Pyrromethene-boron difluoride complex derivative and preparation method thereof
A technology of boron difluoride and pyrromethine, which is applied in the field of pyrromethine-boron difluoride complex derivatives and its preparation, can solve the problems of low laser efficiency, difficult boron difluoride complex modification compounds, Narrow laser tuning range and other issues, to achieve the effect of simple synthesis method, rich structure and functional diversity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
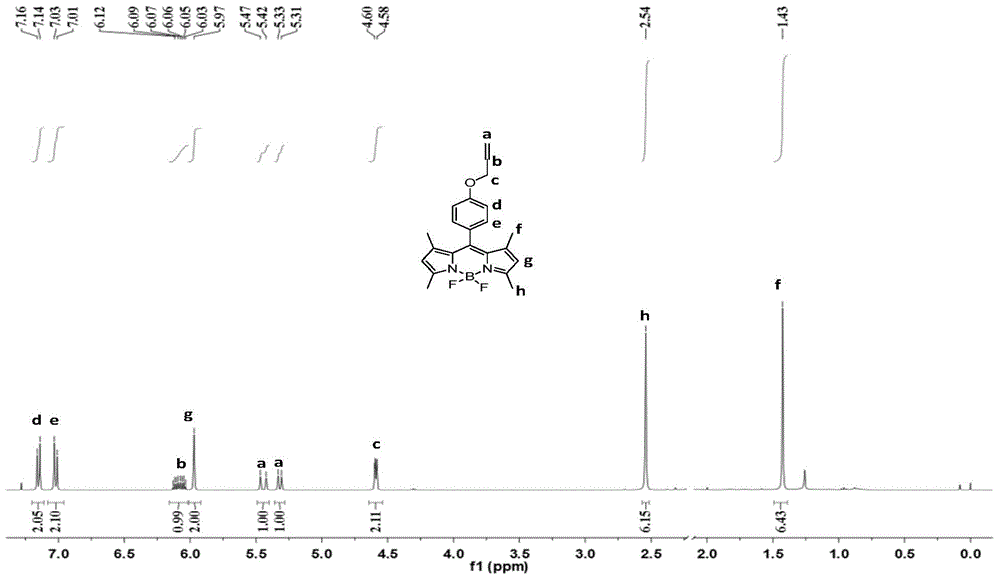
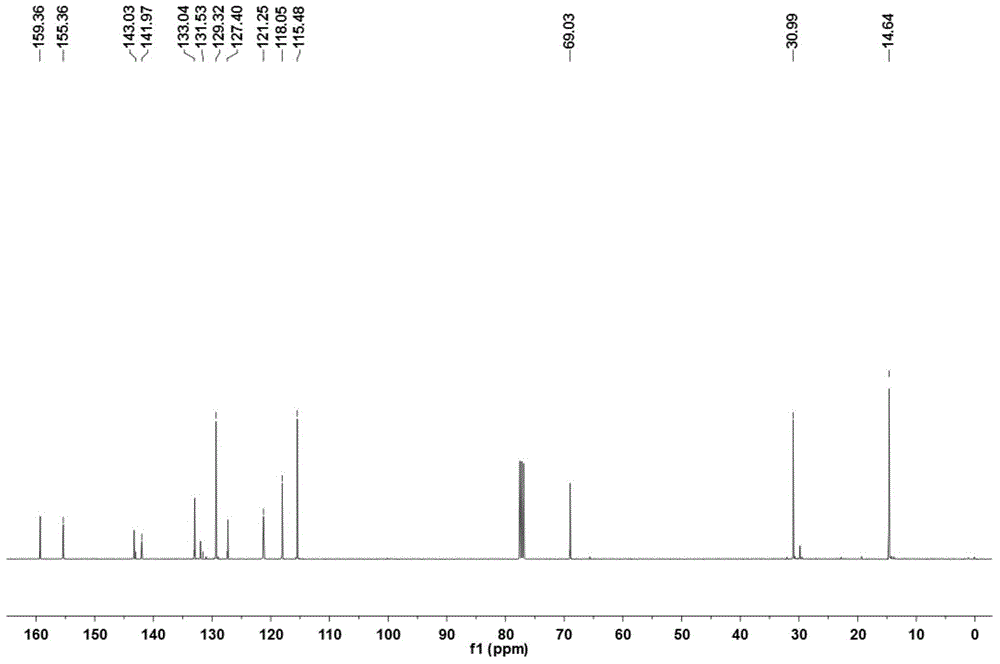
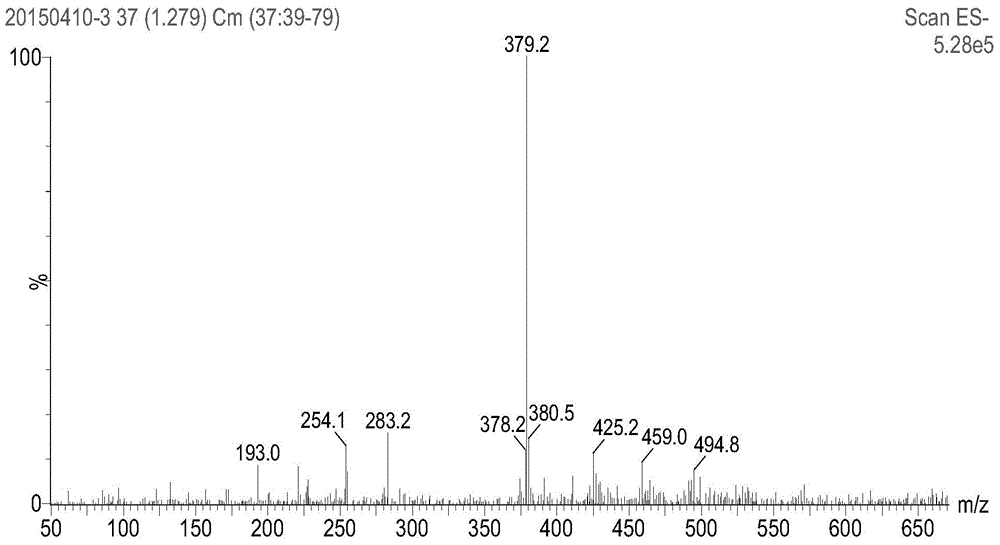
[0038]1,3,5,7-Tetramethyl-8-(4-allyloxyphenyl)pyrromethene-boron difluoride complex was used as a probe for detecting proteins, and the experiment was as follows: In No. 1 and Add concentration is the Tris-Hcl buffer solution of 0.5mol / L and the NaCl solution of 0.4mol / L in No. 2 absorption pool, adjust pH value to 7.4, then add the 1 that concentration is 0.03mmol / L in No. 1 absorption pool, 3,5,7-Tetramethyl-8-(4-allyloxyphenyl)pyrromethene-boron difluoride complex solution 2mL, scan its absorption spectrum in the wavelength range of 300-800nm, the excitation wavelength Fix around the wavelength of the maximum absorption peak, add 2mL of r-globulin solution with a concentration of 0.03mmol / L to No. 1 and No. 2 absorption pools respectively, and measure the fluorescence intensity F of No. 1 absorption pool 1 , the fluorescence intensity of No. 2 absorption cell is F 2 ; while the fluorescence intensity of 1,3,5,7-tetramethyl-8-(4-allyloxyphenyl)pyrromethene-boron difluoride ...
example 1
[0042] Step 1: Under the condition of protecting from light and nitrogen at room temperature, add 100 mL of anhydrous dichloromethane to a reactor equipped with a stirrer, then add 419 mg (4.4 mmol) of 2,4-dimethylpyrrole and 4-ene After propoxybenzaldehyde 546mg (2.0mmol), the stirrer was turned on, and then 20 μL of catalyst was added to react with trifluoroacetic acid for 10 hours;
[0043] Step 2: Dissolve 454mg of the oxidizing agent 2,3-dichloro-5,6-dicyano-1,4-p-benzoquinone in 10mL of dichloromethane, then add it into the reaction kettle, and stir at room temperature for 30min;
[0044] Step 3: Add 3 mL of triethylamine, stir at room temperature for 10 min, slowly add 5 mL of boron trifluoride ether in an ice bath, and continue stirring for 3 h;
[0045] Step 4: sequentially wash with water, dry, evaporate the solvent under reduced pressure, use n-hexane:ethyl acetate=10:1 (volume ratio) as the eluent, and conduct column chromatography separation with silica gel as the...
example 2
[0049] Step 1: Under the condition of protecting from light and nitrogen at room temperature, add 100 mL of anhydrous dichloromethane to a reaction kettle equipped with a stirrer, then add 419 mg (4.4 mmol) of 2,4-dimethylpyrrole and 4-ene After propoxybenzaldehyde 546mg (2.0mmol), the stirrer was turned on, and then 20 μL of catalyst was added to react with trifluoroacetic acid for 10 hours;
[0050] Step 2: Dissolve 454mg of the oxidizing agent 2,3-dichloro-5,6-dicyano-1,4-p-benzoquinone in 10mL of dichloromethane, then add it into the reaction kettle, and stir at room temperature for 30min;
[0051] Step 3: Add 3 mL of triisopropylamine, stir at room temperature for 10 min, slowly add 5 mL of boron trifluoride diethyl ether in an ice bath, and continue stirring for 8 h;
[0052] Step 4: sequentially wash with water, dry, evaporate the solvent under reduced pressure, use n-hexane:ethyl acetate=10:1 (volume ratio) as the eluent, and conduct column chromatography separation wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com