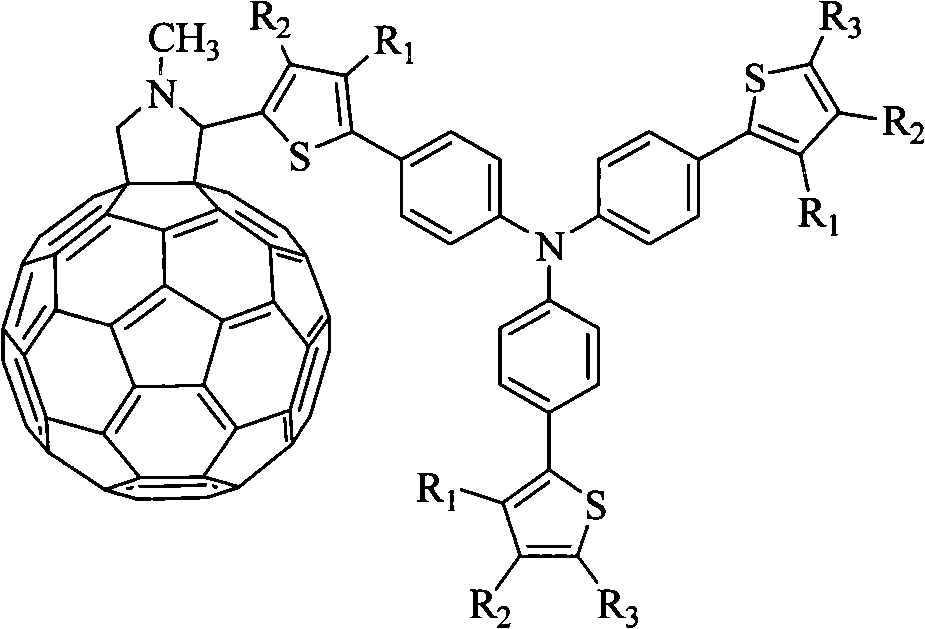
An organic solar cell material and the preparation thereof belong to the field of organic photoelectric materials. The invention discloses an organic solar cell material which contains C60-triphenylamine-thiofuran ternary system which is a fullerene-contained D-A (Donor-Accepter) type difunctional material, wherein the triphenylamine and the thiofuran are in stellated structure. Compared with the fullerene, the compound has greatly improved solubility in organic solvent, so that the process for manufacturing the large-area solar cell is simplified, stronger absorption is ensured in a visible region with a maximum of about 500 nm that is similar to the maximum of solar radiation energy of 475 nm, matching of the compound and solar spectrum radiation is enhanced, solar spectrum response of the material, as well as the photoelectric conversion efficiency is improved. In the invention, the compound can be polymerized on a macromolecule through simple ligand modification, and is made into PLED by spin coating, so as to overcome the defects of possible decomposition by heating and poor crystallization-resistant performance of the small-molecule luminescent material in the evaporation process.
Owner:JIANGNAN UNIV
Method and apparatus for photochemical degradation of organic gas
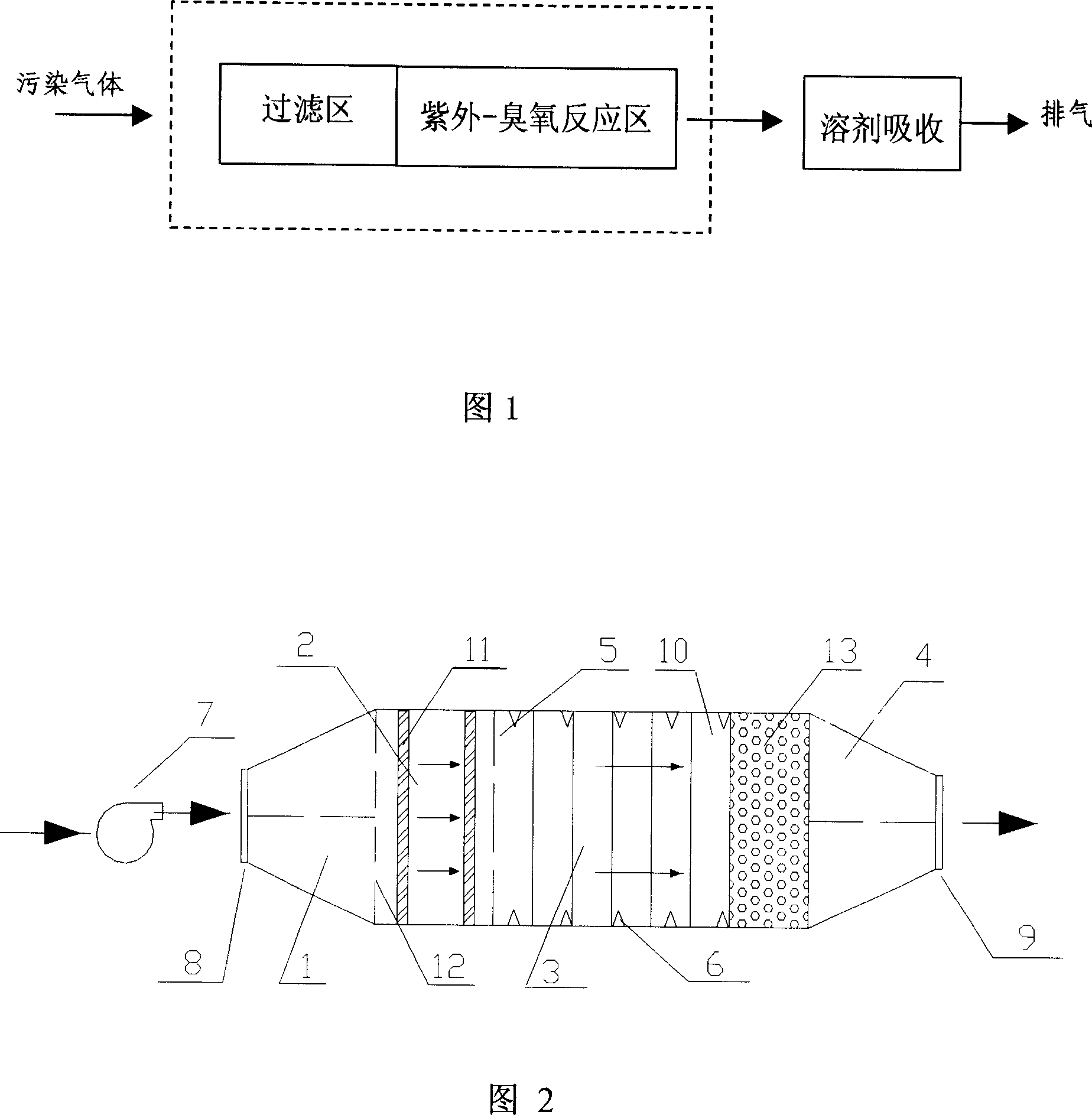
InactiveCN1951544AAchieve degradationSynergistic effect, strong oxidation abilityDispersed particle separationEnergy based chemical/physical/physico-chemical processesPhotochemical degradationSolvent based
The invention relates to a method for using photochemistry method to degrade organic gas. Wherein, it uses ozone, ultraviolet or ozone-ultraviolet to oxidize the organic gas; the processed gas is filtered with particles; then enters into ultraviolet-ozone reaction area, to be radiated and reacted with ozone; then passes the solvent absorber to be purified, and discharged at gas outlet. The invention can effectively degrade organic gas as triphenylamine, without pollution.
Owner:FUJIAN NEWLAND ENTECH CO LTD
Fluorescent compound and application thereof in detecting trace methylamphetamine
ActiveCN101899021AImprove luminous efficiencyThe synthesis method is simpleOrganic chemistryFluorescence/phosphorescenceFluorescent quenchingPyrene
The invention relates to a fluorescent compound and application thereof in detecting trace methylamphetamine, which is characterized in that the structural general formula of the fluorescent compound is shown in the specification, wherein R1 and R2 in the formula are respectively one fluorescent substituent group and the derivative thereof, the fluorescent substituent group comprises triphenylamine, benzene, naphthalene, anthracene, pyrene, fluorine, dinaphthalene, carbazole, benzothiophene, phenothiazinyl and rhodamine, and R3 is alkyl substituent. The steam action of the fluorescent compound provided by the invention and the methylamphetamine can carry out obvious fluorescent quenching in short time, and the methylamphetamine can be detected according to the fluorescent intensity quenching. The fluorescent compound is a novel fluorescent sensing material for detecting methylamphetamine. The detection limit reaches 100 ppb.
Owner:SHANGHAI INST OF MICROSYSTEM & INFORMATION TECH CHINESE ACAD OF SCI
Electro luminescent element
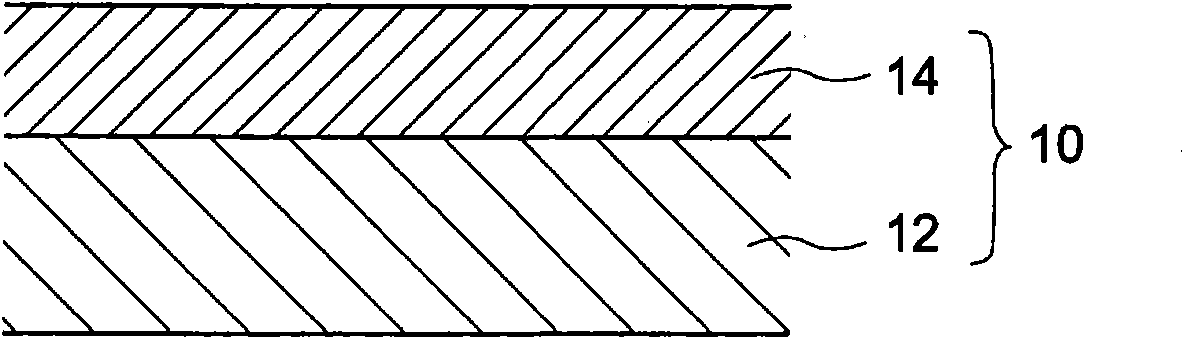
InactiveUS6777111B1Organic chemistryDischarge tube luminescnet screensElectronic transmissionCrystallinity
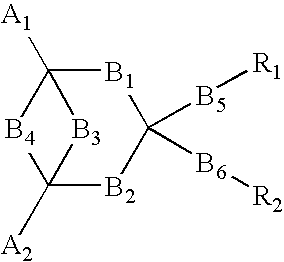
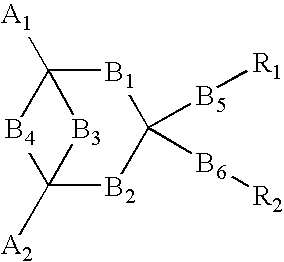
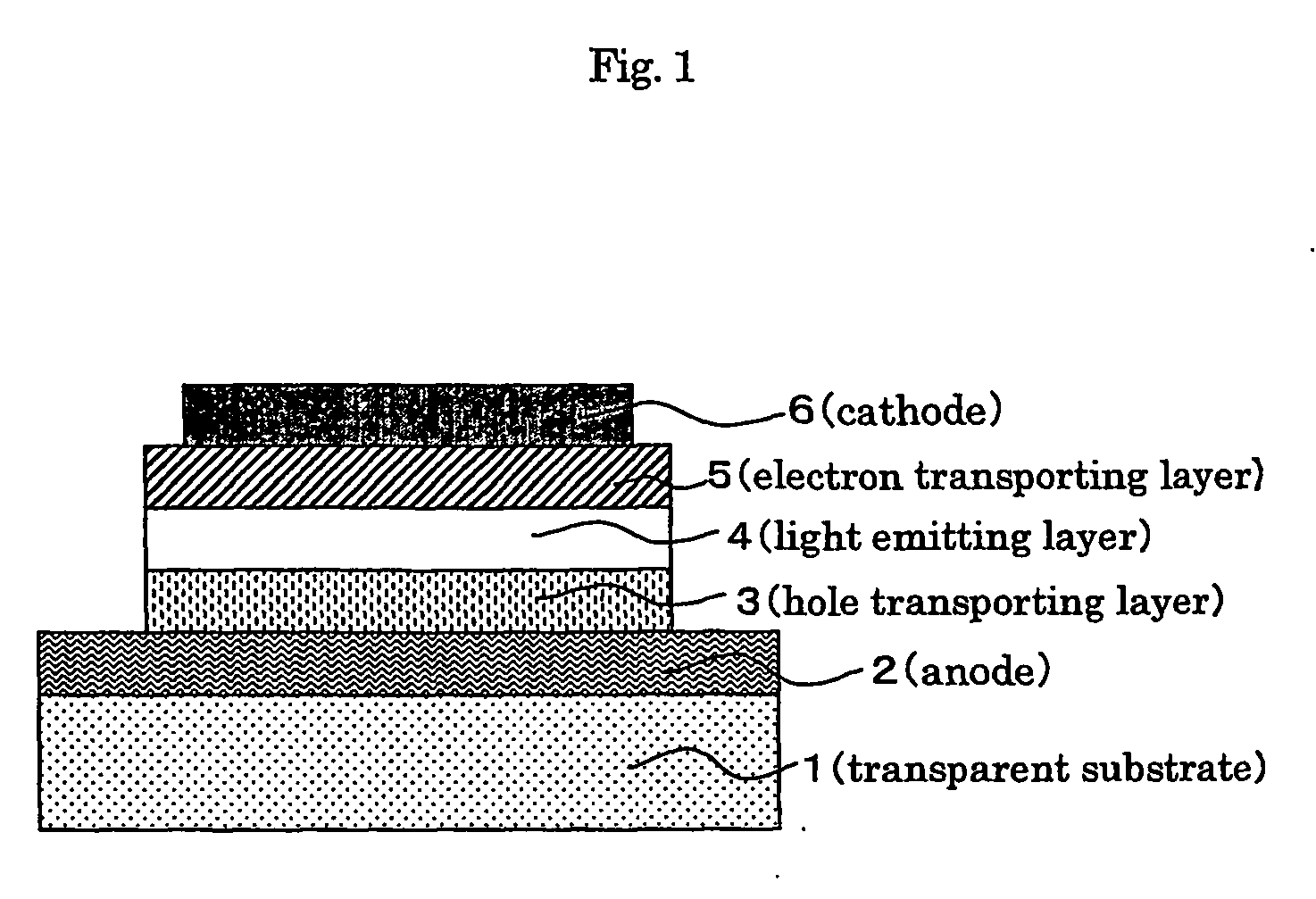
In order to provide an electro luminescent element with high heat endurance and low crystallinity using functional molecules having functions of hole transporting, luminescence, and electron transporting, an electro luminescent element according to the present invention comprises one or more organic compound layers 14 between a first electrode 12 and a second electrode 16, wherein at least one of the organic compound layers 14 is a condensed ring compound derivative represented by the following chemical formula,in which A1 and A2 represent substituents, B1 through B6 represent directly connected or di functional substituents, and R1 and R2 represent functional units having each of the functions of hole transporting, luminescence, and electron transporting, such as triphenylamine, coumarin, and oxadiazole derivative.
Owner:TOYOTA CENT RES & DEV LAB INC
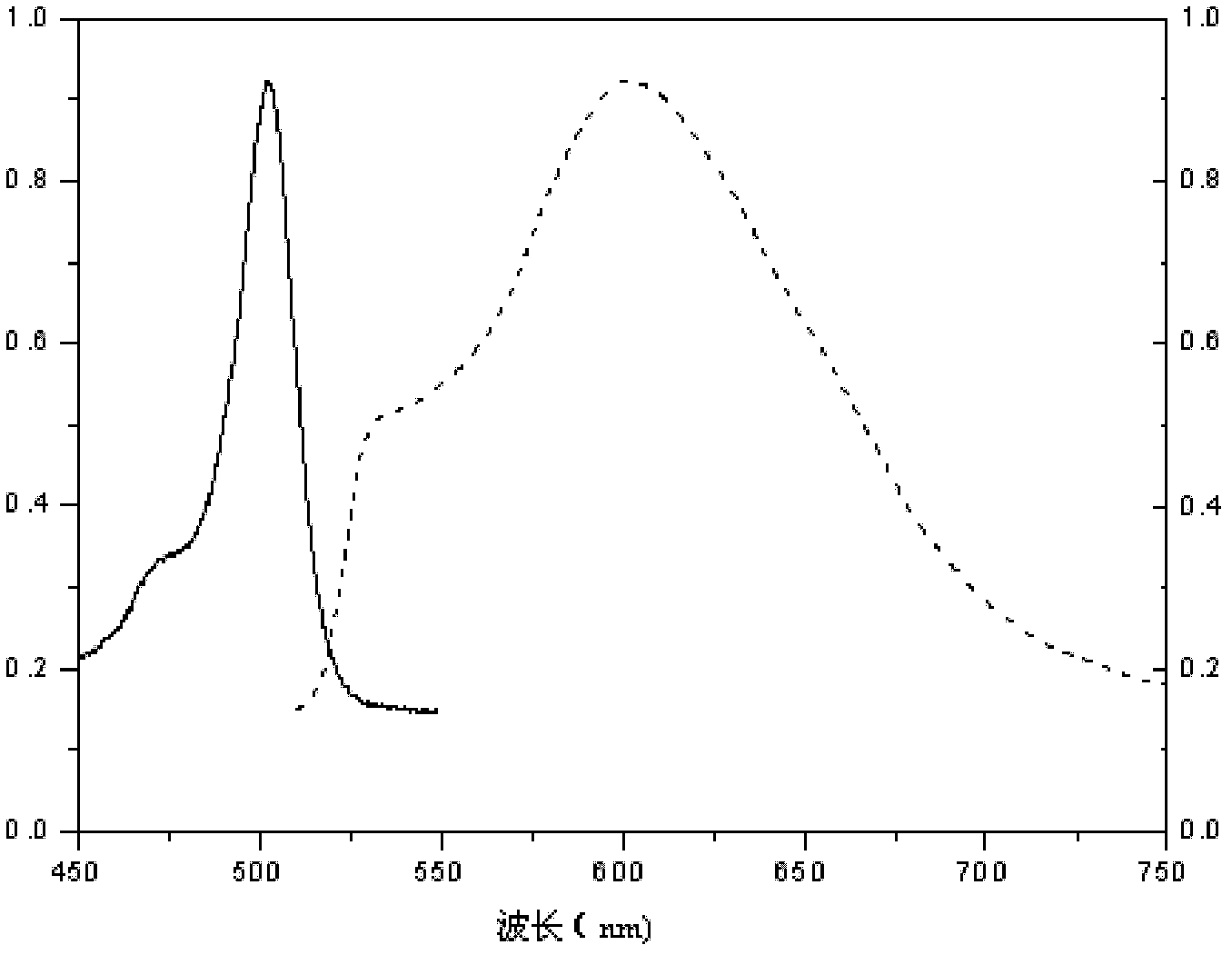
Strong fluorescence fluoro-boron dipyrrole compound containing triphenylamine structure as well as preparation method and application thereof
InactiveCN103172650AGood symmetryImprove laser efficiencyAzo dyesGroup 3/13 element organic compoundsFluorescenceUv vis absorbance
The invention relates to a strong fluorescence fluoro-boron dipyrrole compound containing a triphenylamine structure as well as a preparation method and an application thereof, belonging to the technical fields of organic chemical industry and fine chemical industry. The preparation method comprises the following steps of: weighing and dissolving formyl substituted triphenylamine and 2,4-dimethyl pyrrolo in a dry dissolvent under the protection of inert atmosphere; adding a catalyst; reacting at a room temperature in a dark place for 6-10 hours; then adding an oxidant; reacting at room temperature for 20-40 minutes; and finally adding organic amine and a boron triflouride complex compound and reacting for 3-8 hours, thus obtaining the strong fluorescence fluoro-boron dipyrrole compound containing a triphenylamine structure. The synthetic method is simple and convenient, and the synthetic compound serves as a laser dye and has relatively high solution pumping laser efficiency, narrowed ultraviolet and visible absorption spectrum and fluorescence emission spectrum, high fluorescence quantum efficiency and good light stability.
Owner:CHANGCHUN INST OF OPTICS FINE MECHANICS & PHYSICS CHINESE ACAD OF SCI
Compound for detecting secondary amine, and preparation and application thereof
ActiveCN103694269AHigh fluorescence quantum yieldThe synthesis method is simpleMaterial analysis by observing effect on chemical indicatorGroup 3/13 element organic compoundsAnthraceneBenzene
The invention relates to a compound for detecting secondary amine, and preparation and application thereof. The compound contains boron ester and a BODIPY structure unit in conjugate connection with the boron ester. The compound has a structural general formula shown as in (I), wherein R1 and R2 are independently selected from the group consisting of benzene, naphthalene, anthracene, pyrene, carbazole, triphenylamine, binaphthalene, thiophene, quinoline, imidazole and rhodamine. The preparation method has the advantages of simpleness, high yield, easy separation and high purity. The compound provided by the invention has a novel structure and rapid and sensitive detection of secondary amine; and when the compound reacts with secondary amine, the film turns into purple from red, and the fluorescence is quenched. The test paper and film prepared from the compound react with secondary amine, and significant changes of the color and fluorescence take place in a few minutes and are visible to the naked eyes, so as to conveniently identify secondary amine.
Owner:SHANGHAI INST OF MICROSYSTEM & INFORMATION TECH CHINESE ACAD OF SCI
Phosphorescent polymer compound and organic light emitting device using the same
InactiveUS20070155928A1Increase the driving voltageLow power efficiencyIndium organic compoundsDischarge tube luminescnet screensLow voltageOrganic light emitting device
The present invention relates to a phosphorescent polymer compound comprising a phosphorescent monomer unit and a hole transporting monomer unit having a triphenylamine structure represented by the formula (1): (in the formula, the symbols have the same meanings as defined in the Description), and an organic light emitting device using the compound. Use of the phosphorescent polymer compound of the present invention enables production of organic light emitting device with a high light emitting efficiency at a low voltage, which is suitable for increasing the emission area and mass production.
Owner:SHOWA DENKO KK
Organic electroluminescent device
ActiveUS20110073852A1Improve efficiencyReduce the driving voltageOrganic chemistrySolid-state devicesEngineeringAniline
A problem of the invention is to provide an organic EL device having a high efficiency, a low driving voltage and a long life, by combining various materials for organic EL device, which are excellent in an injection or transportation performance of holes or electrons, and in stability and durability in a thin film, so as to enable the respective materials to effectively reveal their characteristics. The invention relates to an organic electroluminescent device including at least an anode electrode, a hole-injecting layer, a hole-transporting layer, an emitting layer, an electron-transporting layer and a cathode electrode in this order, in which the hole-injecting layer contains an arylamine compound having, in its molecule, a structure in which three or more triphenylamine structures are connected through a single bond or a hetero atom-free divalent group; and the hole-transporting layer contains an arylamine compound having two triphenylamine structures in its molecule.
Owner:HODOGOYA CHEMICAL CO LTD
Carrier transmission material, carrier transmission layer and organic light-emitting device
The invention relates to a carrier transmission material which is a thermal activation delayed fluorescence material. The thermal activation delayed fluorescence material is selected from compounds formed by matching benzonitrile and carbazole or triphenylamine and compounds formed by matching triazine and carbazole, indole carbazole or triphenylamine. The carrier transmission material, namely thethermal activation delayed fluorescence material (TADF) is small in energy gap among mono-triplet state and high in triplet state, and excitons can be avoided being diffused to a carrier transmissionlayer from an organic light-emitting layer, so that light-emitting efficiency is improved. In addition, the carrier transmission material is high in glass transition temperature Tg and thermostability. Due to bipolarity, electron and hole transmission capacity of the carrier transmission material can be adjusted conveniently by adjusting number and proportion of donor groups and receptor groups.The invention further provides a carrier transmission layer and an organic light-emitting device.
Owner:KUNSHAN GO VISIONOX OPTO ELECTRONICS CO LTD +1
Triphenylamine derivative, a method for manufacturing triphenylamine derivates and electronic photographic photoreceptor
InactiveCN101676260AExcellent sensitivity characteristicsLower ionization potentialOrganic compound preparationElectrography/magnetographySolubilityAniline
The present invention provides a triphenylamine derivative having excellent solubilization of a solvent and capable of dissolving with binding resin and improving effectively light sensitivity when the triphenylamine derivative is used as a hole transport agent of electronic photographic photoreceptor, a method for manufacturing the triphenylamine derivative and an electronic photographic photoreceptor. The invention employs triphenylamine derivative represented by formula (1) and (6).
Owner:KYOCERA DOCUMENT SOLUTIONS INC
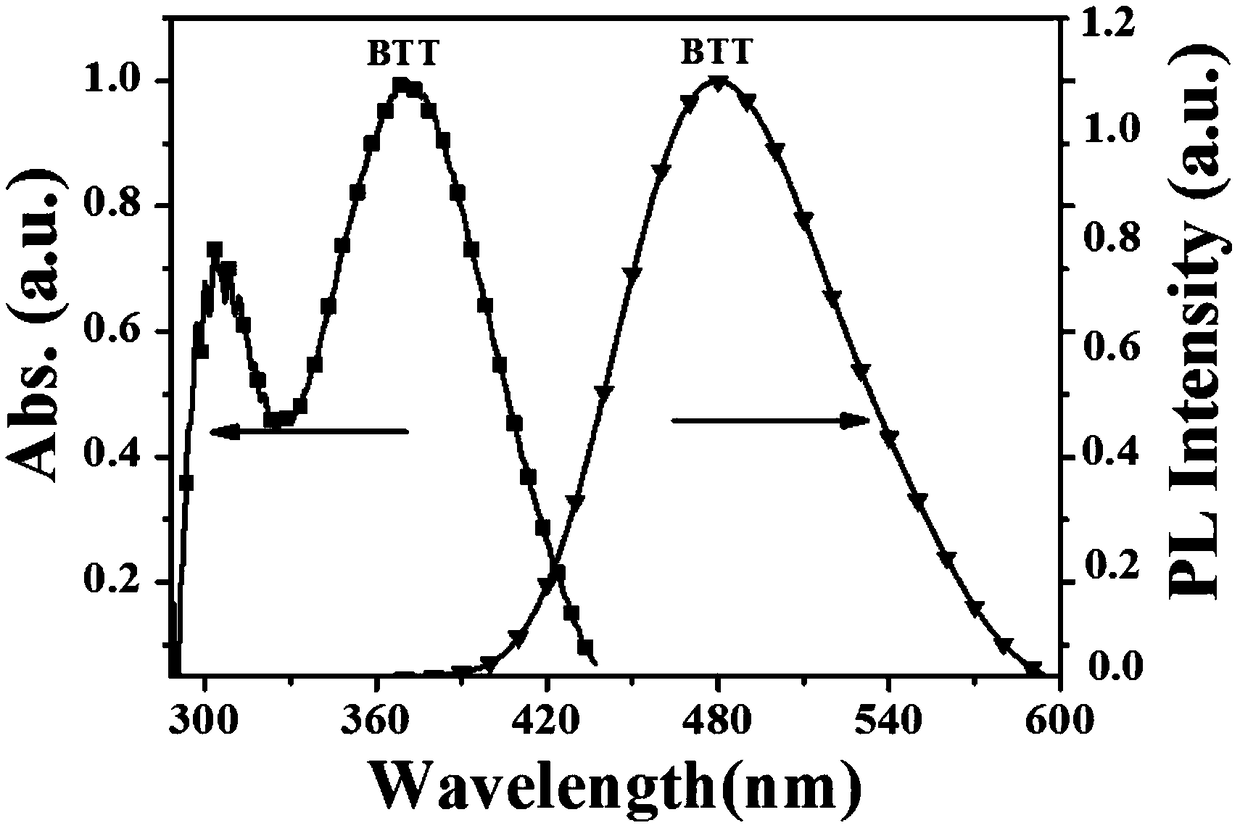
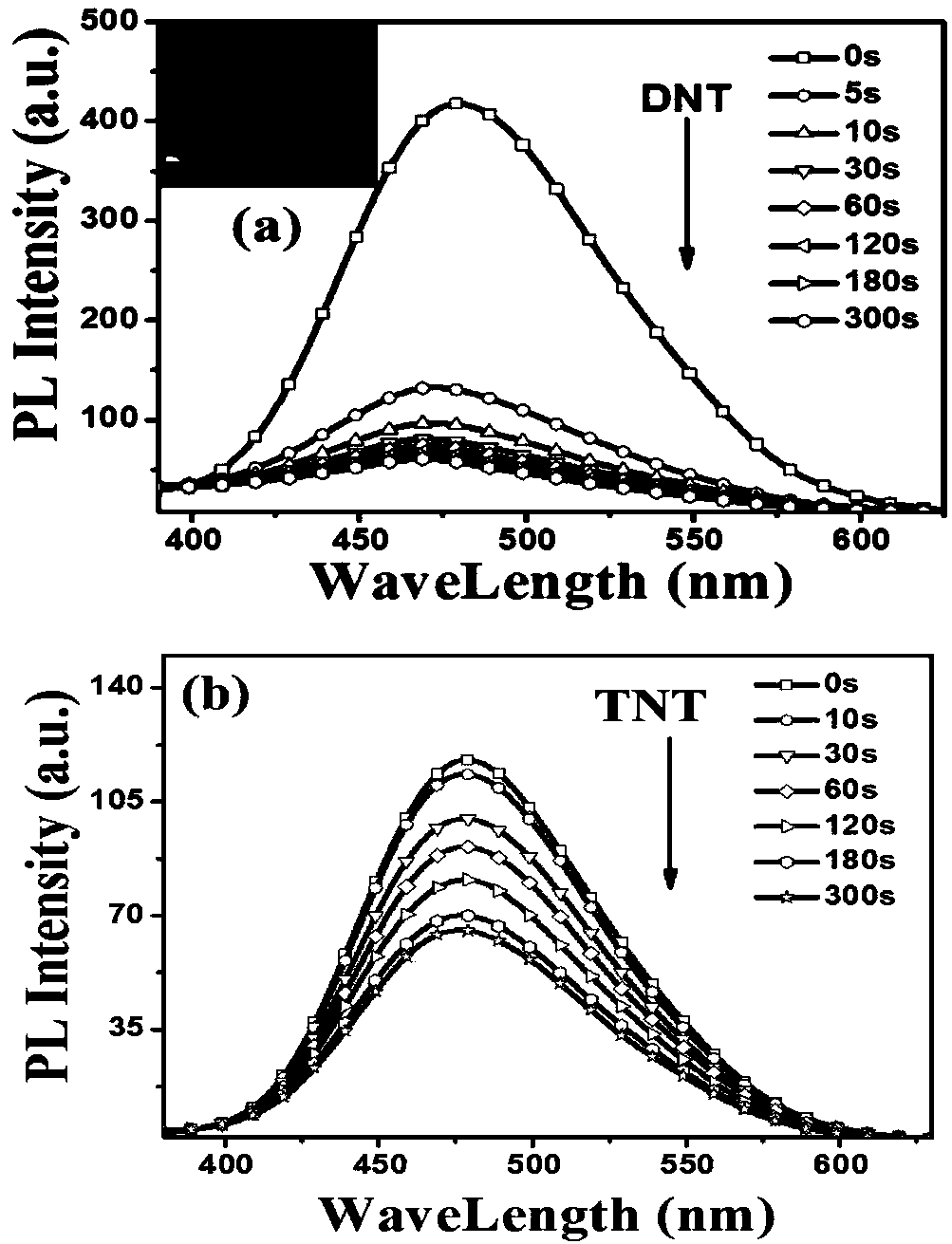
Aggregation-induced luminescent organic fluorescent small-molecule material and application thereof in DNT and TNT gas fluorescence detection
The invention provides an aggregation-induced luminescent organic fluorescent small-molecule material and application thereof in DNT and TNT gas fluorescence detection and belongs to the technical field of fluorescent sensing. The invention particularly relates to an AIE type fluorescent sensing material which takes 3,4,5-triphenyl-4H-1,2,4-triazole as a structural center and is connected with tetraphenyl ethylene, triphenylamine or carbazole. The material shows weak fluorescence in a dilute solution and shows strong fluorescence in an aggregation process or a solid state. As a core construction unit of the fluorescent molecule, the tetraphenyl ethylene enables the material to have AIE properties, and a benzene ring capable of freely rotating has a promoting effect on constructing a film with a porous structure. The fluorescent material can not only form the film with the porous structure and but also form Pi-Pi stacking with NACS explosives, thereby ensuring that the fluorescent material not only has extremely high selectivity and sensitivity for DNT and TNT detection but also has fast response.
Owner:JILIN UNIV
Preparation method of doping type fluorescent micron-nano fibers
InactiveCN101962818AUniform diameterSmooth diameterFilament/thread formingMonocomponent polyolefin artificial filamentFiberAviation
The invention relates to a preparation method of doping type fluorescent micron-nano fibers, relating to a preparation method of fluorescent fibers and solving the problems of complex method realizing fluorescent light with different colors through the traditional organic fluorescent fibers of a monogenic dye, poor spectrum stability and short service life of physically blended organic fluorescent fibers and large diameters of the traditional organic fluorescent fibers. The preparation method of the doping type fluorescent micron-nano fibers comprises the following steps of: dissolving dialdehyde triphenylamine and poly [2-methoxyl-5(2'-ethylhexoxy) para-phenylacetylene] into a polymer solution according to different mass ratios; and then carrying out electrostatic spinning to obtain the doping type fluorescent micron-nano fibers. The doping type fluorescent micron-nano fibers have good fluorescence spectrum stability, long service life reaching two years and diameters of 10 nanometers-3 micrometers, and can be used in the life and highly technical fields of textile clothing, aviation, navigation, national defense industry, building decoration, transportation, night operation, daily life, amusement, leisure, optical-fiber communication, laser waveguide, and the like.
Owner:HEILONGJIANG UNIV
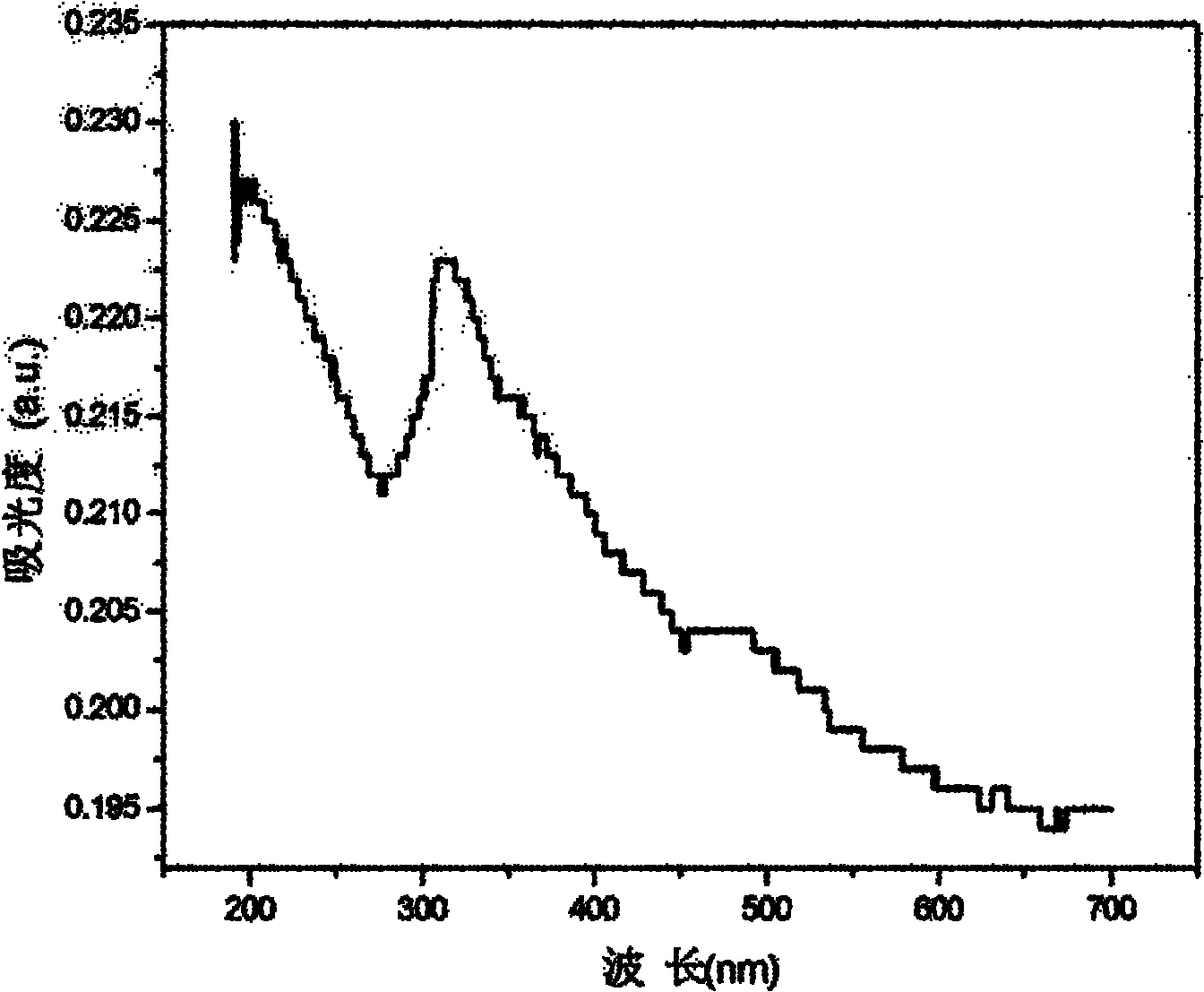
Triphenylamine-thiophene organic dyestuff as well as preparation method and application thereof
InactiveCN103554957AInhibitory complexIncrease the open circuit voltageLight-sensitive devicesOrganic chemistryOrganic dyeThiophene derivatives
The invention provides a triphenylamine-thiophene organic dyestuff. The structural general formula of the triphenylamine-thiophene organic dyestuff is as shown in a formula (I), wherein in the formula (I), R1 is C1-C6 alkoxy, the structural formula of Ar is as shown in a formula (II) or a formula (III), and R2 in the formulas (II) and (III) is C1-C6 alkyls. A preparation method of the triphenylamine-thiophene organic dyestuff comprises the following steps: sequentially carrying out Suzuki coupled reaction and Knoevenagel condensation reaction by using aryl bromal, tetrakis(triphenylphosphine)palladium(0), potassium carbonate and alkoxy substituted triphenylamine boron ester as raw materials, thus obtaining a target product. The triphenylamine-thiophene organic dyestuff can serve as a photosensitizer of a dye-sensitized solar cell. The triphenylamine-thiophene organic dyestuff has the advantages that the distortion spatial structure of alkyl triphenylamine is capable of effectively suppressing the electron recombination and increasing the open-circuit voltage; a thiophene derivative serving as a dyestuff molecule conjugated bridge is capable of ensuring the high molar absorption coefficient and has relatively strong power supply performance, so that the ideal photoelectric conversion efficiency is ensured.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Polyurethane with electrochromism performance and preparation method thereof
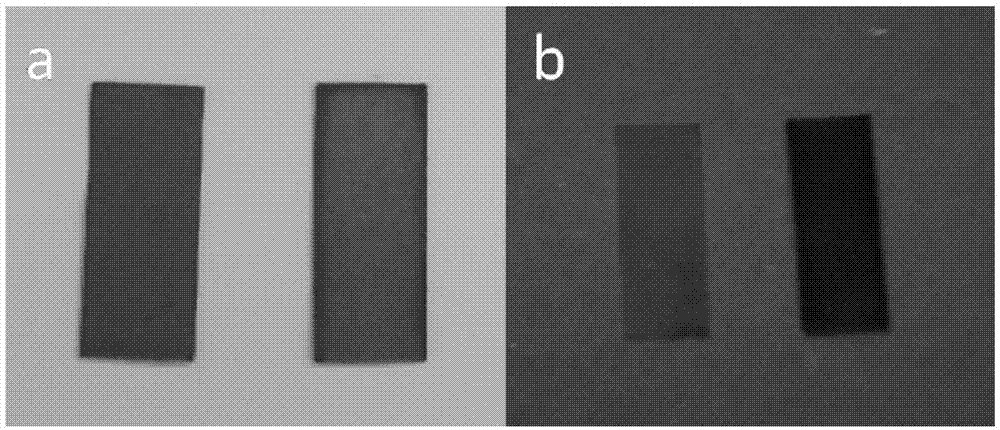
InactiveCN102702461AGood electrochemical redox reversibilityShort response timeCarbamic acid derivatives preparationOrganic compound preparationPolymer scienceBenzaldehyde
The invention discloses polyurethane with electrochromism performance and a preparation method of the polyurethane, and relates to polyurethane and a preparation method thereof. The invention solves the problem that the prior polymer film is easy to drop in the organic solution, so that the electrochromism performance of the prior polymer film is difficult to detect. The structural formula of polyurethane is as follows: the method comprises the following steps of: enabling a diamido-triphenylamine derivative and p-hydroxy benzaldehyde to react to synthesize a monomer; enabling the monomer and isocyanate mixed liquid to react for 12-14 hours at 120-140 DEG C; and filtering and drying the product. The polyurethane disclosed by the invention has good electrochemical oxidation reduction reversibility, fast response time of color change and better chemical stability. The invention is applied in the electrochromism field.
Owner:HEILONGJIANG UNIV
Preparation method and application of triphenylamino metal organic framework compound capable of catalyzing carbon dioxide-epoxy compound cycloaddition
ActiveCN106423282AEasy to synthesizeSynthetic and easy to operateOrganic-compounds/hydrides/coordination-complexes catalystsOrganic chemistry methodsSolventCarbon dioxide
The invention belongs to the technical field of catalytic materials, and relates to a preparation method and application of a triphenylamino metal organic framework compound capable of catalyzing carbon dioxide-epoxy compound cycloaddition. The preparation method comprises the following steps: 1. adding a connection ligand L and a transition metal salt Tm in a mole ratio of 1:(3-5) into a mixed solvent composed of ethanol and N,N-diethyl formamide in a volume ratio of 1:(2-2.5) or a mixed solvent composed of concentrated nitric acid and N,N-dimethylformamide in a volume ratio of 1:(6-8), and uniformly stirring; and 2. putting the solution prepared in the step 1 into a drying oven, drying for 60-90 hours while controlling the temperature at 60-120 DEG C, shutting down the drying oven, cooling to room temperature to precipitate a crystal, filtering and drying to obtain the target material Tm-L. The catalyst synthesis process is simple and easy to operate, and has the advantages of low raw material price for the catalyst and catalytic reaction, and high yield. The obtained functional material has stable chemical properties, and can easily implement large-area popularization and application.
Owner:DALIAN UNIV OF TECH
Triphenylamine two-photon fluorescence probe compound and preparation method and application thereof
InactiveCN103396789AEnhanced two-photon absorption cross sectionImprove the coordination effectOrganic chemistryColor/spectral properties measurementsSolubilitySilver ion
The invention belongs to the field of organic nonlinear optical materials, and particularly relates to a triphenylamine two-photon fluorescence probe compound and a preparation method and an application thereof. The preparation method of the triphenylamine two-photon fluorescence probe compound comprises the following steps of: injecting anhydrous benzene into 4-nitrapyrin-2, 6-dimethyl isophthalate and triphenylphosphine under the protection of nitrogen; increasing temperature, refluxing, and cooling; filtering and washing to obtain chlorination-4-methylene triphenylphosphine group-pyridine-2,6-dimethyl isophthalate, and dissolving into methanol; mixing and stirring tri(4-formylphenyl)amine and absolute methanol; adding the methanol solution of the chlorination-4-methylene triphenylphosphine group-pyridine-2,6-dimethyl isophthalate and the methanol solution of sodium methylate; stirring at 0-5 DEG C for 1-2 hours, stirring at normal temperature for 3-4 hours; separating through column chromatography. The triphenylamine two-photon fluorescence probe compound disclosed by the invention has the advantages of outstandingly enhanced two-photon absorption section, good water solubility, higher reaction activity and very high coordination capacity and can be applied to silver ion detection and pH detection.
Owner:SHANGHAI NORMAL UNIVERSITY
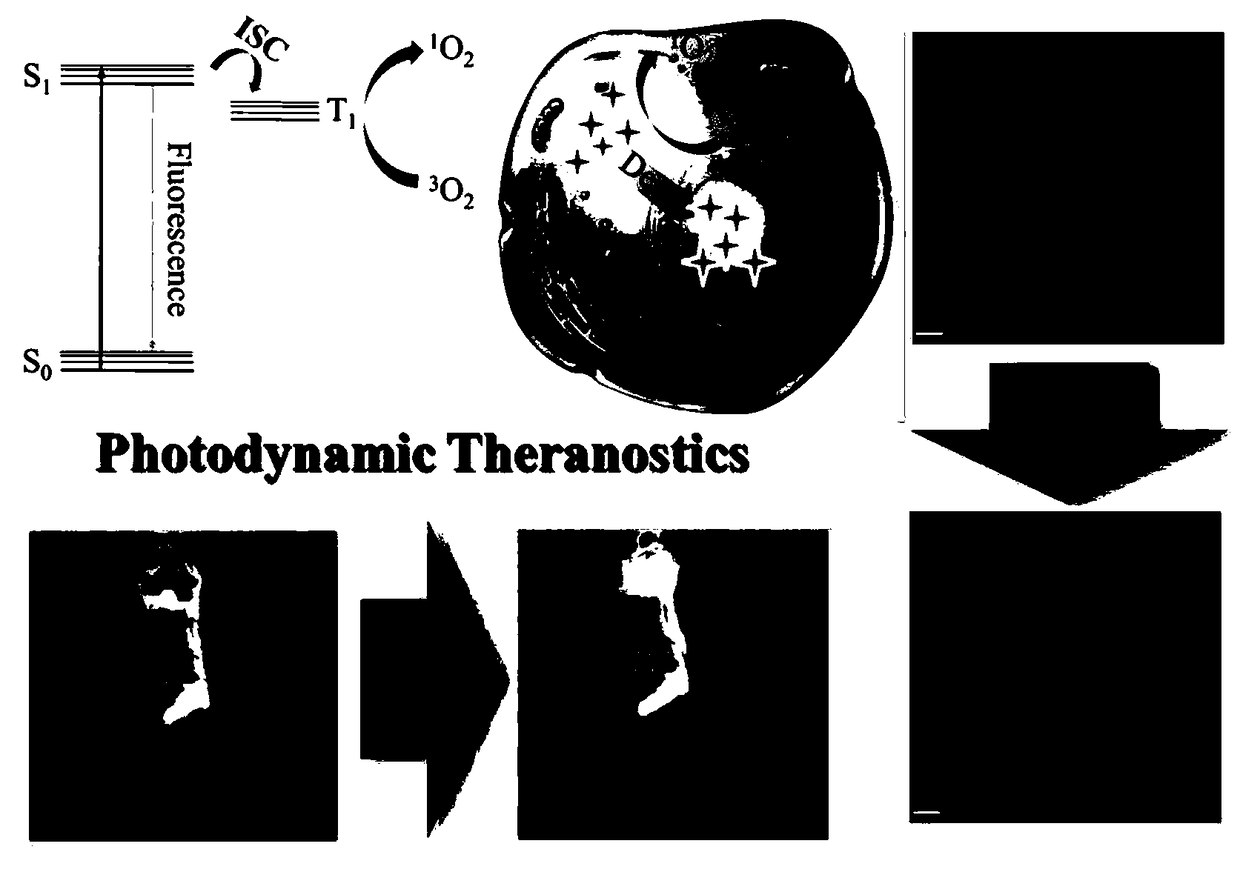
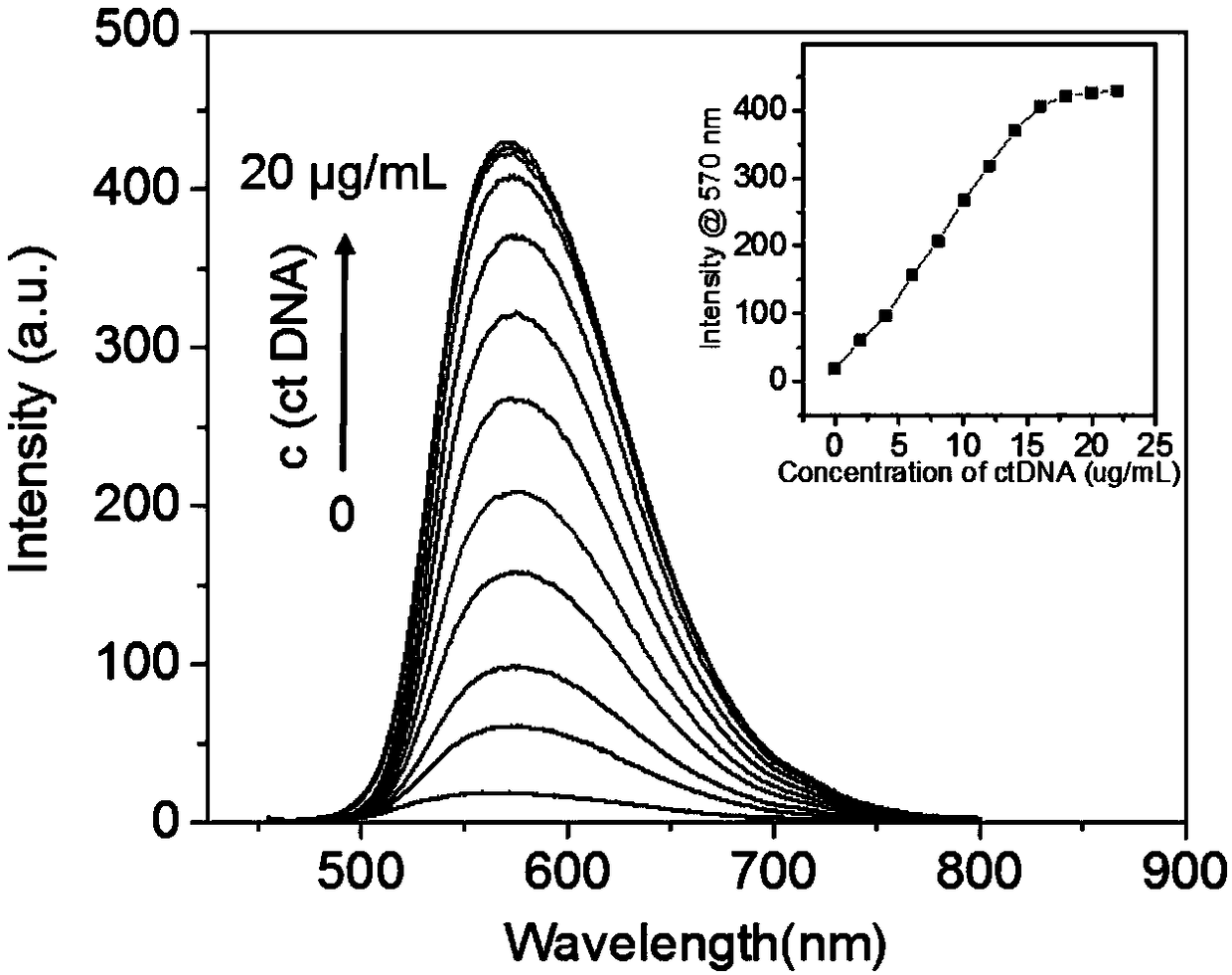
Triphenylamine polypyridine salt based photosensitizer and preparation method and application of triphenylamine polypyridine salt based photosensitizer
ActiveCN108727256AEfficient killingCause deathOrganic chemistryPhotodynamic therapy4-pyridineboronic acidSinglet oxygen
The invention discloses a triphenylamine polypyridine salt based photosensitizer and a preparation method and an application of the triphenylamine polypyridine salt based photosensitizer, and belongsto the technical field of biomedical engineering. The preparation method comprises enabling triphenylamine monoaldehyde diiodine and 4-pyridineboronic acid to have a coupling reaction, generating triphenylamine monoaldehyde bipyridine, then enabling the triphenylamine monoaldehyde bipyridine and an electron withdrawing group to have a condensation reaction, enabling an obtained product to have a salt forming reaction with iodomethane, and obtaining the photosensitizer. The photosensitizer has the electron withdrawing group, the singlet oxygen is produced efficiently under the illumination condition, the purpose of rapidly efficiently killing a tumor cell is fulfilled; the photosensitizer not only efficiently kills the tumor cell and inhibits the growth of the tumor, but also monitors the death of the tumor cell and a tumor tissue in real time, and therefore, a photosensitizer medicine can be used to judge the effect of the photosensitizer on the tumor cell. The preparation method of the photosensitizer is simple, and the cost is low.
Owner:HUAZHONG UNIV OF SCI & TECH
Image forming apparatus and image forming method
An image forming apparatus comprising an electrophotographic photoreceptor to form a toner image by developing an electrostatic latent image with a developer containing a toner and a developing device, in which the photoreceptor contains a charge transport compound having a triphenylamine structure and the developing device supplies a toner having a total content of aromatic volatile compounds of 5 to 30 ppm; an image forming method employing the foregoing electrophotographic photoreceptor and developing device.
Owner:KONICA MINOLTA INC
Electrochromatic material made of isocyanate-triphenylamine and method for preparing electrochromatic material
InactiveCN102675589AGood electrochemical redox reversibilityShort response timeTenebresent compositionsSolid reactionNitrogen
The invention relates to electrochromatic materials and preparation methods thereof, particularly relates to an electrochromatic material made of isocyanate-triphenylamine and a method for preparing the electrochromatic material, and aims to solve the problem that films made of existing electrochromatic materials are prone to shed during test and poor in resistance to solvent corrosion. The structural formula of the electrochromatic material made of isocyanate-triphenylamine is shown as follows. The method includes: adding diisocyanate- triphenylamine or triisocyanate- triphenylamine into anhydrous dichloroethane, stirring under the shielding of nitrogen to enable the diisocyanate- triphenylamine or triisocyanate- triphenylamine to be completely dissolved and well mixed, heating the obtained mixture, adding dihydroxyl monomer, performing condensation backflow reaction at the temperature after heating, then cooling to the room temperature, and finally drying the solid reaction product obtained after filtering separation to constant weight to obtain the electrochromatic material. The method is mainly used for electrochromatic materials made of isocyanate-triphenylamine.
Owner:HEILONGJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com