Non-serum culture medium for multiple animal cell large-scale culture
A serum-free medium and large-scale culture technology, applied to animal cells, etc., can solve problems such as increased costs, differences between product batches, and reduced recovery rates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The above components are mixed and dissolved in pyrogen-free ultrapure water to obtain a culture medium.
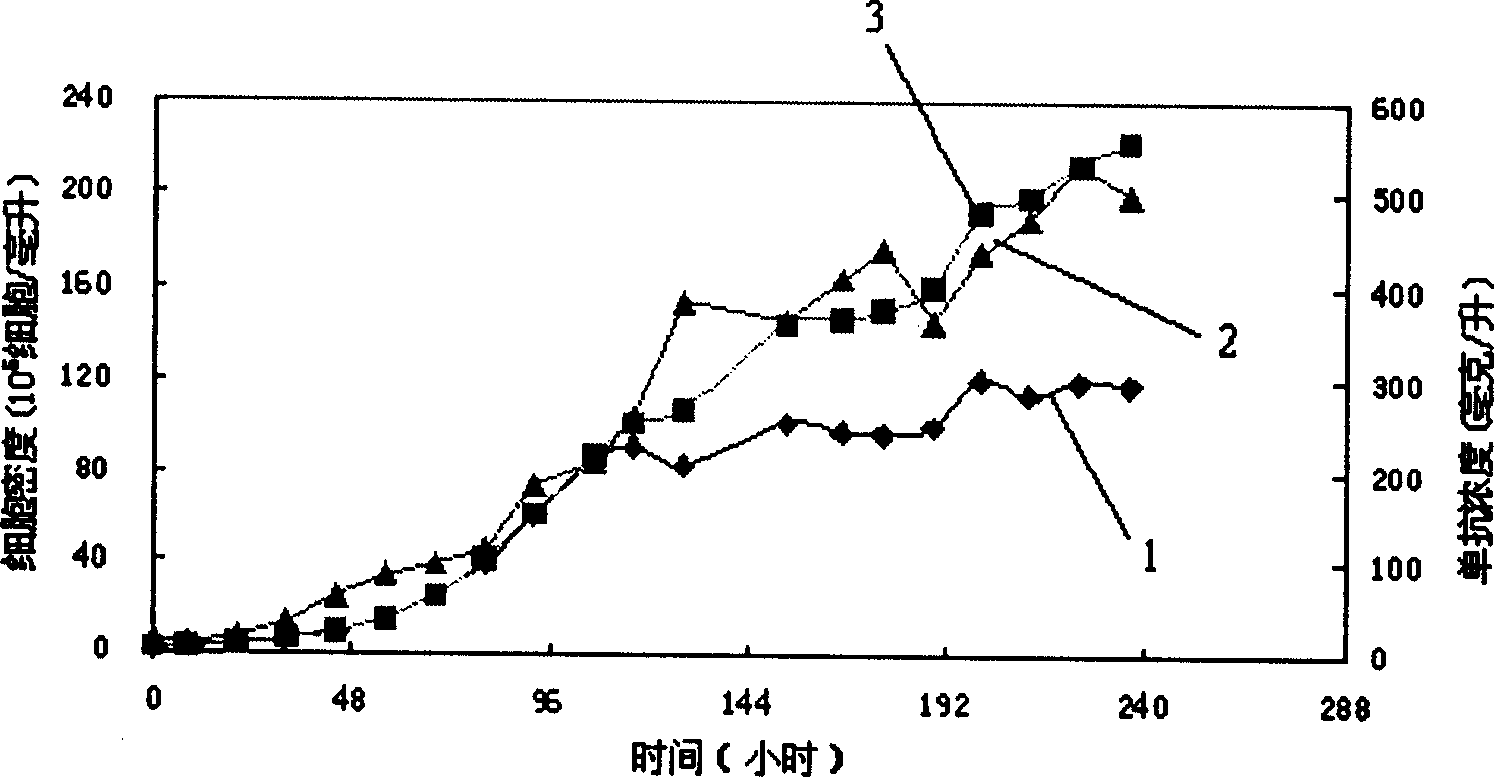
[0034] After the HB58 hybridoma cells (obtained from ATCC) were subcultured and adapted in the serum-free medium of the present invention, they were inoculated in 2 liters of bioreactors of BF-2 (Germany B.BRAUN company), and the inoculation density was 2.0×10 5 cells / ml, start perfusion after 56 hours of cultivation, the perfusion rate is 0.5 (1 / day), the perfusion medium is the serum-free medium of the present invention, and the cell density is maintained at 1.2×10 after 200 hours of cultivation. 7 cells / ml, the monoclonal antibody concentration is maintained at about 500mg / L (see figure 1 ), compared with the results of ordinary culture medium batch culture, the cell density and monoclonal antibody concentration were increased by more than 8 times. In the figure, curve 1 is the viable cell density, curve 2 is the total cell density, and curve 3 is the ex...
Embodiment 2
[0036] Element
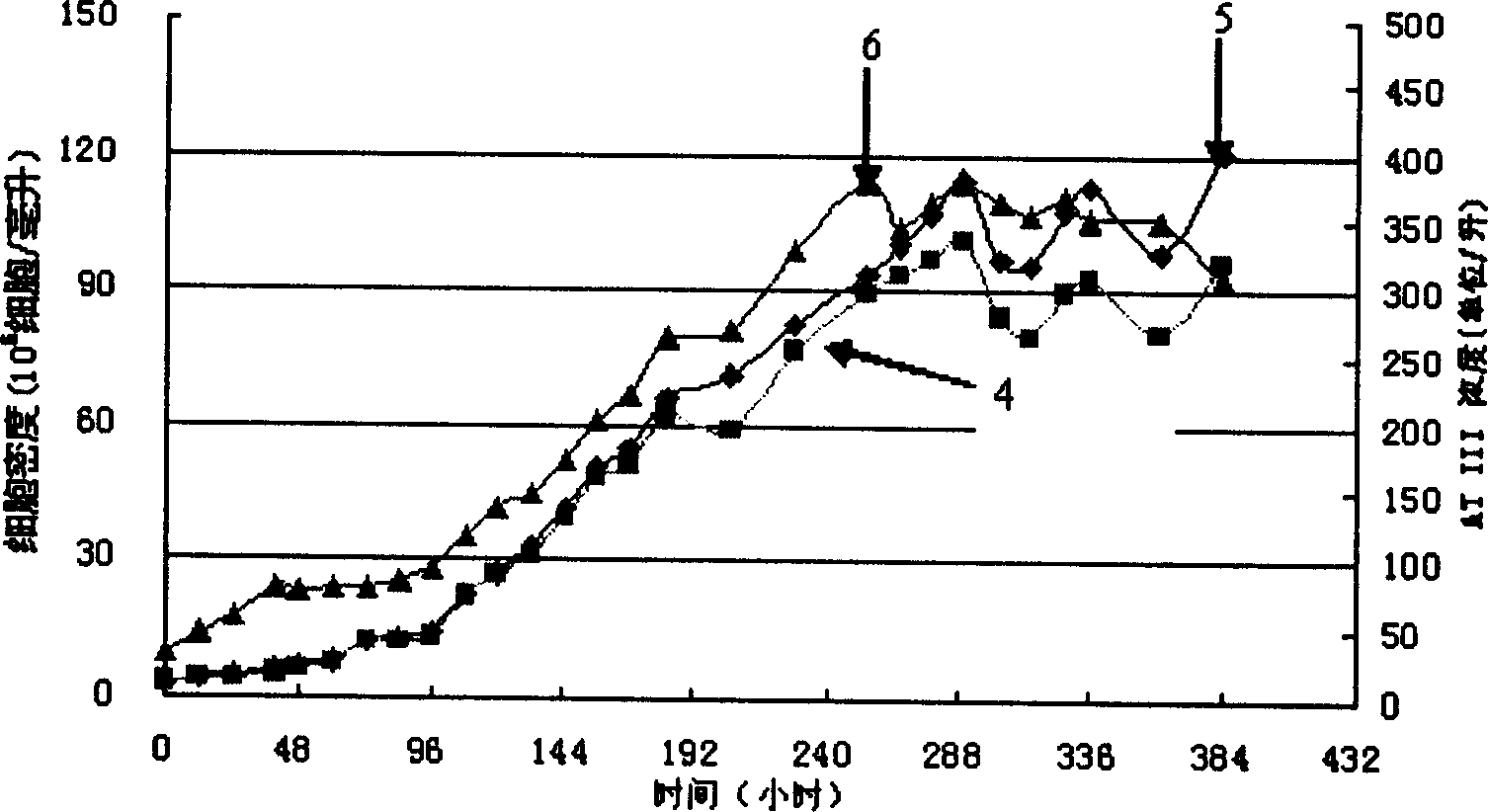
[0037] rCHO cells (rCHO SS3 A2, expressing human anticoagulant factor III) were subcultured and adapted in the serum-free medium according to the present invention, and then inoculated in a B.BRAUN 2-liter bioreactor with an inoculation density of 2.0×10 5 cells / ml, start perfusion after 40 hours of cultivation, the perfusion rate is 0.58 (1 / day), the perfusion medium is the serum-free medium of the present invention, and the cell density is maintained at 0.9-1.0×10 after 255 hours of cultivation 7 cells / ml, the product concentration is maintained at about 350-380U / L (see figure 2 ), compared with the results of ordinary culture medium batch culture, the cell density and monoclonal antibody concentration were increased by 6 times and 5 times, respectively. In the figure, curve 4 is the living cell density, the curve is the total cell density, and curve 6 is the expression level of the product.
Embodiment 3
[0039] Element
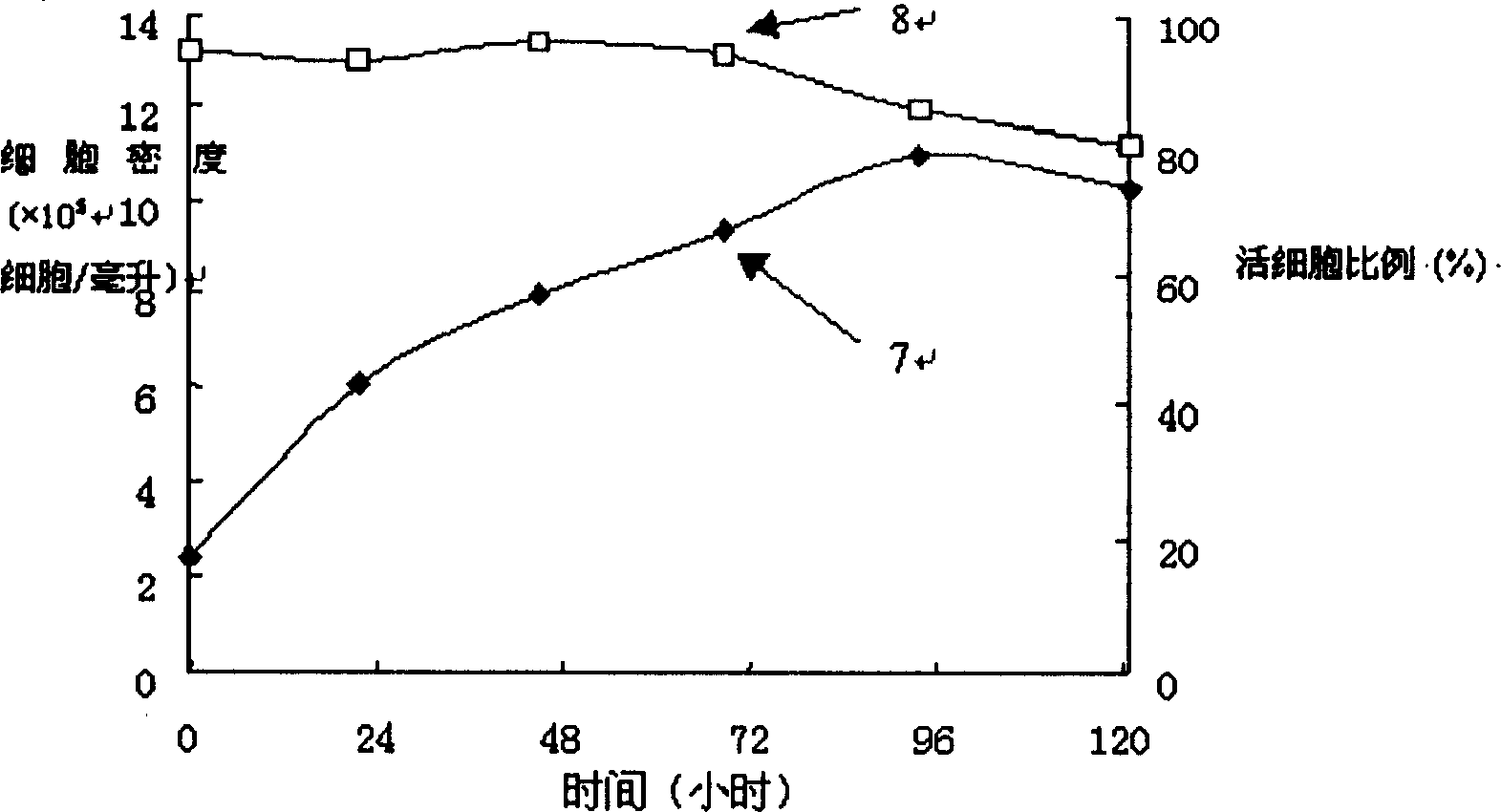
[0040] After the 293 cells were subcultured and adapted in the serum-free medium of the present invention, they were inoculated in a 2-liter bioreactor of B.BRAUN for batch culture, and the inoculation density was 2.45×10 5 cells / ml, by image 3 It can be seen that there is almost no lag phase in the growth of the cells, and they enter the exponential growth phase after inoculation, with an average specific growth rate of 0.46day -1 , with a maximum viable cell density of 11.0×10 5 cells / ml. In the figure, curve 7 is the living cell density, and curve 8 is the ratio of living cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com