Patents
Literature
334 results about "Viable cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Viable cell count. A measure of the number of cells that are viable (i.e. alive and capable of growth) in a given area or volume. Viable cell count can be determined by the total cell count minus the count of nonviable or dead cells. One use of viable cell count is to estimate cytotoxicity.
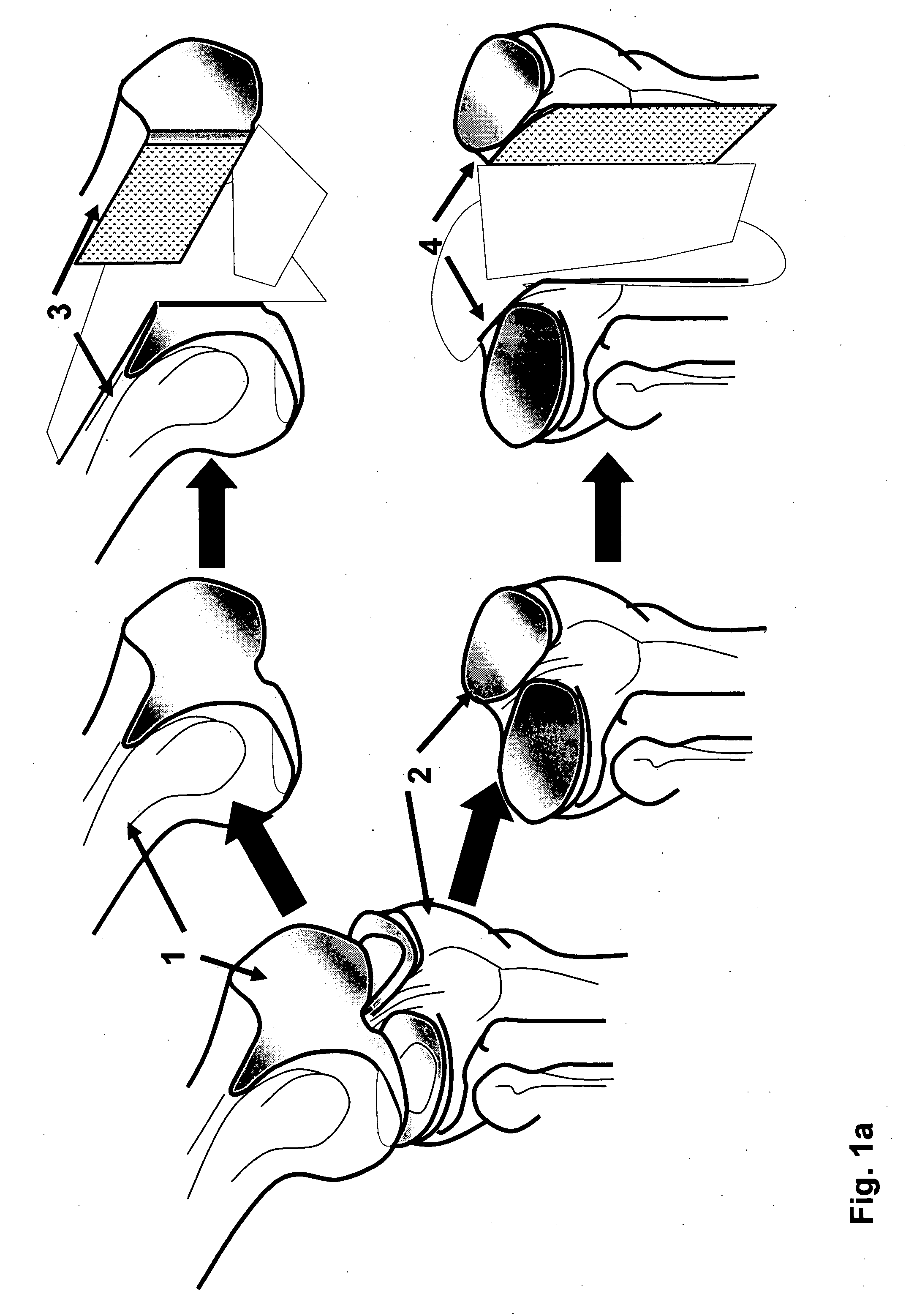
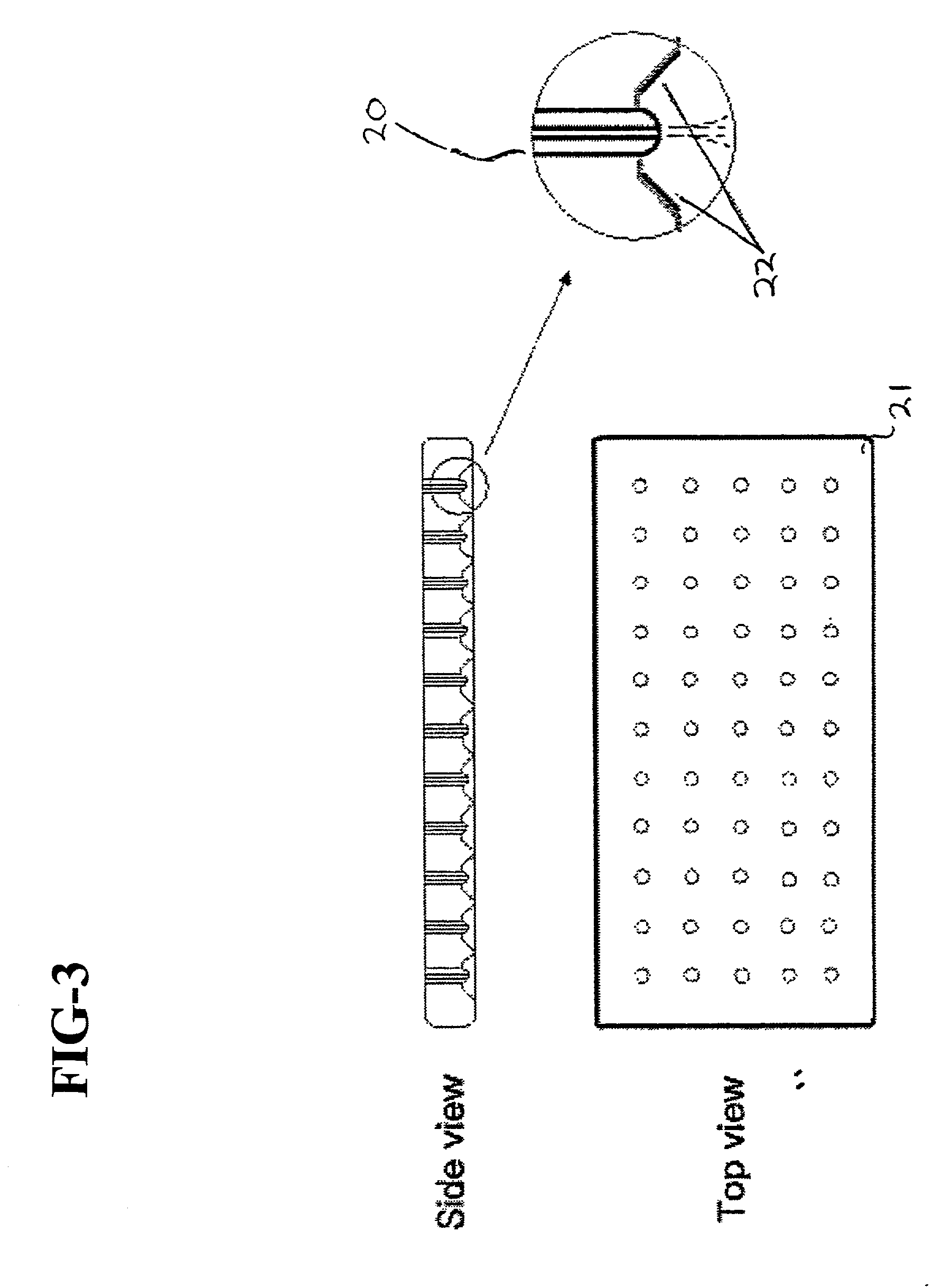
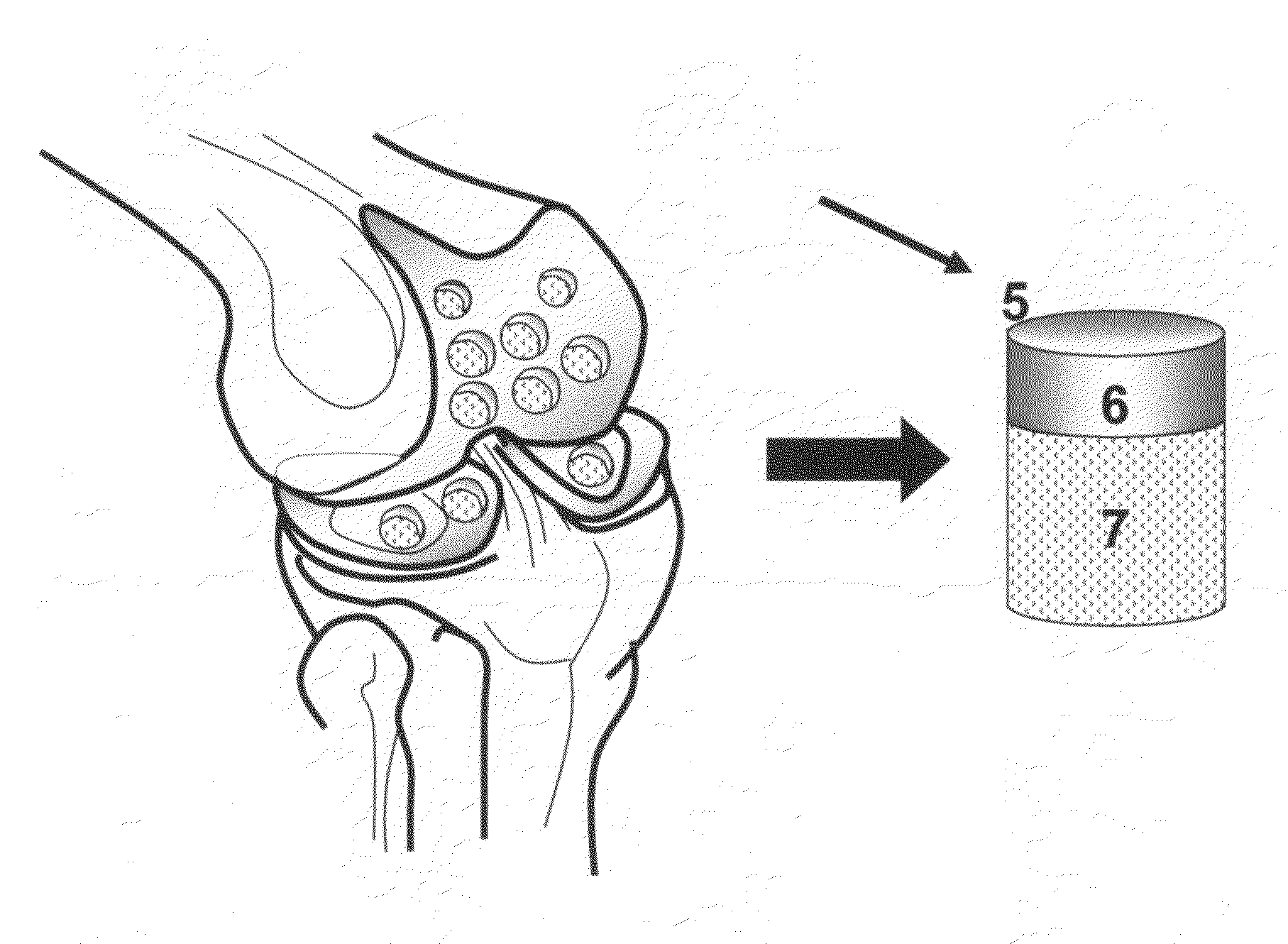
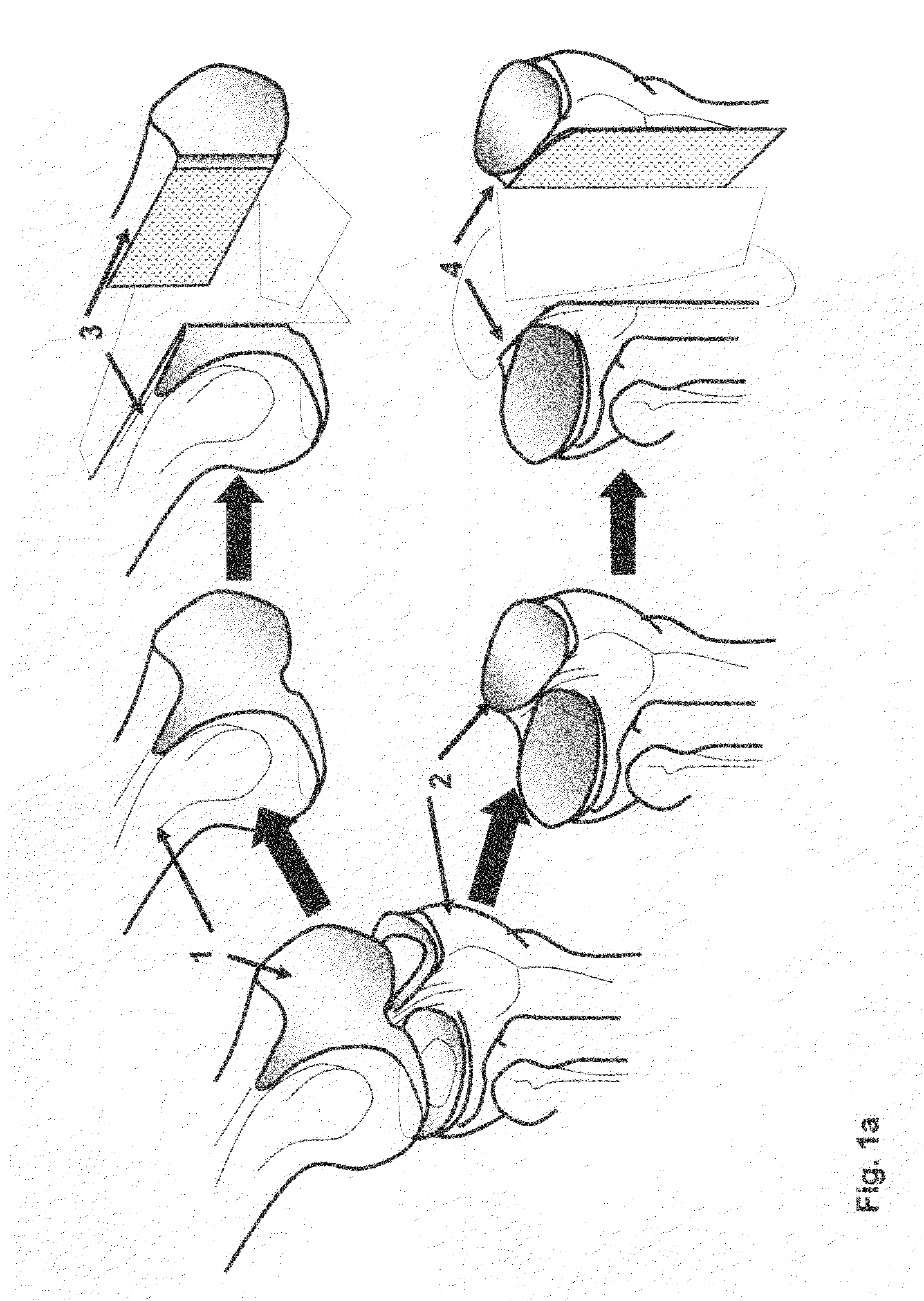
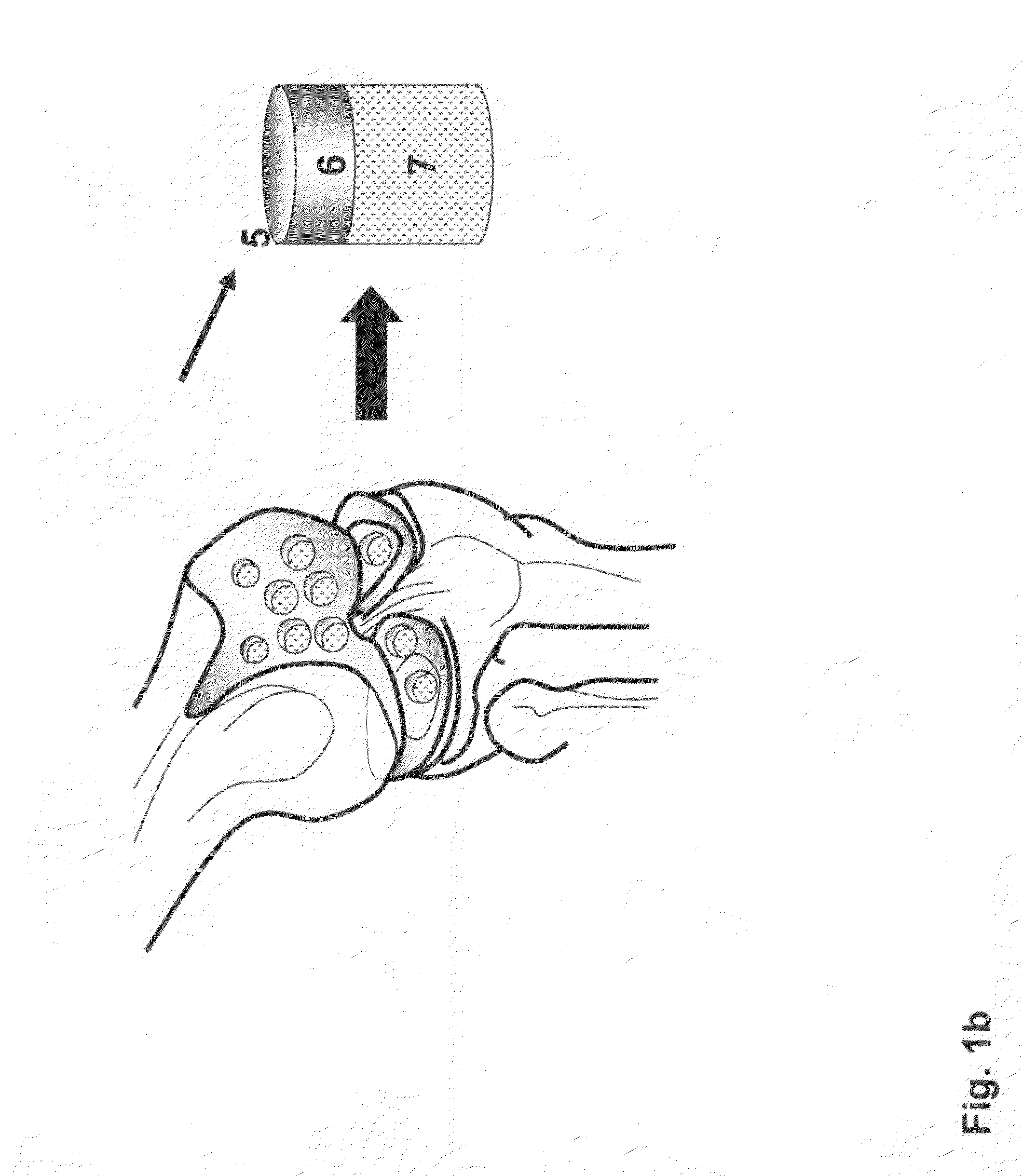
Method and apparatus for resurfacing an articular surface
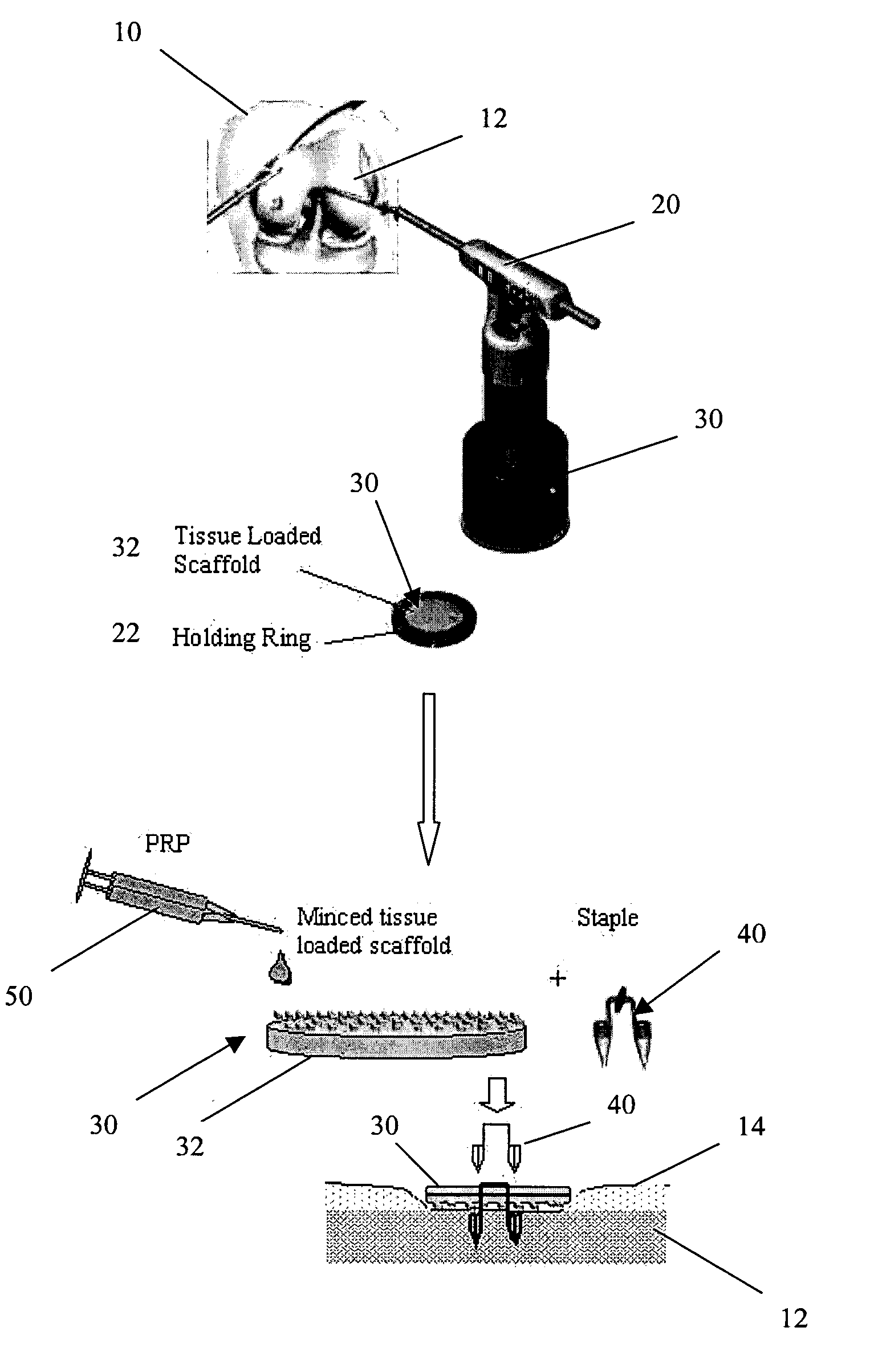
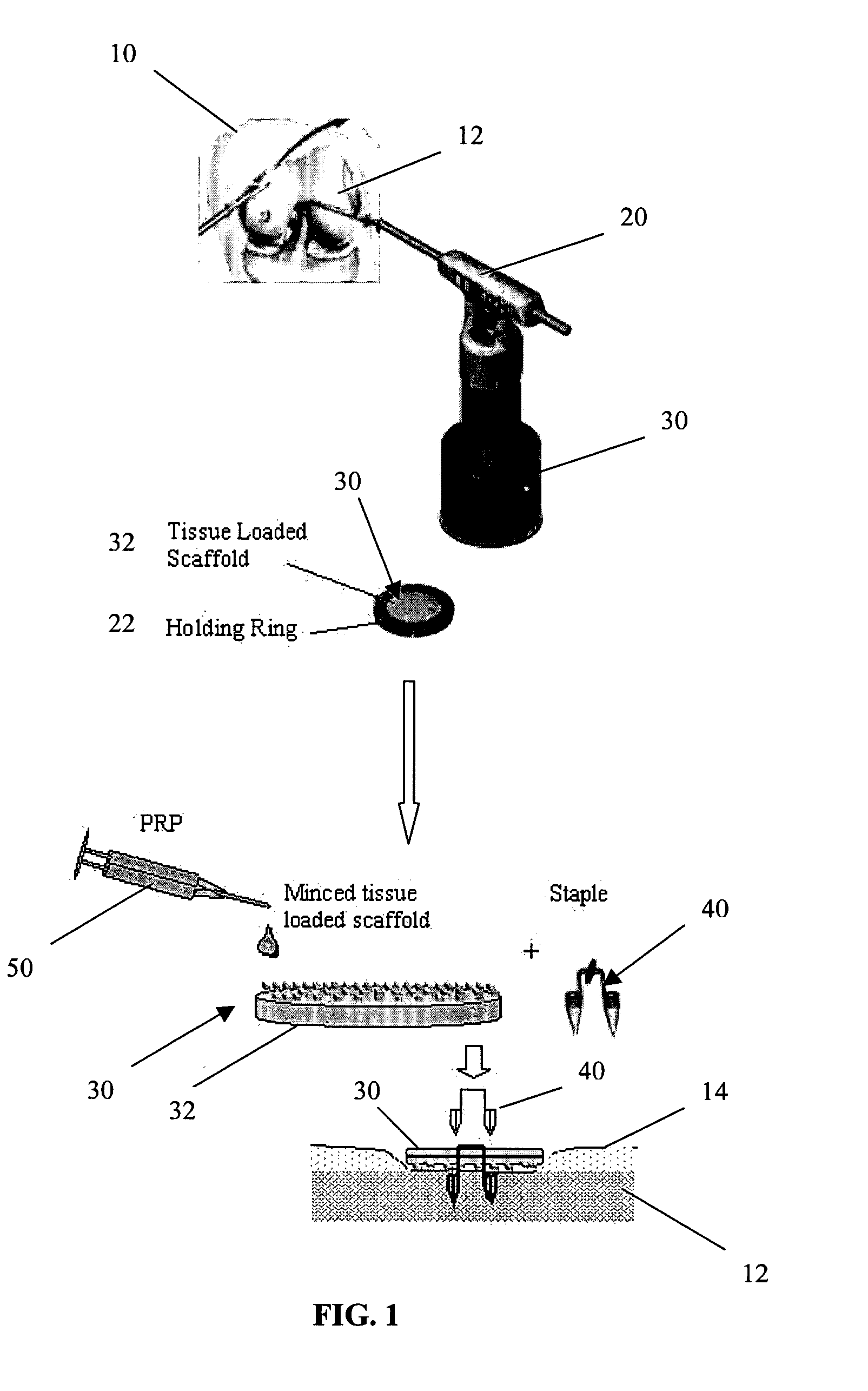
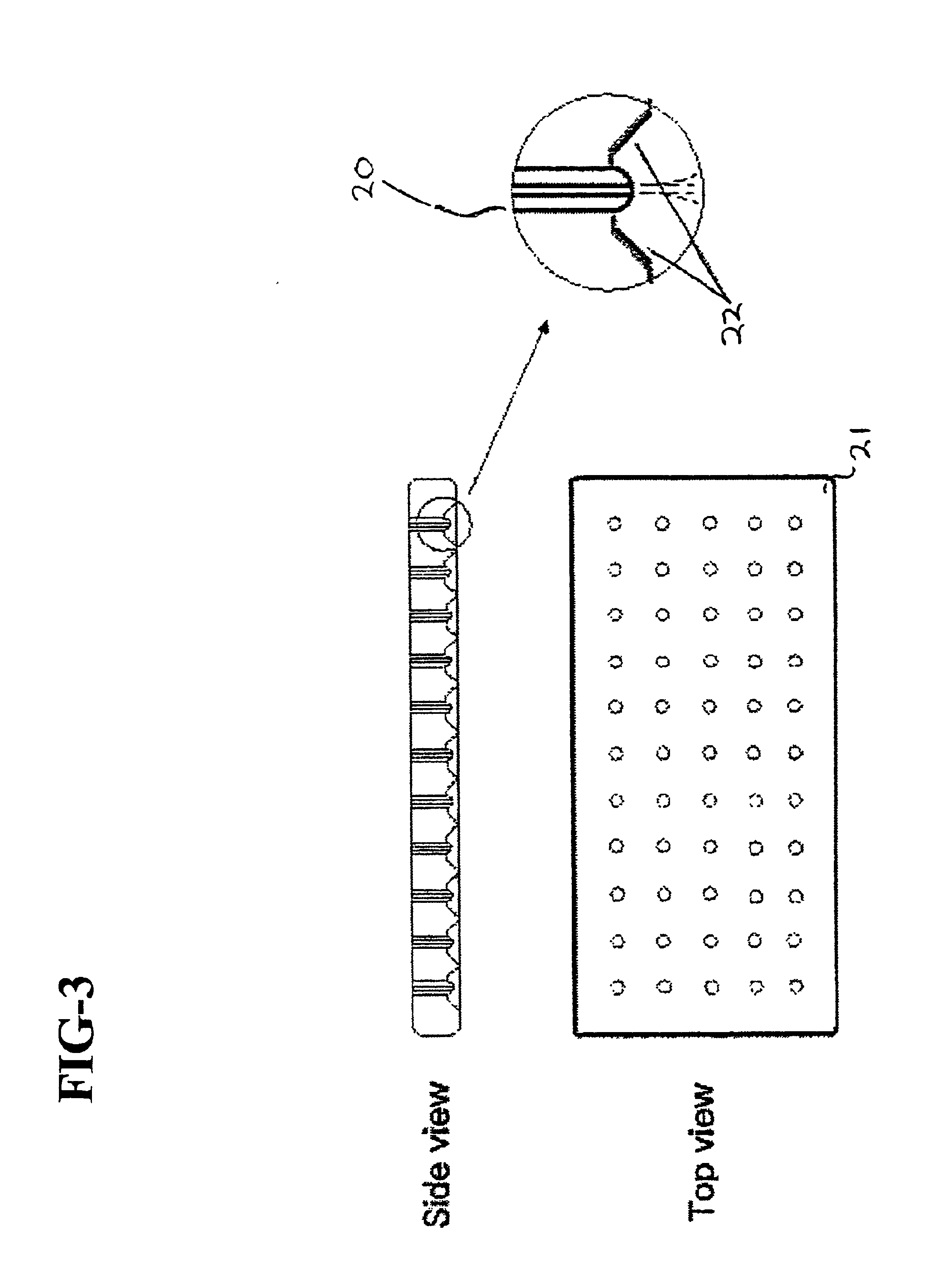
A biocompatible, bioresorbable tissue repair implant or scaffold device is provided for use in repairing a variety of cartilage tissue injuries, and particularly for resurfacing and / or repairing damaged or diseased cartilage. The repair procedures may be conducted with tissue repair implants that contain a biological component that assists in delaying or arresting the progression of degenerative joint diseases and in enhancing tissue healing or repair. The biocompatible, bioresorbable tissue repair implants include a scaffold and particles of viable tissue derived from cartilage tissue, such that the tissue and the scaffold become associated. The particles of living tissue contain one or more viable cells that can migrate from the tissue and populate the scaffold.
Owner:DEPUY SYNTHES PROD INC
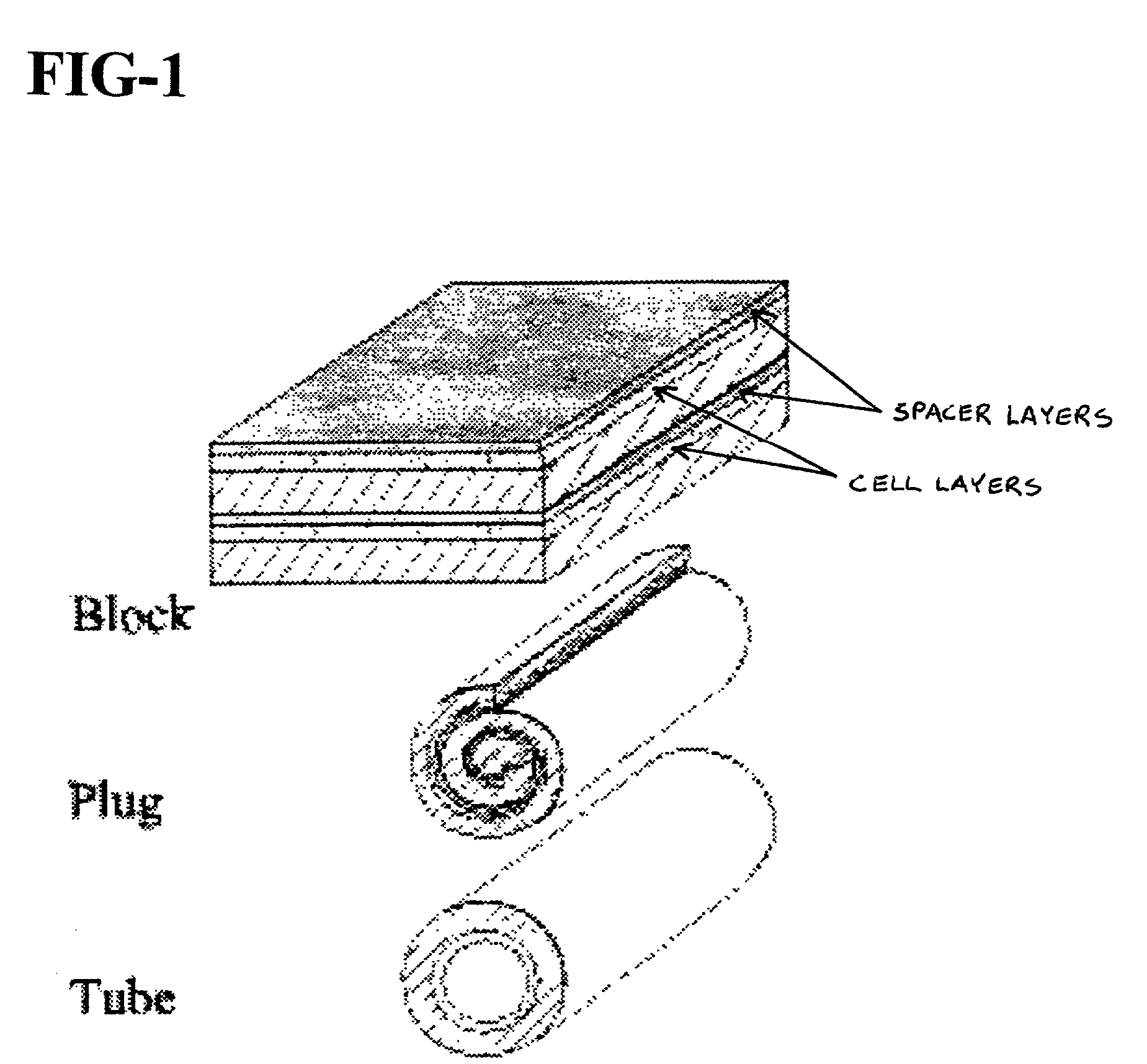
Cell delivery system comprising a fibrous matrix and cells
Cell storage and delivery systems and methods for storing and delivering viable cells to a mammal are disclosed. The cell storage and delivery systems include a biodegradable and / or bioabsorbable fibrous matrix physically associated with viable cells to contain and release the cells at a controlled rate. The biodegradable and / or bioabsorbable matrix can be formed by electrospinning fibers of biodegradable and / or bioabsorbable fiberizable material. The methods include methods for storing viable cells and for delivering viable cells to a mammal using the cell storage and delivery system.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Viable tissue repair implants and methods of use
InactiveUS20050125077A1Good retention of cellEasy to integrateBone implantSkeletal disorderTissue repairRepair tissue
Biocompatible tissue implants are provided for repairing a tissue injury or defect. The tissue implants comprise a biological tissue slice that serves as a source of viable cells capable of tissue regeneration and / or repair. The biological tissue slice can be harvested from healthy tissue to have a geometry that is suitable for implantation at the site of the injury or defect. The harvested tissue slice is dimensioned to allow the viable cells contained within the tissue slice to migrate out and proliferate and integrate with tissue surrounding the injury or defect site. Methods for repairing a tissue injury or defect using the tissue implants are also provided.
Owner:DEPUY SYNTHES PROD INC
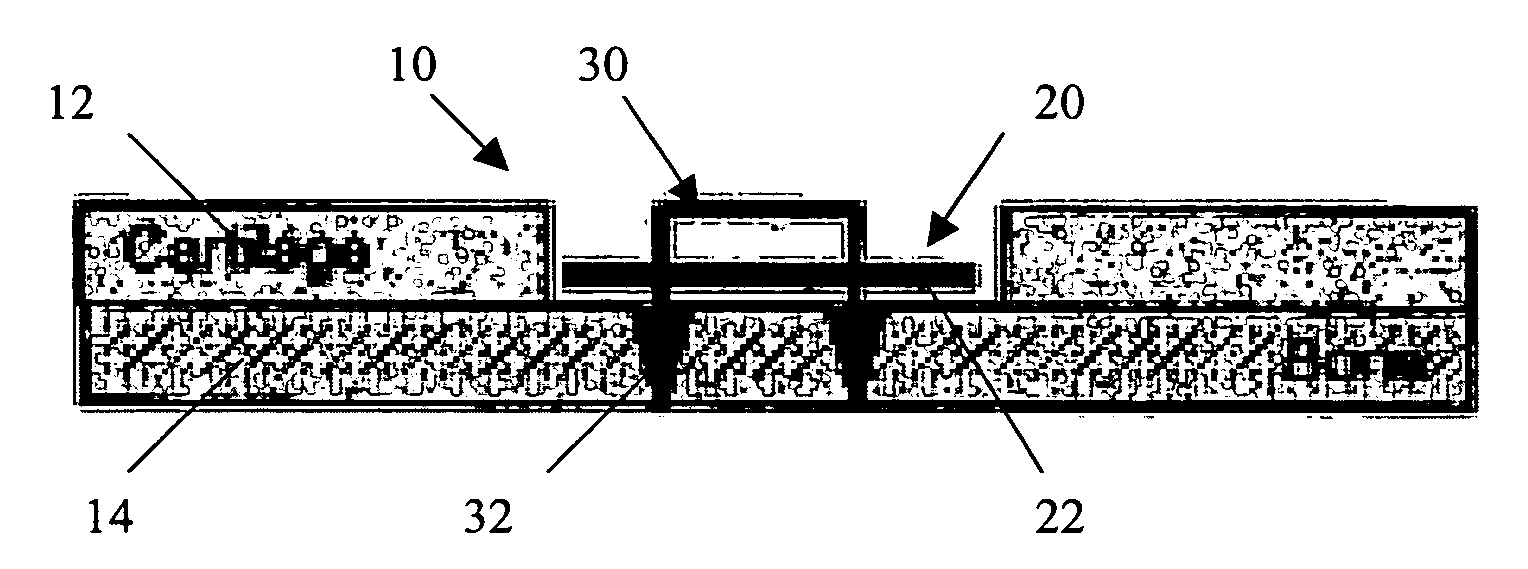
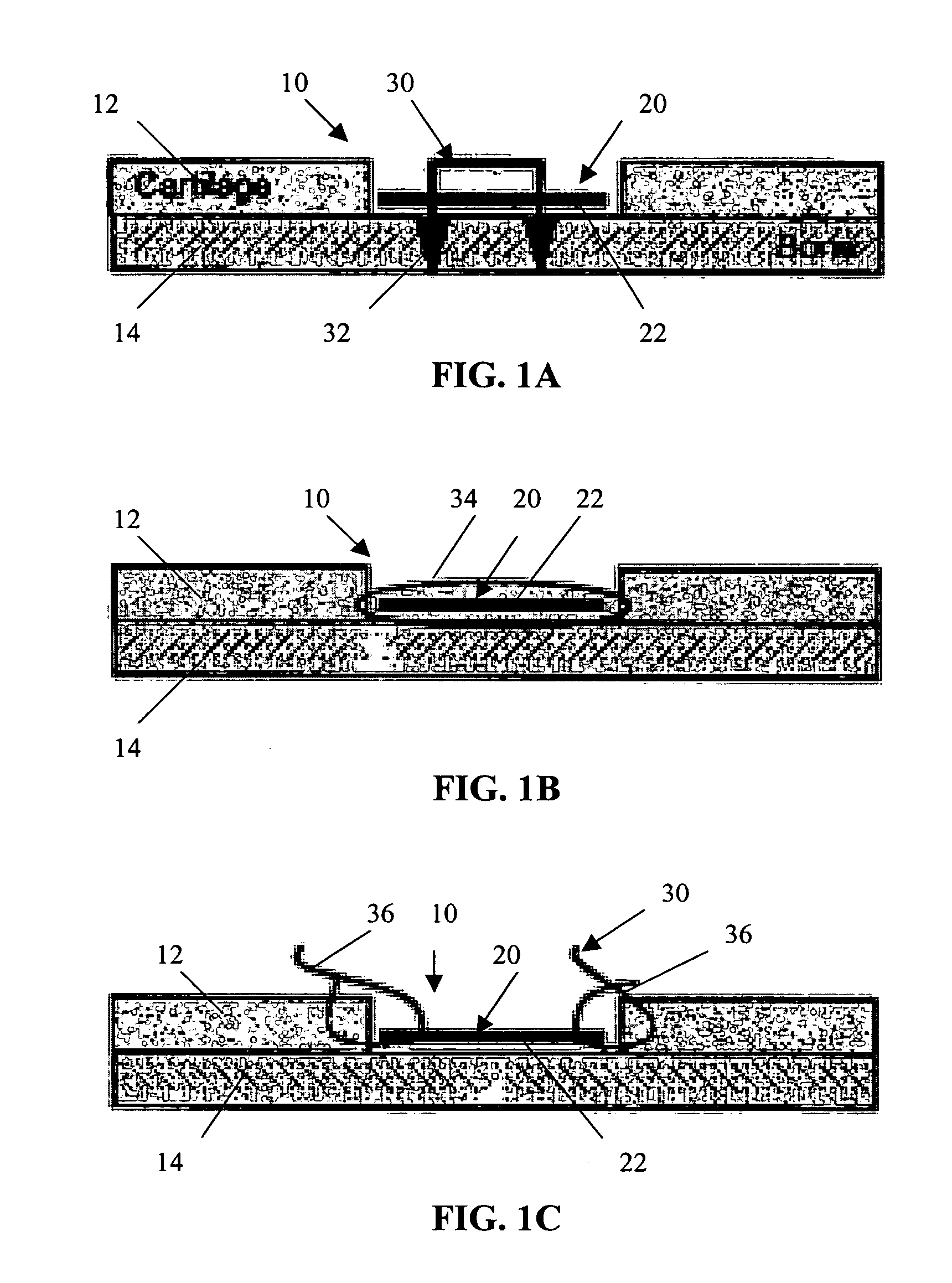
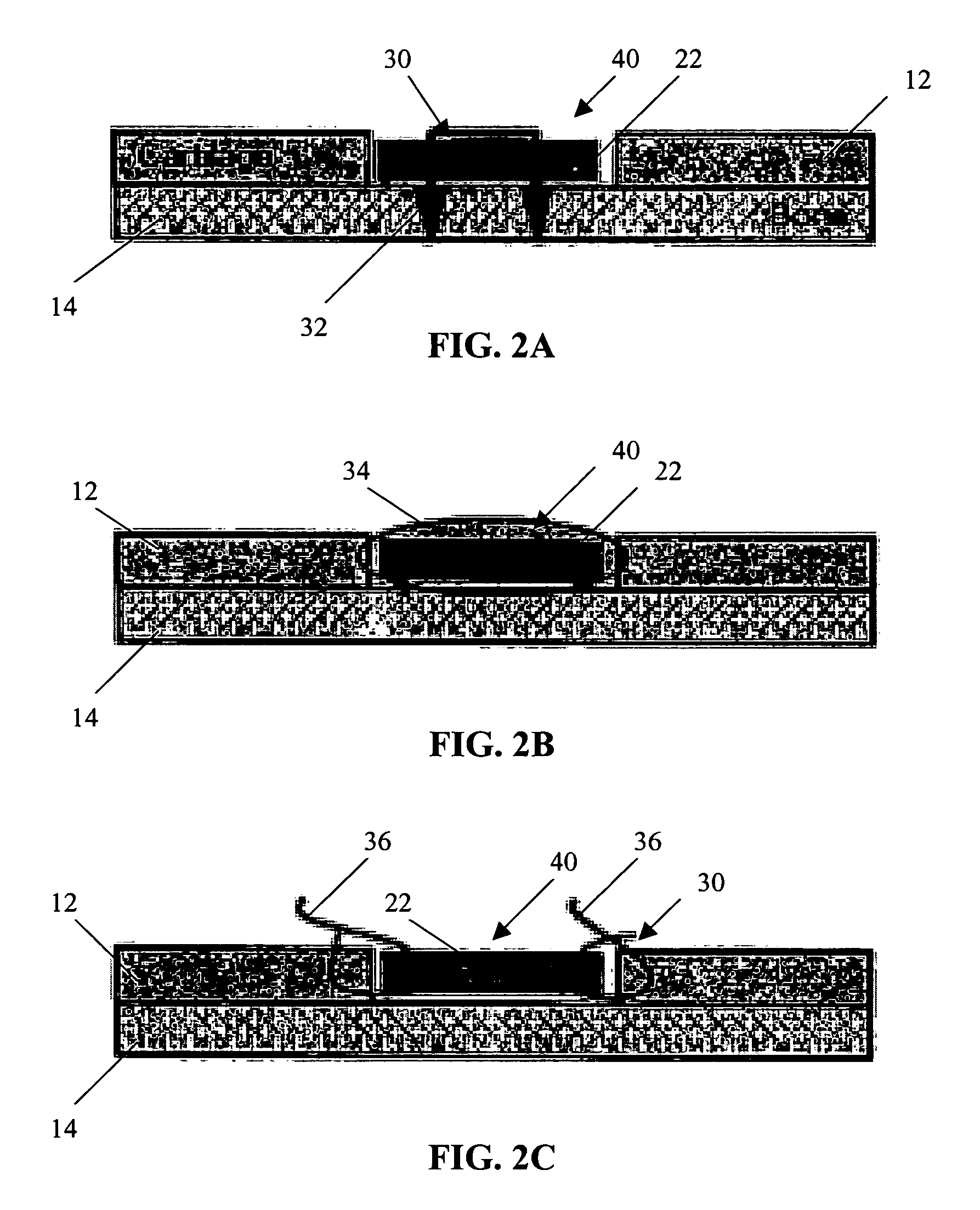
Conformable tissue repair implant capable of injection delivery
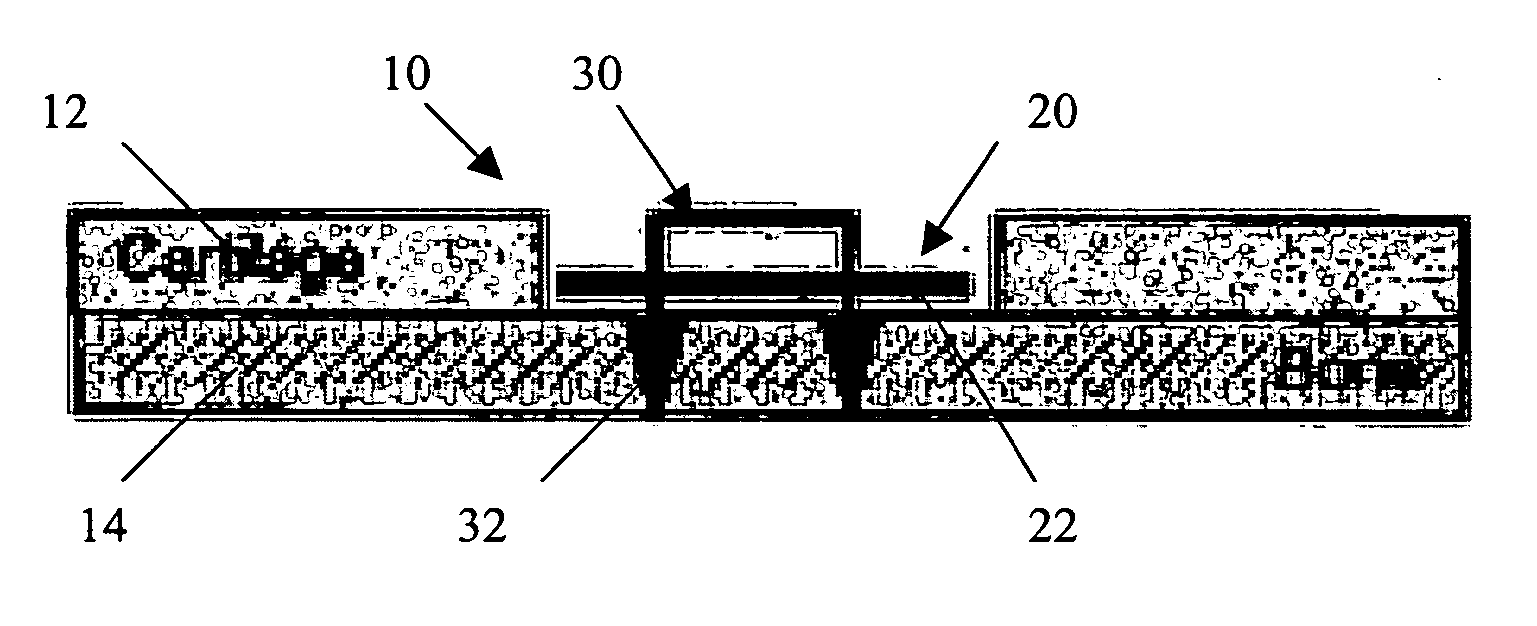
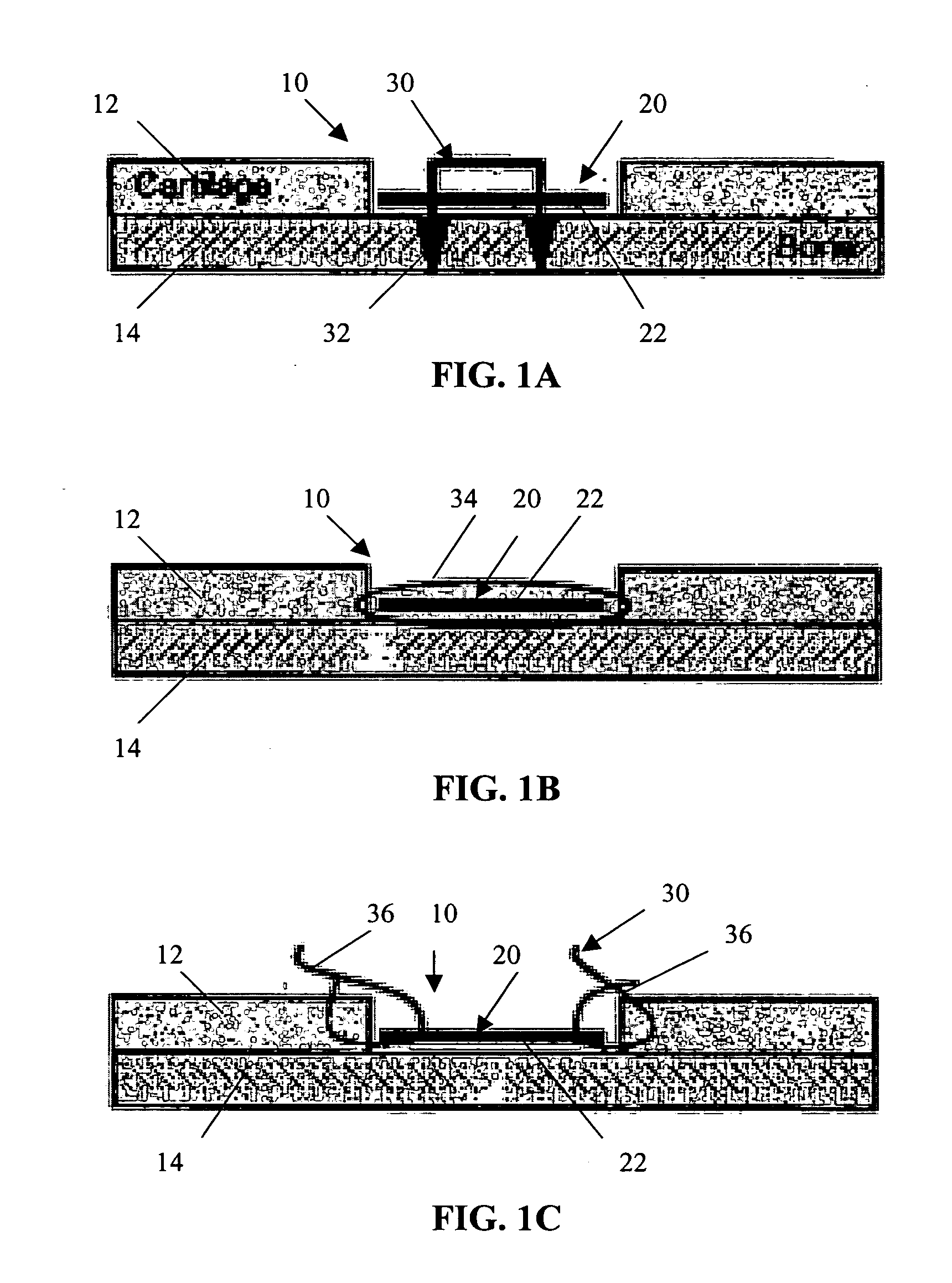
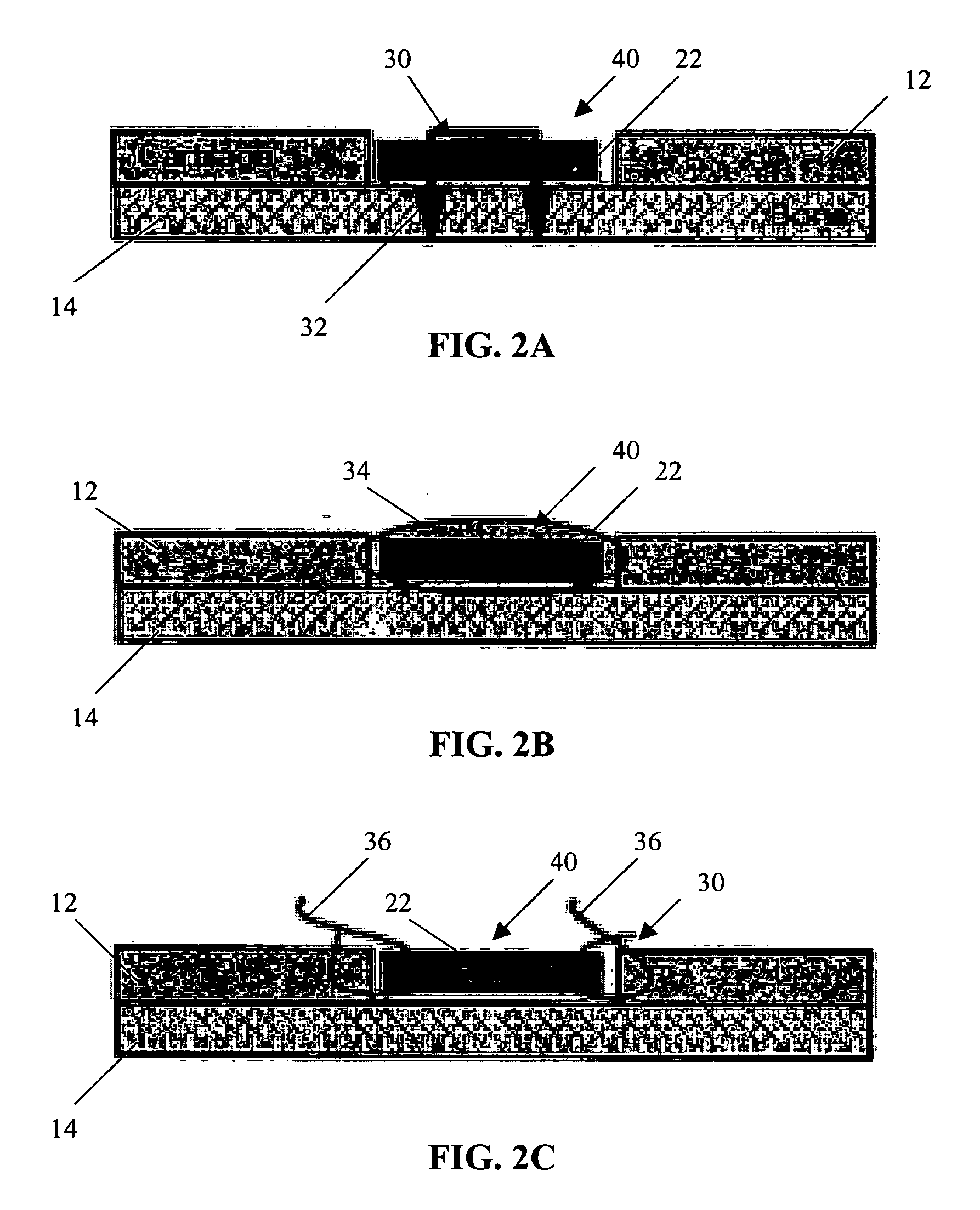
A conformable tissue implant is provided for use in repairing or augmenting a tissue defect or injury site. The tissue implant contains a tissue carrier matrix comprising a plurality of biocompatible, bioresorbable granules and at least one tissue fragment in association with the granules. The tissue fragment contains one or more viable cells that can migrate from the tissue and populate the tissue carrier matrix. Also provided is a method for injectably delivering the tissue implant.
Owner:DEPUY SYNTHES PROD INC
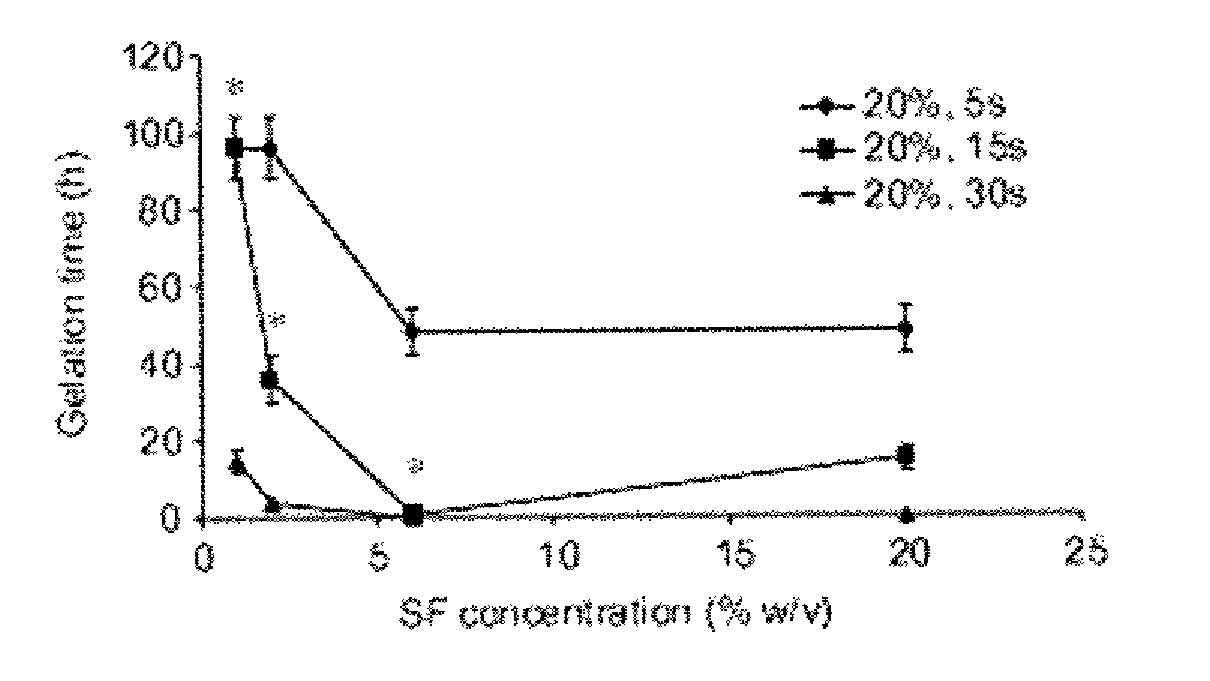
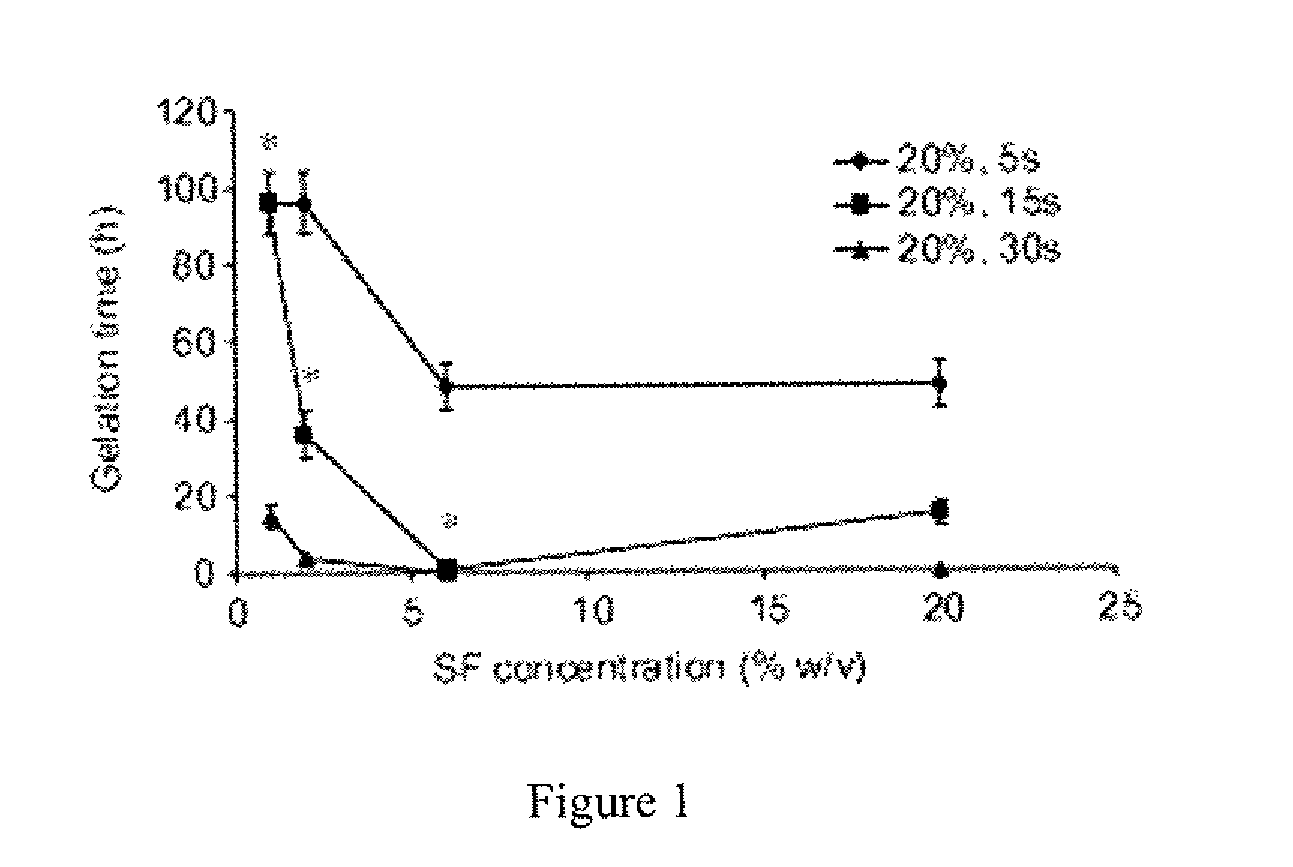
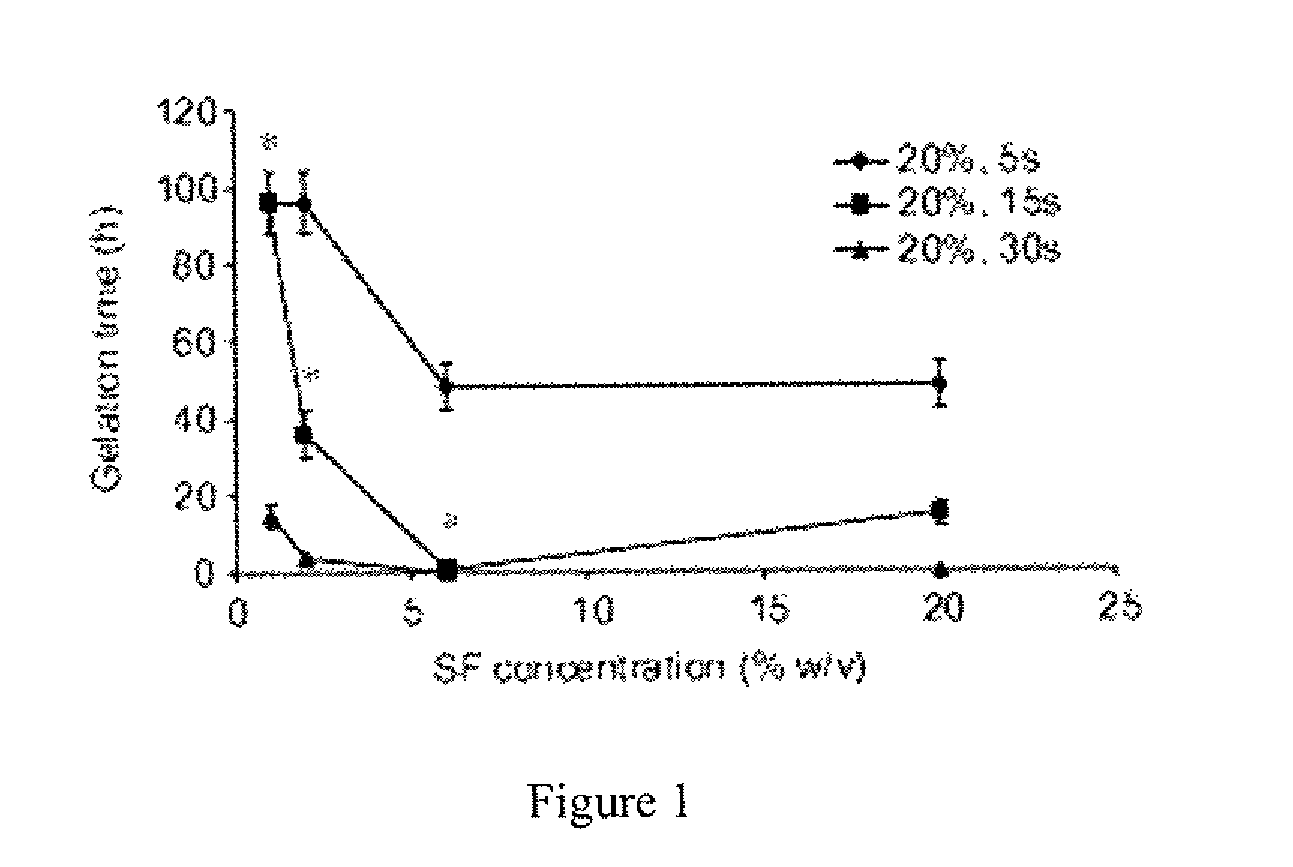
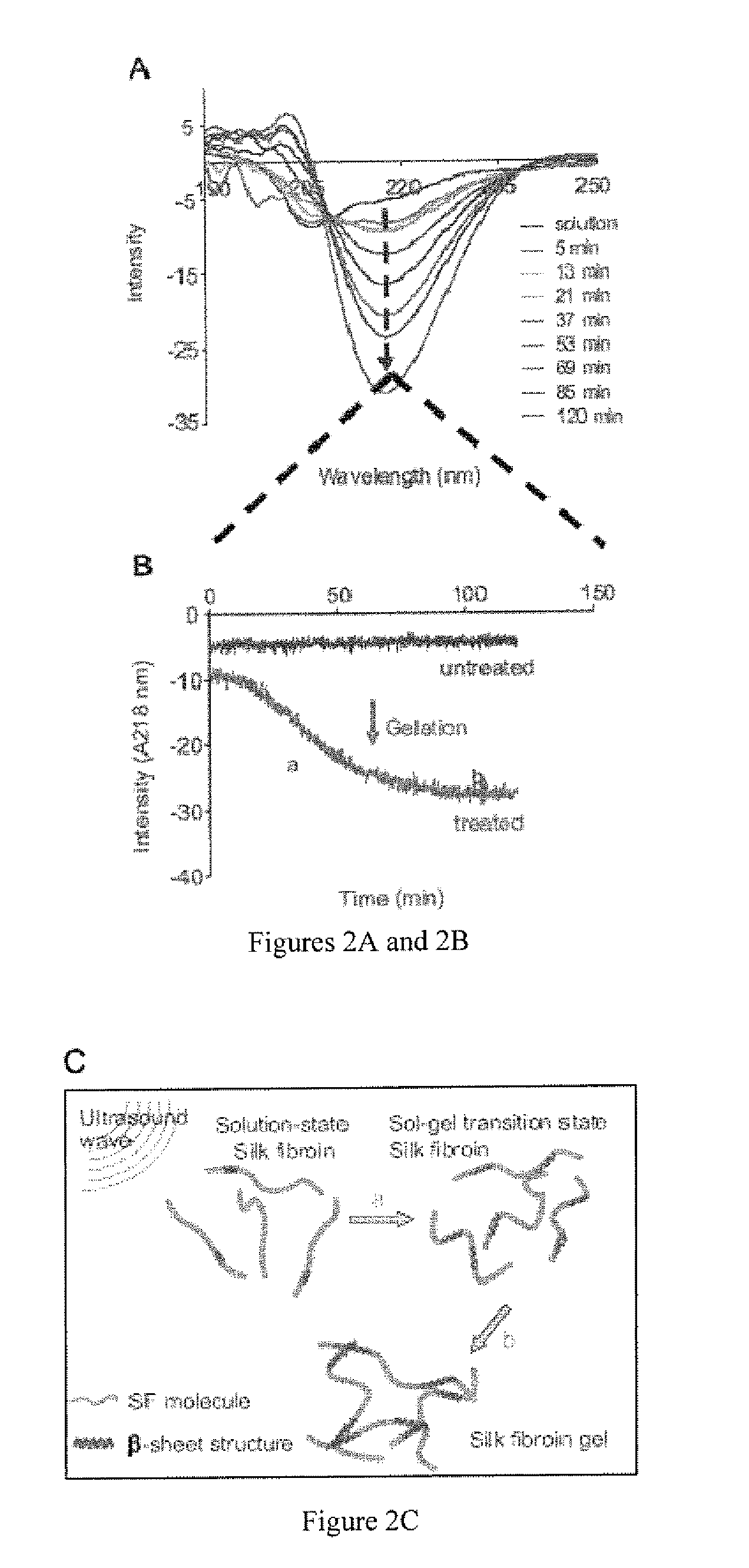
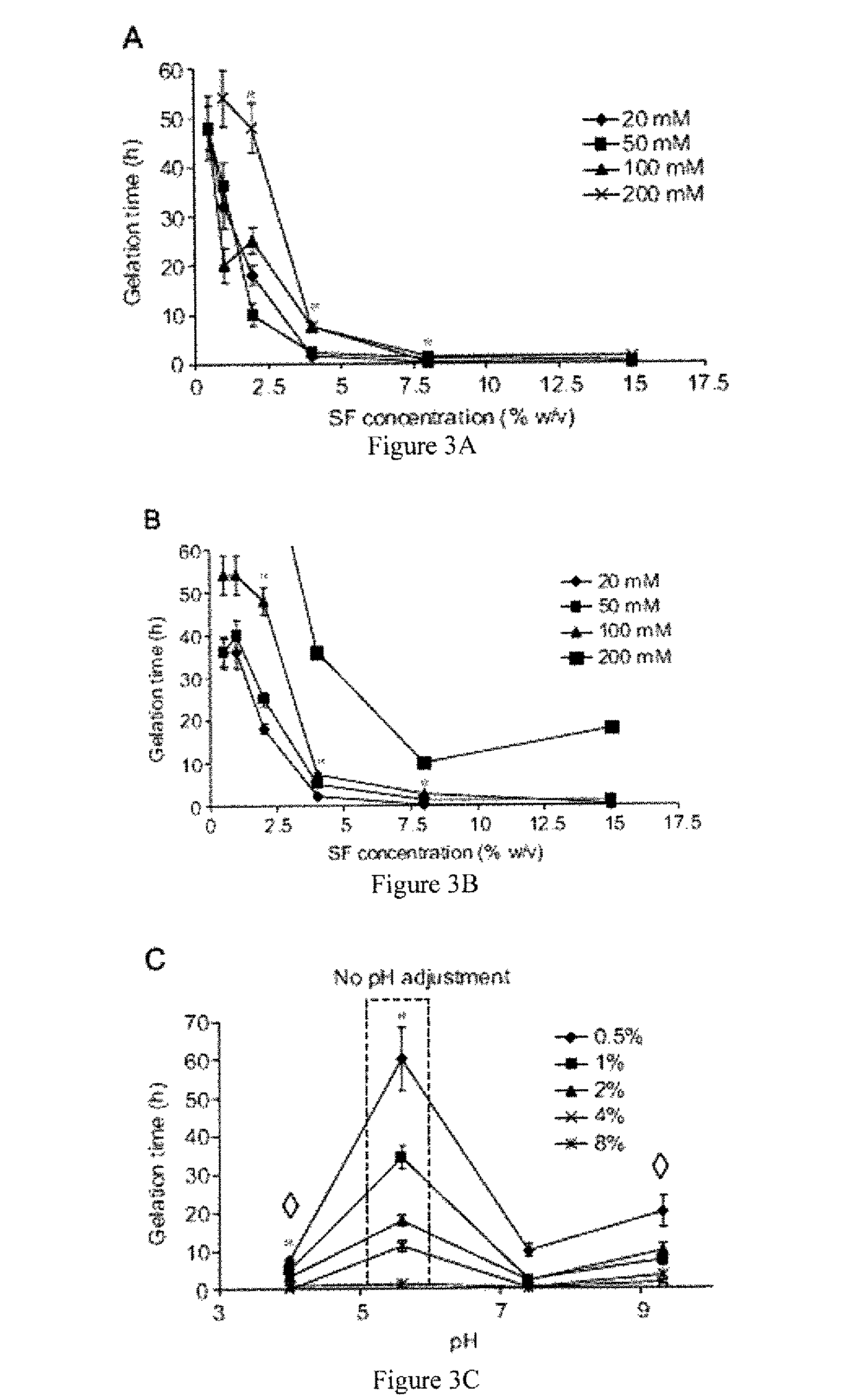
Method for silk fibroin gelation using sonication
ActiveUS20100178304A1Fast gelationCell from functioningBiocideOrganic active ingredientsDelivery vehicleViable cell
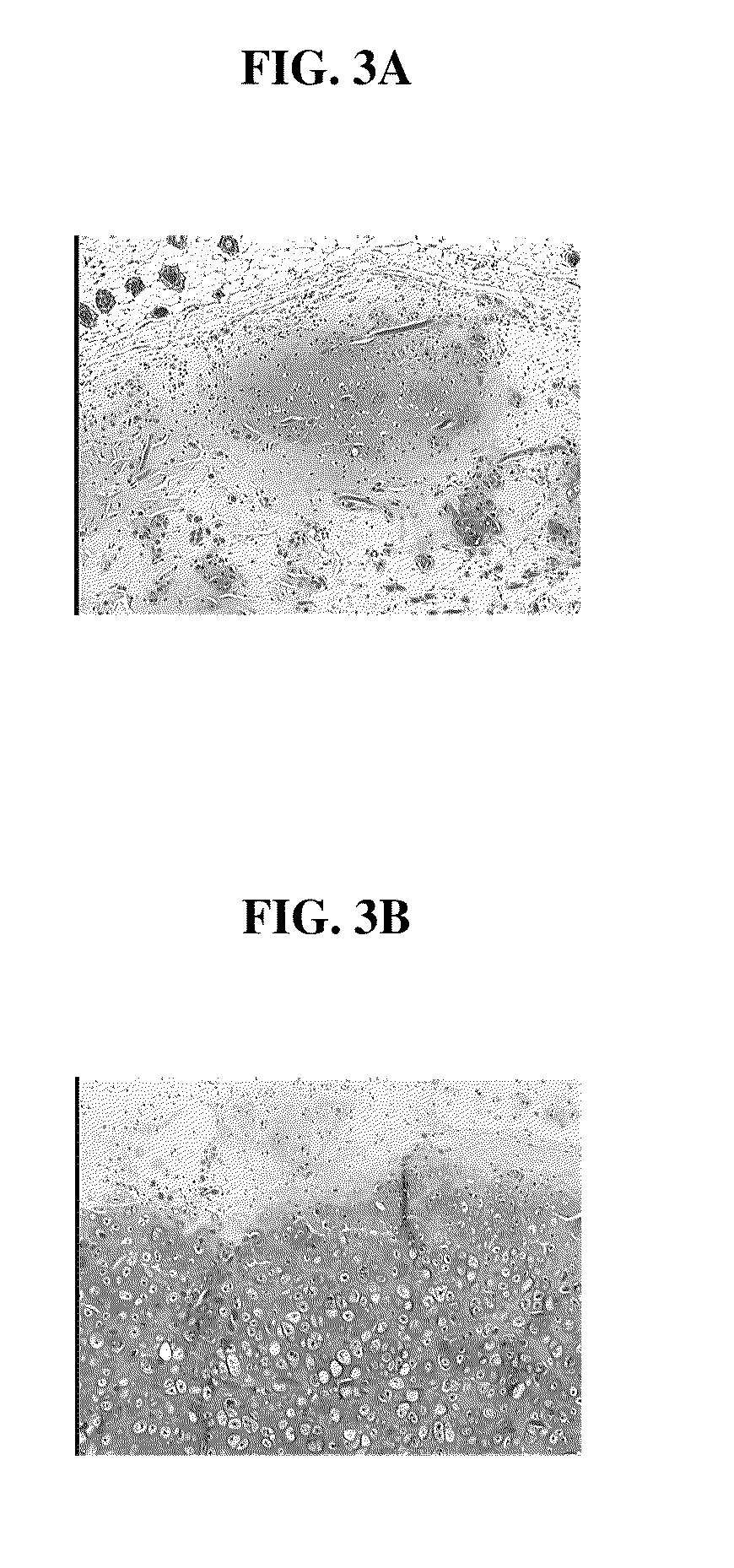
This invention provides for a process of rapidly forming silk fibroin gelation through ultrasonication. Under the appropriate conditions, gelation can be controlled to occur within two hours after the ultrasonication treatment. Biological materials, including viable cells, or therapeutic agents can be encapsulated in the hydrogels formed from the process and be used as delivery vehicles.
Owner:TRUSTEES OF TUFTS COLLEGE TUFTS UNIV
Method for processing and preserving collagen-based tissues for transplantation
InactiveUS20060210960A1Minimize surface tension damageAugment selective preservationSsRNA viruses positive-senseViral antigen ingredientsOrganismBiology
A method for processing and preserving an acellular collagen-based tissue matrix for transplantation is disclosed. The method includes the steps of processing biological tissues with a stabilizing solution to reduce procurement damage, treatment with a processing solution to remove cells, treatment with a cryoprotectant solution followed by freezing, drying, storage and rehydration under conditions that preclude functionally significant damage and reconstitution with viable cells.
Owner:LIFECELL
Conformable tissue repair implant capable of injection delivery
ActiveUS20050113937A1Promote healingPromote new cell growthAntipyreticAnalgesicsTissue repairTissue defect
A conformable tissue implant is provided for use in repairing or augmenting a tissue defect or injury site. The tissue implant contains a tissue carrier matrix comprising a plurality of biocompatible, bioresorbable granules and at least one tissue fragment in association with the granules. The tissue fragment contains one or more viable cells that can migrate from the tissue and populate the tissue carrier matrix. Also provided is a method for injectably delivering the tissue implant.
Owner:DEPUY SYNTHES PROD INC
Ink-jet printing of tissues
A method of forming an array of viable cells is carried out by ink-jet printing a cellular composition containing said cells on a substrate. At least two different types of viable mammalian cells are printed on the substrate, the at least two different types of viable mammalian cells selected to together form a tissue. In some embodiments at least three or four different viable mammalian cells are printed on the substrate, the cells selected to together form a tissue. In some embodiments one of the viable mammalian cell types is a stem cell. In some embodiments the method further comprises printing at least one support compound on the substrate, the support compound selected to form a tissue together with said cells. In some embodiments the method further comprises printing at least one growth factor on the substrate, the growth factor selected to cause the cells to form a tissue.
Owner:WAKE FOREST UNIV HEALTH SCI INC
Cleaning and devitalization of cartilage
InactiveUS20080077251A1Improve recellularizationBone implantDead animal preservationCellular DebrisMedicine
The invention is further directed to producing a cleaned, disinfected, and devitalized cartilage graft by optionally cleaning and disinfecting the cartilage graft; treating the cartilage graft in a pretreatment solution; treating the cartilage graft in an extracting solution; washing the extracted cartilage graft with a rinsing solution; and subsequently soaking the devitalized cartilage graft in a storage solution. The devitalized cartilage graft is essentially free from metabolically viable and / or reproductively viable cells and the rinsing solution is hypotonic solution or isotonic solution. The present invention is further directed to a cleaned, disinfected, and devitalized cartilage graft and a process for cleaning, disinfecting, and devitalizing cartilage grafts. The invention also relates to a process for repairing a cartilage defect and implantation of a cartilage graft into a human or animal by crafting the cartilage matrix into individual grafts, disinfecting and cleaning the cartilage graft, applying a pretreatment solution to the cartilage graft, removing cellular debris using an extracting solution to produce a devitalized cartilage graft, implanting the cartilage graft into the cartilage defect with or without an insertion device, and sealing the implanted cartilage graft with recipient tissue. The devitalized cartilage graft is optionally recellularized in vitro, in vivo, or in situ with viable cells to render the tissue vital before or after the implantation. The devitalized cartilage graft is also optionally stored between the removing cellular debris and the recellularizing steps.
Owner:LIFENET HEALTH
Cell storage and delivery system
Cell storage and delivery systems and methods for storing and delivering viable cells to a mammal are disclosed. The cell storage and delivery systems include a biodegradable and / or bioabsorbable fibrous matrix physically associated with viable cells to contain and release the cells at a controlled rate. The biodegradable and / or bioabsorbable matrix can be formed by electrospinning fibers of biodegradable and / or bioabsorbable fiberizable material. The methods include methods for storing viable cells and for delivering viable cells to a mammal using the cell storage and delivery system.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Viable tissue repair implants and methods of use
InactiveUS7901461B2Enhance tissue regrowthImprove regenerative abilityBone implantSkeletal disorderTissue repairRepair tissue
Biocompatible tissue implants are provided for repairing a tissue injury or defect. The tissue implants comprise a biological tissue slice that serves as a source of viable cells capable of tissue regeneration and / or repair. The biological tissue slice can be harvested from healthy tissue to have a geometry that is suitable for implantation at the site of the injury or defect. The harvested tissue slice is dimensioned to allow the viable cells contained within the tissue slice to migrate out and proliferate and integrate with tissue surrounding the injury or defect site. Methods for repairing a tissue injury or defect using the tissue implants are also provided.
Owner:DEPUY SYNTHES PROD INC
Biocompatible scaffold for ligament or tendon repair
A biocompatible ligament repair implant or scaffold device is provided for use in repairing a variety of ligament tissue injuries. The repair procedures may be conducted with ligament repair implants that contain a biological component that assists in healing or tissue repair. The biocompatible ligament repair implants include a biocompatible scaffold and particles of viable tissue derived from ligament tissue or tendon tissue, such that the tissue and the scaffold become associated. The particles of living tissue contain one or more viable cells that can migrate from the tissue and populate the scaffold.
Owner:DEPUY SYNTHES PROD INC
Process and composition for the manufacture of a microbial-based product
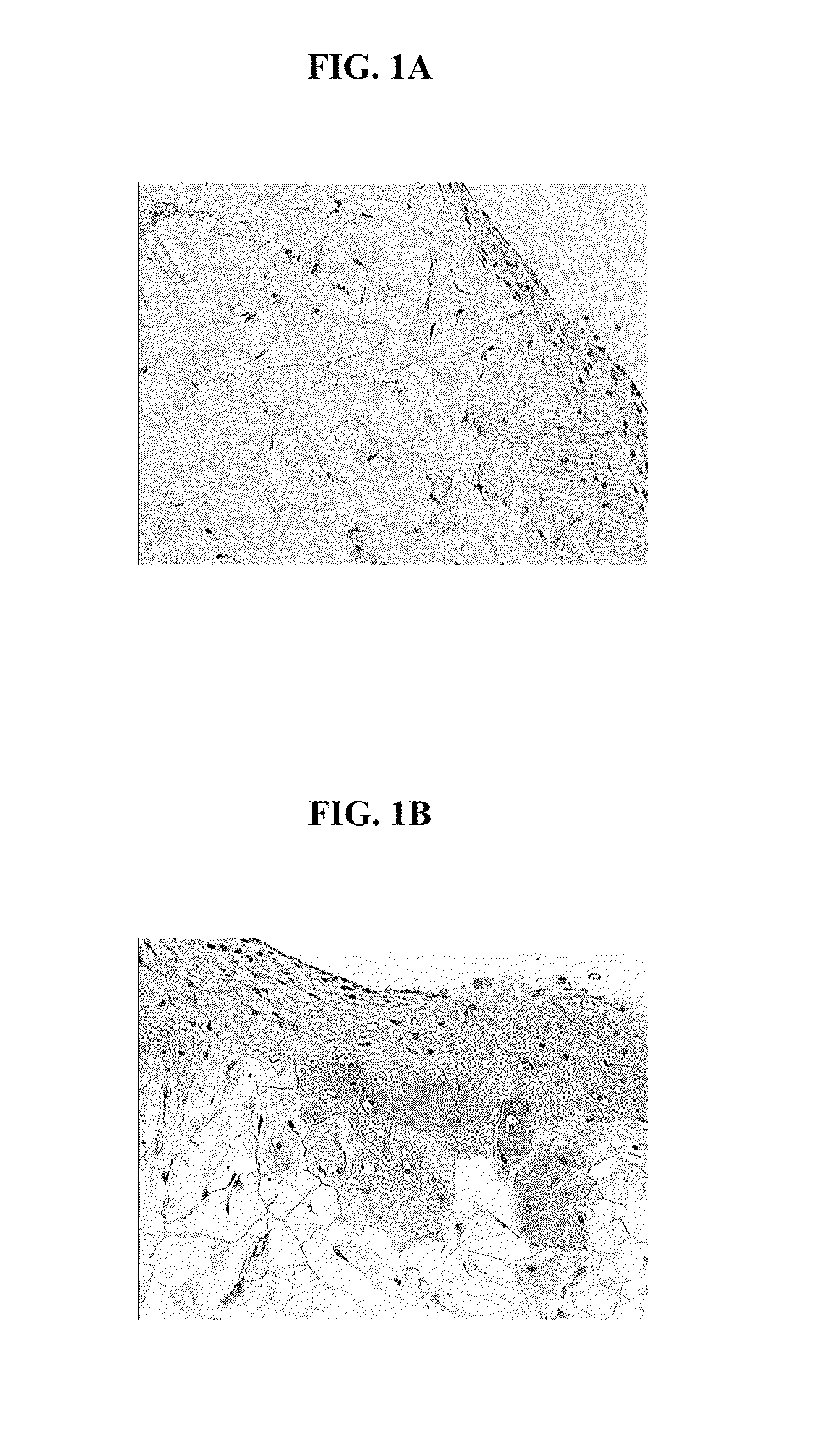
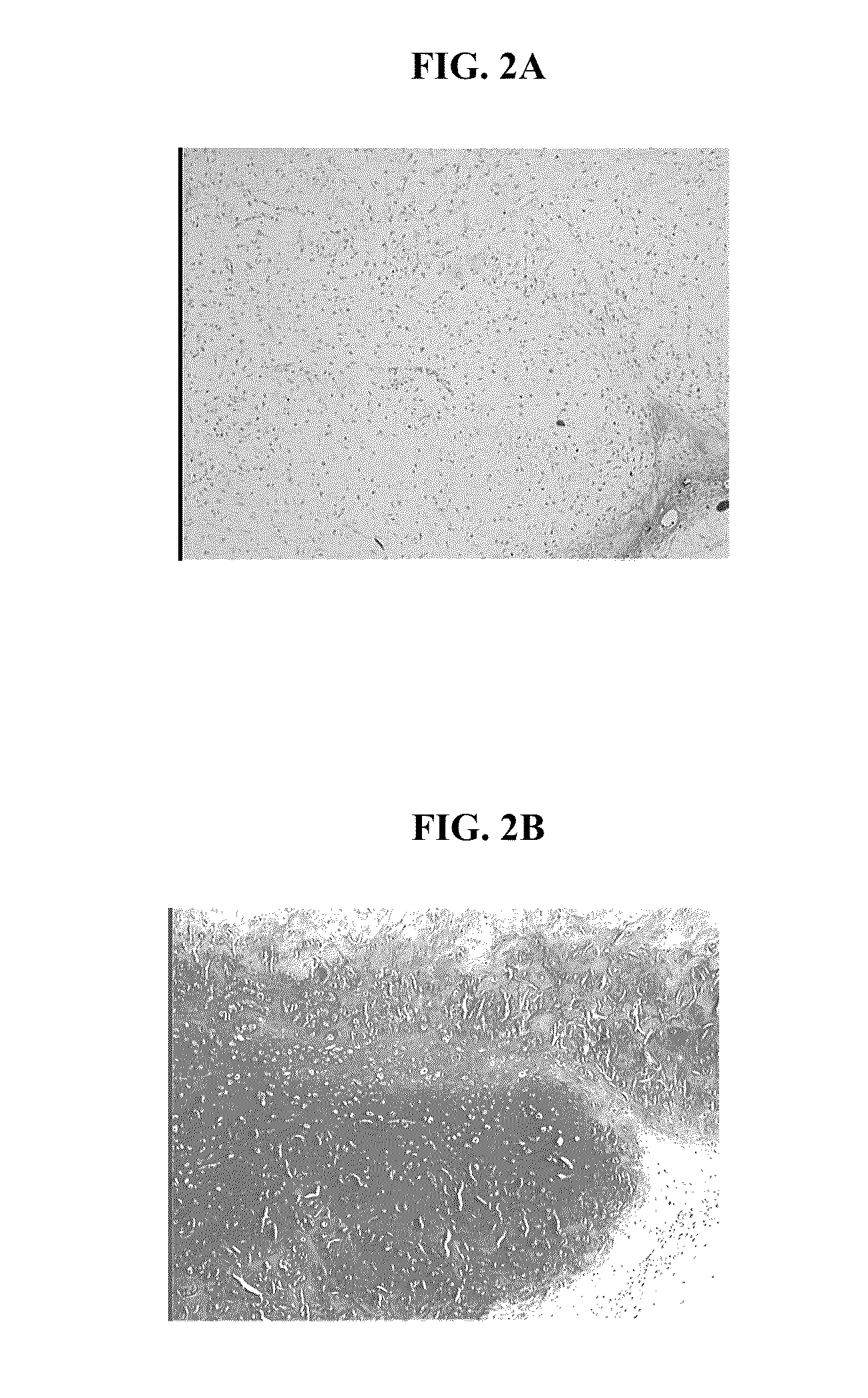
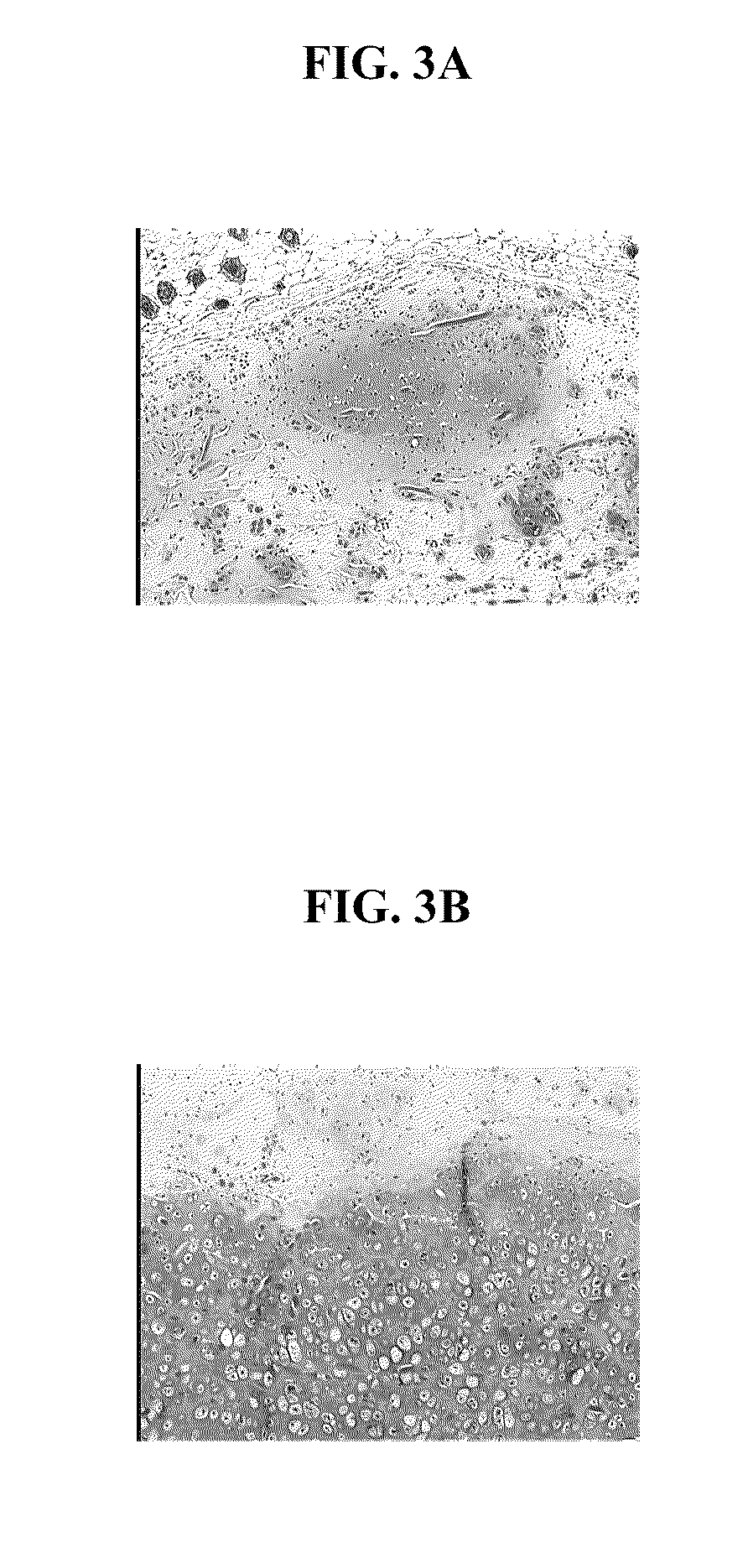
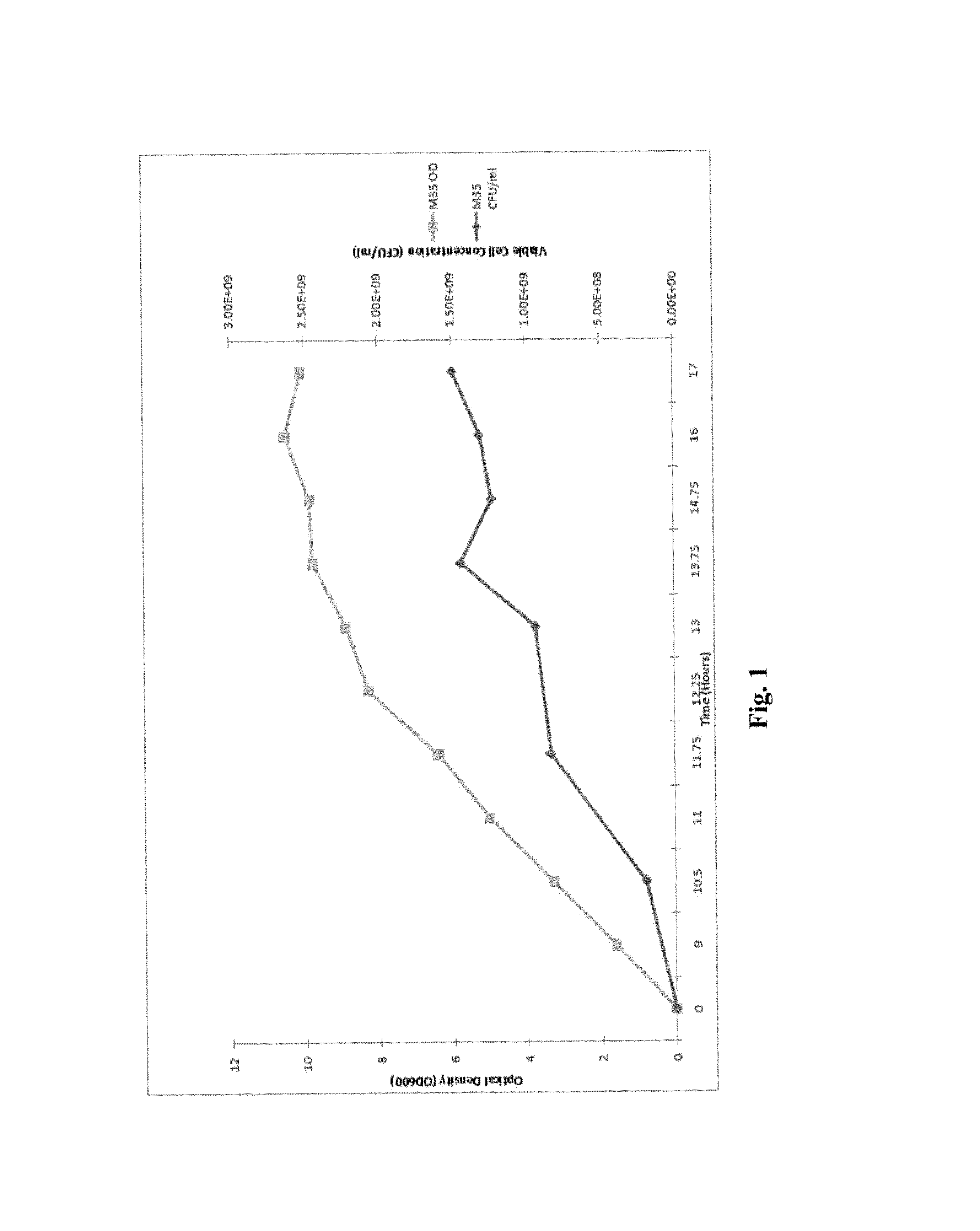
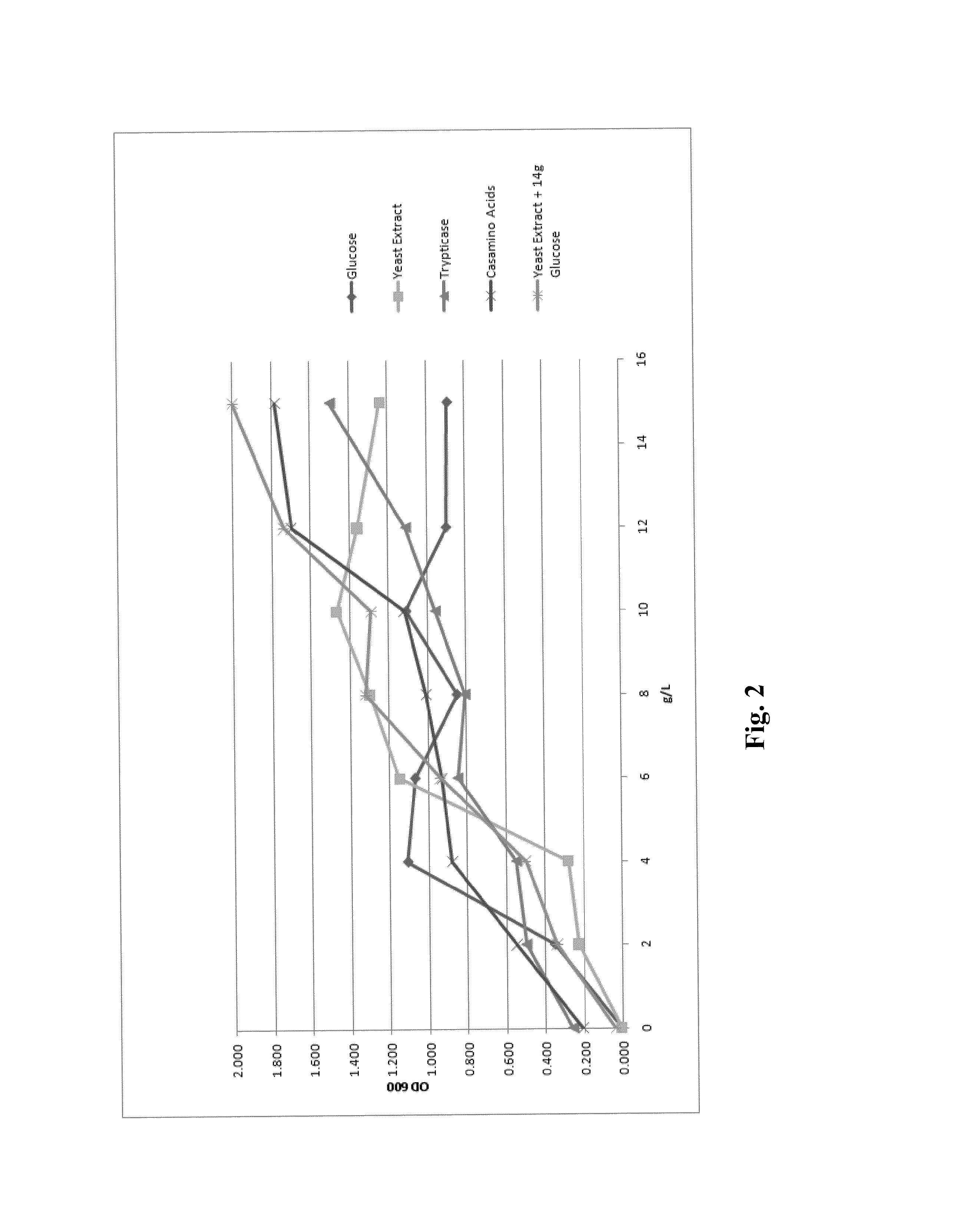
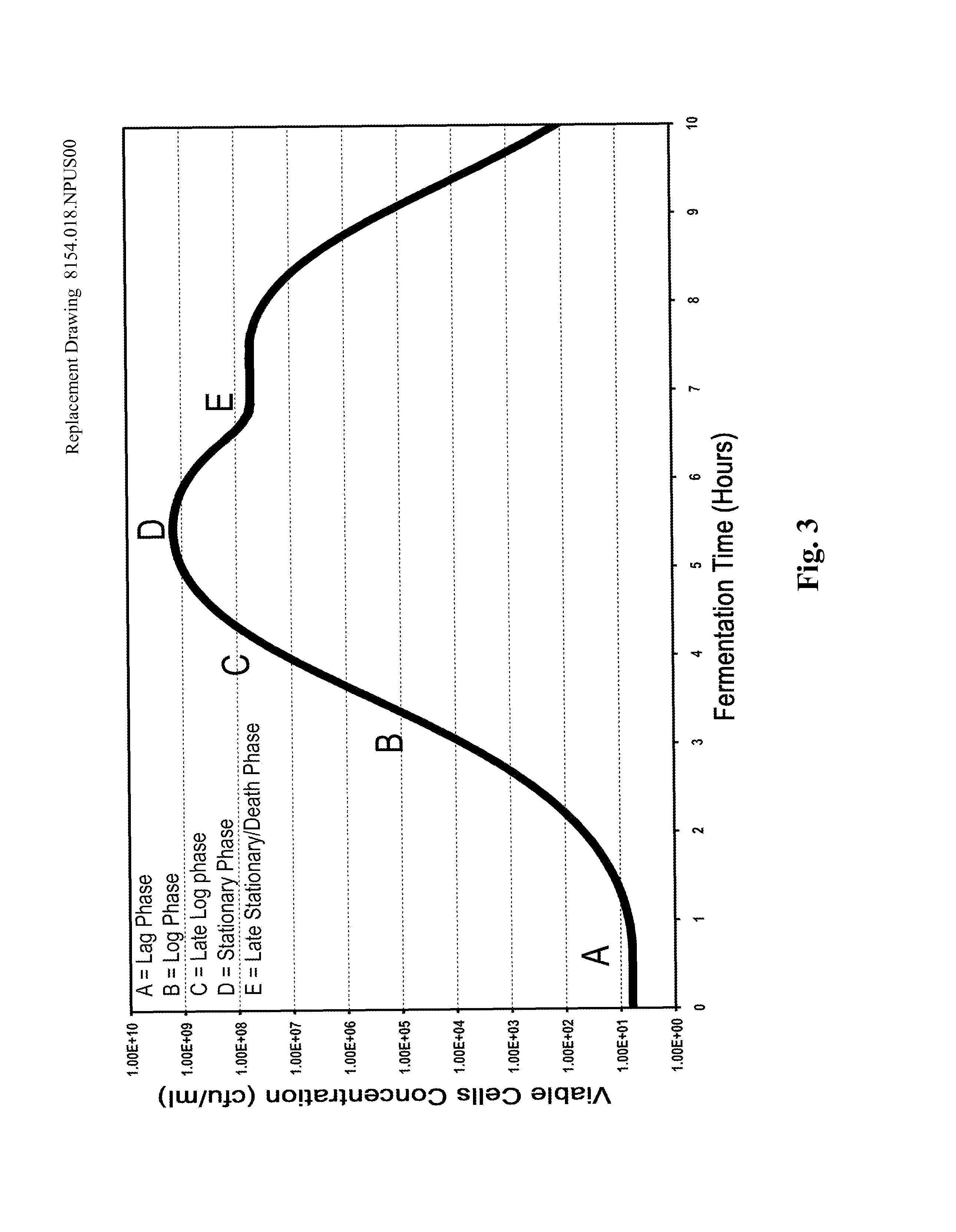
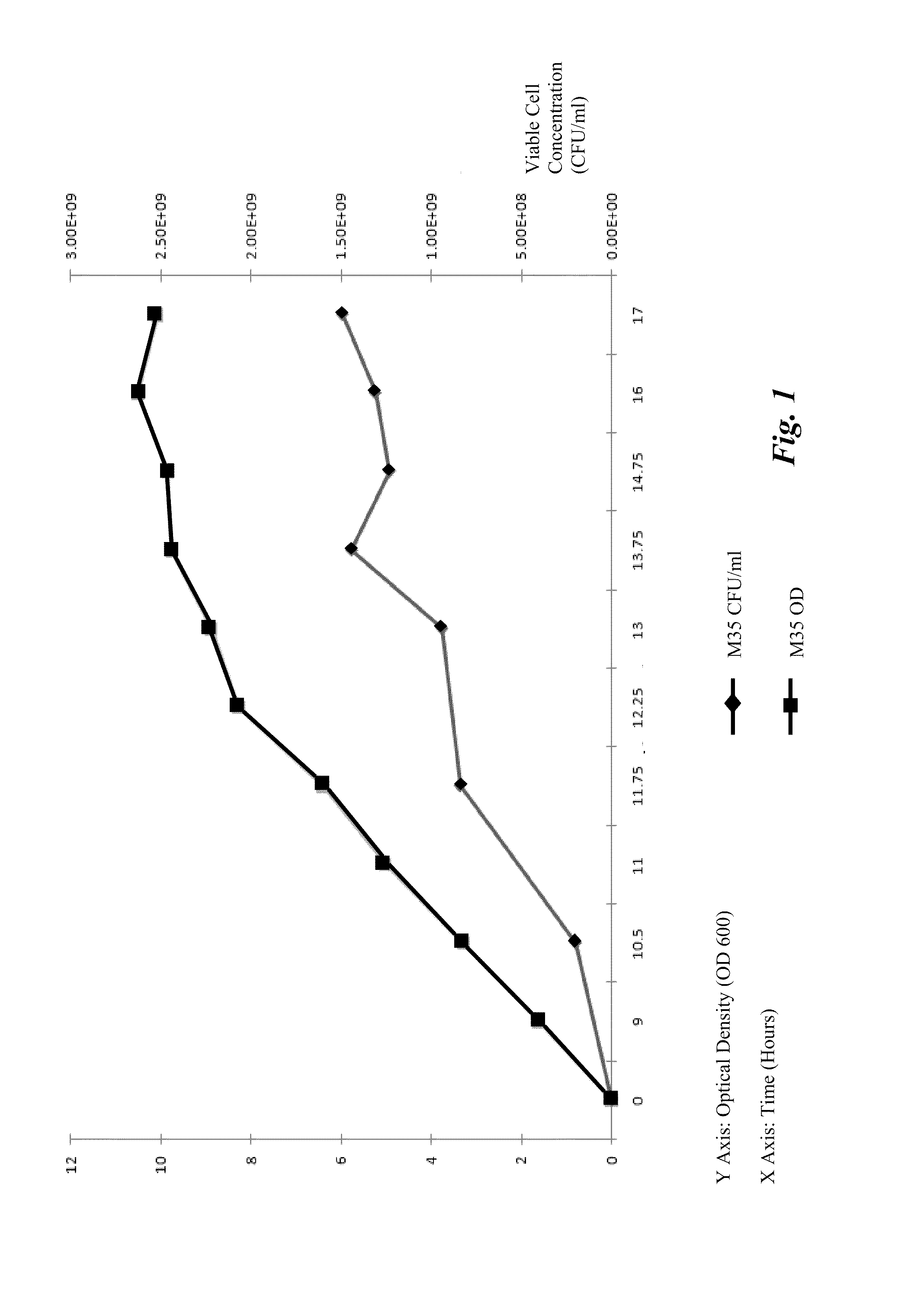
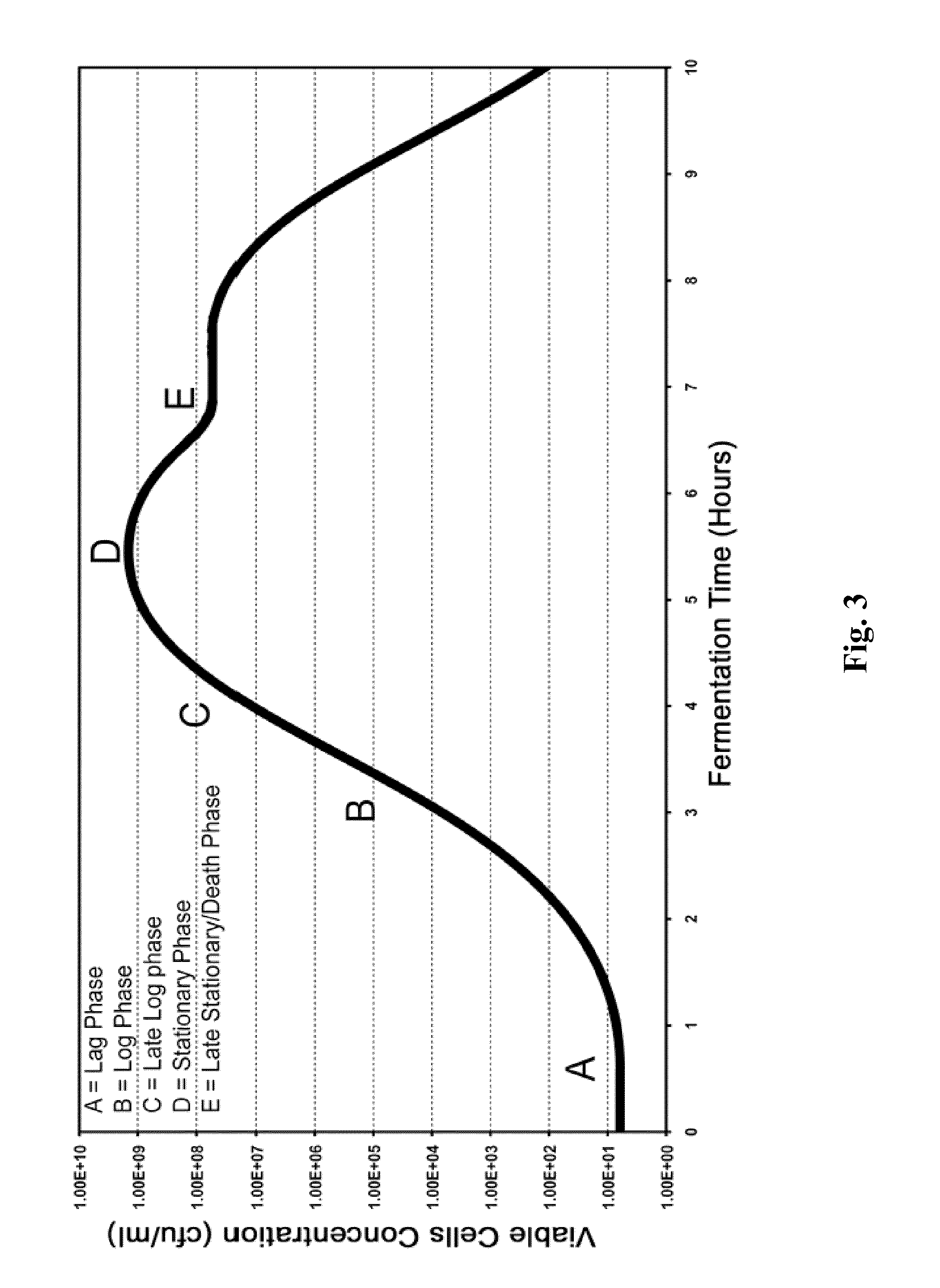
Processes to produce microorganisms that can be incorporated into a microbial-based product that results in high viable cell yields and shelf-stable products are disclosed. These microbial-based products are useful for inhibiting pathogenic growth and as a food additive. A preferred microorganism is the lactic acid producing bacteria, Lactobacillus amylovorus M35. In one embodiment, the process comprises inoculating a lactobacillus fermentation medium with M35 cells, harvesting the M35 cells at mid to late log phase, concentrating the M35 cells, and preserving the M35 cells at a concentration of at least 5×109 cfu / ml.
Owner:MICROBIOS
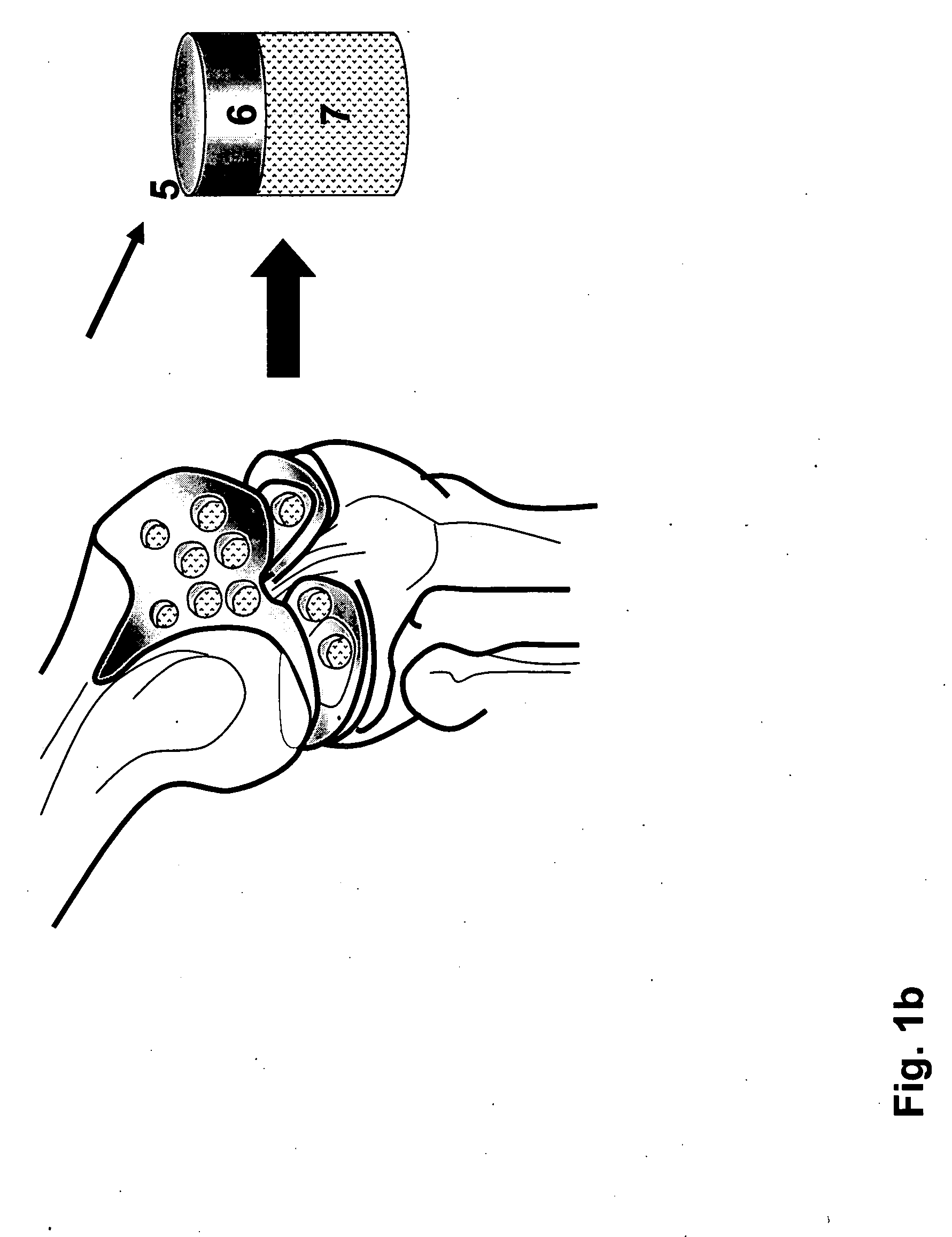
Biocompatible scaffolds with tissue fragments
A biocompatible tissue repair implant or scaffold device is provided for use in repairing a variety of tissue injuries, particularly injuries to cartilage, ligaments, tendons, and nerves. The repair procedures may be conducted with implants that contain a biological component that assists in healing or tissue repair. The biocompatible tissue repair implants include a biocompatible scaffold and particles of living tissue, such that the tissue and the scaffold become associated. The particles of living tissue contain one or more viable cells that can migrate from the tissue and populate the scaffold.
Owner:DEPUY SYNTHES PROD INC
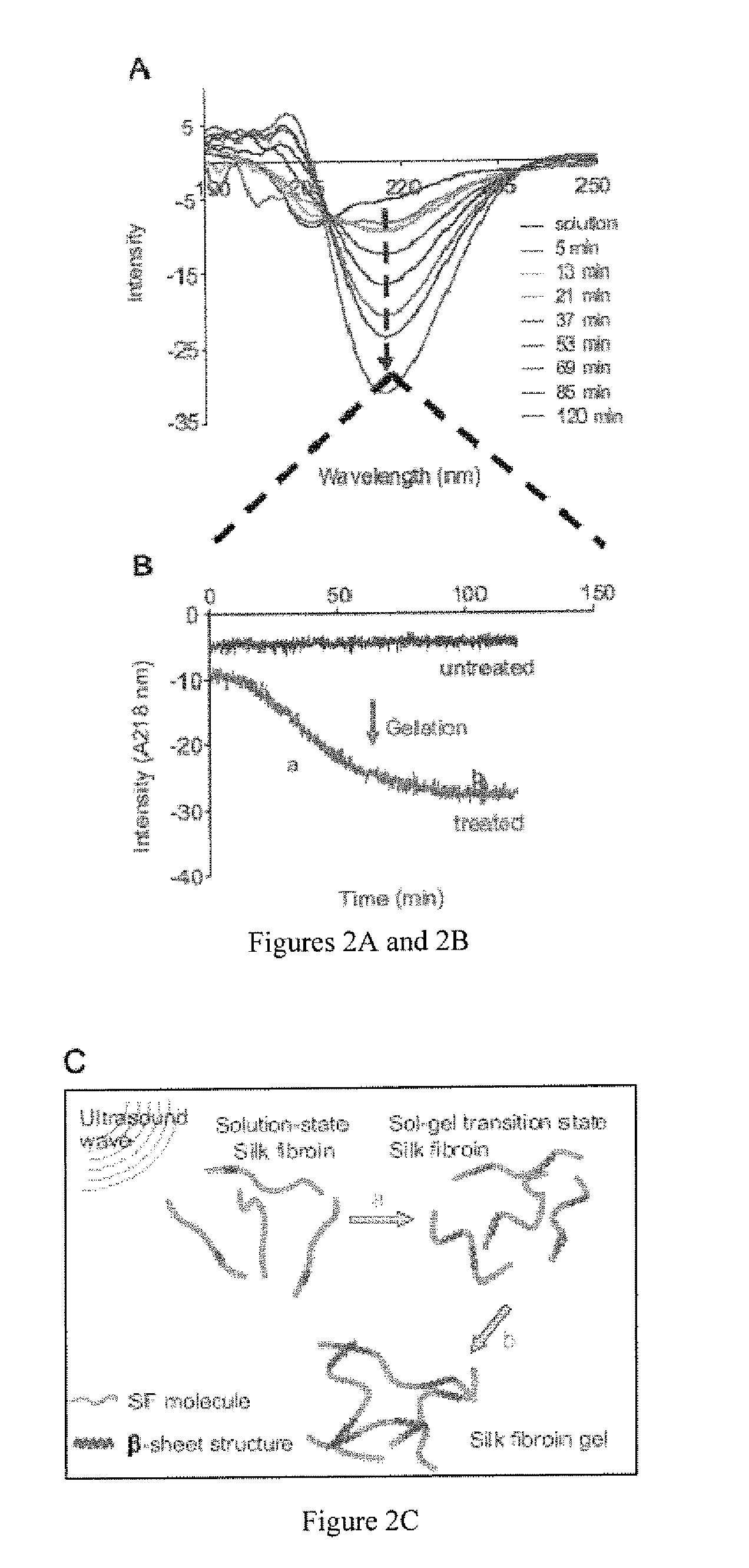
Vortex-induced silk fibroin gelation for encapsulation and delivery
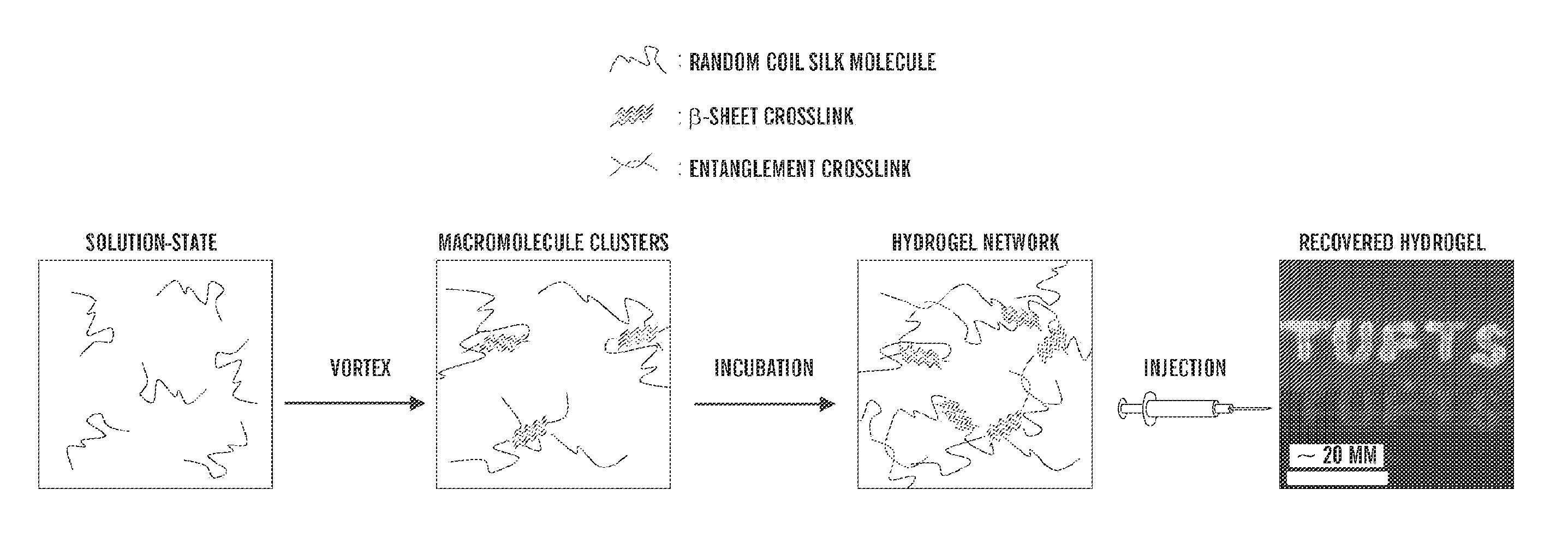
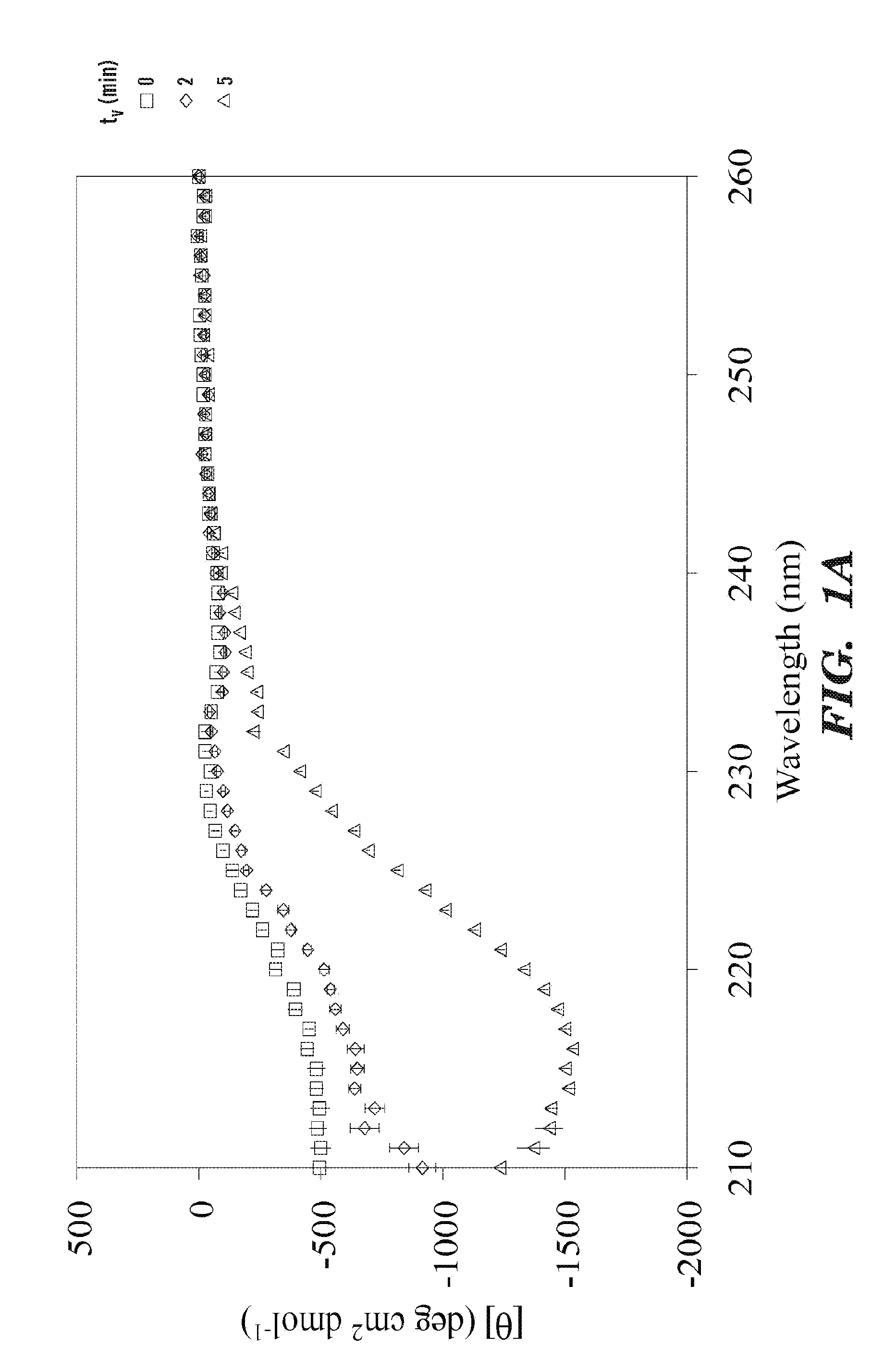
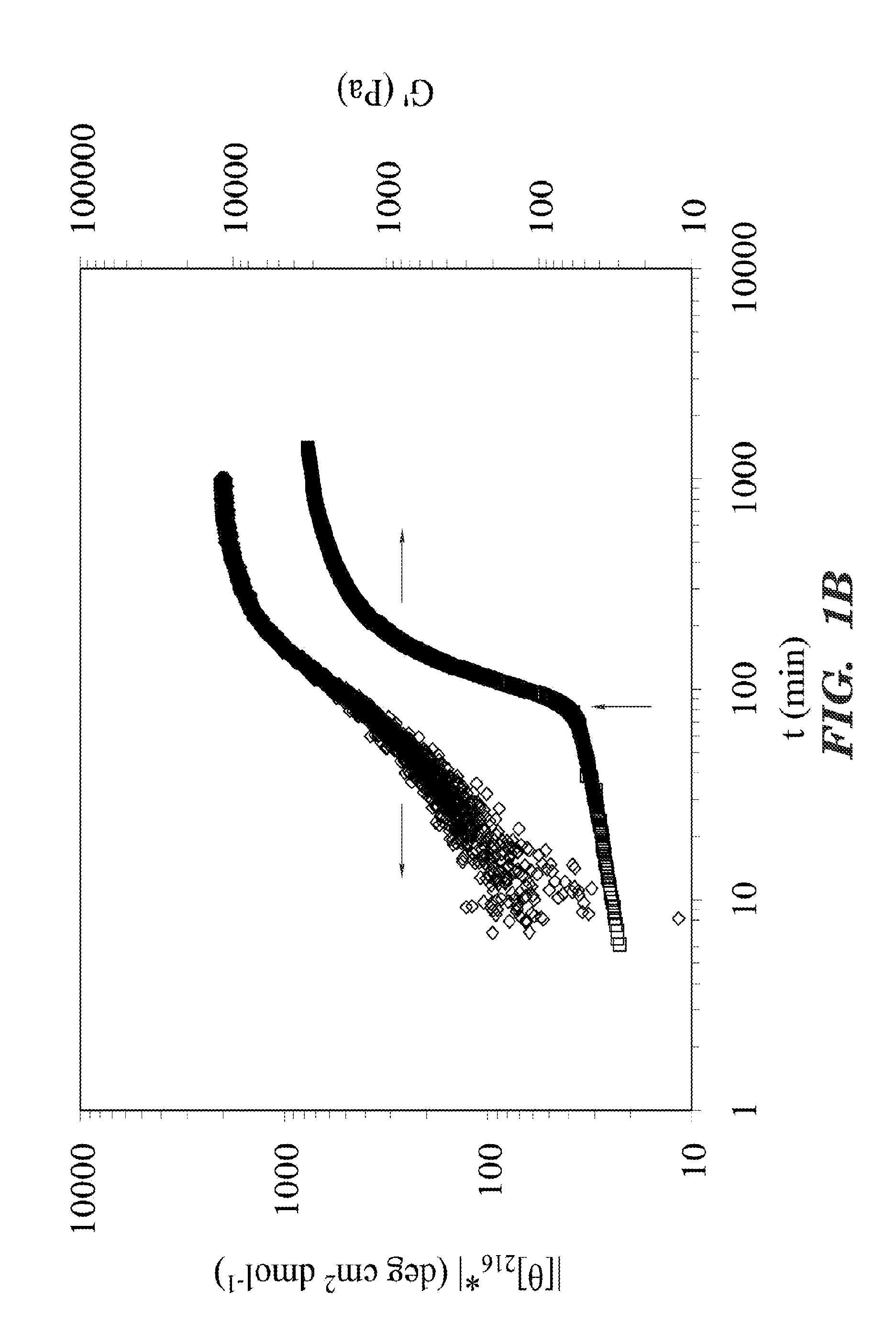
The present invention provided for a novel process of forming silk fibroin gels, and controlling the rate of β-sheet formation and resulting hydrogelation kinetics, by vortex treatment of silk fibroin solution. In addition, the vortex treatment of the present invention provides a silk fibroin gel that may be reversibly shear-thinned, enabling the use of these approach for precise control of silk self-assembly, both spatially and temporally. Active agents, including biological materials, viable cells or therapeutic agents, can be encapsulated in the hydrogels formed from the processes, and be used as delivery vehicles. Hence, the present invention provide for methods for silk fibroin gelation that are useful for biotechnological applications such as encapsulation and delivery of active agents, cells, and bioactive molecules.
Owner:TRUSTEES OF TUFTS COLLEGE TUFTS UNIV
Virtual flow cytometry on immunostained tissue-tissue cytometer
InactiveUS20070020697A1Bioreactor/fermenter combinationsBiological substance pretreatmentsStainingCancers diagnosis
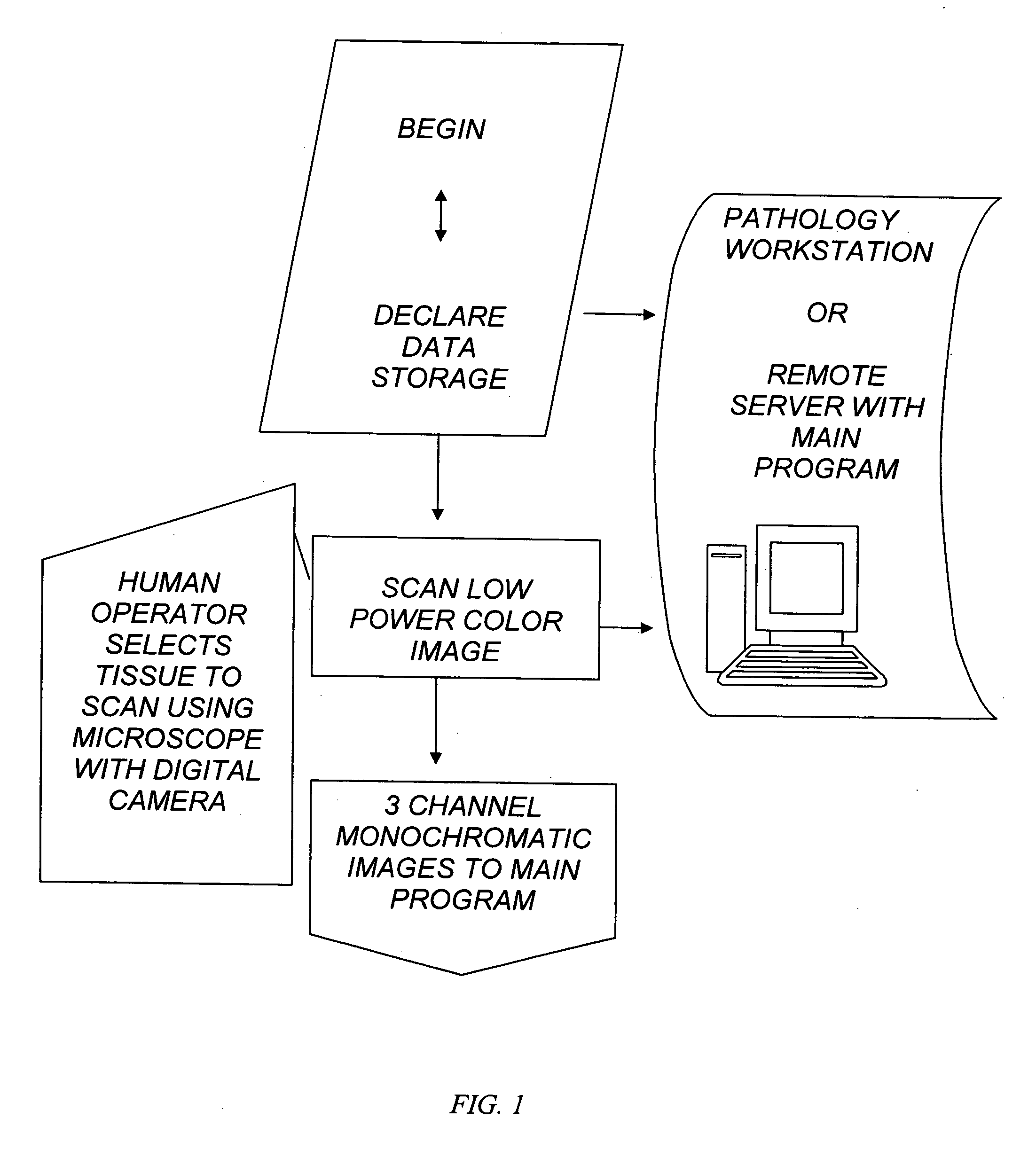
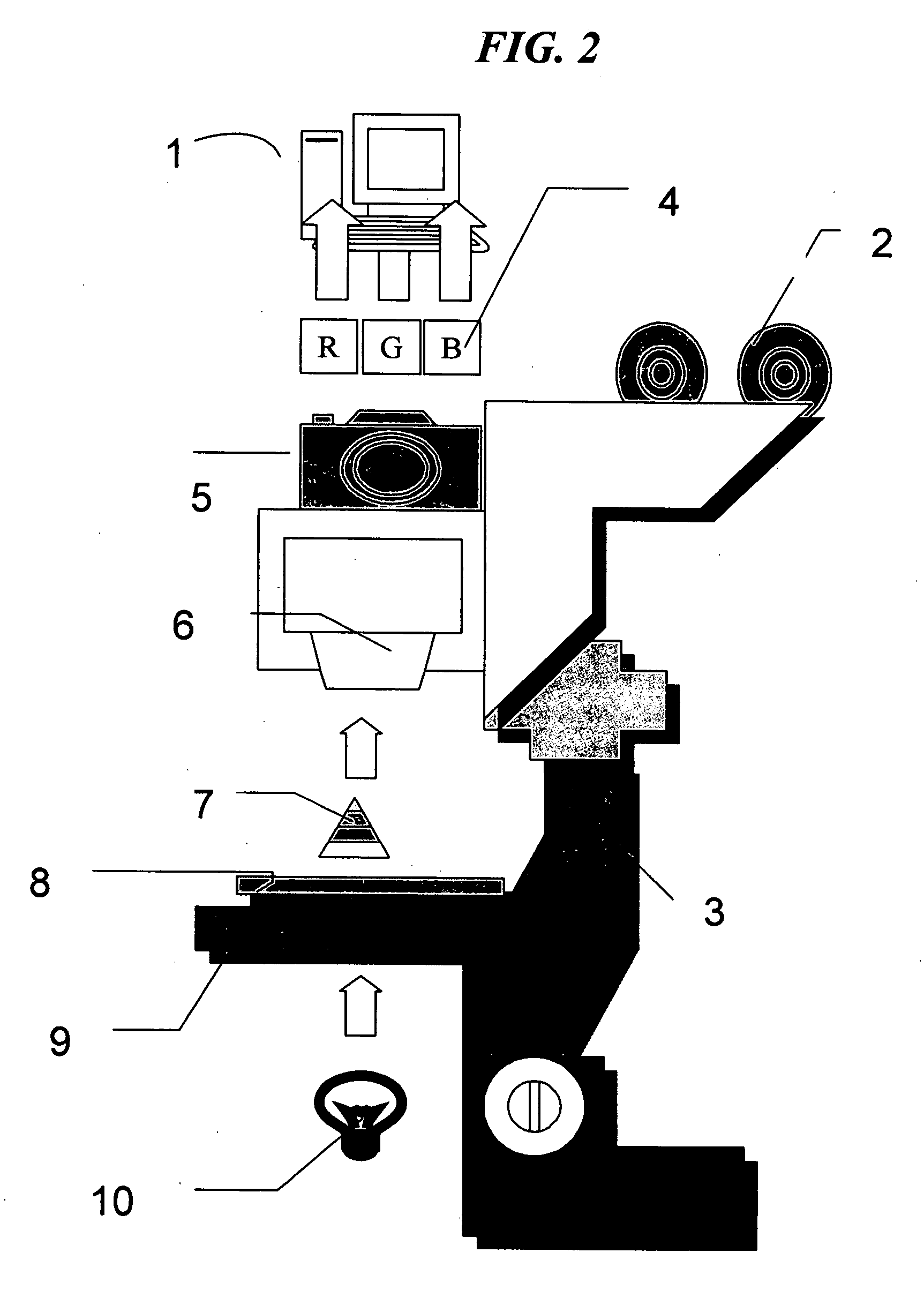
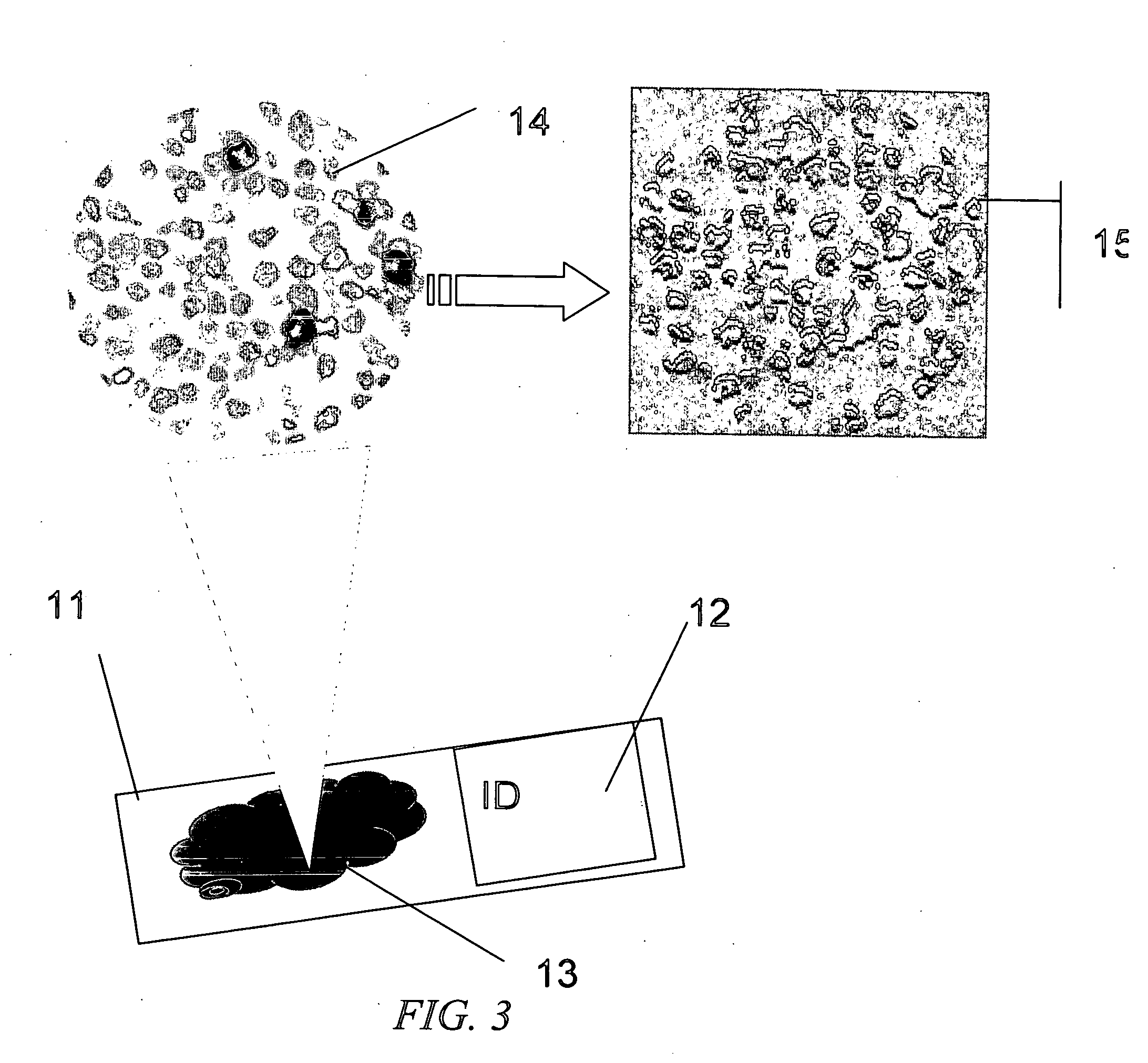
The invention provides an automated method of single cell image analysis which determines cell population statistic, applicable in the field of pathology, disease or cancer diagnosis, in a greatly improved manner over manual or prior art scoring techniques. By combining the scientific advantages of computerized automation and the invented method, as well as the greatly increased speed with which population can be evaluated, the invention is a major improvement over methods currently available. The single cells are identified and displayed in an easy to read format on the computer monitor, printer output or other display means, with cell parameter such as cell size and staining distribution at a glance. These output data is an objective transformation of the subjective visible image that the pathologist or scientist relies upon for diagnosis, prognosis, or monitoring therapeutic perturbations. Using our novel proposed technology, we combine the advantages provided by the clinical standard tool of flow cytometry in quantifying single cells and also retain the advantages of microscopy in retaining the capability of visualizing the immunoreactive cells. Unlike flow cytometry however, the invention uses commonly available formalin fixed immunostained tissue and not fresh viable cells. To accomplish this aim, we resort to new and improved advanced image analysis using a unique, useful, and adaptive process as described herein. The method uses multi-stage thresholding and segmentation algorithm based on multiple color channels in RGB and HS I spaces and uses auto-thresholding on red and blue channels in RGB to get the raw working image of all cells, then refines the working image with thresholding on hue and intensity channels in HS I using an adaptive parameter epsilon in entropy mode, and further separates different groups of cells within the same class, by auto-thresholding within the working image region. The Immunohistochemistry Flow cytometry (IHCFLOW) combination results in a new paradigm that is both useful, novel, and provides objective tangible result from a complex color image of tissue.
Owner:CUALING HERNANI D
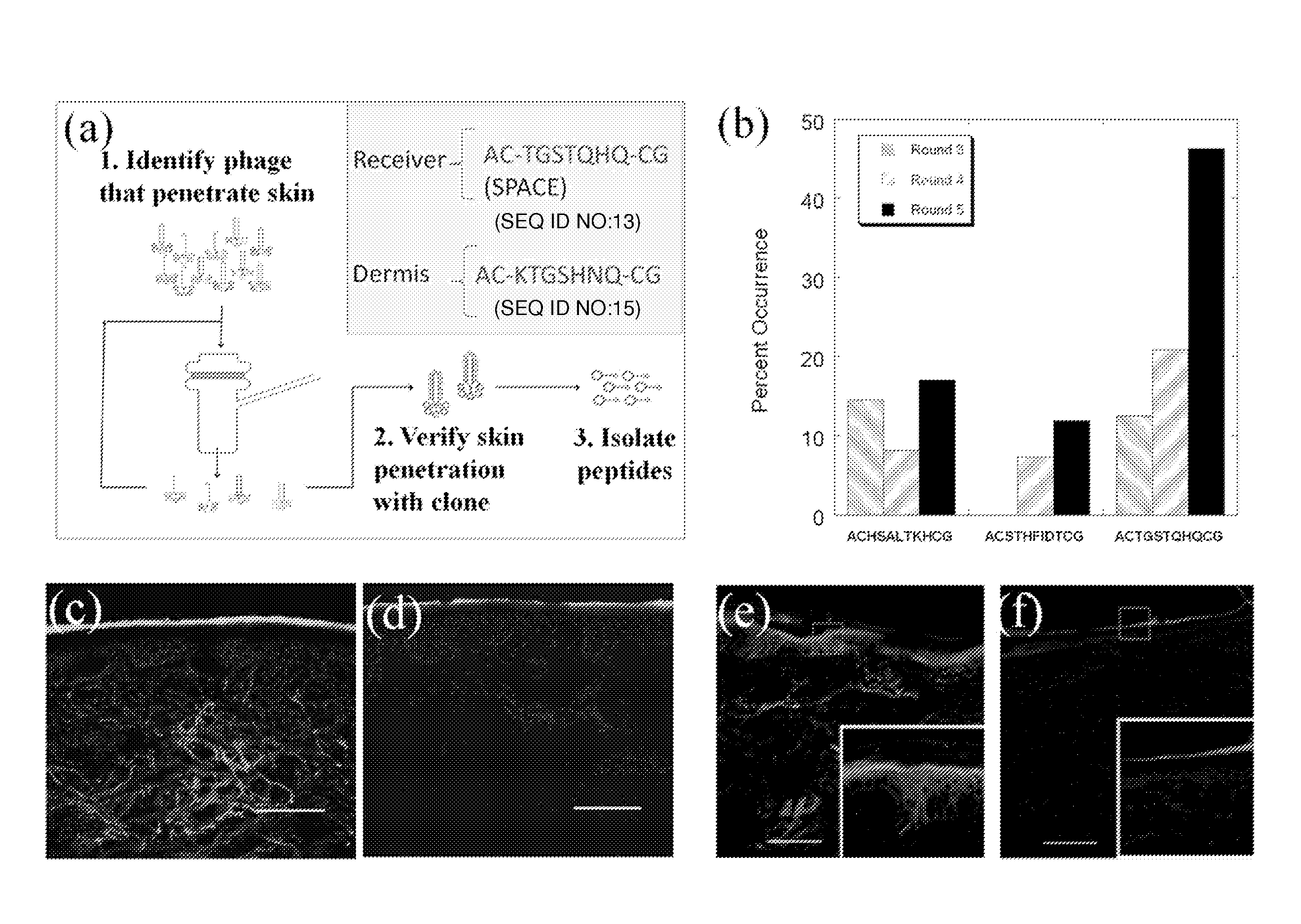
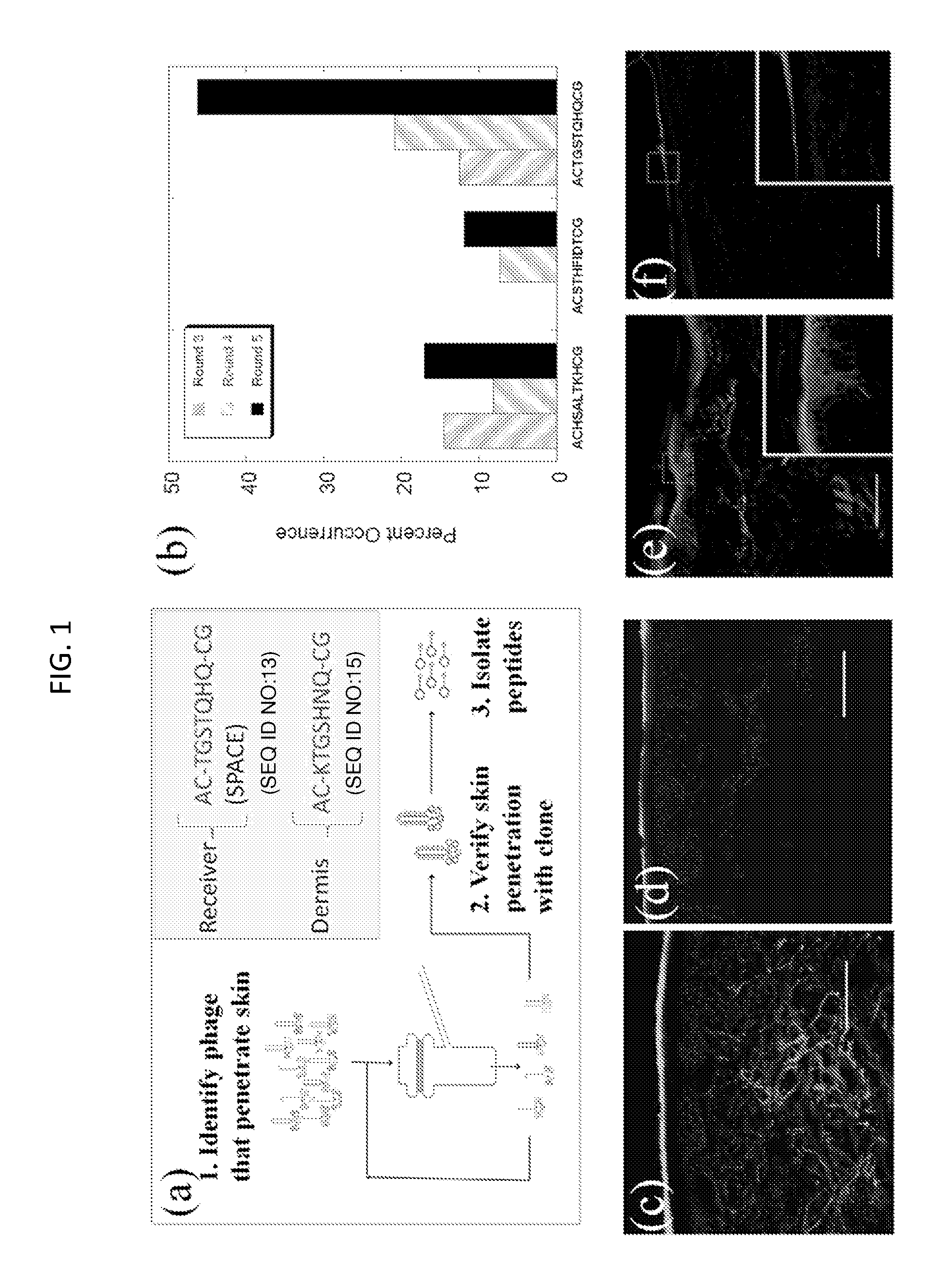
Skin permeating and cell entering (SPACE) peptides and methods of use thereof
The present disclosure provides peptides and peptide compositions, which facilitate the delivery of an active agent or an active agent carrier wherein the compositions are capable of penetrating the stratum corneum (SC) and / or the cellular membranes of viable cells.
Owner:RGT UNIV OF CALIFORNIA
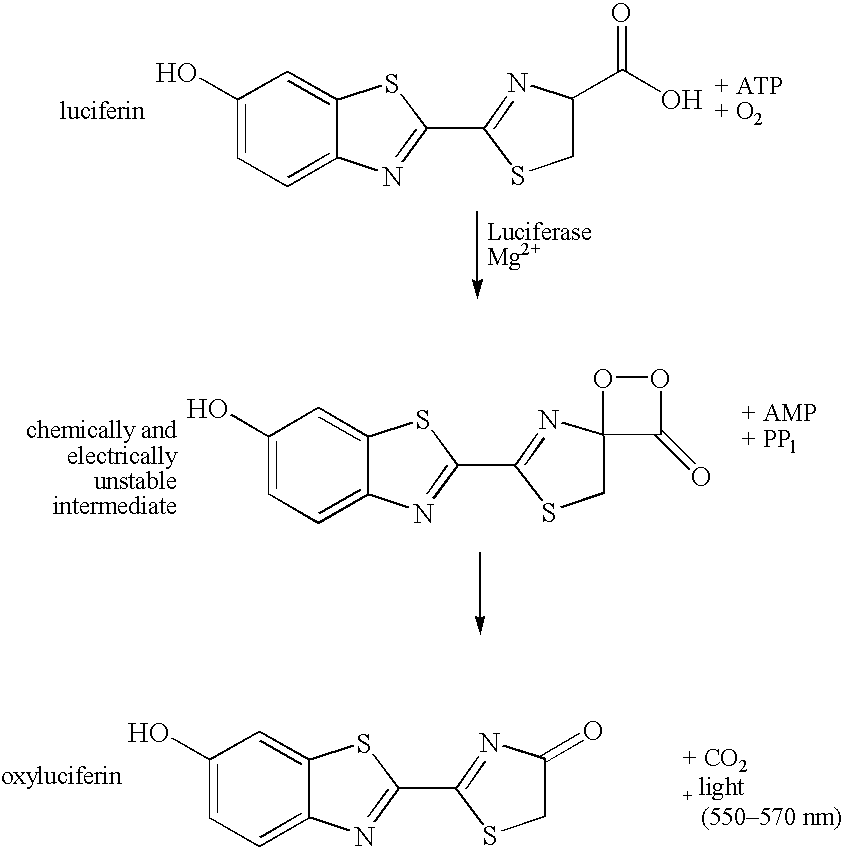
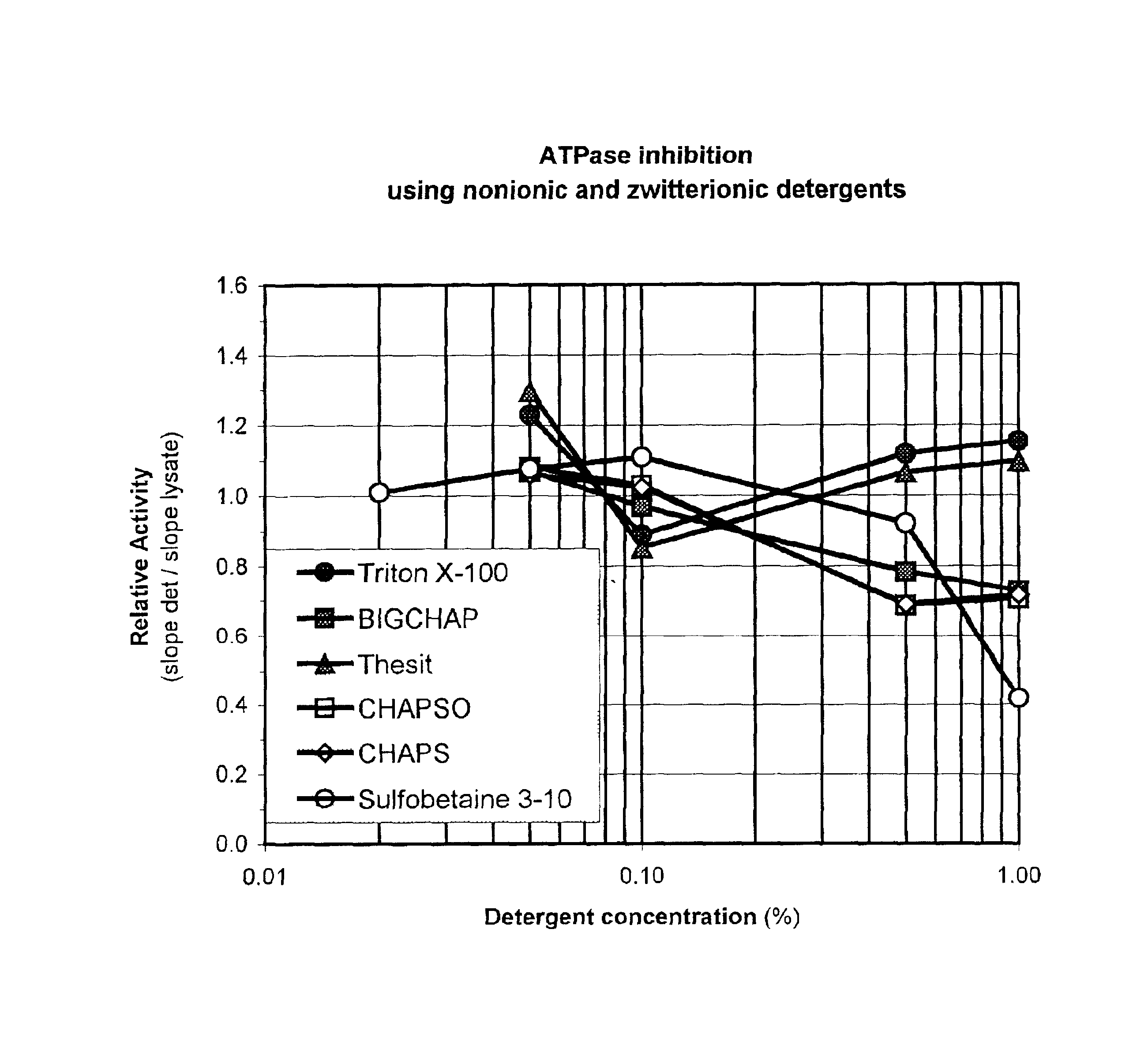
Method for detection of ATP
InactiveUS7083911B2Improve stabilityFacilitate many ATP detectionMicrobiological testing/measurementOxidoreductasesATPaseViable cell
Owner:PROMEGA
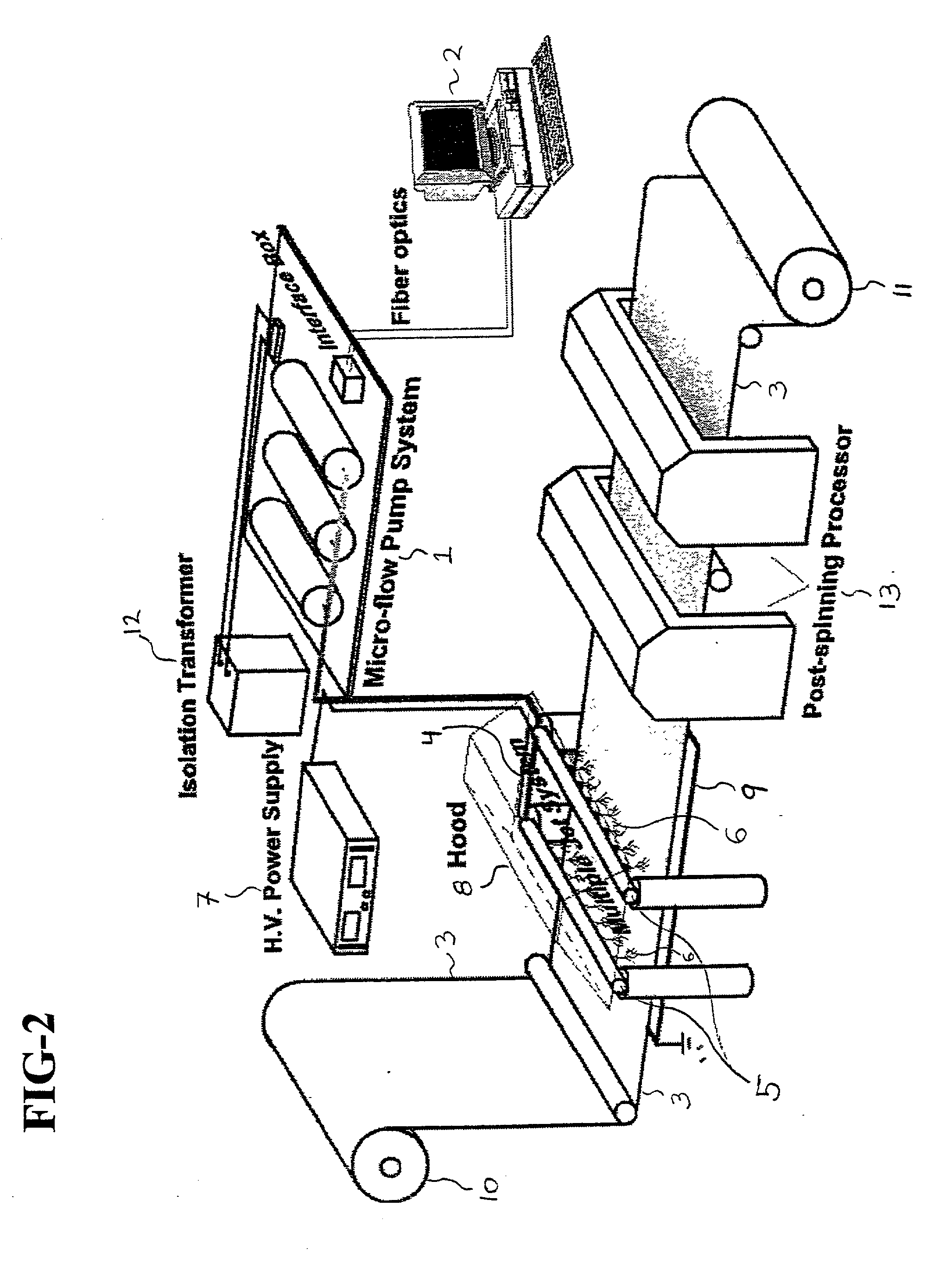
Nano bionic wound-surface cover and preparation method thereof
ActiveCN101507835AAddress barriers to developmentAvoid inconvenienceProsthesisElectrospinningEngineering
The invention provides a nanometer bionic wound-surface cover and a preparation method thereof. The nanometer bionic wound-surface cover comprises a nanometer bionic bracket and hydrosol attached to the bracket, wherein the hydrosol covers one or a plurality of cytokines. The preparation method for the nanometer bionic wound-surface cover provided by the invention comprises the steps of preparingan electrostatic-spinning solution, a cytokine-containing hydrosol solution and a crosslinker solution, preparing the nanometer bionic bracket by use of electrostatic spinning, using an ink-jet printer to print the cytokine-containing hydrosol solution onto the nanometer bionic bracket, and the like, wherein electrostatic spinning and printing can be repeated so as to form the wound-surface covers different in thickness. The preparation method adopts an in-situ autologous stem-cell engineering technique and adopts stem-cell chemotactic factors to attract autologous stem cells to directionally migrate, enter a wound surface and be differentiated according to designed requirements, thereby avoiding inconvenience caused by using viable cells, achieving rehabilitation effects the same with orbetter than that of using the viable cells and having broad application prospects.
Owner:MEDPRIN REGENERATIVE MEDICAL TECH
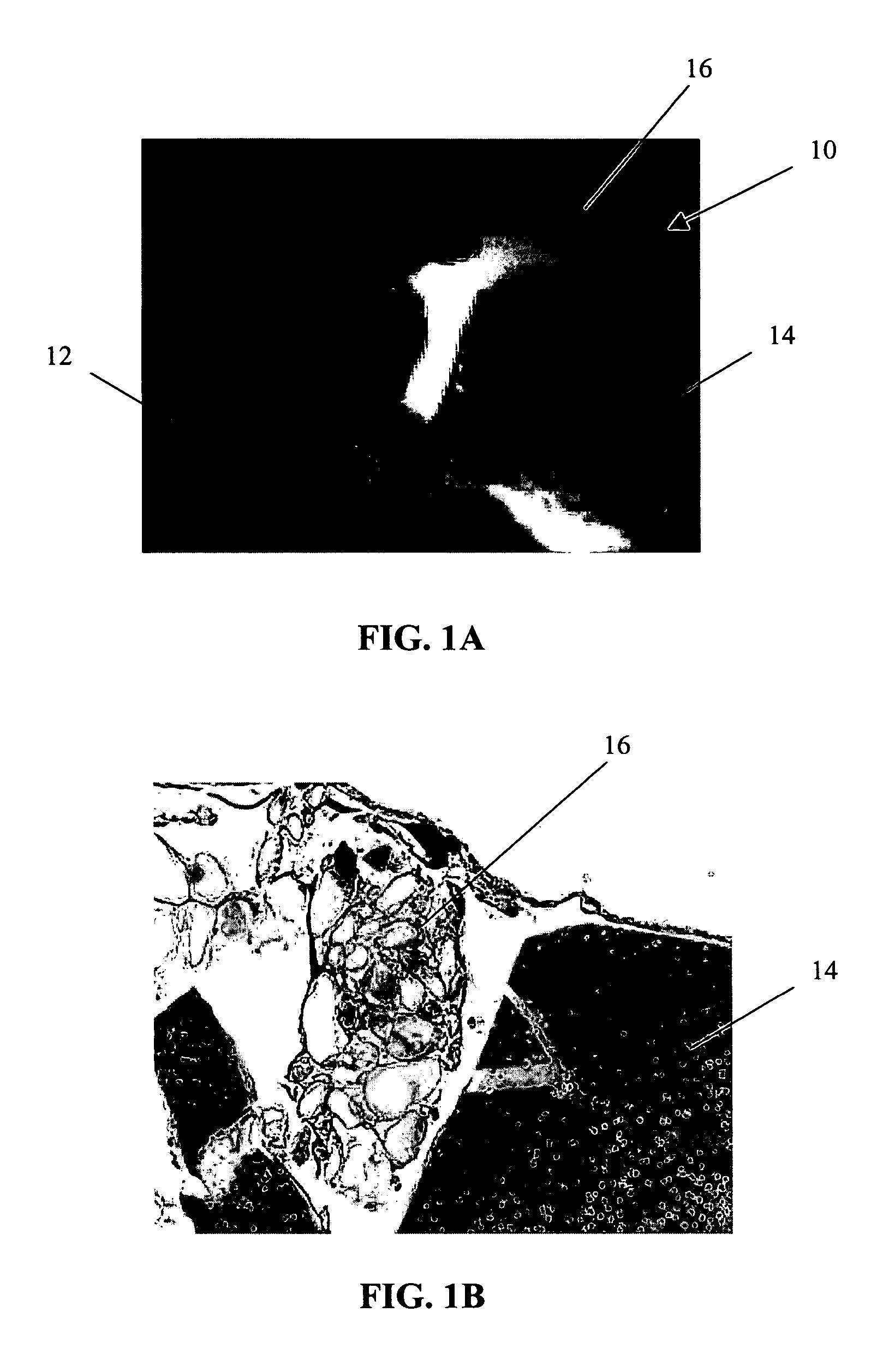
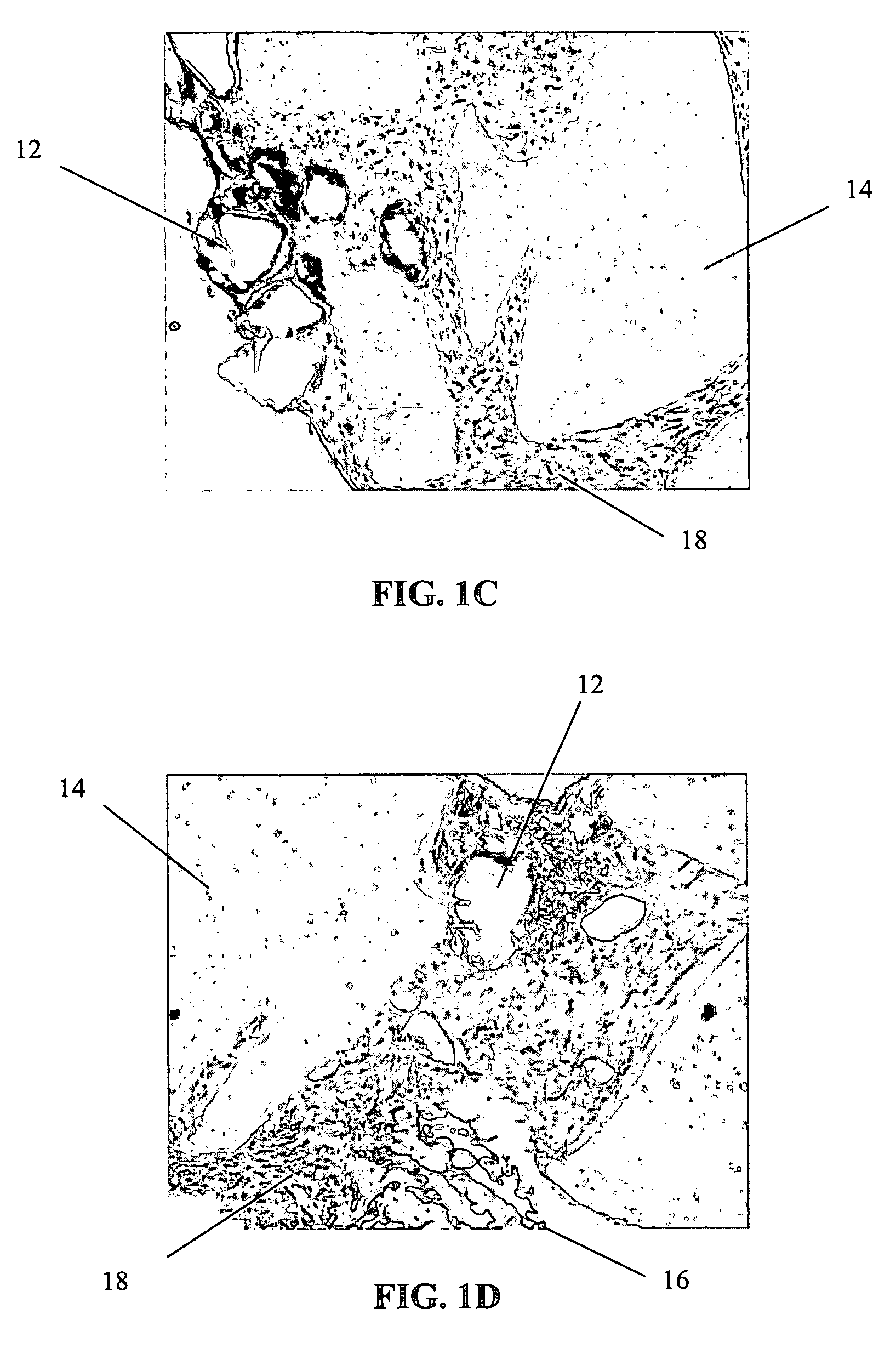
Method of obtaining viable small tissue particles and use for tissue repair
ActiveUS20080153157A1Easy to keepHigh retention ratePowder deliveryPeptide/protein ingredientsTissue repairTissue defect
The invention provides a composition including isolated small living tissue particles, a method of making the tissue particles, and a method of using the composition to ameliorate a tissue defect. The tissue particles are composed of cells and their associated extracellular molecules and are sized, in certain embodiments, to be smaller than about 1 mm. Another aspect of the inventive tissue particles is the large percentage of viable cells. In certain embodiments, the tissue particles are made from cartilage and the composition may also contain additives such as adhesives, solutions, and bioactive agents.
Owner:ZIMMER ORTHOBIOLOGICS
Crafting of cartilage
The invention is directed to producing a shaped cartilage matrix isolated from a human or animal where the cartilage has been crafted to facilitate disinfection, cleaning, devitalization, recellularization, and / or integration after implantation. The invention relates to a process for repairing a cartilage defect and implantation of a cartilage graft into a human or animal by crafting the cartilage matrix into individual grafts, disinfecting and cleaning the cartilage graft, applying a pretreatment solution to the cartilage graft, removing cellular debris using an extracting solution to produce a devitalized cartilage graft, implanting the cartilage graft into the cartilage defect with or without an insertion device, and sealing the implanted cartilage graft with recipient tissue. The devitalized cartilage graft is optionally recellularized in vitro, in vivo, or in situ with viable cells to render the tissue vital before or after the implantation. The devitalized cartilage graft is also optionally stored between the removing cellular debris and the recellularizing steps.
Owner:LIFENET HEALTH
Method for silk fibroin gelation using sonication
ActiveUS8187616B2Fast gelationCell from functioningBiocidePeptide/protein ingredientsDelivery vehicleBiological materials
This invention provides for a process of rapidly forming silk fibroin gelation through ultrasonication. Under the appropriate conditions, gelation can be controlled to occur within two hours after the ultrasonication treatment. Biological materials, including viable cells, or therapeutic agents can be encapsulated in the hydrogels formed from the process and be used as delivery vehicles.
Owner:TRUSTEES OF TUFTS COLLEGE
Device for culturing and transporting cells
InactiveUS20070166819A1Low volumeBioreactor/fermenter combinationsBiological substance pretreatmentsCulture cellReady to use
A disposable device is provided for culturing and / or packaging and transporting ready-to-use viable cells cultured on membranes, gels or micro porous substrata. The device includes a housing base defining an interior for culturing cells. The membrane to be used for culturing the cells is placed inside the housing base and fixed with a ring. The base is closed by a lid, which is designed to protect the underlying cells during transport as well as minimize the volume of media used during culture, as well as transport. Media leakage is prevented by the silicon gasket as well as the snaps provided in the device.
Owner:RELIANCE LIFE SCI PVT
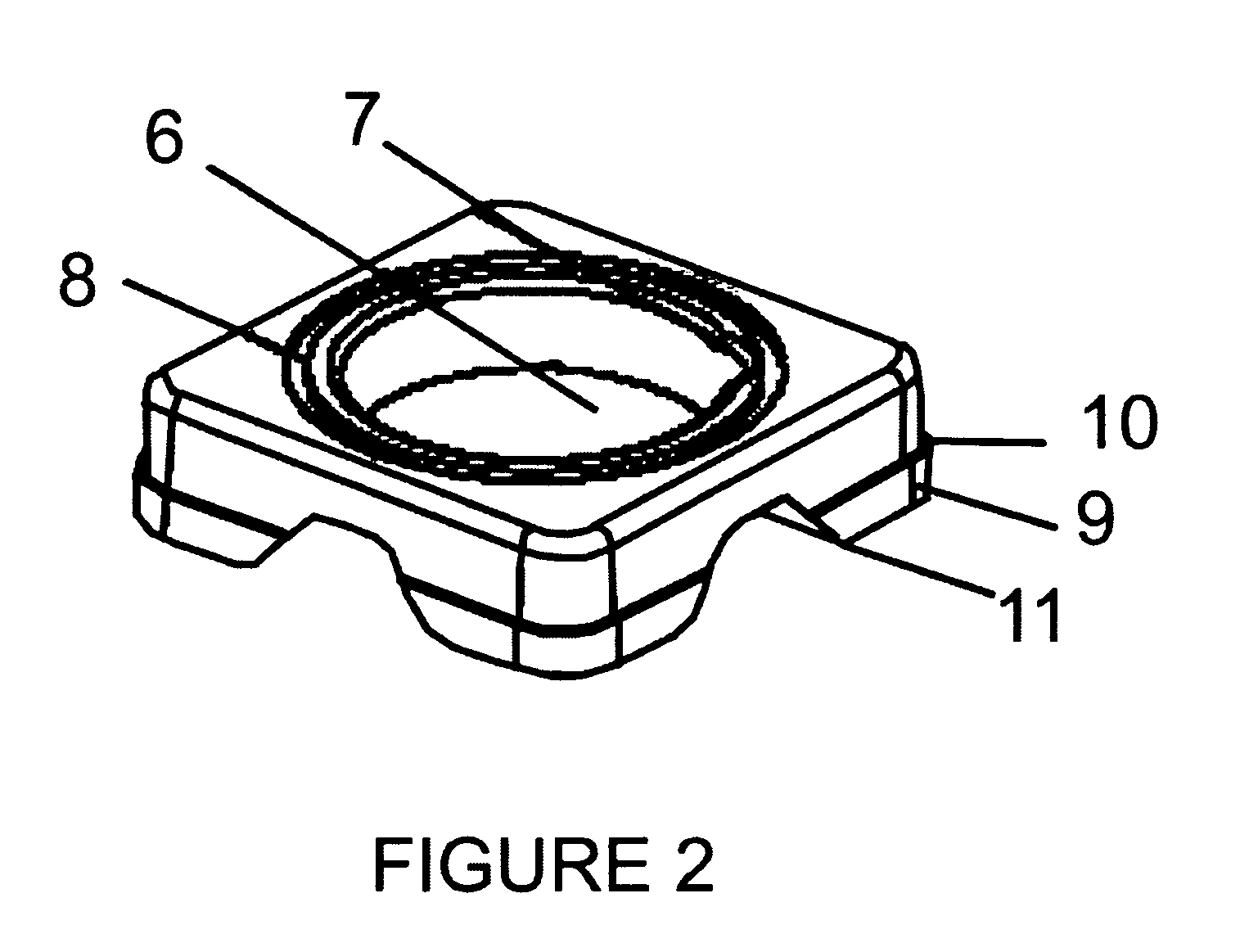
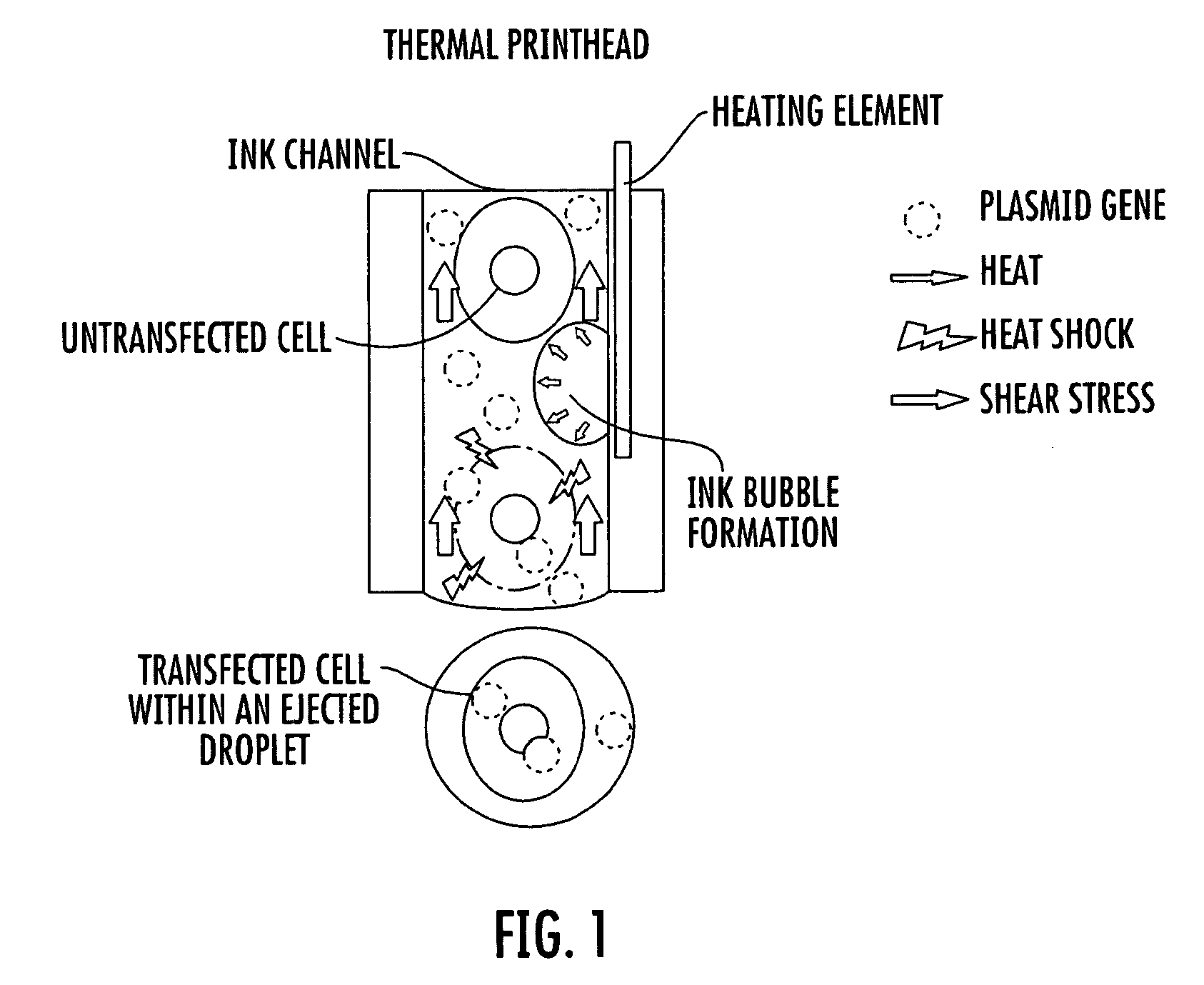
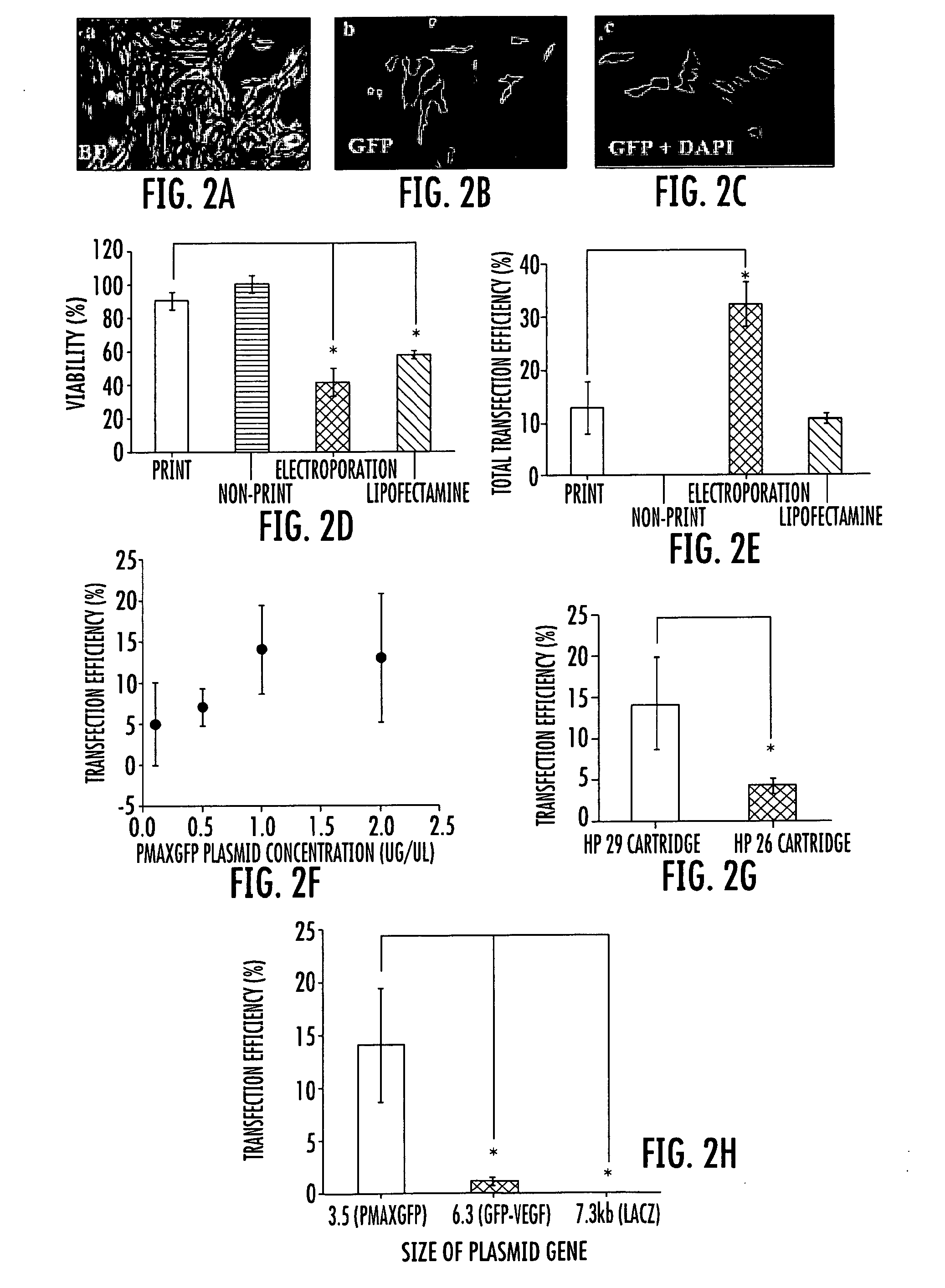
Inkjet gene printing
Provided herein are methods and apparatuses for transfecting a cell with a compound of interest by stressing the cell, e.g. with shear stress. The compound of interest may be nucleic acids, proteins, molecules, nanoparticles, drugs, etc., or any combination thereof. Methods of printing cells with an inkjet printing device are also provided, wherein at least a portion of viable cells (preferably at least 1%) are transfected with a compound of interest. Preferably, at least 25% of the cells are viable after printing. In addition, methods of forming an array of viable cells are provided wherein at least a portion of the viable printed cells (preferably at least 1%) are transfected with at least one compound of interest.
Owner:WAKE FOREST UNIV HEALTH SCI INC
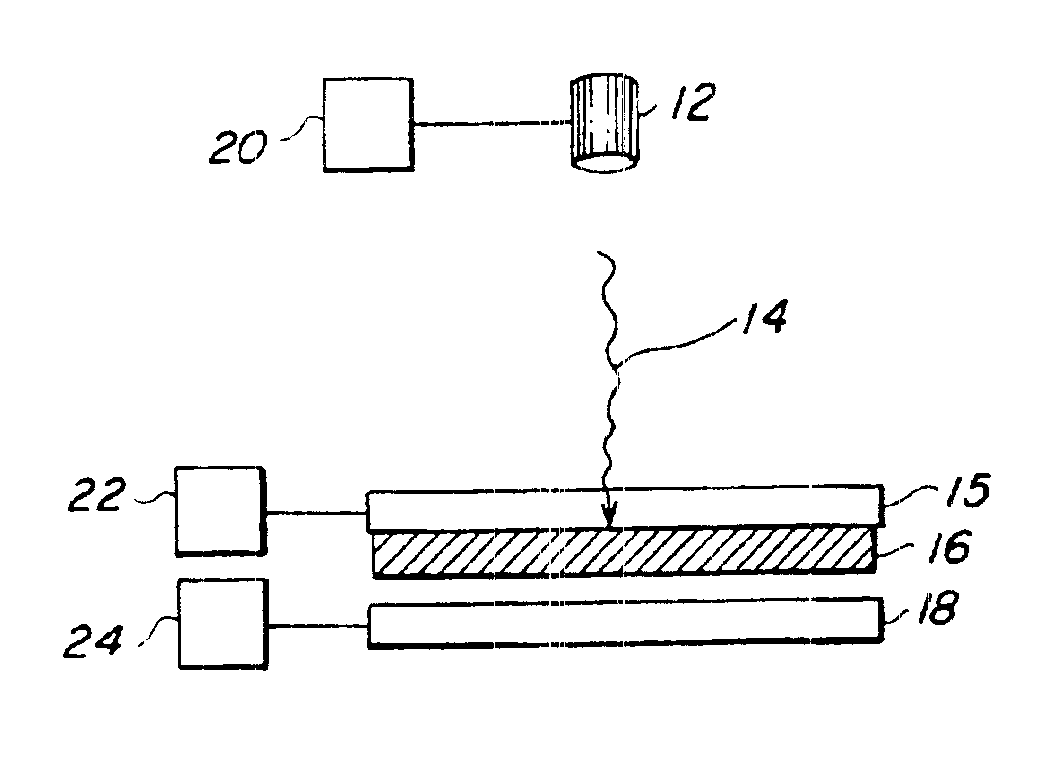
Generation of viable cell active biomaterial patterns by laser transfer
InactiveUS6905738B2Excessive transferMaterial nanotechnologySequential/parallel process reactionsOptoelectronicsBiological materials
A method for depositing a transfer material onto a receiving substrate uses a source of laser energy, a receiving substrate, and a target substrate. The target substrate comprises a laser-transparent support having a laser-facing surface and a support surface. The target substrate also comprises a composite material having a back surface in contact with the support surface and a front surface. The composite material comprises a mixture of the transfer material to be deposited and a matrix material. The matrix material is a material that has the property that, when it is exposed to laser energy, it desorbs from the laser-transparent support. The source of laser energy is positioned in relation to the target substrate so that laser energy is directed through the laser-facing surface of the target substrate and through the laser-transparent support to strike the composite material at a defined target location. The receiving substrate is positioned in a spaced relation to the target substrate. The source of laser energy has sufficient energy to desorb the composite material at the defined target location, causing the composite material to desorb from the defined target location and be lifted from the support surface of the laser-transparent support. The composite material is deposited at a defined receiving location on the receiving substrate. The method is useful for creating a pattern of biomaterial on the receiving substrate.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
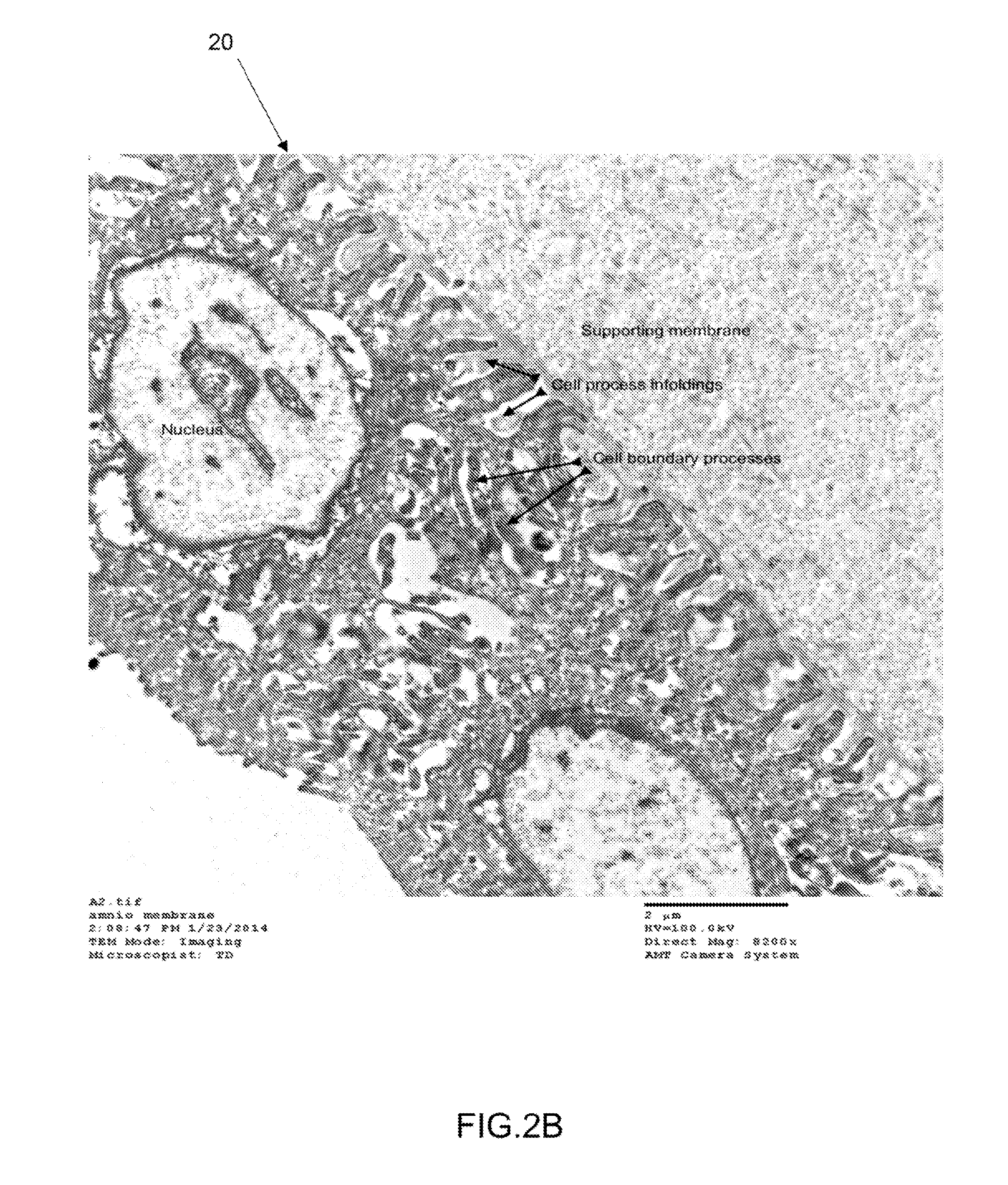
Acellular amnion derived therapeutic compositions
ActiveUS9132156B1Reduce in quantityInduce and responsePowder deliveryCosmetic preparationsAmniotic fluidViable cell
Acellular amnion derived therapeutic compositions are described having a number of various compositional embodiments. An acellular amnion derived therapeutic composition has essentially no live or active amniotic stems cells. The amniotic stem cells may be destroyed, and the cells and cell debris may be removed from the acellular amnion derived therapeutic composition. An acellular amnion derived therapeutic composition may comprise micronized amniotic membrane particles, and / or amniotic fluid. An acellular amnion derived therapeutic composition may be a dispersion of micronized amniotic membrane combined with a fluid, such as plasma, saline, amniotic fluid, combinations thereof and the like. An acellular amnion derived therapeutic composition may be combined with a matrix component to form a composite. An acellular amnion derived therapeutic composition may be used in conjunction with a composition comprising viable cells, such as stem cells.
Owner:AMNIO TECH +1
Augmentation and repair of vocal cord tissue defects
InactiveUS7412978B1Reduce the possibilityLower potentialBiocidePeptide/protein ingredientsCorrective surgeryTissue defect
A method for corrective surgery in a human subject of a vocal cord defect amenable to rectification by the augmentation of tissue subadjacent to the vocal cord defect, the method comprising: placing an effective amount of autologous in vitro cultured cells into a vocal cord tissue of the subject in a position subadjacent to the vocal cord defect, wherein the vocal cord tissue is selected from the group consisting of a scar, Reinke's space, a muscle of the vocal cord, and the lamina propria, wherein the in vitro cultured cells are obtained by culturing a plurality of viable cells retrieved from the subject, and wherein the cultured cells are suspended in a collagen or modified collagen solution prior to injection of the cultured cells. The in vitro cultured cells can be fibroblasts, e.g., fibroblasts derived from dermis, fascia, connective tissue, or lamina propria.
Owner:CASTLE CREEK BIOSCIENCES LLC
Process and composition for the manufacture of a microbial-based product
Processes to produce microorganisms that can be incorporated into a microbial-based product that results in high viable cell yields and shelf-stable products are disclosed. These microbial-based products are useful for inhibiting pathogenic growth and as a food additive. A preferred microorganism is a lactic acid producing bacteria. In one embodiment, the process comprises inoculating a lactobacillus fermentation medium with lactic acid producing bacterial cells, harvesting the lactic acid producing bacterial cells at mid to late log phase, concentrating the lactic acid producing bacterial cells, and preserving the lactic acid producing bacterial cells at a concentration of at least 5×109 cfu / ml.
Owner:MICROBIOS
Implantable chamber for biological induction or enhancement of muscle contraction
InactiveUS20050283218A1Improve conductivityAvoid overgrowthTransvascular endocardial electrodesExternal electrodesDiseaseSinoatrial node
A percutaneously implantable chamber for the treatment of a cardiac condition is disclosed herein, the chamber capable of delivery and maintenance of viable cells comprising a pacemaker gene or other genes intended to impart a specific function via a host cell. An artificial sinoatrial node and artificial atrial ventricular node for the restoration of the pacemaker function of the heart of a subject comprises a chamber comprising cells expressing a pacemaker gene. Further, a chamber may be used for the implantation and maintenance of viable, responsive, immunoisolated cells to induce or enhance muscle contraction of a subject for the treatment of a disorder.
Owner:SYNECOR LLC
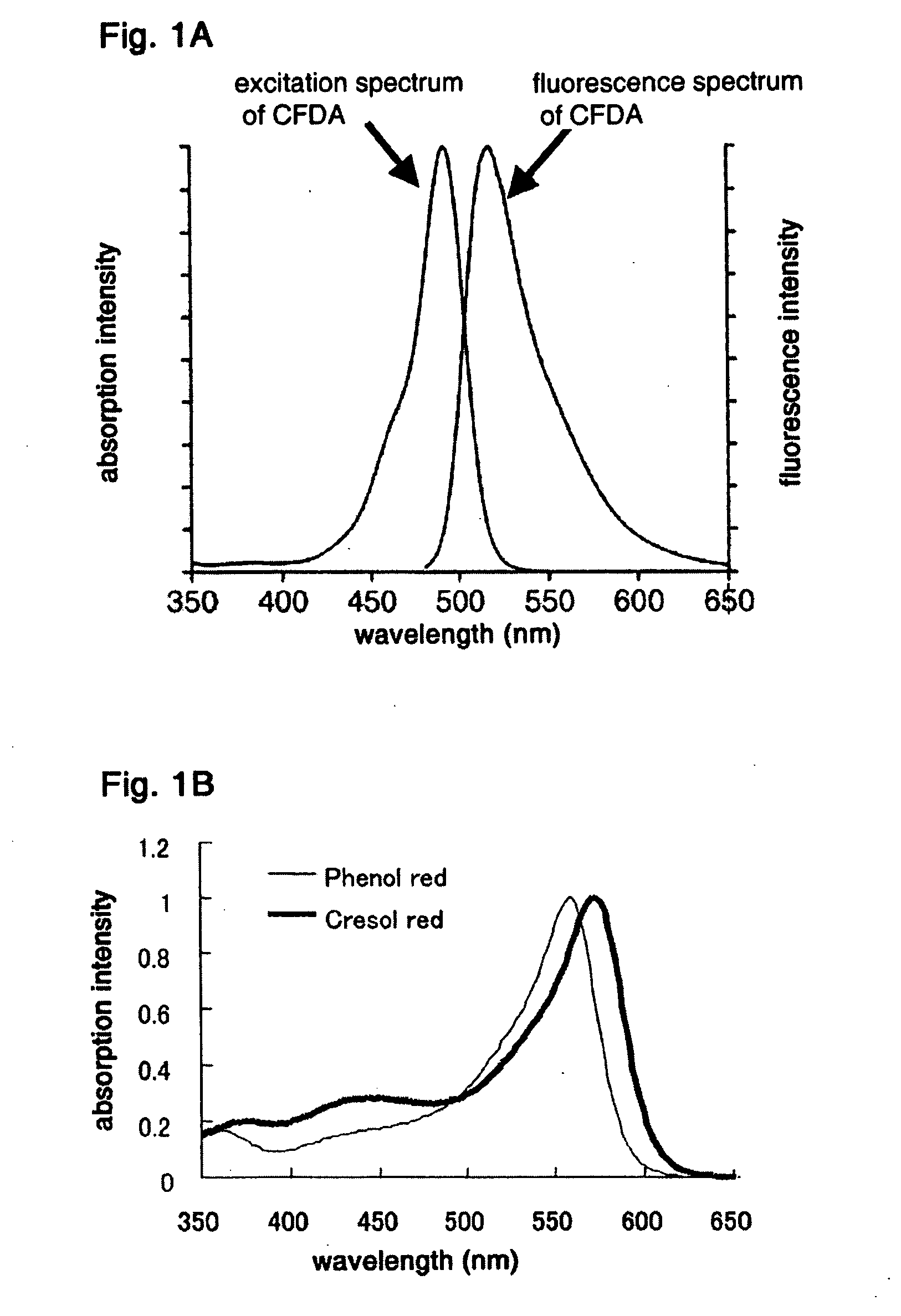
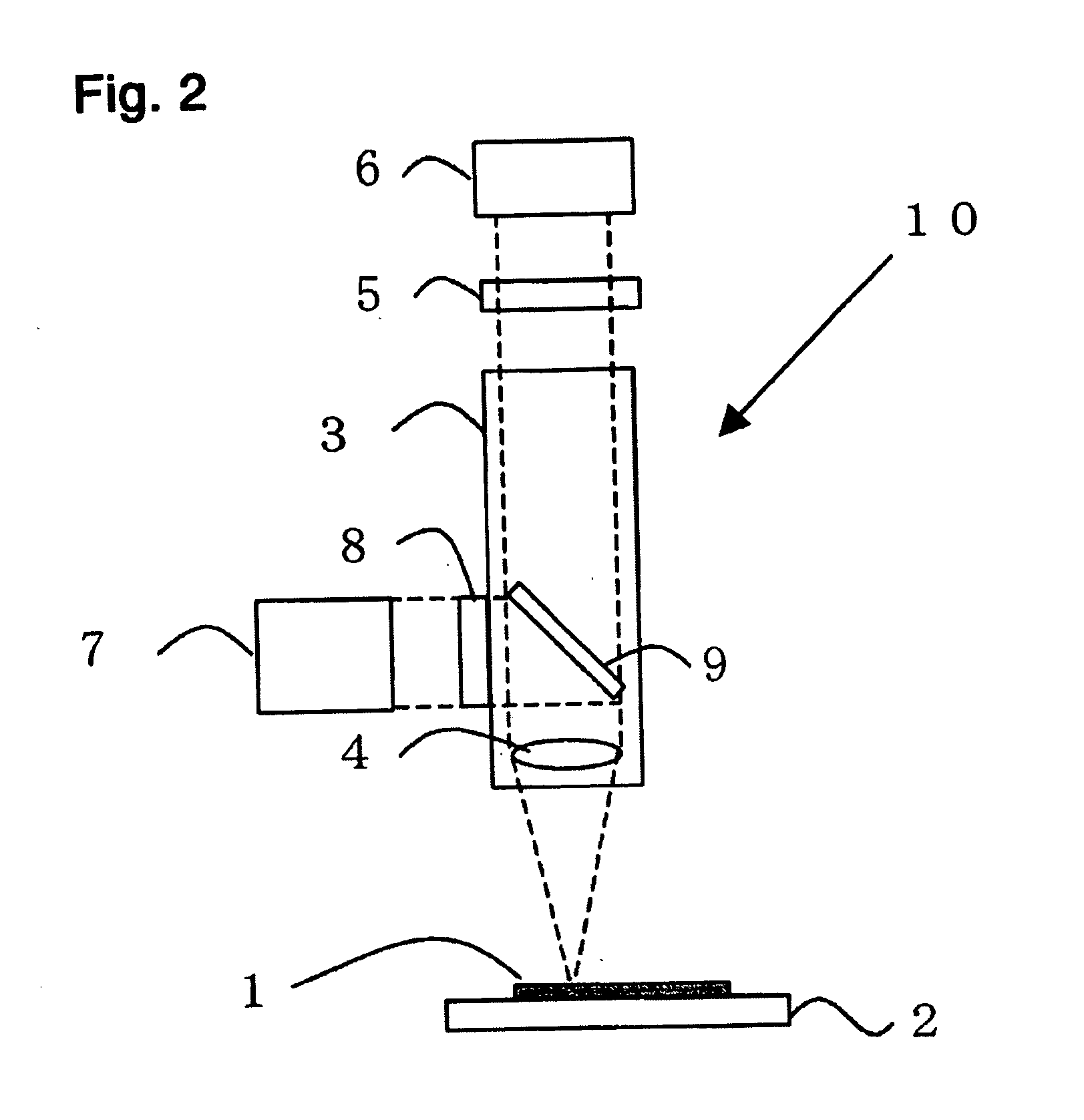
Method of detecting viable cells
InactiveUS20060040400A1Exclude influenceEfficiently quenchedMicrobiological testing/measurementChemiluminescene/bioluminescenceFluorescent stainingFluorescence
A method of detecting and quantifying viable cells in a sample. The method includes fluorescently staining the cells by adding a fluorescent dye into the sample or putting the sample in contact with the fluorescent dye. A quenching dye is then added to the stained sample, or the sample is put into contact with the quenching dye, at a pH different from the pH in the viable cells. The quenching dye used is permeable through the membrane of a viable cell and does not readily absorb fluorescence of the fluorescent dye at the pH in the viable cells, but absorbs the fluorescence of the fluorescent dye at the pH of the fluorescent dye. Next, the sample, now stained with the fluorescent dye and the quenching dye, is illuminated by an excitation light for the fluorescent dye at a pH different from the pH in the viable cells and the fluorescence emitted from the sample is collected and detected.
Owner:FUJI ELECTRIC CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com