Patents
Literature
42 results about "Lamina propria" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
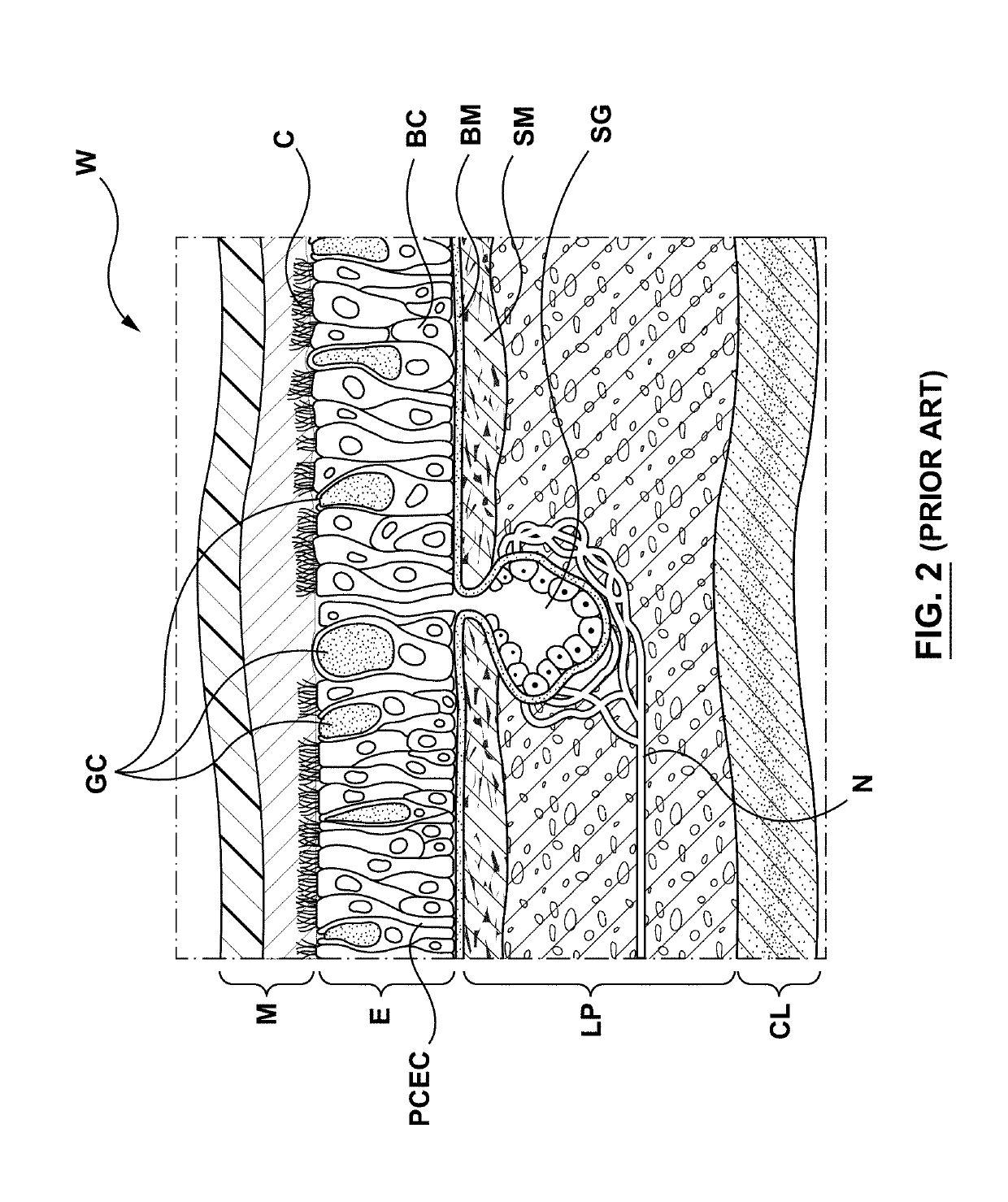
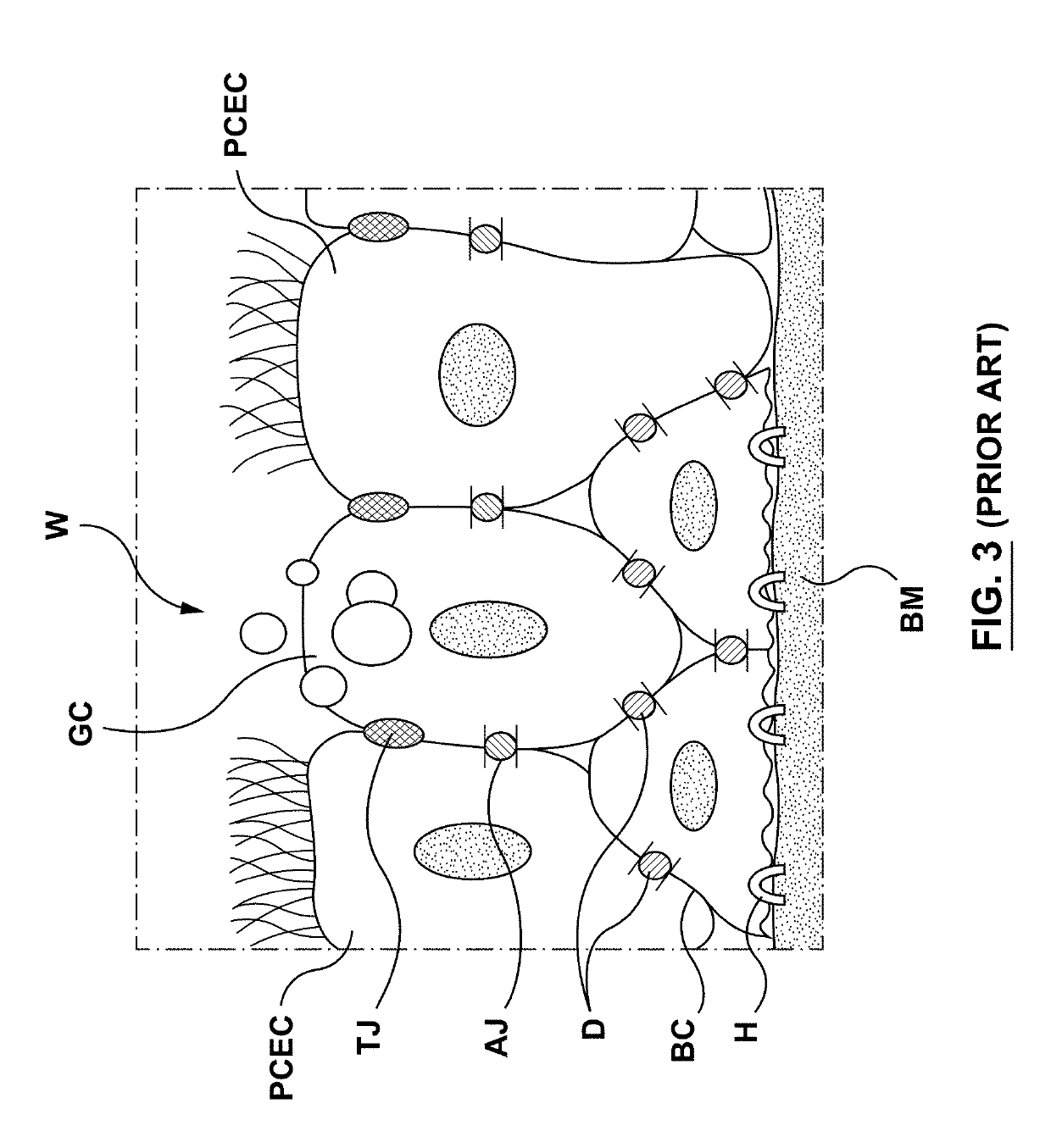
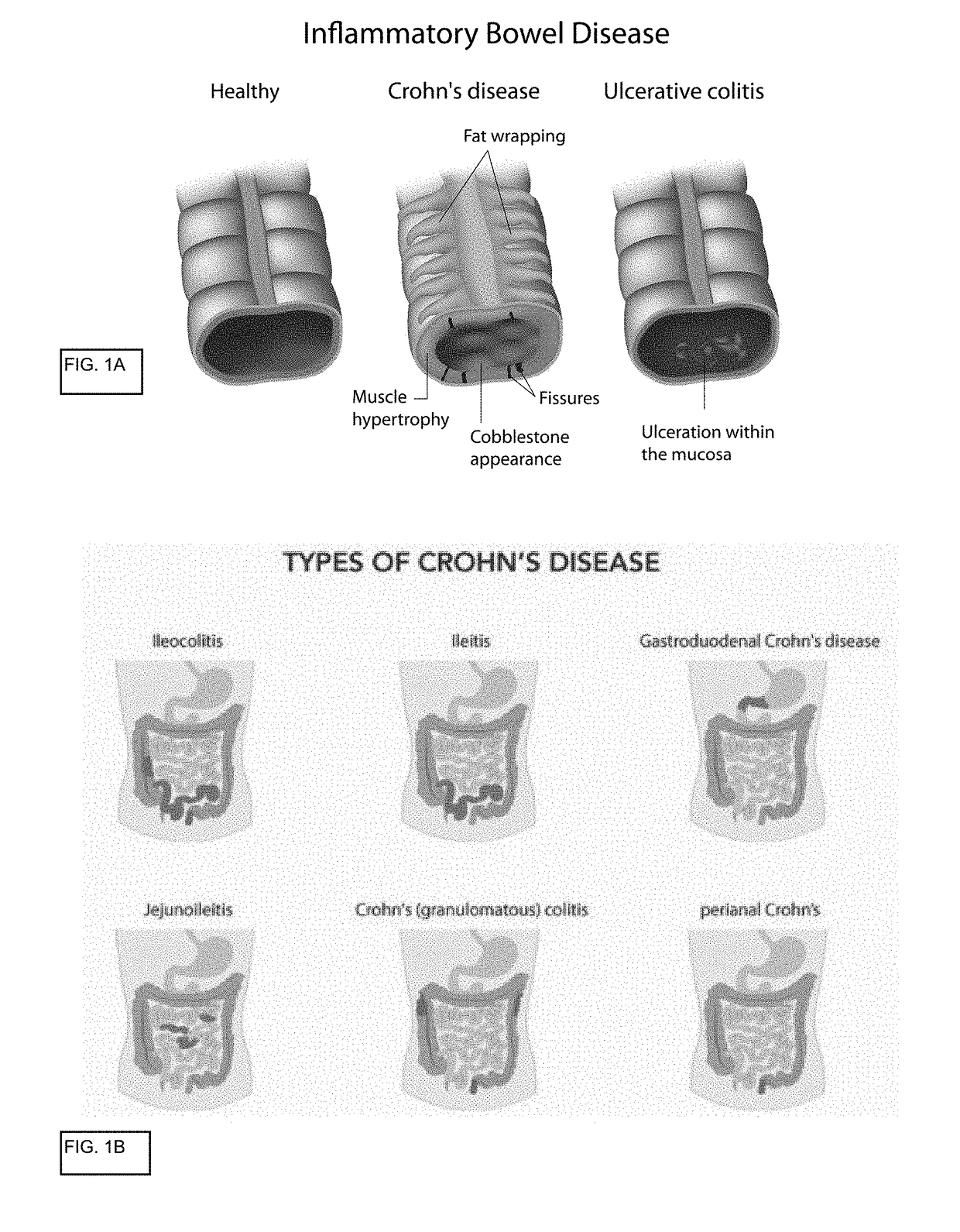
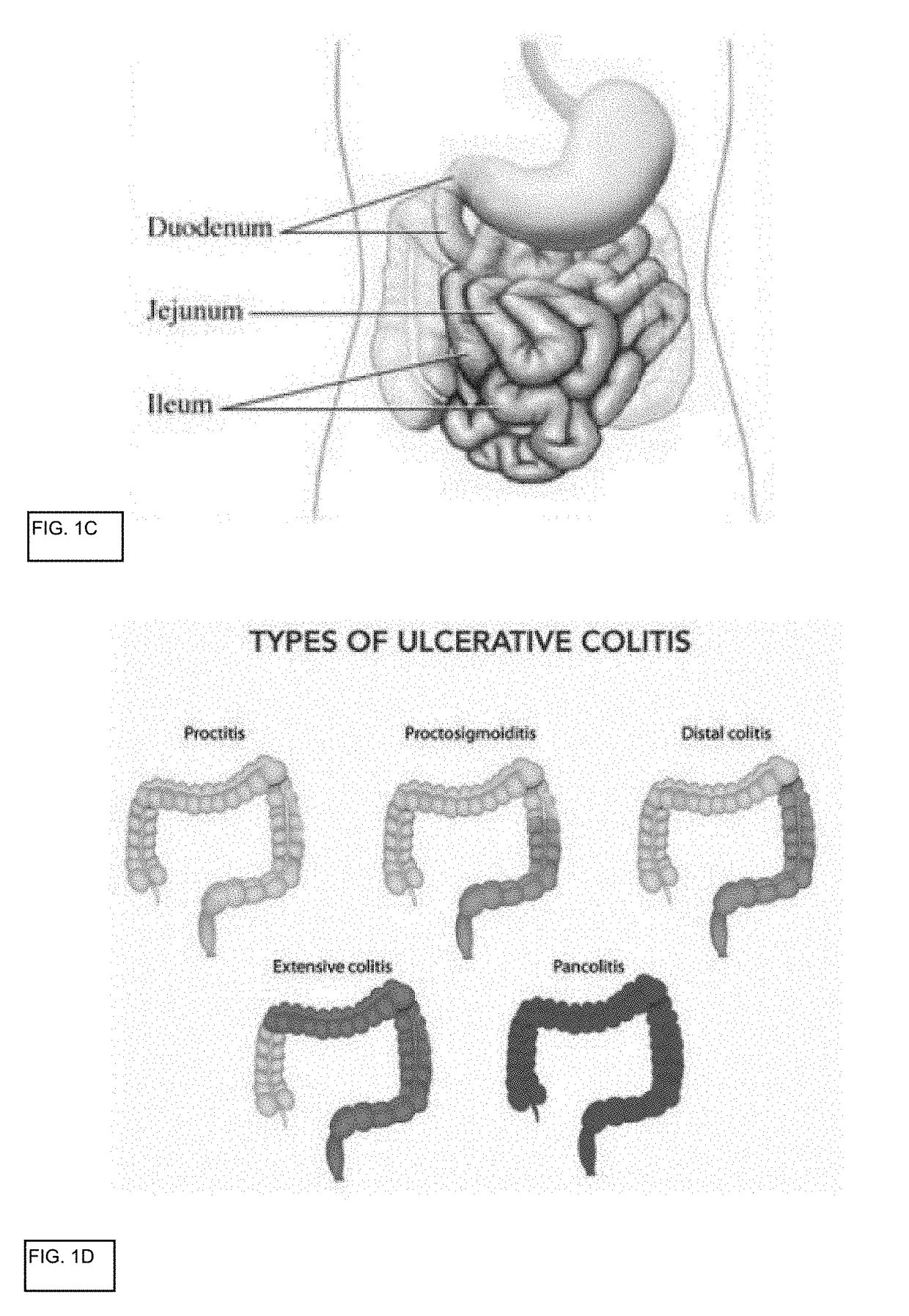
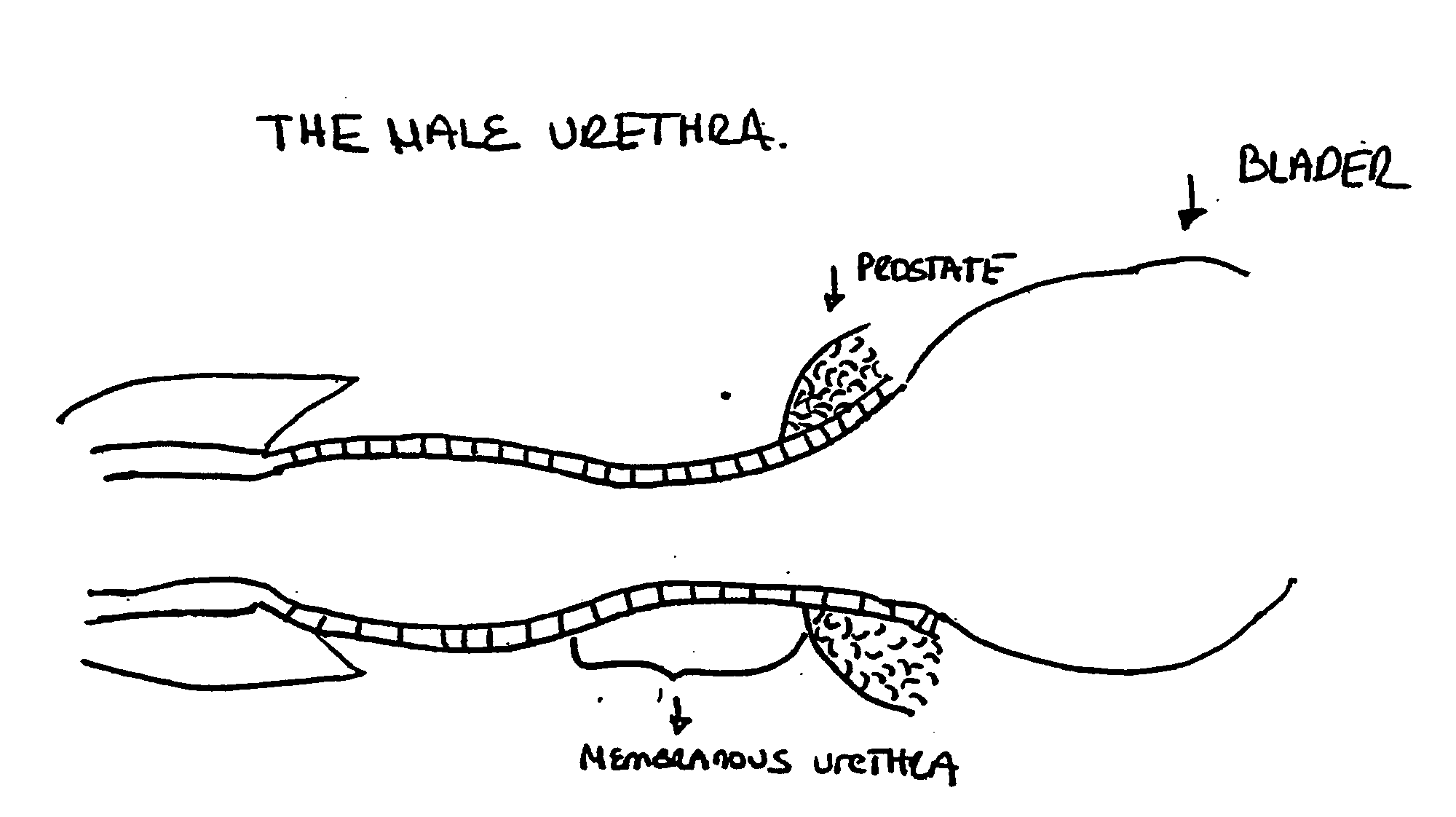
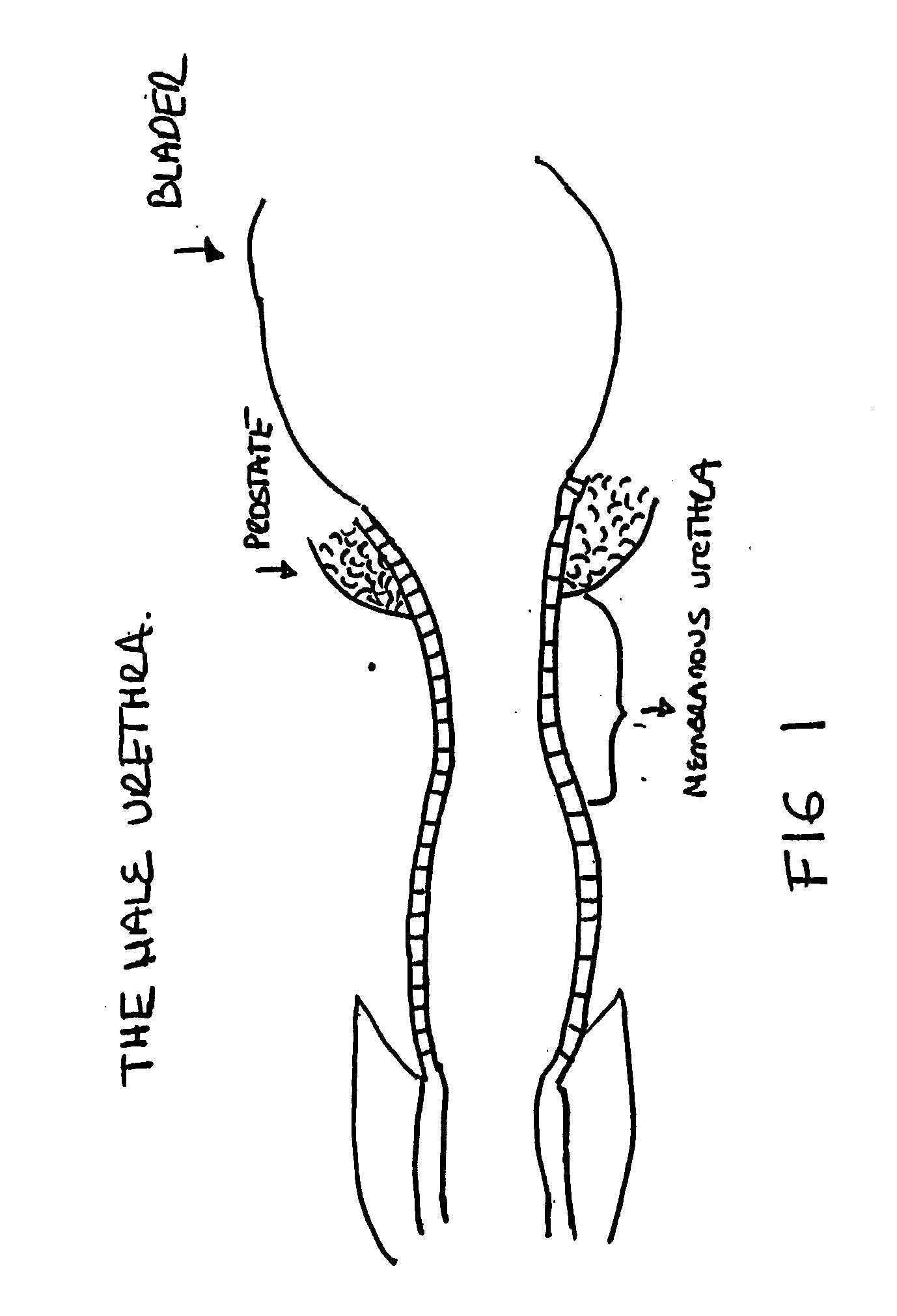
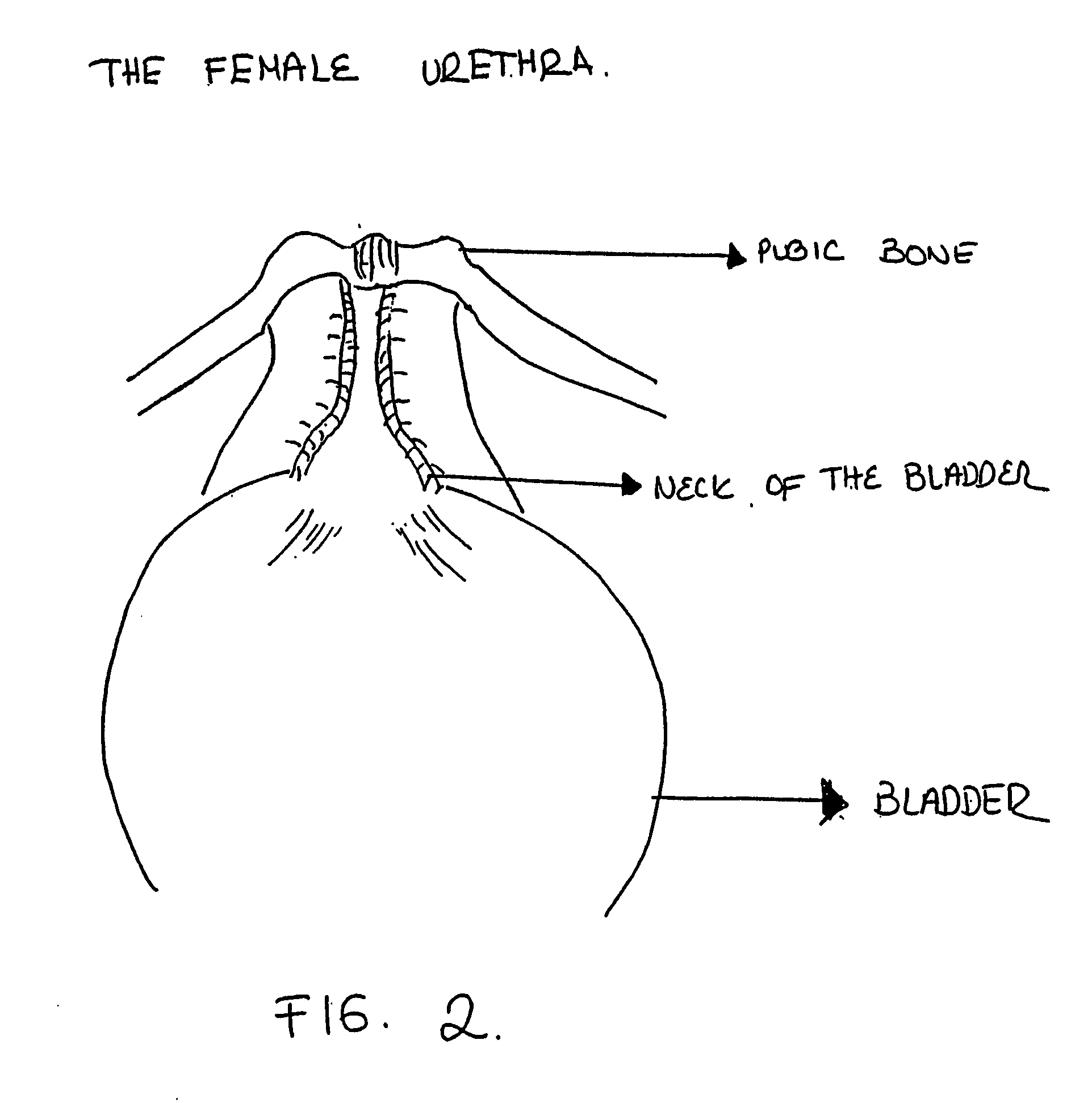
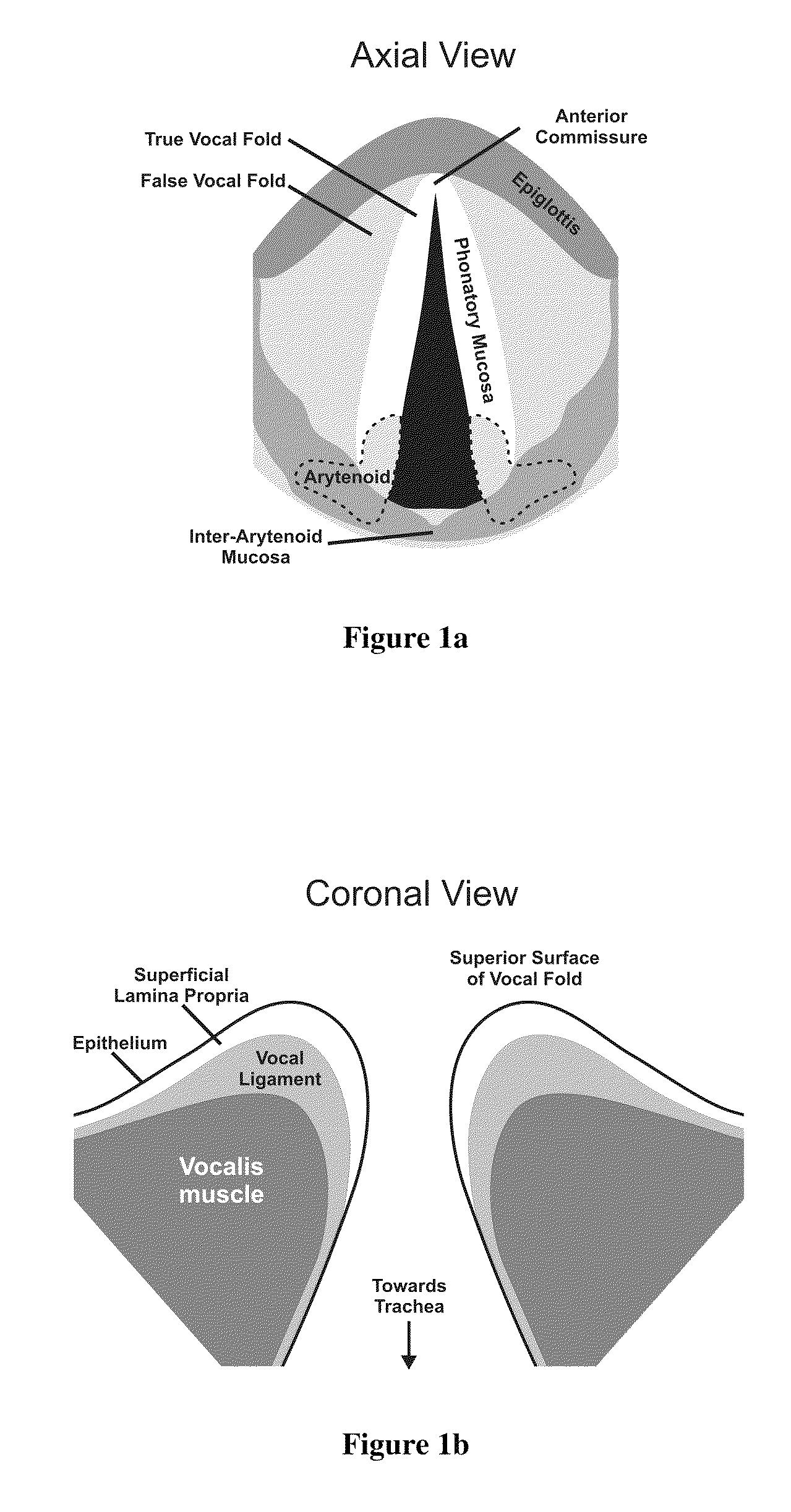
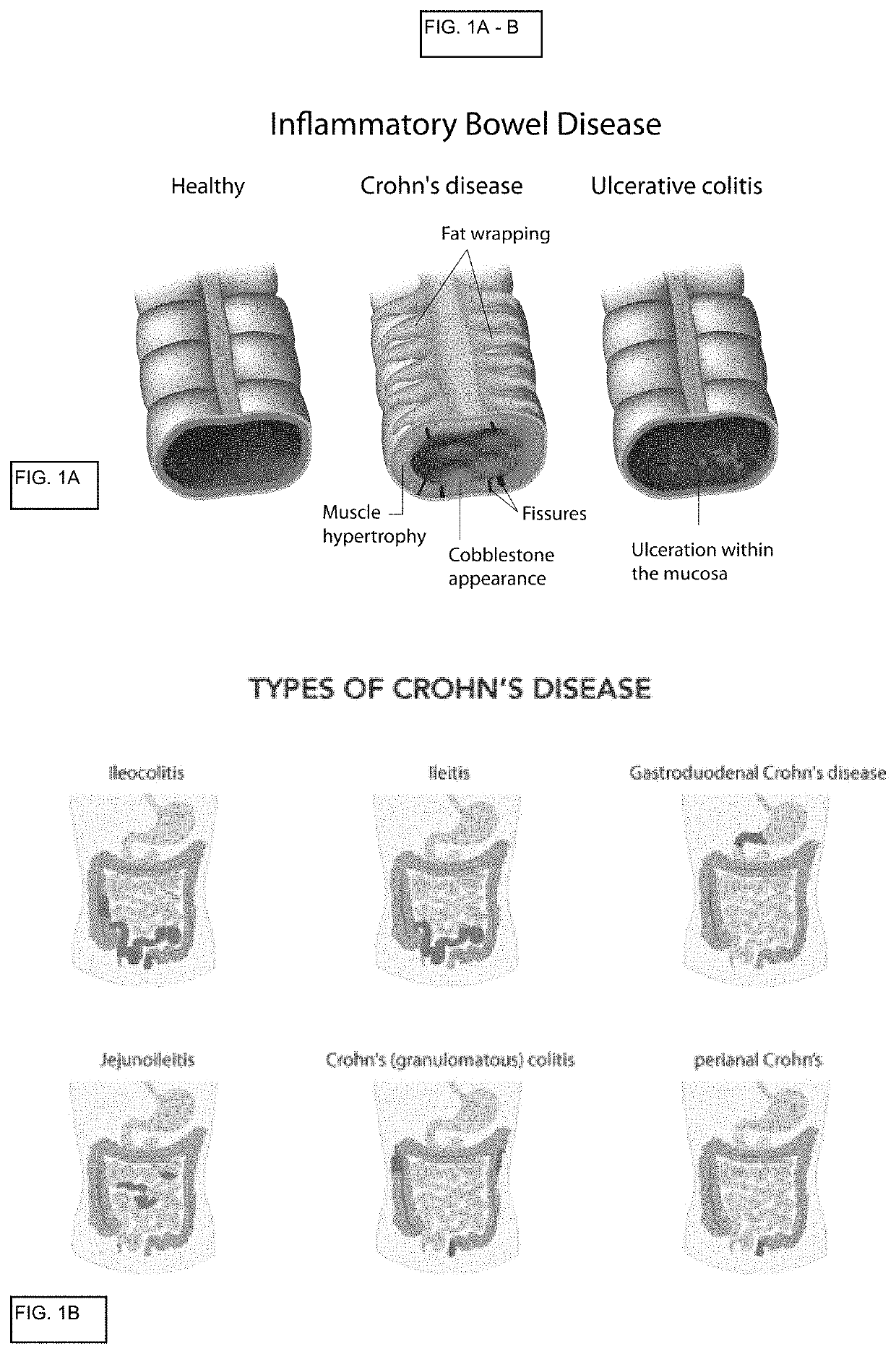
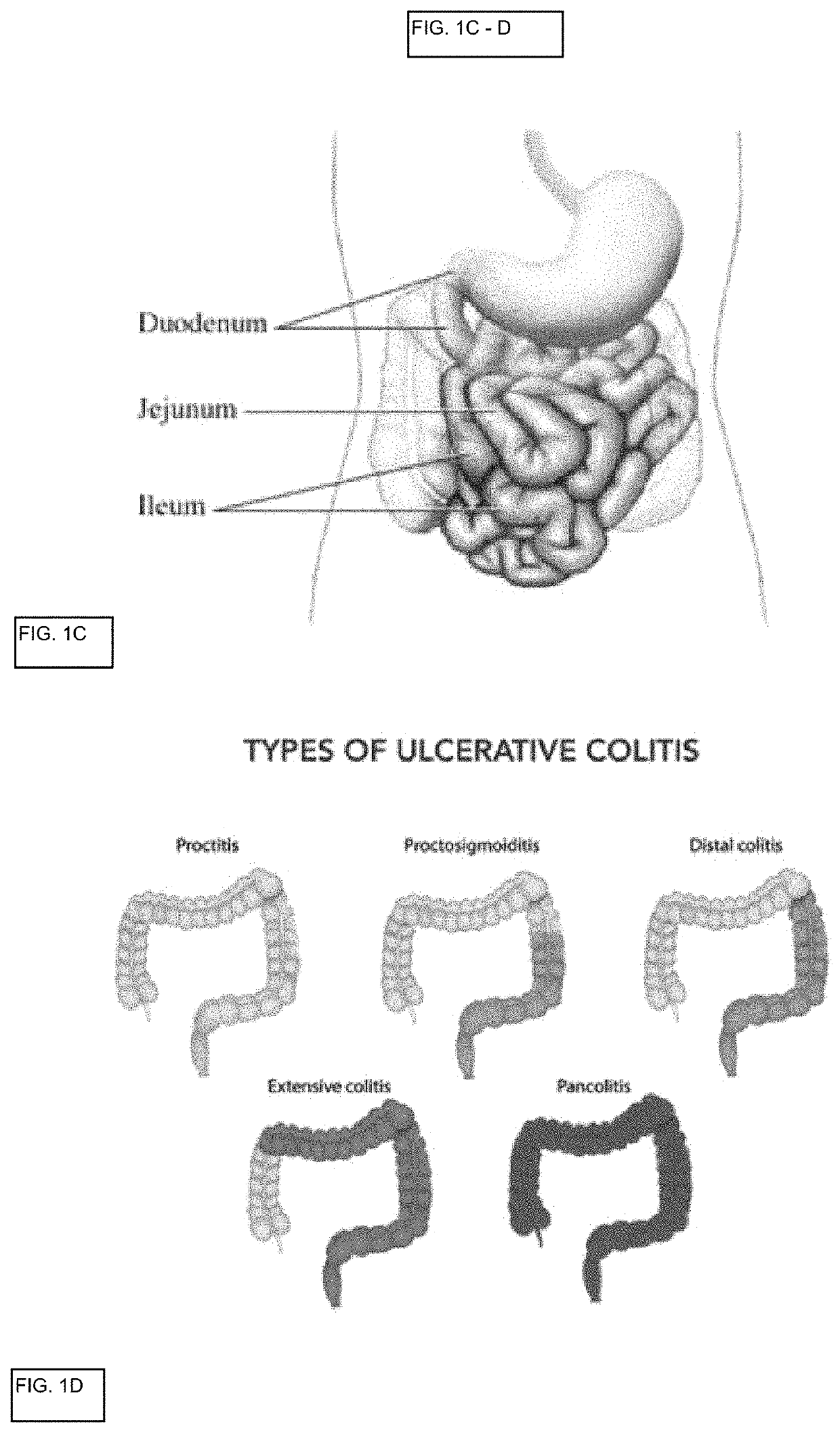
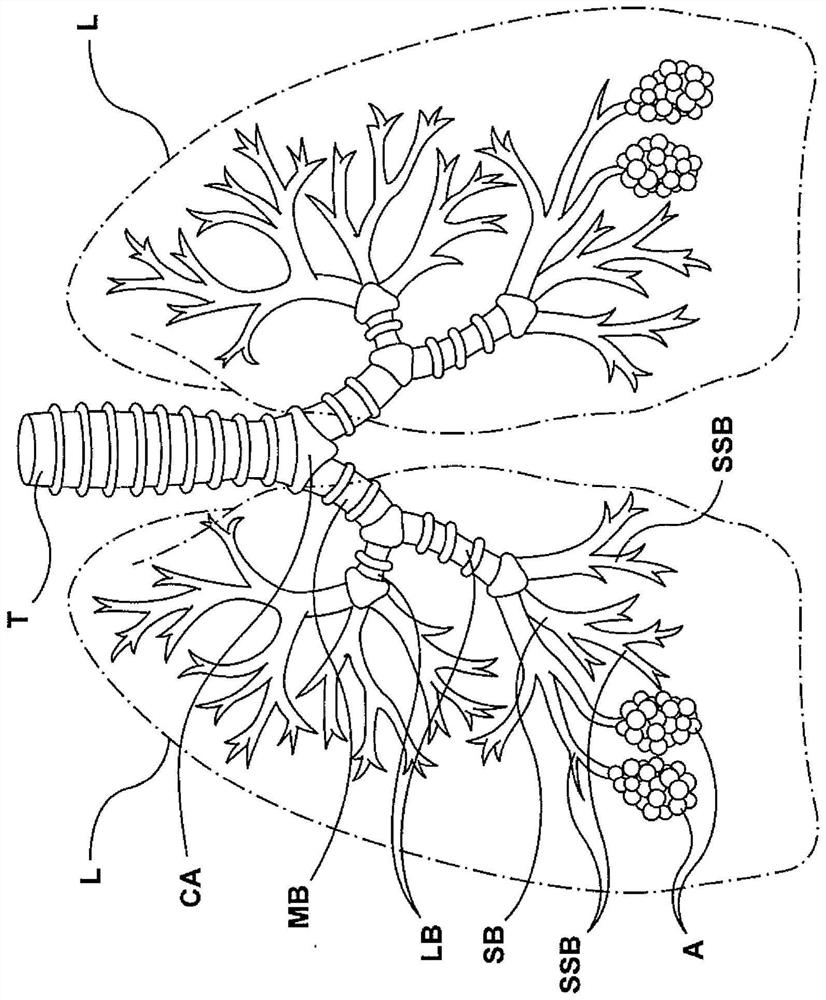
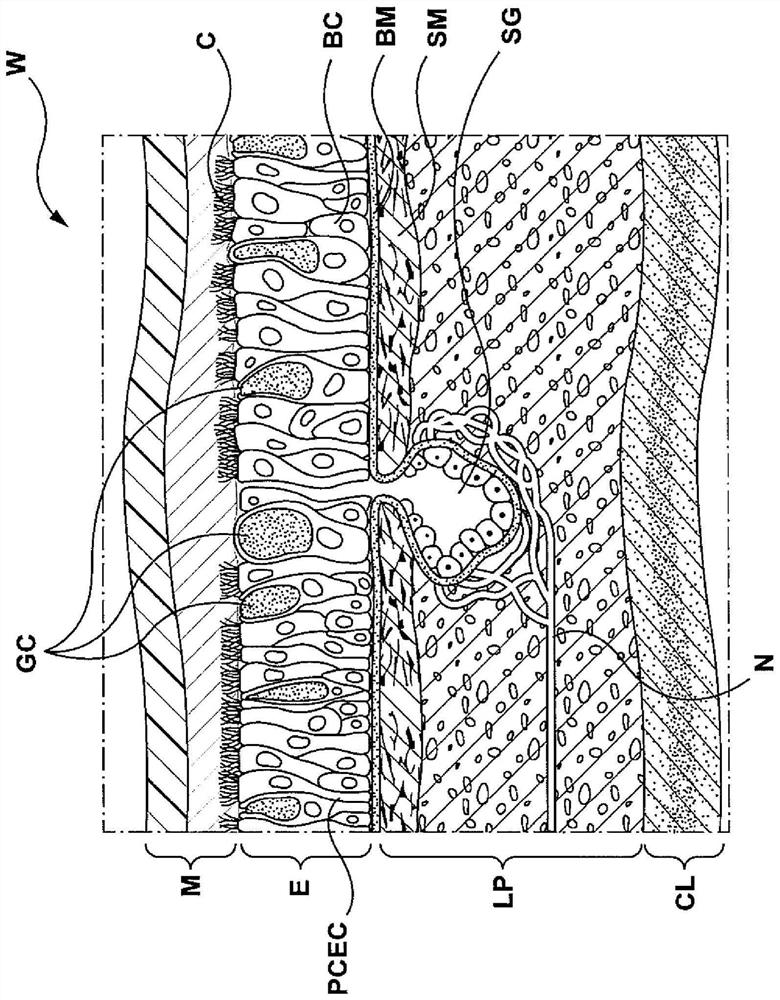
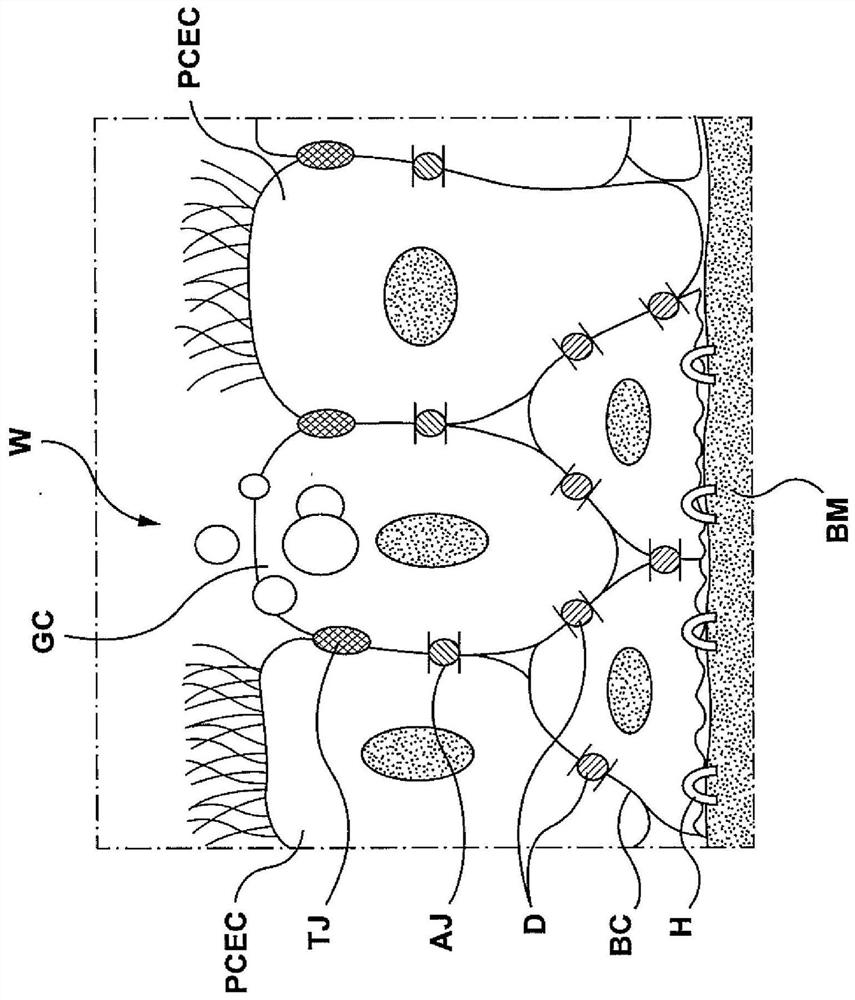
The lamina propria is a thin layer of connective tissue that forms part of the moist linings known as mucous membranes or mucosa, which line various tubes in the body, such as the respiratory tract, the gastrointestinal tract, and the urogenital tract.
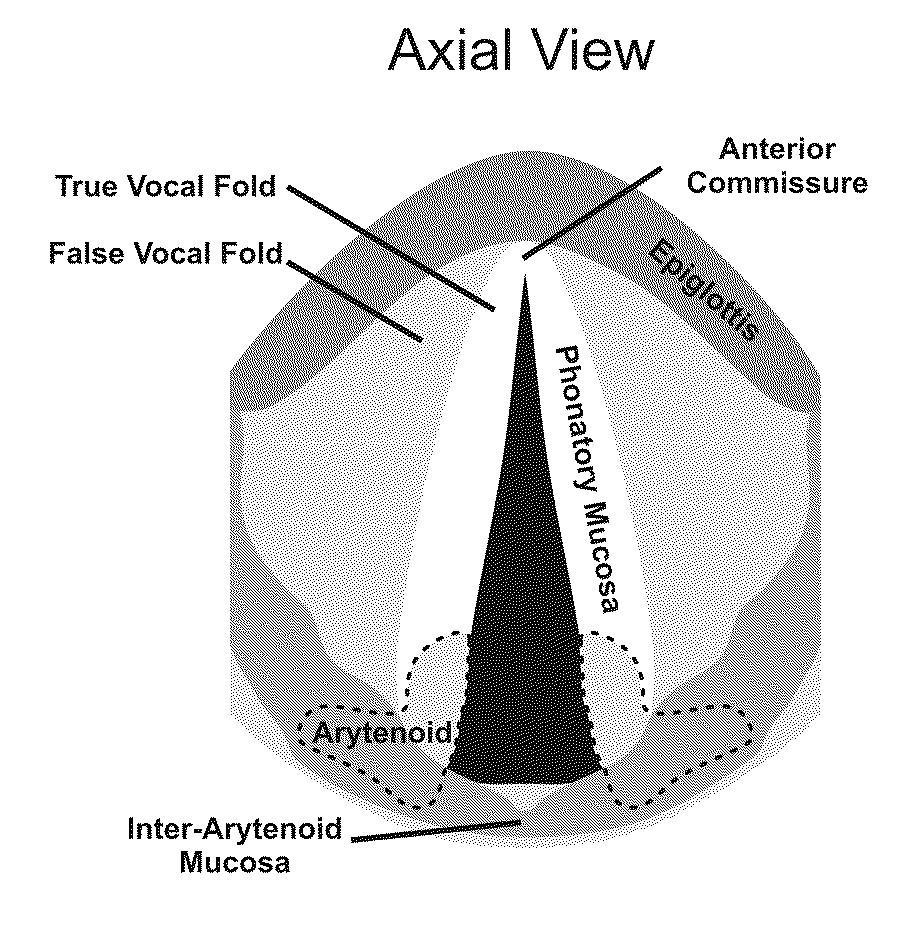
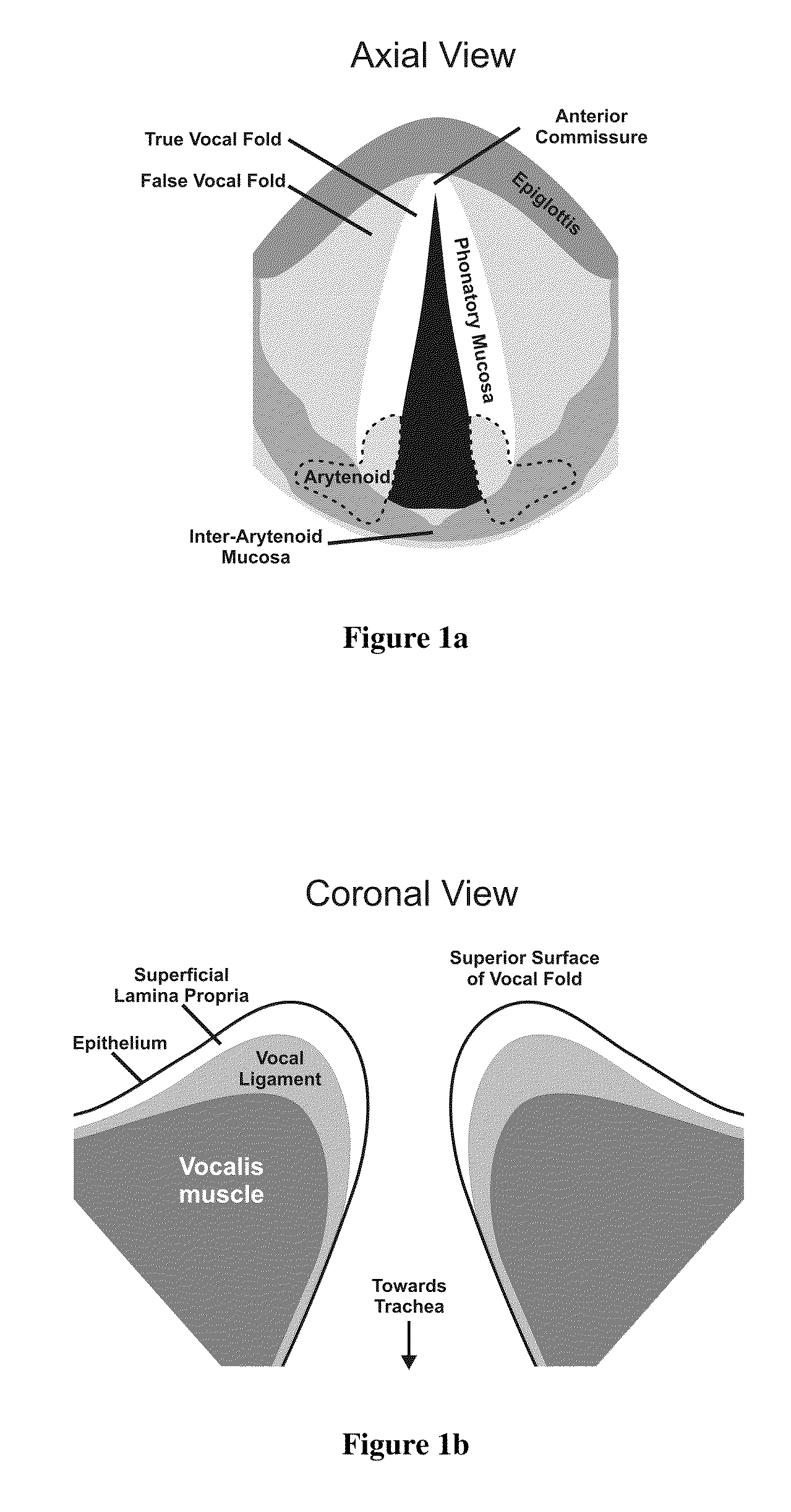
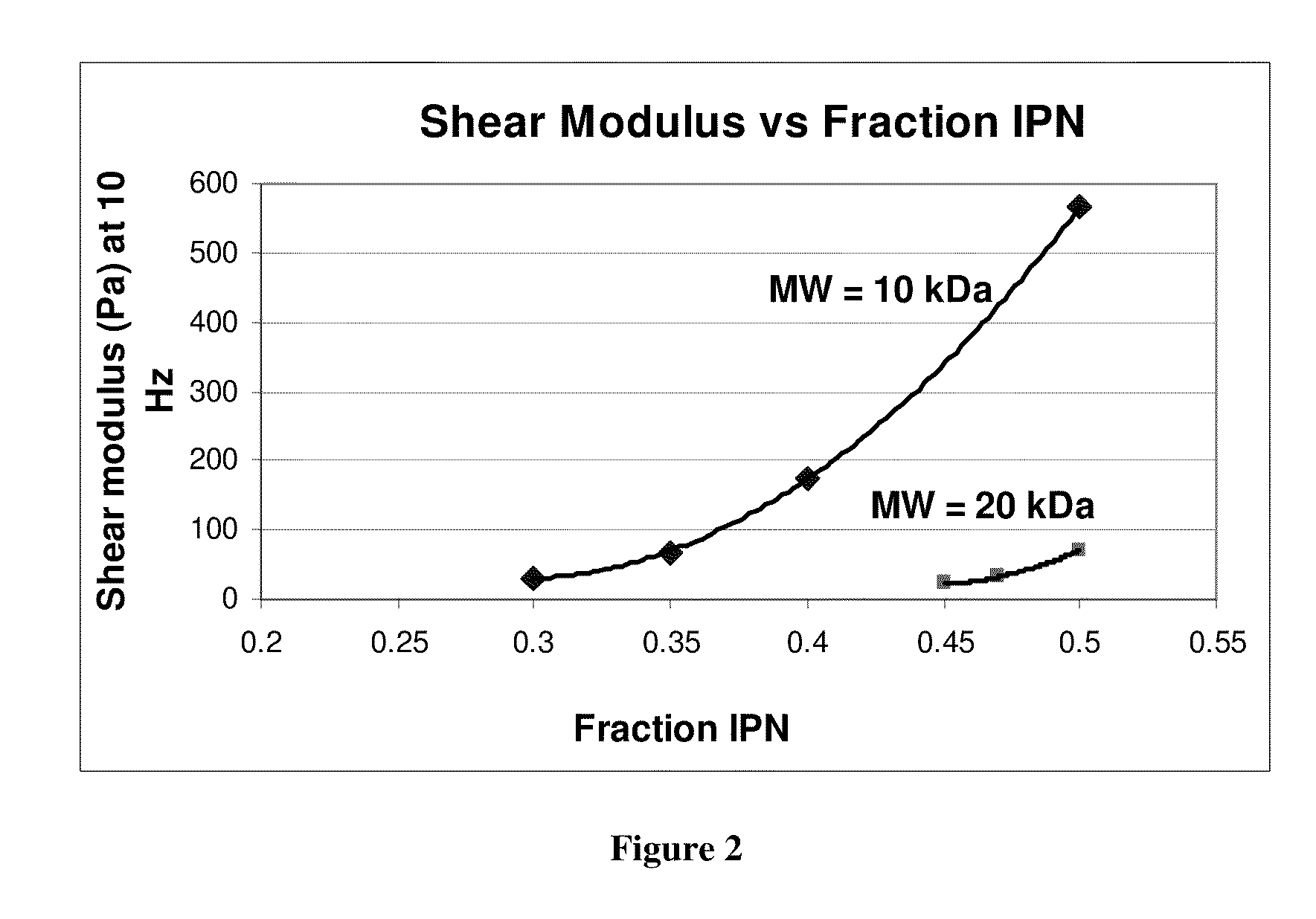
Hydrogels for vocal cord and soft tissue augmentation and repair
ActiveUS20100055184A1Repairing pliabilityDiminished functional vibratory capacityBiocideOrganic active ingredientsEpitheliumBreast implant
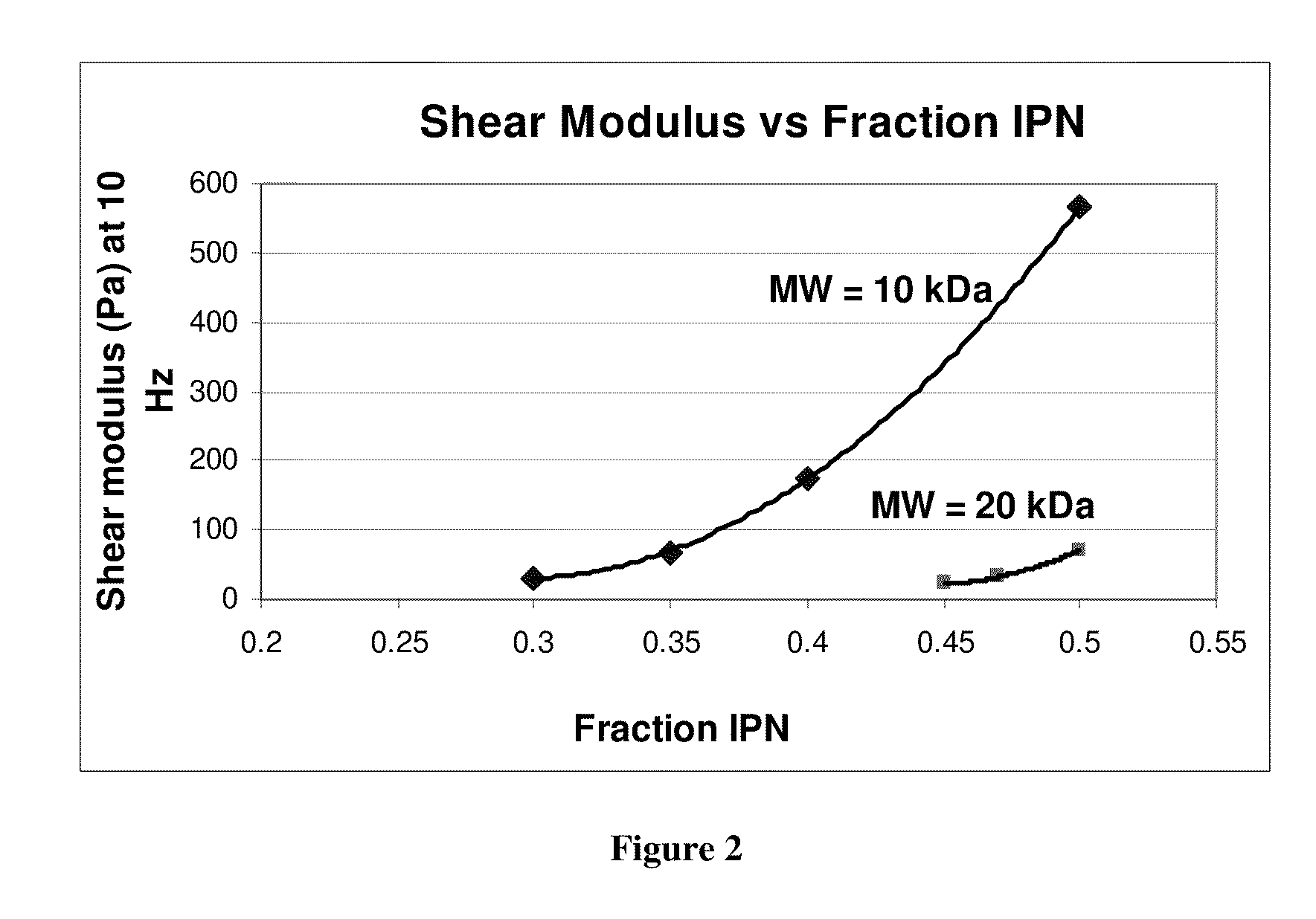
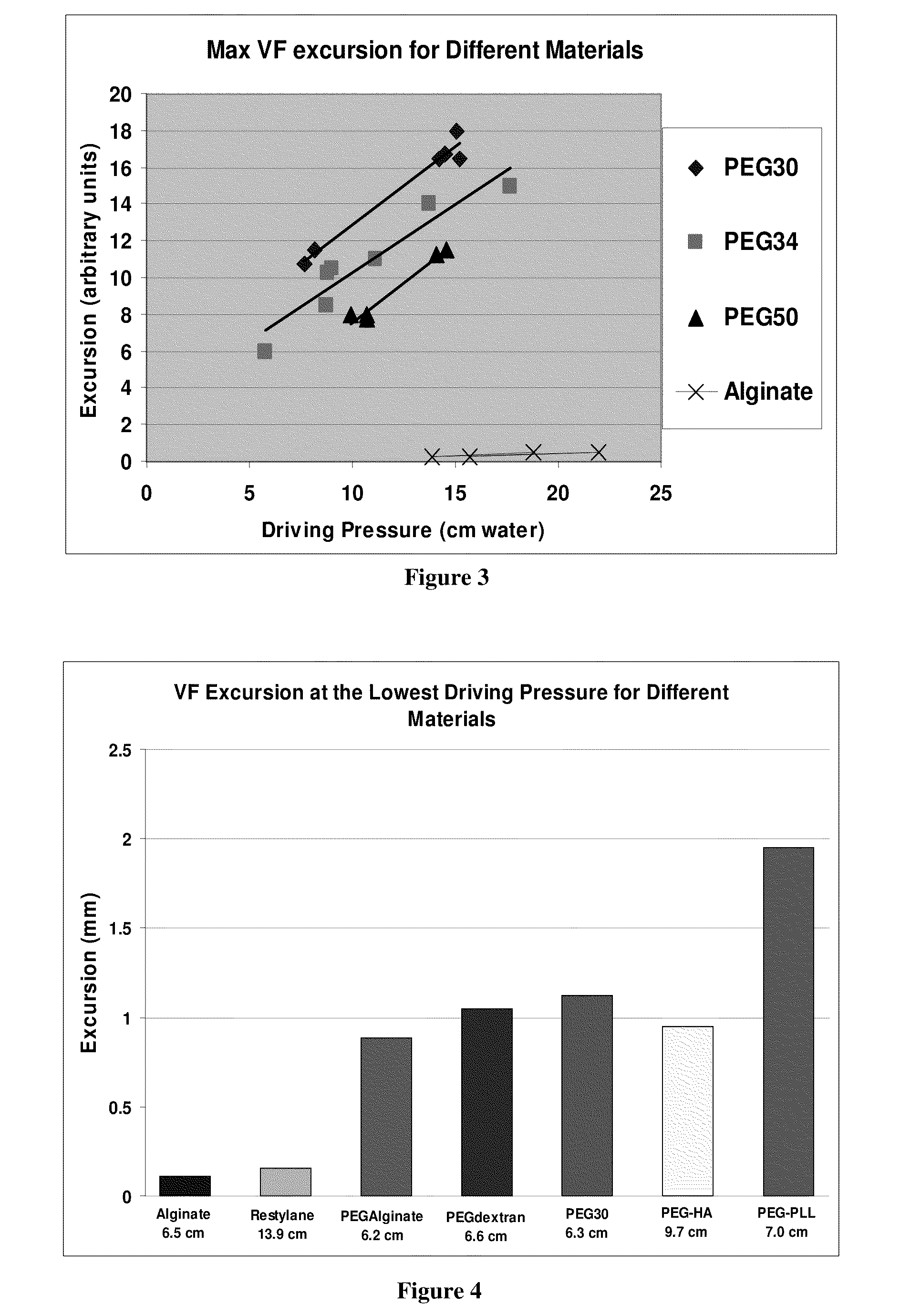
The present invention provides hydrogels and compositions thereof for vocal cord repair or augmentation, as well as other soft tissue repair or augmentation (e.g., bladder neck augmentation, dermal fillers, breast implants, intervertebral disks, muscle-mass). The hydrogels or compositions thereof are injected into the superficial lamina propria or phonatory epithelium to restore the phonatory mucosa of the vocal cords, thereby restoring a patient's voice. In particular, it has been discovered that hydrogels with an elastic shear modulus of approximately 25 Pa are useful in restoring the pliability of the phonatory mucosa. The invention also provides methods of preparing and using the inventive hydrogels.
Owner:MASSACHUSETTS INST OF TECH
Augmentation and repair of vocal cord tissue defects
InactiveUS7412978B1Reduce the possibilityLower potentialBiocidePeptide/protein ingredientsCorrective surgeryTissue defect
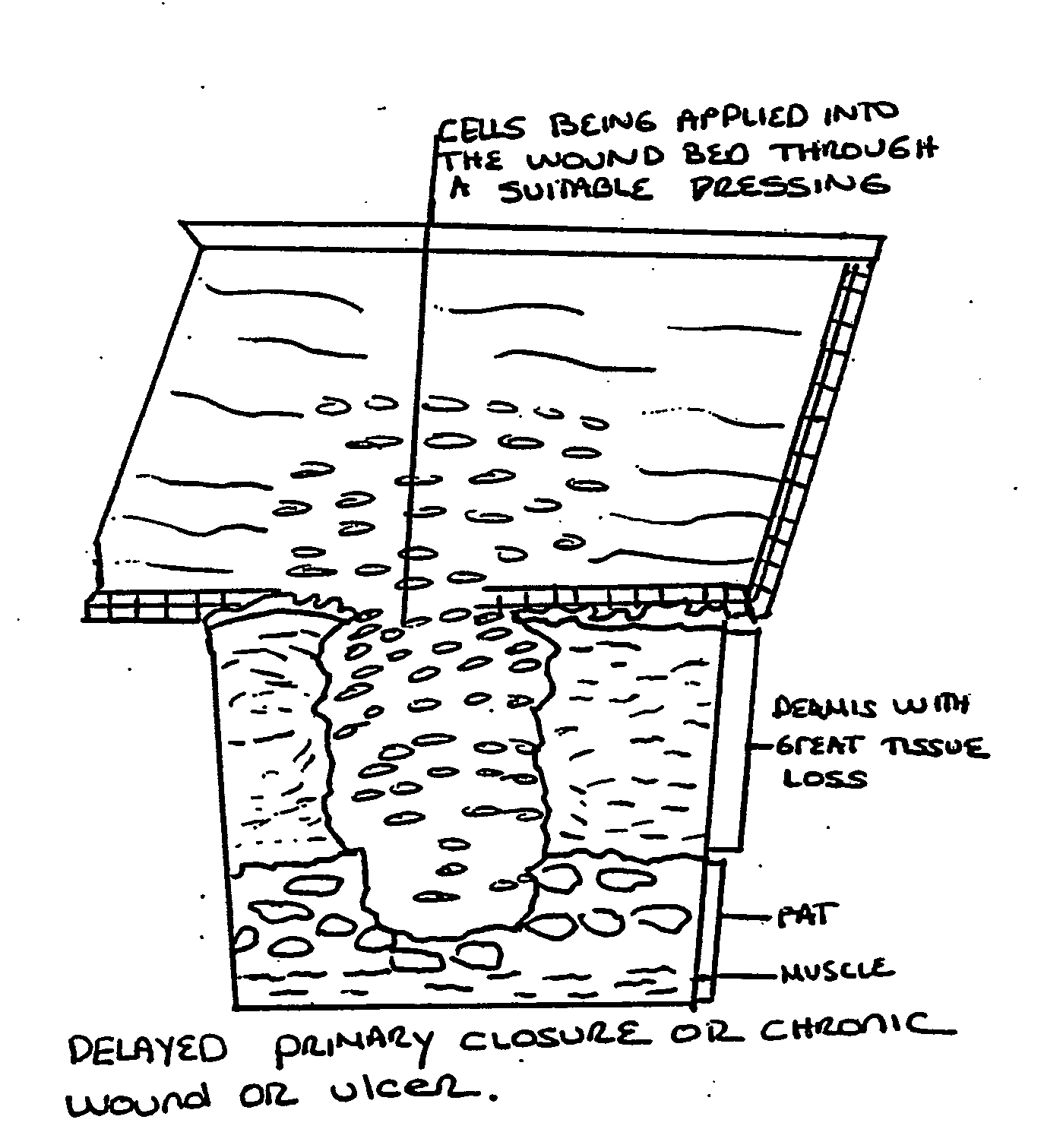
A method for corrective surgery in a human subject of a vocal cord defect amenable to rectification by the augmentation of tissue subadjacent to the vocal cord defect, the method comprising: placing an effective amount of autologous in vitro cultured cells into a vocal cord tissue of the subject in a position subadjacent to the vocal cord defect, wherein the vocal cord tissue is selected from the group consisting of a scar, Reinke's space, a muscle of the vocal cord, and the lamina propria, wherein the in vitro cultured cells are obtained by culturing a plurality of viable cells retrieved from the subject, and wherein the cultured cells are suspended in a collagen or modified collagen solution prior to injection of the cultured cells. The in vitro cultured cells can be fibroblasts, e.g., fibroblasts derived from dermis, fascia, connective tissue, or lamina propria.
Owner:CASTLE CREEK BIOSCIENCES LLC
Augmentation and repair of spincter defects with cells including adipocytic cells
InactiveUS20080299213A2Promote resultsPeptide/protein ingredientsMammal material medical ingredientsLigament structureSurgical department
An embodiment of the invention includes methods for the long-term augmentation and / or repair of skin defects (scars, skin laxness, skin thinning, and skin augmentation), cellulite, breast tissue, wounds and burns, urological and gastroesophageal sphincter structures, hernias, periodontal disease and disorders, tendon and ligament tears and baldness, by the injection or direct surgical placement / implantation of autologous cultured cells and / or cultured cell-produced extracellular matrix that is derived from connective tissue, dermis, fascia, lamina propria, stroma, adipose tissue, muscle, tendon, ligament or the hair follicle. The corrective application is done on tissue proximal or within the area of the defect. The method involves retrieving viable cells from the subject, a neonate or human fetus. Alternatively, the corrective application involves the cells placed in a matrix, preferably comprised of autologous extracellular matrix constituents as a three-dimensional structure or as a suspension, prior to placement into a position with respect to the subject's defect. In a further embodiment, the preferable autologous extracellular matrix constituents are collected from culture and placed in a position with respect to the subject's defect.
Owner:KLEINSEK DONALD +1
Methods, apparatuses, and systems for the treatment of pulmonary disorders
ActiveUS20190231425A1Reduce in quantityElectrotherapySurgical instruments for heatingDiseaseAcute bronchitis
Apparatuses, systems and methods are provided for treating pulmonary tissues via delivery of energy, generally characterized by high voltage pulses, to target tissue using a pulmonary tissue modification system (e.g., an energy delivery catheter system). Example pulmonary tissues include, without limitation, the epithelium (the goblet cells, ciliated pseudostratified columnar epithelial cells, and basal cells), lamina propria, submucosa, submucosal glands, basement membrane, smooth muscle, cartilage, nerves, pathogens resident near or within the tissue, or a combination of any of these. The system may be used to treat a variety of pulmonary diseases or disorders such as or associated with COPD (e.g., chronic bronchitis, emphysema), asthma, interstitial pulmonary fibrosis, cystic fibrosis, bronchiectasis, primary ciliary dyskinesia (PCD), acute bronchitis and / or other pulmonary diseases or disorders.
Owner:GALVANIZE THERAPEUTICS INC
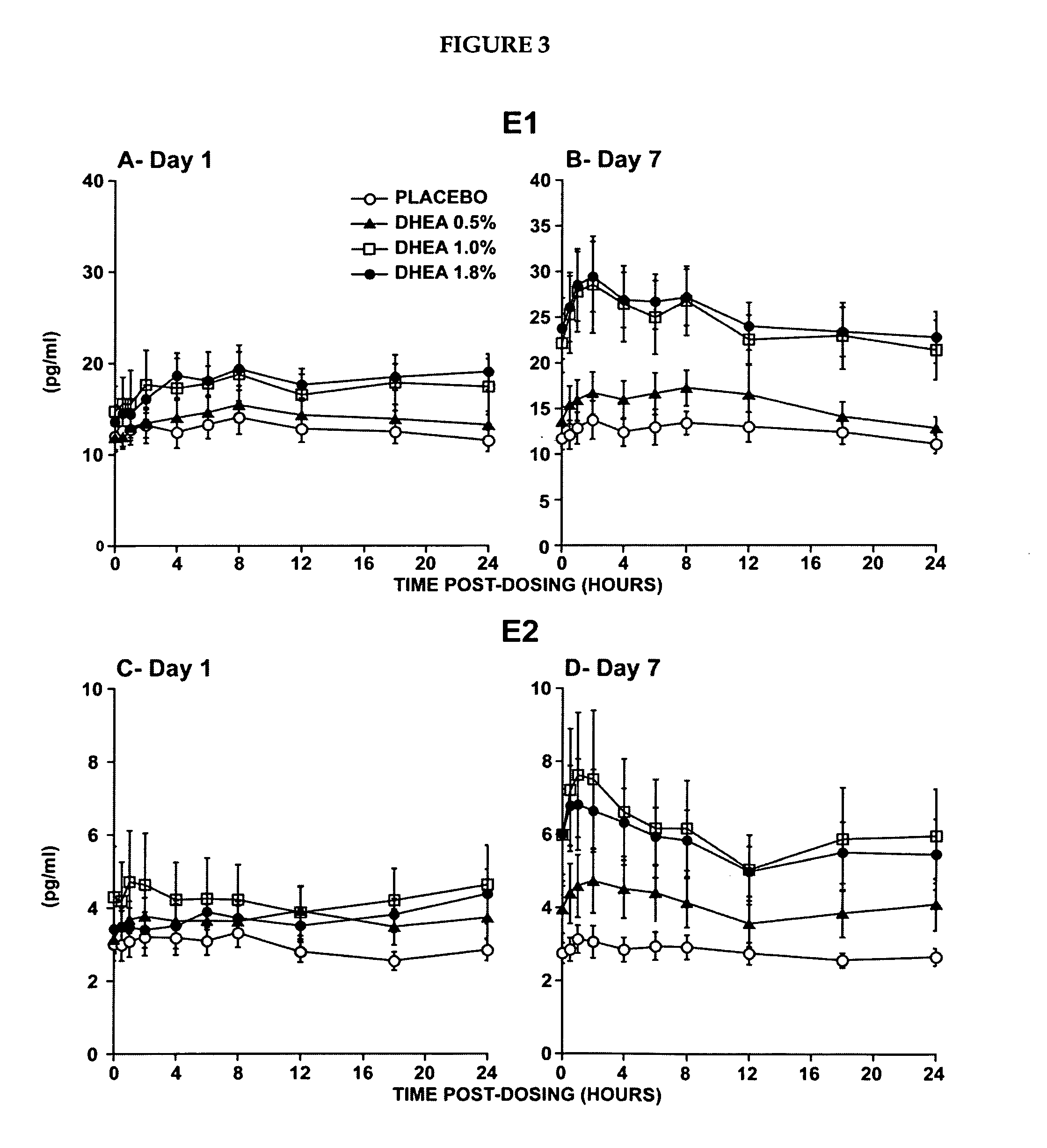
Pharmaceutical compositions
ActiveUS20090054383A1Achieve beneficial effectPrevent adverse side effectsBiocideOrganic active ingredientsDiseaseEstrogenic Effects
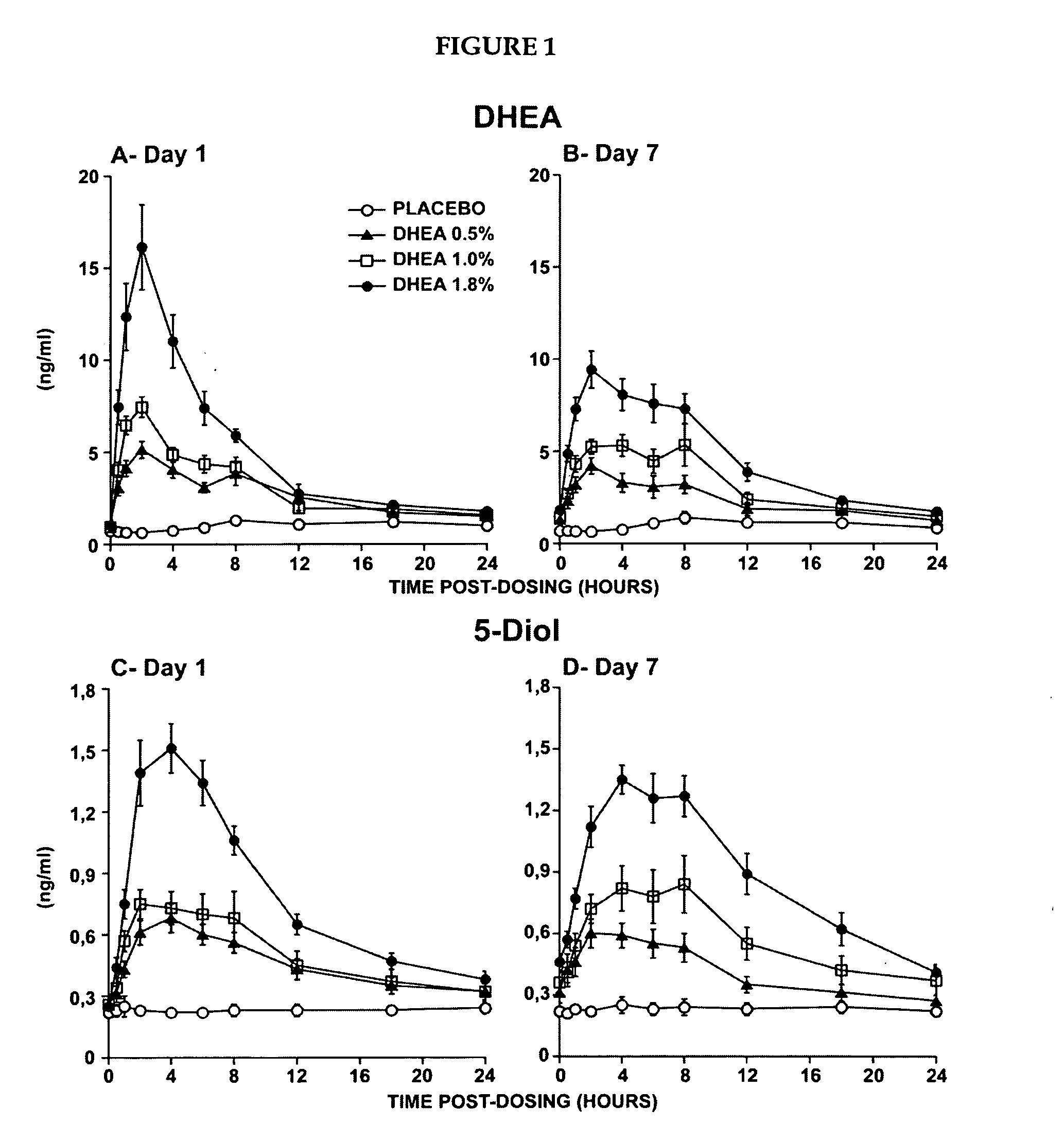
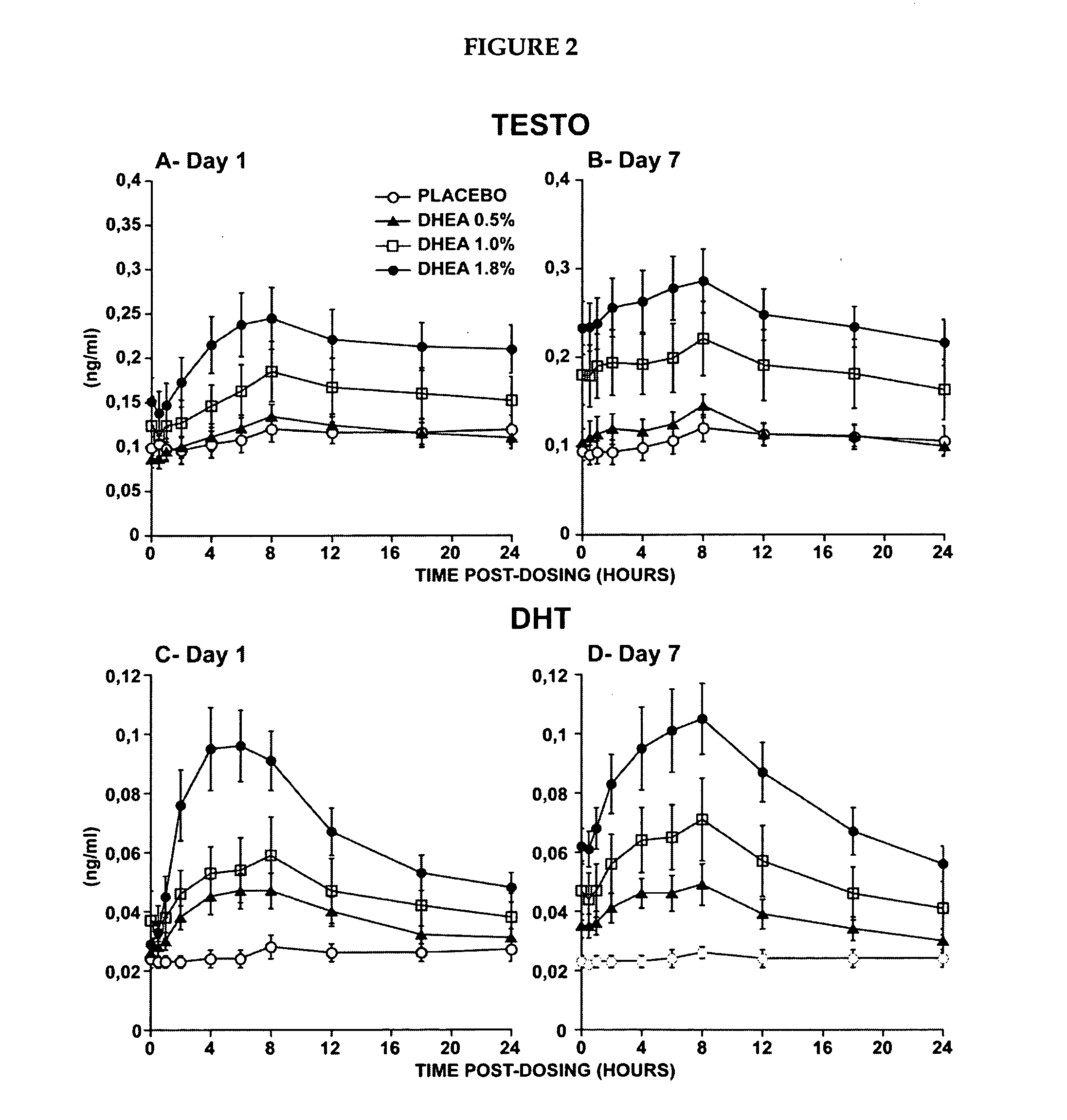
Novel methods for treating or reducing the likelihood of acquiring symptoms or diseases due to the menopause, in postmenopausal women, particularly osteoporosis, vaginal atrophy and dryness, hypogonadism, diminished libido, skin atrophy, connective tissue disease, urinary incontinence, breast, endometrial, ovarian and uterine cancers, hot flashes, loss of muscle mass, insulin resistance, fatigue, loss of energy, aging, physical symptoms of menopause, in susceptible warm-blooded animals including humans involving administration of a sex steroid precursor are disclosed. Said method comprising novel ways of administering and dosing dehydroepiandrosterone (DHEA) in order to take advantage of positive androgenic effects in the vaginal layers lamina propia and / or the layer muscularis, without undesirably causing systemic estrogenic effects in order to avoid the risk of breast and uterine cancer. Pharmaceutical compositions for delivery of active ingredient(s) useful to the invention are also disclosed.
Owner:MYRIEL PHARM LLC
In vitro epithelial models comprising lamina propria-derived cells
ActiveUS20180185844A1Reducing inflammation effecting epithelial regionCell culture mediaLaboratory glasswaresEpitheliumDisease area
An in vitro microfluidic “organ-on-chip” is described herein that mimics the structure and at least one function of specific areas of the epithelial system in vivo. In particular, a multicellular, layered, microfluidic culture is described, allowing for interactions between lamina propria-derived cells and the associated tissue specific epithelial cells and endothelial cells. This in vitro microfluidic system can be used for modeling inflammatory tissue, e.g., autoimmune disorders involving epithelia and diseases involving epithelial layers. These multicellular, layered microfluidic “organ-on-chip”, e.g. “epithelia-on-chip” further allow for comparisons between types of epithelia tissues, e.g., lung (Lung-On-Chip), bronchial (Airway-On-Chip), skin (Skin-On-Chip), cervix (Cervix-On-Chip), blood brain barrier (BBB-On-Chip), etc., in additional to neurovascular tissue, (Brain-On-Chip), and between different disease states of tissue, i.e. healthy, pre-disease and diseased areas. Additionally, these microfluidic “organ-on-chips” allow identification of cells and cellular derived factors driving disease states in addition to drug testing for reducing inflammation effecting epithelial regions.
Owner:EMULATE INC
Methods of preparing olfactory ensheathing cells for transplantation
InactiveUS20020127716A1Efficient purificationBiocideCell dissociation methodsSurgical biopsyLamina propria
A method is described for isolating ensheathing cells, in particular those from olfactory lamina propria and use of the isolated ensheathing cells and lamina propria respectively in transplantation. Isolated lamina propria and ensheathing cells from the olfactory mucosa are well suited for autologous transplantation, where the donor and recipient are the same, as surgical biopsy of the olfactory mucosa is less damaging than isolating tissue from other location of a person's body, for example the olfactory bulb. Transplantation is particularly directed to neural regions (for example the brain, spinal cord and / or peripheral nerves) of a human to assist recovery of acute and chronic nerve damage following surgery or trauma.
Owner:GRIFFITH UNIVERSITY
Augmentation and repair of spincter defects with cells including mesenchymal cells
InactiveUS20080152628A1Promote resultsBiocidePeptide/protein ingredientsLigament structureGastroesophageal sphincter
An embodiment of the invention includes methods for the long-term augmentation and / or repair of skin defects (scars, skin laxness, skin thinning, and skin augmentation), cellulite, breast tissue, wounds and burns, urological and gastroesophageal sphincter structures, hernias, periodontal disease and disorders, tendon and ligament tears and baldness, by the injection or direct surgical placement / implantation of autologous cultured cells and / or cultured cell-produced extracellular matrix that is derived from connective tissue, dermis, fascia, lamina propria, stroma, adipose tissue, muscle, tendon, ligament or the hair follicle. The corrective application is done on tissue proximal or within the area of the defect. The method involves retrieving viable cells from the subject, a neonate or human fetus. Alternatively, the corrective application involves the cells placed in a matrix, preferably comprised of autologous extracellular matrix constituents as a three-dimensional structure or as a suspension, prior to placement into a position with respect to the subject's defect. In a further embodiment, the preferable autologous extracellular matrix constituents are collected from culture and placed in a position with respect to the subject's defect.
Owner:KLEINSEK DONALD +1
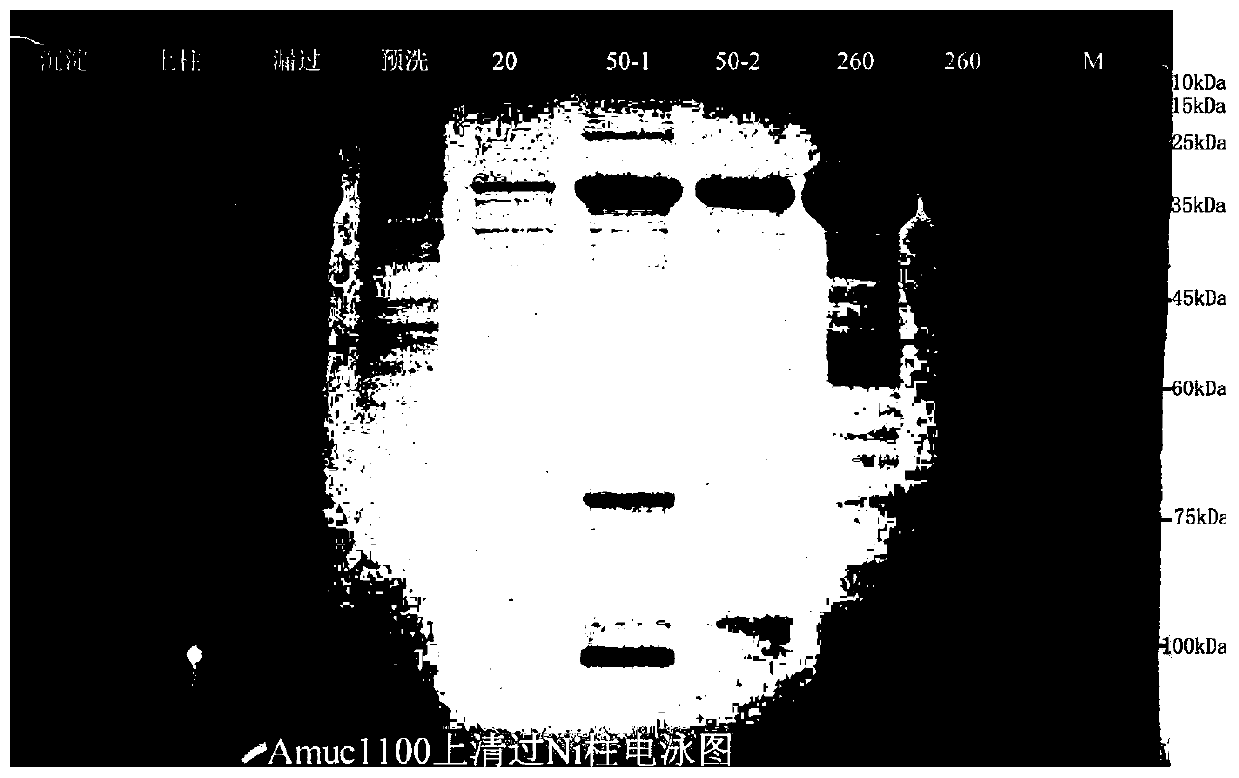
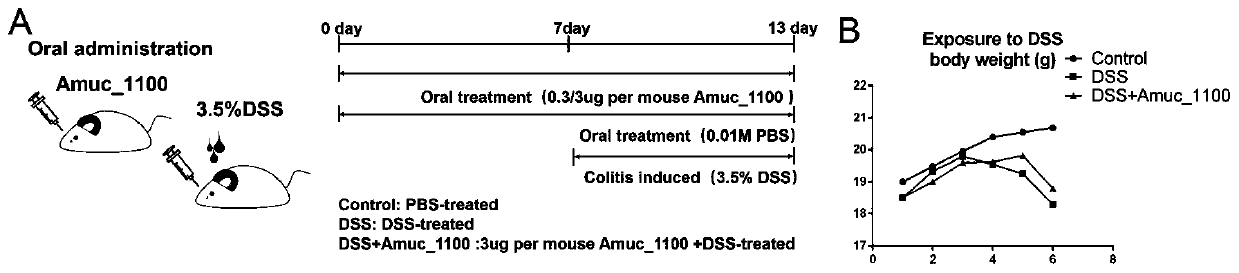
Recombinantly-expressed Akkermansia membrane protein Amuc_1100 and application thereof
ActiveCN110698547ARegulate immune responseStimulate immune responseBacteriaPeptide/protein ingredientsLamina propriaColonic lamina propria
The invention discloses recombinantly-expressed Akkermansia membrane protein Amuc_1100 and an application thereof. The invention recombinantly expresses the Akkermansia membrane protein Amuc_1100, through animal experiments, we find that oral administration of the Akkermansia membrane protein Amuc_1100 can significantly improve the body weight of mice, increase colon length and crypt depth, and activate proliferation of intestinal stem cells to protect mice from colitis damage. Isolation of lymphocytes from the lamina propria of the colon shows that the proportion of Treg cells is significantly up-regulated and that of Th17 cells is significantly down-regulated, proving that Amuc_1100 can restore colon damage by adjusting the proportion of immune cells Treg / Th17. Therefore, the protein isexpected to be developed into a protein product for preventing or treating colitis.
Owner:NANJING AGRICULTURAL UNIVERSITY
Separation, purification and primary culture methods of intestinal macrophages in Ctenopharyngodon idella
ActiveCN107523542AEasy to separateEasy to operateCell dissociation methodsCulture processIntestinal structureFeces
The invention relates to separation, purification and primary culture methods of intestinal macrophages in Ctenopharyngodon idella. The separation method comprises: taking out the rear middle segment of an intestine by aseptic operation, removing intestinal outer fat and mesentery, longitudinally cutting the intestine to clear excrement, and using aseptic bent-tip tweezers to scrape off the intestinal mucus and epithelial cell layer for 15 min; cutting the segment with the mucus and epithelial cell layer removed to obtain fragments, and digesting the fragments in collagenase IV digestive juice to obtain lamina propria single-cell suspension; using a separation kit of fish organ monocytes to separate the intestinal macrophages. The purification method comprises: using a differential wall attachment method to purify the intestinal macrophages. The primary culture method comprises: using RPMI (Roswell Park Memorial Institute medium) 1640 complete culture solution containing autoserum of Ctenopharyngodon idella to culture the intestinal macrophages, changing the solution once with lipopolysaccharide-containing RPMI 1640 complete culture solution after cells attach to the wall, and changing the solution once every other two days so that cells may survive for at least 20 days. The separation, purification and primary culture methods established herein have good operability and repeatability.
Owner:ANHUI AGRICULTURAL UNIVERSITY
Augmentation and repair of spincter defects with cells including mesenchymal cells
InactiveUS20080286242A2Promote resultsBiocidePeptide/protein ingredientsLigament structureSurgical department
An embodiment of the invention includes methods for the long-term augmentation and / or repair of skin defects (scars, skin laxness, skin thinning, and skin augmentation), cellulite, breast tissue, wounds and burns, urological and gastroesophageal sphincter structures, hernias, periodontal disease and disorders, tendon and ligament tears and baldness, by the injection or direct surgical placement / implantation of autologous cultured cells and / or cultured cell-produced extracellular matrix that is derived from connective tissue, dermis, fascia, lamina propria, stroma, adipose tissue, muscle, tendon, ligament or the hair follicle. The corrective application is done on tissue proximal or within the area of the defect. The method involves retrieving viable cells from the subject, a neonate or human fetus. Alternatively, the corrective application involves the cells placed in a matrix, preferably comprised of autologous extracellular matrix constituents as a three-dimensional structure or as a suspension, prior to placement into a position with respect to the subject's defect. In a further embodiment, the preferable autologous extracellular matrix constituents are collected from culture and placed in a position with respect to the subject's defect.
Owner:KLEINSEK DONALD +1
In vitro gastrointestinal model comprising lamina propria-derived cells
ActiveUS20180224432A1Reduce inflammationAccurate modelingGastrointestinal cellsLaboratory glasswaresDisease areaLamina propria
An in vitro microfluidic gut-on-chip is described herein that mimics the structure and at least one function of specific areas of the gastrointestinal system in vivo. In particular, a multicellular, layered, microfluidic culture is described, allowing for interactions between lamina propria-derived cells and gastrointestinal epithelial cells and endothelial cells. This in vitro microfluidic system can be used for modeling inflammatory gastrointestinal tissue, e.g., Crohn's disease, colitis and other inflammatory gastrointestinal disorders. These multicellular, layered microfluidic gut-on-chip further allow for comparisons between types of gastrointestinal tissues, e.g., small intestinal deuodejeum, small intestinal ileium, large intestinal colon, etc., and between disease states of gastrointestinal tissue, i.e. healthy, pre-disease and diseased areas. Additionally, these microfluidic gut-on-chips allow identification of cells and cellular derived factors driving disease states and drug testing for reducing inflammation.
Owner:EMULATE INC
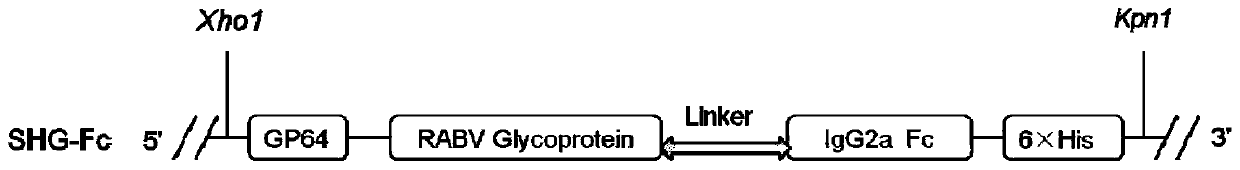
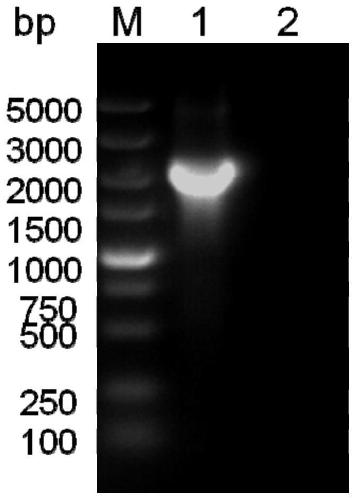
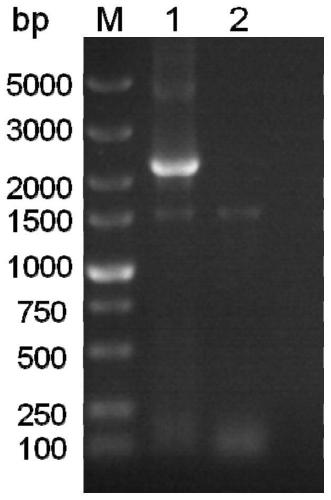
Fusion protein of rabies virus G protein expressing Fc fragment and preparation method thereof
Owner:SOUTH CHINA AGRI UNIV
Hair undifferentiated cells
InactiveUS20080118478A1Promote resultsBiocidePeptide/protein ingredientsCell-Extracellular MatrixLamina propria
An embodiment of the invention includes methods for the long-term augmentation and / or repair of skin defects (scars, skin laxness, skin thinning, and skin augmentation), cellulite, breast tissue, wounds and burns, urological and gastroesophageal sphincter structures, hernias, periodontal disease and disorders, tendon and ligament tears and baldness, by the injection or direct surgical placement / implantation of autologous cultured cells and / or cultured cell-produced extracellular matrix that is derived from connective tissue, dermis, fascia, lamina propria, stroma, adipose tissue, muscle, tendon, ligament or the hair follicle. The corrective application is done on tissue proximal or within the area of the defect. The method involves retrieving viable cells from the subject, a neonate or human fetus. Alternatively, the corrective application involves the cells placed in a matrix, preferably comprised of autologous extracellular matrix constituents as a three-dimensional structure or as a suspension, prior to placement into a position with respect to the subject's defect. In a further embodiment, the preferable autologous extracellular matrix constituents are collected from culture and placed in a position with respect to the subject's defect.
Owner:KLEINSEK DONALD +1
Augmentation and repair of sphincter defects with cells including muscle cells
An embodiment of the invention includes methods for the long-term augmentation and / or repair of skin defects (scars, skin laxness, skin thinning, and skin augmentation), cellulite, breast tissue, wounds and burns, urological and gastroesophageal sphincter structures, hernias, periodontal disease and disorders, tendon and ligament tears and baldness, by the injection or direct surgical placement / implantation of autologous cultured cells and / or cultured cell-produced extracellular matrix that is derived from connective tissue, dermis, fascia, lamina propria, stroma, adipose tissue, muscle, tendon, ligament or the hair follicle. The corrective application is done on tissue proximal or within the area of the defect. The method involves retrieving viable cells from the subject, a neonate or human fetus. Alternatively, the corrective application involves the cells placed in a matrix, preferably comprised of autologous extracellular matrix constituents as a three-dimensional structure or as a suspension, prior to placement into a position with respect to the subject's defect. In a further embodiment, the preferable autologous extracellular matrix constituents are collected from culture and placed in a position with respect to the subjects defect.
Owner:GERIGENE MEDICAL CORP
Separation and purification method of mouse intestinal epithelium mucosa lamina propria dendritic cells
InactiveCN102382802AEfficient removalThe experiment process is simpleBlood/immune system cellsPurification methodsDendritic cell
The invention relates to a separation and purification method of mouse intestinal epithelium mucosa lamina propria dendritic cells, which comprises the following specific steps: (1) the acquisition of mixed cells; (2) the acquisition of individual karyocytes: preparing cells acquired after the digestion of collagenase into a suspension, slowly adding into a mouse lymphocyte separation liquid according to a volume ratio of 1:1, and centrifuging at 1800 rpm for 25 minutes; sucking the middle buffy coats, adding into 1-2 milliliters of MACS buffer, and centrifuging at 1200 rpm for 5 minutes; and removing the supernate, and suspending the cells again, wherein the MACS buffer is a PBS (phosphate buffer solution) containing 2mM mol / milliliter EDTA (ethylene diamine tetraacetic acid) and 0.5% FCS (fetal calf serum); and (3) the acquisition of intestinal epithelium mucosa lamina propria dendritic cells. By using the separation and purification method, a large number of high-purity and high-activity intestinal epithelium mucosa lamina propria dendritic cells can be conveniently, economically and efficiently acquired in short time; and the acquired cells keep a good antigen presentation capability under in vitro conditions, thereby providing favorable conditions for the in vitro simulation of in vivo antigen presenting cell induced lymphocyte differentiation.
Owner:梁廷波
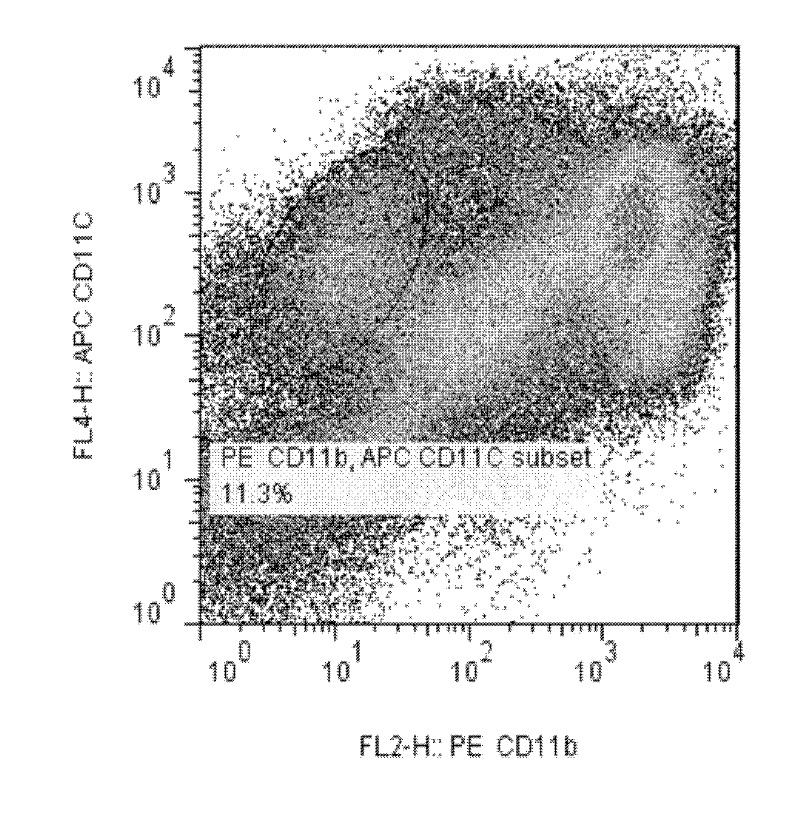
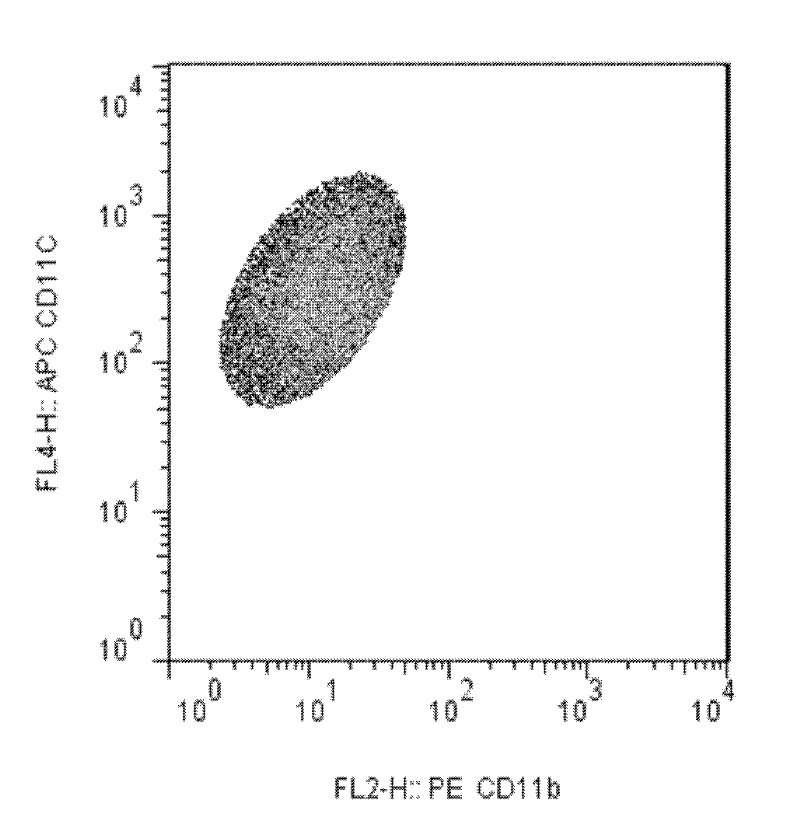
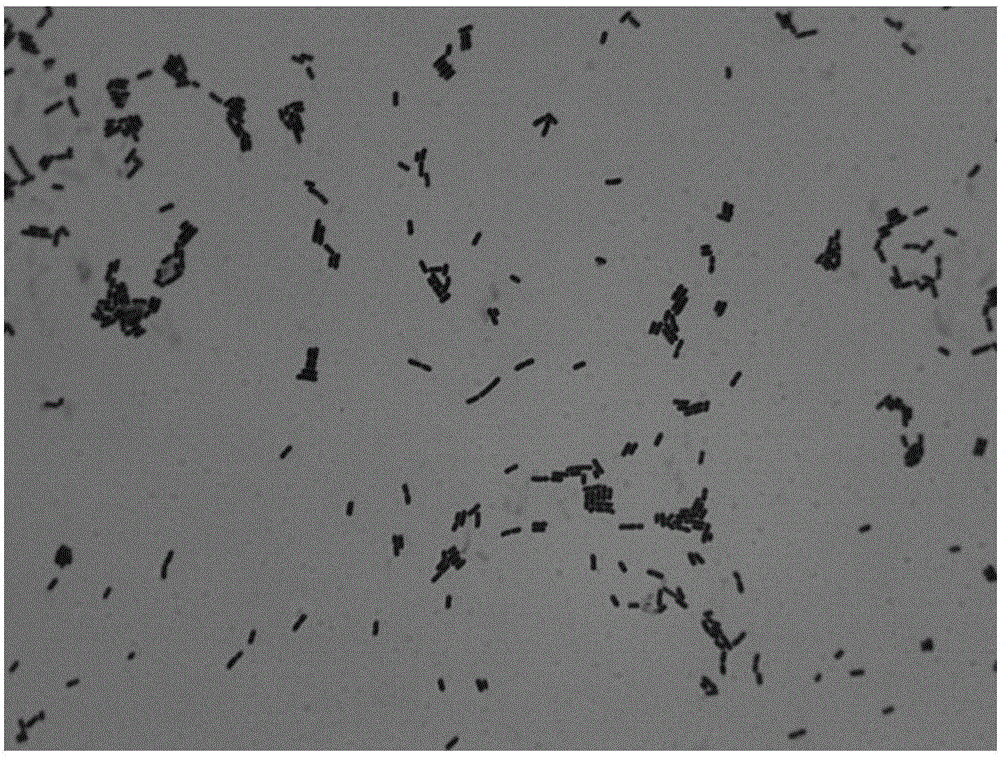
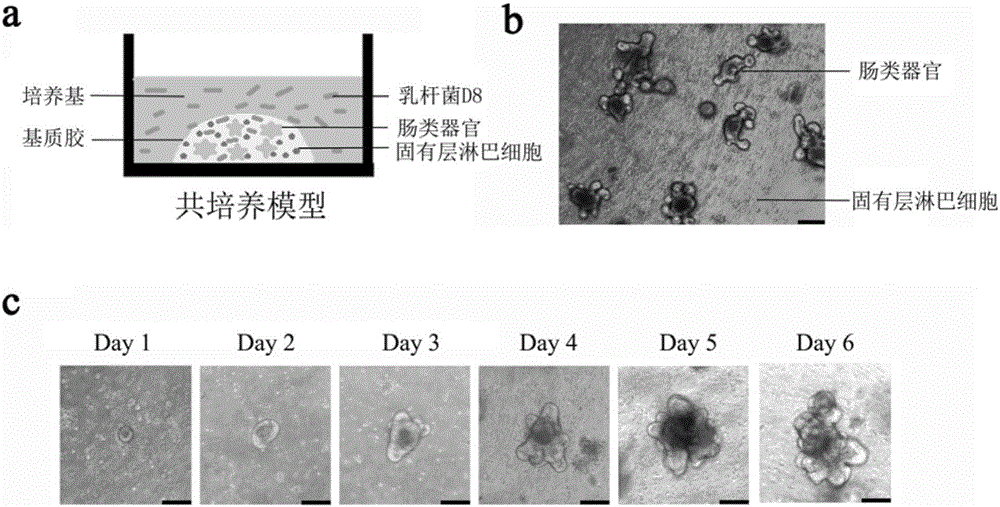
Lactobacillus D8 and application thereof
ActiveCN106497854AIncrease the lengthAdd depthBacteriaGastrointestinal cellsLamina propriaAnimal testing
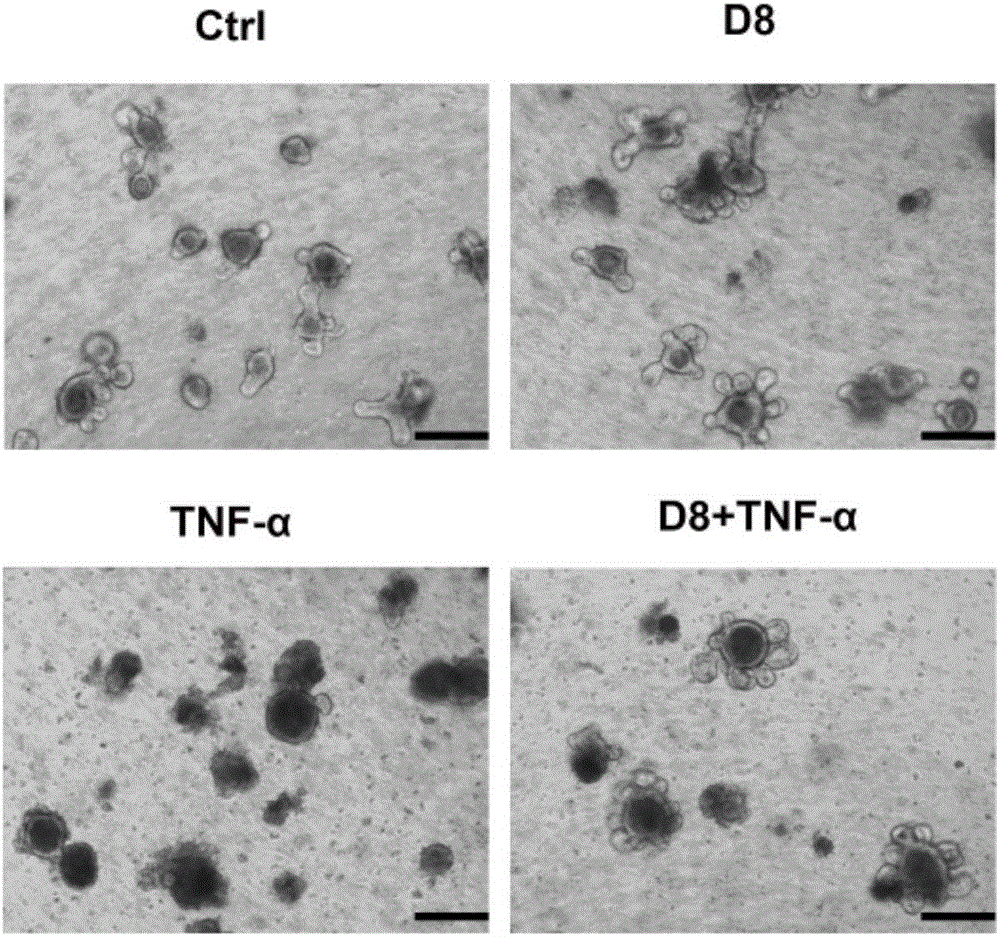
The invention discloses lactobacillus D8 and application thereof. The lactobacillus D8 disclosed by the invention is separated from healthy pigs, the classification name is Lactobacillus sp., and the preservation number is CGMCC No.13112. Construction of an in-vitro lactobacillus-intestinal tract organ-lamina propria lymphocyte co-culture model shows that proliferation and differentiation of intestinal stem cells can be effectively promoted by using the lactobacillus D8, and furthermore completeness of an intestinal mucosa barrier can be maintained. Further animal tests show that the length of intestinal villi and depth of small intestinal crypts can be remarkably improved with orally taken lactobacillus D8, and enteritis symptoms can be alleviated. Therefore, the strain is expected to be developed as probiotics for preventing and / or treating enteritis.
Owner:NANJING AGRICULTURAL UNIVERSITY
Detection model for mouse intestinal mucosa epithelial barrier function and intestinal mucosa immune tolerance cell
InactiveCN107385008AHas immunomodulatory functionUnraveling the intrinsic mechanism of induction of immune tolerancePeptide/protein ingredientsMicrobiological testing/measurementIntestinal structureIntraperitoneal route
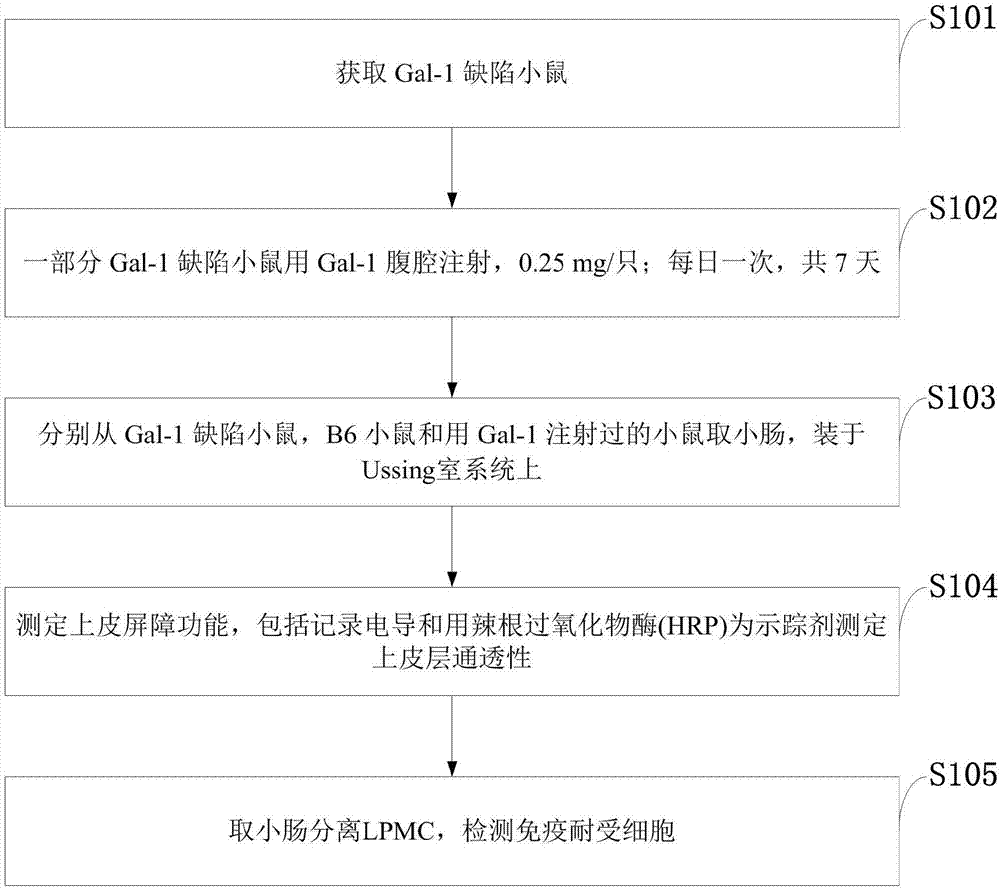
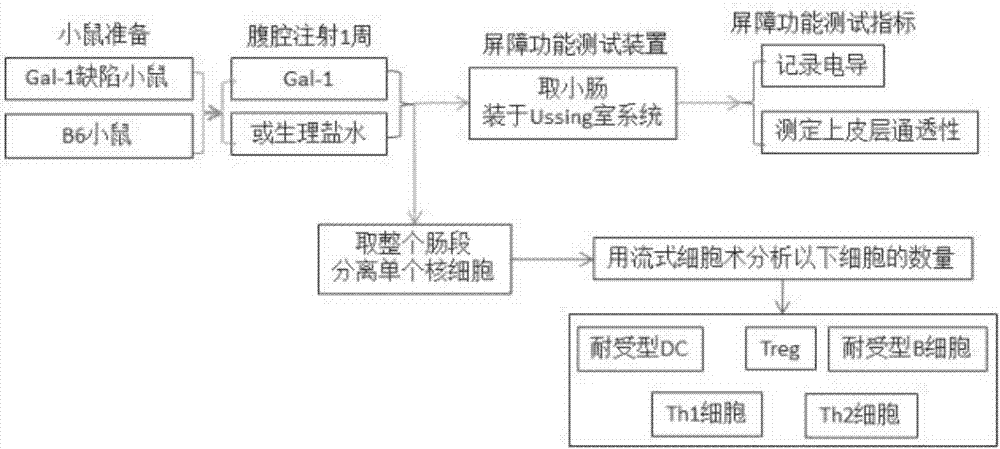
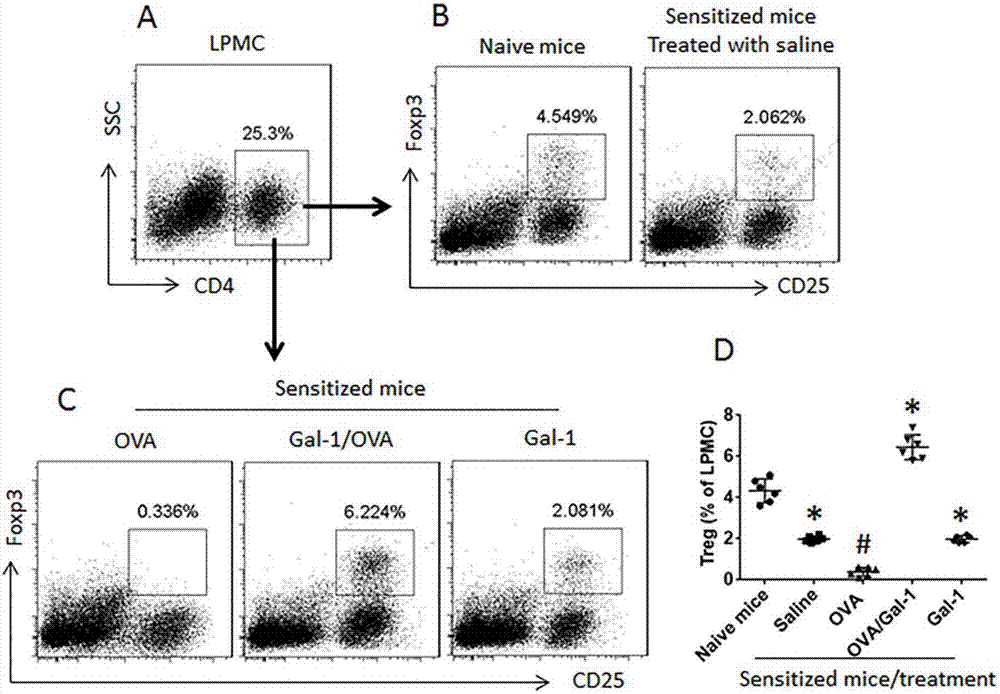
The invention belongs to the technical field of body immunity, and discloses a detection model for a mouse intestinal mucosa epithelial barrier function and an intestinal mucosa immune tolerance cell. The model is characterized in that one part of Gal-1 deficient mice is subjected to Gal-1 intraperitoneal injection, and each mouse is injected with 0.25mg each time per day, and injection is carried out for 7 days; small intestines are respectively taken from the Gal-1 deficient mice, B6 mice and mice injected with Gal-1. The method comprises the following steps: obtaining the Gal-1 deficient mice, wherein the Gal-1 deficient mice are subjected to the Gal-1 intraperitoneal injection; respectively taking the intestines from the Gal-1 deficient mice, the B6 mice and the mice injected with Gal-1, and installing in a Ussing chamber system; measuring an epithelial barrier function; taking the small intestines to separate LPMC (Lamina Propria Mononuclear Cells), and detecting immune tolerance cells. The Gal-1 has an immunoregulation function, the intestinal mucosa is an important organ for forming oral tolerance, and the IEC (Intestinal Epithelial cCells) has an immune tolerance function.
Owner:SHENZHEN UNIV
Augmentation and repair of spincter defects with cells including fibroblasts
InactiveUS20080112935A1Promote resultsBiocidePeptide/protein ingredientsLigament structureSurgical department
An embodiment of the invention includes methods for the long-term augmentation and / or repair of skin defects (scars, skin laxness, skin thinning, and skin augmentation), cellulite, breast tissue, wounds and burns, urological and gastroesophageal sphincter structures, hernias, periodontal disease and disorders, tendon and ligament tears and baldness, by the injection or direct surgical placement / implantation of autologous cultured cells and / or cultured cell-produced extracellular matrix that is derived from connective tissue, dermis, fascia, lamina propria, stroma, adipose tissue, muscle, tendon, ligament or the hair follicle. The corrective application is done on tissue proximal or within the area of the defect. The method involves retrieving viable cells from the subject, a neonate or human fetus. Alternatively, the corrective application involves the cells placed in a matrix, preferably comprised of autologous extracellular matrix constituents as a three-dimensional structure or as a suspension, prior to placement into a position with respect to the subject's defect. In a further embodiment, the preferable autologous extracellular matrix constituents are collected from culture and placed in a position with respect to the subject's defect.
Owner:KLEINSEK DONALD +1
Compositions, formulations and interleukin production and purification
ActiveUS20210154304A1Construction stabilityPeptide/protein ingredientsAntipyreticDimerTherapeutic protein
Described herein are cholix-IL-10 fusion proteins, and methods of use thereof, which can be characterized by a distinct response in an individual when administered. This distinct response can comprise changes in levels of one or more markers in the individual and / or co-localization of IL-10 in the lamina propria of the individual. Further described herein, in some embodiments, are oral formulations of the cholix-IL-10 fusion proteins. Described herein are methods for the purification of an IL-10 delivery construct, including methods for refolding and enrichment, which can result in maintenance of a high percentage of the IL-10 delivery constructs in the biologically active dimer form. Described herein are oral formulations configured for site-specific release of a therapeutic protein in the small intestines or colon. In some cases, the therapeutic protein is in the form of a dimer, such as an IL-10 delivery construct capable of crossing the gut epithelium.
Owner:APPLIED MOLECULAR TRANSPORT INC
In vitro gastrointestinal model comprising lamina propria-derived cells
ActiveUS20180230417A1Reduce inflammationAccurate modelingGastrointestinal cellsApparatus sterilizationDisease areaLamina propria
An in vitro microfluidic gut-on-chip is described herein that mimics the structure and at least one function of specific areas of the gastrointestinal system in vivo. In particular, a multicellular, layered, microfluidic culture is described, allowing for interactions between lamina propria-derived cells and gastrointestinal epithelial cells and endothelial cells. This in vitro microfluidic system can be used for modeling inflammatory gastrointestinal tissue, e.g., Crohn's disease, colitis and other inflammatory gastrointestinal disorders. These multicellular, layered microfluidic gut-on-chip further allow for comparisons between types of gastrointestinal tissues, e.g., small intestinal deuodejeum, small intestinal ileium, large intestinal colon, etc., and between disease states of gastrointestinal tissue, i.e. healthy, pre-disease and diseased areas. Additionally, these microfluidic gut-on-chips allow identification of cells and cellular derived factors driving disease states and drug testing for reducing inflammation.
Owner:EMULATE INC
Hydrogels for vocal cord and soft tissue augmentation and repair
Owner:MASSACHUSETTS INST OF TECH
Hair mesenchymal cells
InactiveUS20090016996A2Promote resultsBiocidePeptide/protein ingredientsLigament structureSurgical department
An embodiment of the invention includes methods for the long-term augmentation and / or repair of skin defects (scars, skin laxness, skin thinning, and skin augmentation), cellulite, breast tissue, wounds and burns, urological and gastroesophageal sphincter structures, hernias, periodontal disease and disorders, tendon and ligament tears and baldness, by the injection or direct surgical placement / implantation of autologous cultured cells and / or cultured cell-produced extracellular matrix that is derived from connective tissue, dermis, fascia, lamina propria, stroma, adipose tissue, muscle, tendon, ligament or the hair follicle. The corrective application is done on tissue proximal or within the area of the defect. The method involves retrieving viable cells from the subject, a neonate or human fetus. Alternatively, the corrective application involves the cells placed in a matrix, preferably comprised of autologous extracellular matrix constituents as a three-dimensional structure or as a suspension, prior to placement into a position with respect to the subject's defect. In a further embodiment, the preferable autologous extracellular matrix constituents are collected from culture and placed in a position with respect to the subject's defect.
Owner:KLEINSEK DONALD A +1
In vitro epithelial models comprising lamina propria-derived cells
ActiveUS10828638B2Bioreactor/fermenter combinationsBiological substance pretreatmentsDisease areaLamina propria
An in vitro microfluidic “organ-on-chip” is described herein that mimics the structure and at least one function of specific areas of the epithelial system in vivo. In particular, a multicellular, layered, microfluidic culture is described, allowing for interactions between lamina propria-derived cells and the associated tissue specific epithelial cells and endothelial cells. This in vitro microfluidic system can be used for modeling inflammatory tissue, e.g., autoimmune disorders involving epithelia and diseases involving epithelial layers. These multicellular, layered microfluidic “organ-on-chip”, e.g. “epithelia-on-chip” further allow for comparisons between types of epithelia tissues, e.g., lung (Lung-On-Chip), bronchial (Airway-On-Chip), skin (Skin-On-Chip), cervix (Cervix-On-Chip), blood brain barrier (BBB-On-Chip), etc., in additional to neurovascular tissue, (Brain-On-Chip), and between different disease states of tissue, i.e. healthy, pre-disease and diseased areas. Additionally, these microfluidic “organ-on-chips” allow identification of cells and cellular derived factors driving disease states in addition to drug testing for reducing inflammation effecting epithelial regions.
Owner:EMULATE INC
In-vitro culture method of vaginal epithelial cells of mouse
InactiveCN105441380AEasy to operateCell dissociation methodsArtificial cell constructsLamina propriaPhosphate
The invention relates to an in-vitro culture method of vaginal epithelial cells of a mouse and belongs to the technical field of cell culture. According to the method, isolated vaginal tissue of the mouse is sheared into pieces in the size of 1 cm*0.5 cm, washed and subjected to overnight culture through Dispase II, the vaginal tissue subjected to overnight culture is separated, lamina propria tissue is discarded, epithelial layer tissue is mixed with 0.25% pancreatin and 0.02% EDTA (ethylene diamine tetraacetic acid) and digested, finally, digestion is stopped through DMEM (Dulbecco modified Eagle medium) / F12 containing 10% fetal calf serum, obtained cell suspension is filtered, centrifugalized, re-suspended with a PBS (phosphate buffer solution) and EPILIFE sequentially and put into a 5% CO2 culture box with the temperature of 37 DEG C to be cultured, and a solution is replaced for the first time after two days and is replaced once every two days later. The method is convenient to operate, a large quantity of cells are obtained, the purity is high, the activity is high, the cells can be subcultured for 4-5 generations stably, and seed cells can be provided for observation of physiological and pathological change and the cancerization process as well as establishment of tissue-engineered vaginas of vaginal epithelium.
Owner:祁文瑾
In vitro gastrointestinal model comprising lamina propria-derived cells
ActiveUS11001795B2Reduce inflammationAccurate modelingGastrointestinal cellsApparatus sterilizationDisease areaLamina propria
An in vitro microfluidic gut-on-chip is described herein that mimics the structure and at least one function of specific areas of the gastrointestinal system in vivo. In particular, a multicellular, layered, microfluidic culture is described, allowing for interactions between lamina propria-derived cells and gastrointestinal epithelial cells and endothelial cells. This in vitro microfluidic system can be used for modeling inflammatory gastrointestinal tissue, e.g., Crohn's disease, colitis and other inflammatory gastrointestinal disorders. These multicellular, layered microfluidic gut-on-chip further allow for comparisons between types of gastrointestinal tissues, e.g., small intestinal deuodejeum, small intestinal ileium, large intestinal colon, etc., and between disease states of gastrointestinal tissue, i.e. healthy, pre-disease and diseased areas. Additionally, these microfluidic gut-on-chips allow identification of cells and cellular derived factors driving disease states and drug testing for reducing inflammation.
Owner:EMULATE INC
Nutritional prophylactic for turbot enteritis
ActiveCN109331028AIncrease heightReduce adhesionClimate change adaptationDigestive systemLamina propriaPhysiology
The invention provides a nutritional prophylactic for turbot enteritis. The nutritional prophylactic contains 20%-30% by mass of mannan oligosaccharide and 70%-80% by mass of sodium butyrate. The invention further provides an application of the nutritional prophylactic to preparation of turbot feed. The nutritional prophylactic for turbot enteritis is used before feeding with feed containing turbot enteritis-inducing anti-nutritional factors and plant protein sources, and can remarkably reduce occurrence of turbot enteritis. Specifically, the nutritional prophylactic can remarkably increase intestinal villus height, reduce intestinal villus adhesion, reduce infiltration of inflammatory cells on lamina propria and submucosa and increase vacuoles absorbed on epithelia cell nuclei. Compared with antibiotics for treatment, the nutritional prophylactic for turbot enteritis is safer and more reliable.
Owner:SHANDONG UNIV
Augmentation and repair of spincter defects with cells including adipocytic cells
InactiveUS20080152721A1Promote resultsPeptide/protein ingredientsMammal material medical ingredientsLigament structureGastroesophageal sphincter
An embodiment of the invention includes methods for the long-term augmentation and / or repair of skin defects (scars, skin laxness, skin thinning, and skin augmentation), cellulite, breast tissue, wounds and burns, urological and gastroesophageal sphincter structures, hernias, periodontal disease and disorders, tendon and ligament tears and baldness, by the injection or direct surgical placement / implantation of autologous cultured cells and / or cultured cell-produced extracellular matrix that is derived from connective tissue, dermis, fascia, lamina propria, stroma, adipose tissue, muscle, tendon, ligament or the hair follicle. The corrective application is done on tissue proximal or within the area of the defect. The method involves retrieving viable cells from the subject, a neonate or human fetus. Alternatively, the corrective application involves the cells placed in a matrix, preferably comprised of autologous extracellular matrix constituents as a three-dimensional structure or as a suspension, prior to placement into a position with respect to the subject's defect. In a further embodiment, the preferable autologous extracellular matrix constituents are collected from culture and placed in a position with respect to the subject's defect.
Owner:KLEINSEK DONALD +1
Methods, apparatuses, and systems for the treatment of disease states and disorders
Apparatuses, systems and methods are provided for treating pulmonary tissues via delivery of energy, generally characterized by high voltage pulses, to target tissue using a pulmonary tissue modification system (e.g., an energy delivery catheter system). Example pulmonary tissues include, without limitation, the epithelium (the goblet cells, ciliated pseudostratified columnar epithelial cells, andbasal cells), lamina propria, submucosa, submucosal glands, basement membrane, smooth muscle, cartilage, nerves, pathogens resident near or within the tissue, or a combination of any of these. The system may be used to treat a variety of pulmonary diseases or disorders such as or associated with COPD (e.g., chronic bronchitis, emphysema), asthma, interstitial pulmonary fibrosis, cystic fibrosis,bronchiectasis, primary ciliary dyskinesia (PCD), acute bronchitis and / or other pulmonary diseases or disorders.
Owner:GALVANIZE THERAPEUTICS INC
Human oral mucosa model and preparation method thereof
InactiveCN109880792AMeet the needs of biological testingArtificial cell constructsSkeletal/connective tissue cellsLamina propriaActive protein
The invention belongs to the technical field of tissue engineering and particularly relates to a human oral mucosa model and a preparation method thereof. The mucosa model comprises a basal layer anda lamina propria, also comprise an epithelial layer and accordingly is highly approximate to the epithelial tissue structure of the human oral mucosa. The model can be applied to safety and efficacy detection of oral care products, small molecular compounds, active proteins, medical devices, chemicals, disposable sanitary products and other products and safety and efficacy evaluation of other materials in contact with the oral mucosa, and the obtained detection data and evaluation results are closer to the evaluation results of the oral mucosa.
Owner:广州舒客实业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com