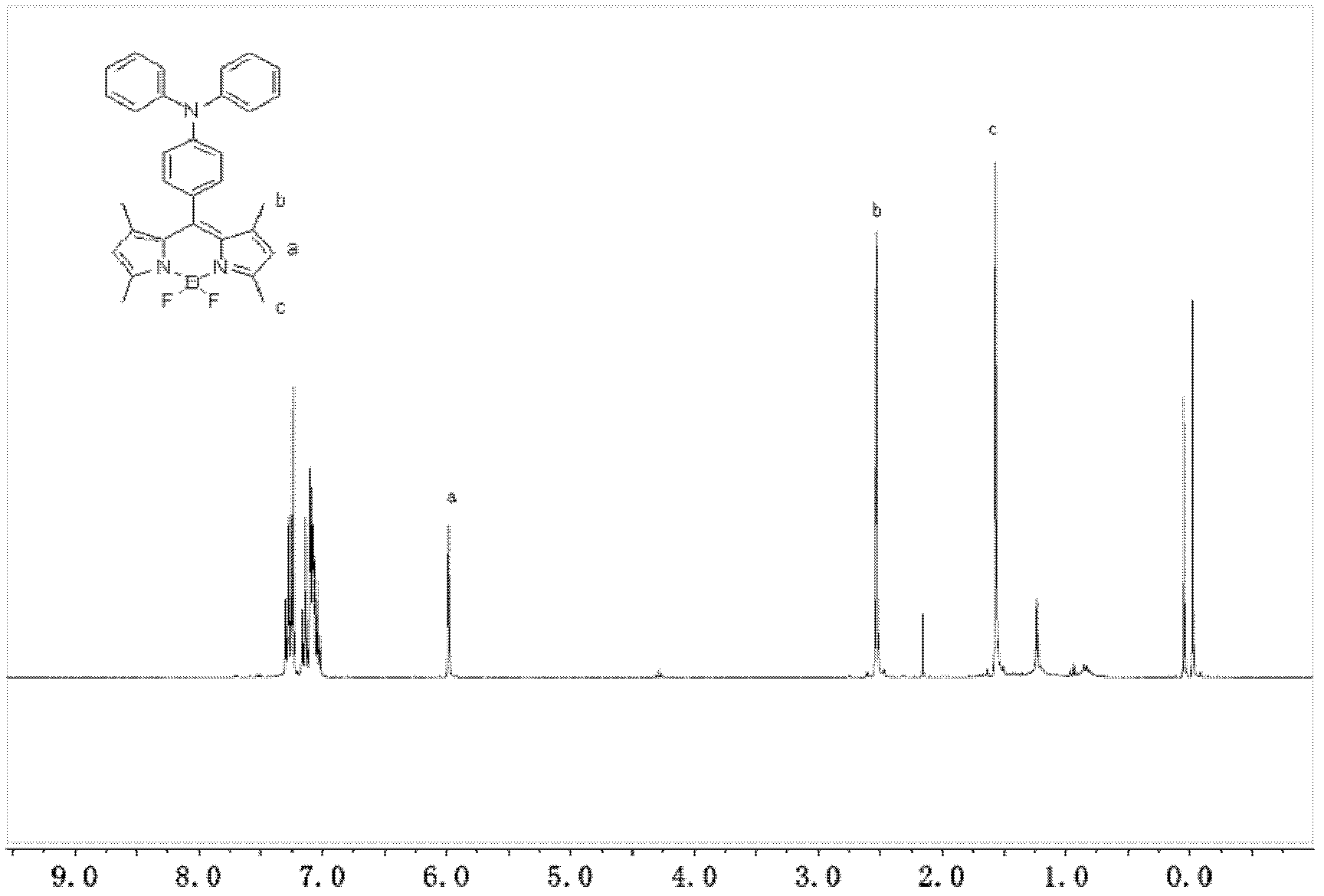
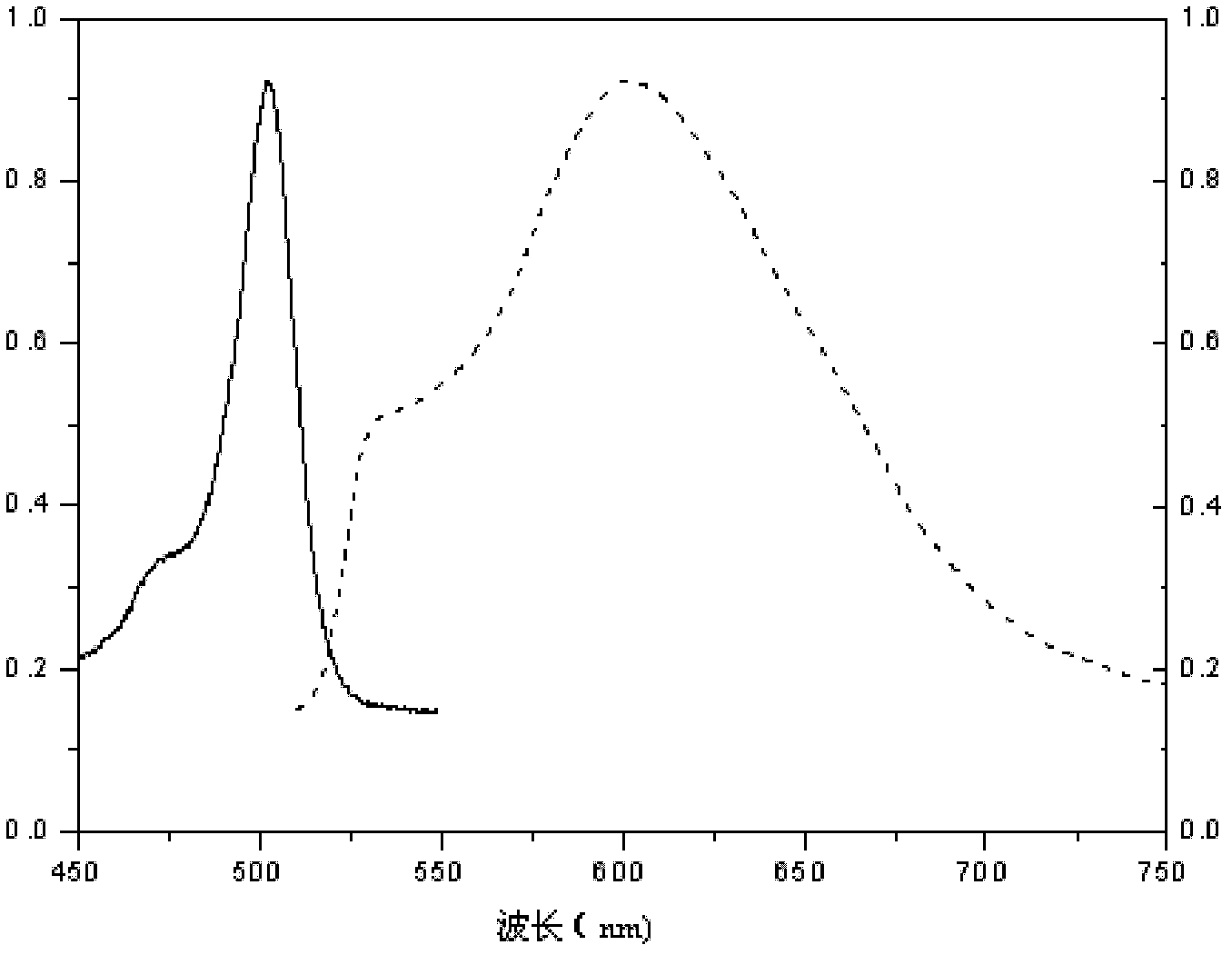
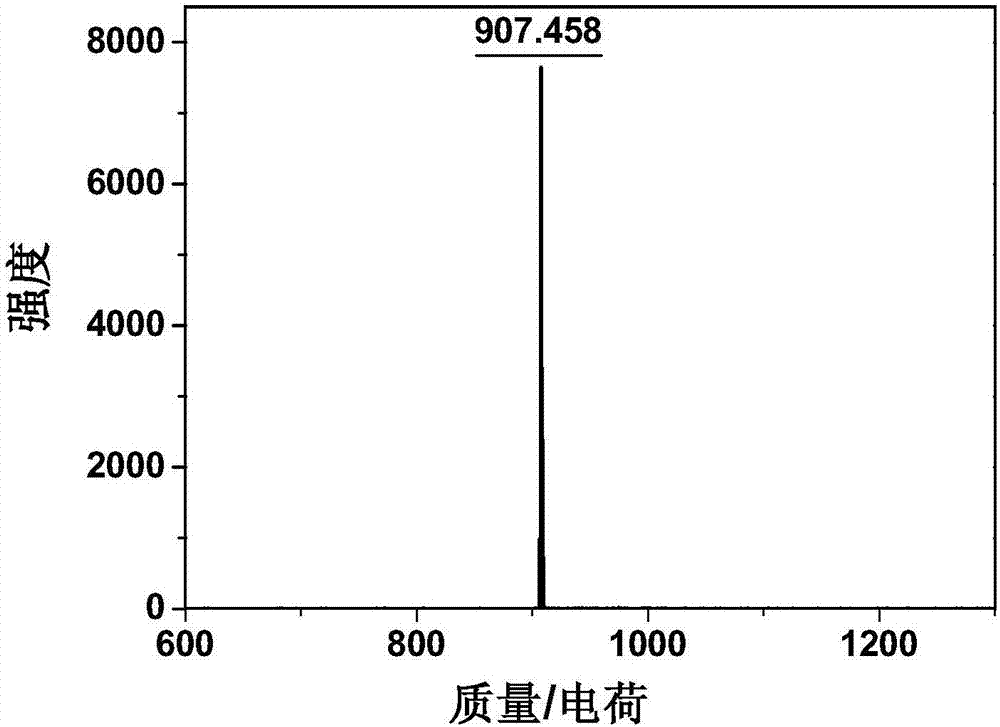
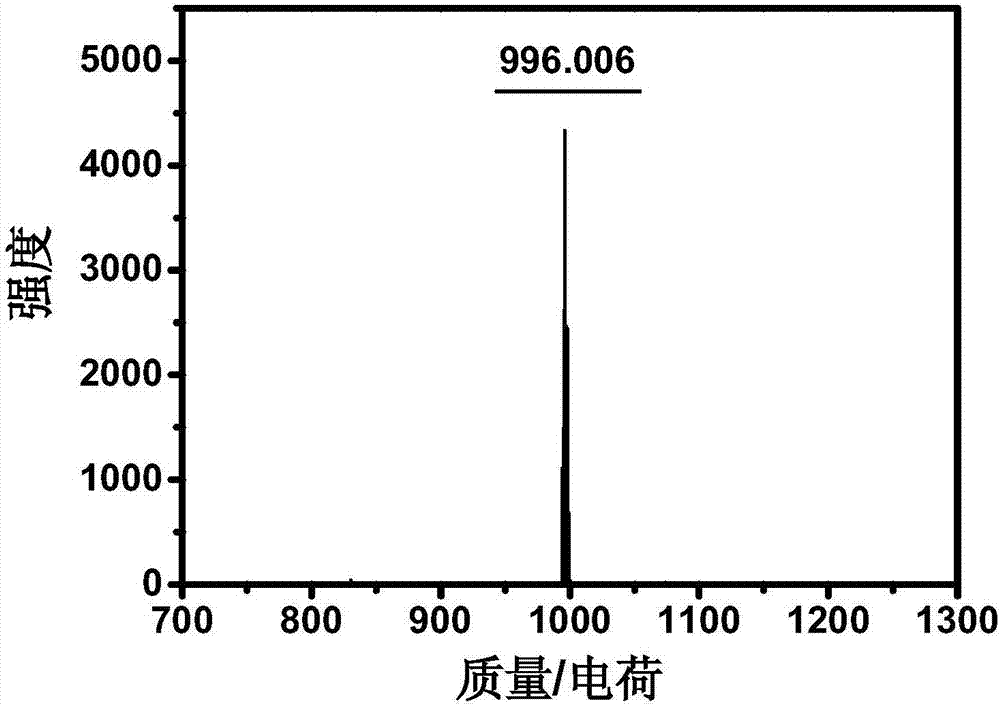
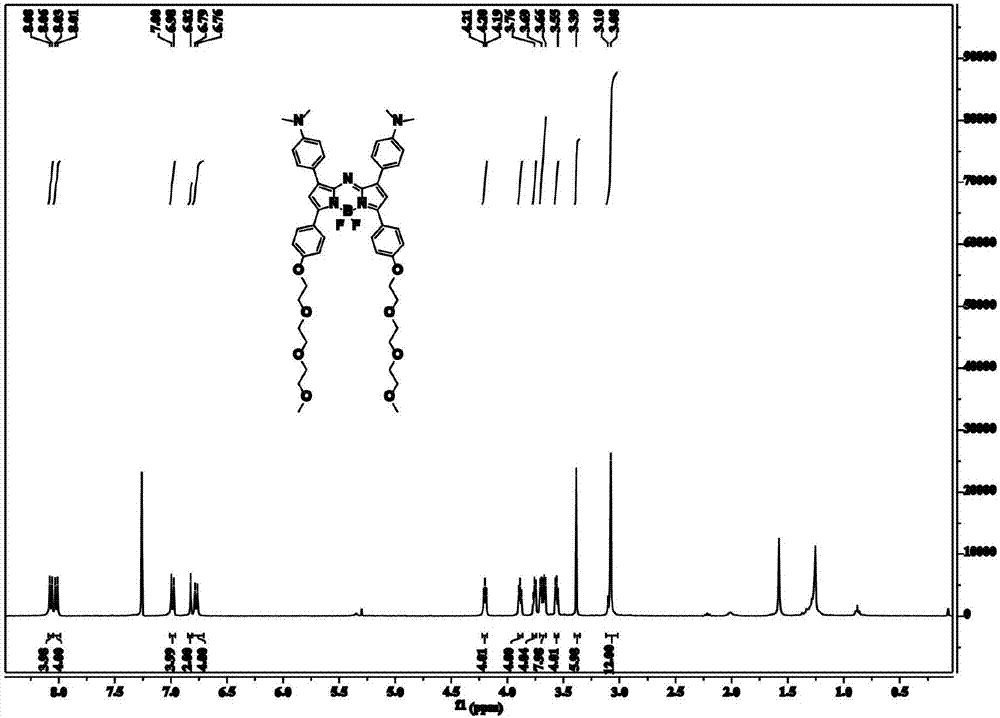
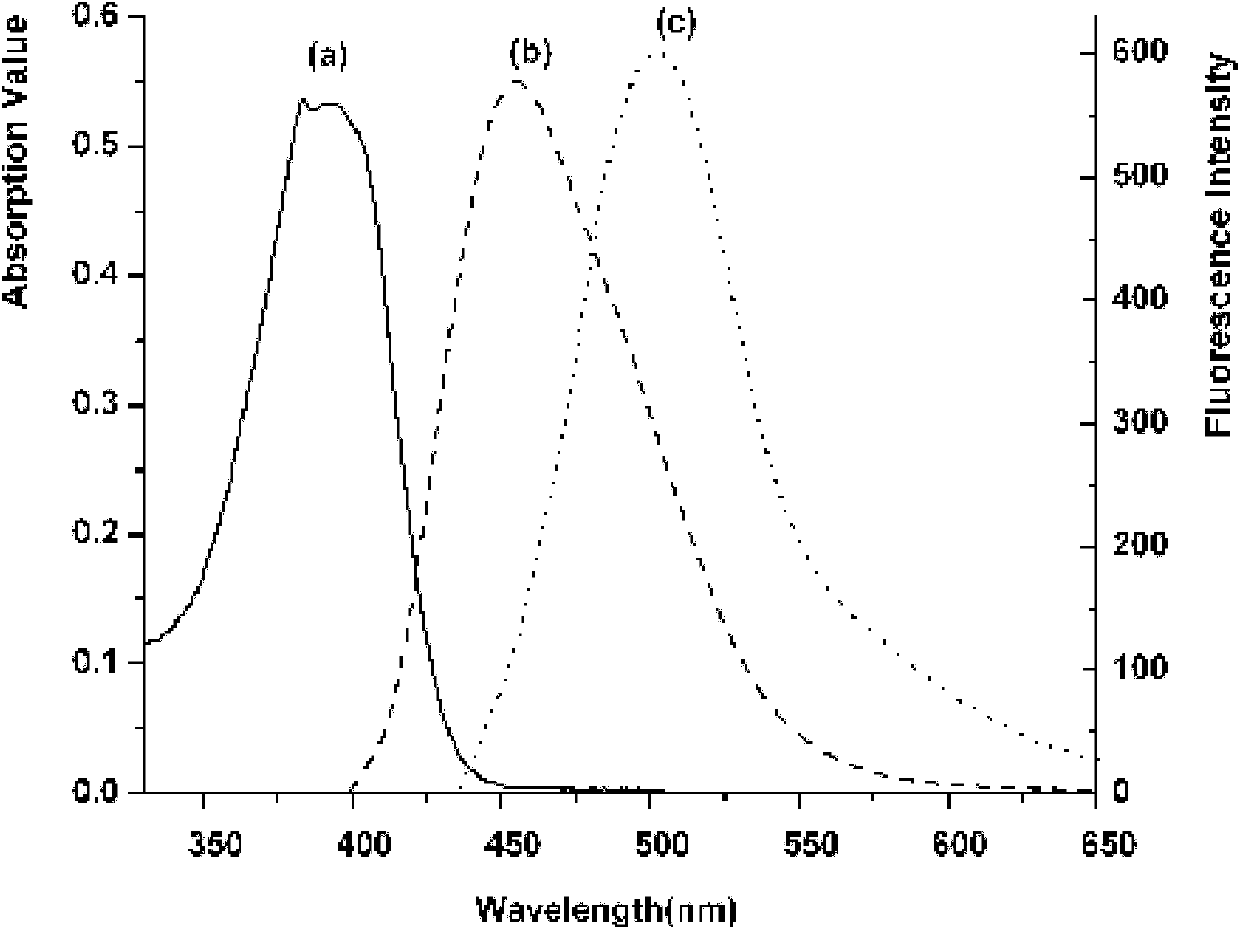
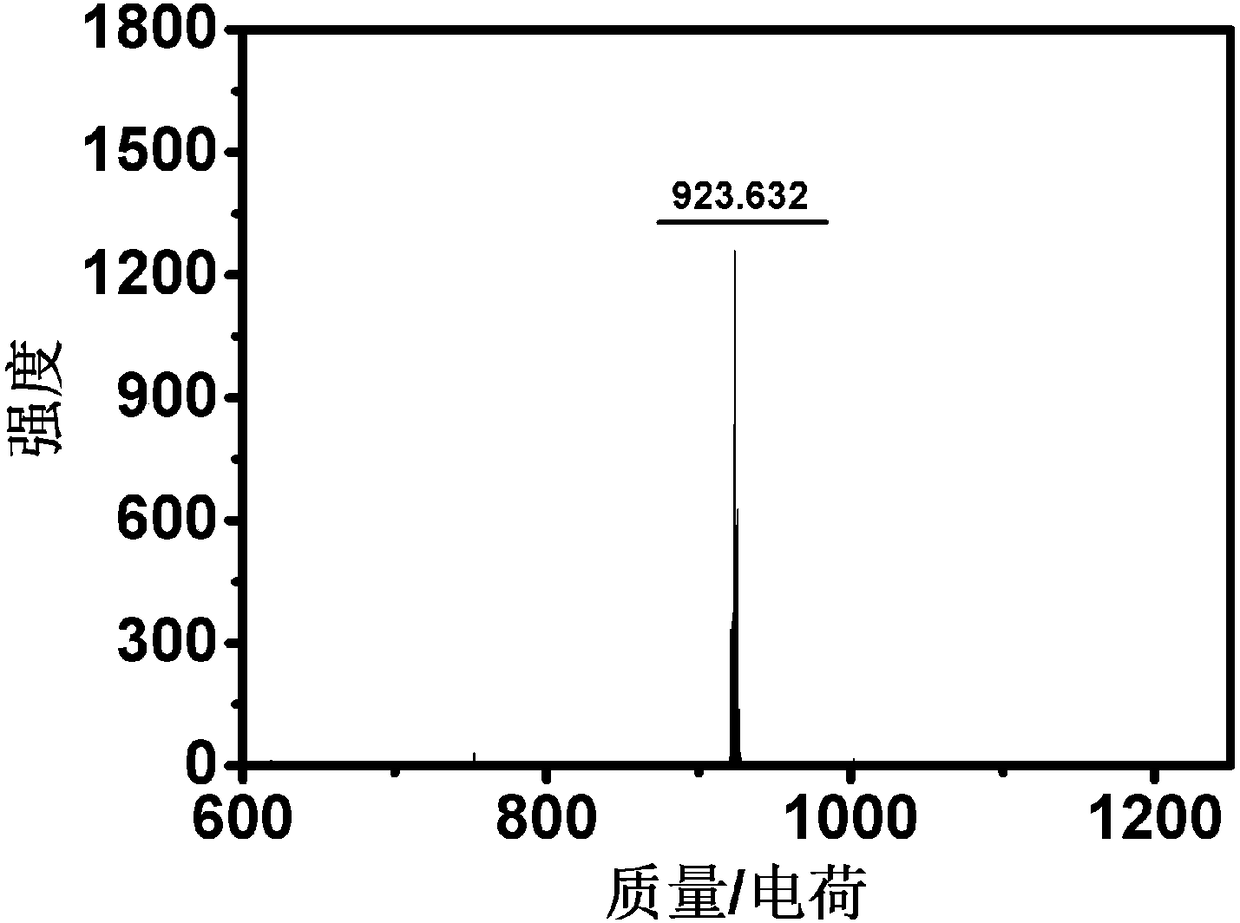
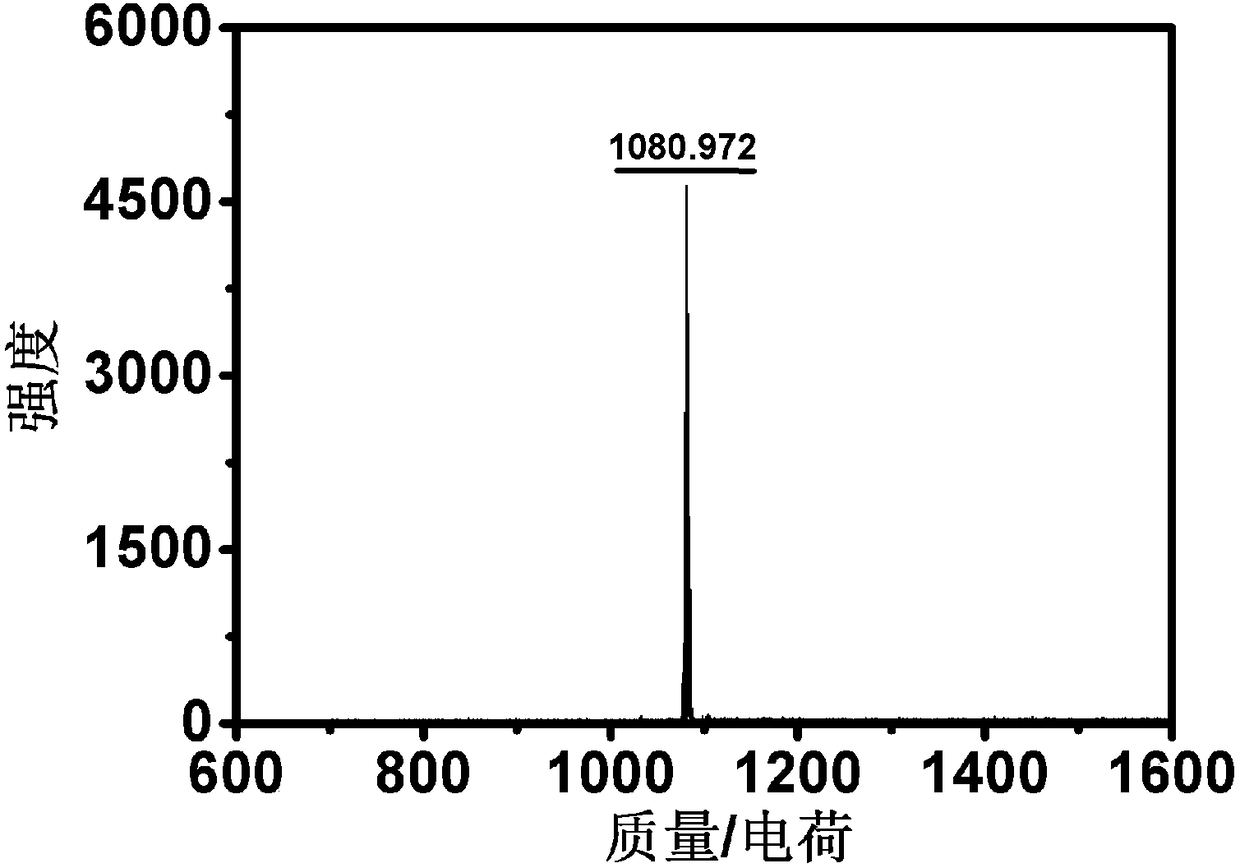
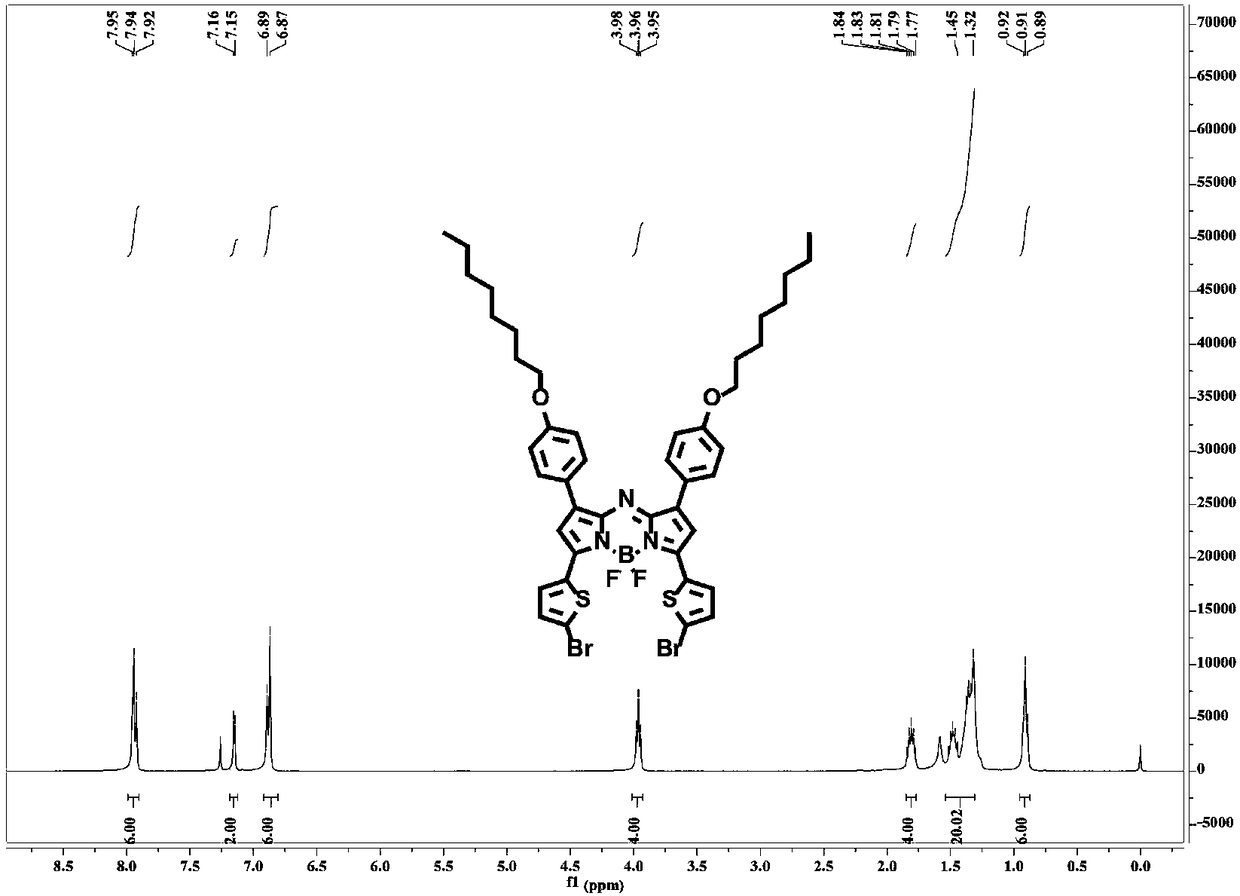
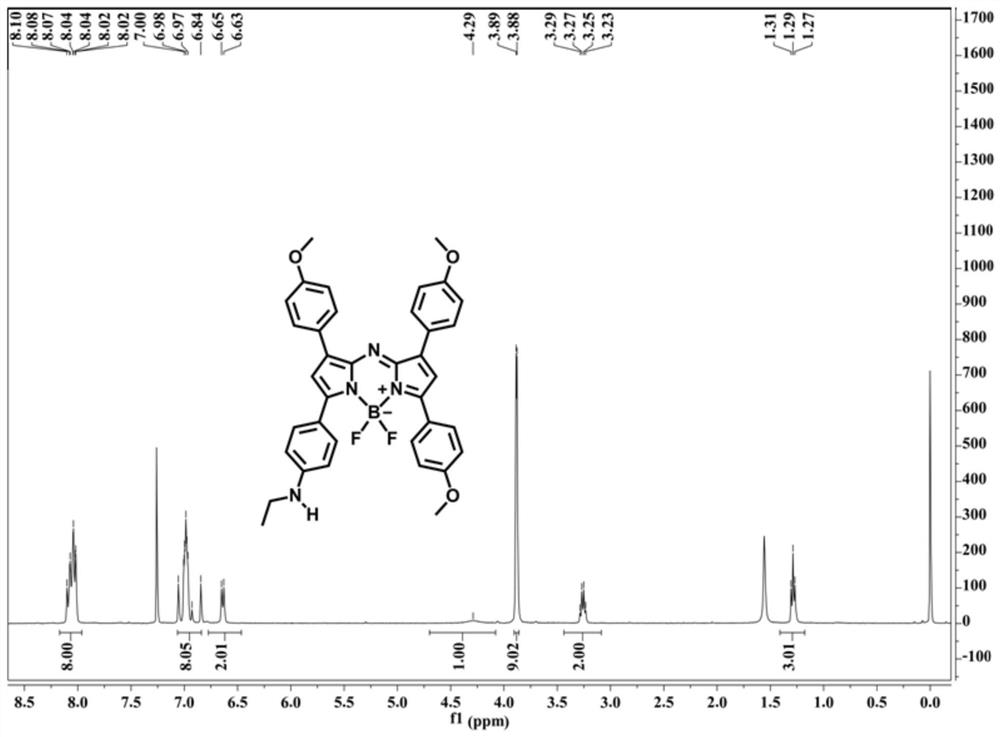
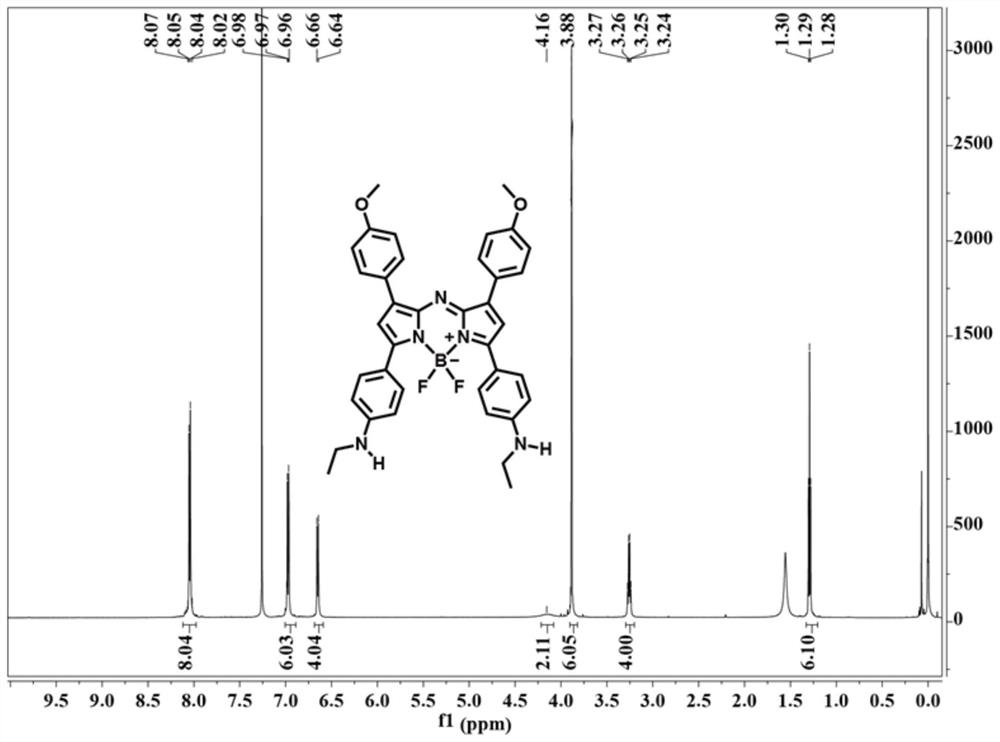
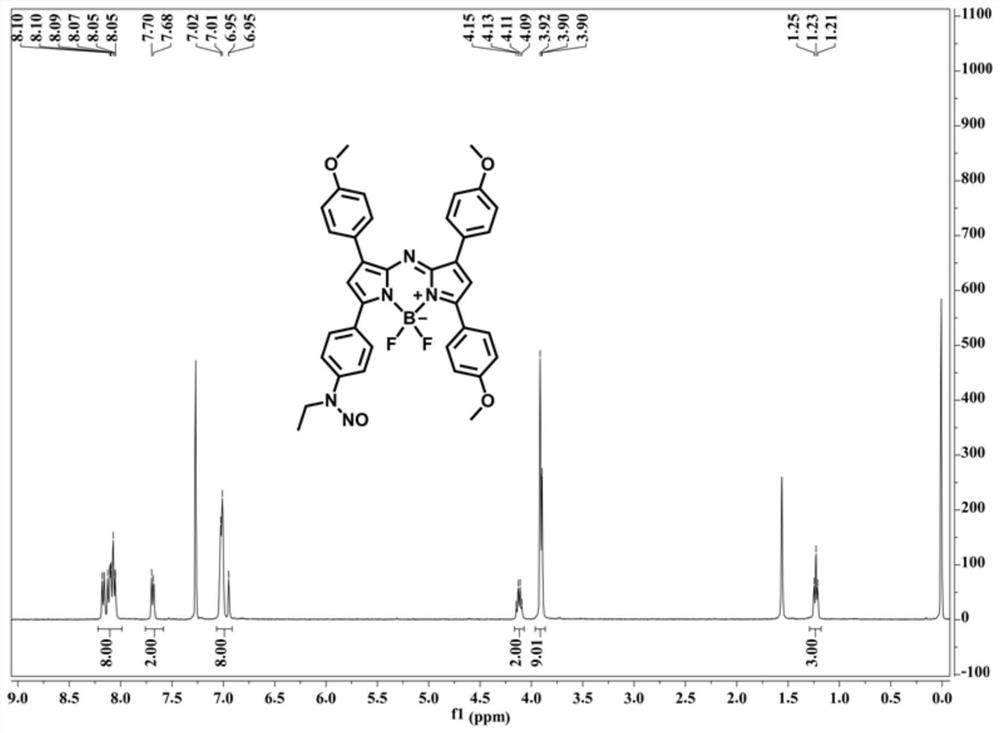
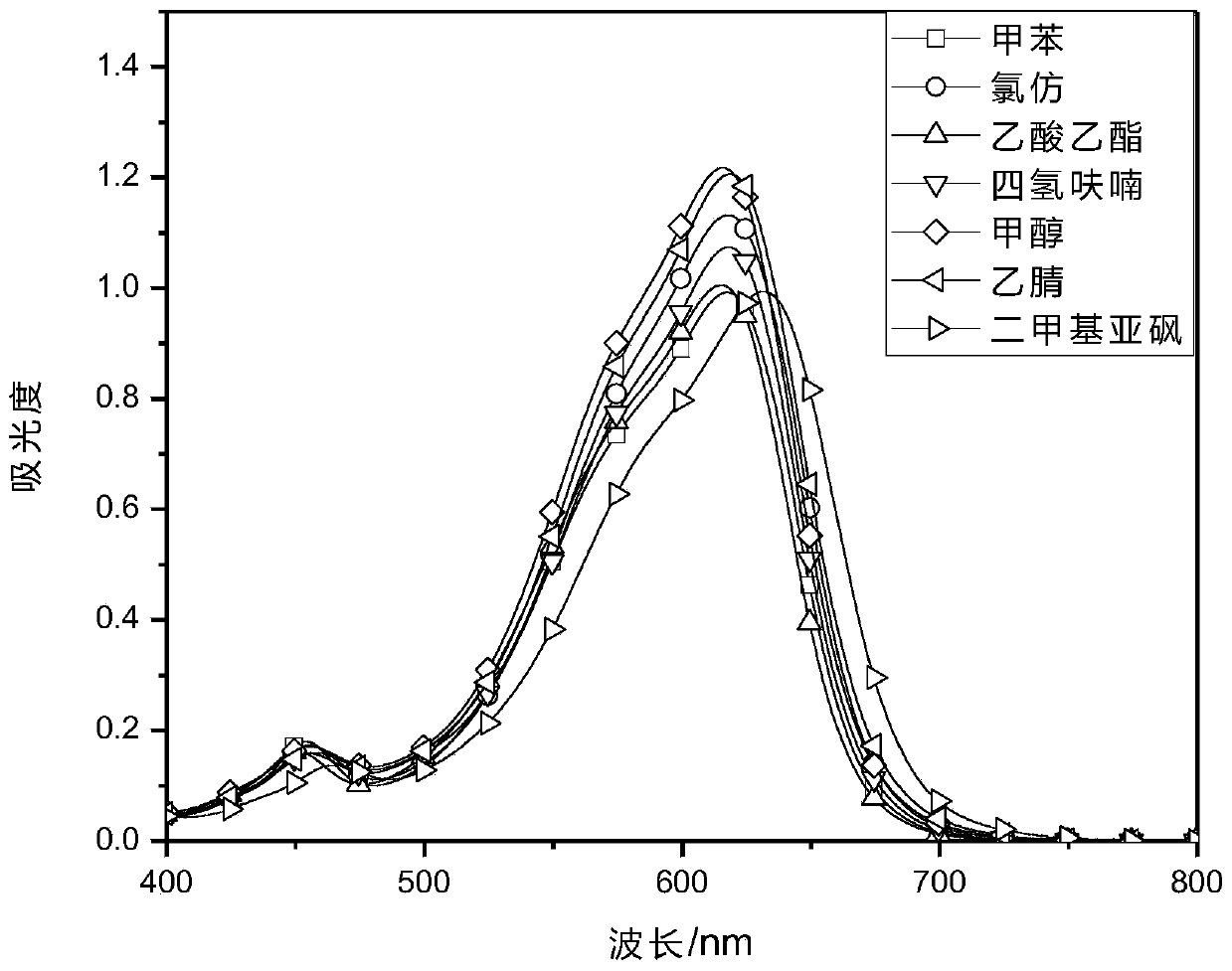
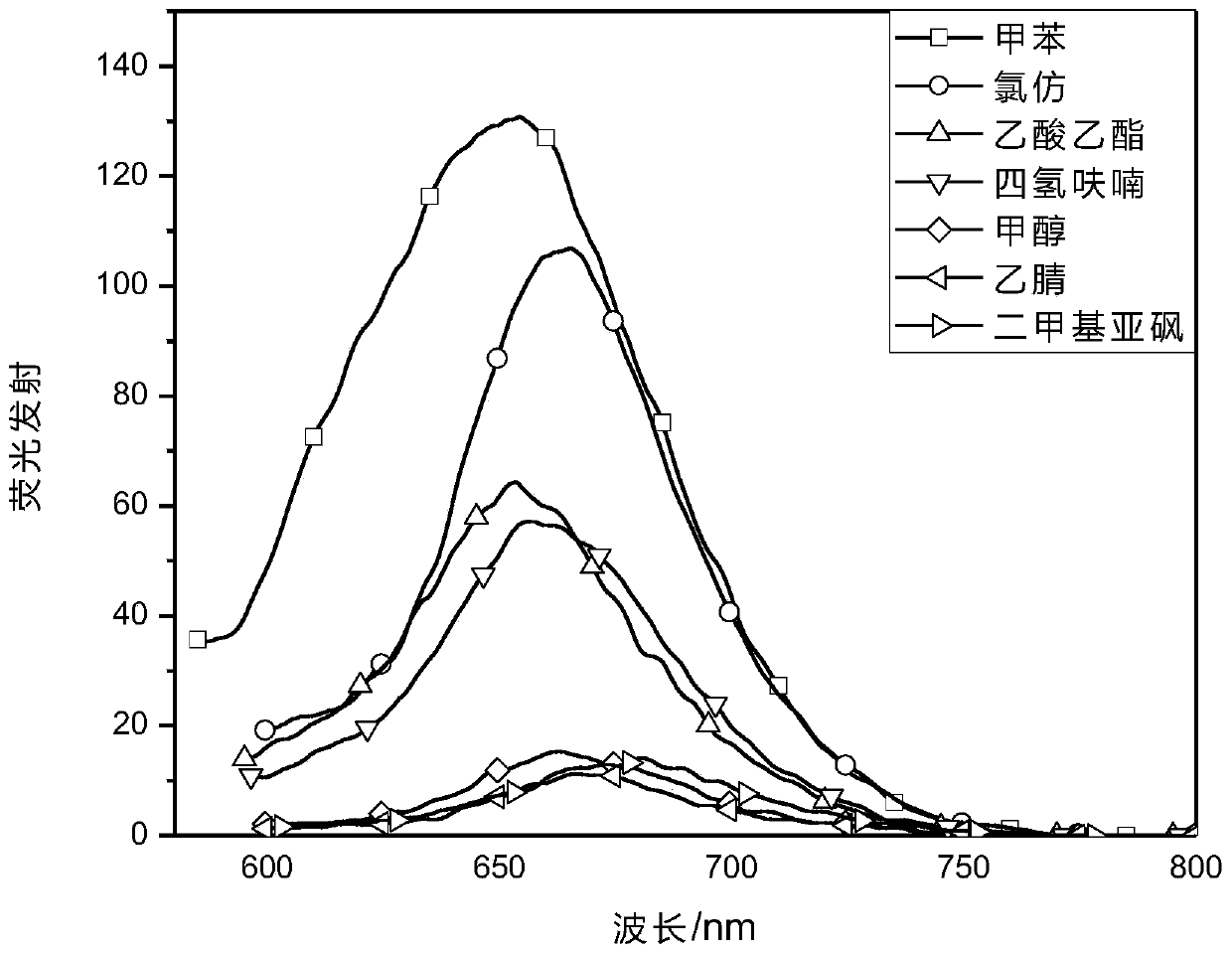
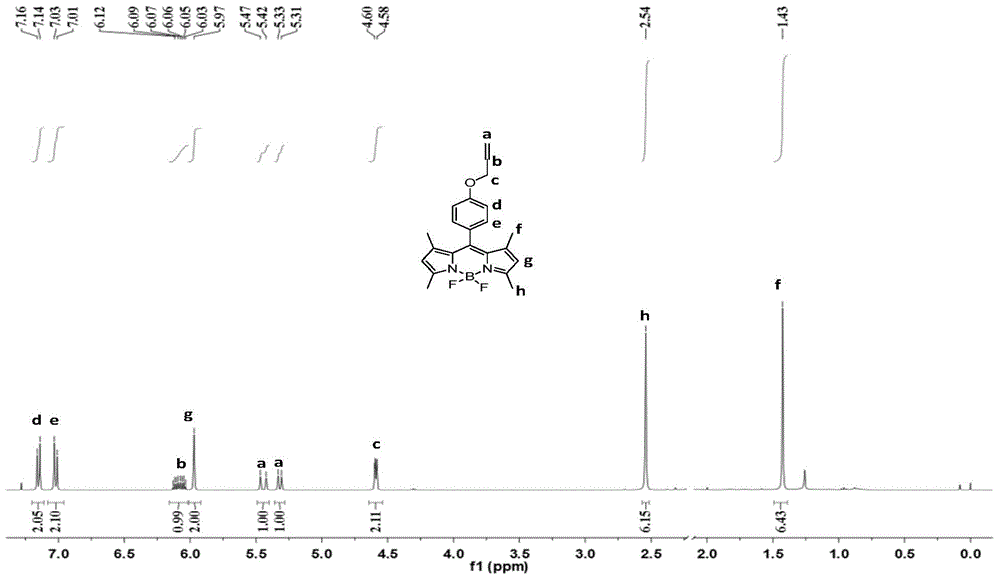
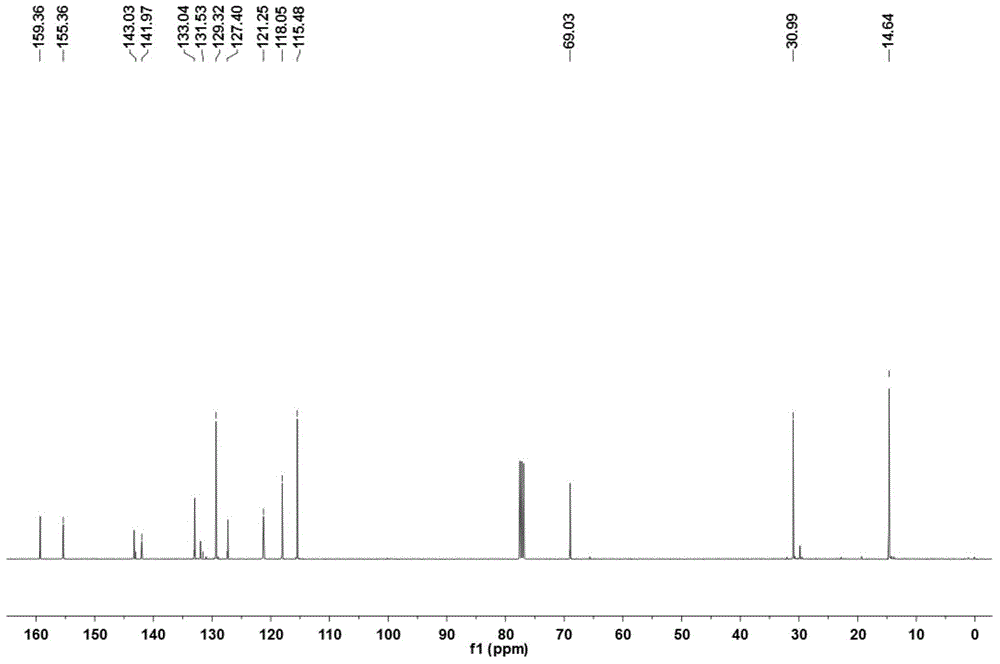
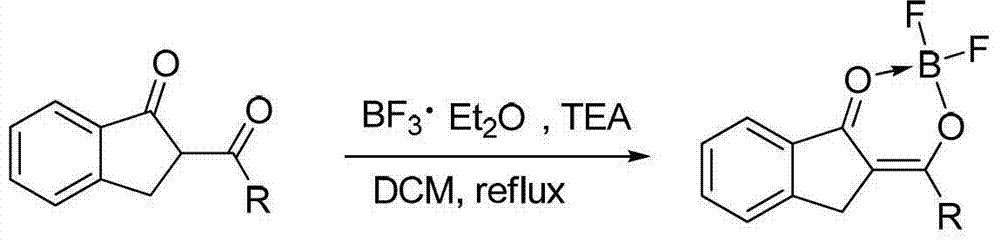
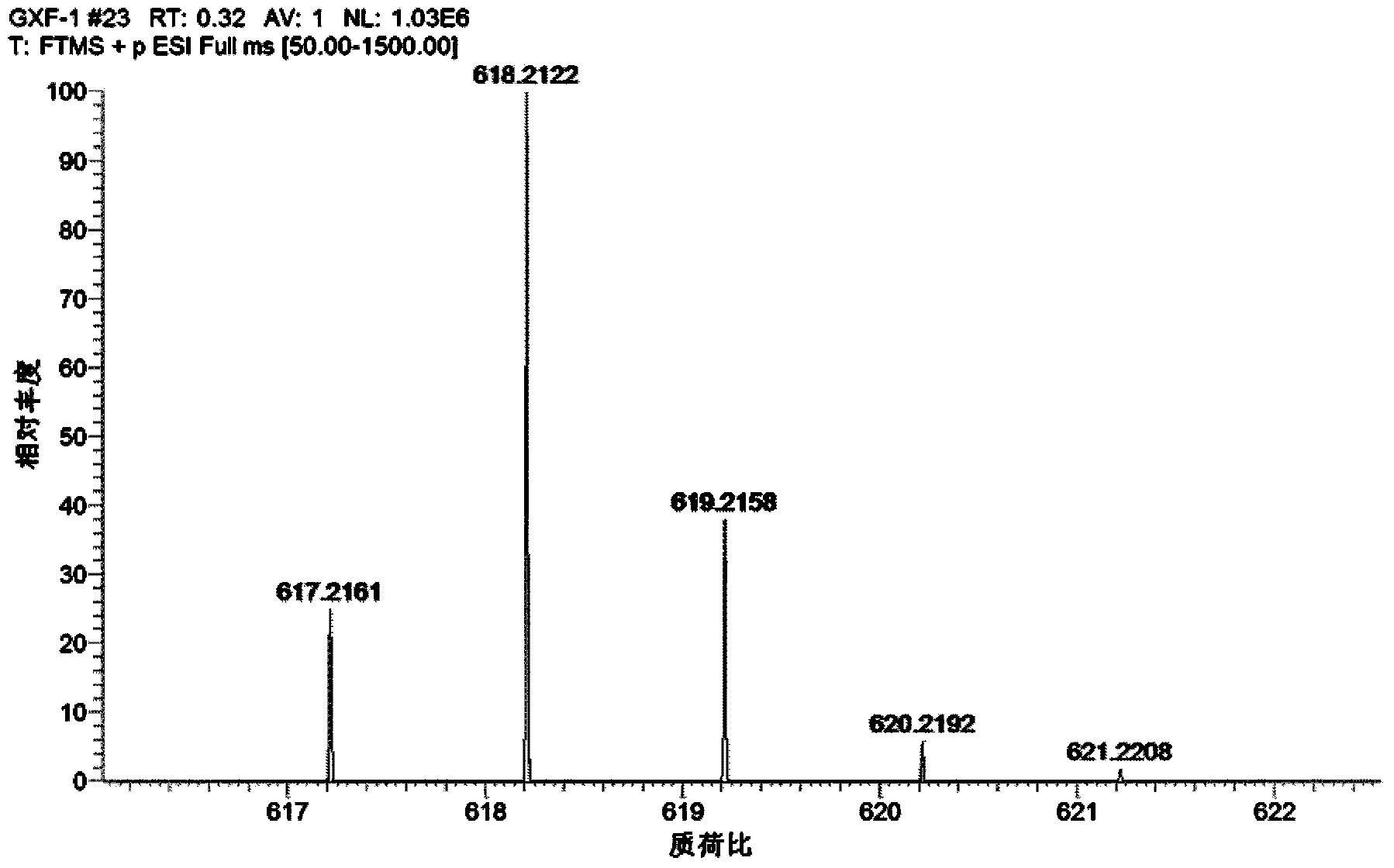
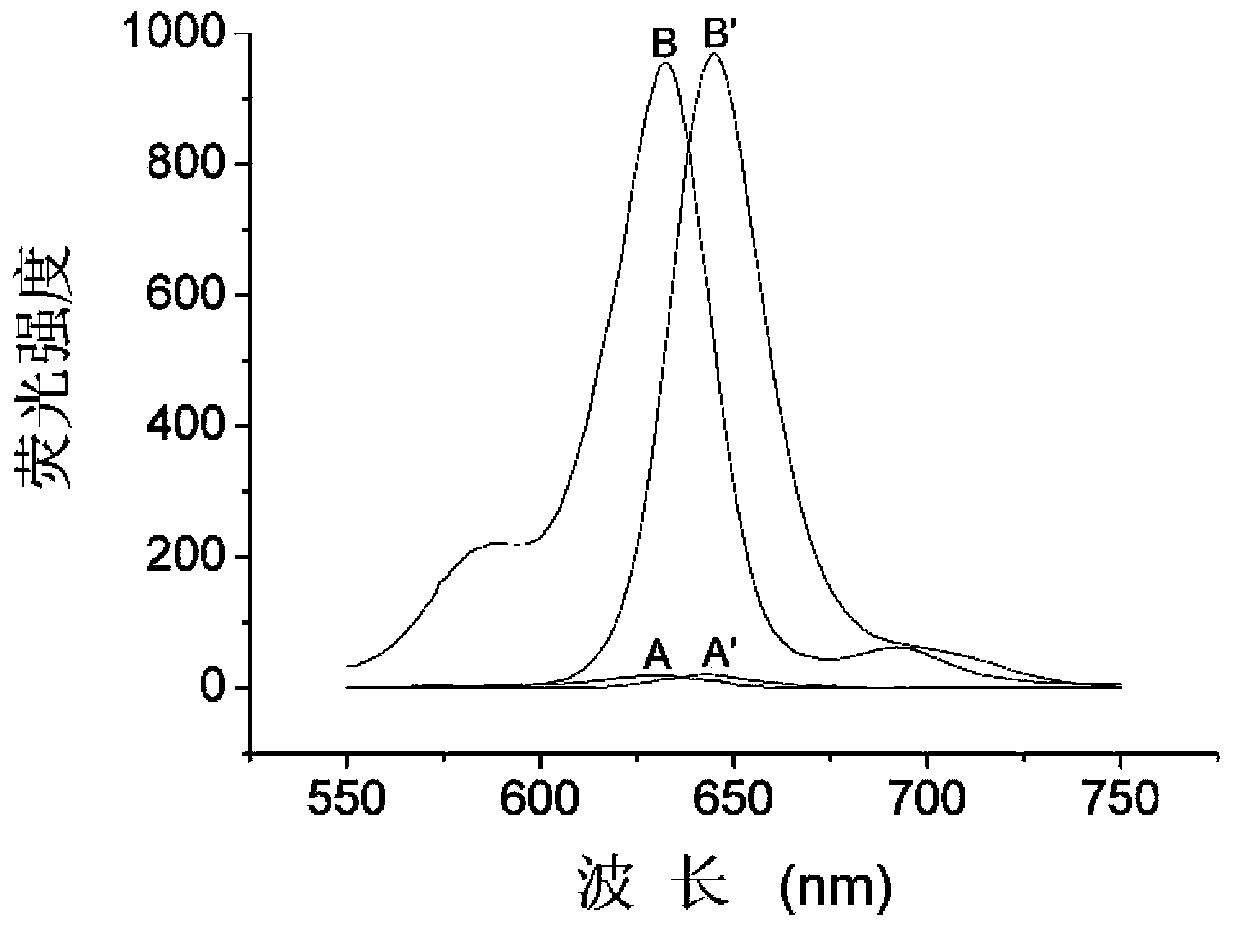
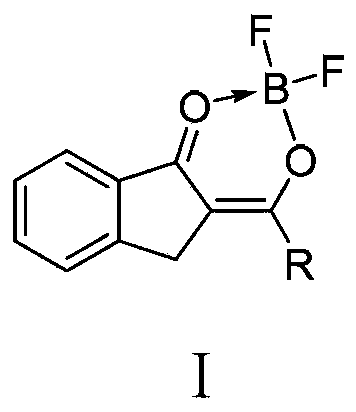
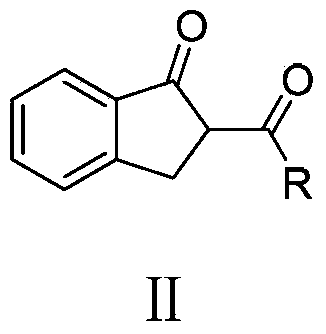
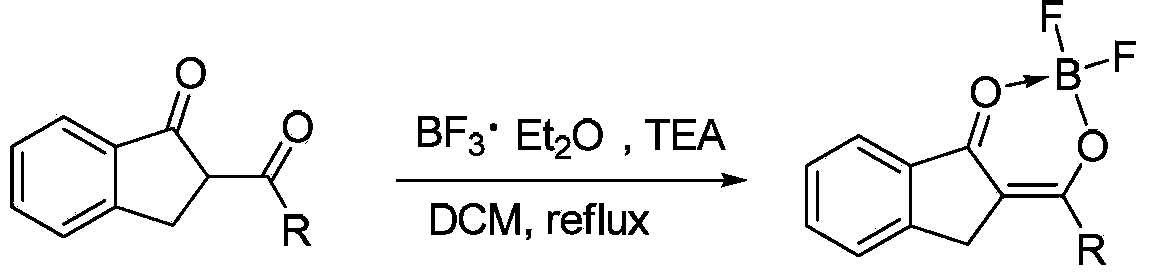
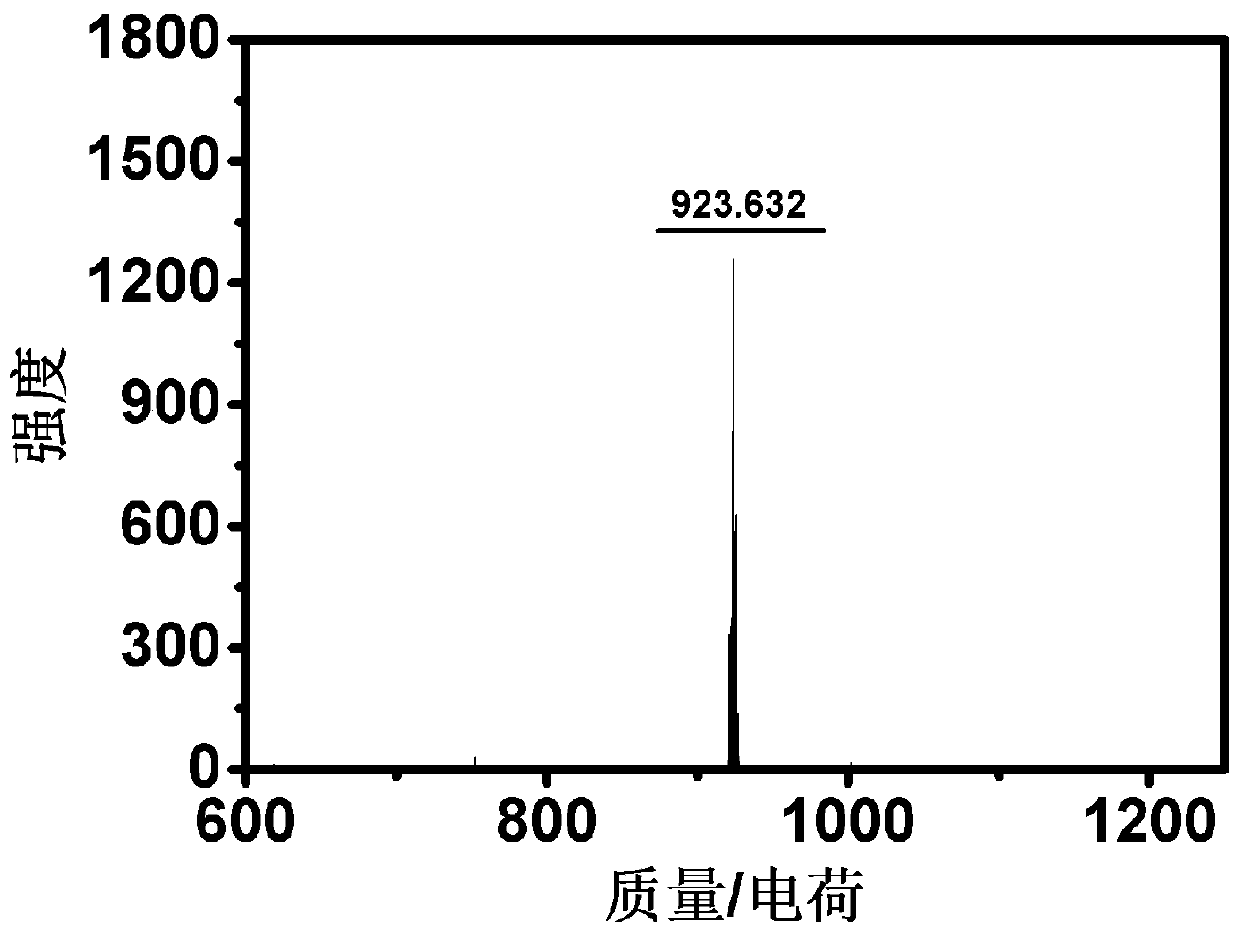
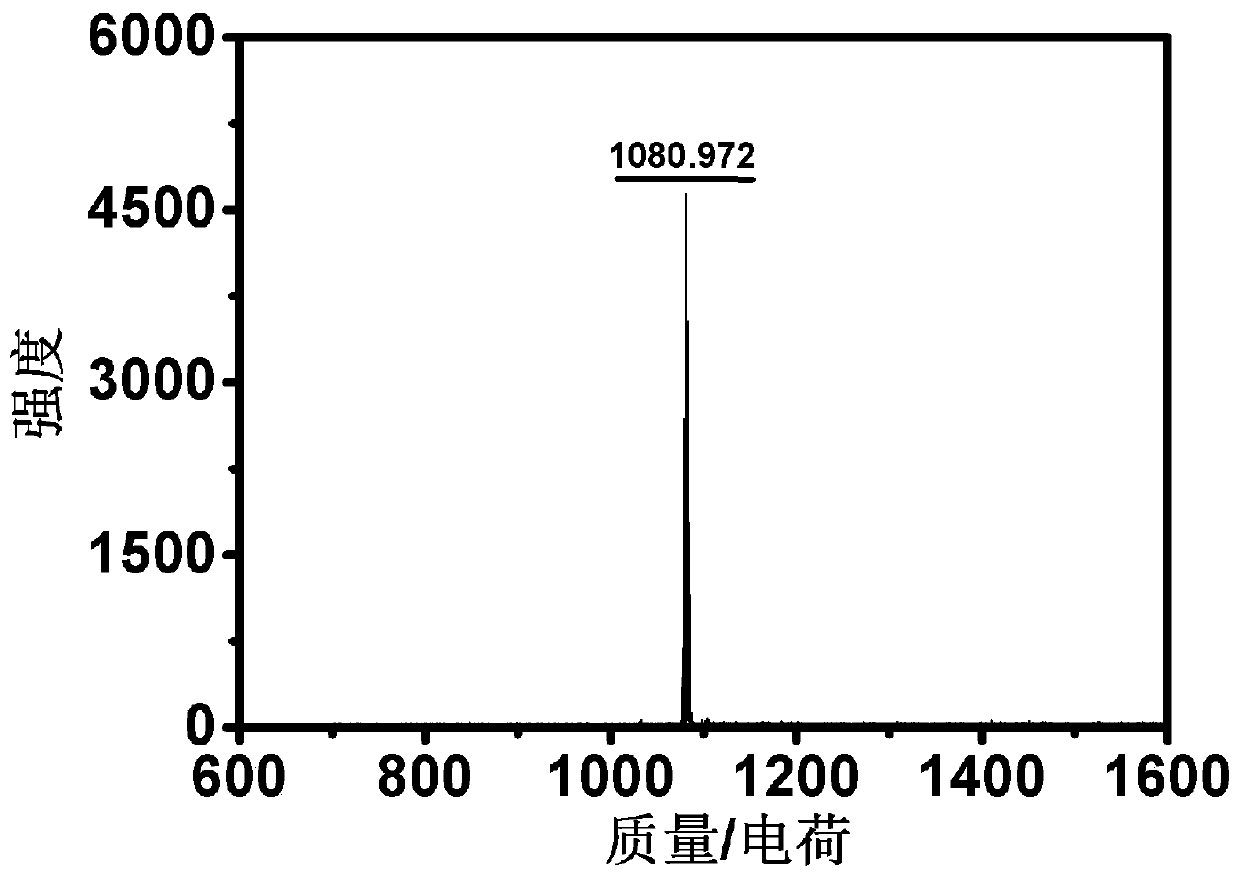
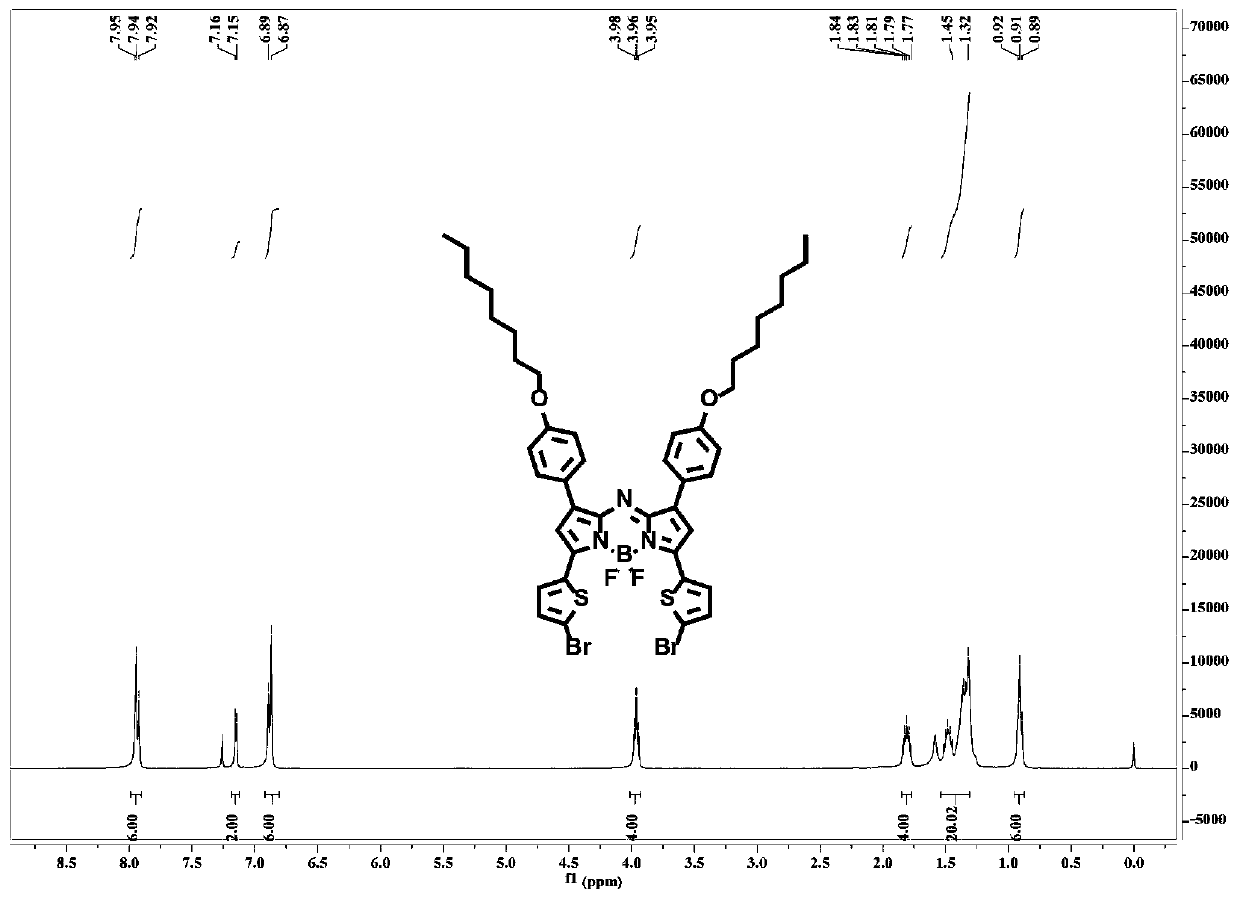
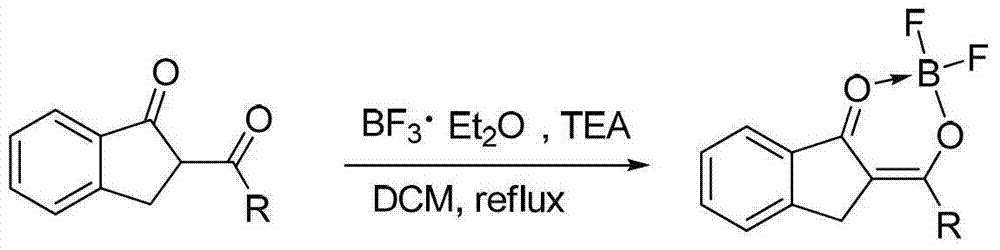
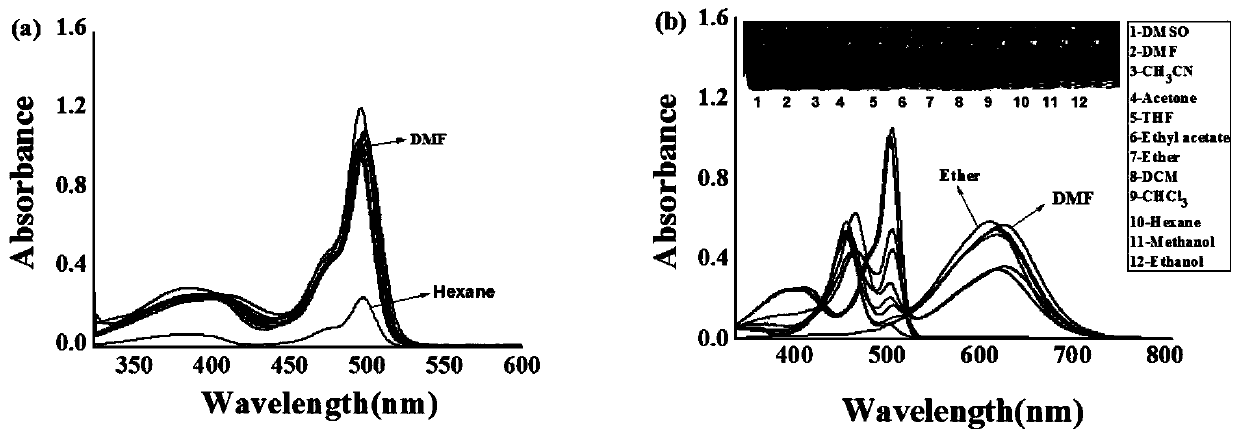
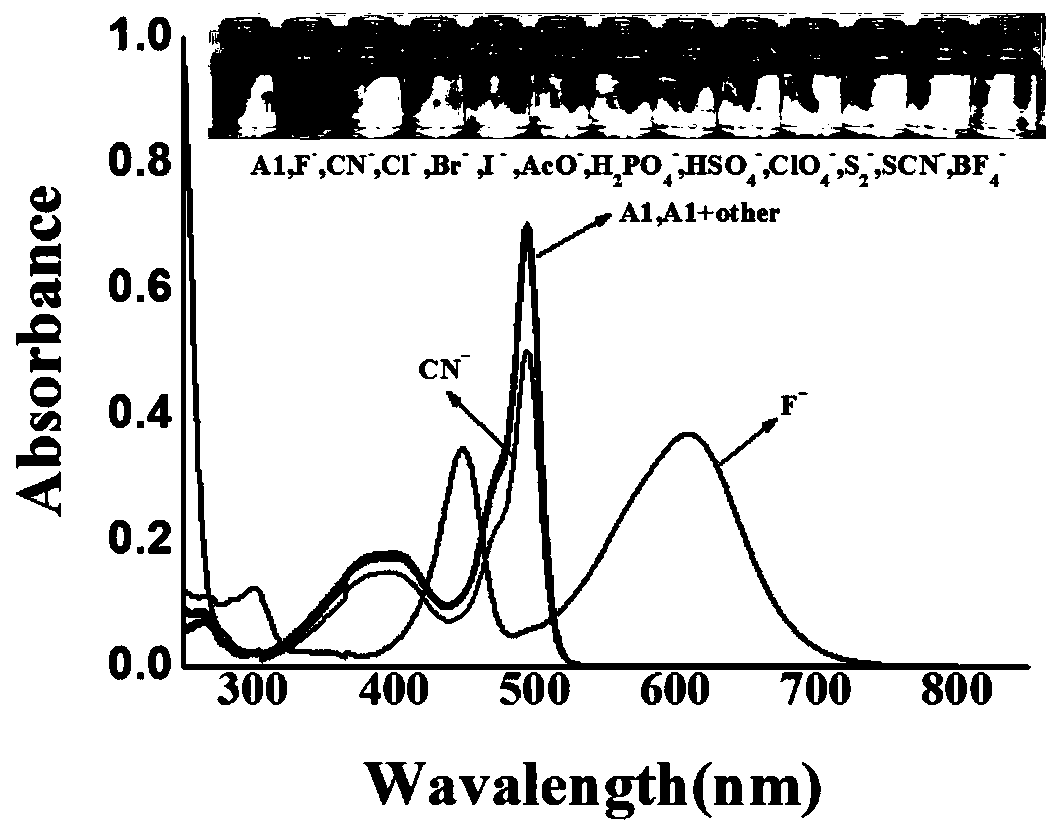
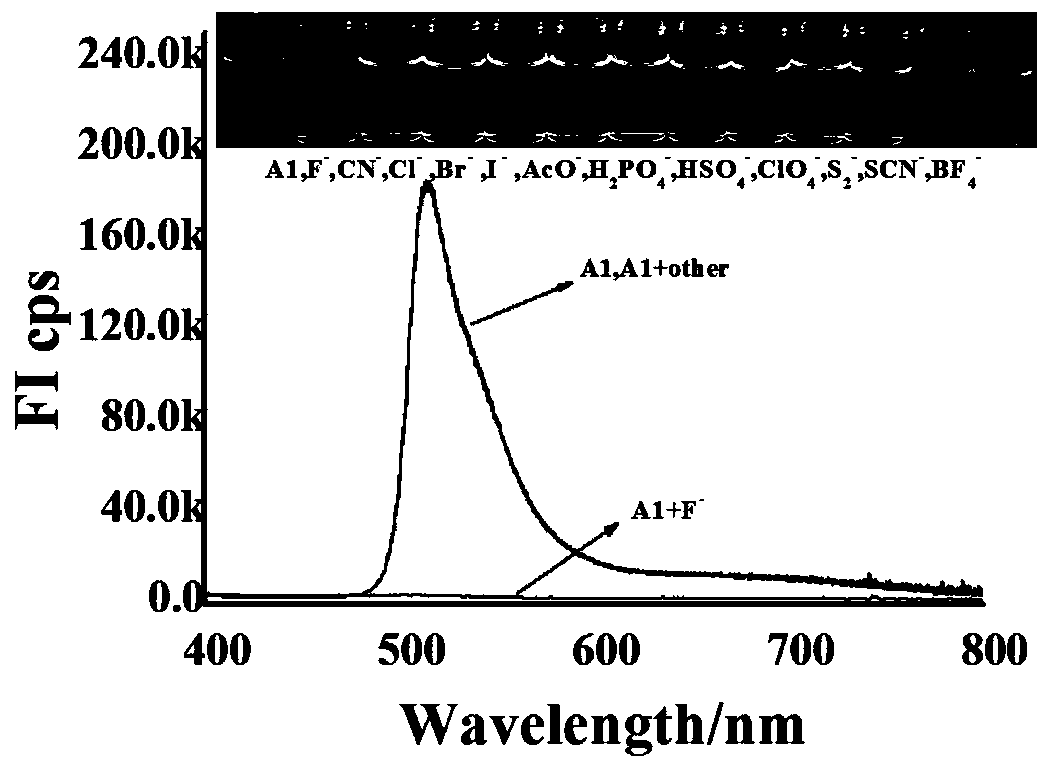
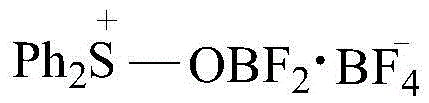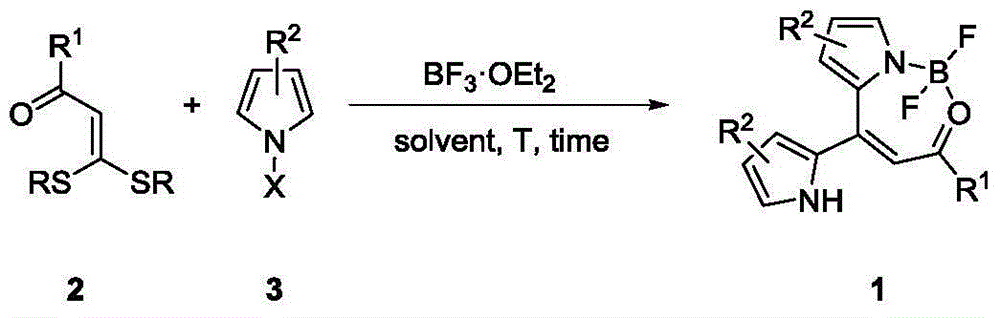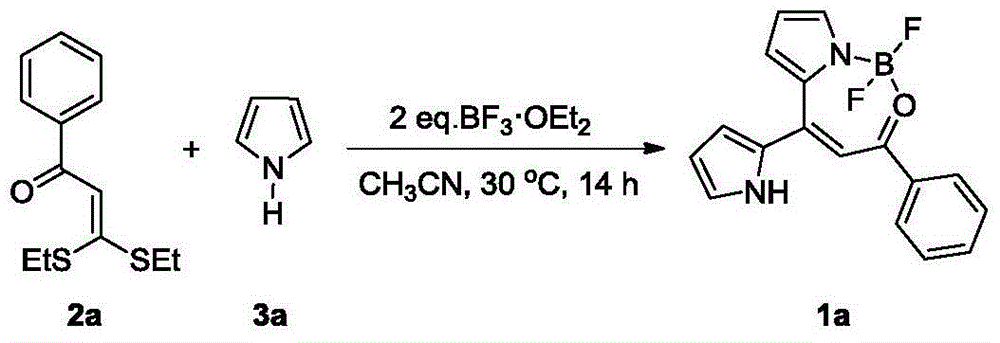Patents
Literature
70 results about "Boron difluoride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Boron monofluoride or fluoroborylene is a chemical compound with formula BF, one atom of boron and one of fluorine. It was discovered as an unstable gas and only in 2009 found to be a stable ligand combining with transition metals, in the same way as carbon monoxide.
Fluorescent probe for detecting biologic thiol and preparation method and usage method thereof
InactiveCN102532178AAvoid interferenceHigh sulfhydryl detection sensitivityGroup 3/13 element organic compoundsFluorescence/phosphorescenceThiolNitrobenzene
The invention discloses a fluorescent probe for detecting a biologic thiol and a preparation method and a usage method thereof. The fluorescent probe for detecting the biologic thiol consists of two parts: namely, a 2, 4-bi-nitrobenzene sulfonyl group which is a recognizing group and a boron difluoride-dipyrryl methane (BODIPY) derivative which is an information reporting functional group. The molecule of the probe can simply and quickly enter a living cell, generates a specificity reaction with the thiol in the cell, and causes to obviously enhance fluorescence intensity, so that the fluorescent probe further can be used for the fluorescence detection and the imaging of the active thiol in the living cell. The fluorescent probe has good stability, can be stored and used for a long time, is applicable to various environments in which the living cell grows, and has higher detection sensitivity for the thiol, strong anti-interference capability, excellent selectivity and no action on other common biologic interfering molecules. The fluorescent probe can simply enter the living cell and a living tissue; the single recognition of the thiol in a biologic system can be achieved effectively; and therefore, the fluorescent probe can be used for the fluorescence imaging of the living cell.
Owner:ZHEJIANG SCI-TECH UNIV
1,3,5,7-tetramethyl-8-triphenylamine base pyrrole methane-boron difluoride complex compound and preparation method thereof
InactiveCN102321109AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsMethylene DichlorideOrganic synthesis
The invention relates to a 1,3,5,7-tetramethyl-8-triphenylamine base pyrrole methane-boron difluoride complex compound and a preparation method thereof, which belong to the technical field of organic synthesis. The existing photocatalysts have few varieties and low conversion rate, and the existing bodipy dye has low infrared fluorescent efficiency and small Stokes displacement. The invention provides the 1,3,5,7-tetramethyl-8-triphenylamine base pyrrole methane-boron difluoride complex compound. The preparation method comprises the steps that: 4-formoxyl tetramethyl and 2,4-dimethyl pyrrole are dissolved in organic solvents, trifluoroacetic acid or monoprop is used as catalysts, and a reaction system is formed; 2,3-dichloro-5,6-dicyan-1,4-para-benzoquinone with the same mol ratio as the 4-formoxyl tetramethyl is taken, is dissolved in the methylene dichloride and is added into the reaction system; and one of triethylamine, triisopropyl amine and N,N-diisopropylethylamine is added into the reaction system, boron trifluoride etherate is added under the ice bath, and final products are generated.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Near-infrared photo-thermal dye based on aza-fluoro borane, preparation and application
InactiveCN107501313AExcellent photothermal performanceGood photoacoustic imagingEnergy modified materialsEchographic/ultrasound-imaging preparationsKetoneNitromethane
The invention discloses a near-infrared photo-thermal dye based on aza-fluoro borane, preparation and application. The photo-thermal dye comprises an electron-donating group containing lone pair electrons and a basic aza-fluoro borane skeleton. The near-infrared photo-thermal dye based on aza-fluoro borane is prepared through steps as follows: (1), ketene synthesis: ketene is prepared from aldehyde ketone by an addition-elimination reaction under the alkaline condition; (2), an addition reaction of ketene and nitromethane; (3), a cyclization reaction; (4), a coordination reaction: a product obtained in the step (3) coordinates with boron difluoride, and a target product is obtained. The photo-thermal dye can be applied to the technical fields of photodynamic therapy and photo-thermal therapy under the guidance of photo-thermal imaging and photo-acoustic imaging, bio-marker and detection and the like.
Owner:NANJING UNIV OF POSTS & TELECOMM
Organic boron difluoride complex and preparation method thereof
InactiveCN102040617AHigh molar absorptivityChemically stableGroup 3/13 element organic compoundsLuminescent compositionsQuantum yieldOrganic base
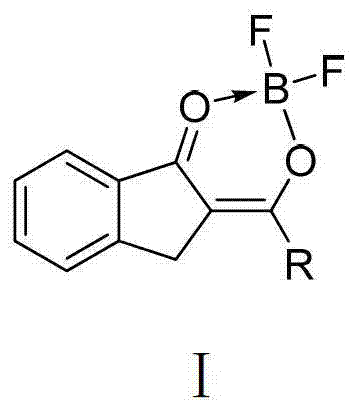
The invention discloses an organic boron difluoride complex which is a compound the structural formula I of which is shown in the specification, wherein R1 and R2 are OCH3, H or Cl independently respectively; R3 and R4 are H, NO2, Br, CH3 or OCH3 independently respectively; the complex has stable chemical properties, strong thermal stability, high fluorescence quantum yield and large molar absorption coefficient; furthermore, the complex not only has strong solution fluorescence, but also has significant solid fluorescence. The invention further discloses a preparation method of the organic boron difluoride complex, and the organic boron difluoride complex is prepared by carrying out room temperature or reflux reaction on a ligand and boron trifluoride diethyl etherate in an anhydrous organic solvent in the presence of organic base; and the method has the advantages of mild reaction conditions, short reaction time, high product yield and fastness and simpleness in separation and purification.
Owner:ZHEJIANG SCI-TECH UNIV
Organic boron difluoride complex
InactiveCN102993224AChemically stableImprove thermal stabilityGroup 3/13 element organic compoundsLuminescent compositionsLaser dyePhotosensitizer
The invention discloses an organic boron difluoride complex. The complex is shown in the structural formula I in the specification, wherein R1 is H or OCH3; and R2 is C6H5, 4-HOC6H4, 4-(CH3)2NC6H4 or 2-C4H4S. The complex can be used for preparing luminescent materials, fluorescent probes, fluorescent tracers, information storage media, laser dyes and photodynamic cancer treatment photosensitizers. The invention also discloses a preparation method of the complex. The complex is prepared through reaction of a corresponding ligand and boron trifluoride diethyl etherate. The complex has the advantages of mild reaction conditions, short reaction time, high product yield, rapidness, simpleness and convenience in separation and purification and high product purity.
Owner:ZHEJIANG SCI-TECH UNIV
Organic boron difluoride complex containing N,O-bidentate ligand
InactiveCN102993223AChemically stableImprove thermal stabilityGroup 3/13 element organic compoundsLuminescent compositionsLaser dyePhotosensitizer
The invention discloses an organic boron difluoride complex containing an N,O-bidentate ligand. The complex is shown in the structural formula I in the specification, wherein R is 2,6-dimethoxyphenyl, oxethyl, 2-methoxyphenyl or 2-thienyl. The complex can be used for preparing luminescent materials, fluorescent probes, fluorescent tracers, information storage media, laser dyes and photodynamic cancer treatment photosensitizers. The invention also discloses a preparation method of the complex. The complex is prepared through reaction of a corresponding ligand and boron trifluoride diethyl etherate. The complex has the advantages of mild reaction conditions, short reaction time, high product yield, rapidness, simpleness and convenience in separation and purification and high product purity.
Owner:ZHEJIANG SCI-TECH UNIV
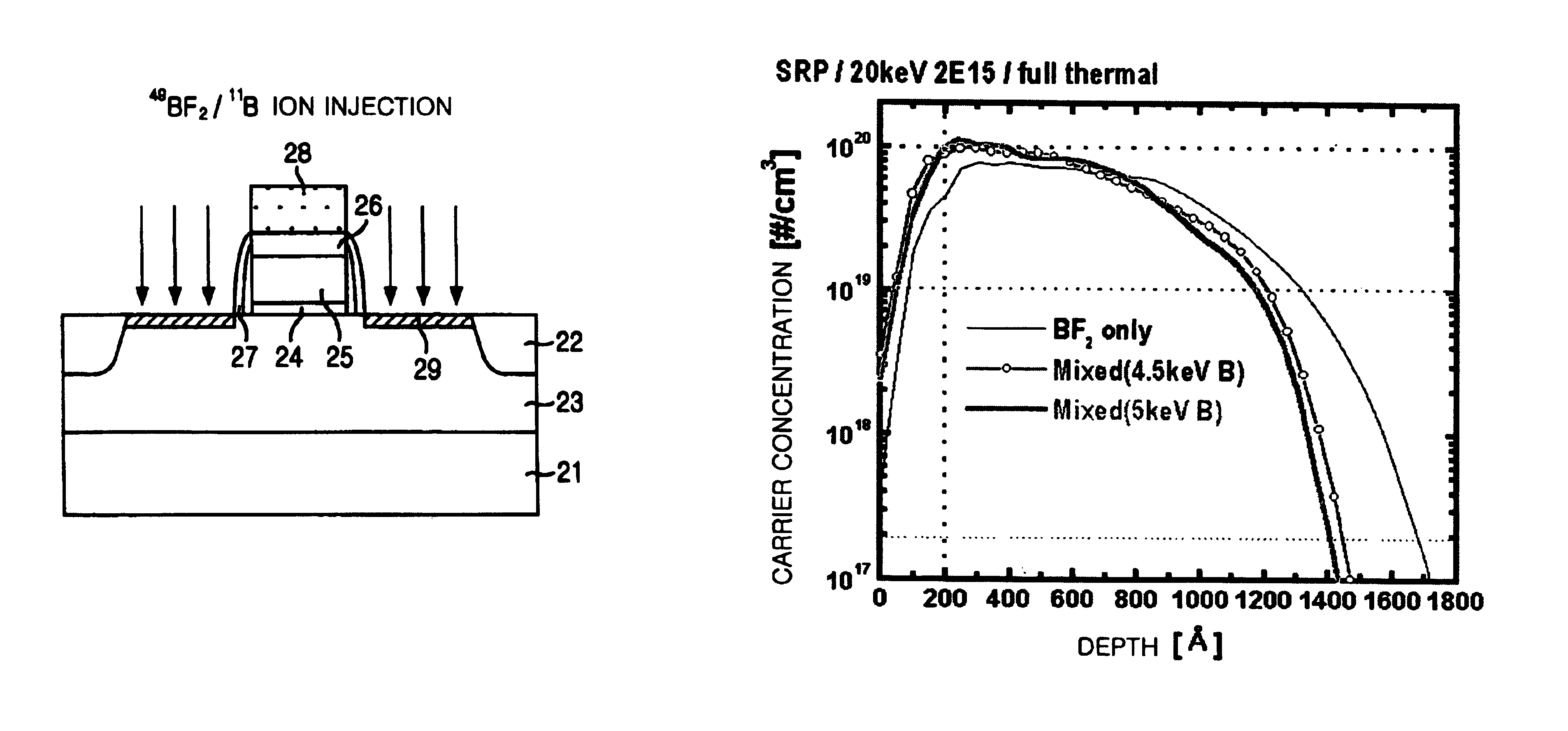
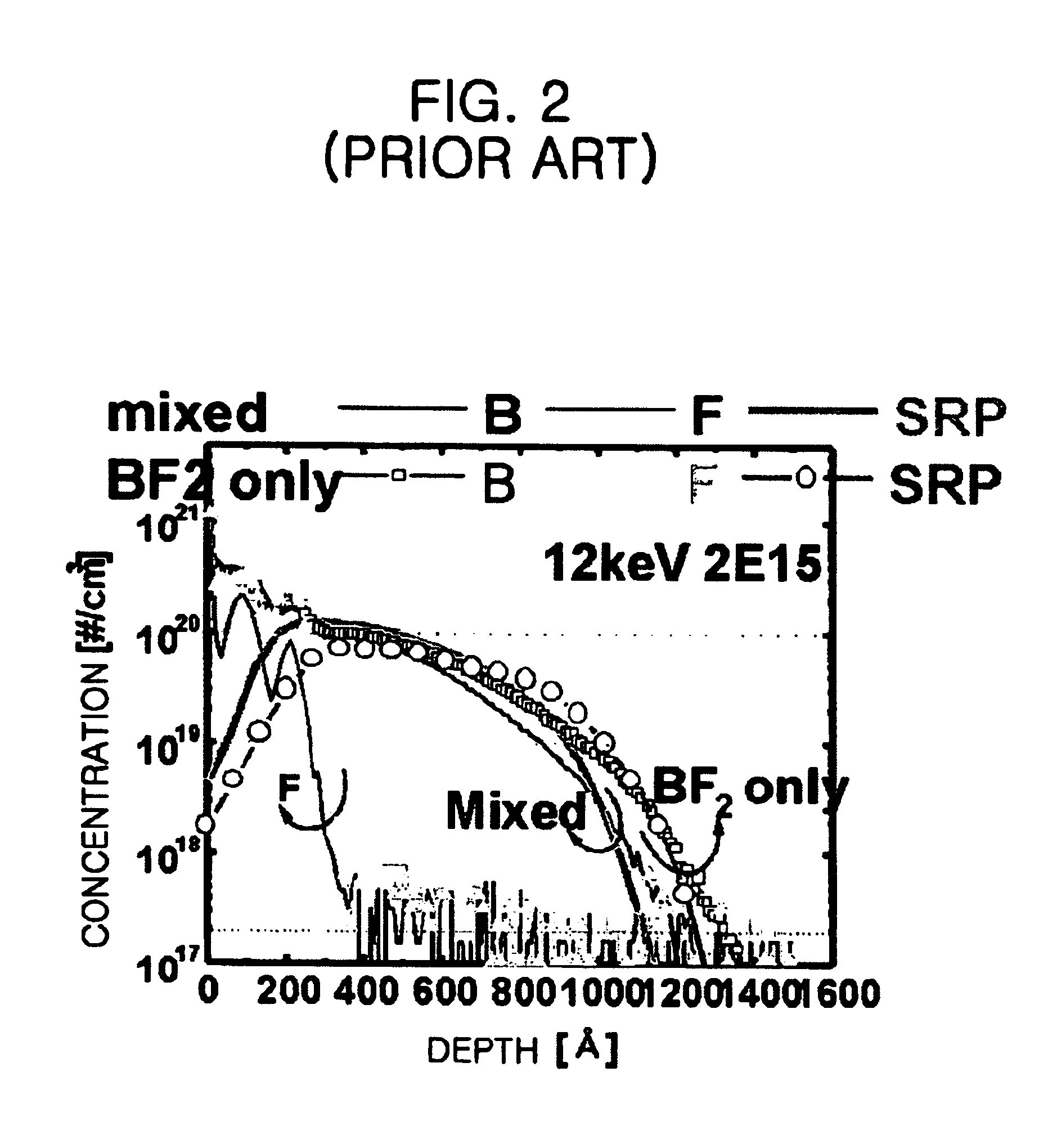
Method for manufacturing a semiconductor device with using double implanting boron and boron difluoride
InactiveUS6939769B2Ion beamImprove productivitySemiconductor/solid-state device manufacturingSemiconductor devicesProduction rateThermal treatment
The present invention provides a method for manufacturing a semiconductor device capable of acquiring productivity when a p-type source / drain is formed by the implantation of a BF2 and B ions. The method for manufacturing a semiconductor device includes the steps of: implanting a BF2 ion in a p-type source / drain region on a silicon substrate with an ion implantation energy of from about 10 keV to about 20 keV; implanting B ion in the p-type source / drain region with an ion implantation energy of from about 5 keV to about 10 keV; and forming a p-type source / drain by carrying out a thermal treatment.
Owner:SK HYNIX INC
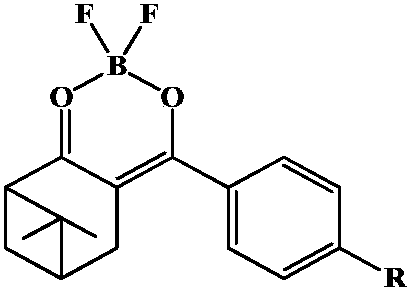
Nopinic alkyl beta-dione boron difluoride complexes and preparation method and application thereof
InactiveCN108409765AIncrease productionLow priceSolid-state devicesSemiconductor/solid-state device manufacturingQuantum yieldClaisen condensation
The invention discloses nopinic alkyl beta-dione boron difluoride complexes and a preparation method and application thereof. The natural reusable resource beta-pinene oxidation product, namely nopinone, is used as a raw material to prepare novel nopinic alkyl beta-dione boron difluoride complexes 3a and 3b; nopinone is subjected to Claisen condensation with methyl p-methoxybenzoate 1a and methyl4-bromobenzoate 1b to separately obtain nopinic alkyl beta-dione compounds 2a and 2b; the compounds 2a and 2 b are subjected to complexing with boron trifluoride etherate to separately obtain nopinicalkyl beta-dione boron difluoride complexes 3a and 3b. The nopinic alkyl beta-dione boron difluoride complexes 3a and 3b have good fluorescent performance and high fluorescent quantum yield; an electroluminescent device made with the nopinic alkyl beta-dione boron difluoride complexes can emit blue-green light. It is shown that the nopinic alkyl beta-dione boron difluoride complexes as light-emitting materials have good application value in the preparation of electroluminescent devices.
Owner:NANJING FORESTRY UNIV
Preparation of near-infrared dye based on aza-fluoroborane and application
ActiveCN108102408AStrong near-infrared absorptionGood photoacoustic imagingPhotodynamic therapyAzo dyesSinglet oxygenNitromethane
The invention discloses preparation of near-infrared dye based on aza-fluoroborane and application. The near-infrared dye is prepared from thiophene-containing groups and a basic aza-fluoroborane framework. The aza-fluoroborane dye is prepared by the following steps: (1) synthesis of ketene: the ketene is obtained by addition-elimination reaction of aldehyde and ketone under an alkali condition; (2) ketene and nitromethane conduct addition reaction under the alkali condition; (3) ring-forming reaction: under the condition with existence of an ammonia source, two times of equivalent ketene andnitromethane conduct ring-forming reaction; (4) coordination reaction: products obtained in the step (3) and metal or boron difluoride are coordinated to obtain a target product. The preparation and the application disclosed by the invention have the advantages that the target dye has strong near-infrared absorption (the molar light absorption coefficient is more than 100000M<-1>cm<-1>), high singlet oxygen yield and good photothermal effect, and can be used for photodynamic and photothermal synergy with single-wavelength excitation for tumor treatment under the guidance of photothermal imaging, photoacoustic imaging and fluorescence imaging.
Owner:NANJING UNIV OF POSTS & TELECOMM
PMOS source and drain region ion implantation method and PMOS device manufacturing method
InactiveCN104241106AImprove reliabilityInhibition of warming effectSemiconductor/solid-state device manufacturingSemiconductor devicesPhotoresistBoron difluoride
The invention provides a PMOS source and drain region ion implantation method and a PMOS device manufacturing method. The PMOS source and drain region ion implantation method comprises the steps that a substrate is provided, wherein a grid electrode structure is formed on the surface of the substrate, and side walls are formed on the two sides of the grid electrode structure; with the side walls serving as masks, PMOS source and drain region ion implantation is performed on the two sides of the grid electrode structure so that ions can be implanted into the substrate, wherein the implanted ions contain fluorine and boron difluoride, and the implantation of the fluorine and the boron difluoride is performed in the environment at the temperature ranging from -100 DEG C to -50 DEG C. The implantation of the fluorine and the boron difluoride is performed in the environment at the temperature ranging from -100 DEG C to -50 DEG C, and the environment at the temperature ranging from -100 DEG C to -50 DEG C can inhibit the temperature rise effect of silicon wafers during implantation of the fluorine and the boron difluoride; accordingly, the escape speed of the fluorine element can be lowered, and corrosion of photoresist is inhibited.
Owner:SHANGHAI HUALI MICROELECTRONICS CORP
Near-infrared fluorescent probe and preparation method and application thereof
ActiveCN111778016AAchieve gas therapyEvenly distributedEnergy modified materialsInorganic active ingredientsTumor therapyFluorophore
The invention provides a near-infrared fluorescent probe and a preparation method and application thereof. According to the near-infrared fluorescent probe provided by the invention, a functional group capable of releasing nitric oxide (NO) gas is introduced into a boron difluoride-dipyrromethene (BODIPY) fluorophore skeleton, and the near-infrared fluorescent probe can release NO under the irradiation of near-infrared light. According to the near-infrared fluorescent probe provided by the invention, the synergistic effect of gas therapy, photothermal therapy and immunotherapy on tumors is realized, the near-infrared fluorescent probe has photoacoustic imaging, photo-thermal imaging and fluorescence imaging effects, the material after releasing NO gas can improve the signal-to-noise ratioof photoacoustic imaging, photo-thermal imaging and fluorescence imaging, so that the material can be used for providing reference and guidance for tumor treatment based on the imaging effect, and tumor cooperative treatment under the guidance of photoacoustic imaging, photo-thermal imaging and fluorescence imaging is realized.
Owner:SHENZHEN UNIV
Pretreatment method for ion implanter
ActiveCN104465292AGuaranteed uniformityReduce preprocessing timeElectric discharge tubesSemiconductor/solid-state device manufacturingPretreatment methodTerminal voltage
The invention discloses a pretreatment method for an ion implanter. The method includes two pretreatment stages, in the first pretreatment stage, pretreatment gas flows into an ion source reaction chamber of the ion implanter, and the ion source reaction chamber is cleaned; in the second pretreatment stage, ion source energy is adjusted to 50 keV, and the terminal voltage is adjusted to 10 kV, so that preset beams are generated, emitted along a beam channel of the ion implanter and used for cleaning the ion implanter. According to the method, boron difluoride is adopted for replacing Ar gas and serves as the pretreatment gas for cleaning treatment, pretreatment time is shortened, a production period is shortened, cleaning efficiency and source change efficiency are improved, uniformity of ion beams is guaranteed, and cleaning efficiency is as high as 80% to 95%.
Owner:SHANGHAI HUALI MICROELECTRONICS CORP
Synthetic Method of 5,5-Dimethyl-2,4-Adipaldehyde-0,0-Boron Difluoride
InactiveUS20120071695A1Mild reaction conditionsSimple procedureOrganic compounds purification/separation/stabilisationDispersed particle separationAfter treatmentBoron trifluoride
This invention, which involves synthetic method of 5, 5- dimethyl-2, 4-adipaldehyde-0, 0-Boron difluoride, belongs to the field of organic synthesis. Synthetic method of 5, 5-dimethyl-2, 4-adipaldehyde-0, 0-Boron difluoride is to react pinacolone and boron trifluoride diethyl ether at low temperature, and then add aqueous alkaline solution in after treatment to extract product from ether, after that, separate fluid, condense organic phase and final product is obtained. Yield of this method is 2 to 3 times higher than that in literature, and apart from that, mild reaction condition, simple procedures, easy operation, and low cost make it easy for industrial production. The product can be used directly in next step reaction without any special purification.
Owner:BEIJING AGLAIA TECH DEV
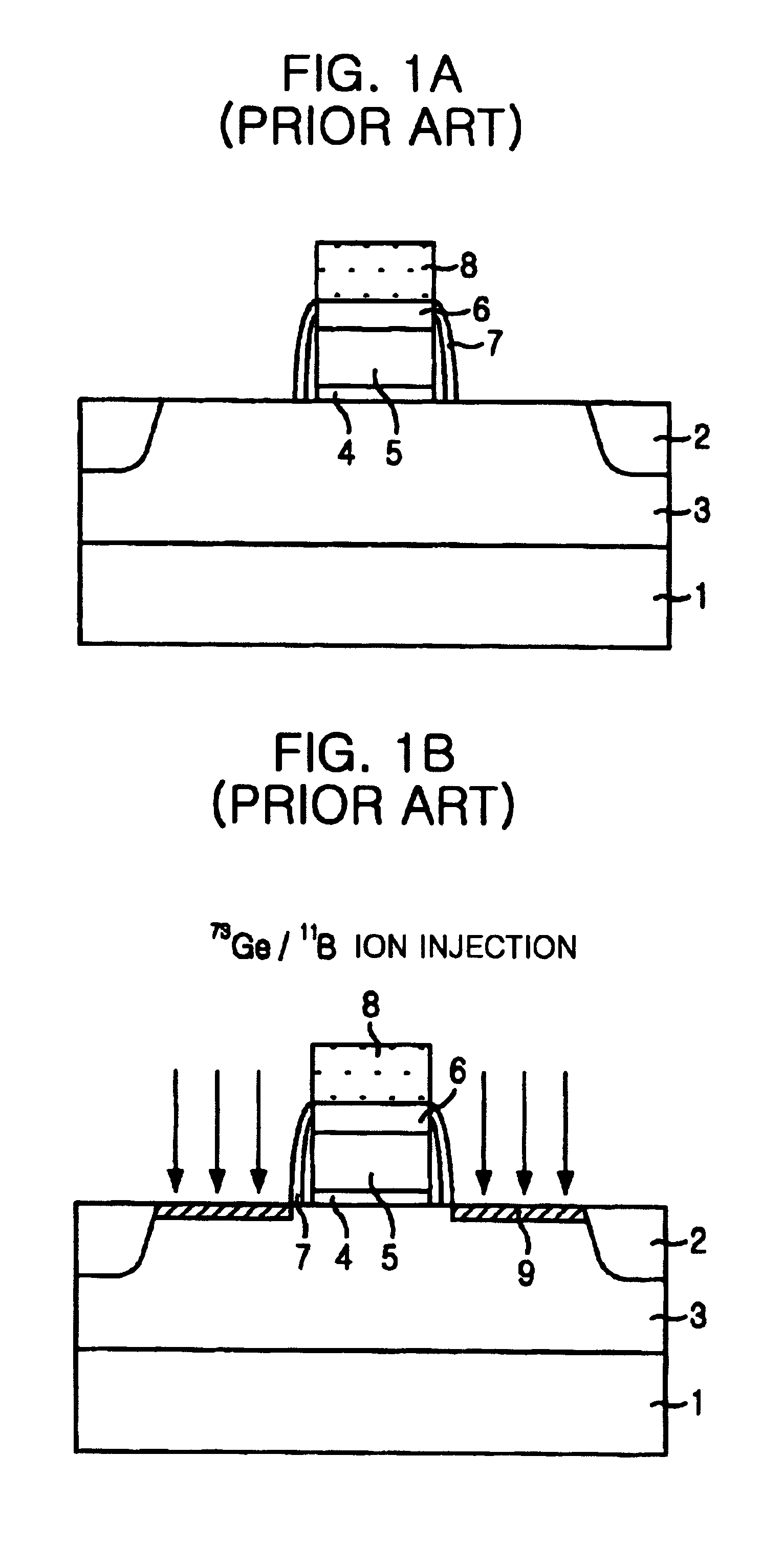
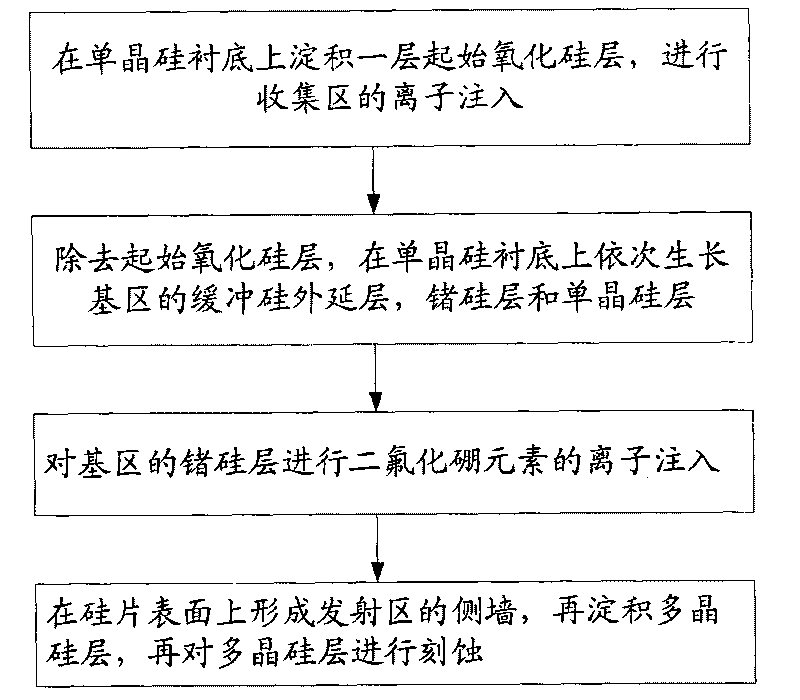
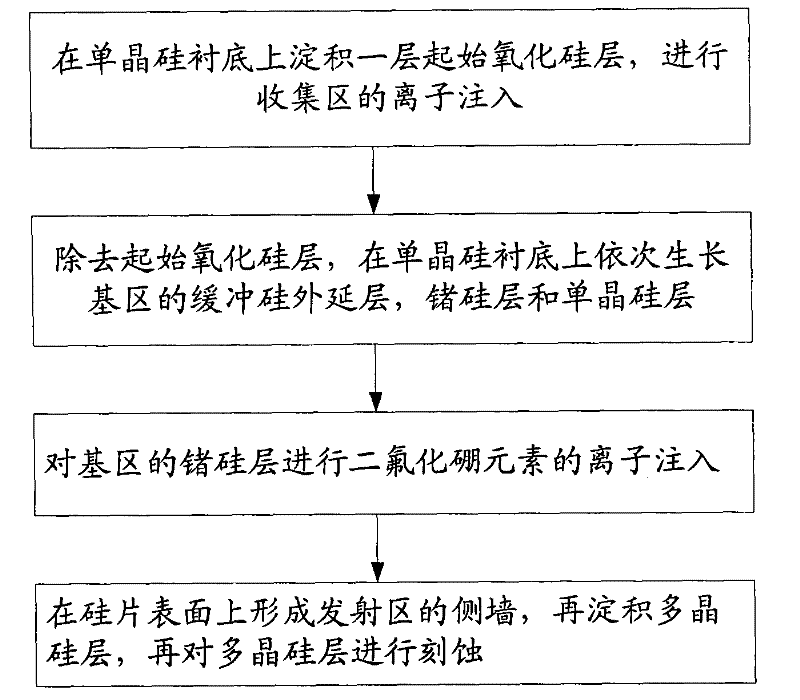
Method for inhibiting boron diffusion in base region of heterojunction bipolar transistor
ActiveCN101707182APrevent barrierImprove performanceSemiconductor/solid-state device manufacturingIn situ dopingBoron difluoride
The invention relates to a method for inhibiting boron diffusion in a base region of a heterojunction bipolar transistor, which comprises the step that when a buffer silicon layer, a germanium-silicon layer and a monocrystal silicon layer of the base region are grown on a silicon substrate, ion injection of a boron difluoride element into the germanium-silicon layer instead of in-situ doping of a boron element of impurity elements on the germanium-silicon layer is performed after the buffer silicon layer, the germanium-silicon layer and the monocrystal silicon layer of the base region are grown completely. The method is simple and easy to operate and can prevent potential barrier from occurring and ensure that the performance of products is stable.
Owner:SHANGHAI HUAHONG GRACE SEMICON MFG CORP
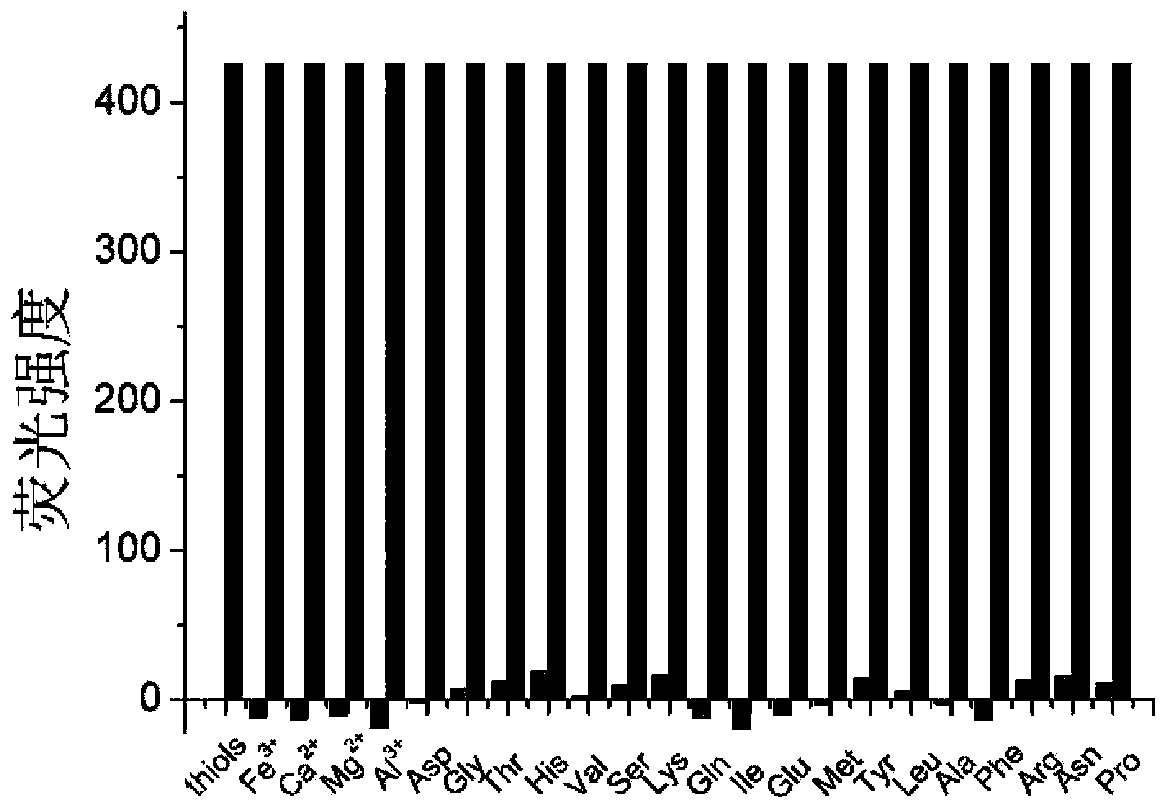
Synthesis and application of fluorescence molecular probes for detecting mercaptoamino-acids by virtue of naked eyes and fluorescence enhancement
InactiveCN103804401AHigh sensitivityGood optical performanceMaterial analysis by observing effect on chemical indicatorGroup 3/13 element organic compoundsSolubilityNitromethane
The invention relates to a preparation method and an application of naked eye identification and fluorescence enhancement type mercaptoamino-acid fluorescence probes, and specifically relates to preparation based on boron difluoride-dipyrromethane derivatives and an application thereof in detecting mercaptoamino-acids. The fluorescence probes are synthesized through direct reaction of the boron difluoride-dipyrromethane derivatives with nitromethane, and the pure probe product can be obtained through column chromatography analysis. The fluorescence probes are simple and convenient to synthesize, mild in reaction conditions, easy to purify and high in synthesis yield. The maximum absorption wavelength of the probe molecules is at 519nm; the probe molecules are good in solubility in a 50% acetonitrile aqueous solution and stable in optical properties; the absorption wavelength of the probe molecules is transferred to 506nm from 519nm as the mercaptoamino-acids are added; the fluorescence spectrum of the probe molecules is continuously enhanced at 520nm, and enhanced by 150 times at most, and in the meantime, the corresponding solution color turns to yellow from pink. The naked eye identification and fluorescence enhancement type mercaptoamino-acid fluorescence probes overcome the shortcoming that the response of the existing fluorescence probes to the mercaptoamino-acids is only based on the change of the fluorescence intensity. The probe molecules are high in sensitivity, high in identification ability to the mercaptoamino-acids and high in response speed, the response range of the probe molecules is from 0 to 1000 micrometers, the detection limits of the probe molecules to Cys, CSH and Hcy are 0.45 micrometer, 0.21 micrometer and 0.12 micrometer, and therefore, the detection range is wide and the detection limits are low; in short, the probes of the type have important practical application value in the fields such as biochemistry and environmental science.
Owner:SUZHOU ROWLAND BIOTECH
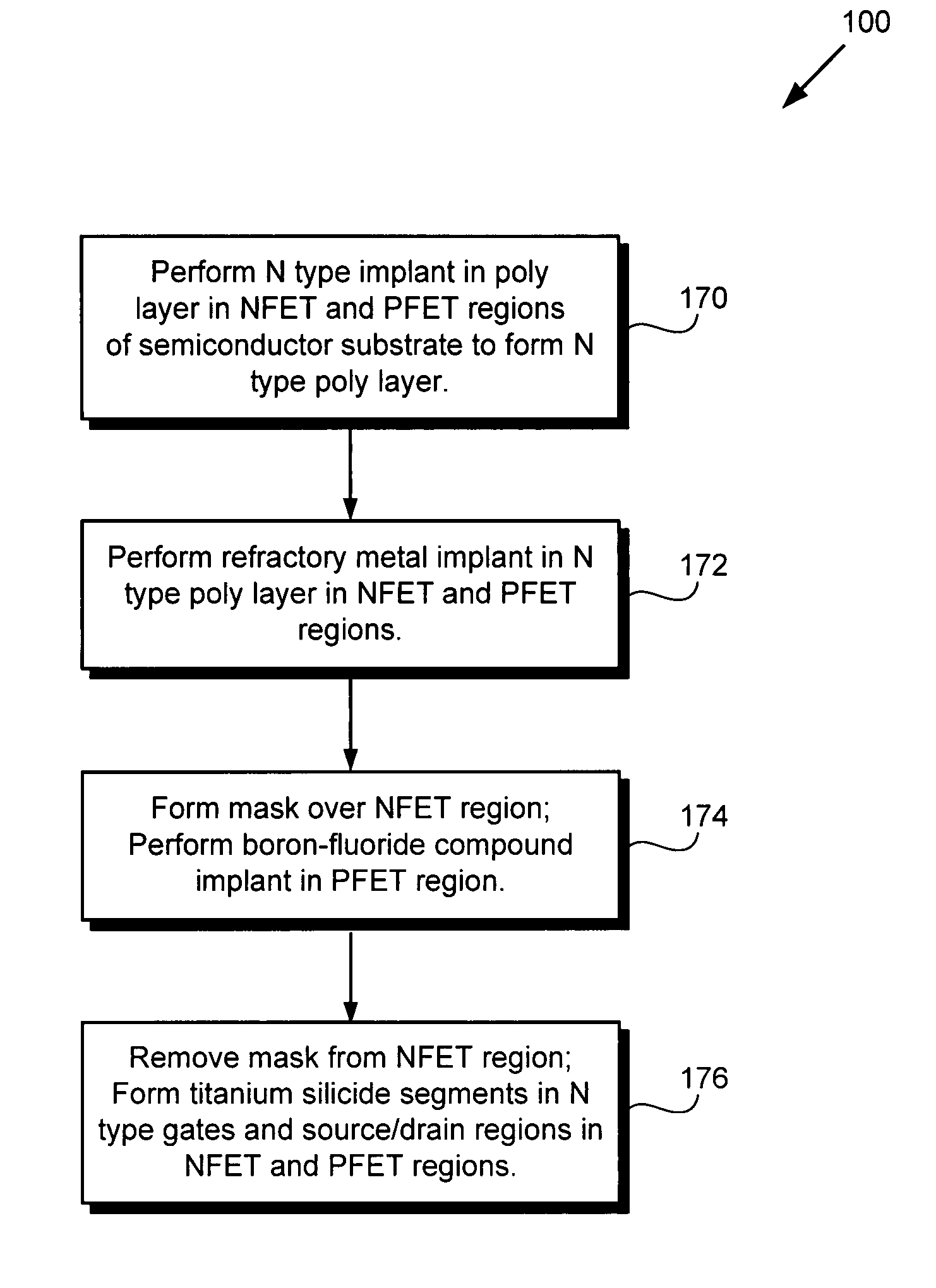
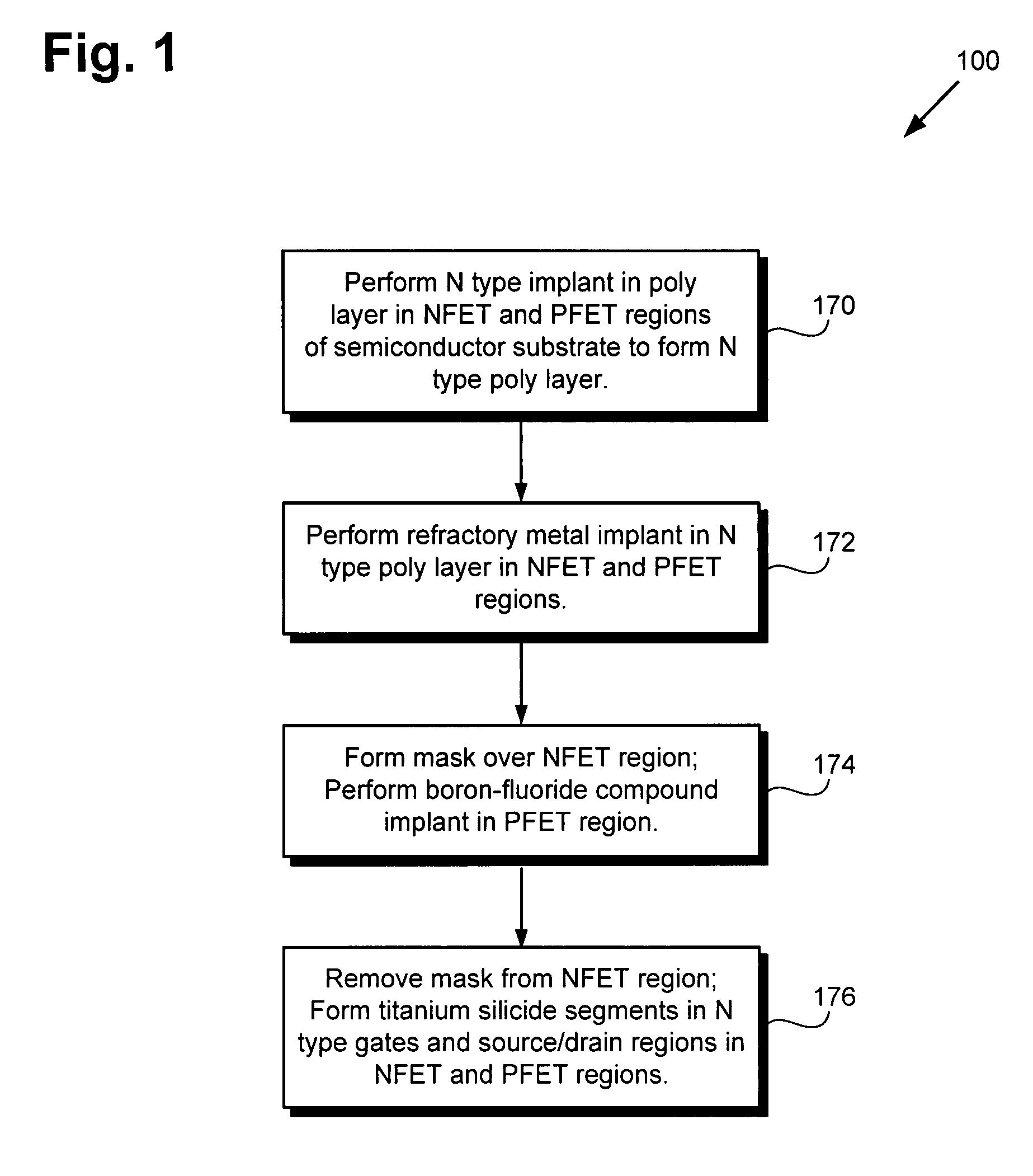
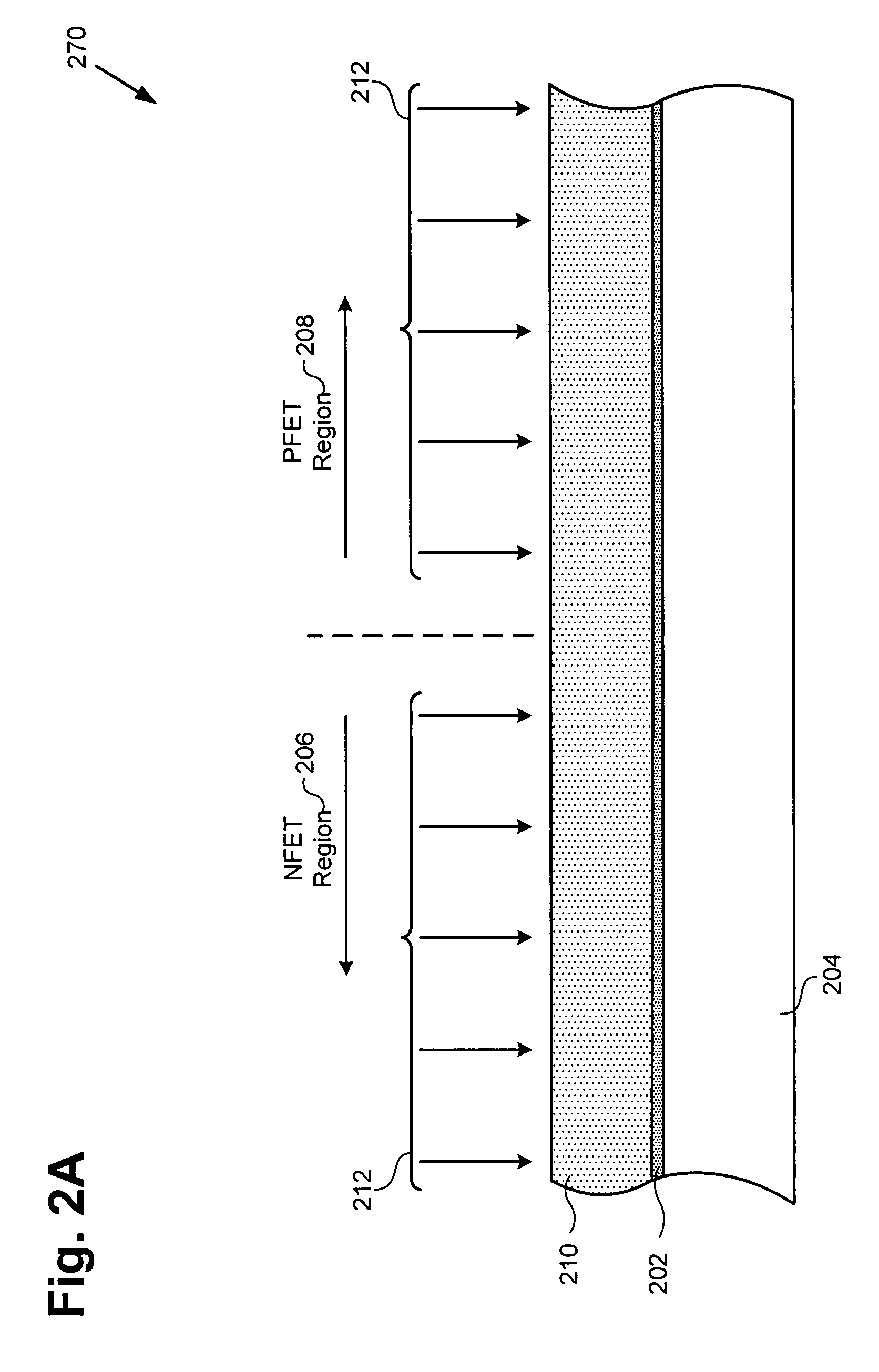
Method for forming a reduced resistivity poly gate and related structure
ActiveUS7776675B1Low resistivitySemiconductor/solid-state device manufacturingSemiconductor devicesFluorideBoron difluoride
According to an exemplary embodiment, a method for forming a reduced resistivity poly gate includes a step of implanting a refractory metal, such as molybdenum, in an N type poly layer in a PFET region of a semiconductor substrate. The method further includes a step of implanting a boron-fluoride compound, such as boron difluoride, in an N type gate in the PFET region, where the N type gate comprises a portion of the N type poly layer. The method further includes a step of forming a titanium silicide segment in the N type gate. The refractory metal reduces a resistivity of the titanium silicide segment, thereby forming the reduced resistivity poly gate.
Owner:NEWPORT FAB
Synthesis of cyanine, coumarin and dicarbonyl boron fluoride hybrid fluorochrome and application thereof
ActiveCN110183478AHigh yieldEasy to purifyMethine/polymethine dyesGroup 3/13 element organic compoundsCyanineIndoline
The invention discloses a synthetic method of a new cyanine, coumarin and difluoro boron hybrid dye with a fluorescent property, which has a general molecular formula shown in formula I. The dye is synthesized through design with the 7th site being a lignocaine electron-donating group and the 3rd site being a 6-methyl boron difluoride-beta-dicarbonyl hexatomic ring, wherein the methyl of the hexatomic ring is activated under the electrophilic effect and subjects to two times of Knoevenagel condensation with 1,3,3-trimethyl-2-methylene indoline acetaldehyde under different reaction conditions,the length of a conjugated chain is increased and a cyanine structure is introduced at the same time, so that the new cyanine, coumarin and difluoro boron hybrid dye I is formed. The synthetic methodis simple, and has wide application prospect in the fields such as fluorescence probes, life analysis and photoelectric materials.
Owner:QINGDAO UNIV OF SCI & TECH
Pyrromethene-boron difluoride complex derivative and preparation method thereof
InactiveCN104860974AEasy to introduceMolecular structure diversificationGroup 3/13 element organic compoundsPhenyl groupMethyl group
The invention provides a pyrromethene-boron difluoride complex derivative. A 1,3,5,7-tetramethyl-8-(4-allyloxy phenyl) pyrromethene-boron difluoride complex is formed by substituting allyloxy phenyl for a group on C8 of the pyrromethene-boron difluoride complex, the derivative contains specific 4-allyloxy phenyl group, so that the compound reactivity is improved greatly, and introduction of the 1,3,5,7-tetramethyl-8-(4-allyloxy phenyl) pyrromethene-boron difluoride complex as a group to a to-be-detected matter molecule is facilitated. The invention further provides a preparation method of the pyrromethene-boron difluoride complex derivative. The synthetic method is simple, intermediate separation is not required, and multiple steps of reactions are finished in one pot.
Owner:NORTHEAST NORMAL UNIVERSITY
Method for inhibiting boron diffusion in base region of heterojunction bipolar transistor
ActiveCN101707182BPrevent barrierImprove performanceSemiconductor/solid-state device manufacturingIn situ dopingBoron difluoride
The invention relates to a method for inhibiting boron diffusion in a base region of a heterojunction bipolar transistor, which comprises the step that when a buffer silicon layer, a germanium-silicon layer and a monocrystal silicon layer of the base region are grown on a silicon substrate, ion injection of a boron difluoride element into the germanium-silicon layer instead of in-situ doping of aboron element of impurity elements on the germanium-silicon layer is performed after the buffer silicon layer, the germanium-silicon layer and the monocrystal silicon layer of the base region are grown completely. The method is simple and easy to operate and can prevent potential barrier from occurring and ensure that the performance of products is stable.
Owner:SHANGHAI HUAHONG GRACE SEMICON MFG CORP
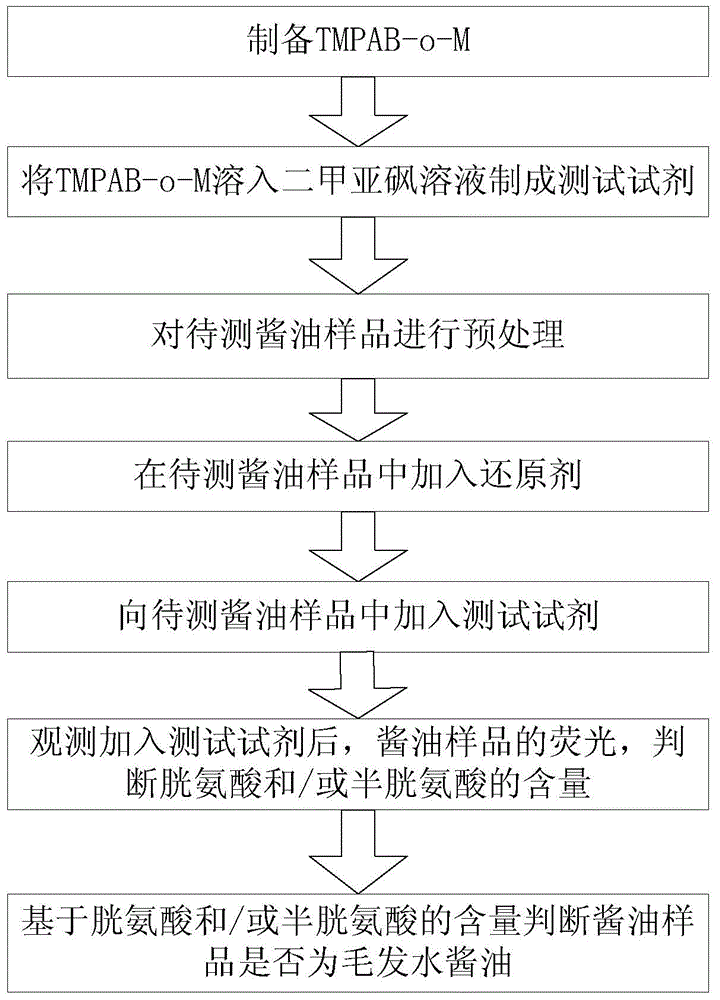
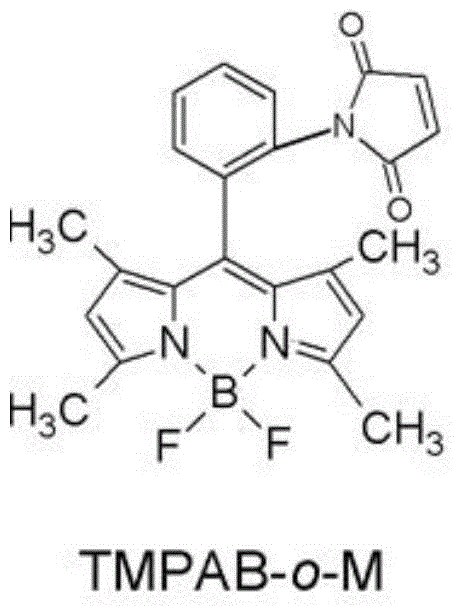
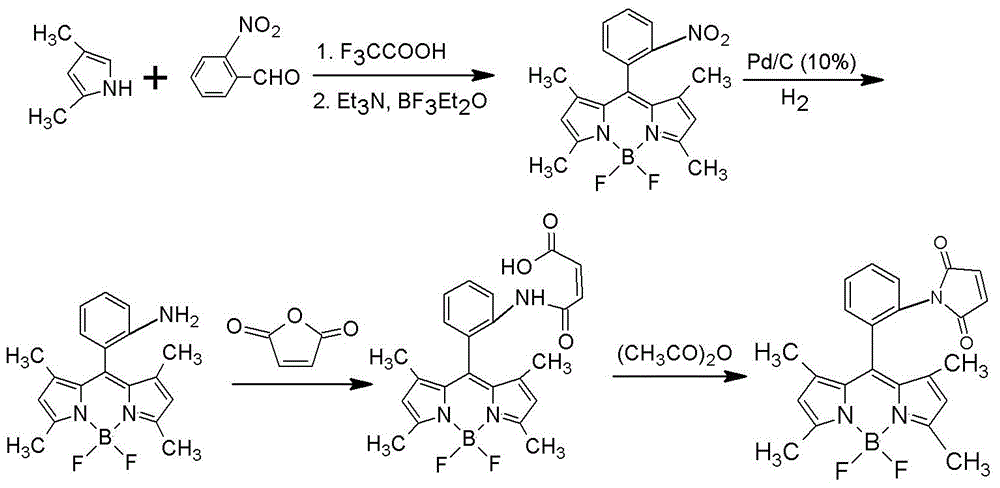
Method for detecting hair hydrolyzed soybean sauce
InactiveCN104597017AEnsure safetyShort derivation timeGroup 3/13 element organic compoundsFluorescence/phosphorescenceFluorescence spectrometryPre treatment
The invention provides a method for detecting hair hydrolyzed soybean sauce. The method comprises the following steps: (1) preparing 1,3,5,7-tetramethyl-8-phenyl-(2-maleimide)-boron difluoride dipyrrylmethane, and preparing a solution of preset concentration to serve as a test reagent; (2) pretreating a soybean sauce sample, and reducing cystine therein; and (3) dripping the test reagent into the soybean sauce sample, observing or detecting fluorescence of the soybean sauce sample after the test reagent is dripped, and judging whether the soybean sauce sample is hair hydrolyzed soybean sauce based on the intensity of the fluorescence. According to the test method disclosed by the invention, the hair hydrolyzed soybean sauce can be effectively detected. Moreover, according to the test method, judgment can be performed by virtue of naked eyes only, and the method is simple and feasible. If the cystine in the hair hydrolyzed soybean sauce needs to be quantitatively detected, only the intensity of a fluorescence spectrum needs to be subjected to quantitative analysis.
Owner:HUBEI INSPECTION & QUARANTINE TECH CENT
O,O two-tooth type organic boron difluoride fluorescent dye and preparation method thereof
InactiveCN103242674AGood effectOvercoming the lack of fluorescence emissionAzo dyesLuminescent compositionsBoron trifluorideDiethyl ether
The invention discloses an O,O two-tooth type organic boron difluoride fluorescent dye. The dye is the compound shown in the structural general formula I in the specification and is prepared by complexing a beta-dicarbonyl compound and boron trifluoride diethyl ether. The dye has the advantages of simplicity in reaction operation, easiness in obtaining reaction reagents and high product yield. The prepared red fluorescent dye in the general formula I has the characteristic of fluorescence emission in both solution and solid states and can be used for preparing fluorescent probes, OLED (organic light emitting diode) display materials and dye-sensitized solar cells. In particular, the dye also has the characteristic of fluorescence emission in the solid state, and the characteristic greatly widens the application range of the organic boron fluorescent dye.
Owner:张庆峰
Long-wavelength fluorescence probe, and preparation method and application thereof
InactiveCN103710020AThe influence of environmental pH value is smallGood choiceGroup 3/13 element organic compoundsFluorescence/phosphorescenceAcetic anhydrideBenzaldehyde
The invention discloses a long-wavelength fluorescence probe, and a preparation method and application thereof. The preparation method comprises the following steps: reacting 1,3,5,7-tetramethyl-8-phenyl-(2'-nitro)-boron difluoride-dimethyl pyrrole and benzaldehyde to generate a conjugated system with a larger 3,5-distyryl group, reducing -NO2 into -NH2 with zinc powder, carrying out addition reaction with maleic anhydride, and reacting with acetic anhydride to obtain the long-wavelength fluorescence probe 1,7-dimethyl-3,5-distyryl-8-phenyl-(2'-maleimido)-boron difluoride dipyrromethene. The preparation method is simple; the prepared long wavelength fluorescence probe has the advantages of small influence by environmental pH value, favorable selectivity for sulfhydryl compounds, and high sensitivity; the autofluorescence intensity of the fluorescence probe is very weak, but can be enhanced after reaction with sulfhydryl group, and thus, the fluorescence probe can be used for detecting sulfhydryl compounds; and the fluorescence probe can generate small damage to organisms, and thus, has wide application prospects in the fields of biological tissues, cell fluorescence imaging and the like.
Owner:WUHAN UNIV
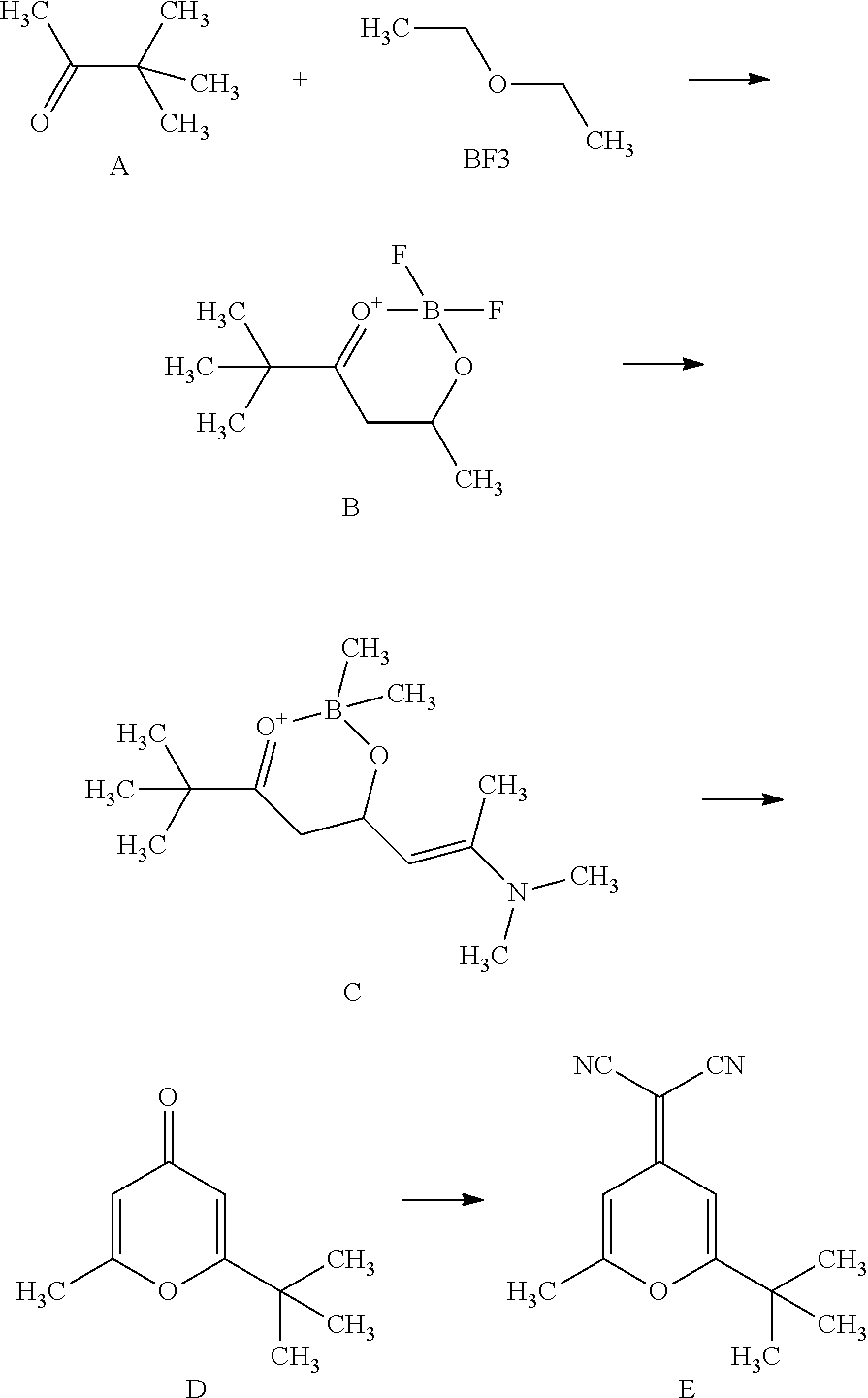
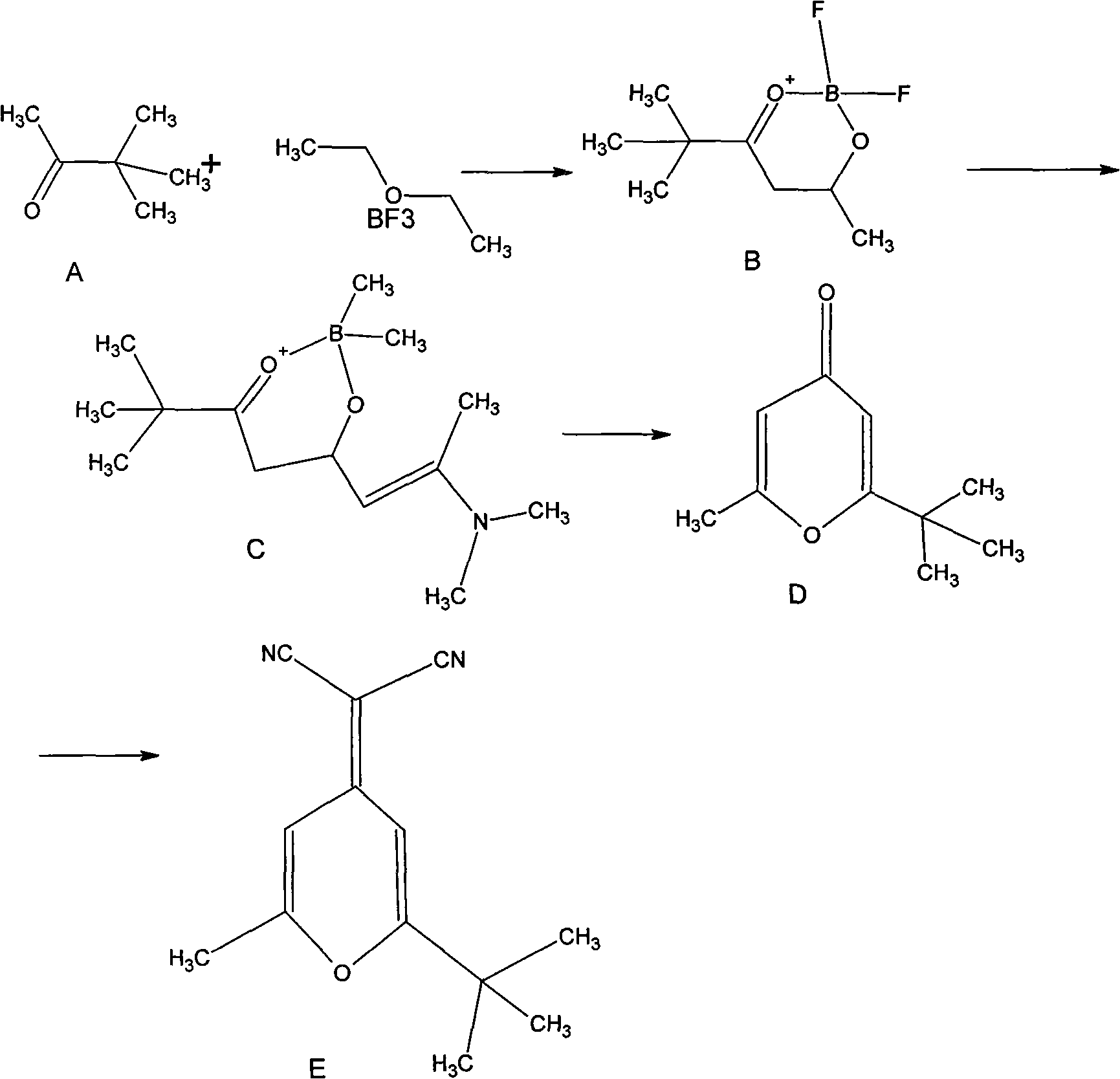
2-methyl-6-tert-butyl-4-dicyanmethylene-4H-pyran synthesis method
ActiveCN101891725AHigh process yieldMild reaction conditionsOrganic chemistrySynthesis methodsOrganic synthesis
The invention relates to a 2-methyl-6-tert-butyl-4-dicyanmethylene-4H-pyran synthesis method, belonging to the field of organic synthesis. The synthesis method comprises the steps of: preparing 5,5-dimethyl-2,4-adipaldehyde-O,O-boron difluoride through the reaction of pinacotone and boron trifluoride diethyl ether; preparing 7-dimethyl amino-2,2-dimethyl-6-ene-3,5-suberic aldehyde-O,O-boron difluoride through the aldolization reaction of the ,5-dimethyl-2,4-adipaldehyde-O,O-boron difluoride and N,N-dimethylacetylamide dimethyl; preparing 2-methyl-6-tertiary butyl-pyrone through perchloric acid cyclization; and reacting 2-methyl-6-tertiary butyl-pyrone with malononitrile to prepare the 2-methyl-6-tert-butyl-4-dicyanmethylene-4H-pyran. The invention is characterized in that alcohol solvents are utilized in the last step for recrystallization. The invention has the advantages of simple operation technology and easy large-scale production and can ensure that the total yield is far higher than the yield provided by the literature and the purity of the final product is very high. The invention can be directly used for preparing DCJTB (4-(Dicyanomethylene)-2-tert-butyl-6-(1,1,7,7-tetramethyljulolidin-4-yl-vinyl)-4H-pyran).
Owner:GUANGDONG AGLAIA OPTOELECTRONICS MATERIALS
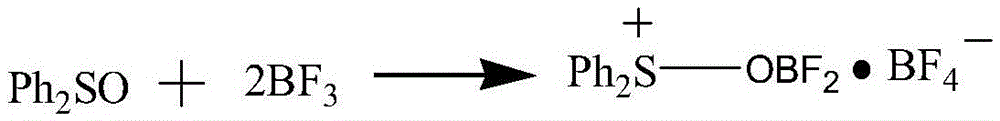
Preparation method for boron difluoride diphenyl sulphur tetrafluoroborate compound
InactiveCN105541889AEasy to makeMild reaction conditionsGroup 3/13 element organic compoundsTetrafluoroborateReaction temperature
The invention relates to a preparation method for a boron difluoride diphenyl sulphur tetrafluoroborate compound. The method includes the steps that diphenyl sulfoxide is dissolved in an organic solvent, boron trifluoride is slowly dripped with stirring, and after dripping is finished, the solution is stirred for 10-50 min to ensure a complete reaction; an excess precipitator is added into the obtained solution, separation is carried out, and the boron difluoride diphenyl sulphur tetrafluoroborate compound is obtained. Compared with the prior art, the method has the advantages that the boron difluoride diphenyl sulphur tetrafluoroborate compound prepared through the method is brown liquid, the preparation process is simple, reaction conditions are mild, reaction time is short, the yield of the reaction product is high, the stability of the product is good, and the product can be applied to ring-opening polymerization of tetrahydrofuran and copolymers thereof.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
O,O two-tooth type organic boron difluoride fluorescent dye and preparation method thereof
InactiveCN103242674BGood effectOvercoming the lack of fluorescence emissionAzo dyesLuminescent compositionsBoron trifluorideSolar cell
Owner:张庆峰
Preparation and application of a near-infrared dye based on azafluoroborane
The invention discloses preparation of near-infrared dye based on aza-fluoroborane and application. The near-infrared dye is prepared from thiophene-containing groups and a basic aza-fluoroborane framework. The aza-fluoroborane dye is prepared by the following steps: (1) synthesis of ketene: the ketene is obtained by addition-elimination reaction of aldehyde and ketone under an alkali condition; (2) ketene and nitromethane conduct addition reaction under the alkali condition; (3) ring-forming reaction: under the condition with existence of an ammonia source, two times of equivalent ketene andnitromethane conduct ring-forming reaction; (4) coordination reaction: products obtained in the step (3) and metal or boron difluoride are coordinated to obtain a target product. The preparation and the application disclosed by the invention have the advantages that the target dye has strong near-infrared absorption (the molar light absorption coefficient is more than 100000M<-1>cm<-1>), high singlet oxygen yield and good photothermal effect, and can be used for photodynamic and photothermal synergy with single-wavelength excitation for tumor treatment under the guidance of photothermal imaging, photoacoustic imaging and fluorescence imaging.
Owner:NANJING UNIV OF POSTS & TELECOMM
Synthesis method of dipyrromethene N, O-boron difluoride derivative
InactiveCN105693752AEasy to makeEasy to purifyGroup 3/13 element organic compoundsEthyl EthersBoron difluoride
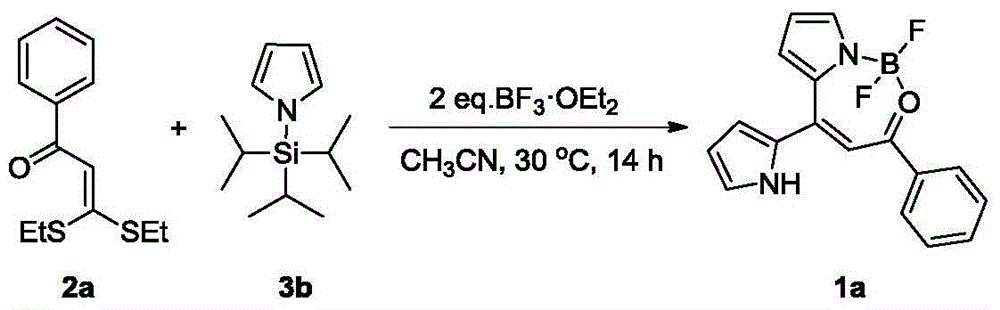
The invention discloses a synthesis method of a dipyrromethene N, O-boron difluoride derivative. The method takes ketene dithioacetals, a pyrrole compound and boron trifluoride ethyl ether as reaction raw materials to carry out one-step reaction to obtain a dipyrromethene N, O-boron difluoride derivative. Compared with reported synthesis methods of dipyrromethene boron difluoride analogues, the method provided by the invention has the advantages of easily available raw materials, simple operation, and mild synthesis reaction conditions, etc.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
O,O two-tooth type organic boron difluoride fluorescent dye and preparation method thereof
InactiveCN103242823AGood effectOvercoming the lack of fluorescence emissionAzo dyesLuminescent compositionsBoron trifluorideDiethyl ether
The invention discloses an O,O two-tooth type organic boron difluoride fluorescent dye. The dye is the compound shown in the structural general formula I in the specification and is prepared by complexing a beta-dicarbonyl compound and boron trifluoride diethyl ether. The dye has the advantages of simplicity in reaction operation, easiness in obtaining reaction reagents and high product yield. The prepared red fluorescent dye in the general formula I has the characteristic of fluorescence emission in both solution and solid states and can be used for preparing fluorescent probes, OLED (organic light emitting diode) display materials and dye-sensitized solar cells. In particular, the dye also has the characteristic of fluorescence emission in the solid state, and the characteristic greatly widens the application range of the organic boron fluorescent dye.
Owner:李艳琴
Fluorescent probe for detecting fluorine ions and preparation method and application thereof
PendingCN111423467ADecreased fluorescence intensityIncrease conjugate planeGroup 4/14 element organic compoundsFluorescence/phosphorescenceFluoProbesMeth-
The invention relates to a fluorescent probe for detecting fluorine ions and a preparation method and application of the fluorescent probe, the chemical name of the fluorescent probe is 8-(4-((4-((tert-butyldiphenylsilyl)oxy)phenyl)methoxy)phenyl)-dipyrromethene boron difluoride. The preparation method comprises (1) synthesis of 4-(di(1H-pyrrole-2-yl)methyl)phenol, (2) synthesis of p-hydroxyphenyl-dipyrromethene boron difluoride, and (3) synthesis of 8-(4-((4-((tert-butyldiphenylsilyl)oxy)phenyl)methoxy)phenyl)-dipyrromethene boron difluoride. The fluorescent probe is applied to detection of fluorine ions under a solid state condition. Compared with the prior art, the fluorescent probe has the advantages of good specificity, strong anti-interference capability, excellent sensitivity, low fluorescence detection limit, capability of detecting fluorine ions under a solid state condition and the like.
Owner:SHANGHAI INSTITUTE OF TECHNOLOGY
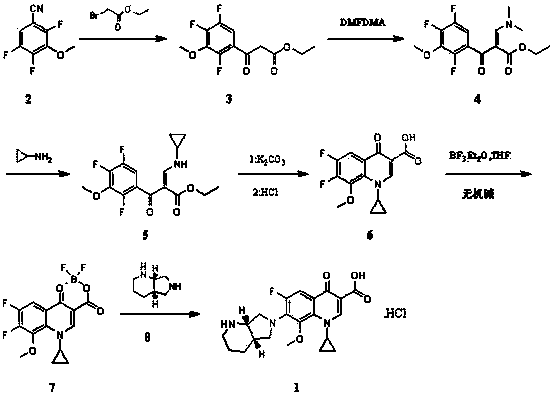
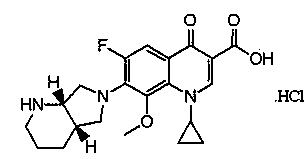
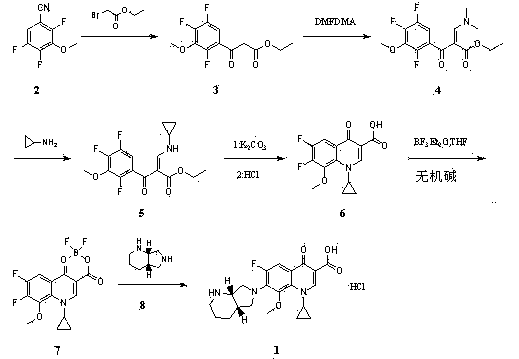
Stable moxifloxacin hydrochloride compound and preparation method thereof
The invention discloses a moxifloxacin hydrochloride compound, which is prepared by the steps of: performing Reformatsky reaction on 3-methoxyl-2,4,5-trifluoro benzonitrile to obtain ethyl 3-methoxyl-2,4,5-trifluoro benzoyl acetate, performing enamine formation, amine exchange, nucleophilic substitution, hydrolysis and chelating to obtain (8-methoxyl-1-cyclopropyl-6,7-difluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate) boron difluoride, and performing nucleophilic substitution reaction to obtain moxifloxacin hydrochloride. The preparation method of moxifloxacin hydrochloride compound has the benefits that the operation is easy and simple, and the intermediates obtained in each step are high in purity; the reaction is sufficient, the subsidiary reactions are few, and the purification is easy; and the reaction steps are reduced and the industrialized production is easy to realize.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com