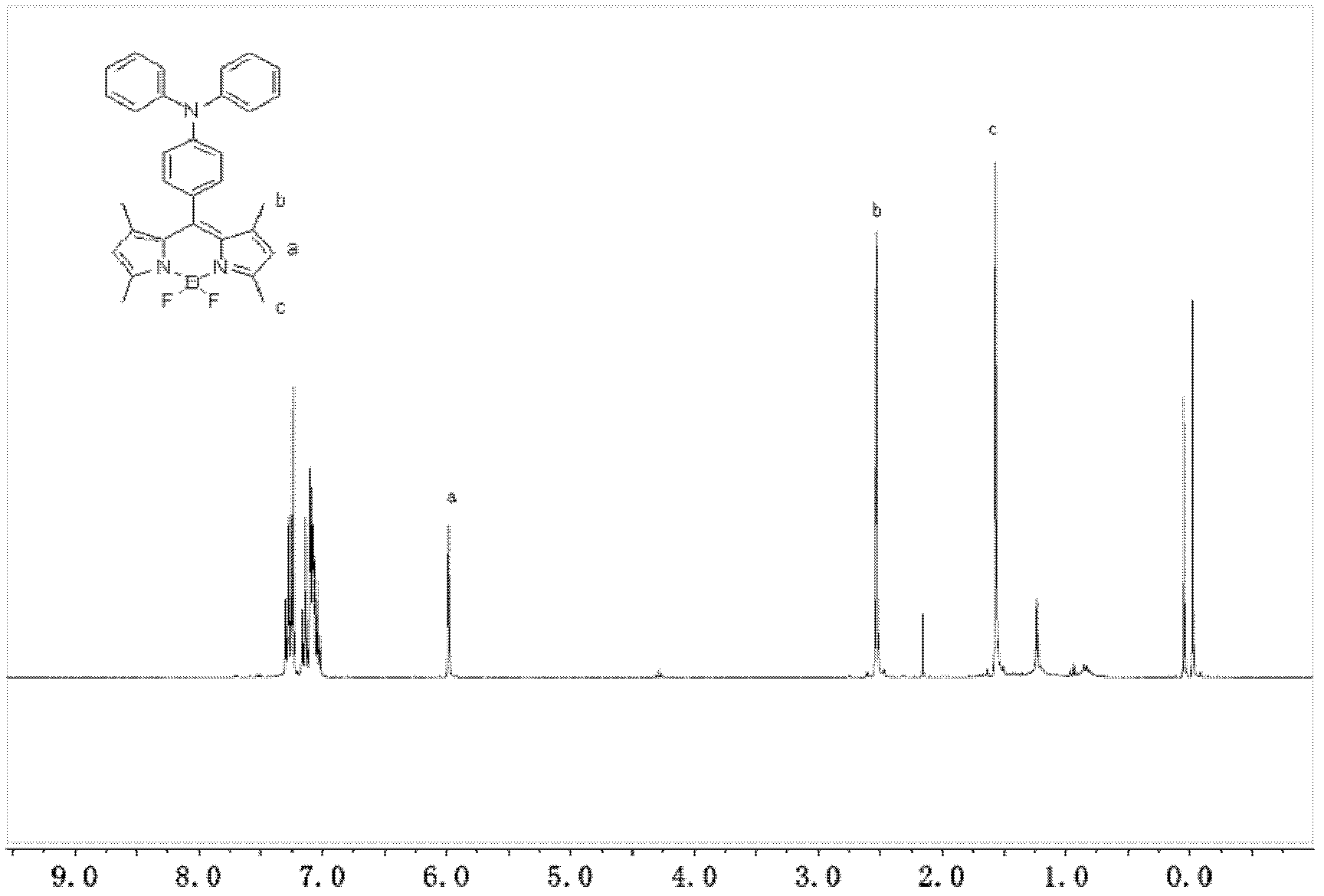
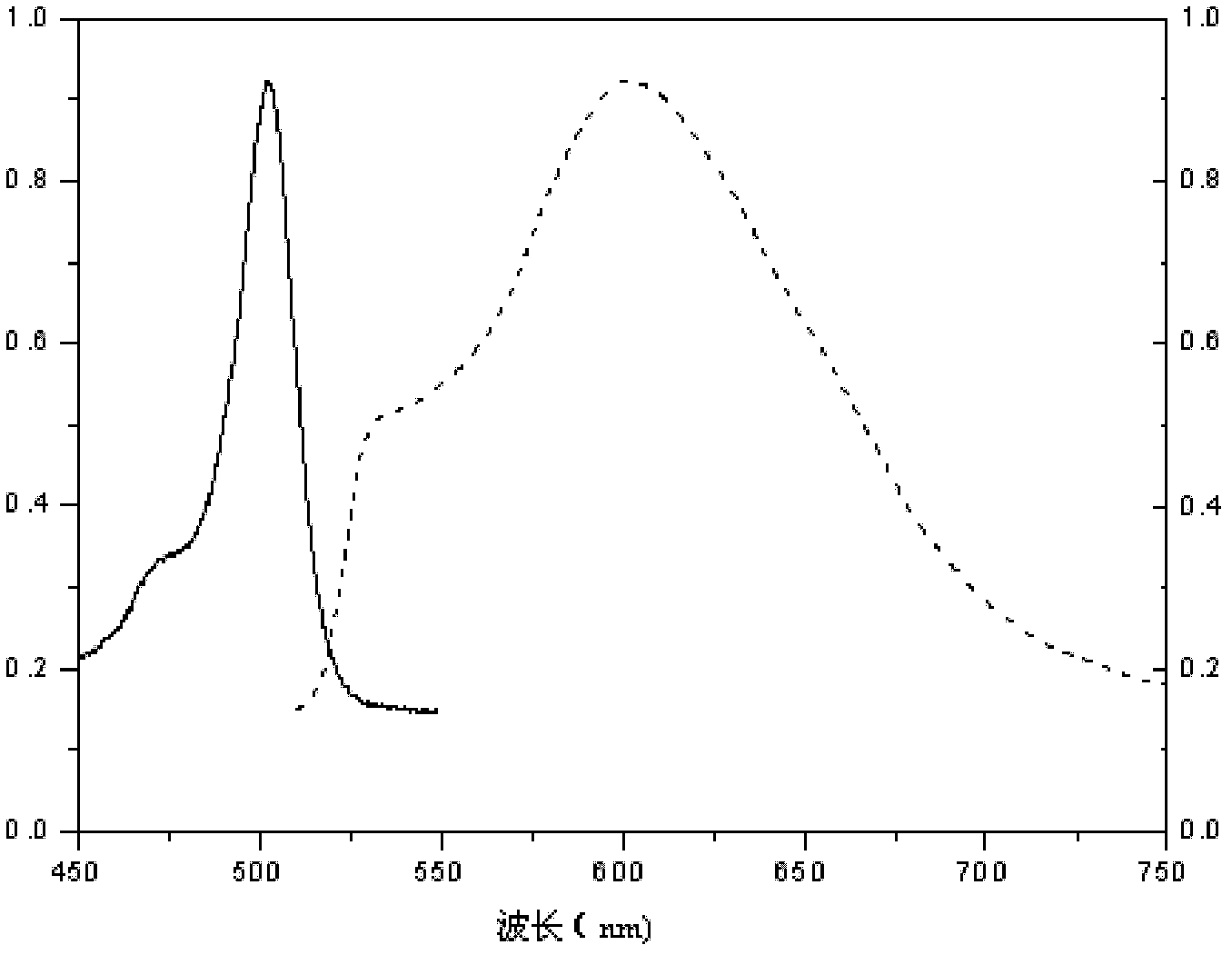
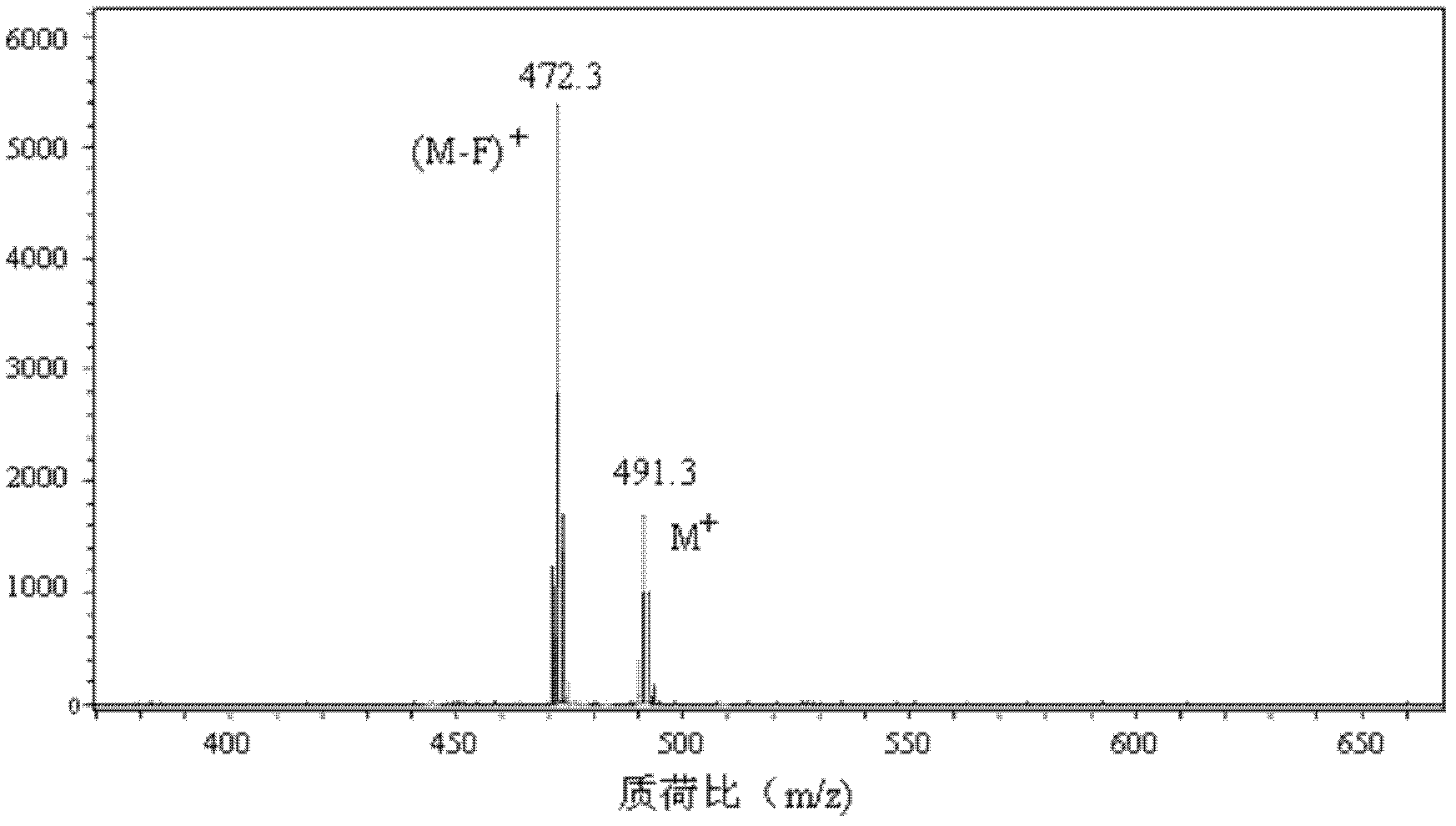1,3,5,7-tetramethyl-8-triphenylamine base pyrrole methane-boron difluoride complex compound and preparation method thereof
A technology of triphenylaminopyrromethene and boron difluoride, which is applied in the field of organic synthesis, can solve the problems of unfavorable detection or observation, increased self-absorption of fluorescent dyes, unfavorable application of live imaging, etc., to achieve wide application and reduce self-absorption Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0041] The preparation method of the present invention will be specifically described below.
[0042] The first step: 546 mg (2.0 mmol) of 4-formyl triphenylamine and 419 mg (4.4 mmol) of 2,4-dimethylpyrrole were dissolved in 100 mL of dried dichloromethane, and then 20 μL of trifluoroacetic acid was added as a catalyst, A reaction system is formed, and the room temperature is protected from light and stirred overnight to generate the first intermediate;
[0043] The second step: take 454mg (2.0mmol) of oxidant 2,3-dichloro-5,6-dicyano-1,4-p-benzoquinone according to the equimolar ratio with 4-formyltriphenylamine and dissolve it in 10mL In methyl chloride, add it to the reaction system that the first intermediate is generated, and stir at room temperature for 30 min to generate the second intermediate;
[0044] The third step: add 3 mL of triethylamine to the reaction system with the second intermediate, stir at room temperature for 10 min, slowly add 3 mL of boron trifluori...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com