Triphenylamine polypyridine salt based photosensitizer and preparation method and application of triphenylamine polypyridine salt based photosensitizer
A technology of benzothiadiazole, triphenylamine, pyridine, and triphenylamine, which is applied in the field of biomedical engineering, can solve the problems of inability to monitor the cell state in real time, low singlet oxygen quantum yield of photosensitizer, etc., to promote cancer cell death, nuclear The effect of enhanced membrane permeability and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the synthesis of photosensitizer JP2
[0035] (1) Synthesis of compound III
[0036]
[0037] In a 100ml two-necked flask, add triphenylamine monoaldehyde diiodide IV (4.2g, 8mmol), cesium carbonate (5.21g, 16mmol), catalyst tetraphenylphosphine palladium (0.92g, 0.8mmol) and 50ml toluene, nitrogen protection , heated to 60°C, stirred for 10min, injected ethanol dissolved with 4-pyridineboronic acid (2.96g, 24mmol) into the two-necked flask, raised the temperature to 90°C, and refluxed for 10h. After the reaction was completed, it was cooled to room temperature and filtered with celite to remove the catalyst. The filtrate was spin-dried, extracted with ethyl acetate and saturated saline solution, and the crude product was spin-dried for column chromatography (eluent: DCM:Ethanol=50:1). Obtained III, yield: 3.15 g (92%). 1 H NMR (400MHz, cdcl 3 )δ9.87(s,1H),8.66(dd,J=4.5,1.6Hz,4H),7.89–7.71(m,2H),7.68–7.59(m,4H),7.50(dd,J=4.5, 1.6Hz, 4H), 7.37–7.25(...
Embodiment 2
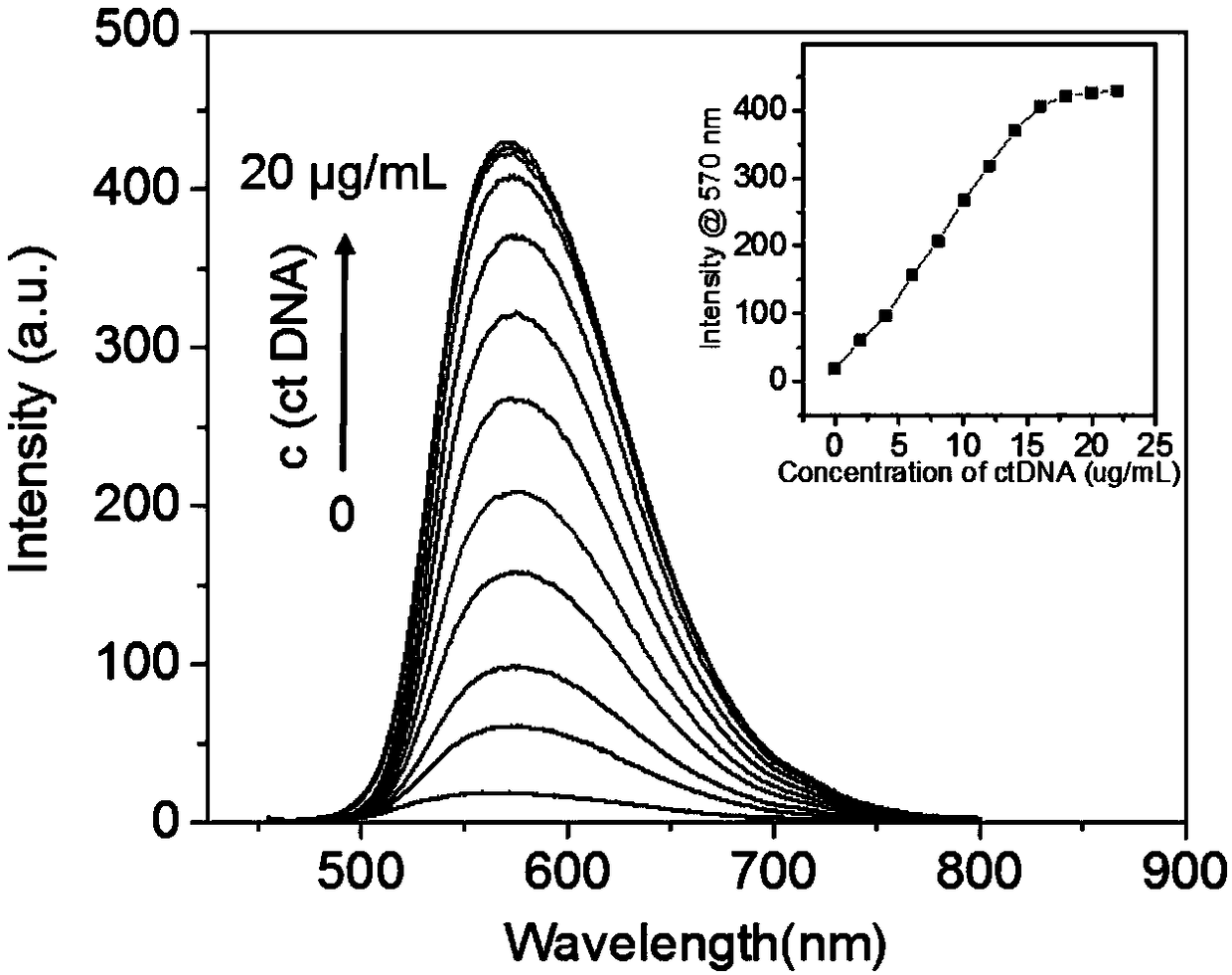
[0044] Example 2: Fluorescence changes of photosensitizer JP2 in different concentrations of ctDNA
[0045] At a concentration of 10 μmol L -1 Different concentrations of ctDNA were added to the PBS solution of photosensitizer JP2. -1 , the excitation wavelength is 445nm, with the increase of ctDNA concentration, the fluorescence intensity is continuously enhanced, such as figure 2 shown. It shows that JP2 and ctDNA have a certain binding ability, which can stimulate the aggregation-induced luminescent performance of JP2 itself, thereby enhancing the fluorescence. This is also the main reason why JP2 lights up the nucleus.
Embodiment 3
[0046] Example 3: Cell death of living HeLa cancer cells under illumination at different times
[0047] After HeLa cells were incubated with 10 μM photosensitizer JP2 for 2 h, the residual dye was washed away with PBS solution and then incubated with mitochondrial dye Mito Tracker Deep Red for 30 min. Observe the fluorescence distribution of the photosensitizer in the cells under a microscope, and under the irradiation of the confocal built-in light source (488nm, 10mW), the obtained image 3 the result of which image 3 (a), image 3 (e) and image 3 (i) are the bright field images of the cells at 0 seconds, 8 seconds and 15 seconds after adding the photosensitizer JP2, respectively; image 3 (b) for, image 3 (f) and image 3 (j) are the fluorescence images of the cells at 0 seconds, 8 seconds and 15 seconds after adding the photosensitizer JP2, respectively; image 3 (c) for, image 3 (g), image 3 (k) are respectively the staining diagrams of mitochondria in the ce...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com