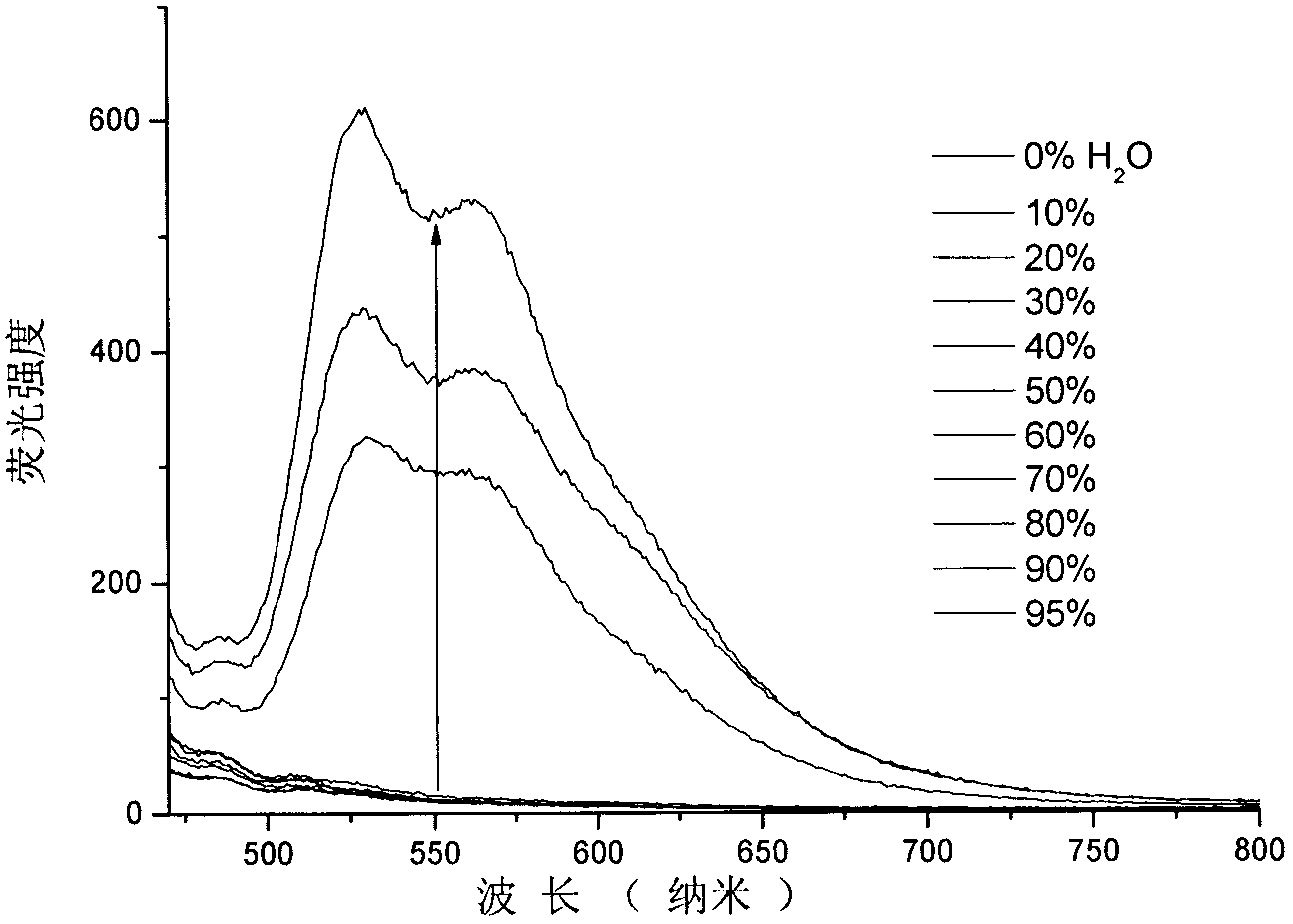
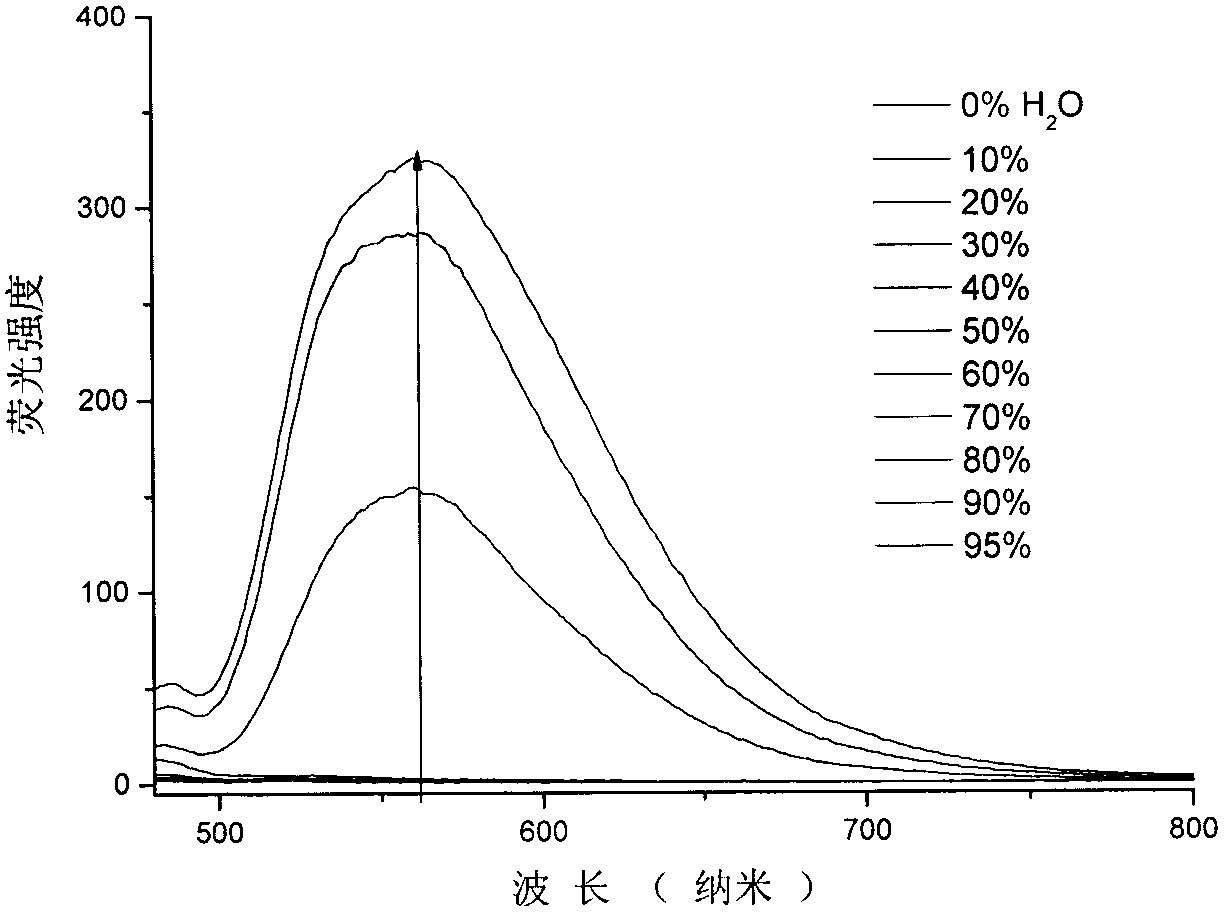
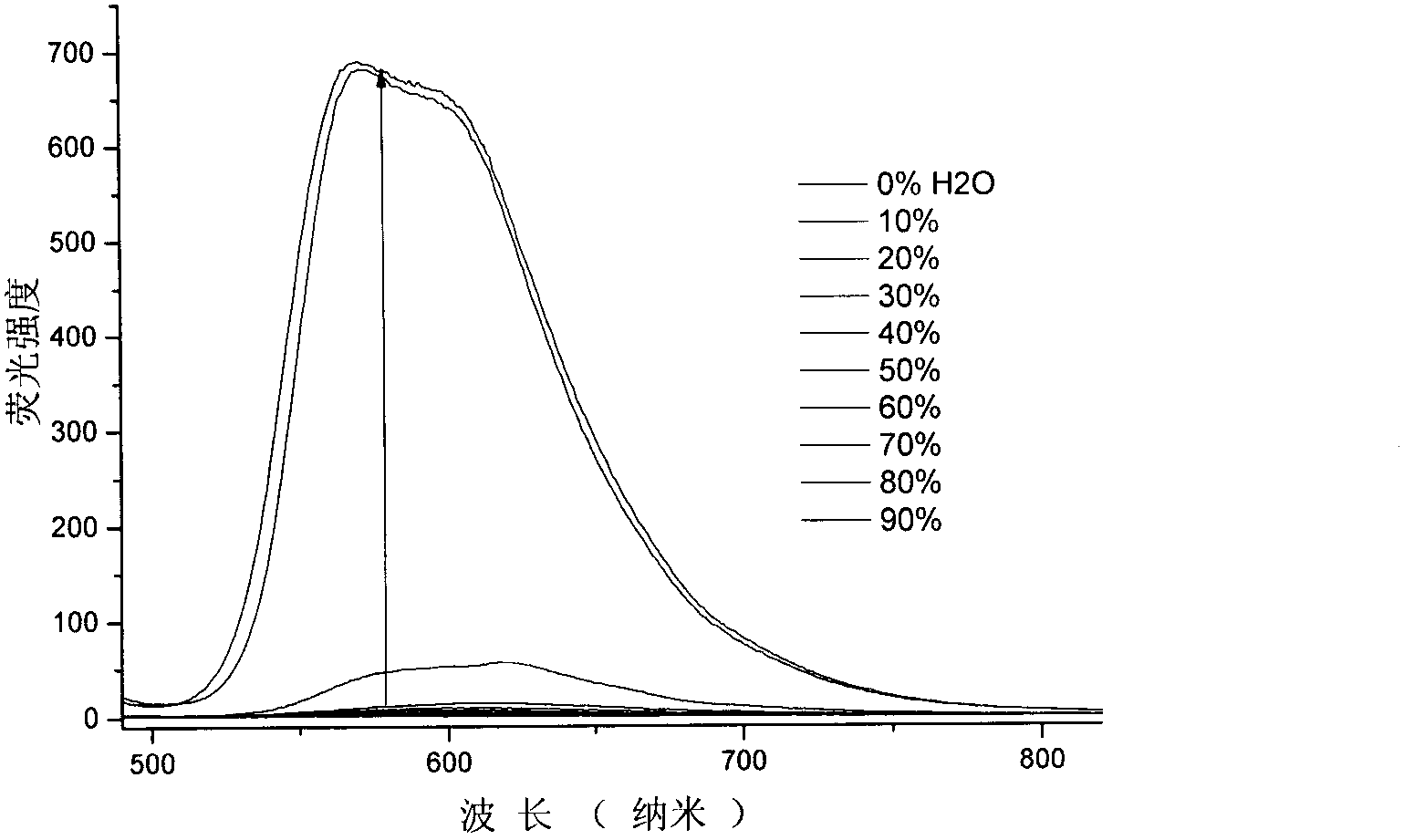
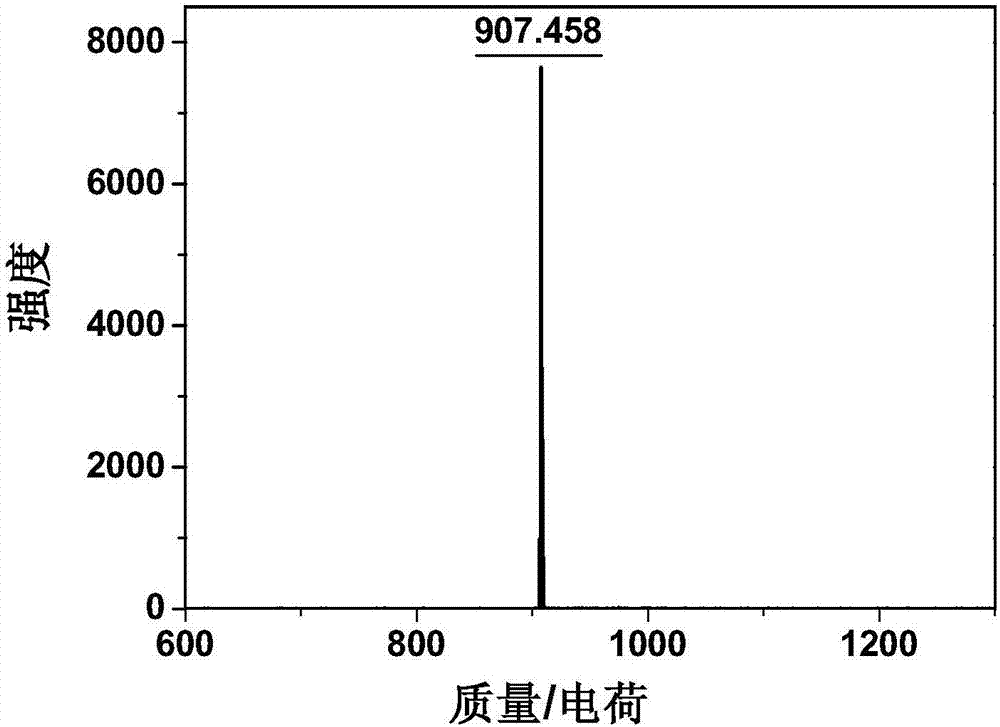
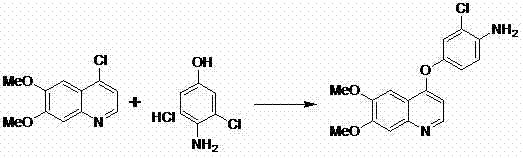
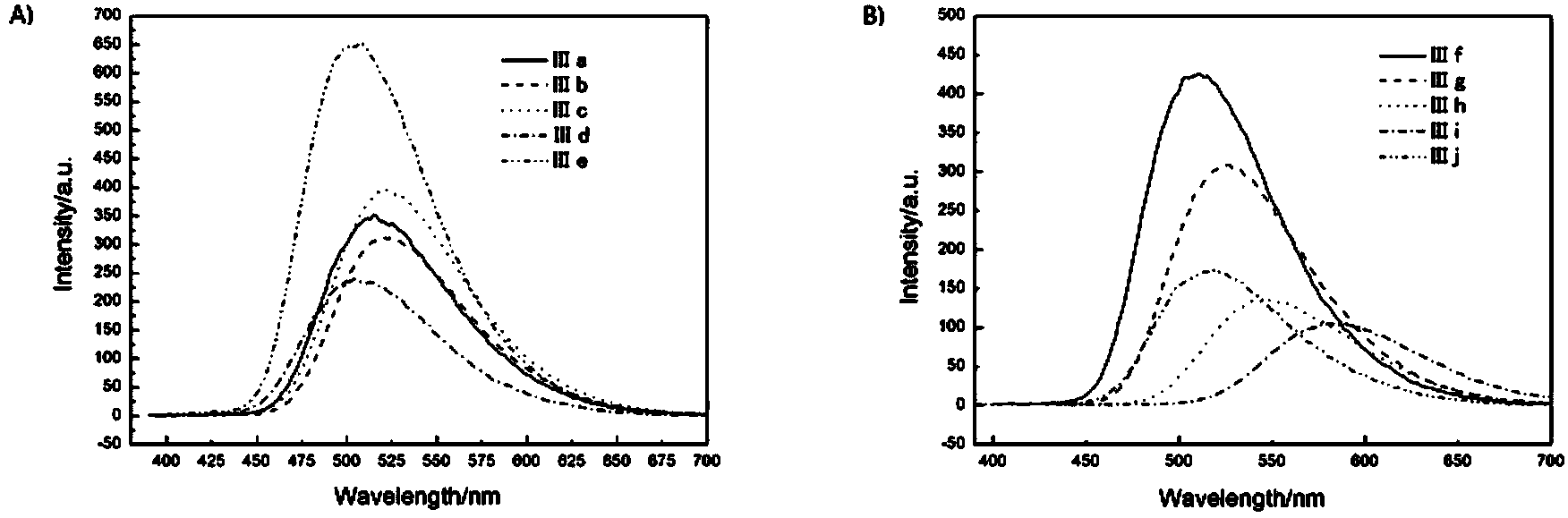
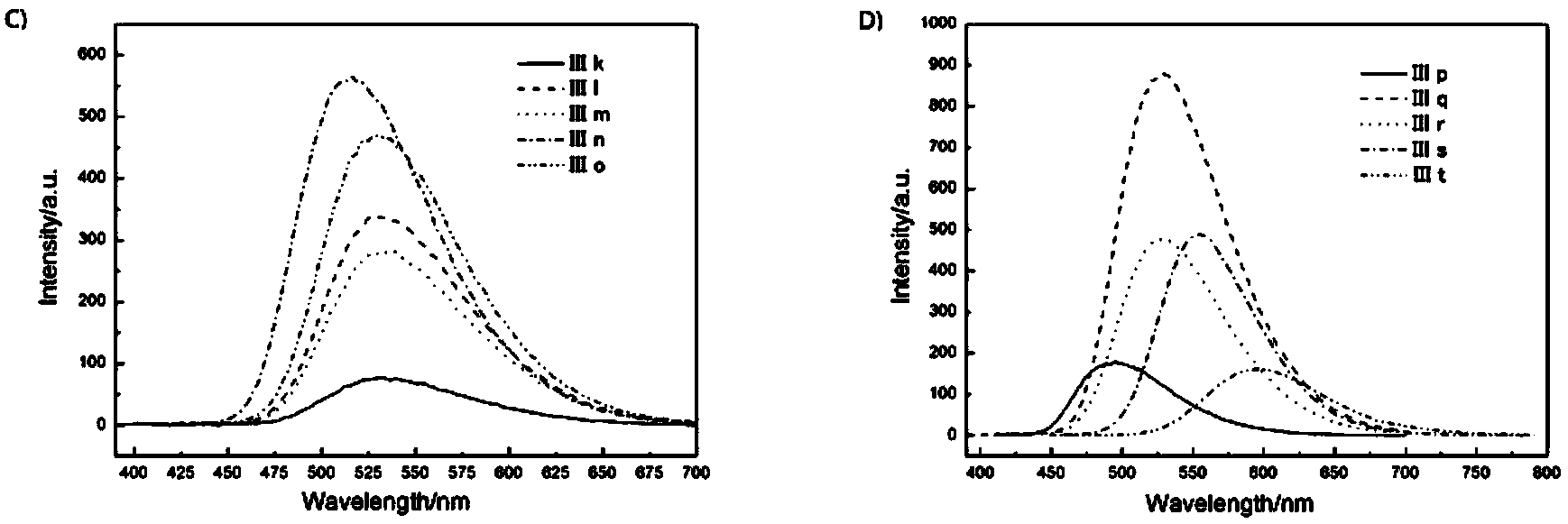
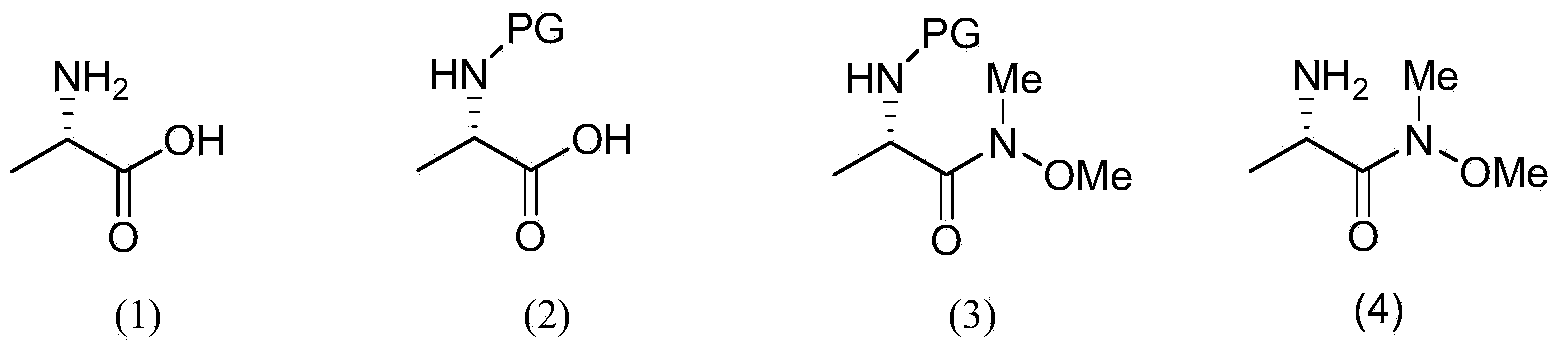
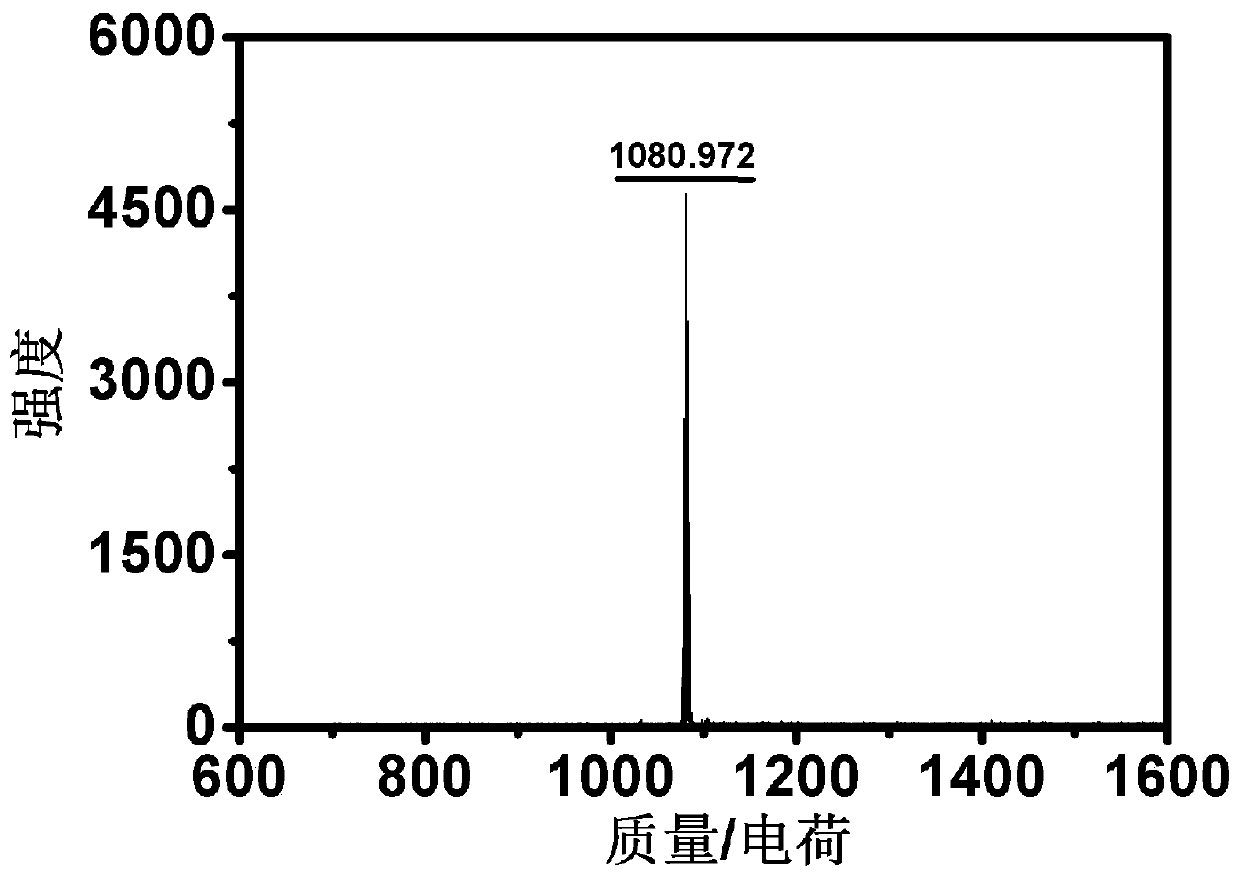
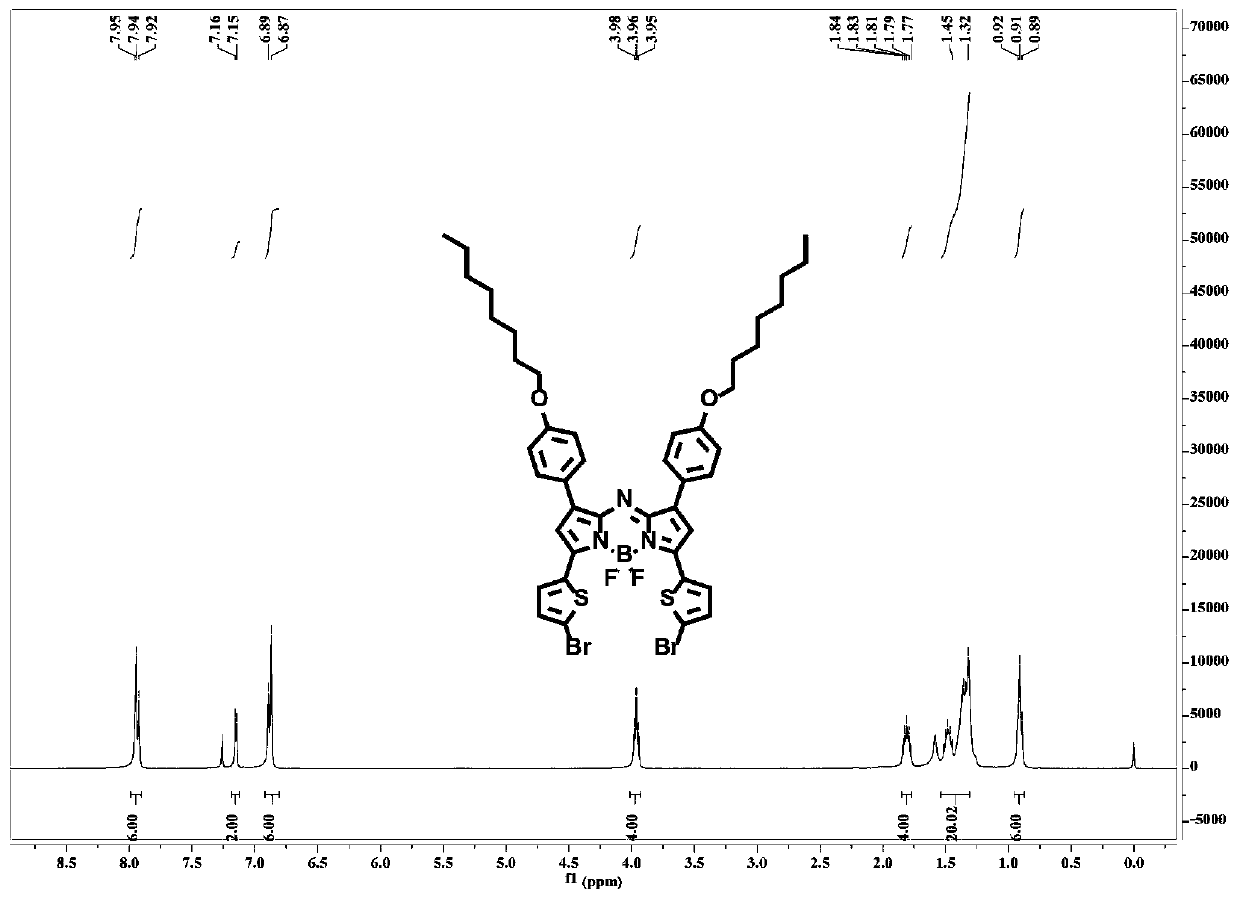
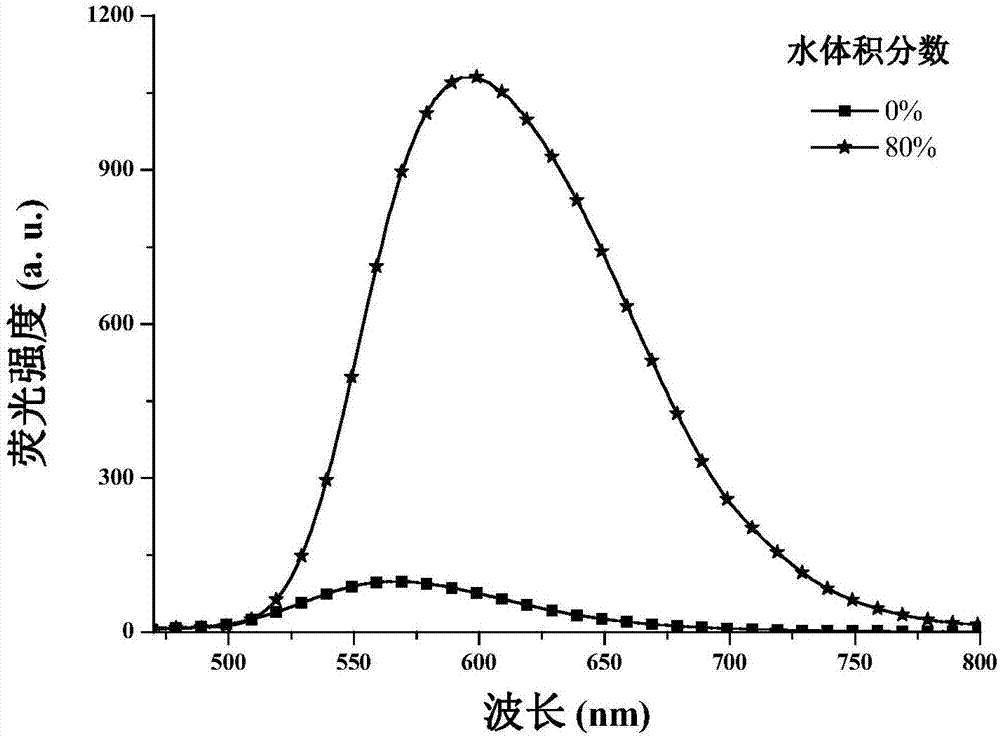
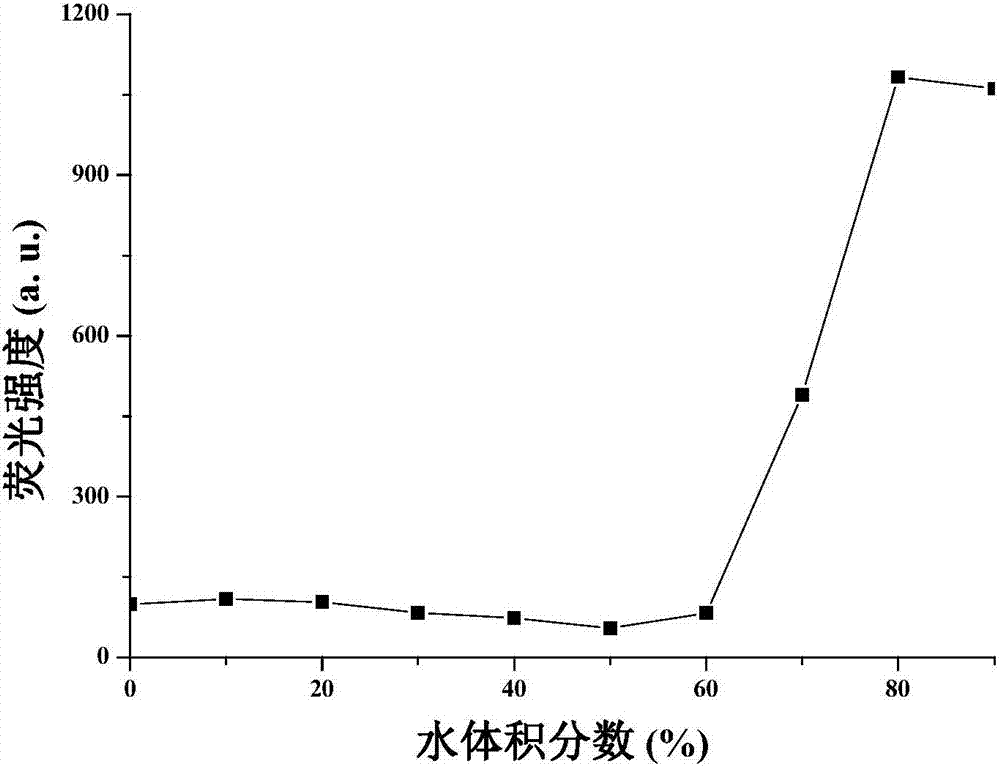
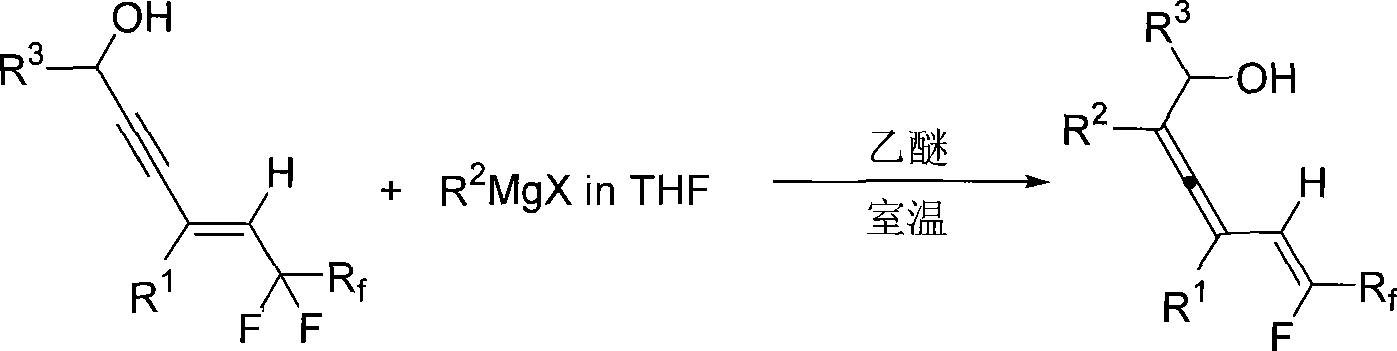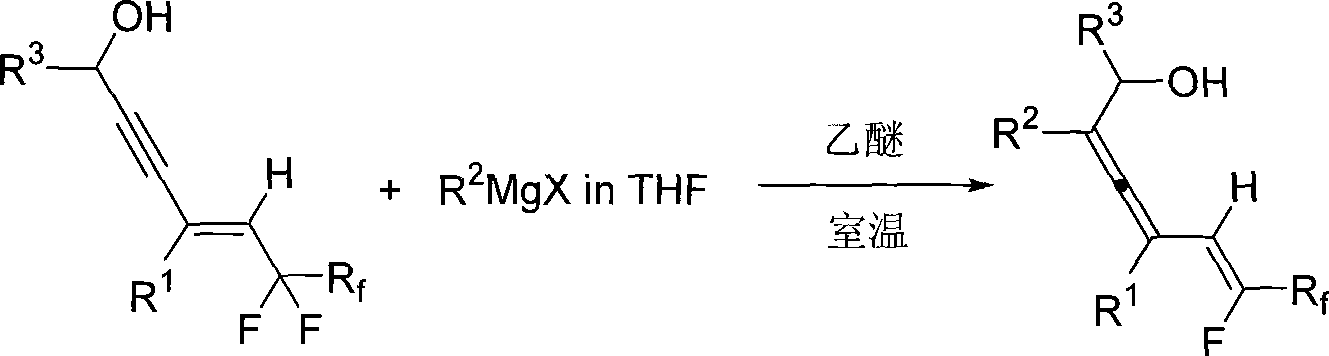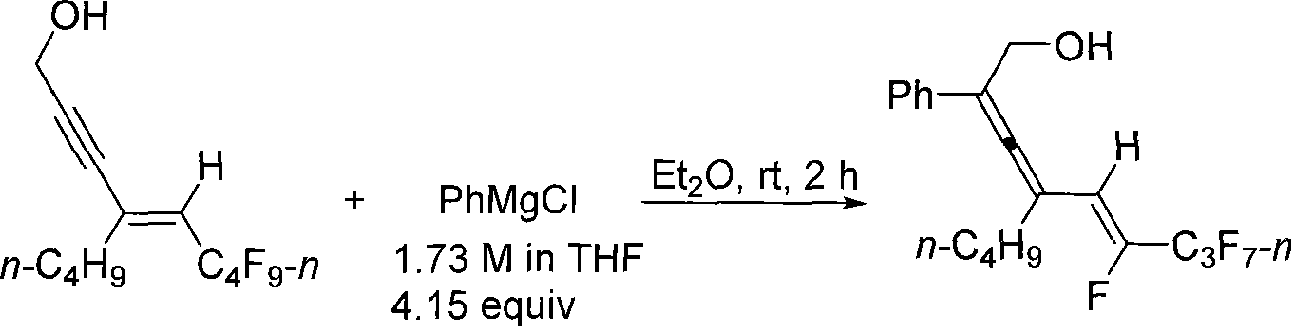Patents
Literature
44 results about "Addition elimination" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The overall mechanism of an addition-elimination reaction is known as an addition-elimination mechanism. Some nucleophilic aromatic substitution reactions occur via a two-step mechanism in which the first step, by definition, is an addition and the second step an elimination. The overall mechanism is known as an addition-elimination mechanism.
Quinoline nitrile derivative with aggregation-induced emission performance
ActiveCN102702096AStrong fluorescenceSignificant aggregation-induced luminescence characteristicsOrganic chemistryLuminescent compositionsQuinolineOrganism
The invention relates to a quinoline nitrile derivative. A method comprises the following steps of: reacting 2-methylquinoline serving as an initial raw material with the corresponding alkyl halide to obtain quaternary ammonium salt, performing Michael addition-elimination reaction on the quaternary ammonium salt and malononitrile, and performing Knoevenagel condensation reaction on the obtained compound and the corresponding aromatic aldehyde to obtain a target product. The aggregate or solid substance of the derivative has strong fluorescence and large wavelength, is a good aggregation-induced emission material, and has considerable application prospects in the fields of electroluminescent devices, fluorescent probes, intelligent materials, organism imaging and the like.
Owner:EAST CHINA UNIV OF SCI & TECH
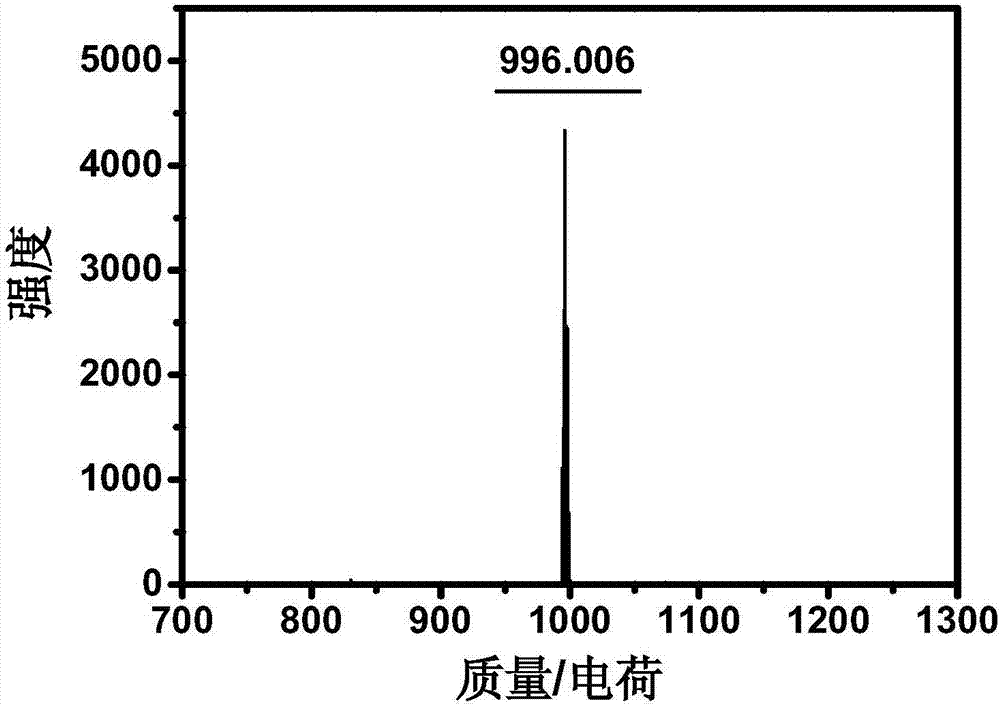
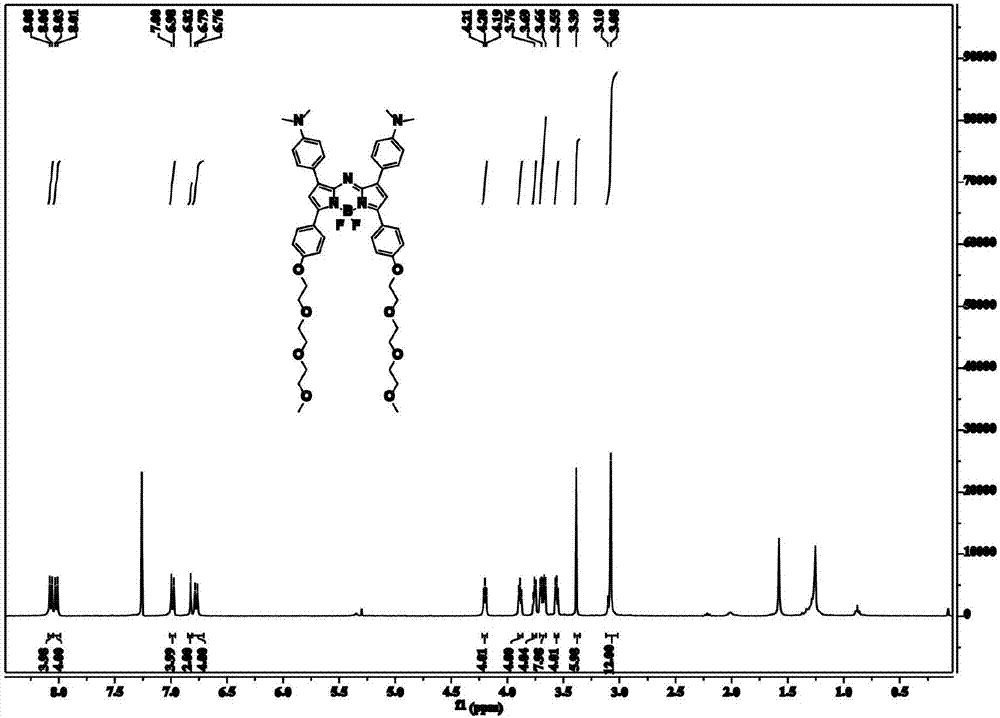
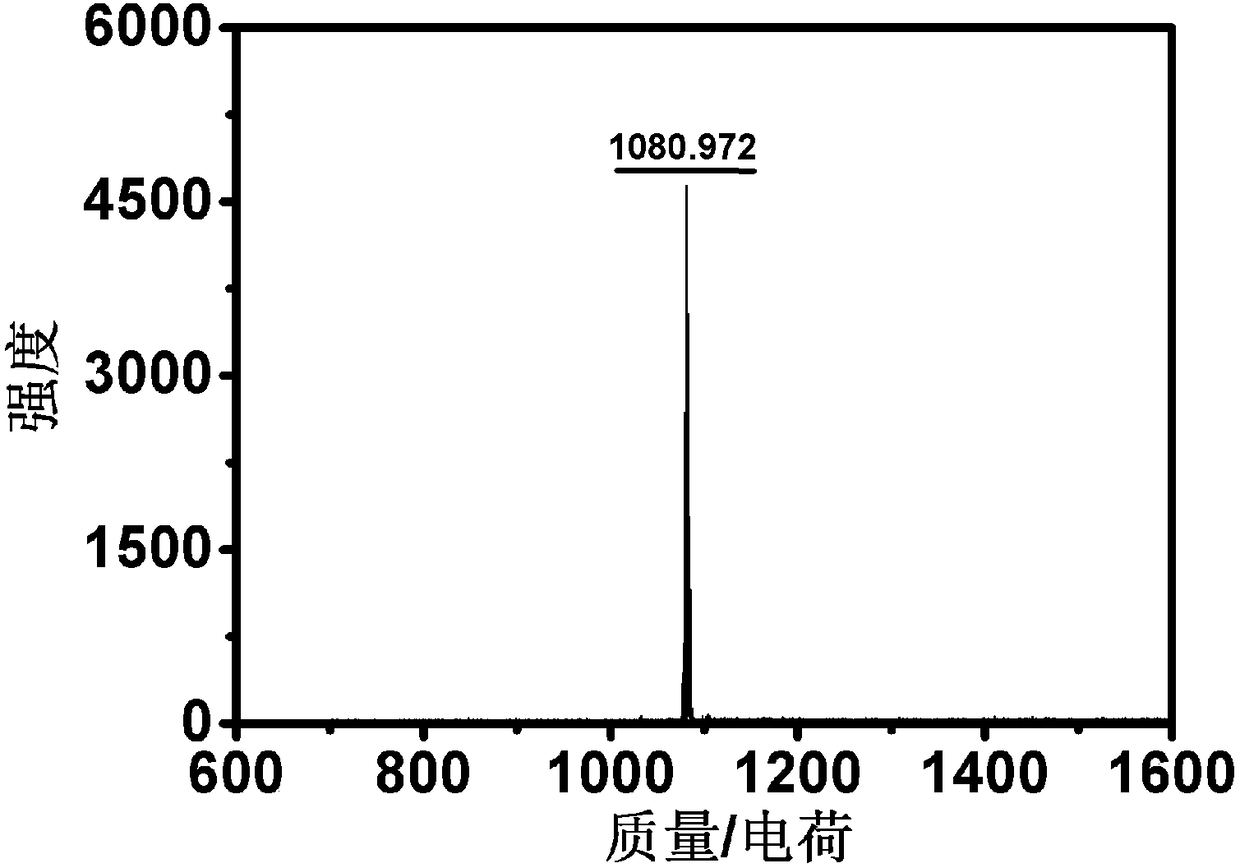
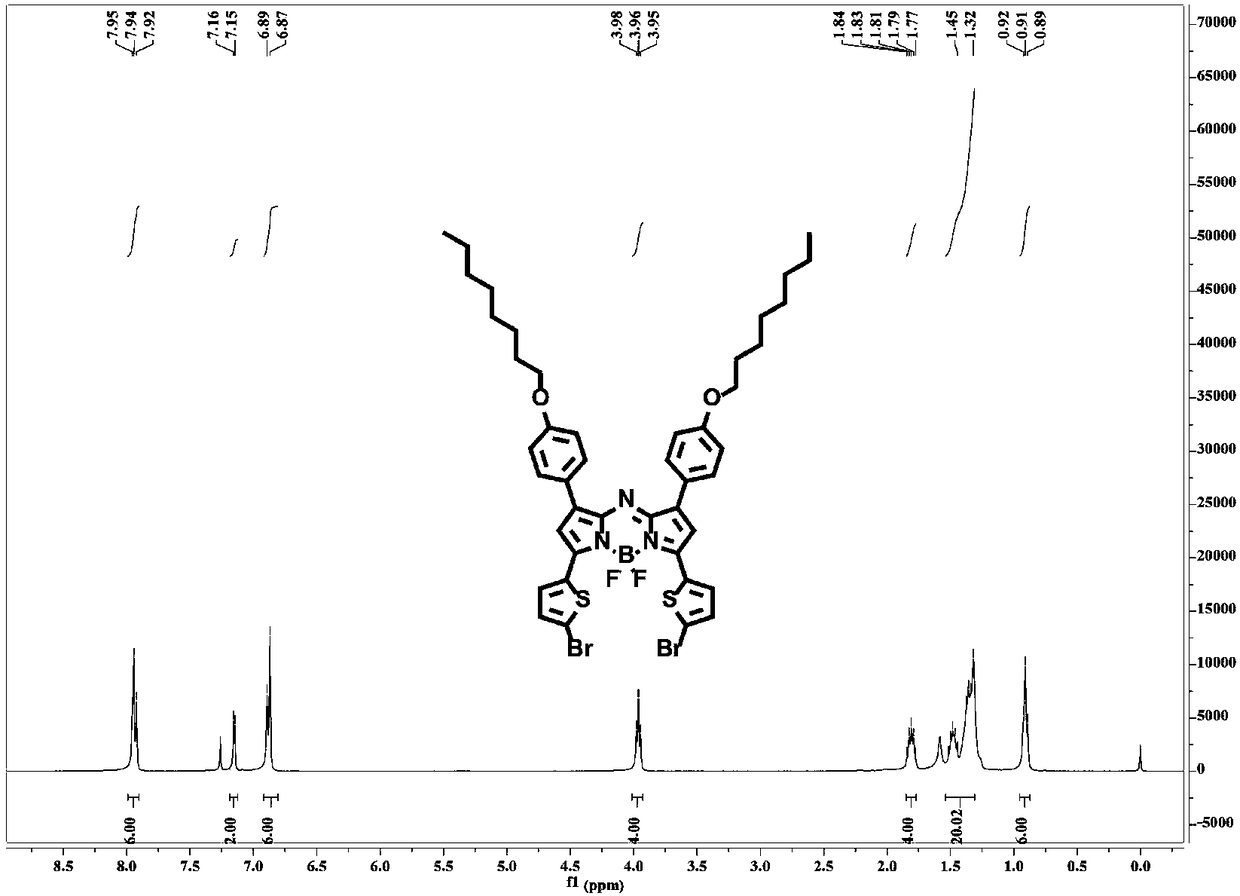
Near-infrared photo-thermal dye based on aza-fluoro borane, preparation and application
InactiveCN107501313AExcellent photothermal performanceGood photoacoustic imagingEnergy modified materialsEchographic/ultrasound-imaging preparationsKetoneNitromethane
The invention discloses a near-infrared photo-thermal dye based on aza-fluoro borane, preparation and application. The photo-thermal dye comprises an electron-donating group containing lone pair electrons and a basic aza-fluoro borane skeleton. The near-infrared photo-thermal dye based on aza-fluoro borane is prepared through steps as follows: (1), ketene synthesis: ketene is prepared from aldehyde ketone by an addition-elimination reaction under the alkaline condition; (2), an addition reaction of ketene and nitromethane; (3), a cyclization reaction; (4), a coordination reaction: a product obtained in the step (3) coordinates with boron difluoride, and a target product is obtained. The photo-thermal dye can be applied to the technical fields of photodynamic therapy and photo-thermal therapy under the guidance of photo-thermal imaging and photo-acoustic imaging, bio-marker and detection and the like.
Owner:NANJING UNIV OF POSTS & TELECOMM
Synthesis method of anti-tumor targeted therapeutic drug tivozanib
ActiveCN102532116AMild reaction conditionsSimple and fast operationOrganic chemistryTumor targetTumor targeting
The invention relates to a synthesis route of an anti-tumor targeted therapeutic drug tivozanib, which comprises the following steps that: o-dimethoxy benzene used as a starting material is subjected to Friedel-Crafts acylation, nitration, reduction, addition elimination, chlorination, and condensation and substitution reactions to synthesize the tivozanib. The synthesis method provided by the invention has the advantages that: the synthetic raw materials are cheap and easy to obtain, the reaction conditions are mild, the process is stable, the cost is low, the yield is high and the like, and therefore, the method provided by the invention is suitable for industrial production of tivozanib.
Owner:WUHAN MAIDESEN MEDICAL TECH
Dicyanomethylene-4H-pyran derivatives as well as preparation method and application thereof
ActiveCN107382982AThe synthesis process is simpleEasy to purifyOrganic chemistryTenebresent compositionsColor changesStructural unit
The invention particularly relates to dicyanomethylene-4H-pyran derivatives as well as a preparation method and an application thereof. The preparation method of the dicyanomethylene-4H-pyran derivatives comprises the following steps: (1) 2,6-dimethyl-4H-pyran-4-one 1 as a starting material is subjected to an addition-elimination reaction with malononitrile, and an intermediate 2 is obtained; (2) the intermediate 2 is subjected to an addition-elimination reaction with indolal with different alkyl chains, and the dicyanomethylene-4H-pyran derivatives are obtained. Indole structural units with different alkyl groups and dicyanomethylene-4H-pyran structural units are introduced into the field of organic solid luminescent materials, novel piezoluminescence color change materials are developed, and the derivatives play an important role in the field of photoelectricity. The provided method adopts a simple synthesis process, purification is easy, and the synthesized piezoluminescence materials are quite applicable to various fields of preparation of pressure sensors, anti-counterfeiting trademarks and the like and have good scientific research value and industrialized application potential.
Owner:WENZHOU UNIVERSITY
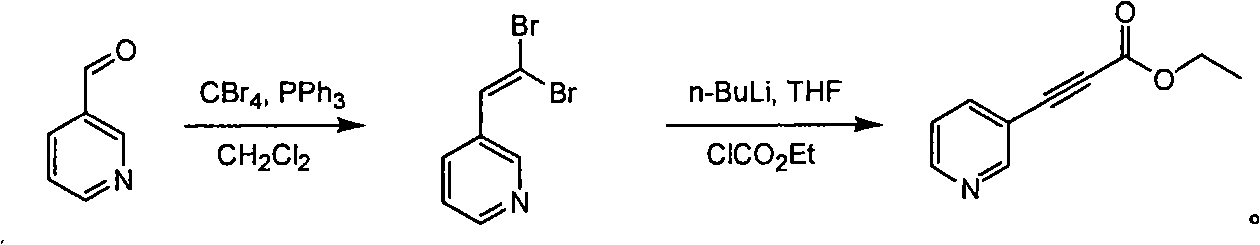
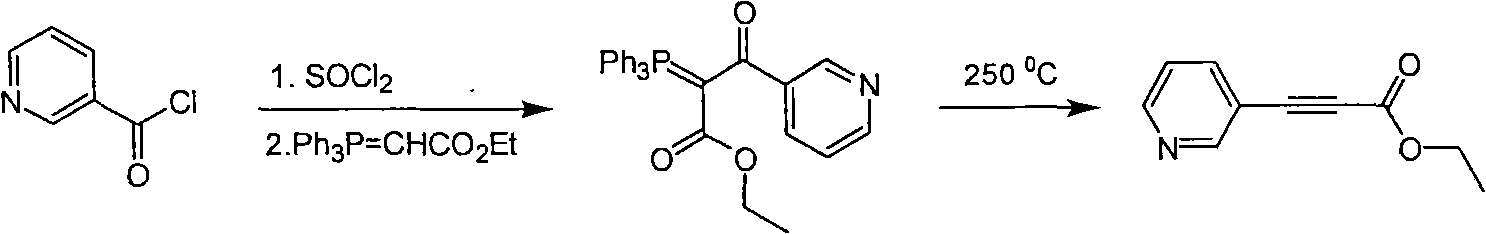
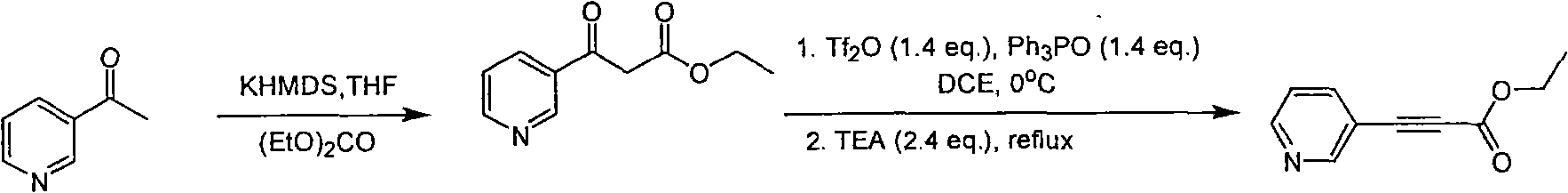
Method for synthesizing pyridine propiolic acid ester
InactiveCN101654431AReasonable choice of reaction processNo purification requiredOrganic chemistryChemical reactionChloroformate
The invention relates to a method for preparing pyridine propiolic acid ester and mainly solves the technical problem of high temperature requirement in the prior process for synthesizing the pyridinepropiolic acid ester. In the method, pyridylaldehyde serving as a readily available and large-scale production material undergoes a witting reaction, and the resulting product undergoes an addition-elimination reaction with chloroformate to obtain the corresponding pyridine propiolic acid ester. The chemical reaction formula is shown as above. The invention provides the method for synthesizing the pyridine propiolic acid ester, which has the advantages that: the raw materials are low in price and readily available, the total yield is 50 to 60 percent, and the intermediate is unnecessarily purified.
Owner:上海药明康德新药开发有限公司
Preparation of near-infrared dye based on aza-fluoroborane and application
ActiveCN108102408AStrong near-infrared absorptionGood photoacoustic imagingPhotodynamic therapyAzo dyesSinglet oxygenNitromethane
The invention discloses preparation of near-infrared dye based on aza-fluoroborane and application. The near-infrared dye is prepared from thiophene-containing groups and a basic aza-fluoroborane framework. The aza-fluoroborane dye is prepared by the following steps: (1) synthesis of ketene: the ketene is obtained by addition-elimination reaction of aldehyde and ketone under an alkali condition; (2) ketene and nitromethane conduct addition reaction under the alkali condition; (3) ring-forming reaction: under the condition with existence of an ammonia source, two times of equivalent ketene andnitromethane conduct ring-forming reaction; (4) coordination reaction: products obtained in the step (3) and metal or boron difluoride are coordinated to obtain a target product. The preparation and the application disclosed by the invention have the advantages that the target dye has strong near-infrared absorption (the molar light absorption coefficient is more than 100000M<-1>cm<-1>), high singlet oxygen yield and good photothermal effect, and can be used for photodynamic and photothermal synergy with single-wavelength excitation for tumor treatment under the guidance of photothermal imaging, photoacoustic imaging and fluorescence imaging.
Owner:NANJING UNIV OF POSTS & TELECOMM
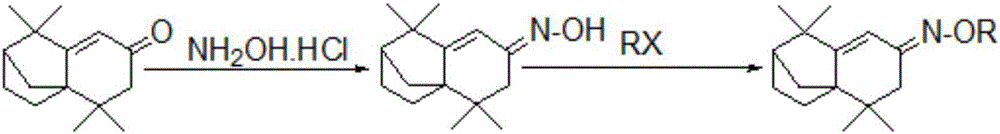
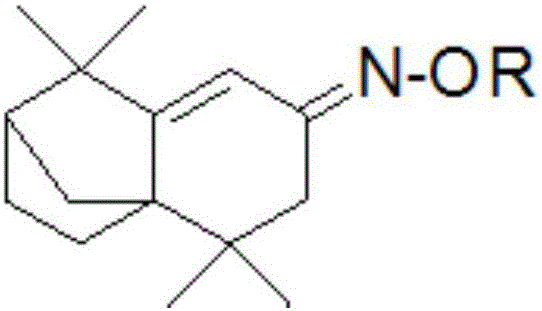
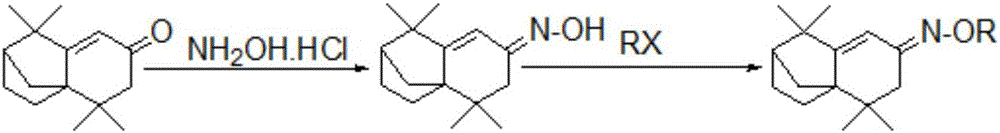
Isolongifolenone oxime ether derivative, and preparation method and application thereof
An isolongifolenone oxime ether derivative is prepared by the following steps of: taking isolongifolenone as the raw material, synthesizing isolongifolenone oxime through addition-elimination reaction, and performing alkylation reaction of the isolongifolenone oxime. Simultaneously, the isolongifolenone oxime ether derivative disclosed by the invention can inhibit bacteria, such as staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, bacillus proteus vulgaris and pseudomonas aeruginosa, and fungus, such as Aspergillus niger, exserohilum turcicum, corn sheath blight, alternaria musae Bovr.et Bat. and colletotrichum musae; and the isolongifolenone oxime ether derivative has the excellent insecticidal action for insect pests, such as aphid and rice planthopper.
Owner:广西鼎弘树脂有限公司
Aqueous-phase synthesis covalent organic framework material and preparation method thereof
The invention discloses an aqueous-phase synthesis covalent organic framework material and a preparation method thereof and belongs to the technical field of covalent organic frameworks. The preparation method disclosed by the invention is a simple and general method for preparing a series of high-crystallinity organic porous frameworks (COFs) with the pore size of 1.59-2.92nm in a water environment system by virtue of a Michael addition-elimination reaction. The prepared covalent organic framework material is high in crystallinity, ensures a surface ordered pore passage structure and has excellent universality, and the structure can derive many covalent organic frameworks with new functional groups; industrial production can be easily facilitated, the environment is not severely polluted, and volatile organic compounds (VOCs) are not produced; and moreover, a novel synthetic route is developed for synthesizing the COFs by virtue of Michael addition. Thermogravimetic analysis shows that the prepared porous material has excellent stability; and nitrogen adsorption analysis shows that the prepared COFs have high specific surface areas.
Owner:JILIN UNIV
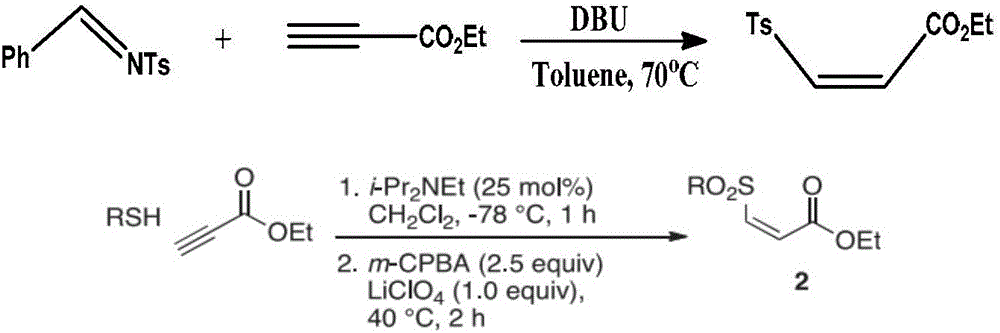
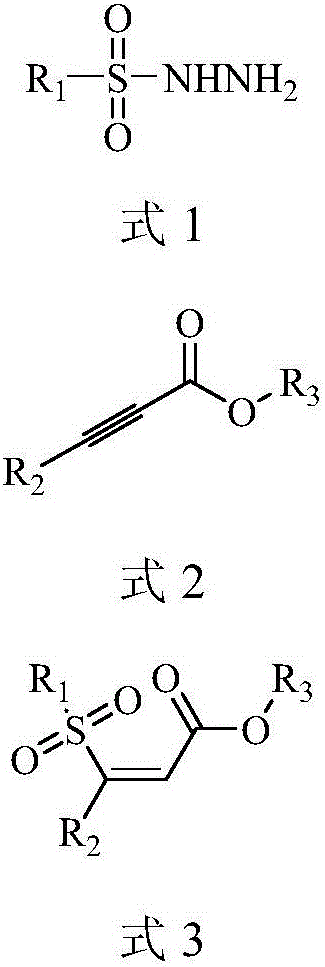
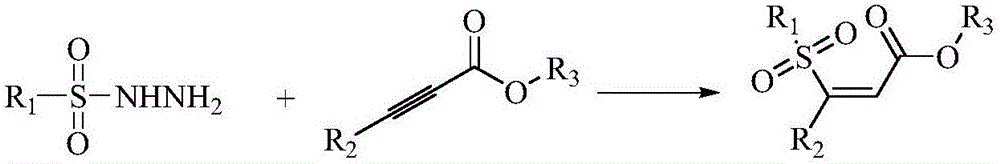
Preparation method of (Z)-sulfonyl olefine acid ester
InactiveCN105884663AThe process steps are simpleMild reaction conditionsOrganic chemistryOrganic compound preparationOne pot reactionAcetylenic acid
The invention discloses a preparation method of (Z)-sulfonyl olefine acid ester. The method includes: allowing a sulfohydrazide compound and an acetylenic acid ester compound to have an addition-elimination reaction through a one-pot method so as to obtain the sulfonyl olefine acid ester. The method has the advantages that the (Z)-sulfonyl olefine acid ester compound is synthesized in a high-yield and high-selectivity manner under mild conditions through the one-pot reaction, and the method is simple to operate, environmentally friendly and beneficial to industrial production and does not need catalysts.
Owner:HUNAN UNIV OF SCI & ENG
Efficient synthesis method of novel fluorescent material 1,3-dihydroisobenzofuran compound
InactiveCN104140409ALarge Stokes shiftAdjustable emission wavelengthOrganic chemistryLuminescent compositionsFuranSynthesis methods
The invention provides a synthesis method for conveniently and rapidly constructing a 1,3-dihydroisobenzofuran fluorescent material. The 1,3-dihydroisobenzofuran compound is large in Stokes shift and adjustable and controllable in fluorescence emission wavelength. The synthesis method comprises one step: carrying out an addition-elimination reaction by taking outer cycloalkene ethers and aromatic aldehydes as raw materials under the catalytic action of potassium tert-butoxide so as to obtain the compound with a structural framework. The 1,3-dihydroisobenzofuran derivatives all have good conjugated structures, so that the compound has strong fluorescence. Especially, the fluorescence property of the compound is more obvious on the basis of the 1,3-dihydroisobenzofuran derivatives containing donor-receptor structures. The maximum emission wavelength of the 1,3-dihydroisobenzofuran derivatives can be adjusted and controlled from 496nm to 597nm by virtue of the introduction of different substituents. Thus, the synthesis method has the characteristics of being high in yield, easily available in raw materials, short in reaction time, simple in aftertreatment and the like. Thus, a foundation for the synthesis of a novel fluorescent compound is provided.
Owner:EAST CHINA UNIV OF SCI & TECH
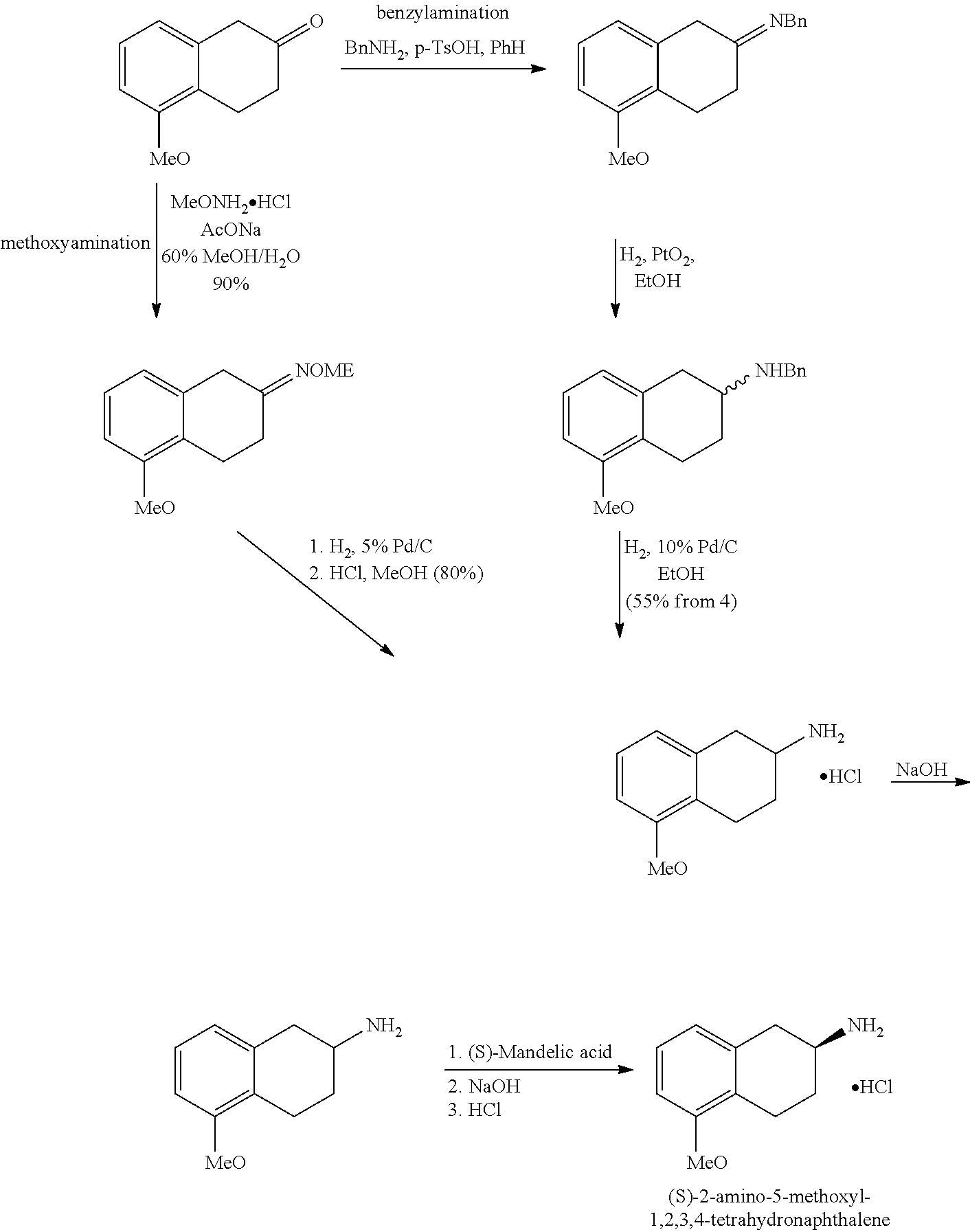
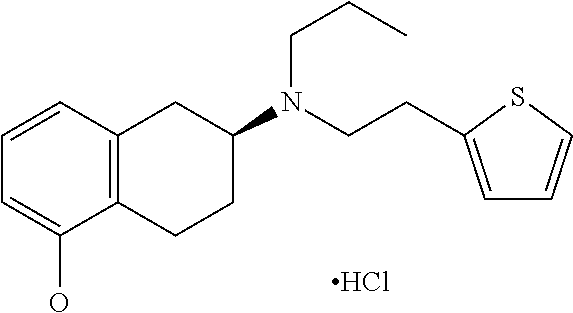
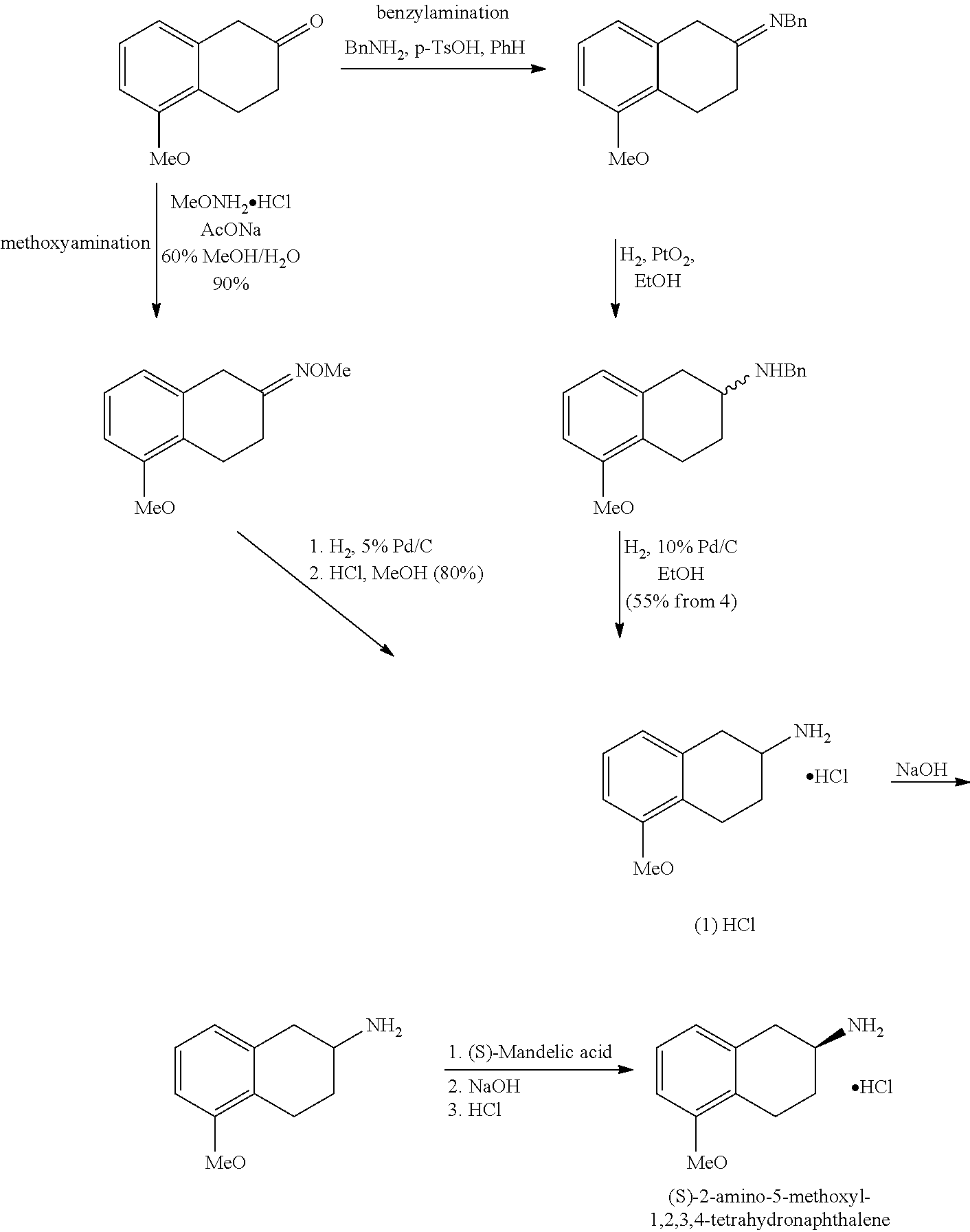
Method of Preparing (S)-2-amino-5-Methoxytetralin Hydrochloride
ActiveUS20140046095A1Increase productionLow yieldIsocyanic acid derivatives preparationOrganic compound preparationTetralin2-Tetralone
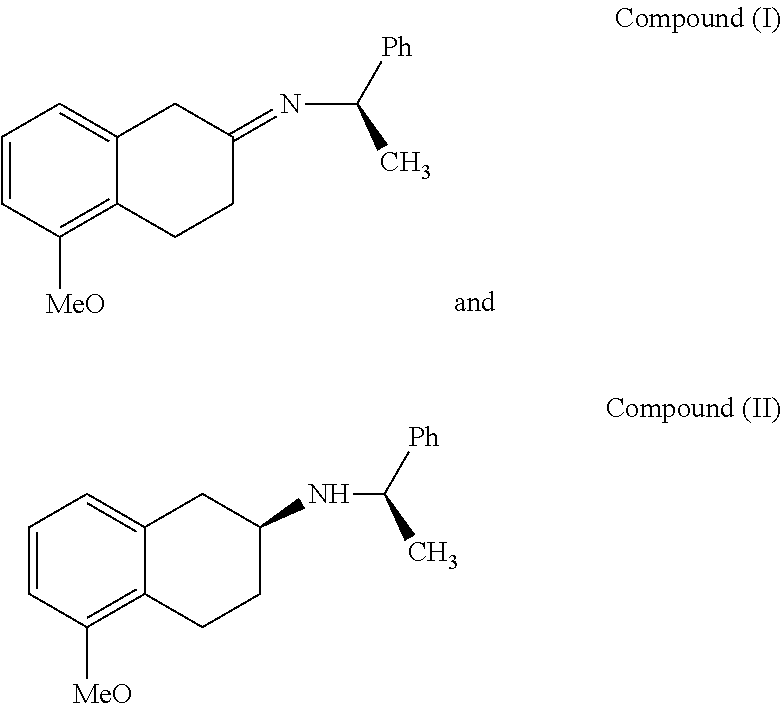
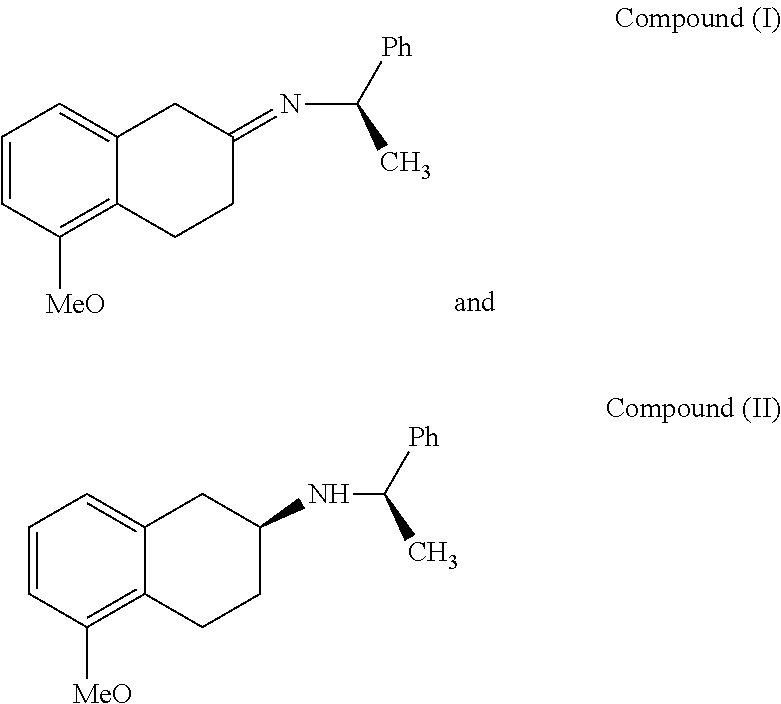
A method of preparing (S)-2-amino-5-methoxytetralin hydrochloride[(S)-2-amino-5-methoxyl-1,2,3,4-tetrahydronaphthalene hydrochloride], comprising the steps of: (1) producing a compound (I) by addition-elimination reaction of 5-methoxy-2-tetralone and R-(+)-a-phenylethylamine; (2) producing a compound (II) by reduction reaction of the compound (I) with a reducing agent; and (3) producing a compound (II) hydrochloride by reacting the compound (II) with a salt-forming agent, then carrying out reduction reaction with a palladium-carbon catalyst to produce (S)-2-amino-5-methoxytetralin hydrochloride. The method can significantly increase the yield of (S)-2-amino-5-methoxytetralin hydrochloride with short synthetic path, low preparation cost and less pollution, which is environmentally friendly and is suitable for medical industrialized production. The structural formulae of the compound (I) and the compound (II) are:resepectively.
Owner:ANHUI QINGYUN PHARMA & CHEM
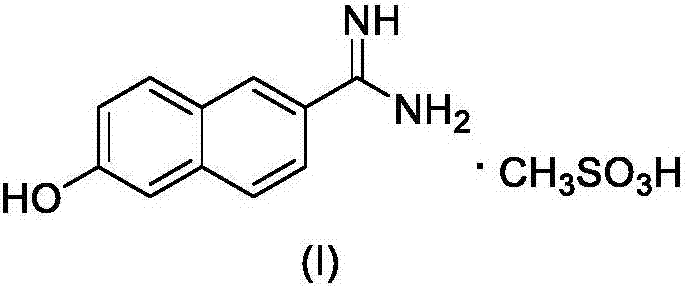
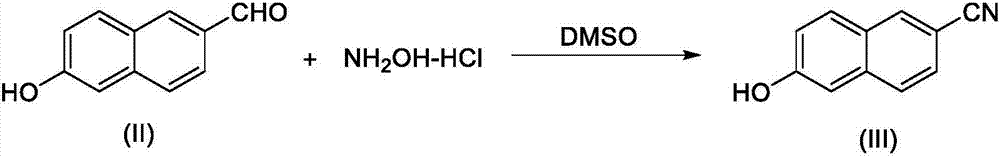
New 6-amidino-2-naphthol methanesulfonate synthesis method
ActiveCN103896809AReduce pollutionMild reaction conditionsSulfonic acids salts preparationSodium bicarbonateHydroxylamine Hydrochloride
The present invention relates to a new synthesis method for a nafamostat mesylate intermediate 6-amidino-2-naphthol methanesulfonate. According to the new method, 6-hydroxy-2-naphthaldehyde is adopted as a raw material, dimethyl sulfoxide is adopted as a reaction solvent, the dimethyl sulfoxide and hydroxylamine hydrochloride are subjected to an addition elimination reaction to obtain 6-cyano-2-naphthol, the 6-cyano-2-naphthol is subjected to a pinner reaction in a HCl / methanol solution to obtain 6-hydroxy-2-naphthalene imino methyl ester hydrochloride, ammonia gas is introduced to carry out an aminolysis reaction to obtain 6-amidino-2-naphthol, and the 6-amidino-2-naphthol sequentially reacts with sodium bicarbonate and methanesulfonic acid to convert into the 6-amidino-2-naphthol methanesulfonate. According to the present invention, in the new synthesis method, the 6-cyano-2-naphthol preparation in the first step adopts the completely-new method, the use of the highly toxic copper cyanide in the traditional method is avoided, the operations are simple, and the conditions are mild; and the second step adopts the improved pinner method, wherein the reaction of acetyl chloride and methanol is adopted to produce HCl to replace direct introduction of HCl gas into the reaction system, such that the improved method has strong operability and industrialization is easily achieved.
Owner:BEIJING LABWORLD BIO MEDICINE TECH
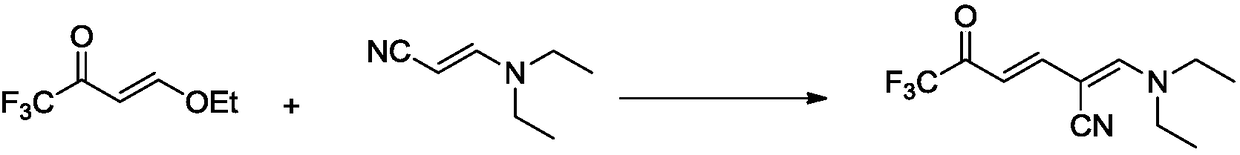
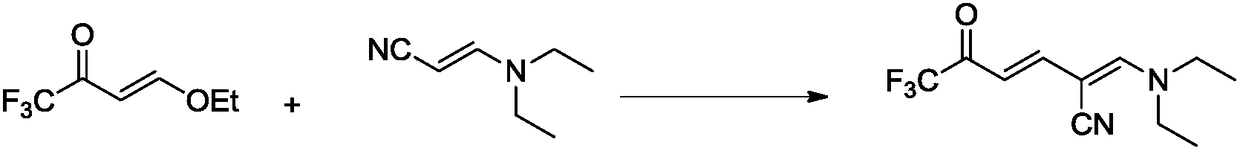
Synthetic method for 6-trifluoromethyl-nicotinic acid
InactiveCN109160897AThe synthesis process is simpleEasy to operateOrganic chemistryCyanoacetic acidAcrylonitrile
The invention discloses a method for synthesizing 6-trifluoromethyl-nicotinic acid. The method comprises the specific steps: carrying out addition-elimination reaction reactions on trifluoroacetic acid and vinyl ethyl ether under the action of phosphorus pentachloride to obtain 4-ethoxyl-1,1,1-trifluoro-3-butene-2-one; carrying out addition-elimination reaction reactions on ethidene diamine and cyanoacetic acid in a heating condition to obtain 3-(diethyl amino) acrylonitrile first; carrying out an Stork alkylation reaction on 3-(diethyl amino) acrylonitrile and 4-ethoxyl-1,1,1-trifluoro-3-butene-2-one to obtain 2-((diethyl amino) methylene)-6,6,6-trifluoro-5-oxo-3-hexenenitrile; carrying out exocondensation on 2-((diethyl amino) methylene)-6,6,6-trifluoro-5-oxo-3-hexenenitrile under the action of ammonium acetate to obtain 6-trifluoromethyl cyanopyridine; and carrying out cyano hydrolysis reaction on the 6-trifluoromethyl cyanopyridine t obtain 6-trifluoromethyl-nicotinic acid. The synthetic method is more economic, environmentally friendly, efficient and simple.
Owner:HENAN NORMAL UNIV
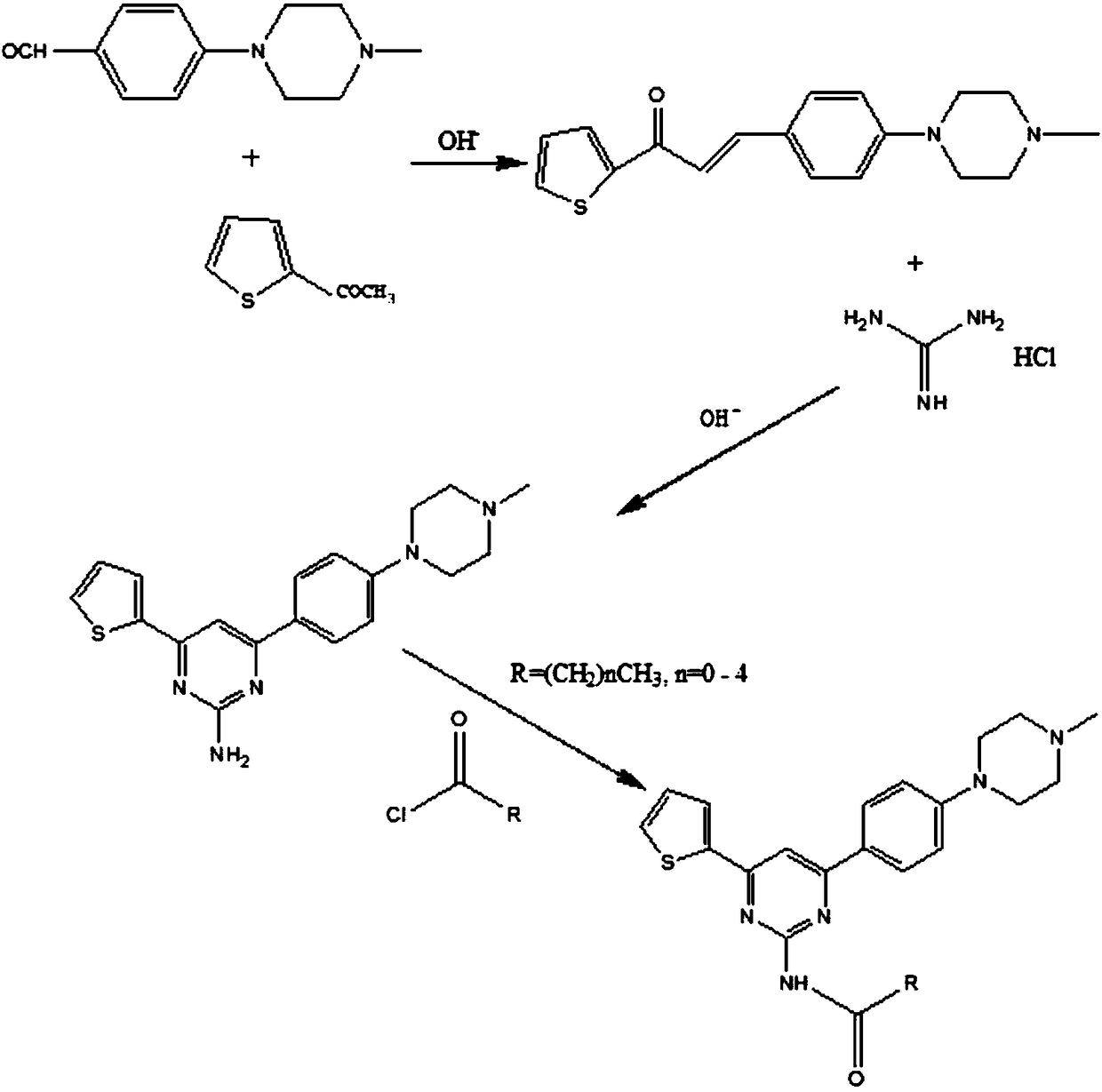
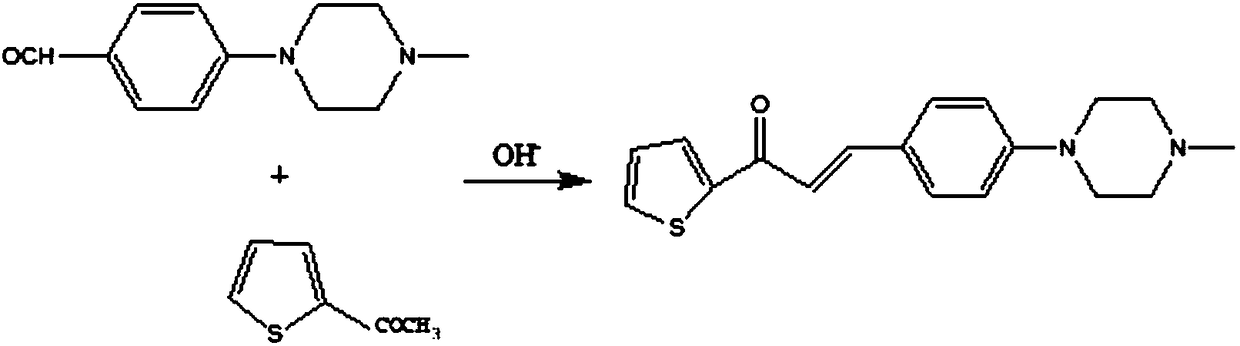
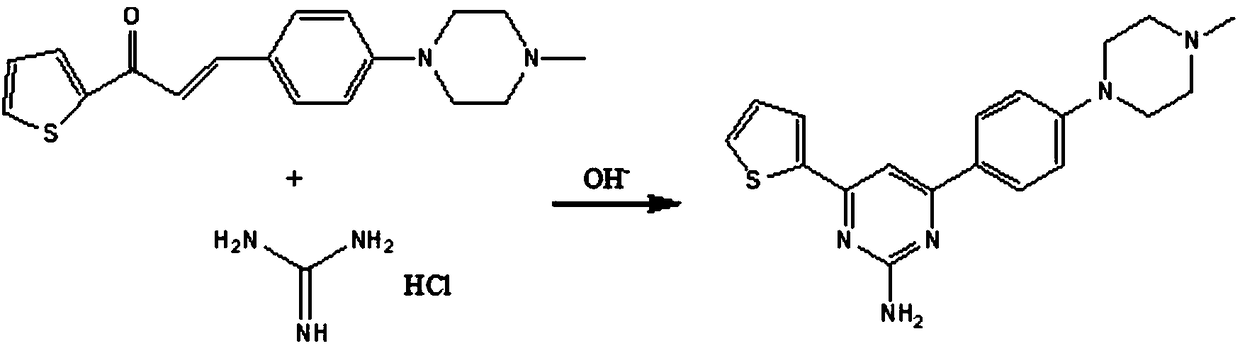
Preparation method of aryl-substituted pyrimidinamine acylated derivative
The invention discloses a preparation method of an aryl-substituted pyrimidinamine acylated derivative. The preparation method comprises the following steps: preparation of 1-(2-thienyl)-3-(4-(4-methylpiperazinyl)phenyl)-acrylketone, preparation of 4-(2-thienyl)-6-(4-(4-methylpiperazinyl)phenyl)-pyrimidin-2-amine and preparation of 4-(2-thienyl)-6-(4-(4-methylpiperazinyl)phenyl)pyrimidin-2-acylated derivative amine; in the preparation method, an ABC superfamily P-gp efflux system and an RND family AcrAB-TolC efflux system are used as research objects, an efflux pump inhibitor of the aryl-substituted pyrimidinamine acylated derivative is designed, and 4-(4-methylpiperazinyl)benzaldehyde is used as a main raw material, to perform three-step reaction of Michael addition elimination, cyclization and acylation to obtain a target compound. The preparation method has the advantages of high selectivity, simple operation, easy availability of raw materials and mild conditions, hopes that the derivative can exert antibacterial and synergistic activity, and has great economic value and far-reaching social significance.
Owner:湖北欣瑞康医药科技有限公司
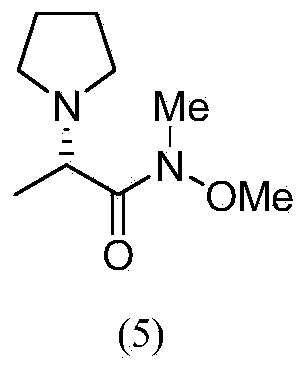
(S)-N-methoxy-methyl-2-(pyrrolidine) propionamide and preparation method and application thereof
ActiveCN104211663AMild reaction conditionsSimple and fast operationOrganic chemistryBulk chemical productionAlkyl transferHydroxylamine Hydrochloride
The invention discloses a (S)-N-methoxy-methyl-2-(pyrrolidine) propionamide shown as a formula (5). A preparation method is as follows: subjecting a starting material L-alanine to amino protection, reaction with N,O-dimethyl hydroxylamine hydrochloride, removal of amino protecting group, and alkylation; and subjecting the prepared compound shown as (5) to addition elimination and reduction to obtain an Efavirenz chiral ligand shown as the formula (7). The synthetic method of Efavirenz chiral ligand provided by the invention has the advantages of mild reaction conditions, simple operation, high yield and low production cost, and is suitable for industrialized production.
Owner:HANGZHOU OULIAN MEDICINE SCI & TECH CO LTD
Quinoline nitrile derivative with aggregation-induced emission performance
ActiveCN102702096BSignificant aggregation-induced luminescence characteristicsEasy to synthesizeOrganic chemistryLuminescent compositionsQuinolineOrganism
The invention relates to a quinoline nitrile derivative. A method comprises the following steps of: reacting 2-methylquinoline serving as an initial raw material with the corresponding alkyl halide to obtain quaternary ammonium salt, performing Michael addition-elimination reaction on the quaternary ammonium salt and malononitrile, and performing Knoevenagel condensation reaction on the obtained compound and the corresponding aromatic aldehyde to obtain a target product. The aggregate or solid substance of the derivative has strong fluorescence and large wavelength, is a good aggregation-induced emission material, and has considerable application prospects in the fields of electroluminescent devices, fluorescent probes, intelligent materials, organism imaging and the like.
Owner:EAST CHINA UNIV OF SCI & TECH
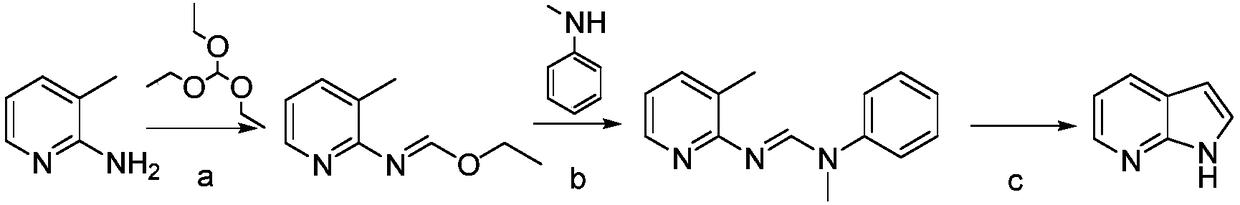
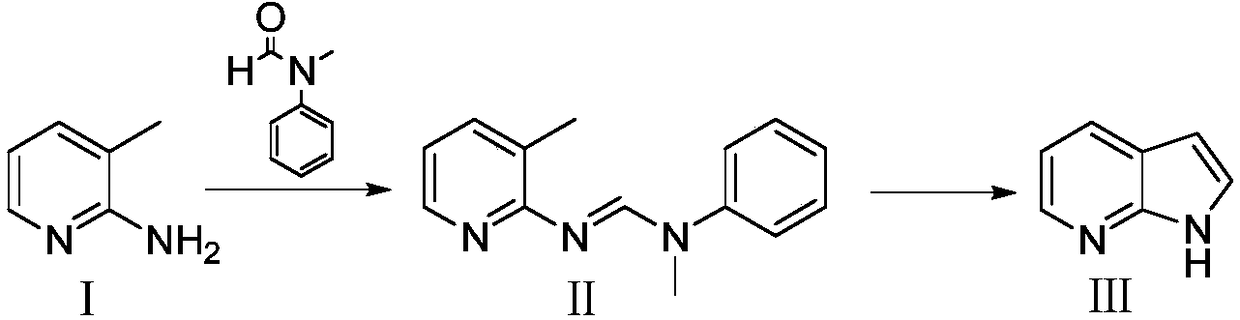
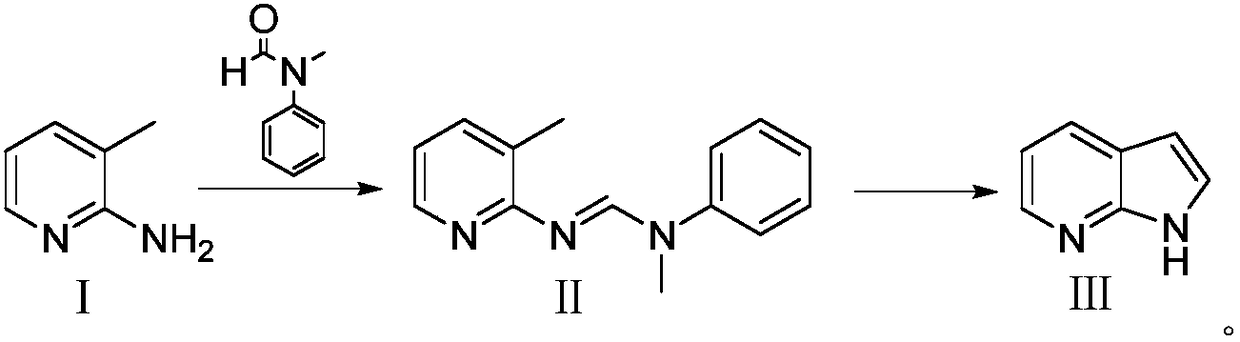
Preparation method for 7-azaindole
InactiveCN108976228AHigh feasibilityThe reaction conditions are mild and controllableOrganic chemistryNitrogenCombinatorial chemistry
The invention discloses a preparation method for 7-azaindole, and belongs to technical field of the medicinal chemistry synthesis. The preparation method comprises the following steps: using a compound I, namely 2-amino-3-methylpyridine and N-methylformanilide, to perform an addition elimination reaction under the action of a dehydrating agent, to obtain a compound II; performing intramolecular ring closure on the compound II under the action of strong base, to obtain a target compound III. The preparation method is capable of acquiring the target product through two-step synthesis only. The method is simple in post-treatment, less in side reaction, high in yield, and in comply with requirements of industrial production. (The formula is shown in the description.).
Owner:EAST CHINA NORMAL UNIV +2
Preparation and application of a near-infrared dye based on azafluoroborane
The invention discloses preparation of near-infrared dye based on aza-fluoroborane and application. The near-infrared dye is prepared from thiophene-containing groups and a basic aza-fluoroborane framework. The aza-fluoroborane dye is prepared by the following steps: (1) synthesis of ketene: the ketene is obtained by addition-elimination reaction of aldehyde and ketone under an alkali condition; (2) ketene and nitromethane conduct addition reaction under the alkali condition; (3) ring-forming reaction: under the condition with existence of an ammonia source, two times of equivalent ketene andnitromethane conduct ring-forming reaction; (4) coordination reaction: products obtained in the step (3) and metal or boron difluoride are coordinated to obtain a target product. The preparation and the application disclosed by the invention have the advantages that the target dye has strong near-infrared absorption (the molar light absorption coefficient is more than 100000M<-1>cm<-1>), high singlet oxygen yield and good photothermal effect, and can be used for photodynamic and photothermal synergy with single-wavelength excitation for tumor treatment under the guidance of photothermal imaging, photoacoustic imaging and fluorescence imaging.
Owner:NANJING UNIV OF POSTS & TELECOMM
1,4-dihydropyridine derivatives as well as preparation method and application thereof
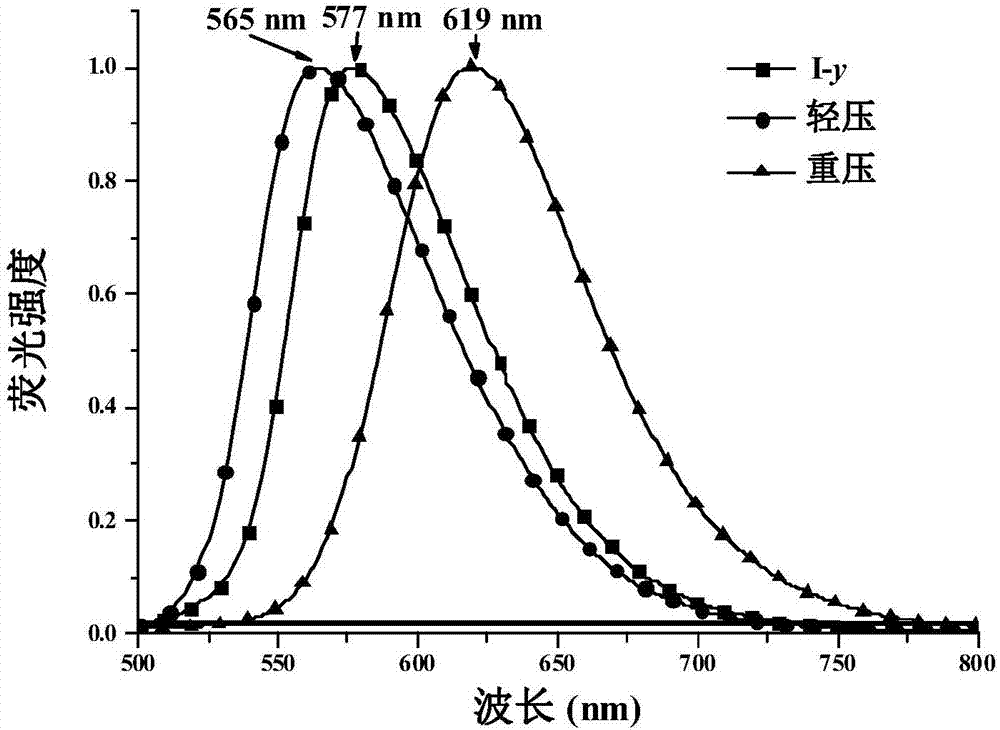
ActiveCN107382978AGreat research valueOrganic chemistry methodsForce measurement by measuring optical property variationChemical synthesisDihydropyridine
The invention belongs to the field of organic chemical synthesis and application and relates to 1,4-dihydropyridine derivatives as well as a preparation method and an application thereof. The preparation method of the 1,4-dihydropyridine derivatives comprises the following steps: (1) preparing an initial sample of the 1,4-dihydropyridine derivatives; (2) recrystallizing the initial sample of the 1,4-dihydropyridine derivatives in different ways to obtain three different crystalline compounds I-y, I-o and I-r, wherein the step (1) comprises the following steps: (11) forming an intermediate 2 from 2,6-dimethyl-4H-pyran-4-one 1 as an initial material as well as Meldrum's acid through an addition-elimination reaction; (12) forming an intermediate 3 from the intermediate 2 and 4-dimethylaminobenzaldehyde through a condensation reaction; (13) synthesizing the initial sample of the 1,4-dihydropyridine derivatives from the intermediate 3 and ethylamine through nucleophilic substitution.
Owner:WENZHOU UNIVERSITY
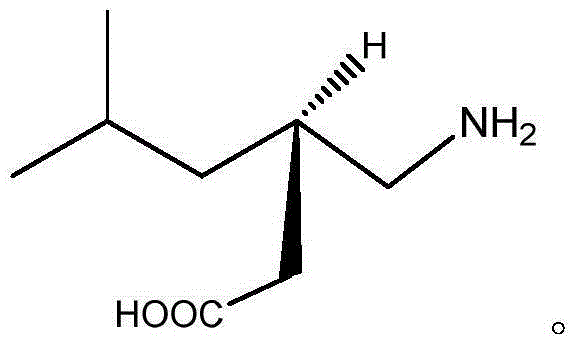
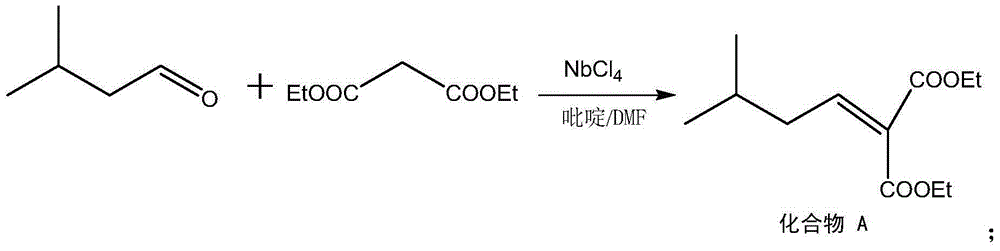
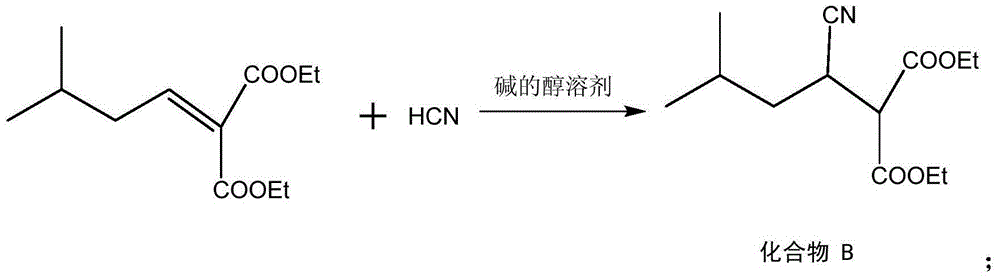
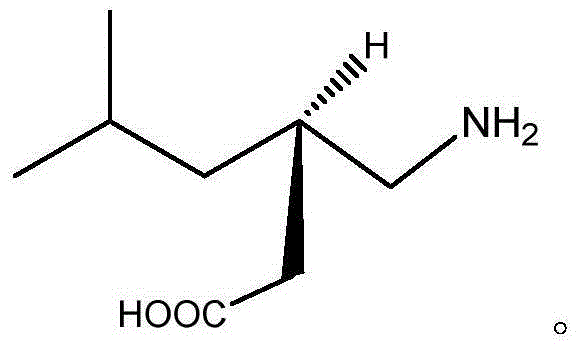
Preparation method for pregabalin
InactiveCN105061234AGuaranteed yieldGuaranteed purityOrganic compound preparationAmino-carboxyl compound preparationHydration reactionNiobium
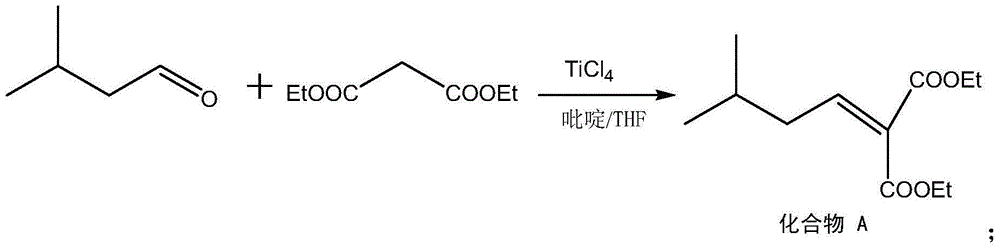
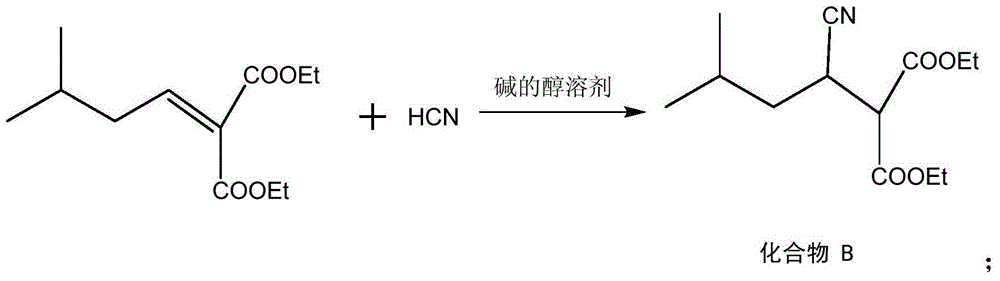
The invention discloses a preparation method for pregabalin. The preparation method comprises the following steps: step 1, performing an addition elimination reaction on isovaleraldehyde and diethyl malonate in a solvent comprising DMF and pyridine by using niobium tetrachloride as a catalyst at the temperature of 5-10 DEG C; step 2, performing Michael addition on a product obtained in the step 1 in an alkaline alcohol solvent; step 3, performing a hydrogenation reaction on a product obtained in the step 2 and hydrogen by using raney nickel as a catalyst; step 4, performing a hydration reaction on a product obtained in the step 3 under the condition of hydrochloric acid; step 5, performing chiral resolution on a product obtained in the step 4 in a mixed solvent comprising alcohol and water by using L-tartaric acid as a resolving agent. According to the preparation method, inexpensive and readily-available isovaleraldehyde is used as a raw material, and pregabalin is obtained through the addition elimination reaction, the Michael addition reaction, the hydrogenation reaction, the hydration reaction and chiral resolution. The reaction route is simple, and the yield of each reaction is relatively high, so that the total yield and the purity of the final product of pregabalin are ensured.
Owner:TAICANG YUNTONG BIOCHEM ENG
Method for synthesizing Pregabalin
InactiveCN105061235AGuaranteed yieldGuaranteed purityOrganic compound preparationAmino-carboxyl compound preparationAlcoholL-Aspartate
The invention discloses a method for synthesizing Pregabalin. The synthesis method comprises the following steps: 1, enabling isovaleraldehyde and diethyl malonate to be subjected to addition elimination reaction in a solvent composed of pyridine and THF at the temperature of 5-10 DEG C by taking titanium tetrachloride as a catalyst; 2, enabling the product obtained in the step 1 to be subjected to Michael addition in an alcohol solvent of alkali; 3, enabling the product obtained in the step 2 and hydrogen to be subjected to hydrogenation reaction by taking Pd as a catalyst; 4, enabling the product obtained in the step 3 to be subjected to hydrolysis reaction under the hydrochloric acid condition; 5, enabling the product obtained in the step 4 to be subjected to chiral resolution in a mixed solvent of alcohol and water by taking L-aspartic acid as a resolving agent. According to the invention, the low-price isovaleraldehyde easy to obtain is used as the raw material, and is subjected to addition elimination, Michael addition, hydrogenation reaction, hydrolysis and chiral resolution, so as to obtain the Pregabalin. The reaction route is simple, and the yield of the reaction in each step is relatively high, so that the total yield and purity of the final Pregabalin are guaranteed.
Owner:TAICANG YUNTONG BIOCHEM ENG
Preparation method of furilazole
The invention discloses a preparation method of furilazole, which comprises the following steps: 1, carrying out a nucleophilic addition reaction on furfural and trimethylsilyl cyanide; 2, reducing a-(2-furan)-a-trimethylsiloxy acetonitrile; 3, carrying out an addition elimination reaction on a-aminomethyl-2-furancarbinol and acetone; and 4, amidating 5-(2-furan)-2,2-dimethyloxazolidine. The preparation method has the advantages of simple process operation, and pureness and high yield of the target product, and provides another approach for the synthesis of like herbicide safeners.
Owner:NANJING UNIV OF SCI & TECH +1
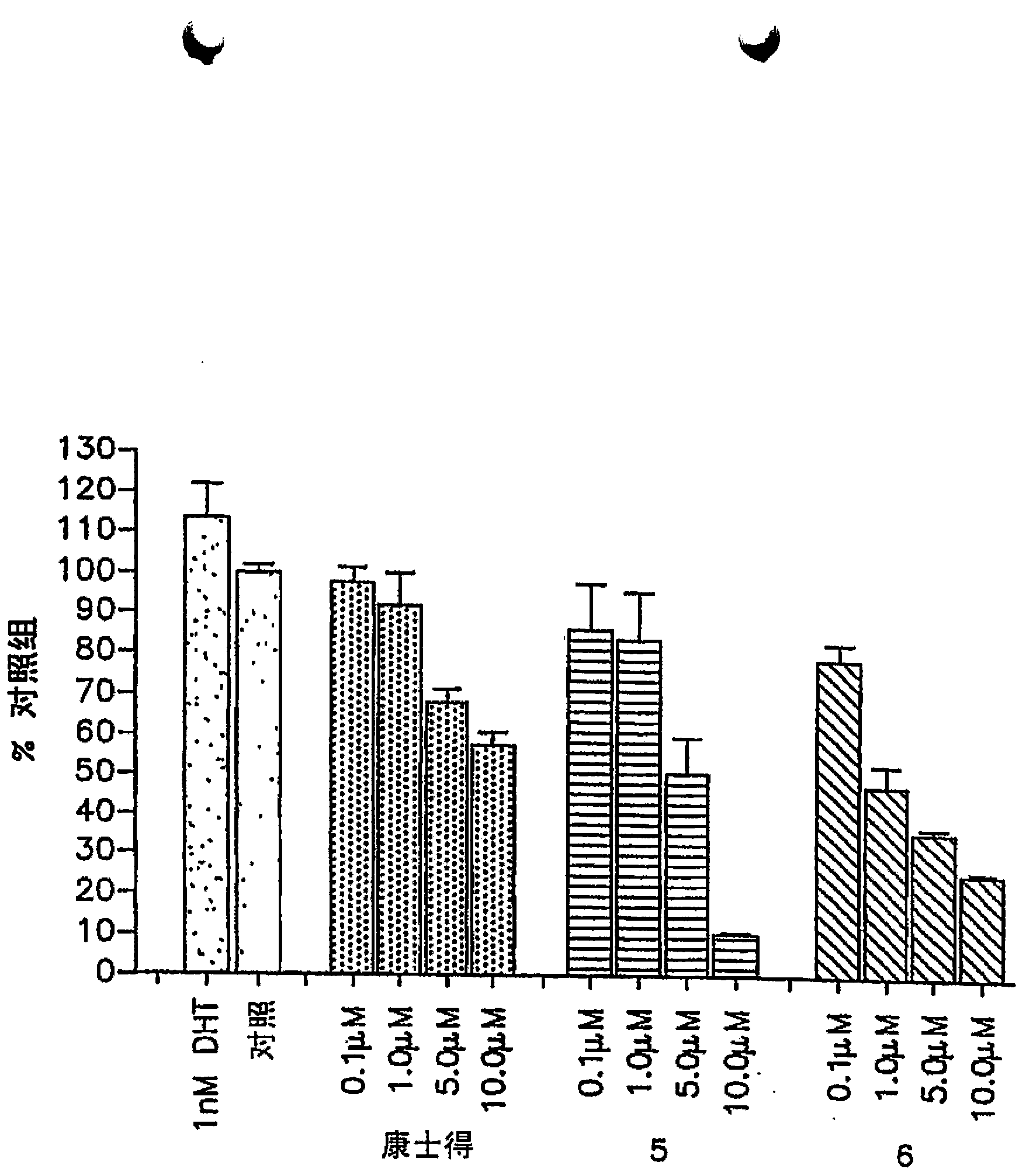
Steroidal c- 17 benzoazoles
Described are steroidal C-17 benzoazoles, pyrimidinoazoles (azabenzoazoles) and diazines. Methods for their synthesis are also described, which include methods having a step of nucleophilic vinylic "addition-elimination" substitution reaction of 3²-acetoxy-17-chloro-16-formylandrosta-5,16-diene or analogs thereof and benzoazole or pyrimidinoazole nucleophiles and methods having a palladium catalyzed cross-coupling reaction of 17-iodoandrosta-5,16-dien-3²-ol or analogs thereof with tributylstannyl diazines. The compounds are potent inhibitors of human CYP 17 enzyme as well as potent antagonists of both wild type and mutant androgen receptors (AR). The compounds are useful for the treatment of human prostate cancer.
Owner:UNIV OF MARYLAND BALTIMORE
Method for synthesizing 2, 3, 5 (Z)-trienol containing fluorine
InactiveCN101519341AEasy to separate and purifyEasy to operateOrganic compound preparationHydroxy compound preparationAlcoholGrignard reagent
The invention relates to a method for synthesizing 2, 3, 5 (Z)-trienol containing fluorine in a high-zone and stereoselectivity way, i.e. 2, 3, 5 (Z)-trienol compound containing fluorine is synthesized through the addition-elimination reaction of Grignard reagent and 5-perfluoroalkyl-4(E)-olefin-2-alkyne-1-alcohol. The method is simple to operate and easy to obtain raw materials and reagent; the reaction has high-zone and stereoselectivity without participation of noble metal, can simultaneously introduce a plurality of substitutional groups, has high yield and easy separation and purification of the product, and is suitable for synthesizing various 2, 3, 5 (Z)-trienols containing fluorine.
Owner:ZHEJIANG UNIV
High-selectivity simple preparation method of apatinib
ActiveCN109810052AStrong methylene activityHigh purityCarboxylic acid nitrile preparationOrganic compound preparationWastewaterPyridine
The invention provides a high-selectivity simple preparation method of apatinib. The high-selectivity simple preparation method comprises the following steps: carrying out first-time amidation reaction on 1-(4-aminophenyl)cyclopentyl carbonitrile II and excessive malonate diester to prepare N-4-(1-cyanocyclopentyl)phenyl monomalonate monoamide III; decompressing, distilling and recycling the excessive malonate diester; adding 4-aminomethylpyridine IV into residues and carrying out second-time amidation reaction to obtain N-(4-pyridine)-methyl-N'-4-(1-cyanocyclopentyl)phenyl malonamide V; carrying out addition-elimination-condensation reaction on a formula shown as a formula V, 2-halogenated acrylaldehyde and ammonia to obtain the apatinib I. The high-selectivity simple preparation method provided by the invention has the advantages that raw materials are cheap and easy to obtain, a technological flow is simple and convenient, the wastewater amount is less, the method is green, safe andenvironmentally friendly and the cost is low; the preparation method provided by the invention has high selectivity and the prepared apatinib has the advantages of few impurities and high purity.
Owner:XINFA PHARMA
The synthetic method of nafamostat mesylate intermediate-6-amidino-2-naphthol mesylate
ActiveCN103896809BReduce pollutionMild reaction conditionsSulfonic acids salts preparationSodium bicarbonateHydroxylamine Hydrochloride
Owner:BEIJING LABWORLD BIO MEDICINE TECH
Isolongifolenone oxime ether derivatives and their preparation methods and applications
Owner:广西鼎弘树脂有限公司
Synthesis method of Acrylodan and analogues thereof
PendingCN112645828AGood effectOvercoming expensive and hard-to-obtain flawsOrganic chemistryOrganic compound preparationFluoProbesTrifluoroacetic acid
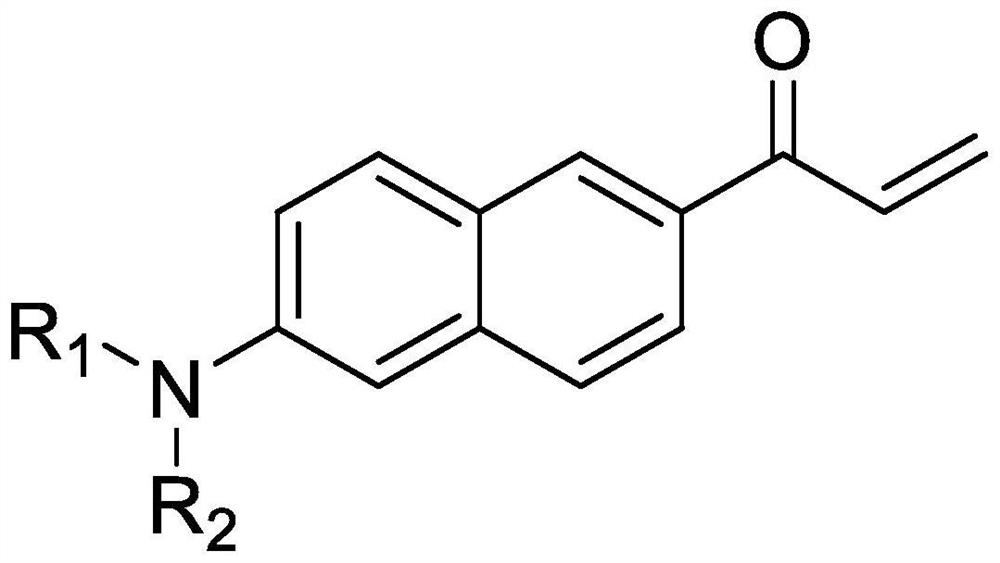
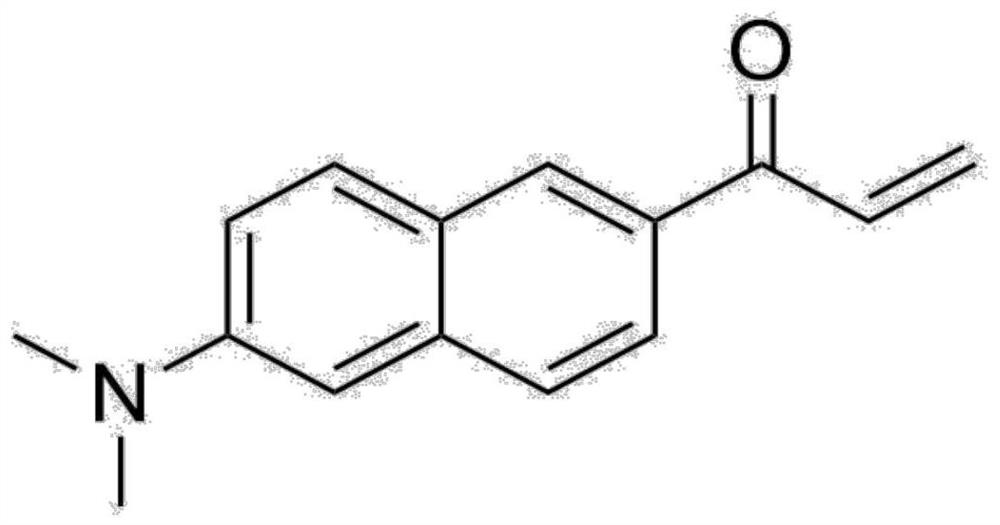
The invention belongs to the technical field of synthesis of fluorescent probes in biochemistry, and particularly relates to an environment-sensitive fluorescent probe and a synthesis process route of Acrylodan and analogues thereof. A method comprises the following steps: taking 6-methoxy-2-acetyl naphthalene as a raw material, carrying out substitution reaction on the 6-methoxy-2-acetyl naphthalene and a lithium amide salt, carrying out addition elimination reaction on an obtained amino-substituted acetyl naphthalene product and trifluoroacetic acid, and then carrying out addition elimination reaction on the obtained amino-substituted acetyl naphthalene product and aldehydes to finally obtain the high-purity Acrylodan compound and analogues. The method has the advantages of simple synthesis steps, short reaction time, high yield, low production cost and the like, and solves the problems of complex synthesis steps, harsh synthesis conditions and expensive synthesis raw materials in original synthesis methods. The optimization and improvement of the synthetic process route of Acrylodan and analogues can greatly promote the application of the environment-sensitive probe in the field of biochemistry.
Owner:LANZHOU UNIVERSITY
Method of preparing (S)-2-amino-5-methoxytetralin hydrochloride
ActiveUS9145353B2Increase productionLow yieldOrganic compound preparationOrganic chemistry methodsTetralin2-Tetralone
Owner:ANHUI QINGYUN PHARMA & CHEM
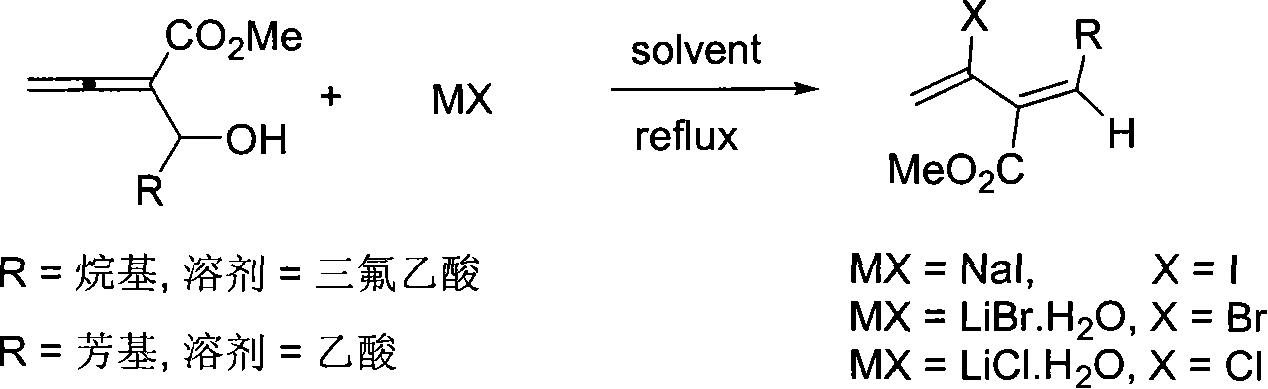
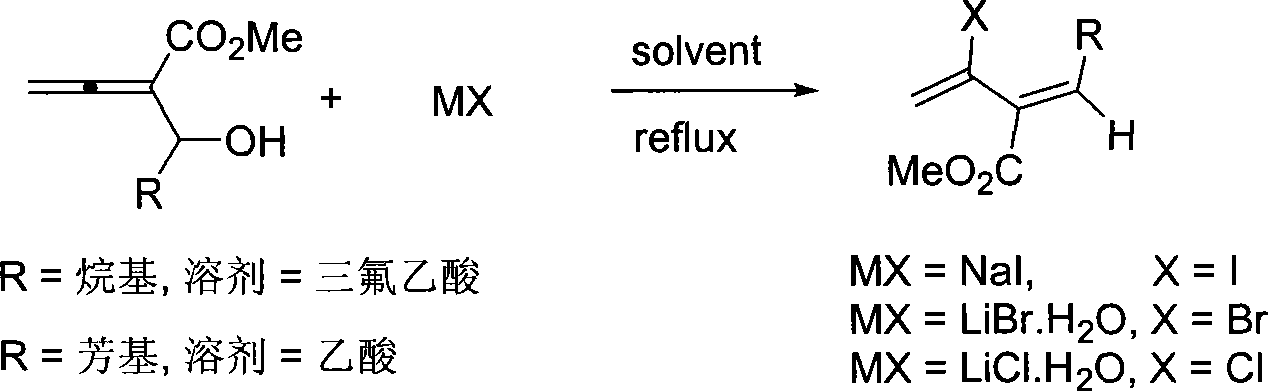
Method of synthesizing 3-carbomethoxy-2-halogen-1,3(Z)-conjugated diolefin
InactiveCN101054347AEasy to separate and purifyHigh stereoselectivityOrganic compound preparationCarboxylic acid esters preparationAcetic acidHalogen
The invention relates to a 3-methyl ester-2-halogen-1,3( Z )-conjugated diene and synthetic method thereof, in which a series of 3-methyl ester-2-halogen-1,3( Z )-conjugated diene compounds is generated through addition-elimination reaction of haloid salt with 3-methyl ester-1,2-allenes-4-alcohol under trifluoroacetic acid or acetic acid counterflow condition. The method is characterized in that it operated simply, its raw material and reagent is obtained easily, it has high stereoselectivity, it is able to induct a plurality of substituent, its products are readily separated and purified simultaneously and it is suitable for synthesizing various substituted 3-methyl ester -2-halogen -1,3( Z )-conjugated diene.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com