Near-infrared photo-thermal dye based on aza-fluoro borane, preparation and application
A near-infrared light, heterofluoroborane technology, applied in the direction of azo dyes, luminescent materials, organic dyes, etc., to achieve the effect of weakening damage, good photothermal performance, and deep tissue penetration depth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
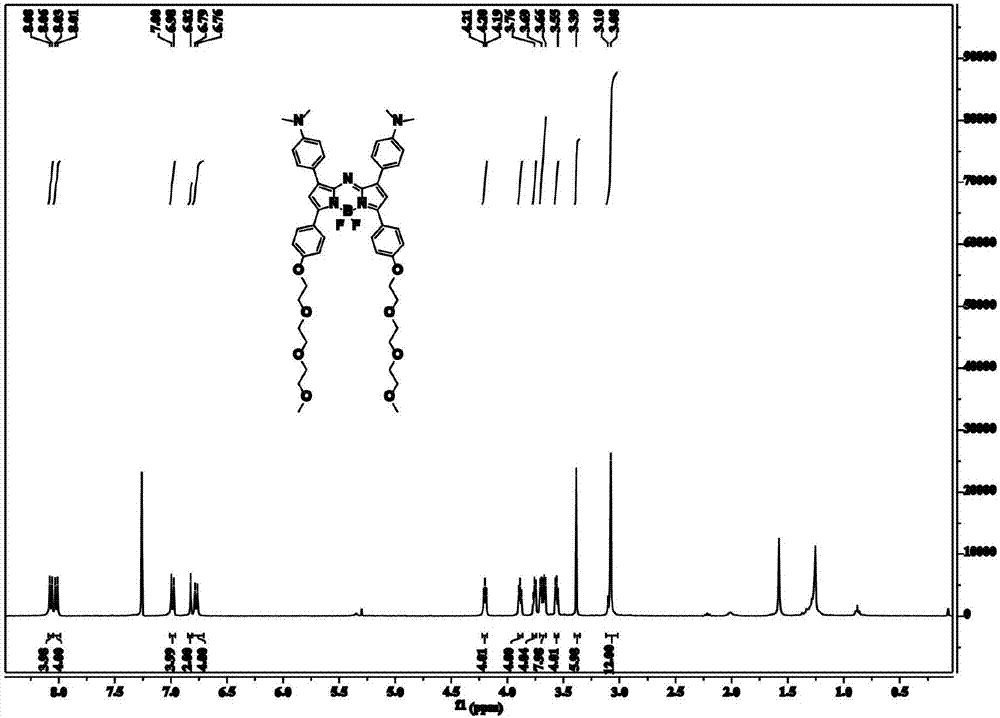
[0035] Example 1: Synthesis of Azafluoroborane Photothermal Dye C
[0036]
[0037] Synthesis of Compound 2
[0038] Take a clean two-necked bottle, add magneton, 1.49g of p-dimethylaminobenzaldehyde (about 10mmol), 2.8g of 1 (about 10mmol) and 40ml of ethanol solution. After stirring until all the solids were dissolved, slowly add 23ml of sodium hydride solution (containing 2.0g of sodium hydroxide). The reaction was stirred at room temperature for 24 h, and a yellow solid was precipitated during the reaction. After the reaction, 1M hydrochloric acid solution was used to adjust the reaction solution to neutrality, and a solid was obtained by suction filtration, and washed three times with deionized water. 2.1 g of a pale yellow solid were dried in vacuo.
[0039] 1 H NMR (400MHz, CDCl 3 ): δ (ppm) = 8.01 (d, J = 8.0Hz, 2H), 7.78 (d, J = 16.0Hz, 1H), 7.53 (d, J = 8.0Hz, 2H), 7.35 (d, J = 16.0 Hz,1H),6.98(d,J=8.0Hz,2H),6.69(d,J=8.0Hz,2H),4.21(t,2H),3.89(t,2H),3.77-3.65...
Embodiment 2
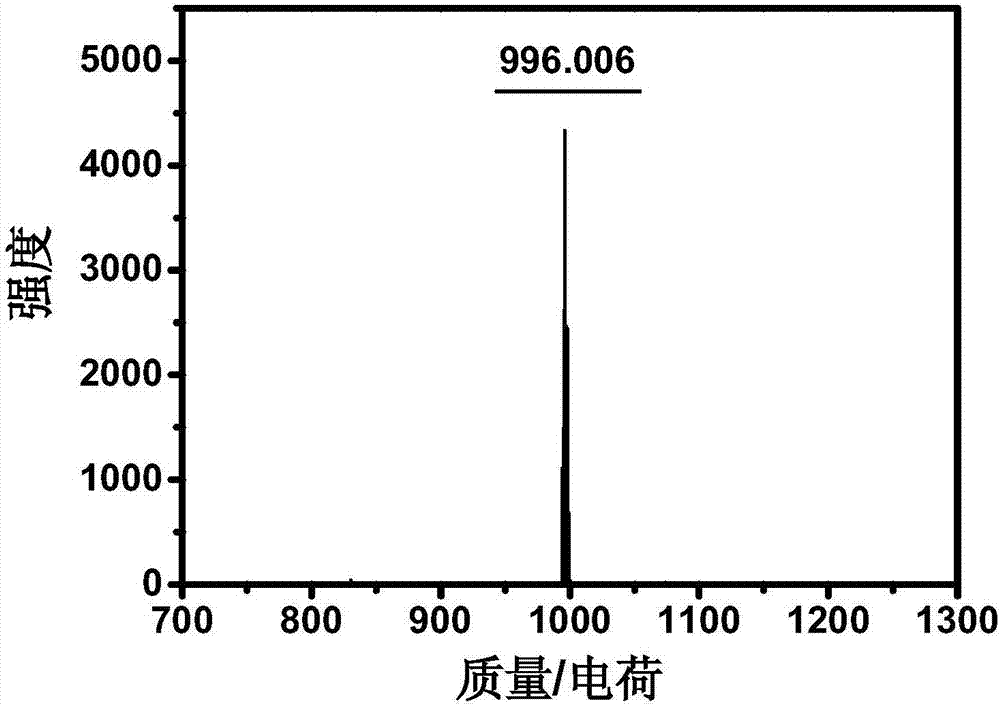
[0049] Example 2: Synthesis of Azafluoroborane Photothermal Dye D
[0050]
[0051] Synthesis of compound 1
[0052] Take a clean two-necked bottle, add magneton, 1.36g of p-hydroxyacetophenone (about 10mmol), 9.66g of bromooctane (about 50mmol), 6.91g of potassium carbonate and 40ml of anhydrous N,N-dimethyl formamide solution. Under magnetic stirring, react at 80°C for 24h. After the reaction was completed, water / dichloromethane was extracted several times, and the organic phases were combined. After separation by chromatography column, 2.46 g of light yellow liquid was obtained (the yield was about 99%).
[0053] Synthesis of Compound 2
[0054] Take a clean two-necked bottle, add magneton, 1.24g of 1 (about 5mmol), 0.75g of p-dimethylaminobenzaldehyde (about 5mmol) and 20ml of ethanol solution. Stir until all the solids are dissolved, then slowly add 5ml of sodium hydride solution (containing 1.00g of sodium hydroxide). The reaction was stirred at room temperature...
Embodiment 3
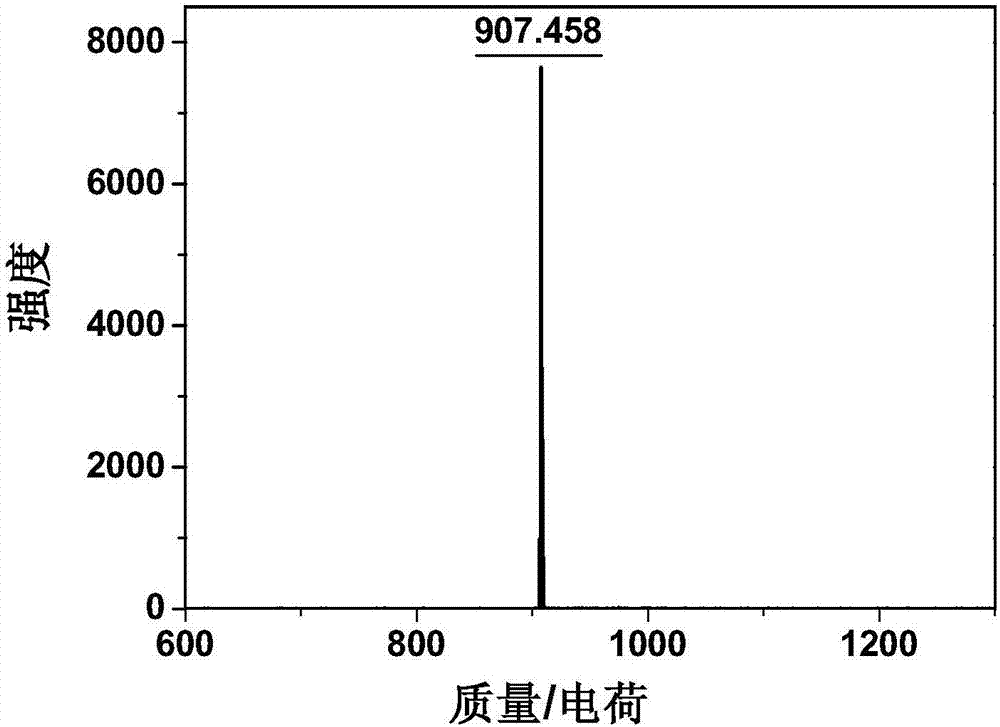
[0067] Embodiment 3: the test of the molecular weight of C
[0068] Take a small amount of sample C, mix it with the matrix, then apply the sample, and measure it with MALDI-TOF / TOF, as shown in Figure 1, which preliminarily proves the correctness of the C molecule.
[0069] [m / e] (M, MALDI-TOF) theoretical value: 907.85, experimental value: 907.46
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com