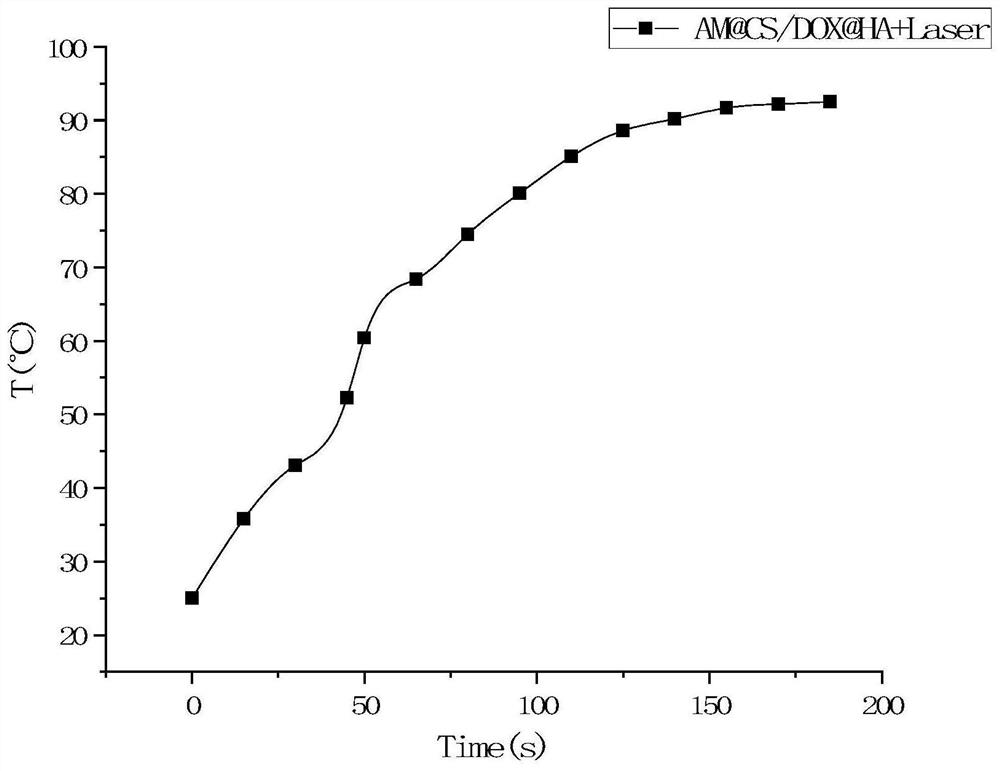
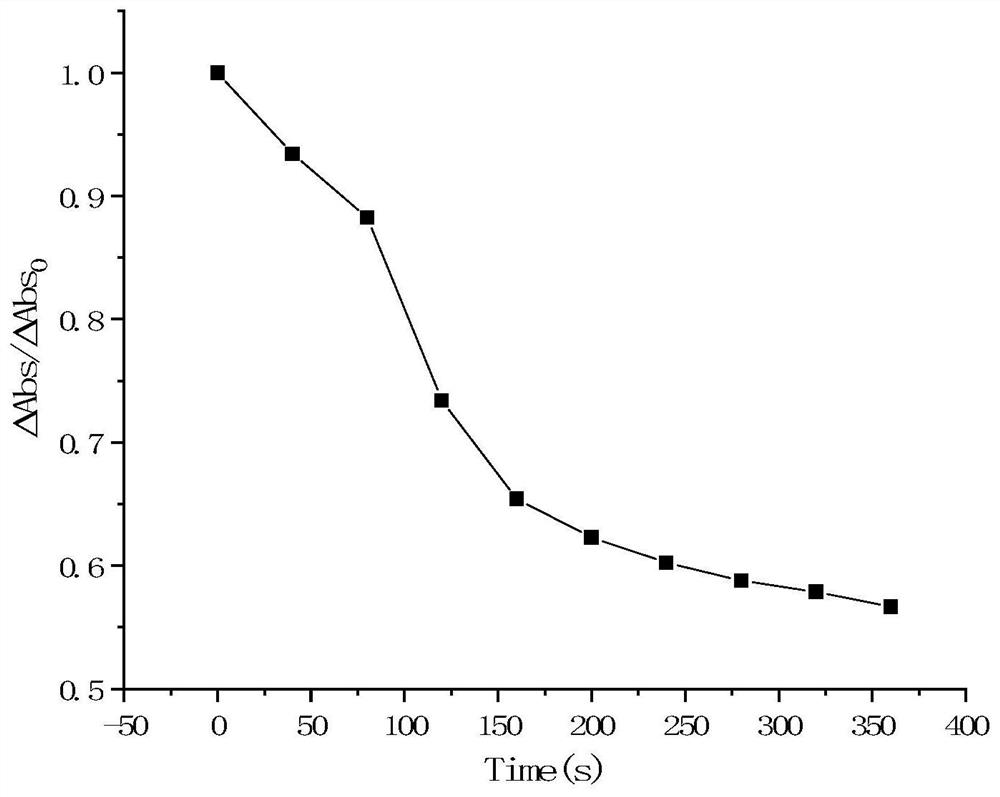
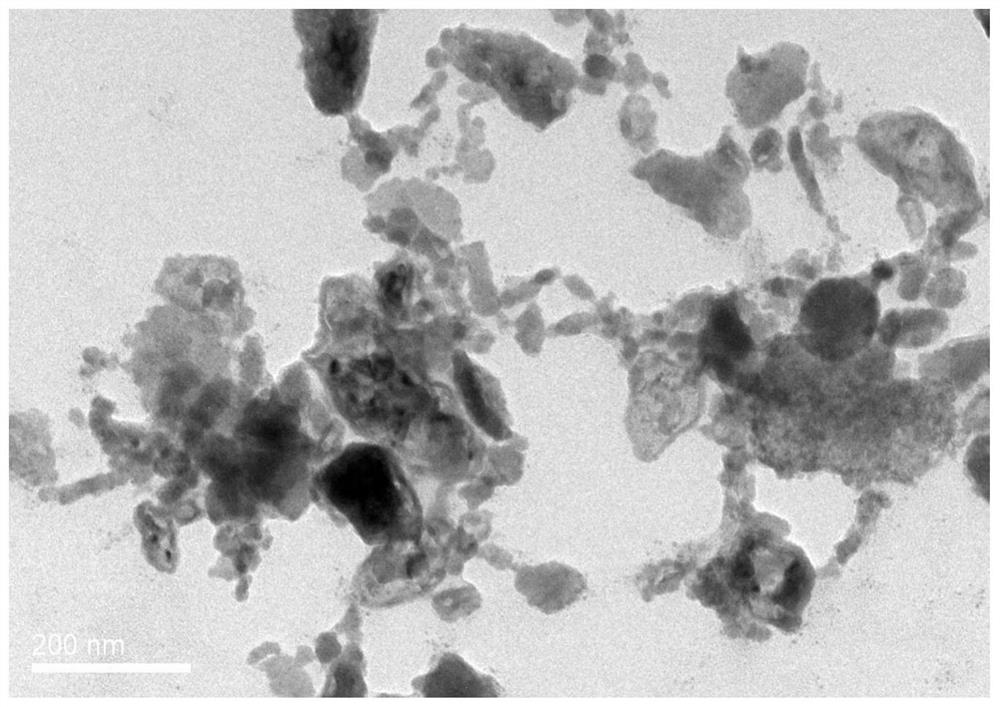
Preparation method of antimonene-based tumor targeted drug-loaded nanoparticles
A drug-loaded nano- and tumor-targeted technology, applied in the field of medicine, can solve the problems of difficult chemical modification, poor product stability, and few AM surface active groups
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Chitosan solution preparation: Weigh 20 mg chitosan and dissolve it in 20 ml acetic acid to prepare a 1 mg / mL chitosan solution, and store it in a refrigerator at 4°C.
[0036] (2) Preparation of hyaluronic acid solution: Weigh 20 mg of hyaluronic acid and dissolve it in 20 ml of deionized water to prepare a 1 mg / mL hyaluronic acid solution, and store it in a refrigerator at 4°C.
[0037] (3) Preparation of doxorubicin doxorubicin solution: Weigh 10 mg of ICG and dissolve in 10 ml of deionized water to prepare a 1 mg / mL doxorubicin solution, wrap it in tin foil and store it in a refrigerator at 4°C.
[0038](4) Preparation of AMNPs: Disperse 2g of antimony powder in a clean beaker containing 40ml of NMP at room temperature, seal it with plastic wrap, and use a φ6 ultrasonic probe to sonicate in an ice bath for 8h under an ultrasonic cell disruptor, with a power of 1000w, and stop for 1s every 3s. Stir the dispersion with a glass rod every 30 minutes to make it evenl...
Embodiment 2
[0044] (1) Chitosan solution preparation: Weigh 20 mg chitosan and dissolve it in 10 ml acetic acid to prepare a 2 mg / mL chitosan solution, and store it in a refrigerator at 4°C.
[0045] (2) Preparation of hyaluronic acid solution: Weigh 20 mg of hyaluronic acid and dissolve it in 10 ml of deionized water to prepare a 2 mg / mL hyaluronic acid solution, and store it in a refrigerator at 4°C.
[0046] (3) Preparation of doxorubicin doxorubicin solution: Weigh 20 mg of ICG and dissolve in 10 ml of deionized water to prepare a 2 mg / mL ICG solution, wrap it in tin foil and store it in a refrigerator at 4°C.
[0047] (4) Preparation of AMNPs: Disperse 4 g of antimony powder in a clean beaker containing 80 ml of NMP at room temperature, seal it with plastic wrap, and use a φ6 ultrasonic probe to sonicate in an ice bath for 10 h under an ultrasonic cell disruptor, with a power of 1000 W, and stop for 1 s every 3 s. Stir the dispersion with a glass rod every 30 minutes to make it evenl...
Embodiment 3
[0052] (1) Preparation of chitosan solution: Weigh 15 mg of chitosan and dissolve it in 10 ml of acetic acid to prepare a 1.5 mg / mL chitosan solution, and store it in a refrigerator at 4°C.
[0053] (2) Preparation of hyaluronic acid solution: Weigh 15 mg of hyaluronic acid and dissolve it in 10 ml of deionized water to prepare a 1.5 mg / mL hyaluronic acid solution, and store it in a refrigerator at 4°C.
[0054] (3) Preparation of doxorubicin doxorubicin solution: Weigh 15 mg of ICG and dissolve in 10 ml of deionized water to prepare a 1.5 mg / mL doxorubicin solution, wrap it in tin foil and store it in a refrigerator at 4°C.
[0055] (4) Preparation of AMNPs: Disperse 3g of antimony powder in a clean beaker containing 50ml of NMP at room temperature, seal it with plastic wrap, and use a φ6 ultrasonic probe to sonicate in an ice bath for 8.5h under an ultrasonic cell disruptor, with a power of 1000w, and stop for 1s every 3s , use a glass rod to stir the dispersion every 30 min...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com