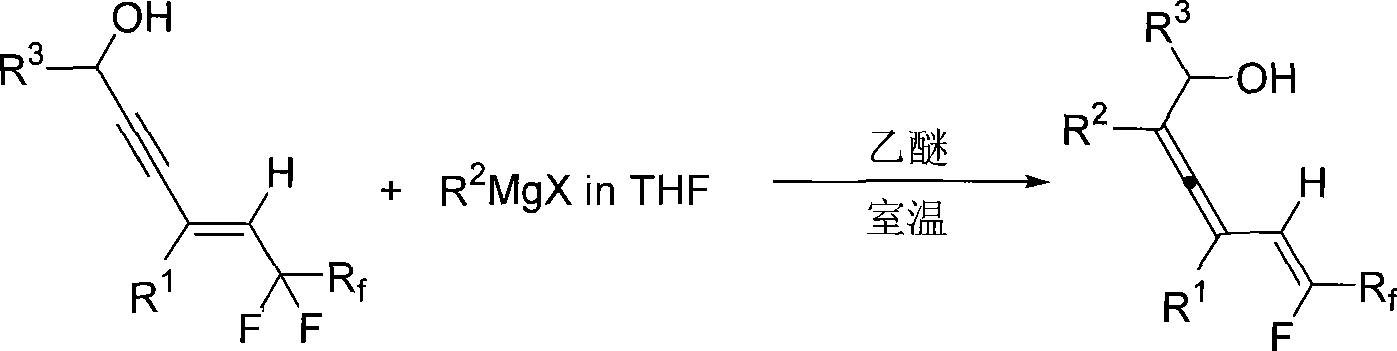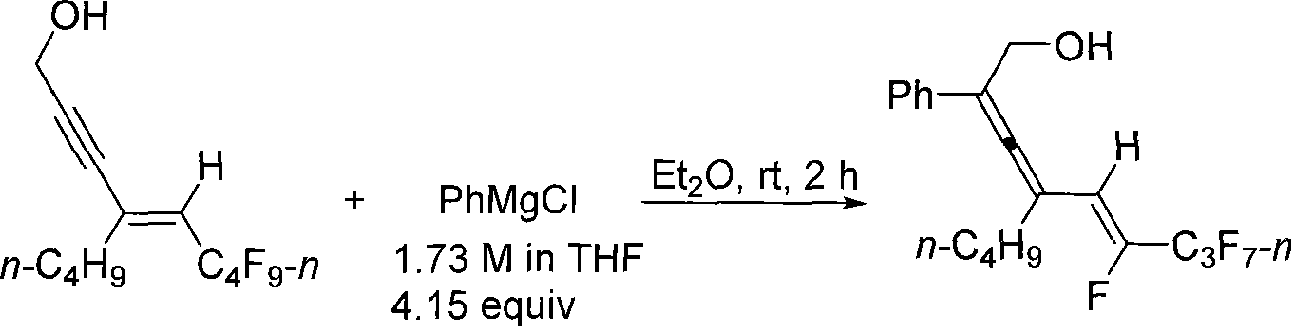Method for synthesizing 2, 3, 5 (Z)-trienol containing fluorine
A technology of trienol and addition, applied in chemical instruments and methods, preparation of organic compounds, preparation of hydroxyl compounds, etc., can solve problems such as limitation and achieve the effect of high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Take a reaction tube, bake it under vacuum, fill it with nitrogen to remove moisture, dry it thoroughly three times, and then cool it to room temperature under nitrogen atmosphere. Add 6,6,7,7,8,8,9,9,9-nonafluoro-4-butyl-4(E)-nonen-2-yn-1-ol (E-18b) into the reaction tube (70.0 mg, 0.197 mmol) and 0.5 mL of anhydrous ether. At room temperature, 0.48 mL of a tetrahydrofuran solution (1.73 M) of phenylmagnesium chloride was added dropwise into the reaction tube with a syringe, and the dropwise addition was completed within 2 minutes, and the reaction was continued for 2 hours at room temperature. Then put the reaction tube into an ice-water bath, and slowly add 5 mL of saturated NH 4 Quenched with Cl solution, extracted with ether (25mL×3), combined the organic phases, washed with saturated NaCl solution (10mL×2), anhydrous NaCl 2 SO 4 After dry filtration, concentration, and flash column chromatography, 75.5 mg of the product was obtained with a yield of 95%. The pr...
Embodiment 2
[0022] According to the method described in Example 1, the difference is that the substrates and reagents used are: 6,6,7,7,8,8,9,9,9-nonafluoro-4-butyl-4(E)- Nonen-2-yn-1-ol (318.9mg, 1.25mmol) and 4-methoxyphenylmagnesium bromide (2.0M in THF, 0.40mL, 0.80mmol) were dissolved in 0.5mL anhydrous diethyl ether at room temperature Under the solvent, reacted for 2 hours to obtain 83.0 mg of the product, and the yield was 93%. The product is a yellow liquid.
[0023]
[0024] 1 HNMR (300MHz, CDCl 3 )δ 7.35-7.27(m, 2H), 6.94-6.87(m, 2H), 5.92(d, J=34.2Hz, 1H), 4.57(s, 2H), 3.81(s, 3H), 2.38(t, J=7.4Hz, 2H), 1.69(bs, 1H), 1.55-1.30(m, 4H), 0.90(t, J=7.2Hz, 3H); 19 F NMR (282MHz, CDCl 3 )δ-80.8-(-80.9)(m, 3F), -118.0-(-118.2)(m, 2F), -127.2-(-127.6)(m, 3F); 13 C NMR (75MHz, CDCl 3 )δ 207.1 (d, J=4.4Hz), 159.2, 144.7 (dt, J 1 =267.8Hz and J 2 =27.8Hz), 127.6, 125.5, 114.2, 112.3-112.0(m), 108.0, 103.4(d, J=3.2Hz), 61.9, 55.3, 31.8(d, J=2.8Hz), 30.4, 22.2, 13.8; IR(neat)v...
Embodiment 3
[0026] According to the method described in Example 1, the difference is that the substrates and reagents used are: 6,6,7,7,8,8,9,9,9-nonafluoro-4-butyl-4(E)- Nonen-2-yn-1-ol and 4-fluorophenylmagnesium bromide (2.0M in THF, 0.40mL, 0.80mmol) were reacted for 2 hours at room temperature in 0.5mL of anhydrous ether as a solvent to obtain the product 78.6 mg, 89% yield. The product is a yellow liquid.
[0027]
[0028] 1 HNMR (300MHz, CDCl 3)δ 7.41-7.31(m, 2H), 7.11-7.00(m, 2H), 5.91(d, J=34.2Hz, 1H), 4.58(s, 2H), 2.38(t, J=74Hz, 2H), 1.72(bs, 1H), 1.54-1.30(m, 4H), 0.90(t, J=72Hz, 3H); 19 F NMR (282MHz, CDCl 3 )δ-80.8-(-80.9)(m, 3F), -114.1-(-114.3)(m, 1F), -118.0-(-118.2)(m, 2F), -126.3-(-126.7)(m , 1F), -127.2-(-127.4)(m, 2F); 13 C NMR (75MHz, CDCl 3 )δ 207.2 (d, J = 3.8Hz), 162.3 (d, J = 246.5), 145.0 (dt, J 1 =266.5Hz and J 2 =27.8Hz), 129.4(d, J=3.5Hz), 128.1(d, J=7.6Hz), 115.7(d, J=22.1Hz), 111.9-111.6(m), 107.5, 103.3(d, J= 3.2Hz), 61.9, 31.8(d, J=2.9Hz), 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com